Abstract
Objective
Prospective data on the association between resistin levels and cardiovascular disease (CVD) events are sparse with conflicting results.
Methods
We studied 3044 aged 70–79 years from the Health, Aging, and Body Composition Study. CVD events were defined as coronary heart disease (CHD) or stroke events. «Hard» CHD events were defined as CHD death or myocardial infarction. We estimated hazard ratio (HR) and 95% confidence intervals (CI) according to the quartiles of serum resistin concentrations and adjusted for clinical variables, and then further adjusted for metabolic disease (body mass index, fasting plasma glucose, abdominal visceral and subcutaneous adipose tissue, leptin, adiponectin, insulin) and inflammation (C-reactive protein, interleukin-6, tumor necrosis factors-α).
Results
During a median follow-up of 10.1 years, 559 patients had «hard» CHD events, 884 CHD events and 1106 CVD Events. Unadjusted incidence rate for CVD events was 36.6 (95% CI 32.1–41.1) per 1000 persons-year in the lowest quartile and 54.0 per 1000 persons-year in the highest quartile (95% CI: 48.2–59.8, P for trend < 0.001). In the multivariate models adjusted for clinical variables, HRs for the highest vs. lowest quartile of resistin was 1.52 (95% CI 1.20–1.93, P< 0.001) for «Hard» CHD events, 1.41 (95% CI 1.16–1.70, P=.001) for CHD events and 1.35 (95% CI 1.14–1.59, P=0.002) for CVD events. Further adjustment for metabolic disease slightly reduced the associations while adjustment for inflammation markedly reduced the associations.
Conclusions
In older adults, higher resistin levels are associated with CVD events independently of clinical risk factors and metabolic disease markers, but markedly attenuated by inflammation.
Keywords: Cardiovascular Prevention, Biomarkers, Inflammation, Insulin resistance, Cohort studies
Introduction
Resistin has received much attention in recent years as an emerging biomarker involved in pathways of adiposity, insulin resistance and inflammation.1–3 Resistin is mainly secreted by inflammatory cells in humans and associated with the presence of atherosclerosis.2,3 Prospective data on the association between resistin levels and cardiovascular disease (CVD) events are increasing A recent meta-analysis with conflicting results. 4–7 including 7 studies reported that circulating resistin levels were associated with mortality, especially in high-risk individuals, such as patients with diabetes.8 Diabetic patients have been reported to have higher levels of resistin compared to non-diabetic patients, suggesting some specific role of resistin in this high-risk population.9,10 In older adults, inflammatory markers, such as interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-alpha (TNF-alpha) have been independently associated with CVD events with an incremental improvement of prediction.11,12 Some recent data suggest that resistin levels were associated with incident CVD events independently of inflammatory markers.13 However, controversies persist whether the association between circulating resistin levels and CVD events is independent of metabolic disease or inflammatory markers.
A previous study within the Health Aging, and Body Composition Study (Health ABC Study) has shown an association between resistin levels and heart failure (HF) events in the elderly population.14 In this present study, we aim at describing the association between serum resistin levels and CVD events among older adults of the Health ABC Study. We explored whether the association between resistin levels and CVD events might be confounded or attenuated by metabolic disease or inflammatory markers.
Materials
Study Design and Population
We analyzed data from the Health ABC study, a prospective cohort study of 3075 community-dwelling men and women aged 70 to 79 years enrolled between April 1997 and June 1998, and who were without overt physical disability at enrollment. Participants were identified from a sample of white and black Medicare-eligible adults living in designated zip coded areas surrounding Pittsburgh, PA, and Memphis, TN, USA. Details of eligibility and exclusion criteria have been previously described.15 All participants gave written informed consent and the local Institutional Review Boards approved the protocol. We excluded 31 participants with missing data for resistin. The final sample consisted of 3044 participants.
Biomarker Measurements
In the Health ABC Study, baseline blood samples were obtained after overnight fasting, frozen at −70°C, and shipped to the core laboratory at the University of Vermont. Serum resistin concentration was measured on EDTA plasma at the University of Pennsylania using a commercially available human resistin enzyme-linked immunosorbent assay (ELISA) from Linco Research St Louis MO (EZHR-95K). Intra- and interassay coefficients of variation for this assay are 4.5% and 7.4%, respectively ((Millipore website Linco Research, Inc. Human Resistin ELISA. Available at: www.millipore.com/catalogue/item/ezhr-95k.). Each sample was diluted 1:10 before the assay was performed using assay buffer from the kit. The lowest 5% extreme values were repeated. The laboratory technicians who performed the assays were blinded to participant characteristics and the cardiovascular outcomes assessment. Measures of IL-6, CRP and TNF-α were done in duplicate using a high-sensitivity ELISA (R&D Systems, Inc., Minneapolis, Minnesota). Blind duplicate analyses (n=150) for IL-6, CRP and TNF- α showed interassay coefficients of variation of 10.3%, 8.0% and 15.8%, respectively. Serum leptin was measured using the Sensitive Human Leptin RIA Kit (product number SHL-81K) from Linco Research, Inc. (St. Charles, MO). The intra-assay CV was 3.7–7.5 % and the inter-assay CV is 3.2–8.9%. Adiponectin concentrations were measured in duplicate by radioimmunoassay (Linco Research Inc). Insulin was measured from serum at baseline using a Microparticle Enzyme Immunoassay (MEIA) on the Abbot IMx (Abbott Laboratories Diagnostics Division, South Pasadena, CA).
Study Outcomes
All participants were asked direct questions every 6 months to detect the incidence of any interim CVD events which included coronary heart disease (CHD) and stroke events. Using algorithms mirroring those of the Cardiovascular Health Study, CVD events were adjudicated based on interview, review of all hospital records, death certificates and other documents by a panel of experts blinded to the results of resistin. CHD events were defined as acute myocardial infarction [MI], coronary death, hospitalization for angina, or coronary revascularization (angioplasty of coronary arteries and coronary artery bypass graft surgery). As done in previous publications, we also analyzed separately hard CHD events, defined as nonfatal MI and CHD death (including fatal MI) and soft CHD events, defined as hospitalization for angina and coronary revascularization.16 Stroke event was defined as any hospitalization for fatal or nonfatal stroke. Follow-up time was defined by the time from baseline visit until the first event date for those who presented an event or was censored at the last contact date for those who did not have any event or to last follow-up. Among Health ABC participants who were alive at 10 years (median follow-up for this analysis), 73 were lost to follow-up (2.4% of the cohort -- this is the % censored by 10 years due to FU loss -- in other words, 97.6% had complete FU data by 10 years).
Covariates
Covariates included sociodemographic variables (age, gender, race), physical and biological parameters, including smoking status (current, past, or never smokers), body mass index (calculated as weight in kilograms divided by the height in meters squared), total cholesterol, high-density lipoprotein cholesterol, and creatinine (all measured by a calorimetric technique on a Johnson & Johnson Vitros 950 analyzer, New Brunswick, New Jersey). Hypertension was defined by self-report and use of antihypertensive medications, or measured blood pressure, with systolic of 140 mm Hg or higher, diastolic of 90 mm Hg or higher, or both. Participants were considered as having diabetes if they reported a physician diagnosis of diabetes, and/or if they were taking insulin or an oral diabetes medication, and/or if their fasting plasma glucose was ≥126mg/dl in accord with the American Diabetes Association criteria in place near the start of the Health ABC Study (in 2002).17,18 The use of cardiovascular medication (statin, aspirin, angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blocker [ARB]) was identified using Iowa Drug Information System codes. Preexisting CVD was defined as a diagnosis of CHD (angina, prior myocardial infarction [MI], angioplasty of coronary arteries, or coronary artery graft surgery), stroke or transient ischemic attack, peripheral arterial revascularization, carotid artery disease, heart failure. Abdominal visceral and subcutaneous adipose tissue areas at the lumbar (L4-L5) level were measured with computed tomography (CT). Areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area using imaging software (RSI Systems). Visceral fat was manually distinguished from subcutaneous fat by tracing along the fascial plane defining the internal abdominal wall. Body mass index (BMI) was categorized as normal weight if BMI was ≤24.9 kg/m2, overweight if BMI between 25.0 kg/m2 and 29.9 kg/m2 and obesity if BMI ≥30.0 Kg/m2. We defined the inflammation severity according to the number of inflammatory markers (CRP, TNF-α, IL-6) above the 3rd tertile of the distribution within the Health ABC Study.12
Statistical Analysis
Baseline participant characteristics were compared across quartiles of resistin levels using analysis of variance for continuous variables and χ2 for categorical variables.14,19 Cox proportional hazards models were used to assess the hazard ratios (HR) of baseline resistin quartiles with CVD outcomes. We used a sequence of models to test the sensitivity of the results to covariates according to previous described mechanisms:14 (model 1) a crude hazard ratio, (model 2) adjusted for clinical variables (age, gender, race, total cholesterol, HDL-cholesterol, smoking, preexisting hypertension, diabetes at baseline, preexisting CVD), (model 3) additional adjustment for metabolic disease (BMI, fasting plasma glucose, insulin, CT abdominal visceral and subcutaneous fat, leptin, adiponectin) and (model 4) additional adjustment for inflammatory markers (CRP, TNF-α, IL-6). For continuous covariates of the model 2–4, we used restricted cubic splines with knots located at 10th and 50th and 90th in case of violation of linearity. The proportional hazards assumption was verified using graphical methods. We found a significant interaction of resistin quartiles on CVD by diabetes at baseline (P value < 0.05) and therefore presented results accordingly. We further tested for statistical interactions with gender, race, diabetes at baseline, preexisting CVD, BMI categories and inflammation. We computed the multivariate adjusted incidence rate for each CVD event (total CVD events, CHD events, hard CHD events and stroke events) by quartiles of serum resistin using Poisson regression models. We performed Kaplan-Meier estimates of CVD cumulative hazard over time according to quartiles of baseline resistin levels. Analyses were performed using Stata version 12 (Stat Corporation) with a level of significance at 2-sided p value < 0.05.
Results
The mean age of participants was 74 ±3 years with 49% of men, 59% white and 15.2% with diabetes at baseline. The median value of resistin among the 3044 participants was 18.1ng/mL (interquartile range [IQR] 14.0 to 24.3; range 1.9 to 221). Significant correlation was found between circulating resistin levels and metabolic disease, as well as inflammatory markers (P < 0.05), but the magnitude of the rho coefficient was modest (Table 1).
Table 1.
Baseline Participant Characteristics and Correlation With Resistin Levels
| Variable | Value | Rho* | P value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 73.0 (71.0, 76.0) | 0.058 | 0.001 |
| Women, n (%) | 426 (56) | −0.039 | 0.031 |
| White race, n (%) | 419 (55) | 0.044 | 0.164 |
| Clinical variables | |||
| History of diabetes *, n (%) | 243 (32) | 0.090 | <0.001 |
| History of hypertension †, n(%) | 448 (59) | 0.101 | <0.001 |
| History of CVD ‡, n (%) | 182 (24) | 0.054 | 0.003 |
| Current smoker, n (%) | 77 (10) | 0.025 | 0.235 |
| Total cholesterol (mg/dl) | 200.0 (177.0, 226.0) | −0.061 | <0.001 |
| HDL-cholesterol (mg/dl) | 51.0 (42.0, 63.0) | −0.107 | <0.001 |
| Triglycerides (mg/dl) | 118.0 (88.0, 164.0) | 0.089 | <0.001 |
| Metabolic disease markers | |||
| Fasting plasma glucose (mg/dl) | 94.0 (87.0, 106.0) | 0.074 | <0.001 |
| Hemoglobin glycated (%) | 6.1 (5.7, 6.6) | 0.096 | <0.001 |
| Fasting Insulin (UI/ml) | 7.0 (4.9, 10.3) | 0.124 | <0.001 |
| BMI (kg/m2) | 26.9 (24.1, 30.1) | 0.091 | <0.001 |
| CT abdominal visceral fat area | 132.4 (94.6, 181.5) | 0.091 | <0.001 |
| CT abdominal subcutaneous fat area | 267.4 (197.2, 357.1) | 0.059 | 0.002 |
| Adiponectin (μg/ml) | 10.0 (6.0, 15.0) | −0.046 | 0.011 |
| Leptin (ng/ml) | 10.4 (5.2, 20.3) | 0.056 | 0.002 |
| Inflammatory markers | |||
| Interleukin-6 (pg/ml) | 2.0 (1.4, 3.2) | 0.225 | <0.001 |
| C-reactive protein (μg/ml) | 1.7 (1.0, 3.1) | 0.184 | <0.001 |
| TNF-alpha (pg/ml) | 3.2 (2.4, 4.1) | 0.264 | <0.001 |
Values for continuous variables represent median (interquartile range).
Spearman’s rank correlation rho for continuous variables; for binary variables (present=1, absent=0), the z score of the rank sum test is transformed into the corresponding rho value for comparison purposes: rho=1 denotes perfect positive correlation, rho=−1 denotes perfect negative correlation, and rho=0 absent correlation.
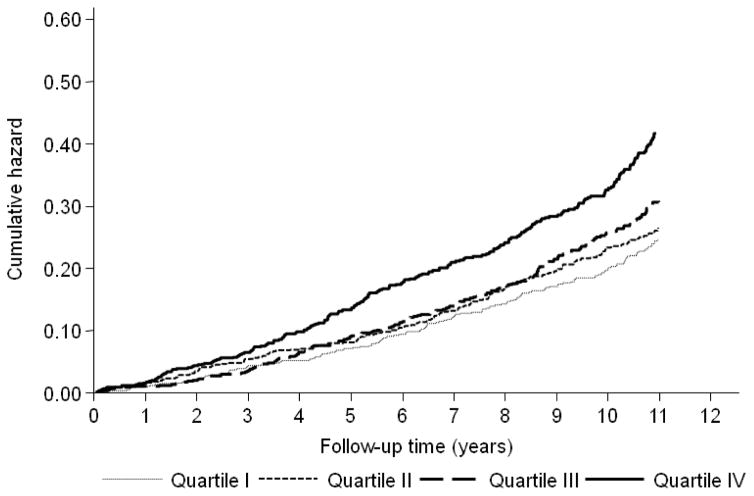
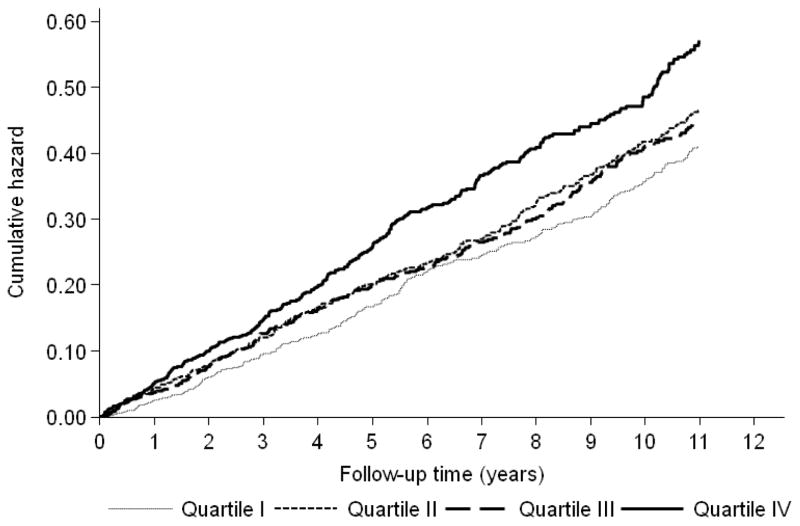
During a median follow-up of 10.1 years (IQR, 5.0 to 11.6) 884 participants (29.0%) had CHD events, including 559 with “hard” CHD events (death and MI), and 333 participants (10.9%) had stroke events, yielding to a total of 1106 CVD events (36.3%) participants. The Kaplan-Meier estimates of “hard” CHD events and CVD cumulative hazard over time according to the quartiles of resistin showed that the curve for the highest quartile was significantly demarcated from other groups, the log rank test for the differences between resistin quartiles was significant (P < 0.001) for both outcomes (Figure 1 and Figure 2).
Figure 1. Kaplan-Meier Estimates of 559 «hard» CHD Events According to Resistin Quartiles in 3044 Older Adults.
Abbreviations: CHD, coronary heart disease. Hazards were unadjusted.
«Hard» CHD was defined as cardiac death or non-fatal myocardial infarction.
Figure 2. Kaplan-Meier Estimates of 1106 CVD Events According to Resistin Quartiles in 3044 Older Adults.
Abbreviations: CHD, coronary heart disease. Hazards were unadjusted
«Hard» CHD was defined as cardiac death or non-fatal myocardial infarction.
In the overall population, unadjusted incidence rate for “hard” CHD events was 17.8 (95% CI 14.6–20.9) per 1000 persons-year in the lowest quartile and 30.2 per 1000 persons-year in the highest quartile (95% CI: 25.8–34.5, P for trend < 0.001). The adjusted incidence rate was 23.5 (95% CI 18.4–28.5) per 1000 persons-year in the lowest quartile and 30.0 (95% CI 24.9–35.0) in the highest quartile (P for trend=0.079). Crude HRs for “hard” CHD events was 1.70 (95% CI 1.35–2.15) and slightly decreased after adjustment for clinical variables and metabolic disease markers (HR 1.46, 95% CI 1.12–1.91), but were strongly attenuated after adjustment for inflammation with HR of 1.24 (95% CI 0.93–1.66, P for trend=0.075, Table 2). The association between higher resistin levels and CHD event was also markedly attenuated after adjustment for inflammation (HR 1.17, 95% CI 0.93–1.47. P for trend=0.293, Table 3).
Table 2.
Association Between Quartiles of Resistin and 559 «hard» CHD Events in 3044 Older Adults
| Hazard Ratios (HR) for Quartile of Resistin (95% CI) | |||||
|---|---|---|---|---|---|
|
| |||||
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | P value 4th vs. 1st Quartile | |
| Number of «hard» CHD Events/Number of Participants | 120/763 | 125/759 | 137/761 | 177/761 | |
| Model 1 : Crude | 1 (ref) | 1.06 (0.83–1.37) | 1.23 (0.97–1.58) | 1.70 (1.35–2.15) | < 0.001 |
| Model 2 : plus clinical variables † | 1 (ref) | 0.98 (0.76–1.27) | 1.09 (0.85–1.41) | 1.52 (1.20–1.93) | <0.001 |
| Model 3 : Model 2 plus metabolic disease ‡ | 1 (ref) | 0.97 (0.73–1.28) | 1.07 (0.81–1.41) | 1.46 (1.12–1.91) | 0.005 |
| Model 4 : Model 3 plus inflammation § | 1 (ref) | 0.86 (0.64–1.16) | 1.00 (0.75–1.34) | 1.24 (0.93–1.66) | 0.075 |
Abbreviations: BMI, body mass index; CI, confidence intervals; CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard ratios
Diabetes at baseline was defined as self-reported medical diagnosis and/or using any hypoglycemic medication and/or fasting plasma glucose 126 mg/dl and/or hemoglobin glycated 6.5%. Preexisting hypertension was defined as self-report and use of anti-hypertensive medications, or measured blood pressure 140 and/or 90 mm Hg. Preexisting CVD was defined as prior CHD, stroke, peripheral arterial revascularization, carotid artery disease and heart failure.
Model 2 included: age, gender, race, total cholesterol, splines high-density lipoprotein cholesterol, smoking, hypertension, diabetes, and preexisting CVD.
Model 3 plus splines BMI, splines fasting plasma glucose, insulin, abdominal and subcutaneous fat area, leptin and splines adiponectin.
Model 4 plus splines C-reactive protein, splines tumor necrosis factor-alpha and splines interleukin-6.
Table 3.
Association Between Quartiles of Resistin and 884 CHD Events in 3044 Older Adults
| Hazard Ratios (HR) for Quartile of Resistin (95% CI) | |||||
|---|---|---|---|---|---|
|
| |||||
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | P value 4th vs. 1st Quartile | |
| Number of «hard» CHD Events/Number of Participants | 195/763 | 215/759 | 215/761 | 259/761 | |
| Model 1 : Crude | 1 (ref) | 1.14 (0.94–1.38) | 1.19 (0.98–1.44) | 1.54 (1.28–1.85) | <0.001 |
| Model 2 : plus clinical variables † | 1 (ref) | 1.04 (0.85–1.27) | 1.07 (0.88–1.31) | 1.41 (1.16–1.70) | 0.001 |
| Model 3 : Model 2 plus metabolic disease ‡ | 1 (ref) | 1.03 (0.83–1.29) | 1.05 (0.84–1.31) | 1.35 (1.09–1.67) | 0.015 |
| Model 4 : Model 3 plus inflammation § | 1 (ref) | 0.96 (0.76–1.21) | 0.99 (0.78–1.25) | 1.17 (0.93–1.47) | 0.293 |
Abbreviations: BMI, body mass index; CI, confidence intervals; CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard ratios
Diabetes at baseline was defined as self-reported medical diagnosis and/or using any hypoglycemic medication and/or fasting plasma glucose 126 mg/dl and/or hemoglobin glycated 6.5%. Preexisting hypertension was defined as self-report and use of anti-hypertensive medications, or measured blood pressure 140 and/or 90 mm Hg. Preexisting CVD was defined as prior CHD, stroke, peripheral arterial revascularization, carotid artery disease and heart failure.
Model 2 included: age, gender, race, total cholesterol, splines high-density lipoprotein cholesterol, smoking, hypertension, diabetes, and preexisting CVD.
Model 3 plus splines BMI, splines fasting plasma glucose, insulin, abdominal and subcutaneous fat area, leptin and splines adiponectin.
Model 4 plus splines C-reactive protein, splines tumor necrosis factor-alpha and splines interleukin-6.
The unadjusted incidence rate for CVD events was 36.6 (95% CI 32.1–41.1) per 1000 persons-year in the lowest quartile and 54.0 (95% CI 48.2–59.8) in the highest quartile (P for trend < 0.001). The adjusted incidence rate was 47.0 (95% CI 40.1–53.9) per 1000 persons-year in the lowest quartile and 51.2 (95% CI 44.7–57.7) in the highest quartile (P for trend=0.557). The association was increased among participants in the highest resistin quartile with a crude HR of 1.49 (95% CI: 1.26–1.76, P for trend < 0.001, Table 4). HRs slightly decreased after adjustment for clinical variables and metabolic disease markers (HR 1.22, 95% CI 1.01–1.49, P for trend=0.090), but markedly attenuated after adjustment for inflammation (HR 1.04, 95% CI 0.85–1.28, P for trend=0.591). The association between resistin quartiles and stroke events was not significant after adjustment for clinical variables (HR 1.29, 95% CI 0.94–1.75, P for trend=0.110), for metabolic disease markers (HR 1.09, 95% CI 0.77–1.54, P for trend=0.641) and finally for inflammation (HR 0.91, 95% CI 0.63–1.32, P for trend=0.621). For all the models, we did not find additional interactions by gender, race, diabetes at baseline, preexisting CVD, BMI categories and inflammation (P >0.05).
Table 4.
Association Between Quartiles of Resistin and 1106 CVD Events in 3044 Older Adults
| Hazard Ratios (HR) for Quartile of Resistin (95% CI) | |||||
|---|---|---|---|---|---|
|
| |||||
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | P value 4th vs. 1st Quartile | |
| Number of CVD Events/Number of Participants | 247/763 | 276/759 | 266/761 | 317/761 | |
| Model 1 : Crude | 1 (ref) | 1.16 (0.98–1.38) | 1.16 (0.97–1.38) | 1.49 (1.26–1.76) | < 0.001 |
| Model 2 : plus clinical variables † | 1 (ref) | 1.09 (0.91–1.29) | 1.05 (0.88–1.26) | 1.35 (1.14–1.59) | 0.002 |
| Model 3 : Model 2 plus metabolic disease ‡ | 1 (ref) | 1.05 (0.87–1.28) | 0.99 (0.81–1.20) | 1.22 (1.01–1.48) | 0.090 |
| Model 4 : Model 3 plus inflammation § | 1 (ref) | 0.97 (0.79–1.19) | 0.91 (0.74–1.12) | 1.04 (0.85–1.28) | 0.591 |
Abbreviations: BMI, body mass index; CI, confidence intervals; CVD, cardiovascular disease; HR, hazard ratios
Diabetes at baseline was defined as self-reported medical diagnosis and/or using any hypoglycemic medication and/or fasting plasma glucose 126 mg/dl and/or hemoglobin glycated 6.5%. Preexisting hypertension was defined as self-report and use of anti-hypertensive medications, or measured blood pressure 140 and/or 90 mm Hg. Preexisting CVD was defined as prior CHD, stroke, peripheral arterial revascularization, carotid artery disease and heart failure.
Model 2 included: age, gender, race, total cholesterol, splines high-density lipoprotein cholesterol, smoking, hypertension, diabetes, and preexisting CVD.
Model 3 plus splines BMI, splines fasting plasma glucose, insulin, abdominal and subcutaneous fat area, leptin and splines adiponectin.
Model 4 plus splines C-reactive protein, splines tumor necrosis factor-alpha and splines interleukin-6.
Discussion
In this large prospective cohort of older persons, we found that higher levels of resistin were associated with an increased risk of CVD events independently of clinical variables. Further adjustment for metabolic disease markers slightly reduced the associations while adjustment for inflammation (CRP, TNF-α, and IL-6) markedly reduced the associations. Our data support that resistin is implicated in the inflammatory process characteristics of atherosclerosis and associated with clinically relevant CVD events in older adults.
Previous clinical studies with resistin
Our study is the largest available cohort reporting the association between circulating resistin and clinical outcomes. A recent meta-analysis with 4016 patients showed an increased risk of all-cause mortality per one SD increment in resistin concentration (HR 1.21, 95% CI 1.03–1.42), while the association for CVD mortality with 4187 patients was HR 1.05 (95% CI 1.01–1.10).8 Previous published cross-sectional reported conflicting results with limited statistical power, co-variables adjustment and non-standardization of clinical outcomes. 5–7,20 A subanalysis from the European Investigation into Cancer and Nutrition-Postdam case-control Study reported that higher plasma resistin levels were independently associated with an increased risk of MI but not with stroke. 21 In the Heart and Soul study, among 980 participants with a preexisting CHD, elevated serum resistin levels were associated with higher rates of mortality and hospitalization for HF, but the association was no more significant after adjustment for clinical variables.19 In the Health ABC, resistin levels have been independently associated with HF events after adjustment for the adiposity and inflammatory markers.14 And recently, in 1913 participants of the Multi-Ethnic Study of Atherosclerosis, resistin levels were associated independently of inflammatory markers with incident CVD, CHD and HF events.13
Proof-of-concept
Experimental studies in obese mice suggest that resistin belongs to a group of adipose tissue-related hormones, such as leptin or adiponectin, and is a key factor linking obesity to diabetes with mechanisms related to insulin resistance.1 Mechanistic studies in obese mice showed that lack of resistin levels was associated with a diminution in the increase of post-fast blood glucose, suggesting a role in obesity-related hyperglycaemia.22 Interestingly, human resistin is not homolog to rodent resistin and is mainly secreted by inflammatory cells. 9,10,23,24 Several studies have shown an association with other inflammatory markers in human (TNF-α and IL-6). 3,4,25,26 Resistin promotes expression of pro-atherogenic molecules (ICAM-1, VCAM-1, MCP-1 and ET-1), down-regulates anti-atherogenic molecule (TRAF-3, an inhibitor of CD40 ligand) and is implicated in the first stage of the atherosclerosis process through endothelial cell activation. 2,27 Two loci at TYW3/CRYZ and NDST4 gene regions have been both associated with higher circulating resistin levels and higher risk of CHD, suggesting a strong biological proof-of-concept of the current observed association.26 In addition, some genetic variants (single nucleotide polymorphisms) in the resistin gene have been associated with a risk of diabetes type 2 in Caucasians, especially in case of obesity.28 However, more data are needed to assess the association between genetic variants of the resistin gene and the inflammation. These mechanistic findings confirm the complex relationship between adiposity, insulin resistance, inflammation and traditional CVD risk factors.
Future Perspectives
The incremental role of inflammatory markers, such as Il-6, CRP and TNF-α, in the prediction of CVD events has been reported in previous studies, suggesting that new promising markers might improve the clinical risk assessment of CVD. 11,12 This is particularly true in older adults, of whom traditional risk-based models might underestimate the risk of CVD. The role of resistin in clinical practice is still limited, although data suggest that resistin could act as an important mediator in the mechanism of CVD or metabolic disorders. The potential causality of resistin in the manifestation of CVD, as well as the mechanisms linking resistin to inflammation, needs to be explored in large longitudinal prospective cohorts with repeated assessment of marker values and adding previously reported genetic mutations. It is well known that the traditional risk-based models underestimate the risk of CVD in older persons and the addition of inflammatory markers improves the prediction of CHD.12 Currently, there is a lack of consensus in the standardization of resistin levels measurement across studies, as well as in the determination of a specific cut-off that might change risk stratification or medical decision. 14 Those steps are needed in the evaluation of potential novel markers before any clinical recommendations.29
Limitations
Our study has several strengths and limitations. The data were drawn from a well-characterized cohort of older adults with a large number of CVD events over 11 years of follow-up. However, participants were selected on the basis of no disability at baseline and might not be fully be representative of the general older adult population. In addition, our findings are not applicable to participants younger than 70 years old and we cannot exclude that some CVD events might have been missed, especially in case of an event that did not require any hospitalization. The measurement of resistin might present variability due to the assay, which would however bias the results toward the null hypothesis. Collection of resistin concentrations and other inflammatory markers were only performed at baseline, limiting the possibilities to assess the evolution of those markers with time. However, very few prospective data are available with such as large panel of biomarkers. We tried to control for potential confounders or mediating factors, but we cannot exclude the possibility of unmeasured confounders. Finally, we reported only associations and cannot conclude for any causality.
Conclusion
In conclusion, our study suggests that higher serum resistin concentrations are associated with coronary heart disease and cardiovascular disease events in older adults, but markedly attenuated by inflammation.
Acknowledgments
Dr Gencer’s research on cardiovascular prevention is supported by a grant from the Swiss National Science Foundation (SNSF SPUM33CM30-140336, Inflammation and acute coronary syndromes (ACS) – Novel strategies for prevention and clinical management), Swiss Heart Foundation, Geneva University Hospitals, Foundation Schmidheiny, Foundation Gerbex-Bourget and De Reuter Foundation. Prof Rodondi’s research is supported by a grant from the Swiss National Science Foundation (SNSF 320030-150025) and the Swiss Heart Foundation. This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH). National Institute on Aging (NIA), Bethesda Md. This research was supported by NIA Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR01259. The NIA funded the Health ABC Study, reviewed the manuscript, and approved its publication. The authors are solely responsible for the design and conduct of this analysis, all study analyses and drafting end editing of the paper.
Footnotes
Disclosures
All other authors have no conflict of interest to declare
References
- 1.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001 Jan 18;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 2.Verma S, Li SH, Wang CH, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003 Aug 12;108(6):736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 3.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005 Feb 22;111(7):932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 4.Yaturu S, Daberry RP, Rains J, Jain S. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006 May;34(3–4):219–223. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Burnett MS, Devaney JM, Adenika RJ, Lindsay R, Howard BV. Cross-sectional associations of resistin, coronary heart disease, and insulin resistance. The Journal of clinical endocrinology and metabolism. 2006 Jan;91(1):64–68. doi: 10.1210/jc.2005-1653. [DOI] [PubMed] [Google Scholar]
- 6.Ohmori R, Momiyama Y, Kato R, et al. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. Journal of the American College of Cardiology. 2005 Jul 19;46(2):379–380. doi: 10.1016/j.jacc.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Pischon T, Bamberger CM, Kratzsch J, et al. Association of plasma resistin levels with coronary heart disease in women. Obesity research. 2005 Oct;13(10):1764–1771. doi: 10.1038/oby.2005.215. [DOI] [PubMed] [Google Scholar]
- 8.Fontana A, Spadaro S, Copetti M, et al. Association between resistin levels and all-cause and cardiovascular mortality: a new study and a systematic review and meta-analysis. PloS one. 2015;10(3):e0120419. doi: 10.1371/journal.pone.0120419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochemical and biophysical research communications. 2003 Sep 19;309(2):286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochemical and biophysical research communications. 2003 Jan 10;300(2):472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 11.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003 Nov 11;108(19):2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 12.Rodondi N, Marques–Vidal P, Butler J, et al. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. American journal of epidemiology. 2010 Mar 1;171(5):540–549. doi: 10.1093/aje/kwp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muse ED, Feldman DI, Blaha MJ, et al. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015 Mar;239(1):101–108. doi: 10.1016/j.atherosclerosis.2014.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Serum resistin concentrations and risk of new onset heart failure in older persons: the health, aging, and body composition (Health ABC) study. Arteriosclerosis, thrombosis, and vascular biology. 2009 Jul;29(7):1144–1149. doi: 10.1161/ATVBAHA.109.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Archives of internal medicine. 2005 Nov 28;165(21):2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 16.Auer R, Bauer DC, Marques-Vidal P, et al. Association of major and minor ECG abnormalities with coronary heart disease events. Jama. 2012 Apr 11;307(14):1497–1505. doi: 10.1001/jama.2012.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kositsawat J, Kuchel GA, Tooze JA, et al. Vitamin D Insufficiency and Abnormal Hemoglobin A1c in Black and White Older Persons. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014 Aug 11; doi: 10.1093/gerona/glu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaffe K, Falvey CM, Hamilton N, et al. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA internal medicine. 2013 Jul 22;173(14):1300–1306. doi: 10.1001/jamainternmed.2013.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang MH, Na B, Schiller NB, Whooley MA. Association of resistin with heart failure and mortality in patients with stable coronary heart disease: data from the heart and soul study. Journal of cardiac failure. 2011 Jan;17(1):24–30. doi: 10.1016/j.cardfail.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Chan JL, Yiannakouris N, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. The Journal of clinical endocrinology and metabolism. 2003 Oct;88(10):4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- 21.Weikert C, Westphal S, Berger K, et al. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. The Journal of clinical endocrinology and metabolism. 2008 Jul;93(7):2647–2653. doi: 10.1210/jc.2007-2735. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee RR, Rangwala SM, Shapiro JS, et al. Regulation of fasted blood glucose by resistin. Science. 2004 Feb 20;303(5661):1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 23.Savage DB, Sewter CP, Klenk ES, et al. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001 Oct;50(10):2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 24.Yang RZ, Huang Q, Xu A, et al. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochemical and biophysical research communications. 2003 Oct 24;310(3):927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 25.Fargnoli JL, Sun Q, Olenczuk D, et al. Resistin is associated with biomarkers of inflammation while total and high-molecular weight adiponectin are associated with biomarkers of inflammation, insulin resistance, and endothelial function. European journal of endocrinology / European Federation of Endocrine Societies. 2010 Feb;162(2):281–288. doi: 10.1530/EJE-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi Q, Menzaghi C, Smith S, et al. Genome-wide association analysis identifies TYW3/CRYZ and NDST4 loci associated with circulating resistin levels. Human molecular genetics. 2012 Nov 1;21(21):4774–4780. doi: 10.1093/hmg/dds300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manduteanu I, Pirvulescu M, Gan AM, et al. Similar effects of resistin and high glucose on P-selectin and fractalkine expression and monocyte adhesion in human endothelial cells. Biochemical and biophysical research communications. 2010 Jan 15;391(3):1443–1448. doi: 10.1016/j.bbrc.2009.12.089. [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Warram JH, Trischitta V, Doria A. Genetic variants at the resistin locus and risk of type 2 diabetes in Caucasians. The Journal of clinical endocrinology and metabolism. 2002 Sep;87(9):4407–4410. doi: 10.1210/jc.2002-020109. [DOI] [PubMed] [Google Scholar]
- 29.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009 May 5;119(17):2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]