Abstract
Background
Habitual physical activity (HPA) measurement addresses the impact of MS on real-world walking, yet its interpretation is confounded by the competing influences of MS-associated walking capacity and physical activity behaviors.
Objective
To develop specific measures of MS-associated walking capacity through statistically sophisticated HPA analysis, thereby more precisely defining the real-world impact of disease.
Methods
Eighty-eight MS and 38 control subjects completed timed walks and patient-reported outcomes in clinic, then wore an accelerometer for 7 days. HPA was analyzed with several new statistics, including the maximum step rate (MSR) and habitual walking step rate (HWSR), along with conventional methods, including average daily steps. HPA statistics were validated using clinical walking outcomes.
Results
The six-minute walk (6MW) step rate correlated most strongly with MSR (r=0.863, p<10−25) and HWSR (r=0.815, p<10−11) rather than average daily steps (r=0.676, p<10−11). The combination of MSR and HWSR correlated more strongly with the 6MW step rate than either measure alone (r=0.884, p<10−14). The MSR overestimated the 6MW step rate (μ=10.4, p<10−7), whereas the HWSR underestimated it (μ=−18.2, p<10−19).
Conclusions
Conventional HPA statistics are poor measures of capacity due to variability in activity behaviors. The MSR and HWSR are valid, specific measures of real-world capacity which capture subjects’ highest step rate and preferred step rate, respectively.
Keywords: Multiple Sclerosis, Gait Disorders/Ataxia, Outcomes Research, Six-Minute Walk, Habitual Physical Activity, Habitual Walking Performance, Accelerometry
INTRODUCTION
Habitual physical activity (HPA) measurement has emerged as a walking outcome in MS[1] and Parkinson’s disease[2], with broad applications in health care[3],[4]. HPA improves upon available mobility measurements by quantifying the impact of disease on real-world functioning. Currently, HPA is commonly reported as (1) daily step counts or movement counts; or (2) amount of moderate physical activity (MPA) and vigorous physical activity (VPA). However, physical activity in all people, including those with chronic disease, is affected by a multitude of personal, environmental, and social factors[5],[6]. As a result, these statistics fail to distinguish subjects’ real-world walking capacity – which quantifies their ability to walk under real-world conditions, and is directly affected by MS – from physical activity behaviors not immediately attributable to disease. This dilemma that has been recognized[7], but not yet solved. Total daily activity is easy to calculate, but difficult to interpret when both disability and activity behaviors are variable.
Studies evaluating HPA in MS have borne out this concern. Although MS subjects are active less often then controls[8],[9], physical activity statistics (MPA and VPA) are correctly viewed as measures of activity behaviors, not precise measures of the impact of disease on subjects’ ability to be active. Daily step counts are the best known measure of real-world walking capacity, as shown by statistically significant correlations to the six-minute walk (6MW), timed 25-foot walk (T25FW), and MS Walking Scale (MSWS-12)[10],[11]. However, daily counts explain less than half of the variance in these outcomes[10],[11], and they do not reliably change when patient-reported walking ability changes[12]. Although daily counts have been established as valid walking outcomes, they are most accurately viewed as imprecise measures of both walking capacity and activity behaviors.
To address these limitations, we set out to establish new HPA statistics that isolate the direct impact of disease from activity behaviors. We hypothesize that the maximum step rate (MSR) along with the habitual walking step rate (HWSR), a statistic derived through personalized activity modeling (PAM), can meet this objective, providing clinicians with real-world capacity outcomes in MS and potentially other chronic diseases. This hypothesis is validated in MS through correlational analysis to the aforementioned walking outcomes, which in turn sheds new light on the real-world meaning of existing measures.
METHODS
Recruitment and Study Procedures
Study procedures were approved by the University of Virginia (UVa) Institutional Review Board, and written consent was obtained. Subjects with clinically definite MS[13] were recruited from the UVa Neurology Department outpatient clinic population along with healthy controls. All subjects were age 18–64 years and able to ambulate for six minutes, possibly with an assistive device. Those with neurological impairment from other diagnoses, orthopedic limitations, morbid obesity, or known cardiac or respiratory disease were excluded. All fatiguerelated medications were withheld 48 hours prior to their appointment.
Baseline demographics, medical history, and medications were documented. An Expanded Disability Status Scale (EDSS)[14] assessment was performed by Neurostatus-certified staff. MS-associated walking impairment was classified by EDSS as mild (0–2.5), moderate (3.0–4.0), or severe (≥4.5). The timed 25-foot walk (T25FW) was assessed prior to 6MW testing. Additionally, the MS Walking Scale (MSWS-12)[15] (MS only) and Modified Fatigue Impact Scale (MFIS)[16] were collected. The MFIS was analyzed as a single scale, but also broken down into its physical, cognitive, and psychosocial subscales, which measure these respective components of fatigue.
Subjects completed a six-minute walk (6MW) in a 75-foot hospital corridor using the script developed by Goldman et al.[17] while wearing an ActiGraph GT3X accelerometer on their nondominant hip and a Polar S610i heart rate monitor. Distance was manually recorded in 1-minute epochs, and step rates were recorded by ActiGraph (total steps ÷ six minutes).
Following the study visit, subjects wore the same ActiGraph device during waking hours for 7 days. Steps were aggregated into minute-wise step rates using ActiLife software, then exported to Matlab R2015b for subsequent processing. Wear days were declared valid only if steps were detected for at least 10 hours[11], and subjects with fewer than 6 valid days were excluded from the analysis. Other analyses in MS have required as few as 3 valid days[18] and as many as 7[11] for inclusion; we chose a conservative cutoff to ensure there was a consistent and sufficient quantity of data to fit the models described in the next section. For the conventional statistical analysis, step rates were classified as MPA, VPA, or neither using disability-specific cut-points that vary by MSWS-12 Score[19]. Time spent in moderate-to-vigorous physical activity (MVPA) was defined as the sum of time spent in MPA and VPA.
Personalized Activity Modeling
Step rates from valid days were used to fit a personalized activity model (PAM) for each subject. In essence, the PAM uses unsupervised statistical learning to identify subject-specific activity states, which are then used to classify the step rate. The following two assumptions underlie the process: (1) changes between activities can be modeled as a Markov chain; and (2) step rates in each activity follow a negative binomial distribution[20].
Step rates were fitted to hidden Markov models with a “not-worn” state (step rate = 0) and 2–6 additional states with negative binomial output using the Baum-Welch algorithm[21]. States were initialized with means evenly spaced between 0 and the subject’s maximum step rate (MSR), with variance set to MSR ÷ N, the number of states. The model with lowest Bayesian Information Criterion (BIC) was selected as the final model. Step rates were then fitted to this model using the Viterbi algorithm[22].
Active states (e.g. walking, running) were identified by their expected value (>MSR/2) and coefficient of variance (≤8). If only one active state was present, it was identified as walking unless the expected step rate exceeded 130. If two active states were present, the state with higher expected step rate was identified as running, and the other state was identified as walking. No subjects had more than two active states, thus further identification procedures were not needed and additional activities (e.g. stair climbing) were not specifically identified. The habitual walking step rate (HWSR) and habitual running step rate (HRSR) were defined as the expectations of the distributions identified as walking and running, respectively.
Statistical Analysis
Demographics, outcomes, and activity statistics were compared between disability groups by ANOVA and chi-square test as appropriate for numeric and categorical variables, respectively. Pearson correlations have been used to quantify relationships between activity statistics and clinical outcomes, which are continuous or approximately continuous (MFIS and MSWS-12). T25FW measurements have been converted from time to speed (feet/sec) to avoid non-linear relationships to other walking outcomes.
Correlation coefficients were interpreted as strong (>0.6), moderate (0.3–0.6), or weak (<0.3). Significance levels have been adjusted based on the number of comparisons per analysis. A significance level of α = 0.004 was used for demographics and outcomes (m=12); α = 0.001 and α = 0.0001 were used when comparing activity statistics and identifying significant correlations, respectively (m=28 and m=128). Correlations with p<10−8 are also reported.
RESULTS
Subject Demographics and Outcome Measures
In total, 88 subjects with MS[13] and 38 control subjects were recruited (Table 1). Among MS subjects, 52.3% had mild disability (EDSS 0–2.5), 35.2% moderate (EDSS 3.0–4.0), and 12.5% severe (EDSS ≥ 4.5). A higher proportion were female among MS subjects (83.0%) compared to controls (71.1%), though this difference was not statistically significant. Education was similar between groups, but age, employment status, years since symptom onset and diagnosis, and clinical outcomes showed statistically significant differences. In particular, control subjects were younger than MS subjects (p<0.0001). Disease subtype was also significantly different between groups, with 95.7% of mild MS subjects having relapsing-remitting disease compared to 77.4% of moderate MS and 45.5% of severe MS. MSWS-12 responses spanned the full range of possible scores (0–100).
Table 1.
Subject Demographics
| Variable | (1) Control | (2) Mild (EDSS 0 – 2.5) |
(3) Moderate (EDSS 3.0 – 4.0) |
(4) Severe (EDSS 4.5 – 6.5) |
p-value | Group-Wise Differences (Tukey’s qs<0.05) |
|---|---|---|---|---|---|---|
| Number of Subjects | 38 (30.2%) | 46 (36.5%) | 31 (24.6%) | 11 (8.7%) | NA | NA |
| Sex, F:M (%Female) | 27:11 (71.1%) | 38:8 (82.6%) | 25:6 (80.7%) | 10:1 (90.9%) | 0.4194 | none |
| Age, mean±SD | 35.05±12.38 | 41.43±9.94 | 47.19±7.85 | 46.00±8.66 | <0.0001* | 1–2, 1–3, 1–4 |
| Years Since Symptom Onset, mean±SD | NA | 13.17±6.87 | 18.29±8.30 | 18.55±5.07 | 0.005* | 2–3 |
| Years Since Diagnosis, mean±SD | NA | 11.58±6.20 | 13.71±5.37 | 14.27±5.46 | 0.1891 | none |
| Disease Subtype | ||||||
| Relapsing-remitting MS | NA | 44 (95.7%) | 24 (77.4%) | 5 (45.5%) | 0.0007* | NA |
| Secondary progressive MS | NA | 2 (4.3%) | 3 (9.7%) | 3 (27.3%) | ||
| Primary progressive MS | NA | 0 (0.0%) | 2 (6.5%) | 0 (0.0%) | ||
| Progressive-relapsing MS | NA | 0 (0.0%) | 2 (6.5%) | 3 (27.3%) | ||
p<0.01
6MW: Six-Minute Walk; T25FW: Timed 25-Foot Walk; MSWS-12: MS Walking Scale; MFIS: Modified Fatigue Impact Scale
Conventional Habitual Physical Activity Analysis
Average daily steps, MPA, and MVPA were significantly different between controls, mild MS, moderate MS, and severe MS (p<0.0001). Controls had approximately twice as much MVPA as mild MS subjects (196.8%) and over four times that of moderate MS subjects (464.5%). MPA, VPA, and MVPA were zero for all severe MS subjects (Table 2).
Table 2.
Clinical Outcomes and HPA Statistics by Disability Level
| Variable | (1) Controls | (2) Mild MS (EDSS 0 – 2.5) |
(3) Moderate MS (EDSS 3.0 – 4.0) |
(4) Severe MS (EDSS 4.5 – 6.5) |
ANOVA p- value |
Group-Wise Differences (Tukey’s qs<0.05) |
|---|---|---|---|---|---|---|
| Clinical Outcomes | ||||||
| 6MW Distance, mean±SD | 2079.9±258.9 | 1826.5±279.2 | 1619.7±306.6 | 537.2±327.3 | <0.0001* | all pairings |
| 6MW Step Rate, mean±SD | 123.40±10.1 1 | 117.83±15.5 4 | 109.99±18.7 9 | 35.86±27.76 | <0.0001* | 1–3, 1–4, 2–4, 3–4 |
| T25FW, mean±SD | 3.59±0.50 | 4.17±1.02 | 4.49±0.80 | 22.36±17.96 | <0.0001* | 1–4, 2–4, 3–4 |
| MSWS Score, mean±SD | NA | 14.35±19.73 | 30.10±24.81 | 84.85±17.46 | <0.0001* | 2–3, 2–4, 3–4 |
| MFIS Total, mean±SD | 17.22±9.96 | 24.28±17.46 | 39.03±16.35 | 48.73±15.23 | <0.0001* | 1–3, 1–4, 2–3, 2–4 |
| Conventional HPA Statistics | ||||||
| Average Daily Steps | 7952.03 ±3466.61 | 6347.18 ±2961.43 | 5270.82 ±2217.68 | 1703.39 ±1009.68 | <0.0001* | 1–3, 1–4, 2–4, 3–4 |
| Total MPA Time | 113.55±125.35 | 58.57±77.01 | 27.90±37.87 | 0.00±0.00 | 0.0001* | 1–2, 1–3, 1–4 |
| Total VPA Time | 22.97±51.51 | 10.83±28.92 | 1.48±7.36 | 0.00±0.00 | 0.0410 | 1–3 |
| Total MVPA Time | 136.53±147.36 | 69.39±98.89 | 29.39±40.92 | 0.00±0.00 | <0.0001* | 1–2, 1–3, 1–4 |
| New HPA Statistics | ||||||
| Maximum Step Rate | 141.92±24.6 6 | 123.43±25.9 0 | 113.06±23.5 1 | 52.09±30.65 | <0.0001* | 1–2, 1–3, 1–4, 2–4, 3–4 |
| Habitual Walking Step Rate | 104.00±8.70 | 102.23±11.8 2 | 97.52±11.85 | 45.39±40.69 | <0.0001* | 1–4, 2–4, 3–4 |
| Habitual Running Step Rate | 151.05±17.6 7 | 146.80±20.3 2 | NA | NA | 0.6151 | none |
p<10−3
HPA: Habitual Physical Activity; MPA: Moderate Physical Activity; VPA: Vigorous Physical Activity; MVPA:Moderate to Vigorous Physical Activity
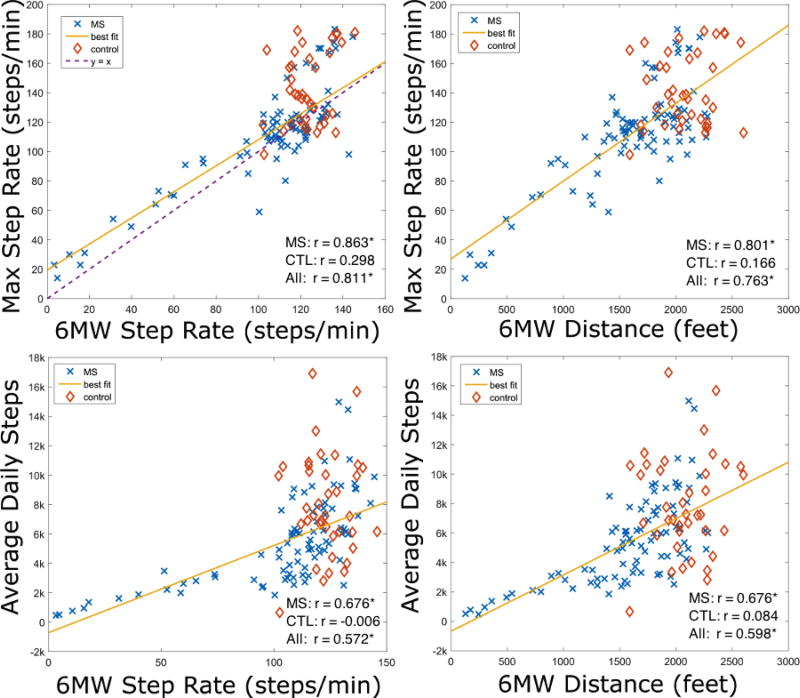
In MS subjects, average daily steps were strongly and significantly correlated with the 6MW step rate (r=0.676, p<10−11) and 6MW distance (r=0.676, p<10−12) (Fig 1, bottom panels). Average daily steps were also significantly correlated with T25FW speed (r=0.675, p<10−12), MSWS-12 (r=−0.627, p<10−10), and MFIS (r=−0.490, p<10−5). Total MVPA was moderately and significantly correlated with 6MW distance (r=0.415, p<10−4), T25FW speed (r=0.436, p<10−4), MFIS (r=−0.413, p<10−4), MFISphy(r=−0.453, p<10−4), and MFISpsych(r=−0.406, p<10−4). Among the demographic characteristics, total MVPA was significantly associated only with age (r=−0.437, p<10−6). Additional correlations may be found in Table 3. None of these correlations were statistically significant in control subjects.
Figure 1.
6MW step rate and distance correlate more strongly with maximum step rate than average daily steps.
Table 3.
Linear Correlations Between Activity Statistics and Clinical Outcomes in MS Subjects
| Activity Statistic | 6MW Distance |
6MW Step Rate |
T25FW Speed |
MSWS Score |
MFIS Total |
MFIS Phys. |
MFIS Cog. |
MFIS Psych. |
|---|---|---|---|---|---|---|---|---|
| Conventional HPA Statistics | ||||||||
| Average Daily Steps | 0.676** | 0.676** | 0.675** | −0.627** | −0.490* | −0.537* | −0.391 | −0.493* |
| Total MPA Time | 0.429* | 0.407 | 0.442* | −0.411* | −0.430* | −0.464* | −0.338 | −0.427* |
| Total VPA Time | 0.264 | 0.261 | 0.304 | −0.251 | −0.254 | −0.303 | −0.165 | −0.239 |
| Total MVPA Time | 0.415* | 0.396 | 0.436* | −0.397 | −0.413* | −0.453* | −0.315 | −0.406* |
| New HPA Statistics | ||||||||
| Maximum Step Rate | 0.801** | 0.863** | 0.755** | −0.756** | −0.504* | −0.569** | −0.383 | −0.564* |
| Habitual Walking Step Rate | 0.701* | 0.815** | 0.670* | −0.717* | −0.237 | −0.279 | −0.189 | −0.121 |
| Habitual Running Step Rate | 0.553 | 0.676 | 0.721 | −0.703 | −0.331 | −0.460 | −0.187 | −0.378 |
p<10-4;
p<10-8
HPA: Habitual Physical Activity; MPA: Moderate Physical Activity; VPA: Vigorous Physical Activity; MVPA: Moderate-to-Vigorous Physical Activity
New Habitual Physical Activity Analysis
The maximum step rate (MSR) was most strongly correlated with clinical walking outcomes among all of the HPA statistics. Specifically, the MSR was strongly correlated with 6MW step rate (r=0.863, p<10−25), 6MW distance (r=0.801, p<10−20), T25FW speed (r=0.755, p<10−16), MSWS-12 (r=−0.756, p<10−16), and MFIS (r=0.504, p<10−25) in MS subjects (Table 3). Figure 1 shows its superior performance over average daily steps when estimating 6MW step rate and distance. The MSR was faster than the 6MW step rate in 68.3% of subjects, resulting in a statistically significant difference between the two measures (μ=10.4, p<10−7) (Fig 1, top left panel).
Fewer total model states were identified in MS subjects (median 4, range 3–6) compared to controls (median 5, range 2–6) (p<0.001). Walking was identified in 76.3% of controls, 60.1% of mild MS, 41.9% of moderate MS, and 18.2% of severe MS (χ2=15.7, p=0.001). Running was identified in 31.6% of controls, 19.6% of mild MS, and no subjects with moderate or severe MS (χ2=14.7, p=0.002). In all subjects, the number of active states was significantly associated with younger age (p<0.001), but not sex (p=0.095), level of education (p=0.333), or employment status (p=0.586). MS subjects with more education had more active states (p=0.026).
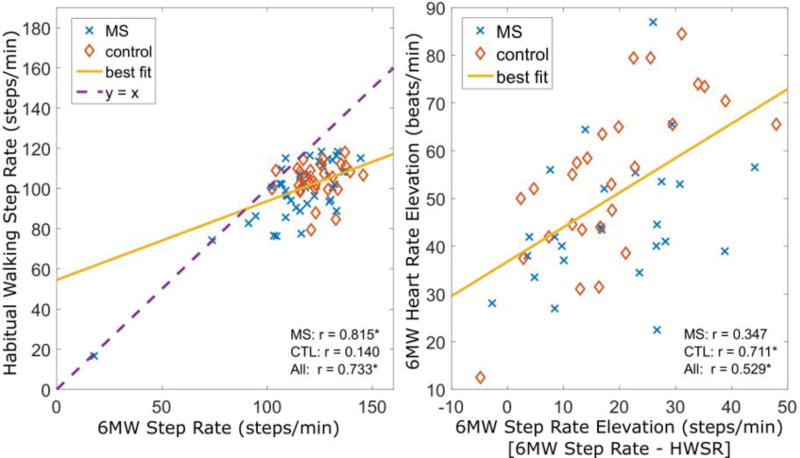
The habitual walking step rate (HWSR) was similar between controls, mild MS, and moderate MS: the decreases between groups was not statistically significant (p=0.196). However, severe subjects had much lower HWSR (p<0.001). The habitual running step rate (HRSR) was similar in controls and MS subjects (p=0.615). HWSR was strongly correlated with 6MW step rate (r=0.815, p<10−11) and 6MW distance (r=0.701, p<10−7) in MS, but not in controls (p > 0.4) (Fig 2). The sum of the MSR and HWSR was a better predictor of 6MW step rate than any individual measure (r=0.884, p<10−14).
Figure 2.
Walking step rates are lower than 6MW step rates (p<10−5), though the two are strongly correlated (left). HR elevation during the 6MW correlates (p<10−4) with the increase in step rate above their habitual walking step rate (right).
In contrast to the MSR, the HWSR was slower than the 6MW step rate in 94.4% of subjects. This difference was statistically significant (μ=−18.2, p<10−19). Moreover, the difference between 6MW step rate and HWSR was moderately correlated with heart rate elevation during the 6MW in all subjects (r=0.529, p<10−4). Step rates during running were significantly higher than the 6MW step rate (μ=21.7, p <10−5).
Differential Correlation by Disability Level
In mild MS, the MSR and average daily steps are moderately to strongly correlated with 6MW step rate (r=0.657, p<10−6; and r=0.601, p<10−4, respectively) and 6MW distance (r=0.467, p=0.001; and r=0.548, p<10−4, respectively). Similarly, T25FW speed is moderately correlated with MSR (r=0.398, p=0.006) and average daily steps (r=0.536, p<0.001) in mild MS. The MFIS, on the other hand, is moderately correlated with the MSR and average daily steps in mild subjects (r=−0.412, p=0.004; and r=−0.358, p=0.014, respectively) and moderate subjects (r=−0.424, p=0.018; and r=−0.443, p=0.013, respectively).
As disability progresses, correlations to walking outcomes increase while correlations to the MFIS decrease. In severe MS, the MSR and average daily steps are almost perfectly correlated with 6MW step rate (r=0.982, p<10−7; and r=0.976, p<10−6, respectively) and 6MW distance (r=0.979, p<10−6; and r=0.962, p<10−5, respectively). T25FW speed also correlates very strongly with MSR (r=0.919, p<10−5) and average daily steps (r=0.944, p<10−5), whereas the MFIS is not significantly correlated with either activity statistic (p>0.8) in severe MS.
DISCUSSION
Our population spanned the full range of ambulatory MS-related disability by EDSS (range 1–6.5) and MSWS-12 (range 0–100), with approximately equal numbers of control subjects, mild MS subjects, and moderate to severe MS subjects. Beyond this range (EDSS>6.5), HPA measurement is not appropriate.
The striking correlations between the MSR and all four walking outcomes support it as a valid measure of walking capacity. Correlation was strongest for 6MW step rate and distance, respectively, followed by MSWS-12 and T25FW, which were similar. This shows that the MSR is a more reliable capacity measure than conventional statistics, and verifies the central hypothesis of this work, namely that average daily steps are a suboptimal measure of capacity. The correlation observed between 6MW distance and average daily steps (r=0.676) is consistent with correlations reported by other studies (r=0.519 and r=0.630)[10],[11]. It is not surprising that the MSR correlates more strongly with 6MW step rate than 6MW distance; this can be explained by variability in stride length, which determines the ratio of step rate to distance traveled.
Fundamentally, one might argue that the MSR is just as valid as the 6MW or T25FW as a direct measure of capacity. The MSR is the highest step rate achieved over a week-long period, whereas the 6MW and T25FW measure speed during in-clinic testing. Each has limitations as a measure of capacity, which might be defined as the highest speed or step rate the subject is able to achieve. All three depend on subject effort in some way, but the 6MW and T25FW are also affected by circumstances and symptoms at the time of testing. More practically, these measures differ in the duration of effort: the MSR approximates the maximum rate subjects can sustain for a minute, compared to the longer 6MW or shorter T25FW. We believe the 6MW is the optimal measure of capacity due to its longer duration and the wealth of supporting results, while the MSR is its real-world compliment.
Interestingly, correlations between HPA and clinical outcomes varied substantially across the MS-disability spectrum. For example, the correlation between 6MW step rate and average daily steps ranged from r = 0.976 in severe MS to r = 0.601 in mild MS. Results suggest that average daily steps are an excellent measure of capacity in severe subjects, but they are significantly influenced by fatigue and behavioral factors in moderate and mild MS. Indeed, the correlation between average daily steps and MFISphy is significant in mild and moderate MS, but not severe MS.
There were no significant relationships between HPA and clinical walking outcomes in control subjects, suggesting that in the absence of disability, HPA is purely behavioral. From a statistical standpoint, the low variability in capacity among controls makes it difficult to identify a correlation.
The PAMs identified walking or running in a majority of subjects (59.5%). The remaining 40% almost certainly walked at some point, but not enough to justify adding a “walking” state to the model. In statistical terms, the improvement to model-likelihood was not significant. This result underscores the profound difference between capacity and behavior: a full 40% of subjects were not active often enough for the model to detect it. This rate differed between controls (23.7%) and mild to moderate MS (45.5%), supporting the finding that MS subjects are less active than controls[8],[9].
While the MSR is the best single measure of walking capacity, the MSR and HWSR can be used together even more effectively. Indeed, their sum has higher correlation to the 6MW step rate than either factor alone. The MSR tends to over-estimate 6MW step rates, whereas the HWSR tends to under-estimate them. In other words, 6MW step rates are faster than subjects walk on a regular basis, but slower than their maximum rate. This result confirms that subjects walk quickly during the 6MW, as instructed, exceeding their habitual pace. In fact, the MSR was lower than the 6MW step rate in almost a third of subjects (31.7%), underscoring the genuine effort they made during testing.
Using a new, statistically sophisticated approach to HPA, we have more accurately defined the impact of disease on real-world walking ability by mitigating the influence of behavioral variability. Conventional HPA statistics are affected by both capacity and activity behaviors, limiting their usefulness as outcome measures. The MSR and HWSR measure capacity by detecting subjects’ highest achieved step rate and habitual step rate, respectively, and their validity is supported by strong, statistically significant correlations to clinical walking outcomes. By this metric, the new statistics markedly outperform conventional HPA statistics. Further, the MSR is calculated as a simple max over the observed counts, making it easy to incorporate in clinical practice.
While the current results hold only in MS, we anticipate similar results in other forms of disability and aim to promote these methods more broadly. In future work, we intend to provide precise, personalized measures of real-world walking behaviors to compliment these new measures of capacity.
Limitations
A first limitation is the younger age of controls compared to MS subjects. This very likely affected group-wise outcomes, such as differences in 6MW distance, and may have partly driven the differential correlations observed between controls and MS. Additionally, further study may be necessary in severe disability (EDSS≥4.5), which comprised only 8.7% of our study population.
The current cross-sectional study could not establish the clinical importance of the new measures proposed. We anticipate that changes in the MSR and HWSR are more closely associated with clinical measures of walking ability compared to existing measures, but this remains to be proven.
While the MSR could be integrated into clinical practice without difficulty, the HWSR requires analysis techniques not commonly available to practitioners.
Most importantly, the influence of specific behavioral factors on physical activity remains uncertain. By showing that the MSR and HWSR are better measures of capacity than daily counts, our results suggest that the latter are affected by factors not related to capacity. However, follow-up work is needed to quantify the influence of specific personal, environmental, and social factors on activity behaviors in MS; and to explore the differential impact of these factors on the new measures we have proposed as compared to older measures of HPA.
Highlights.
Habitual physical activity measures the real-world impact of walking disability.
However, it’s difficult to distinguish the ability to walk from activity behaviors.
New activity statistics are proposed as specific measures of walking capacity.
Their benefit is validated in multiple sclerosis via correlation to timed walks.
Acknowledgments
MDG is funded by the National Institutes of Health – National Institute of Neurologic Disorders and Stroke (K23NS062898). Additional funding was provided by the Broadband Wireless Access and Applications Center, an NSF-supported industry-university cooperative research center (NSF award #1266311), and a gift from the ziMS Foundation. We owe special thanks to Margaret Keller, the CNS of our clinical research program, and Kristina Sheridan, who inspired much of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
MME, SDP, and JCL have nothing to report. MDG reports grants from Biogen Idec, grants from Novartis, other from Acorda, other from Biogen Idec, other from Novartis, personal fees from Novartis, and personal fees from Sarepta outside the submitted work.
References
- 1.Goldman MD, Motl RW, Rudick RA. Possible clinical outcome measures for clinical trials in patients with multiple sclerosis. [Accessed July 29, 2016];Ther Adv Neurol Disord. 2010 3(4):229–239. doi: 10.1177/1756285610374117. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21179614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nero H, Wallén MB, Franzén E, Ståhle A, Hagströmer M. Accelerometer cut points for physical activity assessment of older adults with Parkinson’s disease. Gonzalez-Alegre P, editor. [Accessed July 29, 2016];PLoS One. 2015 10(9):e0135899. doi: 10.1371/journal.pone.0135899. Available at: http://dx.plos.org/10.1371/journal.pone.0135899. [DOI] [PMC free article] [PubMed]
- 3.Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. [Accessed July 29, 2016];BMC Public Health. 2013 13(1):449. doi: 10.1186/1471-2458-13-449. Available at: http://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 5.Giles-Corti B, Donovan RJ. The relative influence of individual, social and physical environment determinants of physical activity. Soc. Sci. Med. 2002;54(12):1793–1812. doi: 10.1016/s0277-9536(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 6.Humpel N. Environmental factors associated with adults’ participation in physical activity A review. [Accessed July 29, 2016];Am. J. Prev. Med. 2002 22(3):188–199. doi: 10.1016/s0749-3797(01)00426-3. Available at: http://www.sciencedirect.com/science/article/pii/S0749379701004263. [DOI] [PubMed] [Google Scholar]
- 7.Weikert M, Motl RW, Suh Y, McAuley E, Wynn D. Accelerometry in persons with multiple sclerosis: Measurement of physical activity or walking mobility? J. Neurol. Sci. 2010;290(1–2):6–11. doi: 10.1016/j.jns.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Klaren RE, Motl RW, Dlugonski D, Sandroff BM, Pilutti LA. Objectively quantified physical activity in persons with multiple sclerosis. Arch. Phys. Med. Rehabil. 2013;94(12):2342–2348. doi: 10.1016/j.apmr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. [Accessed July 29, 2016];Mult. Scler. 2005 11(4):459–63. doi: 10.1191/1352458505ms1188oa. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16042230. [DOI] [PubMed] [Google Scholar]
- 10.Motl RW, Pilutti L, Sandroff BM, et al. Accelerometry as a measure of walking behavior in multiple sclerosis. Acta Neurol. Scand. 2013 doi: 10.1111/ane.12036. [DOI] [PubMed] [Google Scholar]
- 11.Motl RW, Dlugonski D, Suh Y, et al. Accelerometry and its association with objective markers of walking limitations in ambulatory adults with multiple sclerosis. Arch. Phys. Med. Rehabil. 2010;91(12):1942–1947. doi: 10.1016/j.apmr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz CE, Ayandeh A, Motl RW. Investigating the minimal important difference in ambulation in multiple sclerosis: A disconnect between performance-based and patient-reported outcomes? J. Neurol. Sci. 2014;347(1–2):268–274. doi: 10.1016/j.jns.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) [Accessed May 11, 2016];Neurology. 1983 33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. Available at: http://www.neurology.org/cgi/doi/10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 15.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: The 12-Item MS Walking Scale (MSWS-12) [Accessed April 26, 2016];Neurology. 2003 60(1):31–36. doi: 10.1212/wnl.60.1.31. Available at: http://www.neurology.org/content/60/1/31.short. [DOI] [PubMed] [Google Scholar]
- 16.Ritvo P, Fischer JS, Miller DM, et al. MSQLI—Multiple Sclerosis Quality of Life Inventory. A user’s manual. New York Natl. MS Soc. 1997 [Google Scholar]
- 17.Goldman MD, Marrie RA, Cohen Ja. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult. Scler. 2008;14(3):383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 18.Weikert M, Suh Y, Lane A, et al. Accelerometry is associated with walking mobility, not physical activity, in persons with multiple sclerosis. Med. Eng. Phys. 2012;34(5):590–597. doi: 10.1016/j.medengphy.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Agiovlasitis S, Motl RW. Step-rate thresholds for physical activity intensity in persons with multiple sclerosis. Adapt. Phys. Act. Q. 2014;31(1):4–18. doi: 10.1123/apaq.2013-0008. [DOI] [PubMed] [Google Scholar]
- 20.Witowski V, Foraita R, Pitsiladis Y, Pigeot I, Wirsik N. Using hidden Markov models to improve quantifying physical activity in accelerometer data - A simulation study. Bourdon J, editor. [Accessed July 29, 2016];PLoS One. 2014 9(12):e114089. doi: 10.1371/journal.pone.0114089. Available at: http://dx.plos.org/10.1371/journal.pone.0114089. [DOI] [PMC free article] [PubMed]
- 21.Bilmes JA. A gentle tutorial of the EM algorithm and its application to parameter estimation for gaussian mixture and hidden markov models. ReCALL. 1998;4(510):126. Available at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.38.4498&rep=rep1&type=pdf. [Google Scholar]
- 22.Forney GD. The viterbi algorithm. [Accessed August 11, 2016];Proc. IEEE. 1973 61(3):268–278. Available at: http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber=1450960. [Google Scholar]