Abstract
Mitochondrial dynamics, fission and fusion, were first identified in yeast with investigation in heart cells beginning only in the last 5–7 years. In the ensuing time it has become evident that these processes are not only required for healthy mitochondria, but also, that derangement of these processes contributes to disease. The fission and fusion proteins have a number of functions beyond the mitochondrial dynamics. Many of these functions are related to their membrane activities, such as apoptosis. However, other functions involve other areas of the mitochondria, such as OPA1’s role in maintaining cristae structure and preventing cytochrome c leak, and its essential (at least a 10 kDa fragment of OPA1) role in mtDNA replication. In heart disease, changes in expression of these important proteins can have detrimental effects on mitochondrial and cellular function.
Introduction
Overview, History of Mitochondrial Research
Mitochondria perpetually provide fuel to feed the great energy needs of the heart, which is an elegant, self-repairing pump, which functions nonstop for many, many decades. Altman was the first to note the ubiquitousness of the mitochondria, which after viewing under a rudimentary light microscope, he termed bioblasts. He concluded that the bioblasts performed essential function (s), and even considered that they might be small organisms taken up by other organisms (3). Michaelis was the first to associate redox changes with mitochondria, but did not make the link to energy production (127). The first high resolution electron microcopy images of mitochondria were published in the early 1950’s (144). This early work on mitochondria, started from nothing other than intellectual curiosity and a need to understand the natural world, was conducted with rudimentary microscopes in the 1800’s, and laid the foundation for subsequent, robust and diverse research on the mitochondrion (51;161).
Mitochondrial Origins
The endosymbiotic theory was first proposed by biologist Lynn Margulis in the 1960s. Dr. Margulis theorized that mitochondria and their plant counterparts, chloroplasts, arose millions of years ago as a result of the incorporation of prokaryotic organisms by other prokaryotes (116;157). It has been estimated that this event occurred 1.5 giga-years ago (107). Mitochondria have lipid bilayers as membranes and contain circular DNA, like prokaryotes, consistent with this theory. In humans mitochondrial DNA encodes for 13 essential mitochondrial proteins along with tRNAs. Most mitochondrial proteins are encoded by nuclear DNA. It is thought that over time genes migrated to the nuclear genome or were lost. A large amount of energy is required for transcription and translation within the mitochondria, making it inefficient; however, for unclear reasons a subset of genes remain in the mitochondria (107). These genes are primarily involved in oxidative phosphorylation.
Mitochondrial Fission and Fusion Overview
For a long time mitochondria were viewed as important, but static, energy factories, whose main contribution to pathology was over production of ROS. However the mitochondria are now viewed as more dynamic organelles undergoing continuous fission and fusion, which were first described in yeast in the 1990s and found to be essential processes to maintain healthy mitochondria (167). Studies in yeast and cell lines in the 1990’s revealed that mitochondria were very active, continuously dividing and fusing (fission and fusion) (136;157). Importantly, mitochondria were found to exist as interconnected networks, rather than isolated energy factories (25;28). Although many studies demonstrated that loss of fission and fusion proteins was detrimental to lethal for the cell, the exact reasons remained obscure (25). Studies on mitochondrial function in biopsies from patients with inherited mutations of fusion proteins, which cause neuropathies, such as Charcot Marie Tooth disease (discussed below), were confusing, as different mutations had different phenotypes, and some seemed to have no phenotype (6). A newly established database of patients with OPA1 mutations should improve our understanding of the associated mitochondrial pathology (54). However, original reports of OPA1 mutations without phenotype cast doubt on the importance of fission and fusion. This issue became moot when Chen et al. demonstrated that mitochondrial fusion was essential for mitochondrial (mt)DNA stability in skeletal muscle cells, providing some of the first information on an essential process impaired by loss of normal fission or fusion (28).
Mitochondrial Fission
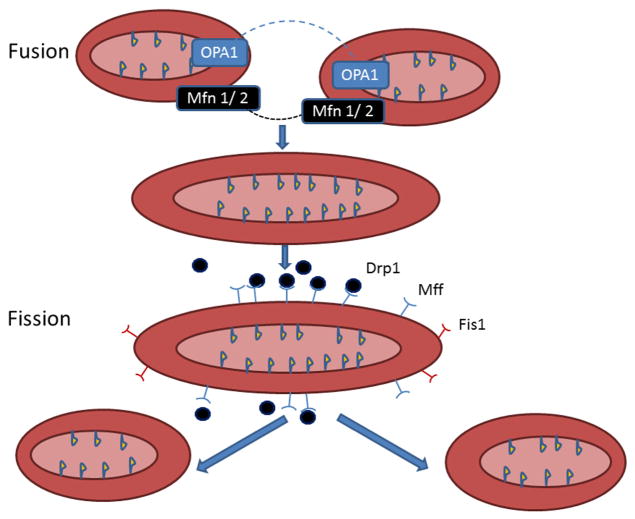
Mitochondrial fission and fusion are dynamic events whereby the mitochondria divide and then fuse with other mitochondria. In eukaryotes, a number of proteins have been identified as being involved in fission (fig. 1). Proteins regulating mitochondrial fission in mammalian cells include dynamin related protein (Drp) 1, mitochondrial fission factor (Mff), and fission (Fis)1 (175;201). Fis 1 was originally identified as the homologue of the yeast fission protein, and was thought to be the only protein required for fission in eukaryotes (133). Fis 1 is a tetratricopeptide repeat, which facilitates protein-protein interaction and is important in protein transport and in chaperone and transcription complexes (16). Although Fis1 had been identified as the only protein required for mitochondrial fission, further investigation revealed that knockout of Fis1 did not impair fission, but knockdown of either Drp1 or Mff compromised mitochondrial fission (143). More recent work suggests that Fis1 has a smaller role in fission, but still participates in this process (111). However, both Fis1 and Mff contribute to the size and number of Drp1 puncta on mitochondria with induction of fission (111). Thus, Fis1 has a lesser role in fission and this may vary by cell type. Fission is more complex in mammalian cells compared to unicellular fungi, and Mff is now recognized as an important mediator of mitochondrial fission.
Figure 1.
Mitochondrial Fusion and Fission: Diagram summarizes mitochondrial fusion and fission. Fusion (top panels) - Mitofusin (Mfn) 1 and 2 together fuse the outer mitochondrial membrane. Expression of these two proteins is unchanged in HF. OPA1 fuses the inner mitochondrial membrane, leading to a single, larger mitochondrion, and OPA1 is decreased in HF. Fission (lower panels) - Fission divides one mitochondrion into two smaller mitochondria. Mff recruits Drp1 to the mitochondria for fission. Drp1 is a cytoplasmic protein, but forms complexes at fission sites on the outer mitochondrial mediating fission.Fis1’s tetratricopeptide repeat motif helps create a scaffold, which facilitates the formation of protein clusters on the outer mitochondrial membrane, but is not essential for fission, as Drp1 can complex on the mitochondrial surface without Fis1, if Mff is present. Mitochondrial fission and fusion are essential for maintenance of normal mitochondria. A key aspect of fusion in the heart may be asymetric fission, as described by Twig et al. (see text). Asymmetric fission generates a smaller mitochondrion with a decreased Δψ m and a larger mitochondrion with a preserved Δψ m. The small, depolarized mitochondrion is removed by mitophagy. Figure updated from Knowlton and Chen (88).
The 81.9 kDa Drp1 is a member of the GTPase superfamily, and is involved in mitochondrial and peroxisomal division. Drp1 also has a role in apoptosis and in mitochondrial fission for mitosis. Drp1 is a cytoplasmic protein, but at key times it creates complexes at fission sites on the outer mitochondrial mediating fission (175). The 16.9 kDa Fis1’s tetratricopeptide repeat motif helps create a scaffold, which facilitates the formation of protein clusters on the outer mitochondrial membrane (181). However, as discussed, Drp1 can complex on the mitochondrial surface in the absence of Fis1, as long as Mff is present. Fis1 has been found to girdle mitochondrial surface, but it does not concentrate at scission sites (181;201). Increased expression of Fis1 increases autophagy, as well as increasing cytochrome c release and apoptosis (64;78;98). Inhibition of Drp1 with P110, which prevents binding of Drp1 to Fis1, but does not effect interactions with Mff or MiD51, ameliorated increased mitochondrial fission after several different toxic stresses (151). Disruption of Fis1 through mutation impairs the removal of damaged mitochondria through autophagy leading to an accumulation of large LC3 aggregates (169). Thus Fis1 has a complex role in maintaining mitochondrial integrity that remains to be fully elucidated.
Mff is a 38.5 kDa protein that has more recently been identified as having a key role in recruiting Drp1 to the mitochondria for fission (58;143). MiD49 and MiD51 also can recruit Drp1 to the mitochondria independent of Mff or Fis1 (145). The increasing number of proteins involved with fission and varying reports of proteins being essential or not essential for fission suggests that there are differences amongst different cell types with regards to the regulation of this important process. It is clear that the regulation of the recruitment and binding of Drp to the mitochondrial surface is complex, and more work will be needed to completely delineate its regulation.
Mitochondrial Fusion
Mitochondrial fusion brings together two different mitochondrion to make a single, larger mitochondrion. In the process, there is mixing of mtDNA, which has been thought to be beneficial (28). Three proteins are essential for fusion (figure 1). Mitofusin (Mfn) 1 and 2 together fuse the outer mitochondrial membrane. Inner mitochondrial membrane fusion depends solely on optic atrophy (OPA)1. Fission and fusion can be impaired under a number of conditions including heart failure and optic neuropathies, including Charcot Marie Tooth Disease and autosomal dominant optic atrophy (ADOA) (76;138;190). Decreased expression of key fusion proteins in ischemic heart failure and the accumulation of small, fragmented mitochondria are consistent with impaired fission and fusion, but no one to date has reported measuring these processes in this setting.
Role of Mitochondrial Fission in Culling Damaged Mitochondria
Once mitochondrial dynamics, with the cycling of fission and fusion events, was identified, many questions arose about its role in the cell (28;104). Although fission and fusion had been shown to be essential processes for cell viability, simple continual mixing of the mitochondrial content would not be sufficient, as dysfunctional mitochondria would be mixed together with normal mitochondria, with no net gain in cellular efficiency and no culling of damaged mitochondria. Significantly, several key studies have delineated how fission can selectively remove impaired mitochondria. Twig et al. were the first to demonstrate that fission events can generate uneven daughter mitochondria with differences in size and membrane potential, Δψm (187). Others have confirmed these findings, showing that a damaged mitochondrion can undergo asymmetric fission with a smaller, damaged mitochondrion being set aside for removal and the healthier remaining mitochondrion continuing to produce energy (194). Uneven daughter mitochondria have differing fates with one daughter mitochondria with high membrane potential and high probability of fusion and the other with low membrane potential, low probability of fusion, and decreased OPA1. This selective fission leads to removal of dysfunctional daughter mitochondria by autophagy. Twig et al. found a lag time of up to several hours between depolarization and removal of the dysfunctional daughter mitochondria by autophagy (187;188).
It is now established that in the normal cell mitochondria normally undergo fission and fusion, dividing and fusing, and these processes are essential for mitochondrial health (figure 1) (28;33;81;104). Fission and fusion are frequent events in some cells, occurring as often as every two minutes in yeast. Fission and fusion are presumed to take place much less frequently in mammalian cells, taking hours or longer (99;167). There has been skepticism that mitochondria in the densely packed cardiac myocyte would actually experience fission and fusion, but it is widely accepted that fission and fusion are critical processes in all cells (33;45;177). It may be that in complex cells, such as cardiac myocytes, more important than mitodynamics, are the other functions of the fission and fusion proteins, such as in mitophagy, which can remove damaged mitochondria without having ongoing repetitive fission and fusion.
Mitochondria and the Endoplasmic Reticulum (ER)
The ERMES (multi-subunit endoplasmic reticulum-mitochondria encounter structure) tethers the ER and mitochondria together (57). Hamasaki and colleagues demonstrated in mammalian cells that autophagosomes form at contact sites between ER and mitochondria and that ATG14 and ATG5 were present at these sites after starvation (70). ERMES has been localized to sites of mitochondrial division in yeast, but an exact counterpart has not been identified in mammalian cells, although Hamaski’s work is an important step (17;70;134). However, inverted formin 2 (INF2, 91), which localizes to the ER, mediates actin accumulation and polymerization between the ER and the mitochondrion at the constriction site, and this is followed by Drp1 recruitment (91). Interestingly, INF2 mutation is associated with the development of Charcot-Marie-Tooth disease accompanied by glomerulosclerosis (190). Mutation of many of the mitochondrial dynamics proteins causes Charcot-Marie-Tooth disease and other related inherited neuropathies (6;190). The interactions of the mitochondria and the endoplasmic reticulum have not been investigated in the cardiovascular system.
Mitochondrial Biogenesis
Mitochondrial Biogenesis, Fusion and Fission
PGC-1α promotes mitochondrial biogenesis, but PGC-1α’s expression was decreased in studies of human heart failure (59;171;193); however others have found PGC-1α to be unchanged (74), or increased (1;85) in heart failure. A moderate increase in PGC1α expression in the heart in mice was associated with increased mortality with TAC surgery and no improvement in mitochondrial function 8 wks. post TAC (82). In fact the PGC1α transgenic mice did worse than non transgenic controls with higher BNP levels, even though expression of PGC1α downstream target genes was improved. Estrogen related receptor (ERR) α, which is not regulated by estrogen, but had some early sequences similarities to estrogen receptors, hence its name, also has a key role in mitochondrial biogenesis. ERRα, which is a downstream target of PGC-1α, is similarly downregulated in heart failure (130;171). mtDNA copy number, a key factor for mitochondrial functional integrity, is decreased in ischemic heart failure (HF, see Table I, (1;83;85). However, one study focusing specifically on dilated, nonischemic cardiomyopathy (DCM) reported higher mtDNA copy numbers in DCM, which evidence suggests is a very different disease from ischemic heart failure (1). Reduced mtDNA copy number corresponded with reduced expression of mtDNA encoded proteins (18;85) Moreover, mtDNA replication was defective in HF (83;85). This is particularly intriguing, given that a 10 kDa fragment of the fusion protein, OPA1, has been identified as being essential for initiation of mtDNA replication, and total OPA1 is decreased in human ischemic cardiomyopathy (ICM or HF, 32;32;47;185).
Table I.
Summary of key proteins involved in mitochondrial protein trafficking, mitophagy and dynamics and alterations in their expression/function in heart failure. (Idiopathic) dilated cardiomyopathy (DCM), Ischemic cardiomyopathy (ICM), Hypertrophic cardiomyopathy (HCM), OMM: outer mitochondrial membrane, IMM: inner mitochondrial membrane.
| Name | Location | Function | ICM | DCM | TAC and Hypertension Induced HF |
|---|---|---|---|---|---|
| Mitochondrial Trafficking | |||||
| TOM/TIM | TOM:OMM TIM:IMM |
Protein import & sorting | Defect in Pam18 (Tim14) is involved in dilated cardiomyopathy with ataxia (DCMA), by human genome mapping (39). | Tom70 was downregulated in pathological hypertrophic hearts from humans and experimental animals (101). | |
| mtHSP70 (Grp75) | Matrix | Chaperone. Interacts with TIM44 (113) Maintains mtDNA |
- | - | - |
| mtHSP40 | Matrix | Chaperone. Maintains mtDNA |
- | Mice deficient in Dnaja3 developed DCM and died before 10 weeks of age, at least in part, through its chaperone mtHSP40 activity (72). | - |
| MPTP | IMM | Non-selective pore for molecules d1.5 kDa | Inhibition of MPTP opening attenuated cardiac ischemic-reperfusion injury in mice (93). | Increased MPTP opening in Drp1 null mitochondria was associated with mitophagy in MEFs and contributed to cardiomyocyte necrosis and dilated cardiomyopathy in mice (178). | Prevention of MPTP opening is protective in patients with obstructive hypertrophic cardiomyopathy (153). |
| HSP60 | Matrix&Cytosol | Increased in IHF in rat and human (89, 105); Abnormal Localization to plasma membrane associated with increased apoptosis (105). Mt HSP60 unchanged in rat IHF(109). Decreased HSP60 in LV cytoplasmic fraction and increased HSP60 in LV mitochondrial fraction in LV (105, 170). Increased in blood in human ICM (115). |
Levels of hsp60 doubled in DCM (89). Decrease of HSP60 level in cytoplasmic fraction and increase of HSP60 in LV mitochondrial fractions (170). Increased HSP60 protein level and mRNA level in patients with DCM (95). |
||
| Autophagy/Mitophagy | |||||
| Parkin | Cytosol | Protein degradation | Dysfunction of PINK-Parkin interaction leads to dilated cardiomyopathy in Mfn2-deficient mouse embryonic fibroblasts and cardiomyocytes and in Parkin-deficient Drosophila heart tubes (34). | - | |
| P53 | Matrix&Cytosol | Transcription factor regulated by cell stress, including DNA damage and hypoxia. Regulates genes involved in cell cycle arrest, DNA repair, senescence, apoptosis |
Inhibition of p53 leads to dilated cardiomyopathy in adult mice cardiomyocytes (198) Accumulation of P53 leads to DCM through CaMKIIδ in mice (184). |
||
| PINK1 | OMM | Mitophagy | - | Dysfunction of PINK-Parkin interaction leads to dilated cardiomyopathy in Mfn2-deficient mouse embryonic fibroblasts and cardiomyocytes and in Parkin-deficient Drosophila heart tubes (34). | PINK1−/− mice develop left ventricular dysfunction and evidence of pathological cardiac hypertrophy as early as 2 mo of age (15) |
| P62 | Cytosol | Mitophagy | Increased in human cardiomyocytes from patients with ICM (35). | Increased in human cardiomyocytes from patients with DCM (35). | Expression of p62 was decreased in Mdivi (mitochondrial division inhibitor) treated TAC mice compared to controls (63). Fission inhibition ameliorates development of HF after TAC (63). |
| Fission & Fusion | |||||
| OPA1 | IMM | Fusion | Expression of OPA1, was decreased in in rat and human ICM (32). | No difference in human DCM (32). | - |
| Mfn1 | OMM | Mitofusins, mitophagy | No difference in ICM rat. Increased in human ICM (32). |
Increased in human DCM (32). | - |
| Mfn2 | OMM | Mitofusins, mitophagy Mfn2 functions as a mitochondrial receptor for Parkin and is required for quality control of cardiac mitochondria | No difference in IHF rat. Increased in human ICM (32). |
Increased in human DCM (32). | Mfn2 was decreased in phenylephrine induced hypertrophy in neonatal rat ventricular myocytes, hypertrophied hearts from spontaneously hypertensive rats, and mice with pressure-overload induced hypertrophy by TAC (1 and 3 weeks). Mfn2 was not decreased in hypertrophied hearts after 15 weeks of TAC, nor in hypertrophied non-infarcted myocardium following MI (52) |
| Drp1 | OMM | Fission | No difference in IHF rat. Increased in human ICM (32). |
Increased in human DCM (32). Conditional cardiomyocyte-specific Drp1 ablation evoked mitochondrial enlargement, lethal dilated cardiomyopathy, and cardiomyocyte necrosis (178). |
Drp1 was phosphorylated in pressure overload induced hypertrophy in TAC mouse hearts and phenylephrine (PE)-treated rat neonatal cardiomyocytes (27). Mutation of Drp1 leads to myocyte hypertrophy (8). |
| Fis1 | OMM | Fission | No difference in IHF rat. No difference in human ICM (32). | No difference in DCM (32). | |
| Oxidative Respiration | |||||
| NDUFA5 | IMM | Complex I ETC |
Decreased in mitochondria in LV of IHF rat (109). | ||
| NDUFV1 | IMM | Complex I ETC |
Decreased in mitochondria in LV of IHF rat (109). | Decreased in patients with DCM (141). | Decreased significantly in TAC mice, and restored in TAC after stem cell treatment (199). |
| Mn-SOD | matrix | Free-radical scavenger | The null mice develop dilated cardiomyopathy and die within 10 days after birth (103). | Zn–SOD was markedly decreased in Copper Deficiency mice which leads to hypertrophic cardiomyopathy (48). | |
Mounting evidence supports that ischemic cardiomyopathy (ICM) and dilated cardiomyopathy (DCM), two common types of heart failure, differ significantly from one another at the cell and molecular level, despite the fact that clinically the two diseases are very similar. Investigations of mitochondria in heart failure demonstrate distinct differences between the two. Frequently studies of heart failure combine together DCM and ICM samples for study and variations in the composition of this mix may explain some of the end-point differences noted above. Most convincing is the comprehensive study by Ahuja and colleagues, which identified marked differences between ICM and DCM mitochondria, including greater mtDNA copy number and more mtDNA deletion mutations in DCM compared to ICM (1).
Mitochondrial Structure
Mitochondria constitute 20–40% of the cardiac cellular volume (117). Intact mitochondrial structure is critical for cellular energy production and overall cardiac function. Mitochondria are dynamic subcellular organelles, undergoing fission and fusion events, and constantly move along microtubules to where they are most needed. This is more apparent in neurons, where mitochondria must move down the axons, than in cardiac myocytes. In the 19th century, the morphologists were not sure that they were looking at the same functionally distinct structure in different cells by light microscopy, because of the variability in the number and shape of mitochondria (3). It was not until the development of high resolution electron microscopy techniques beginning in the 1950’s that mitochondrial structure/morphology could be clearly delineated (79;97).
In adult mammalian cardiac myocytes the mitochondria are intricately organized into three subsets. Most mitochondria are localized between the contractile filaments (interfibrillar), while a minority of mitochondria are localized next to the sarcolemma (subsarcolemmal). A very small amount of mitochondria are perinuclear. In heart failure the interfibrillar mitochondria lose their normal organization (32). Furthermore, size and density of interfibrillar mitochondria decreases in rodent heart failure models (112). OPA1 expression declined in both human and rat ischemic HF, but Mfn1/2, Fis1 and Drp1 are unchanged (32). In contrast, in explanted ischemic failing human hearts, where disease has been present longer due to medical treatment, OPA1 was decreased, Mfn1/2 and Drp1 were increased, and Fis1 unchanged. In dilated, nonischemic cardiomyopathy explanted human hearts had no decrease in OPA1, but an increase in Mfn 1/2 and Drp1(32). Thus, OPA1 expression differs in failing human hearts, depending on type of heart failure. In H9c2 cells, an embryonic cell line, decreasing OPA1 led to more apoptosis at both baseline and after ischemia, through cytochrome c release, consistent with OPA1’s role in maintaining cristae tight junctions and preventing cytochrome c release (32;38;56).
Mfn1/2, OPA1 and Mitochondrial Abundance and Structure - Electron microscopic studies revealed more numerous, but smaller mitochondria in a rat model of ischemic HF (32). In contrast, reduced Mfn2, greater Fis1 and reduced OPA1 expression occurred at 18 weeks in a simple rat myocardial infarction model (80). Mfn2 knockout leads to pleiomorphic and somewhat larger mitochondria in cardiac myocytes (146). In vivo conditional cardiac Mfn2 knockout mice have a low level of cardiac hypertrophy and minor changes reductions in heart function (146). Overall investigations of Mfn1 and Mfn2 support that each compensate for the loss of the other to a degree, but neither Mfn can compensate for loss of OPA1, knockout of which is embryonic lethal (40;43).
Mitochondrial Structure and Mitochondrial Trafficking
The individual mitochondrion is composed of an inner and an outer membrane. The outer membrane is a selectively permeable membrane, containing integral membrane proteins and pores for transporting molecules. Proteins larger than 5000 Daltons require specific signaling sequences, mitochondria targeting sequence (MTS), to be transported across the outer membrane (2;49;60;192). A consequence of this is that the proteins found in this space differ significantly from the proteins found in the cytosol. Energy produced by the electrons move down the electron transport chain powers the maintenance of the hydrogen ion gradient, which powers the conversion of ADP to ATP. Enzymes needed for the citric acid cycle, along with other essential component including dissolved oxygen, water, carbon dioxide, and the recyclable intermediates that serve as energy shuttles, are part of the mitochondrial matrix.
Cristae
The inner membranes, separating mitochondrial matrix from the intermembrane space, convolute into numerous folds, termed cristae, the site of respiration. The number of cristae is proportionate to the metabolic activity of the cell. Thus very metabolically active cells, like cardiac myocytes, have large numbers of cristae in their mitochondria. Cristae, as they are derived from folding of the inner membrane, expand the surface area and enhance ATP production. As discussed above, a 10 kDa fragment of OPA1 keeps cristae junctions tightly closed, preventing the release of cytochrome c and apoptosis. Dysfunctional mitochondria have been frequently found to have disruption and loss of cristae, such as reported with ischemic heart failure (32;109), with reduction in OPA1 expression (31;152), in Huntington’s disease (36), and with reduction in Crif1, as occurs with Alzheimer’s disease (9;23).
The Complexes and the ETC
The protein-rich inner mitochondrial membrane remains sufficiently fluid to provide an ideal environment for each distinct, but connected functional complex comprising the electron transport chain (ETC), which provides essential energy to cells via oxidative phosphorylation. The ETC consists of four membrane-bound multi-subunit enzyme complexes (I–IV) and an ATP synthase (complex V). Subunits of complexes I, III, IV, and V are encoded either by the mitochondrial DNA (mtDNA) or, for the majority, by the nuclear DNA (nDNA, 121;150). On the other hand, complex II subunits are encoded by entirely by nuclear genes (174).
Morphologic Changes in HF
Mitochondria can change their overall morphology from elongated interconnected mitochondrial networks to a fragmented disconnected arrangement through fusion and fission (139;139). In ischemic heart failure, mitochondria have increased fragmentation (109;77;176). Others have found a reduction in size and density of interfibrillar mitochondria in rodent models of heart failure (112). Non ischemic heart failure has distinct changes in mitochondria compared to ischemic heart failure (1). Ahuja et al. found distinct differences in mitochondrial morphology and biogenesis and genomic integrity in human ischemic heart failure compared to non ischemic/dilated heart failure. Although mitochondrial dysfunction was present in both types of cardiomyopathy, mitochondria were smaller and increased number in non-ischemic cardiomyopathy vs. both normal hearts and ischemic cardiomyopathy. In contrast dilated cardiomyopathic hearts had a higher mtDNA copy number and mitochondrial density, but a marked increase in mtDNA deletions compared to both normal hearts and ICM hearts (1).
Coiled-Coil-Helix-Coiled-Coil-Helix Domain Containing 3 (ChChd3) and Cristae Function/Structure - ChChd3 interfaces with the inner membrane proteins mitofilin and OPA1, which stabilizes cristae morphology, and with the outer membrane protein sorting and assembly machinery (Sam) 50, which is involved in the import and assembly of β-barrel proteins on the outer membrane (38). Knockdown of ChChd3 result in a marked decrease of both mitofilin and Sam50, as well as in several mitochondrial proteins. These results were interpreted as consistent with ChChd3 being a scaffolding protein, stabilizing protein complexes and retaining cristae conformation and protein import. Thus, ChChd3 would be essential for maintaining mitochondrial structure and function (38). In addition, ChChd3 has been identified as a transcription factor, repressing Bag1 expression (108).
Mitochondrial function
Mitochondria modulate cardiac energetics, reactive radical biology, calcium homeostasis, and apoptosis, which are essential for the function of a normal heart (129;156). The mitochondria are the primary source of the abundant high energy phosphates needed to maintain uninterrupted cardiac contraction, ion flux and membrane potential (197). Protein phosphorylation has been found to be a key element in regulating mitochondrial function and ROS production (41;94).
Energy production
Cellular respiration takes place in the mitochondria, converting biochemical energy from nutrients into adenosine triphosphate (ATP) which powers the cell, followed by the release of waste products. Mitchell in 1961 first proposed that cellular respiration works by chemiosmotic coupling, a chemical reaction that can drive an osmotic gradient (131). The hydrogen atoms that enter the respiratory chain are split into protons and electrons. The electrons pass down the chain via a succession of redox reactions. The energy released by electron flow is used to pump protons across the membrane. The electrical component generates a potential difference in pH, or acidity, with the outside more acid than the inside. The combination of pH and potential difference across the membrane constitutes proton-motive force, which is the force that drives the synthesis of ATP (131;176). Electrons enter the ETC at either complex I: NADH:ubiquinone oxidoreductase or complex II: succinate dehydrogenase, and are passed to complex III: cytochrome bc1 complex by the carrier ubiquinone Coenzyme Q. Cytochrome c carries electrons from complex III to complex IV: cytochrome c oxidase, where they react with protons and oxygen to form water (26;66). In order to function, the heart must rely heavily on oxidative energy produced by the mitochondria. Fatty acids are the dominant energy source for ATP generation in healthy heart muscle. Other energy sources, such as glycolytic metabolism, are only a minor source of ATP in normal cardiac tissue (117;165). Fatty acid β-oxidation and the oxidation of carbohydrates through the TCA cycle produce most of the intramitochondrial NADH and FADH are the primary origin of electrons for the electron transport chain (173). In diseased heart, such as heart failure or acute ischemia, glycolysis is the primary source of energy.
Complex I Dysfunction
Defects of individual ETC complexes or components of the phosphorylation apparatus have been linked to HF. Complex I is crucial for mitochondria energy production. Complex I extracts energy from NADH, produced by the oxidation of sugars and fats. Complex I mutations are a frequent cause of inherited mitochondrial dysfunction, which occur with a frequency of approximately 1 in 10,000 births (186). Complex I abnormalities cause myopathies, encephalomyopathies, and neurodegenerative disorders, including Parkinson’s disease and Leigh syndrome (158;189). Complex I defects are most clearly manifest by neurologic symptoms, as neurologic tissue is highly dependent on energy production, compared to workhorse cell types, such as fibroblasts. Mitochondrial abnormalities are less evident in the heart, likely because significant neurologic abnormalities lead to such global dysfunction, that any cardiac impairments are concealed. A subset of the mitochondrial abnormalities is secondary to mutations in the mitochondrial genome. However, other mitochondrial abnormalities, which are secondary to a decrease in complex I activity or an increase in the production of reactive oxygen species (ROS), are poorly understood. Rats with ischemic heart failure have decreased complex I activity, as well as proteomic remodeling with more than a 50% decrease in protein levels of NADH Dehydrogenase (Ubiquinone) Flavoprotein 1 (NDUFV1) and NADH Dehydrogenase (Ubiquinone) 1 Alpha Subcomplex, 5 (NDUFA5) (109). Complex I dysfunction has been found to lead to increased ROS and protein acetylation, as well as worsening of heart failure (84). In contrast to ischemic heart failure, in a TAC model (mouse) of heart failure based on mitochondrial respiration measurements, complex I activity was not impaired (22). Similarly there was no evidence of complex 1 dysfunction in a rat TAC model of heart failure (168). However, these investigators found a decrease in mitochondrial proteins and respiratory capacity in IFM, but not in SSM mitochondria (168).
Apoptosis
Human heart failure is associated with pathologic cardiac remodeling leading to progressive dilation of the ventricle, increased wall stress and depressed contractility. Apoptosis of cardiac muscle cells is a fundamental process contributing to the progression to heart failure (95;135). It has been shown that mitochondrial dysfunction and apoptosis contribute to the ongoing cell loss in progression of the failing heart (135). OPA1 has a role in protecting cells from apoptosis, at least in part by preventing cytochrome c release from the cristae into the cytosol (32;137). Prohibitins also have a role in regulating cristae structure and OPA1 localization, and thus are indirectly anti-apoptotic (126;142;195).
The mitochondrial death pathway is effectuated by both the intracellular and extracellular death-signals through activation of mitochondrial permeability transition pore (MPTP) formation, which leads to the release of pro-apoptotic proteins, including cytochrome c and apoptosis-inducing factor (AIF), resulting eventually in the disruption of normal mitochondrial physiology (37;61). The Bcl-2 family also controls apoptosis by regulating mitochondrial permeability. The proteins are located on the outer mitochondrial membrane and the anti-apoptotic members of the Bcl-2 family can also inhibit cytochrome c release (21;106).
VDAC and mPTP
The mitochondrial permeability transition pore (mPTP) is a non-selective pore permeable to any molecule < 1.5 kDa between the cytosol and the mitochondrial matrix. Its primary components are the voltage-dependent anion channel (VDAC), the adenine nucleotide translocase (ANT) and cyclophilin D (191). The mPTP is a non-selective pore transporting small molecules (< 1.5 kDa) between the cytosol and the inner membrane. The mPTP opens during conditions of pathologically high [Ca2+]m (67). An increase in [Ca2+]m alone is a relatively inefficacious at triggering pore opening; however, the sensitivity to [Ca2+]m can be markedly increased by adenine nucleotide depletion, high [Pi] and most significantly, oxidative stress (69). The resulting increased proton permeability leads to a dissipation of the proton motive force as a result of a reduction in both the pH gradient and the membrane potential. Subsequently, mitochondrial ATP synthesis by oxidative phosphorylation drops. Myocytes cannot maintain their ATP levels, therefore undergo necrotic cell death. The mPTP is widely studied in neuronal excitotoxicity, where over stimulation of glutamate receptors leads to excessive calcium entry into the cell (163). It has also been found that decreased NAD+/NADH ratio secondary to complex I deficiency enhances MPTP sensitivity (84). This has important implications for ischemic heart failure, where complex 1 function is impaired.
VDAC
VDAC, provides the aqueous pathway across the outer membrane for the transfer of the substrates generating ATP through oxidative phosphorylation from the cytosol to the inner membrane (100;148). VDAC, with a single pore 2.5–3 nm wide when fully open, allows the flux of metabolites including Pi, ATP/ADP, and Ca2+, across the outer membrane. It is thought that phosphorylation is one factor among many others, that controls the open and closed states of this channel(155). VDAC is reported to be involved in apoptosis of cell lines carrying the mitochondrial A4263G tRNAIle gene mutation, which is a cause of maternally-inherited hypertension (204). This mutation is thought to lead to mischarging of VDAC secondary to amino acid substitutions. Cell studies of this mutation demonstrated that VDAC associated with Bax and that the mitochondrial membrane potential Δψm was decreased and apoptosis increased. Cyclosporin A treatment restored Δψm and decreased apoptosis (204).
Energy production
Mitochondrial Energetics, Respiration and Mfn1 and Mfn2
There is a direct relationship between maintenance of mitochondrial fusion and maintenance of normal mitochondrial function with preserved oxidative phosphorylation (205). Inhibiting mitochondrial fusion results in reduced oxygen consumption (28). Likewise, Mfn2 expression inhibition markedly decreases pyruvate, glucose, and fatty acid oxidation. Intriguingly, skeletal muscle from both obese human and animal models has strikingly less Mfn2 expression (149). In fibroblasts Mfn2 knockdown reduced oxygen consumption and glucose oxidation (14). Compatible with these observations, MEFs with knockout of both Mfn1 and Mfn2 had reduced mitochondrial membrane potential, less respiration, and decreased maximal respiration (29). On the other hand, increased Mfn2 expression results in greater respiratory complex activity, more glycolysis and enhanced mitochondrial biogenesis (149). Similarly, Mfn2 expression increases in settings of intense energy demand, like exercise, and as a response to apoptotic stimuli (29). Hence, Mfn1 and Mfn2 have important effects on mitochondrial respiration and energetics.
Mitochondrial Energetics, Respiration and OPA1
OPA1 has critical role in maintaining cristae structure, and disruption of OPA1 greatly changes cristae morphology (56;124). Furthermore, cristae structure/OPA1 oligomerization varies under different nutritional states (44;56;114). For example, OPA1 is more oligomerized and cristae junctions tighter in states of starvation (124). OPA1 oligomerization also changes with different metabolic substrates.
Complex I and II substrates are associated with much less oligomerization of OPA1 (75). Furthermore, neither rotenone nor the mitochondrial uncoupler, CCCP, affected OPA1 oligomerization, supporting that this is regulated by upstream substrate availability (147). Studies using RNAi to deplete OPA1 in MEFs demonstrated that reduction in OPA1 led to a reduction in basal respiration and loss of the ability to increase oxygen consumption in the presence of the uncoupler 2,4-dinitrophenol (maximal respiration) (29). In studies of the fibroblasts of patients with ADOA, certain OPA1 mutations had impaired complex I driven ATP synthesis, as well as, not unexpectedly, reduced mitochondrial fusion (205). Lodi and colleagues assessed post-exercise phospho-creatinine in the calf muscles of patients with ADOA with OPA1 mutations(heterozygotes for c.2708–2711 deletion TTAG in exon 27, most common OPA1 mutation for ADOA) and controls using phosphor-magnetic resonance spectroscopy (110). With this approach the investigators identified that basal levels of PCr were significantly lower in the OPA1-ADOA patients by about 10%. Furthermore, after exercise the recovery time of PCr levels was markedly prolonged (28 vs. 19 sec, p<0.01). Some OPA1 mutations did not alter mitochondrial activity or bioenergetics (122). Thus, there are select OPA1 domains required to maintain normal mitochondrial function. More comprehensive knowledge of post-translational modifications and identification of proteins complexing with OPA1 will lead to greater insight into the mechanism(s) by which select ADOA OPA1 mutations cause disease without impairing measured mitochondrial function.
Mitochondrial Fission Proteins and Mitochondrial Energetics
Mitochondrial fission protein changes can alter mitochondrial metabolism. Drp1 reduction lowered the basal rate of oxygen consumption, reduced coupled respiration, and slowed ATP synthesis (13). Likewise, a dominant negative Drp1 led to a marked reduction in respiratory capacity (187). Hyperglycemia induced fission and cell death, but prevention of this with a dominant negative Drp1 significantly decreased the mitochondria’s ability to increase respiration (202;203). Likewise, Fis1 depletion decreased the maximal respiratory activity, and increasing Fis1 expression restored the phenotype (187). Thus, both DRP1 and Fis1 influence mitochondrial metabolism.
Mitochondrial Protein Trafficking
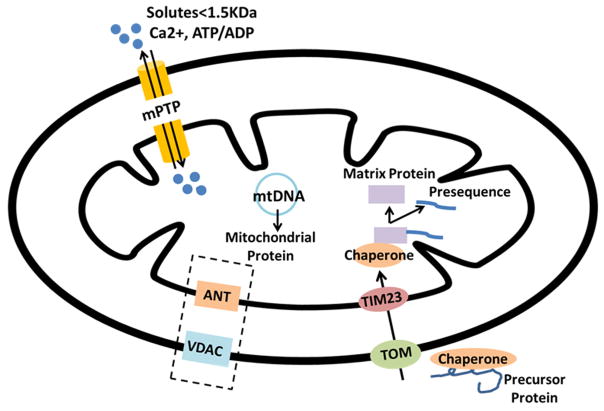
The outer and inner membrane of mitochondria define the separation from the cytosol and from each other. Ions, nutrient molecules, ATP, ADP, and other small molecules can readily move through the outer membrane. Proteins more than 5000 Daltons in size must have a specific signaling sequence, the mitochondrial targeting sequence (MTS), to be transported across the outer membrane (102). While pores in the outer membrane formed by VDAC make this membrane highly permeable to most small molecules (<4–5 kDa), the inner mitochondrial membrane is a functional permeability barrier between the cytosol and the mitochondrial matrix. Trafficking of proteins and small solutes in and out of the mitochondria is essential for normal mitochondrial function (figure 2). The traffic of metabolites and ions tightly links mitochondria to the many other cellular activities.
Figure 2.
Mitochondrial Protein Trafficking: Diagram summarizes key pathways for protein and solute import into the mitochondria. 1) The TIM/TOM complex is the major mitochondrial proteins importing machinery. Proteins that are encoded in the nucleus and synthesized in the cytoplasm are transported into the mitochondrion through TIM/TOM assisted by chaperones. 2) VDAC and ANT have roles in small solute trafficking, but in the stressed cell they are part of the mPTP, which opens leading mitochondrial depolarization. 3) mPTP, spanning the IMM and OMM, consists of membranous elements, including VDAC on the OMM, ANT on the IMM. Under conditions like high Ca+2 concentration, increased oxidative stress, low ATP, and mitochondrial depolarization, mPTP is allows free diffusion of solutes across the membranes, which ultimately leads to mitochondrial swelling, and apoptosis.
Mitochondrial Protein Importation
The coding capacity of the small mitochondrial genome is quite limited. The mitochondrial genome encodes approximately 1% of mitochondrial proteins. Thus, 99% of mitochondrial proteins are expressed by nuclear genes, synthesized on cytosolic ribosomes and then imported into mitochondria through specialized sorting machineries (164;200). Most synthesized precursor proteins are imported through the translocase of the outer mitochondrial membrane (TOM) complex, and this is facilitated by the chaperones in the aqueous compartments operating along the import pathways (50). Once passed through the TOM channel, the precursor proteins are direct to different sorting machineries by their targeting signals. Almost all proteins imported into mitochondria are targeted to the organelle via a cleavable presequence or mitochondrial transport signal (MTS) (113;159). The MTS is proteolytically removed during import into the mitochondria.
TOM/TIM: the Protein Import Machinery of the Mitochondria
Intensive investigation has led to the identification and structural characterization of the large family of transporters involved in mitochondrial protein trafficking. However work remains to be done, particularly with regards to characterizing the specificity of many of the transporters. TOM/TIM are the major mitochondrial protein import machinery: TOM and TIM complexes is designed to conduct translocation of a polypeptide across both the outer and inner mitochondrial membranes (160). The translocase of the outer membrane (TOM) complex is the main entry gate used by most nucleus-encoded mitochondrial precursor proteins. The translocases of the inner membrane(TIMs) are small proteins localized in the inter membrane space, which form specific aggregates and function as chaperones for unfolded proteins in transit through the intermembrane space (90). The TOM complex and the TIM23 complex in the inner membrane align to form a channel through both the outer and inner mitochondrial membranes to import proteins into the matrix. After entering TOM, the precursor proteins with MTS’s are imported by the presequence -TIM23 complex, and then bind to chaperone-like proteins in the intermembrane space, which will remove the MTS.
Role of Heat Shock Proteins in Mitochondrial Protein Trafficking
The HSP70 family members are chaperones for mitochondrial protein import. Macromolecules need to be unfolded for import into the mitochondria and other organelles and the HSP70 group of proteins is responsible for this. HSP75, also known as GRP75, mortalin, mtHSP70, and HSPA9, is an HSP70 family member, which primarily localizes to the mitochondria. HSP75 is also found in the endoplasmic reticulum, the plasma membrane and cytoplasmic vesicles. Schekman originally identified the key role of HSP75 in facilitating the translocation of proteins into the mitochondria (42). Interestingly, HSP75 was significantly decreased in interfibrillar mitochondria in type 1 diabetic heart failure patients (12).
A cytosolic protein is maintained in an unfolded state by association with HSP70 proteins and is thus able to present the MTS to the receptor on the outer mitochondrial surface. When import is initiated, the chaperone proteins are sequentially released as the peptide is transferred into the mitochondrion (162). The dissociation of the chaperone from the imported peptide requires ATP hydrolysis (128). Within the mitochondrial matrix, HSP60 forms a heptameric barrel along with HSP10, which forms the heptameric barrel lid. Within this barrel imported proteins are refolded in a process requiring ATP hydrolysis (119). HSP60 and its prokaryote homologue, GroEL, also protect mitochondrial proteins from denaturation (118;125). In addition, HSP75 has recently been identified as having a key role in assembly of cytochrome c oxidase (complex IV) (19). Thus, heat shock proteins have a critical set of functions with regards to protein import into the mitochondria. First they unfold proteins for transfer, and then other HSPs refold the proteins within the mitochondria. In heart ischemic failure, although mitochondrial levels of HSP60 remain the unchanged, HSP60’s overall localization within the cell changes. In the normal heart about 75% of HSP60 is in the mitochondria, with the rest in the cytosol, but in ischemic heart failure, about 8% of HSP60 is located in the plasma membrane fraction (105). At least some of this HSP60 is actually on the surface of the cardiac myocytes, where it may bind toll-like receptor (TLR4), leading to activation of NFκB and production of inflammatory cytokines, including TNFα (87;105;183).
Small Solutes Trafficking in Mitochondria
Ions and small solutes, such as H+,Pi, ADP/ATP, Ca+2 are trafficking through mitochondria constantly. The transportation of H+,Pi, ADP/ATP is essential to build the proton gradient and generate energy. Calcium signaling through ion channels is key to cardiac function, regulating the pace and strength of the beat of the heart. In the normal heart mitochondrial calcium concentrations ([Ca+2]m) have an essential role in regulating oxidative phosphorylation to keep ATP levels matching the demand for ATP as work load changes. Overall, mitochondrial free calcium is thought to be an important mediator of a range of metabolic activities (154).
Autophagy and Mitochondrial Fission and Fusion
Autophagy, including mitochondrial autophagy (mitophagy), is an essential cellular process for removing irreversibly damaged proteins and organelles (figure 3). There is limited work on autophagy in heart failure, but it does suggest that autophagy is impaired in HF (65;86). The failing heart with increased inflammation and increased ROS would be expected to require a higher rate of autophagy than normal heart. Autophagy, including mitophagy, is an essential cellular process for removing irreversibly damaged proteins and organelles. Autophagy is a highly conserved cellular process responsible for the regulation of cellular degradation. It is referred as a destructive process in which a double membrane envelops cytoplasm and organelles before targeting them to lysosomes for destruction. Ashford and Porter in 1962, first described autophagy as the membrane “shielding the rest of the cell from the general spread of the degradative process” (7). Mitochondria are the center of oxidation and a major source of ROS. Consequently mitochondria are more subject to injury than other organelles, as they are exposed to more ROS than other organelles. Similarly, mtDNA has greater ROS exposure than nuclear DNA, making it more vulnerable to the threat of mutation than nuclear DNA damage leading to mutation. This may be in part why many genes migrated from the mitochondria to the nucleus, as discussed above. Given the dangerous nature of the damaged mitochondrion, the timely removal of this organelle is vital to maintain normal homeostasis. In recent years, an increasing number of studies have shown that mitophagy is a specific process which involves mitochondrial dynamics, or fission and fusion (196).
Figure 3.
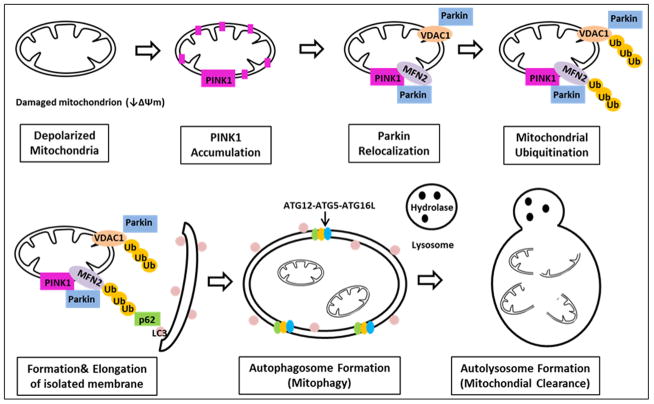
Mitophagy: Summary of steps involved in mitophagy. The depolarized mitochondrion leads to accumulation of PINK1, which phosphorylates Mfn2, which acts as a lure for Parkin. Parkin binding Mfn2 triggers mitophagy. Outer membrane proteins, including both Mfn’s and VDAC, are ubiquitinated. P62 is recruited, binding the ubiquitinated proteins, linking them to LC3. The isolation membrane elongtes and eventually engulfs the mitochondrial pieces destined for autophagy, forming the autophagosome, which eventually fuses with the lysosome, leading to degradation of the enclosed mitochondria.
Mitophagy is a specialized autophagy governing selective removal of damaged mitochondria by autophagosomes (figure 3). Damaged mitochondria exhibit depolarized membrane potential, triggering accumulation of PINK1, which phosphorylates Mfn2, which then acts as a draw for Parkin (34). Parkin binding Mfn2 triggers mitophagy (figure 3). Others have found a role for mtHSP70 in facilitating the interaction of Parkin and Mfn2 in muscle cells (46). The exact molecular mechanisms involved in the important steps leading to activation of mitophagy remain to be completely defined, and may differ in different cell and tissue types. Following recruitment of Parkin, ubiquitination of OMM proteins, such as Mfn1, Mfn2, and VDAC1 occurs. P62, is recruited and binds the Parkin-ubiquitinated substrates, linking these to LC3 for autophagic degradation. Mitochondria are engulfed after elongation of the isolation membrane. Eventually the autophagosome fuses with lysosomes to form the autolysosomes in which the lysosomal hydrolases degrade the damaged mitochondria.
Mitophagy overlaps with ERMES, discussed above, where tethering of mitochondria to ER leads to formation of autophagosome. Fission is needed to chop up mitochondria to fit in the autophagosome. Twig was the first to show that an asymmetric fission occurred, generating a normal polarized daughter and a smaller depolarized daughter, in which the small depolarized daughter is removed by autophagy (187). Twig et. al observed that fission events often generated uneven daughter units, and one daughter with decreased membrane potential has a lower probability of subsequent fusion. The subpopulation of non-fusing mitochondria generated that were found to have reduced Δψm and decreased levels of the fusion protein OPA1. Thus fission followed by selective fusion segregates dysfunctional mitochondria and permits their removal by autophagy (187). Twig et. al also found that the probability for fusion is influenced by organelle motility, instead of contact duration and organelle dimensions; a previous fusion event of the individual mitochondrion influenced the likelihood for a subsequent fusion event, as well as the site where the fusion occurred (188).
Cardiomyopathy, Increased ROS, Inflammation and Mitochondria
Heart Failure, cardiomyopathy and ROS/Inflammation
ROS are persistently elevated in both ischemic heart failure and many cardiomyopathies. The mitochondria are considered to be the primary source of the augmented ROS as well as the major target, leading to a concatenation of events with more ROS, more oxidation of mitochondrial proteins, greater mitochondrial dysfunction and the production of even more ROS. Regular fission and fusion are necessary for healthy mitochondria (figure 1) (28;33;81;104). As discussed, a damaged mitochondrion can undergo asymmetric fission with a smaller, damaged mitochondrion being set aside for removal and the healthier remaining mitochondrion continuing to produce energy (194). We have previously shown that optic atrophy (OPA)1, a critical mitochondrial fusion protein responsible for fusing the inner mitochondrial membrane, is decreased 50% in both rat and human IHF, but not in nonischemic dilated HF (32). Mutations of fusion proteins are associated with inherited optic neuropathies, but have not been previously implicated in heart disease (4;6). This may be because the optic neuropathies associated with fusion protein mutation lead to visual problems and loss of sight. Gradual onset of cardiac impairment is less likely to be noticed as overall activity declines with vision impairment. Knockout of OPA1, modeling one form of ADOA, is lethal. We have shown that the heterozygote mice, hereafter referred to as OPA1 mice, at 3 months have increased ROS, decreased mtDNA copy number, and depressed complex IV activity (31). By 12 months they develop a cardiomyopathy with decreased fractional shortening, decreased heart weight/tibia length ratio, depressed LV developed pressure, decreased cardiac output, increased BNP, increased ROS, decreased mtDNA, depressed complex activity (I and IV), decreased state III and FCCP respiration, and loss of contractile reserve (31). These mice had no response to an infusion of isoproterenol. Most striking was decreased expression of a wide range of anti-oxidant genes, all encoded by the nuclear genome. This was seen as early as 3–4 mo and was more pronounced at 12 mo. Protein levels of transcription factors, including Nrf2, involved in regulation of mitochondrial proteins (nuclear encoded) were not changed, but protein levels do not necessarily translate into transcription factor activity.
Mitochondrial Dynamics in Heart Failure
Fission and Fusion in Disease
Mutations involving fusion and fission proteins most often manifest as neurological disease, likely because of the high energy requirements of the nervous system and the high sensitivity of this tissue to any perturbations. Neurologic impairment may mask cardiac abnormalities, as blindness and other neurologic changes reduce activity such that exercise intolerance and other symptoms will not be induced. Charcot-Marie-Tooth (CMT) disease and ADOA, both inherited neuropathies causing blindness, are both associated with mutations in fusion proteins, Mfn2 for CMT and OPA1 for ADOA (6;43), although the list of genes associated with CMT extends beyond fusion proteins. Similarly, Parkinson’s disease, another significant neurologic disease, has been found to have abnormal expression of Mfn2 (104). Parkinson’s disease has also been linked to mutations in OPA1 (24). More unexpected is the decrease in Mfn2, which has been observed in patients with diabetes (11). Mutation in the fusion proteins is associated with cardiac abnormalities, more evident for OPA1 than Mfn1 and 2, each of which can compensate somewhat for the loss of the other. This is discussed further below.
Fission and Fusion in Heart Failure
Since the first report of changes in mitochondrial fusion proteins in ischemic HF, there has been a marked increase in interest in mitochondrial dynamics in heart disease. OPA1 expression was depressed both explanted human hearts and rats with ischemic heart failure (32). In contrast, both Mfn1/2 and Drp1 increased in human dilated cardiomyopathy (32) As discussed above, OPA1 mutation modeling ADOA resulted in a late onset cardiomyopathy, which coincided with the onset of blindness (31) The OPA1 mutant heart has increased ROS, but decreased anti-oxidant gene expression. Despite increased ROS and impaired mitochondrial function, expression of TFAM, PGC1α, Mfn1/2, Bax, Bak and Nrf2 were unchanged (31). Given that human ischemic HF has a similar decrease in OPA1, these results raise the possibility that human ischemic heart failure is accompanied by similar changes. It is increasingly apparent that abnormalities in fission and fusion contribute to cardiovascular disease (139). Inhibition of mitochondrial fission reduced ischemia/reperfusion injury in the heart (140). As discussed, fission and fusion proteins also have roles in mitophagy and in apoptosis (120). Thus, these proteins have complex functions in the cell beyond mitochondrial fission and fusion.
In neonatal ventricular myocytes exposed to high glucose conditions, mitochondrial fission inhibition using a dominant negative Drp1 mutant (Drp1-K38A), precluded the expected increase in ROS, opening of the mPTP and cell death (202). Cytosolic Ca2+ overload, with thapsigargin or potassium chloride treatment, caused a rapid increase in mitochondrial fragmentation in cardiac myocytes (73). Calcium overload occurs frequently in the diseased heart, and this could be expected to lead to increased mitochondrial fission and increased mitochondrial fragmentation. This is consistent with the finding that mitochondria in ischemic HF are small and fragmented. This in itself would suggest loss of the balance between fission and fusion (32), and would be expected to further escalate the energetic abnormalities in HF (81). In the Drosophila heart silencing of OPA1 and mitochondrial assembly regulatory factor (MARF) resulted in increased mitochondrial heterogeneity and dilation of the Drosophila heart tube along with loss of contractility (45). Interestingly, human Mfn1/2 was able to rescue the MARF RNAi induced cardiomyopathy in the Drosophila (45).
Fission Inhibition to Mitigate Injury and HF - Mitochondrial fission has not been studied as a target in ischemic heart failure. In an in vivo model of mouse cardiac ischemia, pretreatment with mitochondrial division inhibitor (Mdivi)-1 reduced cardiac injury and preserved mitochondrial elongation and reduced opening of the mPTP (140). Similarly transfection of HL-1 cells with a dominant negative Drp1 reduced mitochondrial fragmentation in HL-1 cells after simulated ischemia (140) In studies of neonatal mouse cardiac myocytes subjected to simulated ischemia, Mdivi-1 treatment cardioprotective with decreased ROS and cell death (166). Parallel studies in a Langendorff rat heart model demonstrated that Mdivi before or after reperfusion was protective, reducing ROS and improving cardiac function, both systolic and diastolic, post-ischemia (166). Interestingly, treatment with the calcineurin inhibitor, FK506, inhibited dephosphorylation at s637, preventing Drp1’s mitochondrial translocation and reducing cardiac injury (166).
Despite these promising studies in mitigating cardiac injury associated with ischemia/reperfusion, inhibition of Drp-1 has not been investigated as a treatment for ischemic heart failure. The balance of fission and fusion is thought to be essential for maintenance of healthy mitochondria. Ischemic HF is a chronic disease, and sustained inhibition of either fission or fusion would be expected would be expected to be detrimental. Thus, neither mitochondrial fission or fusion appear to be good therapeutic targets in IHF as this time. Greater understanding of the role of mitochondrial dynamics in the complex cardiac myocyte may provide new insights into when fission and/or fusion may be dysfunctional in IHF and warrant inhibition.
Cardiomyopathy and Inherited Neuropathies
Fusion protein mutations are a cause of inherited optic neuropathies, which as a rule have a gradual onset. Approximately 20% of Charcot-Tooth-Marie disease type II cases are caused by Mfn2 mutation, and much less often OPA1 mutation leads to CTM (104). In contrast, OPA1 mutations are more often the cause of ADOA, which is characterized by the visual impairment and blindness (40). An OPA1 mutation often associated with ADOA had been found to be homozygous embryonic lethal, and not to cause cardiac abnormalities in the heterozygote, but this was based on basic parameters such as heart weight (40). More intensive investigation with advanced cardiac imaging and functional studies demonstrated that the OPA1 heterozygous mutant mouse develops cardiomyopathy at 12 months (31) Multiple mitochondrial abnormalities were present as discussed above.
Alternate Functions of Fusion/fission Proteins
It is now quite evident that abnormalities in fission and fusion are a factor in cardiovascular disease (139). As detailed above, OPA1 was decreased in ischemic cardiomyopathy, while Mfn1/2 were increased in both ischemic and nonischemic heart failure (32). Deletion of Mfn 2 led to mild cardiac hypertrophy with small functional changes (146). Mfn2 knockout actually slowed opening of the mitochondrial permeability transition pore, protecting cardiac myocytes and leading to better recovery after ischemia/reperfusion (146). Unexpectedly, Mfn2 knockouts had higher levels of the anti-apoptotic Bcl2. Knockout of both cardiac Mfn 1 and Mfn2 was embryonic lethal, while inducible knockdown of the two proteins resulted in greater mitochondrial fragmentation, decreased mitochondrial respiration and a fatal dilated cardiomyopathy (33). Mitochondrial fission inhibition can lessen ischemia/reperfusion injury in the heart (140). As discussed earlier, both fission and fusion proteins have roles in apoptosis (120). Thus, this group of mitochondrial proteins has cellular functions beyond mitochondrial fission and fusion.
Mfn2
Mfn2 levels are reduced in skeletal muscle both in obesity and in diabetes. Exercise can mitigate some of these changes (10;62). Mfn2 was first identified as the hyperplasia suppressor gene (HSG). Mfn2 inhibited vascular smooth muscle cell (VSMC, from arteries of spontaneously hypertensive rats) proliferation in culture, and also in a rat model of balloon injury (30). Overexpression of Mfn2 results in higher levels of p21 and p27 leading to cell cycle arrest. Thus, increased Mfn2 inhibits VSMC proliferation without increasing the amount of apoptosis (30). Likewise, Mfn2 overexpression inhibited LDL provoked VSMC proliferation and ameliorated atherosclerosis in a rabbit model (68). Hence results of several studies support a significant role for Mfn2 in preventing or reducing vascular disease. Further studies of Mfn2 indicate that this protein has a role in metabolic control, an idea that has been originated by the Zorzano group (206).
Regulation of Expression of Fission/fusion Proteins
Despite great interest in fusion and fission in mammalian mitochondria, work investigating the regulation of expression fission and fusion protein genes has been very limited. The Regulation of Mfn2 expression has been studied, and the Mfn2 promoter has been shown to contain common elements in the human, rat, and mouse Mfn2 promoters. These elements include including the binding elements for NFκB, ERRα, C/EBP and GATA-1 (179). Six Sp1 sites were conserved in the promoters for all three species. Furthermore, the Mfn2 promoter did not contain a TATA box. Finally high levels of CpG islands were present, which would not be expected to be methylated in mammalian cells, and thus would lead to greater activity. Multiple Sp1 sites are present in the Mfn2 promoter, and it has been shown by ChIP assay that Sp1 binds to the Mfn2 promoter in VSMCs (179). Knockdown of Sp1 in VSMCs with shRNA led to much less Mfn2 promoter activity. However, although much is now known about regulation of Mfn2 expression, little is known about regulation of the other proteins involved in mitochondrial dynamics, and they remain to be investigated.
Mfn2 and Atherosclerosis
Mfn2 has been found to possess metabolic properties, as discussed above. In studies of Mfn2 and its possible role in the pre-atherosclerotic artery, using the ApoE-KO mouse on a high fat diet, there was a 50% decline in aortic levels of Sp1 mRNA and a 60% drop in aortic Mfn2 mRNA levels at one week (179). Provocatively, these were transient changes, disappearing after extended treatment. Nonetheless, these results provide intriguing insights into vascular disease and early atherosclerosis.
There is increasing data supporting a link between reduced Mfn2 and vascular disease. Both diabetes and obesity are associated with reduced Mfn2. Sorianello and colleagues identified that a high fat diet led to reduced Mfn2; however this drop in Mfn2 disappeared over time, despite continuing the same high fat diet. Other investigators have demonstrated that both diabetics and the obese have less Mfn2 in their skeletal muscle (10;62). Humans have repeat exposure to high fat meals, instead of a sustained exposure, and this may preclude development of an adaptive response. Exercise has been shown to enhance Mfn2 expression, offering one approach to mitigating the decrease in Mfn2 in diabetes and the obesity (62). Mfn2’s positive effects on the vasculature, such as possibly slowing atherosclerosis, make it a strong potential target in diabetes and obesity, both of which greatly enhance the risk of heart failure.
Mitochondria, Free Radicals and Aging
The mitochondrial theory of aging was initially proposed in 1972 by Denham Harman, a pioneer in free radical research. This theory proposes that the rate of aging is a function of the amount mitochondrial free-radical leakage vs. the cell’s native ability to repair any resulting damage (71). Aging and aging related diseases can be attributed to mitochondrial free-radical leakage; this is a natural by product of energy production, but greatly increases when there is mitochondrial damage. Free radicals play multiple roles in the cell. Free radicals released from mitochondria can serve to signal respiration, and can cause cellular damage to the nucleus. The deficient respiration signaled by increased free radicals can be corrected by compensatory changes in the activity of mitochondrial genes. However, if the deficiency is not compensative, the overload of free radicals will oxidize the membrane lipids, and eventually collapse membrane potential (172). The risk of myocardial infarction increases with aging. Although the mortality from myocardial infarction has been reduced through a combination of preventative measures and intervention, heart failure, which can result from coronary disease, continues to have a very high mortality with a 50% five year survival (55;132). Overall there has been an increase in the prevalence of heart failure with better survival from coronary disease (132). It is known that there is an increase in the levels of free radicals, including both superoxide anion and the hydroxyl radical, with cardiac reperfusion, which is a common occurrence in cardiac treatment (5;53;123). During the first minutes of reflow, free radicals increase markedly and remain elevated for a prolonged period after restoration of flow in the coronary (182). Mitochondria have been identified as the major source of free radicals during reperfusion, and a potential site of free radical-mediated dysfunction. During normal cellular metabolism, free radicals are produced at the site of complex I and III (20). Ischemia reduces iron-sulfur and ubiquinone, inhibits complex I (also a source of free radicals in heart failure), and decreases superoxide dismutase activity, which results in increased free radical levels with reoxygenation (182). All of these settings enhance endogenous free radical production, which then compounds any myocardial injury, and this response is increased with aging(92;180).
Conclusions
Mitochondrial fission and fusion are essential processes for maintaining mtDNA and normal mitochondrial function. Changes in the proteins involved in fission and fusion have been reported in a number of diseases, including ischemic heart failure, making them a possible therapeutic target. The fission and fusion protein also have roles in apoptosis and mitophagy. Overall they are essential for maintaining cellular homeostasis. Yet, there are some who are skeptical about the feasibility of repetitive mitochondrial fission and fusion in the densely packed adult cardiac myocyte. However, these processes have been shown essential to maintaining mtDNA and mitochondrial function. Are mitochondrial dynamic processes the only way mtDNA and mitochondrial function are maintained, or are there other mechanisms at work in the heart? Given the high energy needs to the adult heart, the need for these processes would seem greatest in the heart and in the brain. However, processes, such as mitophagy, might substitute for frequent fission and fusion. Much investigation remains to be done to understand the many roles of fission and fusion proteins, some of which extend far beyond the mitochondria. There are many interesting things yet to be explored.
Figure 4.
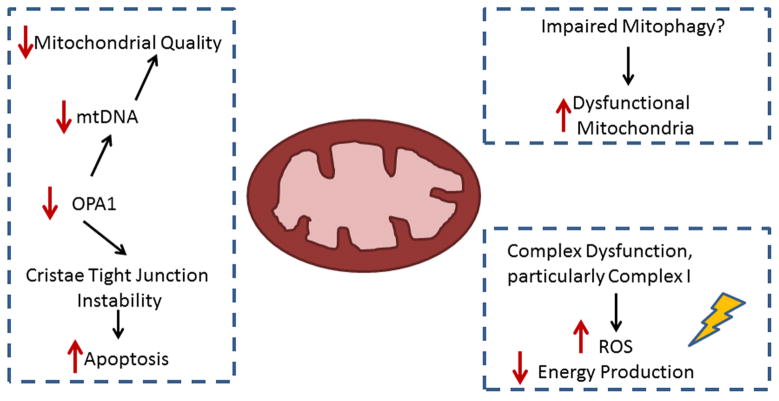
Summary of Mitochondrial Dynamics and Ischemic Heart Failure. Major findings with multiple studies supporting them are shown. The next few years should lead to identification of more roles for fission and fusion in heart failure.
Acknowledgments
Supported by HL077281 and HL07907 both to AAK.
AAK is a Staff Cardiologist at the Mather VA Medical Facility, Sacramento, CA and a Professor of Medicine and Pharmacology at the University of California, Davis.
Footnotes
Disclaimer: This review paper does not represent the views of the U.S. Department of Veterans Affairs or the United States Government
Reference List
- 1.Ahuja P, Wanagat J, Wang Z, Wang Y, Liem DA, Ping P, et al. Divergent Mitochondrial Biogenesis Responses in Human Cardiomyopathy. Circulation. 2013 Apr 15;127:1957–67. doi: 10.1161/CIRCULATIONAHA.112.001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aich A, Shaha C. Novel Role of Calmodulin in Regulating Protein Transport to Mitochondria in a Unicellular Eukaryote. Molecular and Cellular Biology. 2013 Nov 15;33(22):4579–93. doi: 10.1128/MCB.00829-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altmann R. Die Elementarorganismen und ihre Beziehungen zu den Zellen. Leipzig: Veit & Co; 1890. [Google Scholar]
- 4.Amati-Bonneau P, Milea D, Bonneau D, Chevrollier A, Ferré M, Guillet V, et al. OPA1-associated disorders: Phenotypes and pathophysiology. The International Journal of Biochemistry & Cell Biology. 2009 Oct;41(10):1855–65. doi: 10.1016/j.biocel.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. Journal of Biological Chemistry. 1993 Sep 5;268(25):18532–41. [PubMed] [Google Scholar]
- 6.Amiott EA, Lott P, Soto J, Kang PB, McCaffery JM, DiMauro S, et al. Mitochondrial fusion and function in Charcot-Marie-Tooth type 2A patient fibroblasts with mitofusin 2 mutations. Experimental Neurology. 2008;211(1):115–27. doi: 10.1016/j.expneurol.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashford TP, Porter KR. CYTOPLASMIC COMPONENTS IN HEPATIC CELL LYSOSOMES. J Cell Biol. 1962 Jan 1;12(1):198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashrafian H, Docherty L, Leo V, Towlson C, Neilan M, Steeples V, et al. A Mutation in the Mitochondrial Fission Gene Dnm1 Leads to Cardiomyopathy. PLoS Genet. 2010 Jun 24;6(6):e1001000. doi: 10.1371/journal.pgen.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayaz M, Turan B. A Critical Balance Between Oxidative Stress and Antioxidant Defense in Cardiovascular System Under Hyperglycemia: A Summary of Experimental Studies. In: Turan B, Dhalla NS, editors. Diabetic Cardiomyopathy: Biochemical and Molecular Mechanisms. New York: Springer-Verlag; 2014. pp. 123–41. [Google Scholar]
- 10.Bach D, Naon D, Pich S, Sorriano FX, Vega N, Rieusset J, et al. Expression of Mfn2, the Charcot-Marie-Tooth Neuropathy Type 2A Gene, in Human Skeletal Muscle. Diabetes. 2005;54:2685–93. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 11.Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]
- 12.Baseler WA, Dabkowski ER, Williamson C, Croston TL, Thapa D, Powell MJ, et al. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011 Feb 2;300(2):R186–R200. doi: 10.1152/ajpregu.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, et al. Mitochondrial bioenergetics and structural network organization. Journal of Cell Science. 2007 Mar 1;120(5):838–48. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 14.Bhamra G, Hausenloy D, Davidson S, Carr R, Paiva M, Wynne A, et al. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103(3):274–84. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 15.Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. PNAS. 2011 Jun 7;108(23):9572–7. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blatch GL, Lässle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays. 1999 Nov 1;21(11):932–9. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Böckler S, Westermann B. Mitochondrial ER Contacts Are Crucial for Mitophagy in Yeast. Developmental Cell. 2014 Feb 24;28(4):450–8. doi: 10.1016/j.devcel.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Borchi E, Bargelli V, Stillitano F, Giordano C, Sebastiani M, Nassi PA, et al. Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2010 Mar;1802(3):331–8. doi: 10.1016/j.bbadis.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Böttinger L, Guiard B, Oeljeklaus S, Kulawiak B, Zufall N, Wiedemann N, et al. A complex of Cox4 and mitochondrial Hsp70 plays an important role in the assembly of the cytochrome c oxidase. Molecular Biology of the Cell. 2013 Sep 1;24(17):2609–19. doi: 10.1091/mbc.E13-02-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods in Enzymology. 1984;105:429–35. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- 21.Brenner D, Mak TW. Mitochondrial cell death effectors. Current Opinion in Cell Biology. 2009 Dec;21(6):871–7. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, et al. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovacular Research. 2010;85:376–84. doi: 10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- 23.Byun J, Son SM, Cha MY, Shong M, Hwang YJ, Kim Y, et al. CR6-interacting factor 1 is a key regulator in A[beta]-induced mitochondrial disruption and pathogenesis of Alzheimer’s disease. Cell Death Differ. 2014 Nov 7; doi: 10.1038/cdd.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carelli V, Musumeci O, Caporali L, Zanna C, La Morgia C, Del Dotto V, et al. Syndromic parkinsonism and dementia associated with OPA1 missense mutations. Ann Neurol. 2015 Jul;78(1):21–38. doi: 10.1002/ana.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan DC. Mitochondria: Dynamic Organelles in Disease, Aging and Development. Cell. 2006;125:1241–52. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Chance B. The nature of electron transfer and energy coupling reactions. FEBS Letters. 1972;23(1):3–20. doi: 10.1016/0014-5793(72)80272-2. [DOI] [PubMed] [Google Scholar]
- 27.Chang YW, Chang YT, Wang Q, Lin JJ-C, Chen YJ, Chen CC. Quantitative Phosphoproteomic Study of Pressure-Overloaded Mouse Heart Reveals Dynamin-Related Protein 1 as a Modulator of Cardiac Hypertrophy. Molecular & Cellular Proteomics. 2013 Nov 1;12(11):3094–107. doi: 10.1074/mcp.M113.027649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, et al. Mitochondrial Fusion Is Required for mtDNA Stability in Skeletal Muscle and Tolerance of mtDNA Mutations. Cell. 2010;141:280–9. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Chomyn A, Chan DC. Disruption of Fusion Results in Mitochondrial Heterogeneity and Dysfunction. Journal of Biological Chemistry. 2005 Jul 15;280(28):26185–92. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, Guo X, Ma D, Guo Y, Li Q, Yang D, et al. Dysregulation of HSG triggers vascular proliferative disorders. Nature Cell Biology. 2004;6(9):872–83. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Liu TT, Tran AL, Lu X, Tomilov AA, Davies VJ, et al. OPA1 Mutation and Late Onset Cardiomyopathy: Mitochondrial Dysfunction and mtDNA Instability. Journal of the American Heart Association. 2012;1:e003012. doi: 10.1161/JAHA.112.003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovascular Research. 2009 Oct 1;84(1):91–9. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Liu Y, Dorn GW. Mitochondrial Fusion is Essential for Organelle Function and Cardiac Homeostasis. Circ Res. 2011;109(12):1327–31. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Dorn GW. PINK1-Phosphorylated Mitofusin 2 Is a Parkin Receptor for Culling Damaged Mitochondria. Science. 2013 Apr 26;340(6131):471–5. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortes R, Portoles M, Rosello-Lletli E, Almenar L, Salvador A, Rivera M. Nuclear changes and p62 expression in ischemic and dilated cardiomyopathy. Revista espanola de cardiologia. 2007;60:1319–23. doi: 10.1157/13113939. [DOI] [PubMed] [Google Scholar]
- 36.Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D, et al. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington’s disease to apoptotic stimuli. EMBO Molecular Medicine. 2010 Nov 10;2(12):490–503. doi: 10.1002/emmm.201000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crow MT, Mani K, Nam YJ, Kitsis RN. The Mitochondrial Death Pathway and Cardiac Myocyte Apoptosis. Circulation Research. 2004 Nov 12;95(10):957–70. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 38.Darshi M, Mendiola VL, Mackey MR, Murphy AN, Koller A, Perkins GA, et al. ChChd3, an Inner Mitochondrial Membrane Protein, Is Essential for Maintaining Crista Integrity and Mitochondrial Function. Journal of Biological Chemistry. 2011 Jan 28;286(4):2918–32. doi: 10.1074/jbc.M110.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davey KM, Parboosingh JS, McLeod DR, Chan A, Casey R, Ferreira P, et al. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J Med Genet. 2006 May 3;43(5):385–93. doi: 10.1136/jmg.2005.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies V, Hollins A, Piechota M, Yip W, Davies J, White K, et al. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Human Molecular Genetics. 2007;16:1307–18. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- 41.Deng N, Zhang J, Zong C, Wang Y, Lu H, Yang P, et al. Phosphoproteome Analysis Reveals Regulatory Sites in Major Pathways of Cardiac Mitochondria. Molecular & Cellular Proteomics. 2011 Feb 1;10(2) doi: 10.1074/mcp.M110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988;332:800–5. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- 43.Detmer SA, Chan DC. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. Journal of Cell Biology. 2007;176:405–14. doi: 10.1083/jcb.200611080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietrich M, Liu ZW, Horvath T. Mitochondrial Dynamics Controlled by Mitofusins Regulate Agrp Neuronal Activity and Diet-Induced Obesity. Cell. 2013 Sep 26;155(1):188–99. doi: 10.1016/j.cell.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorn GW, Clark CF, Eschenbacher WH, Kang MY, Engelhard JT, Warner SJ, et al. MARF and Opa1 Control Mitochondrial and Cardiac Function in Drosophila. Circ Res. 2011;108:12–7. doi: 10.1161/CIRCRESAHA.110.236745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drew BG, Ribas V, Le JA, Henstridge DC, Zhou Z, Soleymani T, et al. HSP72 is a Mitochondrial Stress Sensor Critical for Parkin Action, Oxidative Metabolism, and Insulin Sensitivity in Skeletal Muscle. Diabetes. 2014 doi: 10.2337/db13-0665. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ElAchouri G, Vidoni S, Zanna C, Pattyn A, Boukhaddaoui H, Gaget K, et al. OPA1 links human mitochondrial genome maintenance to mtDNA replication. Genome Research. 2011;21:12–20. doi: 10.1101/gr.108696.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elsherif L, Ortines RV, Saari JT, Kang YJ. Congestive Heart Failure in Copper-Deficient Mice. Experimental Biology and Medicine. 2003 Jul 1;228(7):811–7. doi: 10.1177/15353702-0322807-06. [DOI] [PubMed] [Google Scholar]
- 49.Emr SD, Vassarotti A, Garrett J, Geller BL, Takeda M, Douglas MG. The amino terminus of the yeast F1-ATPase beta-subunit precursor functions as a mitochondrial import signal. J Cell Biol. 1986 Feb 1;102(2):523–33. doi: 10.1083/jcb.102.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Endo T, Yamano K. Multiple pathways for mitochondrial protein traffic. Biological Chemistry. 2009;390:723–30. doi: 10.1515/BC.2009.087. [DOI] [PubMed] [Google Scholar]
- 51.Ernster L, Schatz G. Mitochondria: A Historical Review. Journal of Cell Biology. 1981;91:227s–55s. doi: 10.1083/jcb.91.3.227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang L, Moore XL, Gao XM, Dart AM, Lim YL, Du XJ. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sciences. 2007 May 16;80(23):2154–60. doi: 10.1016/j.lfs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Ferrari R. The role of mitochondria in ischemic heart disease. Journal of Cardiovascular Pharmacology. 1996;28(Suppl 1):S1–S10. doi: 10.1097/00005344-199600003-00002. [DOI] [PubMed] [Google Scholar]
- 54.Ferré M, Caignard A, Milea D, Leruez S, Cassereau J, Chevrollier A, et al. Improved Locus-Specific Database for OPA1 Mutations Allows Inclusion of Advanced Clinical Data. Human Mutation. 2015;36(1):20–5. doi: 10.1002/humu.22703. [DOI] [PubMed] [Google Scholar]
- 55.Ford ES, Capewell S. Proportion of the Decline in Cardiovascular Mortality Disease due to Prevention Versus Treatment: Public Health Versus Clinical Care. Annu Rev Public Health. 2011 Mar 18;32(1):5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
- 56.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, et al. OPA1 Controls Apoptotic Cristae Remodeling Independently from Mitochondrial Fusion. Cell. 2006 Jul 14;126(1):177–89. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 57.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER Tubules Mark Sites of Mitochondrial Division. Science. 2011 Oct 21;334(6054):358–62. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gandre-Babbe S, van der Bliek AM. The Novel Tail-anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells. Molecular Biology of the Cell. 2008 Jun 1;19(6):2402–12. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garnier A, Zoll J, Fortin D, N’Guessan Bt, Lefebvre F, Geny B, et al. Control by Circulating Factors of Mitochondrial Function and Transcription Cascade in Heart Failure/CLINICAL PERSPECTIVE. Circulation: Heart Failure. 2009 Jul 1;2(4):342–50. doi: 10.1161/CIRCHEARTFAILURE.108.812099. [DOI] [PubMed] [Google Scholar]
- 60.Garrib A, McMurray WC. Cell-free synthesis of a putative precursor to the rat liver mitochondrial glycerol-3-phosphate dehydrogenase. Journal of Biological Chemistry. 1988 Dec 25;263(36):19821–6. [PubMed] [Google Scholar]
- 61.Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Progress in Neurobiology. 2014 Jan;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Gibala MJ, Little JP, MacDonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. Journal of Physiology. 2012;590(pt.5):1077–84. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) Ameliorates Pressure Overload Induced Heart Failure. PLOS One. 2012 Mar 27;7(3):e32388. doi: 10.1371/journal.pone.0032388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomes LC, Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2008 Jul;1777(7–8):860–6. doi: 10.1016/j.bbabio.2008.05.442. [DOI] [PubMed] [Google Scholar]
- 65.Gottlieb R, Mentzer R. Autophagy: an affair of the heart. Heart Fail Rev. 2013;18(5):575–84. doi: 10.1007/s10741-012-9367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Green DE. The electromechanochemical model for energy coupling in mitochondria. Biochimica et Biophysica Acta. 1974;346(1):27–78. doi: 10.1016/0304-4173(74)90011-1. [DOI] [PubMed] [Google Scholar]
- 67.Griffiths E. Mitochondria and Heart Disease. In: Scatena R, Bottoni P, Giardina B, editors. Advances in Mitochondrial Medicine. 942. Springer; Netherlands: 2012. pp. 249–67. [DOI] [PubMed] [Google Scholar]
- 68.Guo Y, Chen K, Gao W, Li Q, Chen L, Wang G, et al. Overexpression of Mitofusin 2 inhibited oxidized low-density lipoprotein induced vascular smooth muscle cell proliferation and reduced atherosclerotic lesion formation in rabbit. Biochemical and Biophysical Research Communications. 2007;363:411–7. doi: 10.1016/j.bbrc.2007.08.191. [DOI] [PubMed] [Google Scholar]
- 69.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2009 Nov;1787(11):1402–15. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 70.Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013 Mar 21;495(7441):389–93. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 71.Harman D. The biologic clock: The mitochondria? Journal of the American Geriatric Society. 1972;20:145–7. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi M, Imanaka-Yoshida K, Yoshida T, Wood M, Fearns C, Tatake R, et al. A crucial role of mitochondrial HSP40 in preventing dilated cardiomyopathy. Nature Medicine. 2006;12:128–32. doi: 10.1038/nm1327. [DOI] [PubMed] [Google Scholar]
- 73.Hom J, Yu T, Yoon Y, Porter G, Sheu SS. Regulation of mitochondrial fission by intracellular Ca2+ in rat ventricular myocytes. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2010 Jun;1797(6–7):913–21. doi: 10.1016/j.bbabio.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu X, Xu X, Lu Z, Zhang P, Fassett J, Zhang Y, et al. AMP Activated Protein Kinase-a2 Regulates Expression of Estrogen-Related Receptor-a, a Metabolic Transcription Factor Related to Heart Failure Development. Hypertension. 2011 Oct 1;58(4):696–703. doi: 10.1161/HYPERTENSIONAHA.111.174128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hua D, Liu My, Cheng Zd, Qin Xj, Zhang Hm, Chen Y, et al. Small interfering RNA-directed targeting of Toll-like receptor 4 inhibits human prostate cancer cell invasion, survival, and tumorigenicity. Molecular Immunology. 2009 Sep;46(15):2876–84. doi: 10.1016/j.molimm.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 76.Hudson G, Amati-Bonneau P, Blakely EL, Stewart JD, He L, Schaefer AM, et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain. 2008 Jan 25;131(2):329–37. doi: 10.1093/brain/awm272. [DOI] [PubMed] [Google Scholar]
- 77.Hwang SJ, Kim W. Mitochondrial Dynamics in the Heart as a Novel Therapeutic Target for Cardioprotection. Chonnam Med J. 2013 Dec;49(3):101–7. doi: 10.4068/cmj.2013.49.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, Grimm S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J. 2011 Feb 2;30(3):556–68. doi: 10.1038/emboj.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jakobs S. High resolution imaging of live mitochondria. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2006 May;1763(5–6):561–75. doi: 10.1016/j.bbamcr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Javadov S, Rajapurohitam V, Killic A, Hunter JC, Zeidan A, Faruq NS, et al. Expression of mitochondrial fusion-fission proteins during post-infarction remodeling: the effect of NHE-1 inhibition. Basic Res Cardiol. 2011;106:99–109. doi: 10.1007/s00395-010-0122-3. [DOI] [PubMed] [Google Scholar]
- 81.Kane L, Youle R. Mitochondrial fission and fusion and their roles in the heart. J Mol Med. 2010;88(10):971–9. doi: 10.1007/s00109-010-0674-6. [DOI] [PubMed] [Google Scholar]
- 82.Karamanlidis G, Garcia-Menendez L, Kolwicz SC, Lee C, Tian R. Promoting PGC-1a-driven biogenesis is detrimental in pressure-overloaded mouse hearts. Am J Physiol Heart Circ Physiol. 2014;307:H1307–H1316. doi: 10.1152/ajpheart.00280.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karamanlidis G, Bautista-Hernandez V, Fynn-Thompson F, del Nido P, Tian R. Impaired Mitochondrial Biogenesis Precedes Heart Failure in Right Ventricular Hypertrophy in Congenital Heart Disease. Circulation: Heart Failure. 2011 Nov 1;4(6):707–13. doi: 10.1161/CIRCHEARTFAILURE.111.961474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz J, Suthammarak W, Gong G, et al. Mitochondrial Complex I Deficiency Increases Protein Acetylation and Accelerates Heart Failure. Cell Metabolism. 2013 Aug 6;18(2):239–50. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, Del Monte F, Tian R. Defective DNA Replication Impairs Mitochondrial Biogenesis In Human Failing Hearts. Circulation Research. 2010 May 14;106(9):1541–8. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kassiotis C, Ballal K, Wellnitz K, Vela D, Gong M, Salazar R, et al. Markers of Autophagy Are Downregulated in Failing Human Heart After Mechanical Unloading. Circulation. 2009 Sep 15;120(11 suppl 1):S191–S197. doi: 10.1161/CIRCULATIONAHA.108.842252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, et al. Extracellular Heat Shock Protein 60, Cardiac Myocytes and Apoptosis. Circ Res. 2009;105(12):1186–95. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knowlton AA, Chen L. Current Advances in Cardiology. Medimond Press; Jul 25, 2013. Mitochondrial Fragmentation and Heart Failure: Fission and Fusion; pp. 119–22. 13 A.D. [Google Scholar]
- 89.Knowlton AA, Kapadia S, Torre-Amione G, Durand JB, Bies R, Young J, et al. Differential expression of heat shock proteins in normal and failing human hearts. Journal of Molecular and Cellular Cardiology. 1998;30:811–8. doi: 10.1006/jmcc.1998.0646. [DOI] [PubMed] [Google Scholar]
- 90.Koehler CM. NEW DEVELOPMENTS IN MITOCHONDRIAL ASSEMBLY. Annual Review of Cell and Developmental Biology. 2004 Oct 8;20(1):309–35. doi: 10.1146/annurev.cellbio.20.010403.105057. [DOI] [PubMed] [Google Scholar]
- 91.Korobova F, Ramabhadran V, Higgs HN. An Actin-Dependent Step in Mitochondrial Fission Mediated by the ER-Associated Formin INF2. Science. 2013 Jan 25;339(6118):464–7. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuka S, Tatarkova Z, Racay P, Lehotsky J, Dobrota D, Kaplan P. Effect of aging on formation of reactive oxygen specires by mitochondria of rat heart. General Physiology and Biophysics. 2013;32(3):415–20. doi: 10.4149/gpb_2013049. [DOI] [PubMed] [Google Scholar]
- 93.Kwong JQ, Davis J, Baines CP, Sargent MA, Karch J, Wang X, et al. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014 Aug;21(8):1209–17. doi: 10.1038/cdd.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex Differences in the Phosphorylation of Mitochondrial Proteins Result in Reduced Production of Reactive Oxygen Species and Cardioprotection in Females. Circulation Research. 2010 Jun 11;106(11):1681–91. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Latif N, Khan MA, Birks E, O’Farrell A, Westbrook J, Dunn MJ, et al. Upregulation of the Bcl-2 family of proteins in end stage heart failure. Journal of the American College of Cardiology. 2000;35:1769–77. doi: 10.1016/s0735-1097(00)00647-1. [DOI] [PubMed] [Google Scholar]
- 96.Latif N, Taylor PM, Khan MA, Yacoub MH, Dunn MJ. The expression of heat shock protein 60 in patients with dilated cardiomyopathy. Basic Research in Cardiology. 1999;94:112–9. doi: 10.1007/s003950050133. [DOI] [PubMed] [Google Scholar]
- 97.Lea PJ, Hollenberg MJ. Mitochondrial structure revealed by high-resolution scanning electron microscopy. American Journal of Anatomy. 1989;184:245–57. doi: 10.1002/aja.1001840308. [DOI] [PubMed] [Google Scholar]
- 98.Lee Y, Jeong S, Karbowski M, Smith CL, Youle RJ. Roles of Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1 and OPA1 in Apoptosis. Molecular Biology of the Cell. 2004;15:5001–11. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial Fusion in Human Cells Is Efficient, Requires the Inner Membrane Potential, and Is Mediated by Mitofusins. Molecular Biology of the Cell. 2002;13:4343–54. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lemasters JJ. Selective Mitochondrial Autophagy, or Mitophagy, as a Targeted Defense Against Oxidative Stress, Mitochondrial Dysfunction, and Aging. Rejuvenation Research. 2005 Mar 1;8(1):3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 101.Li J, Qi M, Li C, Shi D, Zhang D, Xie D, et al. Tom70 serves as a molecular switch to determine pathological cardiac hypertrophy. Cell Res. 2014 Aug;24(8):977–93. doi: 10.1038/cr.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li M, Zhong Z, Zhu J, Xiang D, Dai N, Cao X, et al. Identification and Characterization of Mitochondrial Targeting Sequence of Human Apurinic/Apyrimidinic Endonuclease 1. Journal of Biological Chemistry. 2010 May 14;285(20):14871–81. doi: 10.1074/jbc.M109.069591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995 Dec;11(4):376–81. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 104.Liesa M, Palacin M, Zorzano A. Mitochondrial Dynamics in Mammalian Health and Disease. Physiol Rev. 2009 Jul;89(3):799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 105.Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon S, et al. HSP60 in Heart Failure: Abnormal Distribution and Role in Cardiac Myocyte Apoptosis. American Journal of Physiology. 2007;293:H2238–H2247. doi: 10.1152/ajpheart.00740.2007. [DOI] [PubMed] [Google Scholar]
- 106.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria - Specificity in membrane targeting for death. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2011 Apr;1813(4):532–9. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 107.Lithgow T, Schneider A. Evolution of macromolecular import pathways in mitochondria, hydrogenosomes and mitosomes. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010 Mar 12;365(1541):799–817. doi: 10.1098/rstb.2009.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu H, Li Y, Li Y, Liu B, Wu H, Wang J, et al. Cloning and Functional Analysis of FLJ20420: A Novel Transcription Factor for the BAG-1 Promoter. PLOS One. 2012 May 2;7(5):e34832. doi: 10.1371/journal.pone.0034832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu T, Chen L, Kim E, Tran D, Phinney BS, Knowlton AA. Mitochondrial proteome remodeling in ischemic heart failure. Life Sciences. 2014;101:27–36. doi: 10.1016/j.lfs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lodi R, Tonon C, Valentino M, Iotti S, Clementi V, Malucelli E, et al. Deficit of In Vivo Mitochondrial ATP Production in OPA1-Related Dominant Optic Neuropathy. Ann Neurol. 2004;56:719–23. doi: 10.1002/ana.20278. [DOI] [PubMed] [Google Scholar]
- 111.Loson OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Molecular Biology of the Cell. 2013 Mar 1;24(5):659–67. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lukyanenko V, Chikando A, Lederer WJ. Mitochondria in cardiomyocyte Ca2+ signaling. The International Journal of Biochemistry & Cell Biology. 2009 Oct;41(10):1957–71. doi: 10.1016/j.biocel.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.MacKenzie JA, Payne RM. Mitochondrial protein import and human health and disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2007 May;1772(5):509–23. doi: 10.1016/j.bbadis.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mandal K, Jahangiri M, Mukhin M, Poloniecki J, Camm AJ, Xu Q. Association of Anti-Heat Shock Protein 65 Antibodies With Development of Postoperative Atrial Fibrillation. Circulation. 2004 Oct 26;110(17):2588–90. doi: 10.1161/01.CIR.0000136825.96029.A5. [DOI] [PubMed] [Google Scholar]
- 115.Mandi Y, Hogye M, Talha EM, Skolak E, Csanady M. Cytokine Production and Antibodies against Heat Shock Protein 60 in Cardiomyopathies of Different Origins. Pathobiology. 2000;68(3):150–8. doi: 10.1159/000055916. [DOI] [PubMed] [Google Scholar]
- 116.Margulis L. Symbiosis in Cell Evolution. New York: W.H. Freeman; 1992. [Google Scholar]
- 117.Marin-Garcia J, Goldenthal MJ, Moe GW. Mitochondrial pathology in cardiac failure. Cardiovascular Research. 2001 Jan 1;49(1):17–26. doi: 10.1016/s0008-6363(00)00241-8. [DOI] [PubMed] [Google Scholar]
- 118.Martin J, Horwich AL, Hartl FU. Prevention of protein denaturation under heat stress by the chaperonin HSP 60. Science. 1992;258:995–8. doi: 10.1126/science.1359644. [DOI] [PubMed] [Google Scholar]
- 119.Martin J, Mayhew M, Langer T, Hartl FU. The reaction cycle of GroEL and GroES in chaperonin-assisted protein folding. Nature. 1993;366:228–33. doi: 10.1038/366228a0. [DOI] [PubMed] [Google Scholar]
- 120.Martinou JC, Youle RJ. Mitochondria in Apoptosis: Bcl-2 Family Members and Mitochondrial Dynamics. Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marusich MF, Robinson BH, Taanman JW, Kim SJ, Schillace R, Smith JL, et al. Expression of mtDNA and nDNA encoded respiratory chain proteins in chemically and genetically-derived Rho0 human fibroblasts: a comparison of subunit proteins in normal fibroblasts treated with ethidium bromide and fibroblasts from a patient with mtDNA depletion syndrome. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1997 Dec 31;1362(2–3):145–59. doi: 10.1016/s0925-4439(97)00061-6. [DOI] [PubMed] [Google Scholar]
- 122.Mayorov VI, Lowrey AJ, Biousse V, Newman NJ, Cline SD, Brown MD. Mitochondrial oxidative phosphorylation in autosomal dominant optic atrophy. BMC Biochemistry. 2008;9:22. doi: 10.1186/1471-2091-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCord JM. Free radicals and myocardial ischemia: overview and outlook. Free Radical Biology and Medicine. 1988;4(1):9–14. doi: 10.1016/0891-5849(88)90005-6. [DOI] [PubMed] [Google Scholar]
- 124.Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, et al. Mitochondrial Inner-Membrane Fusion and Crista Maintenance Requires the Dynamin-Related GTPase Mgm1. Cell. 2006;127:383–95. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 125.Mendoza JA, Lorimer GH, Horowitz PM. Chaperonin cpn60 from Escherichia coli protects the mitochondrial enzyme rhodanese against heat inactivation and supports folding at elevated temperatures. Journal of Biological Chemistry. 1992;267:17631–4. [PubMed] [Google Scholar]
- 126.Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Lower B, Wunderlich FT, et al. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes and Development. 2008 Feb 15;22(4):476–88. doi: 10.1101/gad.460708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Michaelis L. Die vitale Fãrbung, eine Darstellungsmethode der zellgranula. Arch f Mikroskop Anat. 1900;55:558–75. [Google Scholar]
- 128.Mihara K, Omura T. Cytosolic Factors in Mitochondrial Protein Import. Experientia. 1996;52:1063–8. doi: 10.1007/BF01952103. [DOI] [PubMed] [Google Scholar]
- 129.Minners J, McLeod CJ, Sack MN. Mitochondrial plasticity in classical ischemic preconditioning—moving beyond the mitochondrial KATP channel. Cardiovascular Research. 2003 Jul 1;59(1):1–6. doi: 10.1016/s0008-6363(03)00337-7. [DOI] [PubMed] [Google Scholar]
- 130.Mirebeau-Prunier D, Le Pennec S, Jacques C, Gueguen N, Poirier J, Malthiery Y, et al. Estrogen-related receptor a and PGC-1-related coactivator constitute a novel complex mediating the biogenesis of functional mitochondria. FEBS Journal. 2010;277:713–25. doi: 10.1111/j.1742-4658.2009.07516.x. [DOI] [PubMed] [Google Scholar]
- 131.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–8. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 132.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics - 2015 Update: A Report From the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 133.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p Gtpase-Mediated Mitochondrial Fission Is a Multi-Step Process Requiring the Novel Integral Membrane Component Fis1p. J Cell Biol. 2000 Oct 16;151(2):367–80. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murley A, Lackner LL, Osman C, West M, Voeltz GK, Walter P, et al. ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. e Life Sciences. 2013 May 14;2:e00422. doi: 10.7554/eLife.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, et al. Apoptosis in heart failure: Release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proceedings of the National Academy of Science, USA. 1999;96:8144–9. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nunnari J, Marshall WF, Straight A, Murray A, Sedat JW, Walter P. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Molecular Biology of the Cell. 1997 Jul 1;8(7):1233–42. doi: 10.1091/mbc.8.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, et al. Loss of OPA1 Perturbates the Mitochondrial Inner Membrane Structure and Integrity, Leading to Cytochrome c Release and Apoptosis. Journal of Biological Chemistry. 2003 Mar 7;278(10):7743–6. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 138.Olichon A, Guillou E, Delettre C, Landes T, Arnaune-Pelloquin L, Emorine LJ, et al. Mitochondrial dynamics and disease, OPA1. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2006 May;1763(5–6):500–9. doi: 10.1016/j.bbamcr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 139.Ong S, Hausenloy DJ. Mitochondrial morphology and cardiovascular disease. Cardiovascular Research. 2010;88:16–29. doi: 10.1093/cvr/cvq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ong S, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting Mitochondrial Fission Protects the Heart Against Ischemia/Reperfusion Injury. Circulation. 2010;121:2012–22. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 141.Ono H, Nakamura H, Matsuzaki M. A NADH Dehydrogenase Ubiquinone Flavoprotein is Decreased in Patients with Dilated Cardiomyopathy. Internal Medicine. 2010;49(19):2039–42. doi: 10.2169/internalmedicine.49.3710. [DOI] [PubMed] [Google Scholar]
- 142.Osman C, Merkwirth C, Langer T. Prohibitins and the functional compartmentalization of mitochondrial membranes. Journal of Cell Science. 2009 Nov 1;122(21):3823–30. doi: 10.1242/jcs.037655. [DOI] [PubMed] [Google Scholar]
- 143.Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, YOULE RJ, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010 Dec 13;191(6):1141–58. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Palade GE. The fine structure of mitochondria. Anat Rec. 1952;114:427–51. doi: 10.1002/ar.1091140304. [DOI] [PubMed] [Google Scholar]
- 145.Palmer CS, Elgass KD, Parton RG, Osellame LD, Stojanovski D, Ryan MT. Adaptor Proteins MiD49 and MiD51 Can Act Independently of Mff and Fis1 in Drp1 Recruitment and Are Specific for Mitochondrial Fission. Journal of Biological Chemistry. 2013 Sep 20;288(38):27584–93. doi: 10.1074/jbc.M113.479873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O’Shea KM, et al. Mitofusin-2 Maintains Mitochondrial Structure and Contributes to Stress-Induced Permeability Transition in Cardiac Myocytes. Molecular and Cellular Biology. 2011;31(6):1309–28. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patten D, Wong J, Khacho M, Soubannier V, Mailloux R, Pilon-Larose K, et al. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 2014;33(22):2676–91. doi: 10.15252/embj.201488349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peixoto PM, Ryu SY, Kinnally KW. Mitochondrial ion channels as therapeutic targets. FEBS Letters. 2010 May 17;584(10):2142–52. doi: 10.1016/j.febslet.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pollak MN. Investigating Metformin for Cancer Prevention and Treatment: The End of the Beginning. Cancer Discovery. 2012 Sep 1;2(9):778–90. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 150.Procaccio V, Mousson B, Beugnot R, Duborjal H, Feillet F, Putet G, et al. Nuclear DNA origin of mitochondrial complex I deficiency in fatal infantile lactic acidosis evidenced by transnuclear complementation of cultured fibroblasts. J Clin Invest. 1999 Jul 1;104(1):83–92. doi: 10.1172/JCI6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. Journal of Cell Science. 2013 Feb 1;126(3):789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ramonet D, Perier C, Recasens A, Dehay B, Bové J, Costa V, et al. Optic atrophy 1 mediates mitochondria remodeling and dopaminergic neurodegeneration linked to complex 1 deficiency. Cell Death and Differentiation. 2013;20(1):771–785. doi: 10.1038/cdd.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rees PSC, Davidson SM, Harding SE, McGregor C, Elliot PM, Yellon DM, et al. The Mitochondrial Permeability Transition Pore as a Target for Cardioprotection in Hypertrophic Cardiomyopathy. Cardiovasc Drugs Ther. 2013;27(3):235–7. doi: 10.1007/s10557-013-6447-z. [DOI] [PubMed] [Google Scholar]
- 154.Roos D, Seeger R, Puntel R, Vargas Barbosa N. Role of Calcium and Mitochondria in MeHg-Mediated Cytotoxicity. J Biomed Biotechnol. 2012 Jul 3;2012:248764. doi: 10.1155/2012/248764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rostovtseva T, Bezrukov S. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr. 2008;40(3):163–70. doi: 10.1007/s10863-008-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sack MN. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovascular Research. 2006 Nov 1;72(2):210–9. doi: 10.1016/j.cardiores.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 157.Sagan L. On the origin of mitosing cells. Journal of Theoretical Biology. 1967;14(3):255–74. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 158.Schapira AHV. Mitochondrial involvement in Parkinson’s disease, Huntington’s disease, hereditary spastic paraplegia and Friedreich’s ataxia. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1999 Feb 9;1410(2):159–70. doi: 10.1016/s0005-2728(98)00164-9. [DOI] [PubMed] [Google Scholar]
- 159.Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–26. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- 160.Schatz G. The Protein Import System of Mitochondria. Journal of Biological Chemistry. 1996 Dec 13;271(50):31763–6. doi: 10.1074/jbc.271.50.31763. [DOI] [PubMed] [Google Scholar]
- 161.Scheffler IE. A century of mitochondrial research: achievements and perspectives. Mitochondrion. 2000;1:3–31. doi: 10.1016/s1567-7249(00)00002-7. [DOI] [PubMed] [Google Scholar]
- 162.Scheffler IE. Mitochondria. 2. Hoboken, N.J: Wiley; 2011. The Protein Import Machinery of Mitochondria. [Google Scholar]
- 163.Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial Dysfunction Is a Primary Event in Glutamate Neurotoxicity. The Journal of Neuroscience. 1996 Oct 1;16(19):6125–33. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schmidt O, Pfanner N, Meisinger C. Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol. 2010 Sep;11(9):655–67. doi: 10.1038/nrm2959. [DOI] [PubMed] [Google Scholar]
- 165.Schultz B, Chan S. Structures and Proton-Pumping Strategies of Mitochondrial Respiratory Enzymes. Annual Review of Biophysics and Biophysical Structure. 2001;30:23–65. doi: 10.1146/annurev.biophys.30.1.23. [DOI] [PubMed] [Google Scholar]
- 166.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, et al. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014 Jan 1;28(1):316–26. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shaw JM, Nunnari J. Mitochondrial dynamics and division in budding yeast. Trends in Cell Biology. 2002;12(4):178–84. doi: 10.1016/s0962-8924(01)02246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shekar KC, Li L, Dabkowski ER, Xu W, Ribeiro J, Hecker PA, et al. Cardiac mitochondrial proteome dynamics with heavy water reveals stable rate of mitochondrial protein synthesis in heart failure despite decline in mitochondrial oxidative capacity. Journal of Molecular and Cellular Cardiology. 2014 Oct;75:88–97. doi: 10.1016/j.yjmcc.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shen Q, Yamano K, Head BP, Kawajiri S, Cheung JTM, Wang C, et al. Mutations in Fis1 disrupt orderly disposal of defective mitochondria. Molecular Biology of the Cell. 2014 Jan 1;25(1):145–59. doi: 10.1091/mbc.E13-09-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sidorik L, Kyyamova R, Bobyk V, Kapustian L, Rozhko O, Vigontina O, et al. Molecular chaperone, HSP60, and cytochrome P450 2E1 co-expression in dilated cardiomyopathy. Cell Biology International. 2005 Jan;29(1):51–5. doi: 10.1016/j.cellbi.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 171.Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1a and ERRa target gene downregulaton is a signature of the failing human heart. Journal of Molecular and Cellular Cardiology. 2009;46:201–12. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sivitz WI, Yorek MA. Mitochondrial Dysfunction in Diabetes: From Molecular Mechanisms to Functional Significance and Therapeutic Opportunities. Antioxidants and Redox Signaling. 2010;12(4):537–77. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Slater EC. Mechanism of oxidative phosphorylation. Annual Review of Biochemistry. 1977;46:1015–26. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smeitink JAM, Loeffen JLCM, Triepels RH, Smeets RJP, Trijbels JMF, van den Heuvel LP. Nuclear Genes of Human Complex I of the Mitochondrial Electron Transport Chain: State of the Art. Human Molecular Genetics. 1998 Sep 1;7(10):1573–9. doi: 10.1093/hmg/7.10.1573. [DOI] [PubMed] [Google Scholar]
- 175.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related Protein Drp1 Is Required for Mitochondrial Division in Mammalian Cells. Molecular Biology of the Cell. 2001 Aug 1;12(8):2245–56. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Soga N, Kinosita K, Yoshida M, Suzuki T. Kinetic Equivalence of Transmembrane pH and Electrical Potential Differences in ATP Synthesis. Journal of Biological Chemistry. 2012 Mar 16;287(12):9633–9. doi: 10.1074/jbc.M111.335356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Song M, Dorn GW. Mitoconfusion: Noncanonical Functioning of Dynamism Factors in Static Mitochondria of the Heart. Cell Metabolism. 2015 Feb 3;21(2):195–205. doi: 10.1016/j.cmet.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW. Mitochondrial Fission and Fusion Factors Reciprocally Orchestrate Mitophagic Culling in Mouse Hearts and Cultured Fibroblasts. Cell Metabolism. 2015 Feb 3;21(2):273–85. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sorianello E, Sorriano FX, FernandeZ-Pascual S, Sancho A, Naon D, Vila-Caballer M, et al. The promoter activity of human Mfn2 depends on Sp1 in vascular smooth muscle cells. Cardiovacular Research. 2012;94(1):38–47. doi: 10.1093/cvr/cvs006. [DOI] [PubMed] [Google Scholar]
- 180.Stice JP, Chen L, Kim SC, Jung JS, Tran AL, Liu TT, et al. 17b-Estradiol, Aging, Inflammation and the Stress Response in the Female Heart. Endocrinology. 2011;152:1589–98. doi: 10.1210/en.2010-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Suzuki M, Jeong SY, Karbowski M, Youle RJ, Tjandra N. The Solution Structure of Human Mitochondria Fission Protein Fis1 Reveals a Novel TPR-like Helix Bundle. Journal of Molecular Biology. 2003 Nov 28;334(3):445–58. doi: 10.1016/j.jmb.2003.09.064. [DOI] [PubMed] [Google Scholar]
- 182.Szweda L, Lucas DT, Humphries KM, Szweda PA. Cardiac Reperfusion Injury: Aging, Lipid Peroxidation, and Mitochondrial Function. In: LeMasters JJ, Nieminen AL, editors. Mitochondria in Pathogenesis. New York: Kluwer; 2002. pp. 95–114. [Google Scholar]
- 183.Tian J, Guo X, Liu XM, Liu L, Weng QF, Dong SJ, et al. Extracellular HSP60 induces inflammation through activating and up-regulating TLRs in cardiomyocytes. Cardiovascular Research. 2013 Jun 1;98(3):391–401. doi: 10.1093/cvr/cvt047. [DOI] [PubMed] [Google Scholar]
- 184.Toko H, Takahashi H, Kayama Y, Oka T, Minamino T, Okada S, et al. Ca(2+)/Calmodulin-Dependent Kinase II-Causes Heart Failure by Accumulation of p53 in Dilated Cardiomyopathy. Circulation. 2010 Aug 31;122(9):891–9. doi: 10.1161/CIRCULATIONAHA.109.935296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tsutsui H, Kinugawa S, Matsushima S. Oxidative Stress and Mitochondrial DNA Damage in Heart Failure. Circulation Journal. 2008;(Suppl A):A31–A37. doi: 10.1253/circj.cj-08-0014. [DOI] [PubMed] [Google Scholar]
- 186.Tuppen HAL, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2010 Feb;1797(2):113–28. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 187.Twig G, Elorza A, Molina AJA, Mohamed H, Wiskstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Twig G, Liu X, Liesa M, Wikstrom JD, Molina AJA, Las G, et al. Biophysical properties of mitochondrial fusion events in pancreatic b-cells and cardiac cells unravel potential control mechanisms of its selectivity. American Journal of Physiology - Cell Physiology. 2010 Aug 1;299(2):C477–C487. doi: 10.1152/ajpcell.00427.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Uehara N, Mori M, Tokuzawa Y, Mizuno Y, Tamaru S, Kohda M, et al. New MT-ND6 and NDUFA1 mutations in mitochondrial respiratory chain disorders. Ann Clin Transl Neurol. 2014 May 28;1(5):361–9. doi: 10.1002/acn3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vallat JM, Mathis S, Funalot B. The various Charcot-Marie-Tooth diseases. Current Opinion in Neurology. 2013;26(5):473–80. doi: 10.1097/WCO.0b013e328364c04b. [DOI] [PubMed] [Google Scholar]
- 191.van Empel VPM, Bertrand ATA, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovascular Research. 2005 Jul 1;67(1):21–9. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 192.van Loon AP, Brändli AW, Pesold-Hurt B, Blank D, Schatz G. Transport of proteins to the mitochondrial intermembrane space: the ‘matrix-targeting’ and the ‘sorting’ domains in the cytochrome c1 presequence. EMBO J. 1987 Aug;6(8):2433–9. doi: 10.1002/j.1460-2075.1987.tb02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1a. Cardiovascular Research. 2008 Jul 15;79(2):208–17. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 194.Vidoni S, Zanna C, Rugolo M, Sarzi E, Lenaers G. Why Mitochondria Must Fuse to Maintain Their Genomic Integrity. Antioxidants and Redox Signaling. 2013;19(4):379–88. doi: 10.1089/ars.2012.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang K, Liu CY, Zhang XJ, Feng C, Zhou LY, Zhao Y, et al. miR-361-regulated prohibitin inhibits mitochondrial fission and apoptosis and protects heart from ischemia injury. Cell Death Differ. 2015;22(6):1058–1068. doi: 10.1038/cdd.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wells WA. Autophagy unveiled. J Cell Biol. 2005 Feb 28;168(5):676–7. [Google Scholar]
- 197.Williamson JR. Mitochondrial Function in the Heart. Annual Review of Physiology. 1979;41:485–506. doi: 10.1146/annurev.ph.41.030179.002413. [DOI] [PubMed] [Google Scholar]
- 198.Xiong S, Van Pelt CS, Elizondo-Fraire AC, Fernandez-Garcia B, Lozano G. Loss of Mdm4 Results in p53-Dependent Dilated Cardiomyopathy. Circulation. 2007 Jun 12;115(23):2925–30. doi: 10.1161/CIRCULATIONAHA.107.689901. [DOI] [PubMed] [Google Scholar]
- 199.Yamada S, Arrell DK, Martinez-Fernandez A, Behfar A, Kane GC, Perez-Terzic CM, et al. Regenerative Therapy Prevents Heart Failure Progression in Dyssynchronous Nonischemic Narrow QRS Cardiomyopathy. Journal of the American Heart Association. 2015 May 26;4(5) doi: 10.1161/JAHA.114.001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yamada Y, Harashima H. Mitochondrial drug delivery systems for macromolecule and their therapeutic application to mitochondrial diseases. Advanced Drug Delivery Reviews. 2008 Oct;60(13–14):1439–62. doi: 10.1016/j.addr.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 201.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The Mitochondrial Protein hFis1 Regulates Mitochondrial Fission in Mammalian Cells through an Interaction with the Dynamin-Like Protein DLP1. Molecular and Cellular Biology. 2003 Aug 1;23(15):5409–20. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovascular Research. 2008;79:341–51. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proceedings of the National Academy of Sciences of the United States of America. 2006 Feb 21;103(8):2653–8. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yuqi L, Lei G, Yang L, Zongbin L, Hua X, Lin W, et al. Voltage-dependent anion channel (VDAC) is involved in apoptosis of cell lines carrying the mitochondrial DNA mutation. BMC Medical Genetics. 2009;10(1):114. doi: 10.1186/1471-2350-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zanna C, Ghelli A, Porcelli AM, Karbowski M, Youle RJ, Schimpf S, et al. OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain. 2008 Feb 1;131(2):352–67. doi: 10.1093/brain/awm335. [DOI] [PubMed] [Google Scholar]
- 206.Zorzano A, Liesa M, Sebastian D, Segales J, Palacin M. Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Seminars in Cell and Developmental Biology. 2010;6:566–74. doi: 10.1016/j.semcdb.2010.01.002. [DOI] [PubMed] [Google Scholar]