Abstract
Background
Early life adversities including harsh parenting, maternal depression, neighborhood deprivation, and low family economic resources are more prevalent in low-income urban environments and are potent predictors of psychopathology, including, for boys, antisocial behavior (AB). However, little research has examined how these stressful experiences alter later neural function. Moreover, identifying genetic markers of greater susceptibility to adversity is critical to understanding biopsychosocial pathways from early adversity to later psychopathology.
Methods
Within a sample of 310 low-income boys followed from age 1.5 to 20, multimethod assessments of adversities were examined at age 2 and age 12. At age 20, amygdala reactivity to emotional facial expressions was assessed using fMRI, and symptoms of Antisocial Personality Disorder were assessed via structured clinical interview. Genetic variability in cortisol signaling (CRHR1) was examined as a moderator of pathways to amygdala reactivity.
Results
Observed parenting and neighborhood deprivation at age 2 each uniquely predicted amygdala reactivity to emotional faces at age 20 over and above other adversities measured at multiple developmental periods. Harsher parenting and greater neighborhood deprivation in toddlerhood predicted clinically-significant symptoms of AB via less amygdala reactivity to fearful facial expressions and this pathway was moderated by genetic variation in CRHR1.
Conclusions
These results elucidate a pathway linking early adversity to less amygdala reactivity to social signals of interpersonal distress 18 years later, which in turn increased risk for serious AB. Moreover, these findings suggest a genetic marker of youth more susceptible to adversity.
Keywords: early life adversity, stress, fMRI, antisocial behavior, Neurogenetics, harsh parenting
Introduction
Early life adversity casts a long shadow on the developing child by predicting multiple negative outcomes, including psychopathology, physical health disparities, and income inequalities across the lifespan and across generations (1). Specific adversities, such as exposure to community violence, poverty, and a parent who is harsh or depressed, each increase risk for psychiatric disorders (2–3). Less is known about the biological mechanisms linking these experiences to psychopathology and how their timing affects the developing brain. Understanding how early life adversity becomes biologically-embedded to increase risk for psychopathology can ultimately lead to the development of more effective strategies to prevent and treat psychiatric disorders (4).
Neural embedding of early experience
The amygdala, a neural structure important for fear learning, emotional responses, and vigilance (5), is critical in responding to stress. A wealth of animal literature indicates that early life adversity leads to structural changes in the amygdala (6,7). Neuroimaging studies in humans suggest that extreme adversity, including maltreatment and social deprivation (8,9), as well common adversities, such as poverty or parental stress (10,11), alter amygdala development. However, little research has examined the impact of variability in more normative experiences (e.g., parenting), nor the unique contributions of various stressors, on later neural functioning (12). Given the role of the amygdala in threat detection and physiological stress responding (5), one specific dimension of parenting likely to undermine amygdala development is harshness, characterized by physical discipline and verbal hostility. Recent empirical evidence suggests that parental harshness is one pathway through which poverty may affect amygdala development (13). However, poverty is associated with a myriad distal and proximal risk factors (e.g., harsh parenting, neighborhood deprivation, low maternal education, maternal depression) that may undermine child development (14,15), highlighting the need to examine the neurodevelopmental effects of community and family-level adversities simultaneously (16). As studies suggest that early childhood (i.e. 0 – 5 years) may be a sensitive period for brain development (17–19), we examined the effects of adversity during toddlerhood on later neural function. However, as adolescence may also be a sensitive period for neural development (20) and many adversities are stable across childhood, we controlled for parallel measures of adversity in early adolescence to identify the unique effect of adversity in toddlerhood on amygdala reactivity during early adulthood.
Implications for psychiatric and behavioral outcomes
Early adversity predicts a range of psychiatric outcomes, yet little research has examined the impact of adversity on brain function as a mechanistic pathway to psychiatric symptoms. Amygdala reactivity to emotional facial expressions is consistently implicated in the pathophysiology of depression and anxiety (21), as well as antisocial behavior (AB) outcomes, including Conduct Disorder, Antisocial Personality Disorder, and psychopathy, which have been linked to deficits in fear processing (22). For boys living in low-income urban environments, harsh parenting, poverty, maternal depression, and neighborhood deprivation, are robust risk factors for AB (3). Thus, a second aim of this study was to test mechanistic pathways linking early adversity to AB via altered amygdala reactivity to emotional faces, particularly to fearful facial expressions. We tested these aims prospectively in a cohort of boys who, based on living in a low-income urban environment and exposed to substantial adversity, were at elevated risk for AB (3).
Genetic susceptibility to early adversity
Finally, although adversity predicts maladaptive outcomes, many children show normative development, highlighting the need to identify markers of who is more susceptible to early adversity. Genetic variation may increase risk by making children more susceptible to poor neurobehavioral outcomes in adverse environments (i.e., diathesis-stress), or may make them more sensitive to both positive and negative outcomes in promotive versus adverse environments, respectively (i.e., differential susceptibility) (23). Animal models suggest that early adversity affects the amygdala via stress-related glucocorticoid signaling (24). Thus, individual variability in cortisol signaling may modulate the effects of stressful experiences on amygdala development. Cortisol signaling is regulated by several receptors, including corticotropin-releasing hormone receptor type 1 (CRHR1), which supports initial activation and negative feedback of the hypothalamic-pituitary-adrenal (HPA) response (25). Extant research linking genetic variation within CRHR1 to stress-related psychopathology (26) and neuroendocrine function (27,28) has focused on single nucleotide polymorphisms (SNPs) rs110402 and rs7209436, where minor allele carriers are at lower risk for stress-related outcomes in the context of adversity (26–28), although these findings are mixed (29,30). Due to the high density of CRHR1 receptors in the amygdala (31) and studies linking these specific SNPs to stress-related outcomes (26–28), our third goal was to examine whether variability in CRHR1 moderated the relationship between early adversity and amygdala reactivity to emotional faces.
The current study
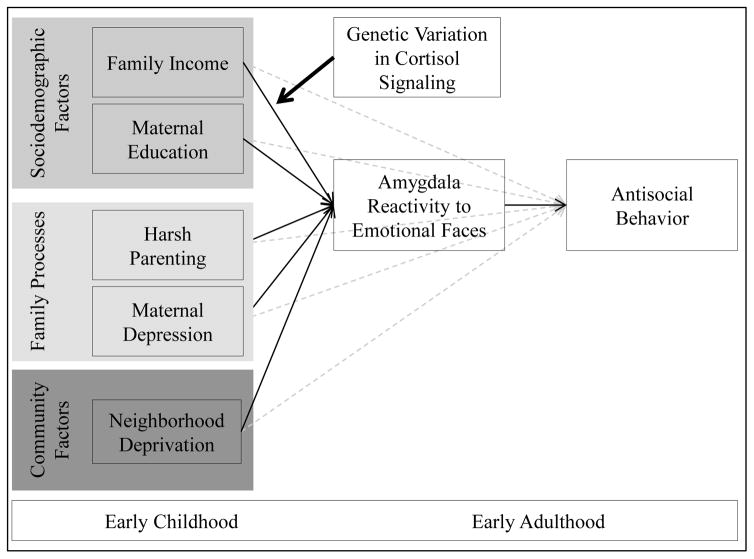
In the present study, we examined pathways from early adversity to amygdala function in a longitudinal study of 310 boys living in low-income, urban families, followed from age 1.5 to 20. We examined the unique impact of sociodemographic-, family-, and community-level adversities in toddlerhood on amygdala reactivity to emotional faces in early adulthood (see Figure 1). Importantly, we tested stringent models that controlled for the overlap and co-occurrence of adversities to test unique effects, and included parallel measures of adversities during early adolescence to isolate the neural effects on adversities during early childhood. We also tested whether early adversity predicted AB via effects on amygdala reactivity to fearful facial expressions. Finally, we explored the moderating role of genetic variation in cortisol signaling, by testing if the relationships between early adversity and amygdala reactivity were stronger among individuals with genetic backgrounds associated with dysregulated HPA-axis function (i.e., CRHR1 SNPs rs7209436, rs110402). We hypothesized that harsh parenting, specifically during early childhood and above and beyond other risk factors, would predict greater amygdala reactivity to fearful, angry, and neutral facial expressions, which would in turn predict greater AB. Moreover, in an exploratory model, we expected that youth with major alleles within CRHR1 (compared to youth with minor alleles) would be more sensitive to the neurobehavioral effects of adversity.
Figure 1. Conceptual model of study aims.
We were guided by a bioecological model of psychopathology (16), process models specifying sociodemographic, family, and community adversities associated with poverty (14,15,53), and neurogenetics and Imaging Gene x Environment interaction models (58,64). We hypothesized that specific adversities present in impoverished contexts put children at differential risk for psychopathology, particularly antisocial behavior in boys, via their effects on brain development and subsequent neural reactivity to emotion and threat. We hypothesized that early adversities would predict antisocial behavior in early adulthood via amygdala reactivity to emotional faces and that this pathway would be stronger among those with genes associated with dysregulated cortisol signaling (24). Specifically, we hypothesized that harsh parenting, as the most proximal factor for young children, would predict greater amygdala reactivity to social signals of threat, distress, and ambiguity, which in turn, would predict antisocial behavior. Moreover, we hypothesized that those with genotypes related to greater cortisol dysregulation would be particularly sensitive to these effects (26–28). Given the need to delineate if these effects are consistent with a diathesis-stress model (i.e., these genes put children at risk for poor outcomes in harsh environments) versus a differential susceptibility model (i.e., these genes mark children who are more sensitive to the environment, for better or worse; 20), we examined the pattern of interactions in Figure 3.
Methods and Materials
Participants
Participants are part of the Pitt Mother & Child Project (PMCP), an ongoing longitudinal study of 310 low-income boys and their families recruited in 1991 and 1992 from Allegheny County Women, Infant and Children (WIC) Nutritional Supplement Clinics when boys were 6–17 months old (3). The sample is low income and ethnically diverse (e.g., 52% European-American, 39% African-American of those included at age 20). Children and mothers were seen almost yearly from age 1.5–20 in the laboratory and/or home with assessments that included questionnaires, psychiatric interviews, and at age 20, an fMRI scanning session. fMRI data were available for 167 men (see Supplement and Table S1).
Measures
Harsh Parenting
Harsh parenting was measured at age 2 via observation and parent interview using items from the Acceptance scale of the Home Observation for the Measurement of the Environment (32). The eight-item scale assessed both verbal (e.g., “parent shouts at child”) and physical (e.g., “parent slaps/spanks child”) harshness.
Contextual Sources of Stress
Additional measures of contextual adversities were collected at age 2: (i) parent-reported monthly family income (dollars), (ii) maternal education (less than a high school diploma versus a high school diploma or higher), (iii) maternal depressive symptoms using the Beck Depression Inventory (33), and (iv) neighborhood deprivation, a composite score of impoverishment using U.S. Census data (e.g., median family income, % households on public assistance) (34).
Antisocial Behavior
To create a dimensional measure of AB, symptoms of Antisocial Personality Disorder and responses to screener questions (i.e., Conduct Disorder symptoms) were collected at age 20 through interviews with the participants by a trained examiner using the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (35). To confirm that associations were specific to AB rather than other related psychopathologies, we controlled for concurrent symptoms of substance use disorder (SUD), alcohol use disorder (AUD) and post-traumatic stress disorder (PTSD), all collected using the SCID-I (36). To control for earlier AB, parent-reported child externalizing behavior at age 2 was measured using the Externalizing factor of the Child Behavior Checklist (37).
Neuroimaging
Amygdala Reactivity Paradigm
Amygdala reactivity was assessed using an emotional faces matching paradigm (38). Trials were presented in counterbalanced blocks of angry, fearful, neutral, or surprised facial expressions interleaved with five blocks of a sensorimotor control (see Supplement).
fMRI Data Acquisition and Preprocessing
Each participant was scanned with a Siemens 3-T Tim Trio. As previously described in this sample (38), preprocessing was conducted using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) with post-processing control for artifacts and exclusion of participants with low amygdala coverage (see Supplement for details).
fMRI Analysis
The general linear model of SPM8 was used to estimate condition-specific (e.g., fearful faces > shapes) BOLD activation for each individual scan. Individual contrast images were then used in second-level random effects models to determine mean expression-specific reactivity using one-sample t-tests. As our goal was to examine amygdala reactivity to specific contrasts, the following were estimated and mean cluster values were extracted from SPM8 to be used in path models: fearful facial expressions > shapes and angry facial expressions > shapes to measure neural reactivity to interpersonal distress and threat, respectively (39), which have been implicated in AB (22). We also examined neutral faces > shapes because recent studies show similar amygdala responses to unmasked neutral faces and other expressions of threat (40,41), suggesting that ambiguity may also be interpreted as threatening (42). Across all three contrasts, we hypothesized that early adversity would be related to greater amygdala reactivity to emotional facial expressions. Contrast-specific BOLD parameter estimates were extracted from activated clusters within anatomically-defined amygdala regions (using the AAL Atlas) that survived the Family-Wise Error (FWE) correction in SPM8 of p<.05 (see Table S2).
DNA Collection and Genotyping
Genomic DNA was collected when youth were age 17 and isolated from saliva samples using the OrageneTM DNA self-collection kit (DNA Genotek Inc., Canada). DNA was extracted using standard methods. SNPs within CRHR1 were identified using TaqMan allelic discrimination assays. All genotypes were in Hardy-Weinberg Equilibrium in the full sample, as well as in the African-American and European-American subsamples. Allelic frequencies did not differ from HapMap expected distributions by race (Table S3). Of the 310 participants in the study, valid genetic data were available for 228 (rs110402) and 225 (rs7209436) participants. To maximize information from both SNPs and to avoid multiple comparisons by looking at each SNP individually, we grouped individuals who were homozygous major at both SNPs into one genotype group and individuals who were heterozygous or homozygous minor into another genotype group, consistent with previous studies (26–28) (see Supplement).
Statistical Analysis
All statistical analyses were performed in Mplus 7.2 (43) using full information maximum likelihood (FIML) estimation. FIML estimation uses the covariance matrix of all available data to produce unbiased estimates and standard errors in the context of missing data (44). Simulation studies indicate that FIML estimation provides unbiased estimates with greater power than listwise deletion even when up to 50% of data are missing (45,46). Thus our analyses used all participants (N=310), except when they were missing data on a grouping variable in multi-group models (i.e., when testing CRHR1 as a moderator; n=214). Using listwise deletion (N=167) produced a similar pattern of results (see Figure S1).
All models controlled for child race and externalizing symptoms at age 2. First, measures of early adversity were entered into six linear regressions predicting amygdala reactivity (i.e., right and left amygdala reactivity to fear>shapes, anger>shapes, and neutral>shapes). We focus on estimates that survived Bonferroni correction for multiple comparisons (p=.05/ six regressions =adjusted p-value of .008). To test developmental specificity, we added measures of adversity from the age 12 assessment to the models (see Supplement). Second, as both AB and multiple measures of adversity were correlated with right amygdala reactivity to fearful facial expressions, for each measure of adversity that predicted right amygdala reactivity to fearful faces, we estimated an indirect effects model linking early adversity to AB via amygdala reactivity using bootstrapping methods to produce unbiased confidence intervals. We could not test any other neural contrasts as mediators in this model because AB was only related to amygdala reactivity to fearful faces, consistent with past work in this sample (38). Finally, we tested whether variation in CRHR1 moderated pathways from adversities to amygdala reactivity and AB by comparing the model fit of a baseline model to a model where the adversity to brain pathway was specified to vary across CRHR1 genotype groups (see Supplement). Given the greater statistical power presented by the entire sample (N=310), we focus on results of the path analyses in the total sample while controlling for child race. However, given potential effects of ancestry (47), we also present results separated by self-reported race in Figures S2 and S3. For models which tested pathways to AB, we also included symptoms of SUD, AUD, and PTSD at age 20 as covariates. Moreover, in a separate model (Figure S4), we included multiple dimensions of psychopathology as outcome variables to ensure the pattern of findings was specific to AB.
Results
Do early childhood adversities predict amygdala reactivity in adulthood?
Zero-order correlations (Table 1) showed that multiple adversities in toddlerhood were associated with amygdala reactivity to angry, fearful, and neutral facial expressions. In particular, harsh parenting was related to lower right amygdala reactivity to both fearful and angry faces and neighborhood deprivation was related to greater bilateral amygdala reactivity to neutral faces. Though only trend-level, harsh parenting and neighborhood deprivation both predicted neural reactivity in the same direction: less amygdala reactivity to fearful, and greater amygdala reactivity to neutral, facial expressions.
Table 1.
Zero-order correlations between toddlerhood adversities and amygdala reactivity to emotional facial expressions at age 20
| Fear | Neutral | Angry | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Right | Left | Right | Left | Right | Left | Mean(SD) | Min | Max | |
|
|
|||||||||
| Adversities (2 years) | |||||||||
|
|
|||||||||
| Harsh parenting | −.21* | −.11 | .153 | .14 | −.163 | −.173 | 4.97(1.93) | 0 | 8 |
| Monthly family income in dollars | .04 | −.01 | .03 | −.11 | .07 | .10 | 1094.60 (695.59) | 249 | 40,000 |
| Neighborhood deprivation | −.133 | −.10 | .16* | .23** | −.10 | −.05 | .38(1.19) | −2.04 | 3.40 |
| Maternal education in years | −.08 | −.09 | −.09 | −.14* | .123 | .03 | 12.62(1.55) | 8 | 18 |
| Maternal depression | .12 | .143 | .002 | .02 | −.17* | −.08 | 7.59(6.28) | 0 | 36 |
| Psychopathology (20 years) | |||||||||
|
|
|||||||||
| Antisocial Behavior | −.20* | −.22* | −.03 | −.02 | .01 | −.04 | 2.43(3.89) | 0 | 18 |
N=310;
p<.10
p<.05,
p<.01,
p<.001.
Which adversities in early childhood have unique effects?
In regression models controlling for all adversities and covariates, harsh parenting in toddlerhood uniquely predicted lower right amygdala reactivity to fearful facial expressions in adulthood (Table 2, Figure 2a). Neighborhood deprivation also uniquely predicted greater bilateral amygdala reactivity to neutral facial expressions (Table 2). Like harsh parenting, maternal education also predicted less left amygdala reactivity to fearful facial expressions and maternal depression predicted lower right amygdala reactivity to angry facial expressions, but neither of these associations survived correction for multiple comparisons. The direction of effects across adversities was consistent: Harsher parenting and greater neighborhood deprivation both predicted lower amygdala response to fearful faces and greater amygdala response to ambiguous neutral faces. Moreover, these results remained when controlling for parallel measures of adversities at age 12 (Table S4), indicating that adversity in toddlerhood exerts unique developmental effects on amygdala function in adulthood.
Table 2.
Early parenting and neighborhood deprivation uniquely predict amygdala reactivity to emotional faces in adulthood
| Fear | Neutral | Angry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Right | Left | Right | Left | Right | Left | |||||||
|
| ||||||||||||
| B(SE) | β | B(SE) | β | B(SE) | β | B(SE) | β | B(SE) | β | B(SE) | β | |
| Harsh parenting | .07(.03) | − .25**# | .05(.02) | −.163 | −.05(.03) | .163 | −.03(.03) | .11 | .03(.02) | −.11 | .05(.03) | −.163 |
| Income | .001(.01) | .001 | −.003(.01) | −.04 | .01(.01) | .13 | .001(.01) | .01 | −.001(.01) | −.02 | .01(.01) | .08 |
| Neighborhood deprivation | −.11(.05) | −.23* | −.09(.05) | −.183 | .20(.05) | .36***# | .13(.05) | .27**# | −.05(.05) | −.09 | −.02(.05) | −.04 |
| Maternal education | −.24(.12) | −.14* | −.23(.13) | −.143 | −.15(.14) | −.08 | −.18(.10) | −.11 | .21(.13) | .12 | −.01(.15) | −.003 |
| Maternal depression | .01(.01) | .15* | .02(.01) | .17* | .001(.01) | .01 | .002(.01) | .02 | −.02(.01) | −.17* | −.01(.01) | −.06 |
N=310,
p<.10
p<.05,
p<.01,
p<.001,
indicates that the estimate survives Bonferroni correction for multiple comparisons (p<.05/6 regressions=.008 adjusted p-value).
Each column presents one linear regression model where all predictor variables are listed in the rows. All predictor variables were collected at age 2 years. Child race was also included as a covariate and emerged as a significant predictor in the linear regression models predicting right fear faces > shapes and right neutral faces > shapes. European Americans had less right amygdala reactivity to fear faces > shapes (β=.22, p<.05) and greater right amygdala reactivity to neutral faces > shapes (β=-.31, p<.001) compared to African American and biracial participants.
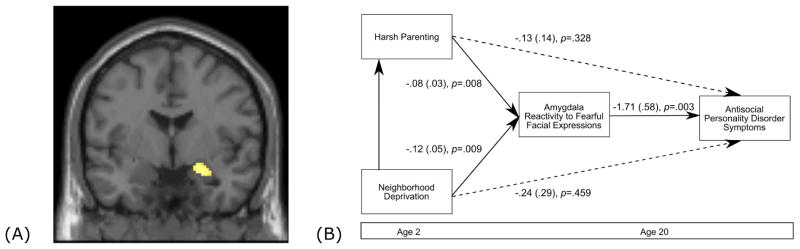
Figure 2. Harsh parenting and neighborhood deprivation predict antisocial behavior via low amygdala reactivity to fearful facial expressions.
(A) Main effect of fearful facial expressions versus shapes in the right amygdala (Table S2). (B) Path model linking harsh parenting and neighborhood deprivation at age 2 to symptoms of Antisocial Personality Disorder at age 20 via right amygdala reactivity to fearful facial expressions. Covariates include child race, toddler externalizing symptoms, maternal education, family income, and maternal depression symptoms at age 2, and at age 20, symptoms of SUD, AUD, and PTSD. Paths are marked with unstandardized coefficients. For indirect effects, we provide the unbiased bootstrapped CI of this effect (p<.05) as well as an estimate of the product coefficients (αβ) (i.e. the ‘sobel test’) as an index of gross effect size .Indirect paths were: Harsh parenting to right amygdala reactivity to fearful facial expressions to Antisocial Personality Disorder symptoms (αβ = .06, p=.046; bootstrapped 95% CI = .13 to .001). Neighborhood deprivation to right amygdala reactivity to fearful facial expressions to Antisocial Personality Disorder Symptoms (αβ = .06, p=.043; bootstrapped 95% CI = .002 to .124). The path model demonstrated good fit, X2 = 2.11 (6) p=.90, CFI = 1.00, RMSEA = .00 CI (.00 to .03), SRMR=.01.
Do early adversities predict AB via amygdala reactivity to fearful facial expressions?
As AB was related to lower amygdala reactivity to fearful faces, we tested an indirect pathway in which harsh parenting and neighborhood impoverishment in toddlerhood predicted AB in adulthood via right amygdala reactivity to fearful faces. To confirm that the indirect pathway was specific to parenting and neighborhood impoverishment, we tested a conservative model that included concurrent measures of all other adversities, early child externalizing behavior, and child race as covariates. The model revealed two significant indirect pathways, in which harsh parenting and greater neighborhood deprivation each uniquely predicted higher AB via lower amygdala reactivity to fearful facial expressions (Figure 2b).
Does variation in CRHR1 moderate the relationship between early adversity and amygdala reactivity to fearful facial expressions?
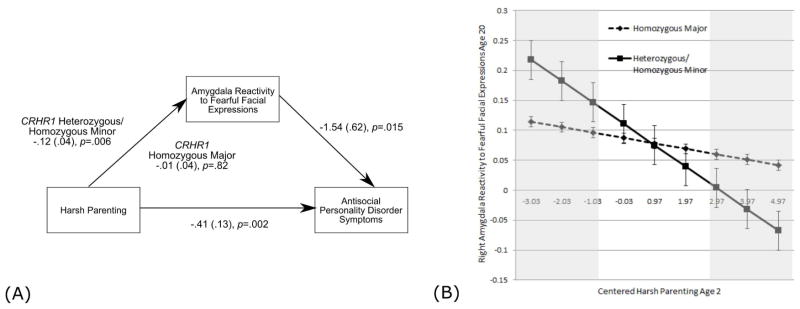
We found a statistically significant moderation of the pathway between harsh parenting, (but not neighborhood deprivation) and right amygdala reactivity by variation in CRHR1. For individuals who were heterozygous or homozygous minor across both SNPs (i.e., 2 or 4 total minor alleles), but not those homozygous for major alleles, harsh parenting predicted amygdala reactivity to fearful faces (Figure 3a). The results suggest a pattern of differential susceptibility rather than diathesis-stress (Figure 3b). Finally, we found that the indirect pathway from harsh parenting to AB via amygdala reactivity was only significant for individuals who were heterozygous or homozygous minor at both SNPs (i.e., moderated mediation; Figure 3a).
Figure 3. CRHR1 genotype moderates the relationship between harsh parenting and amygdala reactivity to fearful facial expressions.
(A) Path model indicates that only among individuals who are heterozygous or homozygous minor at both CRHR1 SNPs is parenting at age 2 related to right amygdala reactivity to fearful faces at age 20. Covariates include child race, toddler externalizing symptoms, neighborhood deprivation, maternal education, family income, and maternal depression symptoms at age 2, and at age 20, symptoms of SUD, AUD, and PTSD. Paths are marked with unstandardized coefficients. Moderation was tested by comparing model fit (X2) of the baseline model (see Supplement) to a model where harsh parenting to amygdala reactivity was allowed to vary across genotype groups, ΔX2=3.88, df=1, p=.04 (indicating significant moderation). In addition, we report a an indirect effect for individuals who are heterozygous or homozygous minor, but no indirect effect for those who were homozygous major, from harsh parenting to amygdala reactivity to Antisocial Personality Disorder symptoms (αβ =.12, p=.042; bootstrapped 95% CI = .01 to .70). The path model demonstrated good fit, X2 = 61.43 (61) p=.47, CFI = 1.00, RMSEA = .10 CI (.00 to .06), SRMR=.06. (B) Predicted values of amygdala reactivity to right fearful facial expressions at each value of harsh parenting for individuals who are homozygous major (n=89) versus heterozygous or homozygous minor (n=125) at both CRHR1 SNPs. The plotted points were calculated using unstandardized beta estimates from a regression model using a continuous interaction term to test moderation by genotype (see Supplement). The shaded gray boxes indicate regions where the standard errors of each plotted point for each genotype group do not overlap (i.e., regions where the expected values of amygdala reactivity are significantly different by genotype group). Thus, this figure depicts a pattern of differential susceptibility to harsh parenting, where heterozygotes and individuals who are homozygous minor at both CRHR1 SNPs show lower amygdala reactivity in the context of high harshness but greater amygdala reactivity in the context of low harshness, with respect to the other genotype group.
Discussion
The findings offer evidence for neurodevelopmental effects of early adversity across 18 years. In a prospectively-followed sample of young men, we found that multiple adversities, including harsh parenting, neighborhood deprivation, low maternal education, and maternal depressive symptoms in toddlerhood predicted amygdala reactivity to emotional facial expressions in adulthood. Importantly, early harsh parenting and neighborhood deprivation predicted amygdala reactivity over and above the effects of other adverse experiences in both toddlerhood and early adolescence, survived correction for multiple comparisons, and predicted clinically-significant AB at age 20. Specifically, these adversities predicted less amygdala reactivity to interpersonal threat and greater amygdala reactivity to interpersonal ambiguity. We also found evidence suggesting that individuals with minor alleles within CRHR1 (rs110402 and rs7209436) exhibited greater neurobehavioral sensitivity to harsh parenting. These findings inform a model of neurodevelopment in which early adversity becomes biologically embedded via altered amygdala reactivity, which in turn, increases risk for AB, potentially only for those with “susceptible” genotypes.
One of our most striking findings is that harsh parenting during toddlerhood predicted amygdala reactivity to interpersonal distress and subsequent AB in early adulthood even after accounting for many other adversities and other co-occurring psychopathology. Prior research linking extreme forms of adversity (i.e., childhood maltreatment) or distal measures of adversity (e.g., socioeconomic status) to amygdala function have not consistently accounted for the correlation between multiple types of adversity including sociodemographic- (i.e., income, maternal education), family- (i.e., maternal depression), and community-level stressors (i.e., neighborhood deprivation) (8–11), a strength of the current study. We also found that harsh parenting in toddlerhood predicted amygdala reactivity even when considering parenting behaviors in early adolescence, suggesting that parenting during early childhood has unique effects on later neural function.
That less amygdala reactivity to interpersonal distress was related to greater AB is consistent with previous work in this sample (38) and a broader literature implicating low amygdala reactivity to interpersonal fear in severe AB (particularly psychopathy; 22,48). However, that harsh parenting was related to lower amygdala reactivity to fearful facial expressions contrasts with our hypotheses and previous studies among adolescents, in which child maltreatment (8) and social deprivation (9) predicted greater amygdala reactivity to angry and fearful facial expressions relative to calm faces, respectively. However, a recent paper using a sample at risk for AB reported that greater childhood family adversity also predicted blunted amygdala reactivity to emotional facial expressions (49), consistent with our results. Thus, it could be that the differences in directionality between our findings and the maltreatment literature (8, 9) are due to sample characteristics including the prevalence of AB and the all-male nature of our sample, with boys being at greater risk for AB (22,48). Second, as research indicates that amygdala reactivity to emotional faces may peak in adolescence and decline into adulthood (50), our findings could also reflect differential developmental trajectories of risk on amygdala reactivity. Finally, we focused on neural contrasts between fearful facial expressions and a non-face shapes stimuli. The neutral facial expressions used in our task were “neutral” as opposed to “calm” (neutral morphed with some happy) faces used in previous studies (8,9). Indeed, we found that multiple adversities predicted greater amygdala reactivity to neutral faces, suggesting that these ambiguous faces may be interpreted as hostile, at least for the males in this study living in dangerous neighborhoods (51), a hypothesis supported by recent studies in similarly high risk contexts (40,41).
Importantly, the direction of findings across different types of adversity was consistent: poorer neighborhood conditions and harsher parenting predicted less amygdala reactivity to fearful facial expressions and greater amygdala reactivity to ambiguous neutral facial expressions. Moreover, neighborhood deprivation during early childhood predicted amygdala reactivity to faces in adulthood even after controlling for neighborhood effects in early adolescence. While this might be surprising because toddlers have more direct contact with parents than the broader neighborhood context (52), impoverished neighborhoods expose families to multiple stressors, including community violence, crowding, and poor quality (pre)schools (15, 53). Neighborhood impoverishment could undermine amygdala development differently at each developmental stage through exposure to various aspects of neighborhood stress (e.g., low quality preschool education early; witnessing community violence later) (53). A recent theory (54) suggests that the effects of adversity on the brain may depend on the type of stress (i.e., threat versus deprivation). Thus, according to this theory, our results could be highlighting the impact of threatening aspects of neighborhood deprivation (e.g., community violence) on the family, child, and amygdala. Clearly more research is needed to understand the ways in which specific aspects of impoverished contexts affect neurodevelopment (14,15).
Previous studies linking early adversity to neural function and psychopathology have examined internalizing outcomes (10,11). Our results extend this literature by describing a mechanistic pathway to AB whereby harsh parenting and neighborhood deprivation were related to higher levels of AB via lower amygdala reactivity to interpersonal distress. Moreover, we tested a stringent model by controlling for comorbidity with substance use and PTSD symptoms (55,56) ,as well as a separate model in which multiple measures of psychopathology were modeled as outcomes in addition to AB (Figure S4), thus demonstrating that these neurodevelopmental effects of early adversity were specific to AB.
Importantly, these pathways showed variability across participants, as there was preliminary evidence that CRHR1 genotype moderated the neural effects of early adversity. Our findings suggested differential susceptibility, such that individuals with minor alleles at two CRHR1 SNPs demonstrated the lowest amygdala reactivity (and greatest risk for AB) when exposed to harsh parenting, but the highest amygdala reactivity (and lowest risk for AB) when exposed to more positive parenting. Though this result is difficult to reconcile with regards to most studies linking greater amygdala reactivity to psychopathology (21), given our findings of the specificity of these results to AB and previous findings in this sample and others that low amygdala reactivity predicts poor outcomes (22,38,48), it may be that amygdala reactivity acts in a different way in predicting risk among high-risk urban young men. That minor allele carriers evinced the greatest risk for poor outcomes was contrary to our hypothesis and some prior research (26–28), though not several other studies (29,30). Our results suggest that among boys living in low-income urban neighborhoods, interventions to reduce harsh parenting and to develop and sustain positive parenting, are critical for long-term neurobehavioral outcomes, but may be particularly effective for youth with “susceptible” alleles within CRHR1 (57).
Although we tested a conservative model by controlling for multiple adversities using observational measures, prospective data at two time points, and correcting for multiple comparisons when examining multiple regressions, our complex imaging gene x environment interaction results need to be replicated in larger samples with greater power to detect smaller and more complex genetic moderation effects (58), and to minimize the risk for false positive associations that can arise in smaller samples (59). As we studied a sample socio-demographically enriched for externalizing psychopathologies, several of the young men could not participate in the MRI (e.g., history of concussion, incarceration), highlighting a broader sampling problem for studies of antisocial participants (60). Further, without ancestry informative markers, we were unable to control for genetic admixture within our sample (47). However, self-reported race is highly correlated with ancestry (61), potentially addressing this limitation. Additionally, though these results suggest that contextual factors may affect neurodevelopment, we only measured amygdala reactivity at one time point and did not examine trajectories of AB or cumulative effects of adversity across childhood. Finally, we cannot exclude potential gene-environment correlations (62). Future studies using genetically-informed designs (63) may be able to parse heritable and non-heritable effects from pathways linking adversity to neurobehavioral outcomes.
Although these limitations call for replication, the current study demonstrates the impact of early adversity on neurobehavioral development using longitudinal measurement of observed environmental adversities from toddlerhood to adulthood, a widely-used fMRI task to probe amygdala reactivity, a cohort of predominantly low-income men exposed to multiple environmental stressors, greater racial diversity than most fMRI studies, an innovative developmental imaging gene x environment interaction model (64), and interviewer-assessed clinically-meaningful antisocial behaviors. These results provide a model by which chronic stressors prevalent in low-income, urban environments increase risk for later AB via their effect on the developing brain, and suggest why only some individuals have poor outcomes under these conditions. These findings inform a model for the development of AB across the lifespan and provide further evidence for the neurotoxic effects of early life adversity within the home and community.
Supplementary Material
Figure S1. Indirect pathway results for the imaging sample only (N=167)
N=167; Path model linking harsh parenting and neighborhood deprivation at age 2 to symptoms of Antisocial Personality Disorder at age 20 via right amygdala reactivity to fearful facial expressions. Covariates include child race, toddler externalizing symptoms, maternal education, family income, and maternal depression symptoms at age 2, and at age 20, symptoms of SUD, AUD, and PTSD. Paths are marked with unstandardized coefficients. For indirect effects, we provide the unbiased bootstrapped CI of this effect (p<.05) as well as an estimate of the product coefficients (αβ) (i.e. the ‘Sobel test’) as an index of gross effect size. Indirect paths were: Harsh parenting to right amygdala reactivity to fearful facial expressions to Antisocial Personality Disorder symptoms (αβ = .05, p=.052; bootstrapped 90% CI=.008 to .09). Neighborhood deprivation to right amygdala reactivity to fearful facial expressions to Antisocial Personality Disorder Symptoms (αβ = .04, p=.04; bootstrapped 95% CI=.003 to .09). The path model demonstrated good fit, X2 = 2.36 (6) p=.88, CFI = 1.00, RMSEA = .00 CI (.00 to .05), SRMR=.01.
Figure S2. Indirect pathway results by race
Path model linking harsh parenting at age 2 to symptoms of Antisocial Personality Disorder at age 20 via right amygdala reactivity to fearful facial expressions. Covariates include child externalizing symptoms, maternal education, family income, and maternal depression symptoms at age 2, and at age 20, symptoms of SUD, AUD, and PTSD. Paths are marked with unstandardized coefficients. Among African American (AA) participants, neighborhood deprivation but not harsh parenting predicted amygdala reactivity to fearful facial expressions, and greater amygdala reactivity to fearful facial expressions significantly predicted less antisocial behavior. There were no indirect pathways linking neighborhood deprivation or harsh parenting to antisocial behavior via amygdala reactivity. Among European American (EA) participants, neighborhood deprivation and harsh parenting did not predict amygdala reactivity to fearful facial expressions and amygdala reactivity to fearful facial expressions did not predict antisocial behavior. There were no indirect pathways linking neighborhood deprivation or harsh parenting to antisocial behavior via amygdala reactivity.
Figure S3. Moderated mediation results by race
Path model linking harsh parenting at age 2 to symptoms of Antisocial Personality Disorder at age 20 via right amygdala reactivity to fearful facial expressions. Covariates include child externalizing symptoms, neighborhood deprivation, maternal education, family income, and maternal depression symptoms at age 2, and at age 20, symptoms of SUD, AUD, and PTSD. Paths are marked with unstandardized coefficients. Moderation was tested by comparing model fit (X2) of the baseline model (see supplemental methods) to a model where harsh parenting to amygdala reactivity was allowed to vary across genotype groups. Among both African American (AA) participants and European American (EA) participants, harsh parenting did not predict amygdala reactivity to fearful facial expressions among individuals who were heterozygous or homozygous minor at both CRHR1 SNPs (AA: n = 54; EA: n = 71) or individuals who were homozygous major at both CRHR1 SNPs (AA: n = 46; EA: n = 43). For AA participants, the pathway from harsh parenting to amygdala reactivity to fearful facial expressions was significantly moderated by genetic variation in CRHR1 (ΔX2=7.95, df=1, p=.004) and there was an indirect effect at trend level for the pathway from harsh parenting to antisocial behavior via amygdala reactivity only for youth heterozygous or homozygous minor (αβ =.24, p=.08; bootstrapped 90% CI = .02 to .46). For EA participants, genetic variation in CRHR1 did not moderate the pathway from harsh parenting to amygdala reactivity (ΔX2=.03, df=1, p=.86).
Figure S4. The effect of early adversities on psychopathology via the brain is specific to antisocial behavior
N=310; Solid lines indicate significant pathways at p<.05 and dotted lines indicate nonsignificant pathways. We tested all indirect effects from harsh parenting and neighborhood deprivation to each measure of psychopathology via right amygdala reactivity to fearful facial expressions. Harsh parenting and neighborhood deprivation predicted symptoms of antisocial personality disorder, but not other measured psychopathologies, via amygdala reactivity. Harsh parenting to amygdala reactivity to antisocial behavior: αβ =.07, p=.066; bootstrapped 90% CI = .007 to .13. Neighborhood deprivation to amygdala reactivity to antisocial behavior: αβ =.07, p=.043; bootstrapped 95% CI = .002 to .128.
Table S1. Summary of available fMRI data for analyses
Table S2. Main effects of amygdala reactivity to emotional faces using fMRI
Table S3. Observed and expected genotype and minor allele frequencies in the total sample and by self-reported race for CRHR1 SNPs
Table S4. Early life adversities predict amygdala reactivity to emotional faces while controlling for adversities at age 12
Acknowledgments
The research reported in this paper was supported by grants to D.S.S. (R01 MH50907, R01 MH01666, and K05 DA25630), D.S.S. and E.E.F (R01 DA026222), L.W.H. (L40 DA036468), and A.M.G (T32 HD00710936) by the National Institutes of Health. A.R.H. received support by National Institutes of Health grant R01 DA033369, R01 DA031579, R01 AG049789, and R21 MH106715. Dr. Hyde’s work is also supported by the Avielle Foundation. We are grateful to the work of the staff of the Pitt Mother & Child Project for their many years of service, and to our study families for sharing their lives with us and making the research possible.
Footnotes
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Supplemental Information: supplemental methods, results; 4 tables, 3 figures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, Garner AS, et al. The Lifelong Effects of Early Childhood Adversity and Toxic Stress. Pediatrics. 2012;129:232–246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 2.Duncan GJ, Ziol-Guest KM, Kalil A. Early-Childhood Poverty and Adult Attainment, Behavior, and Health. Child Dev. 2010;81:306–325. doi: 10.1111/j.1467-8624.2009.01396.x. [DOI] [PubMed] [Google Scholar]
- 3.Shaw DS, Hyde LW, Brennan LM. Early predictors of boys’ antisocial trajectories. Dev Psychopathol. 2012;24:871–888. doi: 10.1017/S0954579412000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 2010;81:357–367. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- 5.Whalen PJ, Phelps EA. The Human Amygdala. New York, NY: Guilford Press; 2009. [Google Scholar]
- 6.Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol and Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 7.Howell BR, Grand AP, McCormack KM, Shi Y, LaPrarie JL, Maestripieri D, et al. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: Relation to amygdala volume: Adverse Caregiving Increases Emotional Reactivity. Dev Psychobiol. 2014;56:1735–1746. doi: 10.1002/dev.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, Viding E. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21 doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation: Neurodevelopment and adversity. Dev Sci. 2011;14: 190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci U S A. 2013;110: 18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, Luby JL, Botteron KN, Dietrich R, McAvoy MP, Barch DM. Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. J Am Acad Child Adolesc Psychiatry. 2014;53: 800–13. doi: 10.1016/j.jaac.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belsky J, de Haan M. Annual Research Review: Parenting and children’s brain development: the end of the beginning: Parenting and children’s brain development. Journal of Child Psychology and Psychiatry. 2011 Apr;52(4):409–28. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 13.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, et al. The Effects of Poverty on Childhood Brain Development: The Mediating Effect of Caregiving and Stressful Life Events. JAMA Pediatr. 2013;167:1135. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conger RD, Ge X, Elder GH, Lorenz FO, Simons RL. Economic stress, coercive family process, and developmental problems of adolescents. Child Dev. 1994;65:541–61. [PubMed] [Google Scholar]
- 15.McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53: 185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 16.Bronfenbrenner U, Morris PA. The bioecological model of human development. In: Damon W, Lerner RM, editors. Handbook of Child Psychology. 6. Hoboken, NJ: John Wiley & Sons, Inc; 2006. pp. 793–828. [Google Scholar]
- 17.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3: 1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callaghan BL, Tottenham N. The Neuro-Environmental Loop of Plasticity: A Cross-Species Analysis of Parental Effects on Emotion Circuitry Development Following Typical and Adverse Caregiving. Neuropsychopharmacology. 2016 Jan;41(1):163–76. doi: 10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomanowska AM, Boivin M, Hertzman C, Fleming AS. Parenting begets parenting: A neurobiological perspective on early adversity and the transmission of parenting styles across generations. Neurosci. 2017;342: 120–139. doi: 10.1016/j.neuroscience.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13: 636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- 21.Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Dev Psychopathol. 2008;20:1231. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- 22.Hyde LW, Shaw DS, Hariri AR. Understanding youth antisocial behavior using neuroscience through a developmental psychopathology lens: Review, integration, and directions for research. Dev Rev. 2013;33: 168–223. doi: 10.1016/j.dr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychol Bull. 2009;135: 885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci U S A. 2012;109: 17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnett MG, Muglia LM, Laryea G, Muglia LG. Genetic approaches to hypothalamic-pituitary-adrenal axis regulation. Neuropsychopharmacology. 2016;41: 245–260. doi: 10.1038/npp.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65: 190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of Childhood Maltreatment with the Corticotropin-Releasing Hormone Receptor Gene: Effects on Hypothalamic-Pituitary-Adrenal Axis Reactivity. Biol Psychiatry. 2009;66: 681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heim C. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Front Behav Neurosci. 2009;3 doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grabe HJ, Schwahn C, Appel K, Mahler J, Schulz A, Spitzer C, et al. Childhood maltreatment, the corticotropin-releasing hormone receptor gene and adult depression in the general population. Am J Med Genet B Neuropsychiatr Genet. 2010;153B: 1483–93. doi: 10.1002/ajmg.b.31131. [DOI] [PubMed] [Google Scholar]
- 30.DeYoung CG, Cicchetti D, Rogosch FA. Moderation of the association between childhood maltreatment and neuroticism by the corticotropin-releasing hormone receptor 1 gene. J Child Psychol and Psychiatry. 2011;52:898–906. doi: 10.1111/j.1469-7610.2011.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiroi N, Wong M, Licinio J, Park C, Young M, Gold P, et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6: 540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 32.Caldwell BM, Bradley RH, et al. Home observation for measurement of the environment. University of Arkansas at Little Rock Little Rock; 1984. [Google Scholar]
- 33.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh JK. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 34.Vanderbilt-Adriance E, Shaw DS. Protective Factors and the Development of Resilience in the Context of Neighborhood Disadvantage. J Abnorm Child Psychol. 2008;36: 887–901. doi: 10.1007/s10802-008-9220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.First MB, Gibbon M, Spitzer RL. American Psychiatric Pub. 1997. User's guide for the structured clinical interview for DSM-IV axis II personality disorders: SCID-II. [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JB. American Psychiatric Pub. 1997. User's guide for the Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. [Google Scholar]
- 37.Achenbach TM. Manual for the Child Behavior Checklist/4-18. University of Vermont Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- 38.Hyde LW, Shaw DS, Murray L, Gard A, Hariri AR, Forbes EE. Dissecting the Role of Amygdala Reactivity in Antisocial Behavior in a Sample of Young, Low-Income, Urban Men. Clin Psychol Sci. 2015:1–18. doi: 10.1177/2167702615614511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- 40.Marusak HA, Carré JM, Thomason ME. The stimuli drive the response: An fMRI study of youth processing adult or child emotional face stimuli. NeuroImage. 2013;83:679–689. doi: 10.1016/j.neuroimage.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Marusak HA, Zundel C, Brown S, Rabinak CA, Thomason ME. Is neutral really neutral? Converging evidence from behavior and corticolimbic connectivity in children and adolescents. Soc Cogn Affect Neurosci. 2016 doi: 10.1093/scan/nsw182. DOI: https://doi.org/10.1093/scan/nsw182. [DOI] [PMC free article] [PubMed]
- 42.Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: Developmental effects of child abuse and neglect. Dev Psychol. 2000;36: 679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- 43.Muthén LK, Muthén B. Mplus user’s guide, Seventh Edition. Los Angeles: Muthén & Muthén; 1998–2011. [Google Scholar]
- 44.McCartney K, Burchinal MR, Bub KL. Best practices in quantitative methods for developmentalists. Monogr Soc Res Child Dev. 2006:i–145. doi: 10.1111/j.1540-5834.2006.07103001.x. [DOI] [PubMed] [Google Scholar]
- 45.Enders C, Bandalos D. The Relative Performance of Full Information Maximum Likelihood Estimation for Missing Data in Structural Equation Models. Struct Equ Modeling. 2001;8: 430–457. [Google Scholar]
- 46.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002;7: 147–177. [PubMed] [Google Scholar]
- 47.Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X, Brown A, et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet. 2005;76: 268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blair RJR, Leibenluft E, Pine DS. Conduct disorder and callous–unemotional Traits in youth. New England Journal of Medicine. 2014;371:2207–2216. doi: 10.1056/NEJMra1315612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holz NE, Boecker-Schlier R, Buchmann AF, Blomeyer D, Jennen-Steinmetz C, Baumeister S, Plichta MM, Cattrell A, Schumann G, Esser G, Schmidt M. Ventral striatum and amygdala activity as convergence sites for early adversity and conduct disorder. Social Cognitive and Affective Neuroscience. 2016 doi: 10.1093/scan/nsw120. Advance online access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20: 1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55: 897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Shaw DS, Bell RQ. Developmental theories of parental contributors to antisocial behavior. J Abnorm Child Psychol. 1993;21: 493–518. doi: 10.1007/BF00916316. [DOI] [PubMed] [Google Scholar]
- 53.Leventhal T, Brooks-Gunn J. The neighborhoods they live in: The effects of neighborhood residence on child and adolescent outcomes. Psychological Bulletin. 2000;126: 309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- 54.McLaughlin KA, Sheridan MA. Beyond cumulative risk a dimensional approach to childhood adversity. Curr Dir Psychol Sci. 2016;25: 239–45. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Comptom WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66: 677–685. doi: 10.4088/jcp.v66n0602. [DOI] [PubMed] [Google Scholar]
- 56.Sareen J, Stein MB, Cox BJ, Hassard ST. Understanding Comorbidity of Anxiety Disorders With Antisocial Behavior: Findings From Two Large Community Surveys. J Nerv Ment Dis. 2004;192: 178–186. doi: 10.1097/01.nmd.0000116460.25110.9f. [DOI] [PubMed] [Google Scholar]
- 57.Belsky J, van IJzendoorn MH What works for whom? Genetic moderation of intervention efficacy. Dev Psychopathol. 2015;27:1–6. doi: 10.1017/S0954579414001254. [DOI] [PubMed] [Google Scholar]
- 58.Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene–environment interactions. Trends Cogn Sci. 2011;15: 417–427. doi: 10.1016/j.tics.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan LE, Keller MC. A Critical Review of the First 10 Years of Candidate Gene-by-Environment Interaction Research in Psychiatry. Am J Psych. 2011;168: 1041–9. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badawi MA, Eaton WW, Myllyluoma J, Weimer LG, Gallo J. Psychopathology and attrition in the Baltimore ECA 15-year follow-up 1981–1996. Soc Psychiatry Psychiatr Epidemiol. 1999;34: 91–98. doi: 10.1007/s001270050117. [DOI] [PubMed] [Google Scholar]
- 61.Novembre J, Johnson T, Bryc K, Kutalik Z, Boyko AR, Auton A, et al. Genes mirror geography within Europe. Nature. 2008;456: 98–101. doi: 10.1038/nature07331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaffee SR, Price TS. Gene–environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12: 432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyde LW, Waller R, Trentacosta CJ, Shaw DS, Neiderhiser JM, Ganiban JM, Reiss D, Leve LD. Heritable and Nonheritable Pathways to Early Callous-Unemotional Behaviors. Am J Psychiat. 2016 doi: 10.1176/appi.ajp.2016.15111381. Advance online access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hyde LW. Developmental psychopathology in an era of molecular genetics and neuroimaging: A developmental neurogenetics approach. Dev and Psychopathol. 2015;27: 587–613. doi: 10.1017/S0954579415000188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Indirect pathway results for the imaging sample only (N=167)
N=167; Path model linking harsh parenting and neighborhood deprivation at age 2 to symptoms of Antisocial Personality Disorder at age 20 via right amygdala reactivity to fearful facial expressions. Covariates include child race, toddler externalizing symptoms, maternal education, family income, and maternal depression symptoms at age 2, and at age 20, symptoms of SUD, AUD, and PTSD. Paths are marked with unstandardized coefficients. For indirect effects, we provide the unbiased bootstrapped CI of this effect (p<.05) as well as an estimate of the product coefficients (αβ) (i.e. the ‘Sobel test’) as an index of gross effect size. Indirect paths were: Harsh parenting to right amygdala reactivity to fearful facial expressions to Antisocial Personality Disorder symptoms (αβ = .05, p=.052; bootstrapped 90% CI=.008 to .09). Neighborhood deprivation to right amygdala reactivity to fearful facial expressions to Antisocial Personality Disorder Symptoms (αβ = .04, p=.04; bootstrapped 95% CI=.003 to .09). The path model demonstrated good fit, X2 = 2.36 (6) p=.88, CFI = 1.00, RMSEA = .00 CI (.00 to .05), SRMR=.01.
Figure S2. Indirect pathway results by race
Path model linking harsh parenting at age 2 to symptoms of Antisocial Personality Disorder at age 20 via right amygdala reactivity to fearful facial expressions. Covariates include child externalizing symptoms, maternal education, family income, and maternal depression symptoms at age 2, and at age 20, symptoms of SUD, AUD, and PTSD. Paths are marked with unstandardized coefficients. Among African American (AA) participants, neighborhood deprivation but not harsh parenting predicted amygdala reactivity to fearful facial expressions, and greater amygdala reactivity to fearful facial expressions significantly predicted less antisocial behavior. There were no indirect pathways linking neighborhood deprivation or harsh parenting to antisocial behavior via amygdala reactivity. Among European American (EA) participants, neighborhood deprivation and harsh parenting did not predict amygdala reactivity to fearful facial expressions and amygdala reactivity to fearful facial expressions did not predict antisocial behavior. There were no indirect pathways linking neighborhood deprivation or harsh parenting to antisocial behavior via amygdala reactivity.
Figure S3. Moderated mediation results by race
Path model linking harsh parenting at age 2 to symptoms of Antisocial Personality Disorder at age 20 via right amygdala reactivity to fearful facial expressions. Covariates include child externalizing symptoms, neighborhood deprivation, maternal education, family income, and maternal depression symptoms at age 2, and at age 20, symptoms of SUD, AUD, and PTSD. Paths are marked with unstandardized coefficients. Moderation was tested by comparing model fit (X2) of the baseline model (see supplemental methods) to a model where harsh parenting to amygdala reactivity was allowed to vary across genotype groups. Among both African American (AA) participants and European American (EA) participants, harsh parenting did not predict amygdala reactivity to fearful facial expressions among individuals who were heterozygous or homozygous minor at both CRHR1 SNPs (AA: n = 54; EA: n = 71) or individuals who were homozygous major at both CRHR1 SNPs (AA: n = 46; EA: n = 43). For AA participants, the pathway from harsh parenting to amygdala reactivity to fearful facial expressions was significantly moderated by genetic variation in CRHR1 (ΔX2=7.95, df=1, p=.004) and there was an indirect effect at trend level for the pathway from harsh parenting to antisocial behavior via amygdala reactivity only for youth heterozygous or homozygous minor (αβ =.24, p=.08; bootstrapped 90% CI = .02 to .46). For EA participants, genetic variation in CRHR1 did not moderate the pathway from harsh parenting to amygdala reactivity (ΔX2=.03, df=1, p=.86).
Figure S4. The effect of early adversities on psychopathology via the brain is specific to antisocial behavior
N=310; Solid lines indicate significant pathways at p<.05 and dotted lines indicate nonsignificant pathways. We tested all indirect effects from harsh parenting and neighborhood deprivation to each measure of psychopathology via right amygdala reactivity to fearful facial expressions. Harsh parenting and neighborhood deprivation predicted symptoms of antisocial personality disorder, but not other measured psychopathologies, via amygdala reactivity. Harsh parenting to amygdala reactivity to antisocial behavior: αβ =.07, p=.066; bootstrapped 90% CI = .007 to .13. Neighborhood deprivation to amygdala reactivity to antisocial behavior: αβ =.07, p=.043; bootstrapped 95% CI = .002 to .128.
Table S1. Summary of available fMRI data for analyses
Table S2. Main effects of amygdala reactivity to emotional faces using fMRI
Table S3. Observed and expected genotype and minor allele frequencies in the total sample and by self-reported race for CRHR1 SNPs
Table S4. Early life adversities predict amygdala reactivity to emotional faces while controlling for adversities at age 12