Abstract
A caprine herd seroprevalence of Coxiella burnetii infection was determined by passive surveillance of domestic goat herds in Washington State. Serum samples (n=1794) from 105 herds in 31 counties were analyzed for C. burnetii antibodies using a commercially available Q fever antibody enzyme-linked immunosorbent assay (ELISA) test kit. The sera were submitted to the Washington Animal Disease Diagnostic Laboratory for routine serologic screening over an approximate 1-year period from November, 2010, through November, 2011. To avoid bias introduced by testing samples from ill animals, only accessions for routine screening of nonclinical animals were included in the study. A standard cluster sampling approach to investigate seroprevalence at the herd level was used to determine optimal study sample size. The results identified C. burnetii antibodies in 8.0% of samples tested (144/1794), 8.6% of goat herds tested (9/105), and 25.8% of counties tested (8/31). Within-herd seroprevalence in positive counties ranged from 2.9% to 75.8%. Counties with seropositive goats were represented in the western, eastern, southeastern, and Columbia basin agricultural districts of the state. To our knowledge this is the first county-specific, statewide study of C. burnetii seroprevalence in Washington State goat herds. The findings provide baseline information for future epidemiologic, herd management and public health investigations of Q fever.
Key Words: : Coxiella burnetii, Q fever, Seroprevalence, Goats
Introduction
Coxiella burnetii, the causative agent of Q fever, is an obligate intracelluar Gram-negative bacterium. The organism is excreted in milk, urine, feces, and uterine discharge of cattle, sheep, and goats (Berri et al. 2001, Woldehiwet 2004, Rodolakis et al. 2007). It is transmitted to humans primarily through inhalation of contaminated aerosols associated with infected animals (Tissot-Dupont et al. 1999, Roest et al. 2011a).
Most C. burnetii infections in domestic goats are subclinical, but acute infections of naïve animals may result in late-term abortions (Maurin and Raoult 1999). The range of reported seroprevalence in domestic goats worldwide varies from 6.5% in Greece to 65% in Iran (Khalili and Sakhaee 2009, Pape et al. 2009). In North America, the reported seroprevalence varies from 3.5% to 19% in Canada (Lang 1989, Hatchette et al. 2002), and an average 24% prevalence has been reported in California (Ruppanner et al. 1978, McQuiston and Childs 2002, Guatteo et al. 2011). The California study also reported specific county seroprevalence ranging between 7% and 100%. There is no seroprevalence data available for C. burnetii in domestic goats in other states, including Washington.
While approximately 60% of all human infections are subclinical, the other 40% result in acute infections presenting as an influenza-like illness that may be accompanied by pneumonia and/or hepatitis (Roest et al. 2011a). Chronic disease occurs in a small percentage of cases from several months to years after acute infection (Arricau-Bouvery and Rodolakis 2005) with 60–70% of chronic infections presenting as endocarditis (Raoult and Marrie 1995). Human seroprevalence in the United States was reported to be 3.1% (95% confidence interval [CI]=2.1–4.3%) in 2003–2004 (Anderson et al. 2009). However, only 117 acute human cases and 15 chronic cases were reported nationwide to the Centers for Disease Control and Prevention in 2008 (www.cdc.gov/qfever/stats/index.html#reading). Many factors are postulated to contribute to the underreporting of the disease, with one being the similarity of Q fever clinical signs to other diseases. In 2011, 20 cases of human Q fever in Montana and Washington were associated with infected goats on or originating from a farm in Washington State (Bjork 2011). Among these cases, 11 were reported in Washington State alone during a 6-month period, compared to one to three cases reported annually through 2010 (Washington State Department of Health, Communicable Disease Report 2010, www.doh.wa.gov/portals/1/Documents/5100/420-004-CDAnnualReport2010.pdf). Lack of baseline data on C. burnetii herd and within-herd prevalence in goats in Washington State made it difficult to assess whether spatial changes might have impacted risk of zoonotic transmission and whether the index herd seroprevalence was high in comparison to other farms. Thus, it was determined that a seroprevalence survey in domestic goats across the state would be a valuable resource for future reference.
Antibodies against Q fever in domestic goats can be detected using several methods including indirect immunofluorescence (IFA), complement fixation (CF), and, more recently, the enzyme-linked immunosorbent assay (ELISA). IFA and ELISA are reported to have comparable diagnostic sensitivity for small ruminant samples, whereas conflicting reports suggest the ELISA may or may not have higher sensitivity than the CF test (Rousset et al. 2007, Kittelberger et al. 2009, Emery et al. 2012). There are three commercially available ELISAs, each using a different source of culture-derived antigen: Nine Mile strain from ticks, or isolates from ovine or bovine sources. The sensitivity of these three ELISAs for detecting C. burnetii antibodies from goat samples varies from 71.4% with the tick-derived antigen to 64.3% with the ovine-derived antigen to 7.1% with the bovine-derived antigen (Horigan et al. 2011). It has been suggested that serological testing is valuable for herd classification, but due to false-negative results it is less useful in identifying infected individuals that may be shedding the organism (Kennerman et al. 2010). The purpose of this study was to determine the herd seroprevalence of C. burnetii in domestic goats across different counties in Washington State using a Q Fever ELISA.
Materials and Methods
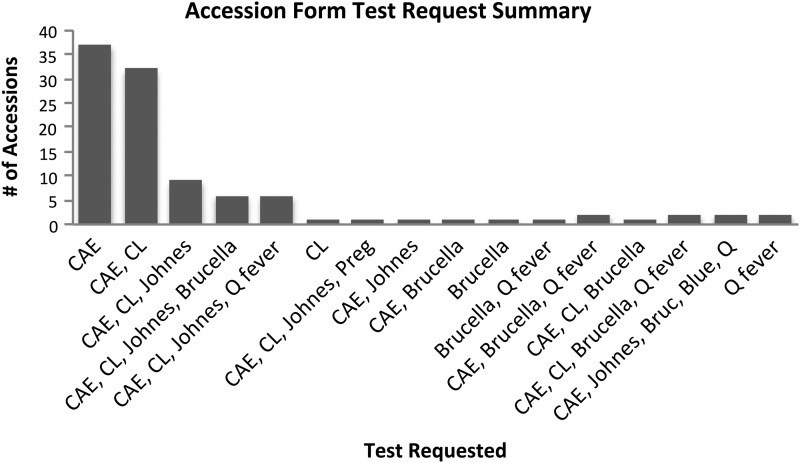
Banked goat sera from submissions to the Washington State University-Washington Animal Disease Diagnostic Laboratory (WADDL) between November, 2010, and November, 2011, were used. To increase the probability that sera were derived from healthy animals, WADDL accession forms were evaluated individually to ensure that only samples submitted for routine biosecurity screening of healthy goats for antibodies to caprine arthritis encephalitis virus (CAE), Mycobacterium avium subsp. paratuberculosis (Johnes disease), Corynebacterium pseudotubercuolosis (caseous lymphadenitis), Bluetongue virus, Coxiella burnetii, or pregnancy testing (Fig. 1) were used in the seroprevalence study. Sample submissions that indicated a clinically ill animal were excluded from the study. Because herd seroprevalence was being investigated, animals were clustered in herds, and a standard cluster sampling approach was used to determine the sample size requirement (Thrusfield 2005). According to the 2007 Census of Agricultural Data in Washington State from the United States Department of Agriculture (USDA) there were 32,840 goats on 3143 farms, or an average of 10.4 goats per farm (http://www.agcensus.usda.gov/Publications/2007/Full_Report/Volume_1,_Chapter_1_State_Level/Washington/wav1.pdf). On the basis of the observed average cluster size of 10 per herd (farm), and assuming a herd prevalence of 0.10 and a between-herd variance in prevalence of 0.06 (Guatteo et al. 2011), a total of 98 clusters were required to achieve a precision of <0.05 and a 95% confidence level in herd prevalence.
FIG. 1.
Summary of the serologic tests requested on the sample submission from healthy goats included in the serosurvey. CAE, caprine arthritis encephalitis; CL, Corynebacterium pseudotuberculosis; Johnes, Mycobacterium paratuberculosis; Brucella, Brucella abortus; Blue, bluetongue virus; Q fever, Coxiella burnetii; Preg, human chorionic gonadotropin hormone.
Herds were classified as seropositive if one or more goats within the herd were seropositive for C. burnetii. In the complete sample set, there were 105 herds that included 1794 animals from 31 counties in Washington. On the basis of the 2007 sensus, and assuming minimal change in the goat census over the past 4 years, this represented approximately 3.3% of the herds and 5.5% of the goats in the state.
Sera were kept frozen at −20°C until ready for testing using a commercial ELISA (CHEKIT Q Fever Antibody ELISA, IDEXX Switzerland AG, Liebefeld-Bern Switzerland) that detects antibodies against Phase I and Phase II antigens from the C. burnetii Nine Mile strain. Samples were thawed at room temperature and the assay performed according to the manufacturer's instructions. Positive and negative controls, provided by the manufacturer, were run in duplicate. Antibody binding was detected by measuring optical density (OD) at a wavelength of 450 nm in each well using an ELISA microplate reader (Biotek Instruments Inc., ELx808 Ultra microplate reader, Winooski, VT), and ODs were used to calculate the percentage of binding relative to controls according to the formula: (ODsample − ODneg)/(ODpos − ODneg) ×100. The cutoff between positive, negative, and suspect samples were based on the manufacturer's recommendations as follows: Sample ODs <30% were classified as negative, sample ODs ≥40% were classified as positive, and sample ODs between ≥30 and <40% were classified as suspect.
Results
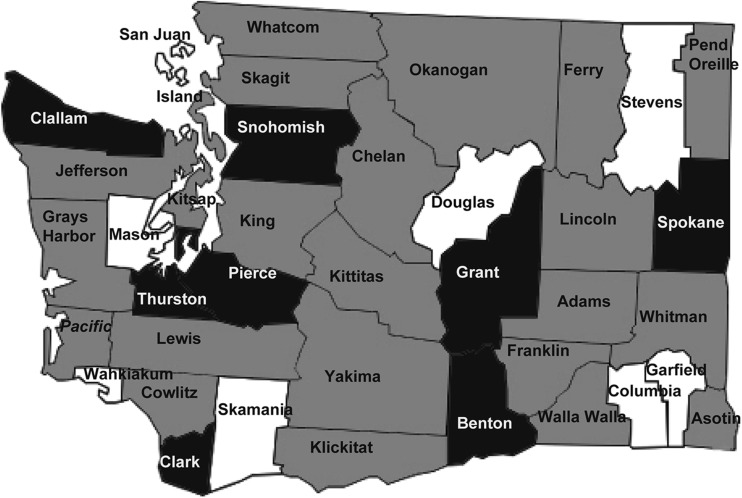
A total of 1794 domestic goat serum samples from 31 counties (out of 39 total counties in Washington State) were tested, with 144 positive samples (8.0%) and two suspect samples (0.1%) identified. Eight of the 31 counties tested had herds with animals seropositive for C. burnetii (25.8%). Eight of the 39 total counties in Washington State were not tested because no samples were received from those counties during the study time period. Seropositive herds were identified in counties from the western, eastern, southeastern, and Columbia basin agricultural districts (www.wsdot.wa.gov/planning/wtp/datalibrary/Economy/AgProduction.htm) (Fig. 2). Only Grant County had more than one seropositive herd, and three positive counties (Benton, Clallam, and Thurston) had only one herd with one positive animal each.
FIG. 2.
Map of 39 counties in Washington State. Eight counties had at least one herd with a seropositive animal (black). Only seronegative animals were detected in 23 counties (grey). Eight counties were not tested (white).
Discussion
Of 105 herds tested, nine (8.6%) had at least one positive animal. The within-herd individual animal seroprevalence varied from 2.9% to 75.8% (Table 1), whereas the overall individual animal seroprevalence of C. burnetii in healthy domestic goat samples from Washington State from November, 2010, through November, 2011, was identified as 8.0% (144/1794). This is lower than the previously reported 24% in California (Ruppanner et al. 1978, McQuiston and Childs 2002). The difference in apparent seroprevalence between Washington and California could be due to multiple undetermined factors. The California study focused on dairy goats, sampled 10 herds per county with an average of 4.5 animals per herd (1054 goats/234 herds), and only 24% (14/58) of the counties. This differs slightly from the study reported herein which focused on all goats in Washington and did not distinguish between dairy, meat, or other animal production types. Our sampling strategy tested between one and 12 herds in the counties with seropositive animals, an average of 17 animals per herd (1794 goats/105 herds), in 79.5% of the counties (31/39). Seropositive animals were distributed throughout all herd sizes in our sample set, such that positive animals were found in herds with small numbers of animals as well as herds with larger numbers of animals.
Table 1.
The Herd Seroprevalence of Domestic Goats in Eight Washington State Counties
| County | Total herds | Total animals | No. positive herds | No. positive/no. in herd | % Within-herd seroprevalence (95% CI)a |
|---|---|---|---|---|---|
| Benton | 8 | 127 | 1 | 1/29 | 3.5 (0.1–17.8) |
| Clallam | 1 | 35 | 1 | 1/35 | 2.9 (0.1–14.9) |
| Clark | 12 | 187 | 1 | 4/9 | 44.4 (13.7–78.8) |
| Grant | 9 | 312 | 2 | 35/54 | 64.8 (50.6–77.3) |
| 75/99 | 75.8 (66.1–83.8) | ||||
| Pierce | 9 | 167 | 1 | 4/38 | 10.5 (2.9–24.8) |
| Snohomish | 4 | 38 | 1 | 3/19 | 15.8 (4.2–39.6) |
| Spokane | 8 | 165 | 1 | 20/44 | 45.5 (30.4–61.2) |
| Thurston | 6 | 70 | 1 | 1/7 | 14.3 (0.4–57.9) |
Only counties with seropositive herds are summarized. Grant County is the only county with more than one seropositive herd.
Fisher exact 95% confidence interval (CI).
If we compare the average within-herd individual animal seroprevalence, herds with less than 40 animals had a mean of 15.2% (standard error of the mean [SEM] 6.24) seropositivity, whereas herds with greater than 40 animals had a mean of 62% (SEM 8.86) seropositivity. When considering herd size alone, without premise location regarded, this observation supports an association between C. burnetii within-herd individual animal seroprevalence and larger herd sizes. However, it is important to note that the two largest herds in our study resided in Grant County, which was the location of a C. burnetii outbreak in 2010 (Bjork 2011). Thus, broadening the comparison to consider the relationship between C. burnetii seroprevalence and both number of goats within a herd and the location of that herd is warranted, as both herd size and geographical location may be determinants of herd seroprevalence. Additional sampling would be required to confirm this preliminary result.
Other possible differences between the Washington and California studies may include the type of antigen in assays used to detect C. burnetii antibodies, breed susceptibility, and management practices of each herd. In the California study, a microagglutination assay using Phase II organisms from the C76 strain was employed, whereas this study used the ELISA with Phase I and II antigens from the Nile Mile strain. It has been reported that goats maintain antibodies to Phase I antigen for longer periods (Hatchette et al. 2003). Thus, the assay used in this study might detect a greater number of true seropositive animals compared to previous reports from California (Ruppanner et al. 1978). A specific breed was identified on the laboratory accession form for only four seropositive herds. These breeds included Boer, Boer-Cross, Toggenburg, Nubian, and LaMancha. All other animals were identified as “domestic goat.” Due to the limited information available, we were unable to determine any association of breed and goat type (meat or dairy) with seropositivity for C. burnetii. Finally, the variation in within-herd seroprevalence among herds in this study may be due to other factors such as differences in the transmissibility phenotype of a specific C. burnetii strain genotype circulating on some farms (Roest et al. 2011b).
This study identified C. burnetii-seropositive goat herds from 25.8% (8/31) of counties tested. This may underrepresent the true number of counties with seropositive goat herds because: (1) The sampling strategy excluded eight counties from which no goat serum samples were submitted to WADDL during November, 2010, to November, 2011; (2) in 11 of 23 counties classified as C. burnetii negative, only one goat herd was analyzed; and (3) only samples from self-identified nonclinical animals were included in this study. No information regarding abortive episodes or reproductive stage of the goats was documented on the accession forms. This study was designed to assess the seroprevalence of clinically normal goats, and conclusions cannot be drawn that correlate shedding with seropositive and seronegative status of the animals. The 2007 agricultural census data do not provide a breakdown of herd distribution by county, and no current data are available to determine how many goat herds are present in the eight counties from which no samples were available. However, the WADDL serves as the reference laboratory within Washington State for goat serological testing, and the absence of submissions from these eight counties suggests that their domestic goat population is limited.
Conclusion
In summary, 8.6% (9/105) of goat herds (95% CI 2.94–75.76%) across Washington State were seropositive to C. burnetii. To our knowledge, this is the first report of C. burnetii seroprevalence in Washington State domestic goat herds. Our sample set is representative of healthy domestic goats in Washington State that use WADDL for diagnostic testing. The C. burnetii herd seroprevalence identified in this study will provide a baseline for future C. burnetii herd seroprevalence studies and public health investigations. The broad range of within-herd seroprevalence is an interesting finding and warrants further investigation to both confirm the significance of our observation and examine the potential reasons.
Acknowledgments
The authors thank Dr. Niles Reichardt for assisting with WADDL database queries, and Josephine Walker for technical assistance in running the ELISA.
Author Disclosure Statement
The authors declare no competing financial interests exist.
References
- Anderson AD. Kruszon-Moran D. Loftis AD. McQuillan G, et al. Seroprevalence of Q fever in the United States, 2003–2004. Am J Trop Med Hyg. 2009;81:691–694. doi: 10.4269/ajtmh.2009.09-0168. [DOI] [PubMed] [Google Scholar]
- Arricau-Bouvery N. Rodolakis A. Is Q fever an emerging or re-emerging zoonosis? Vet Res. 2005;36:327–349. doi: 10.1051/vetres:2005010. [DOI] [PubMed] [Google Scholar]
- Berri M. Souriau A. Crosby M. Crochet D, et al. Relationships between the shedding of Coxiella burnetii, clinical signs and serological responses of 34 sheep. Vet Rec. 2001;148:502–505. doi: 10.1136/vr.148.16.502. [DOI] [PubMed] [Google Scholar]
- Bjork AA A. Q Fever outbreak associated with goat farms. Centers for Disease Control and Prevention MMWR. 2011;60:1393. [Google Scholar]
- Emery MP. Ostlund EN. Schmitt BJ. Comparison of Q fever serology methods in cattle, goats, and sheep. J Vet Diagn Invest. 2012;24:379–382. doi: 10.1177/1040638711434943. [DOI] [PubMed] [Google Scholar]
- Guatteo R. Seegers H. Taurel AF. Joly A. Beaudeau F. Prevalence of Coxiella burnetii infection in domestic ruminants: A critical review. Vet Microbiol. 2011;149:1–16. doi: 10.1016/j.vetmic.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Hatchette T. Campbell N. Whitney H. Hudson R, et al. Seroprevalence of Coxiella burnetii in selected populations of domestic ruminants in Newfoundland. Can Vet J. 2002;43:363–364. [PMC free article] [PubMed] [Google Scholar]
- Hatchette T. Campbell N. Hudson R. Raoult D, et al. Natural history of Q fever in goats. Vector Borne Zoonotic Dis. 2003;3:11–15. doi: 10.1089/153036603765627415. [DOI] [PubMed] [Google Scholar]
- Horigan MW. Bell MM. Pollard TR. Sayers AR, et al. Q fever diagnosis in domestic ruminants: comparison between complement fixation and commercial enzyme-linked immunosorbent assays. J Vet Diagn Invest. 2011;23:924–931. doi: 10.1177/1040638711416971. [DOI] [PubMed] [Google Scholar]
- Kennerman E. Rousset E. Golcu E. Dufour P. Seroprevalence of Q fever (coxiellosis) in sheep from the Southern Marmara Region, Turkey. Comp Immunol Microbiol Infect Dis. 2010;33:37–45. doi: 10.1016/j.cimid.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Khalili M. Sakhaee E. An update on a serologic survey of Q Fever in domestic animals in Iran. Am J Trop Med Hyg. 2009;80:1031–1032. [PubMed] [Google Scholar]
- Kittelberger R. Mars J. Wibberley G. Sting R, et al. Comparison of the Q-fever complement fixation test and two commercial enzyme-linked immunosorbent assays for the detection of serum antibodies against Coxiella burnetti (Q-fever) in ruminants: Recommendations for use of serological tests on imported animals in New Zealand. N Z Vet J. 2009;57:262–268. doi: 10.1080/00480169.2009.58619. [DOI] [PubMed] [Google Scholar]
- Lang GH. Q fever: An emerging public health concern in Canada. Can J Vet Res. 1989;53:1–6. [PMC free article] [PubMed] [Google Scholar]
- Maurin M. Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston JH. Childs JE. Q fever in humans and animals in the United States. Vector Borne Zoonotic Dis. 2002;2:179–191. doi: 10.1089/15303660260613747. [DOI] [PubMed] [Google Scholar]
- Pape M. Mandraveli K. Arvanitidou-Vagiona M. Nikolaidis P, et al. Q fever in northern Greece: Epidemiological and clinical data from 58 acute and chronic cases. Clin Microbiol Infect. 2009;15(Suppl 2):150–151. doi: 10.1111/j.1469-0691.2008.02163.x. [DOI] [PubMed] [Google Scholar]
- Raoult D. Marrie T. Q fever. Clin Infect Dis. 1995;20:489–495. doi: 10.1093/clinids/20.3.489. quiz 496. [DOI] [PubMed] [Google Scholar]
- Rodolakis A. Berri M. Hechard C. Caudron C, et al. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine, and ovine herds. J Dairy Sci. 2007;90:5352–5360. doi: 10.3168/jds.2006-815. [DOI] [PubMed] [Google Scholar]
- Roest HI. Tilburg JJ. van der Hoek W. Vellema P, et al. The Q fever epidemic in The Netherlands: History, onset, response and reflection. Epidemiol Infect. 2011a;139:1–12. doi: 10.1017/S0950268810002268. [DOI] [PubMed] [Google Scholar]
- Roest HI. Ruuls RC. Tilburg JJ. Nabuurs-Franssen MH, et al. Molecular epidemiology of Coxiella burnetii from ruminants in Q fever outbreak, the Netherlands. Emerg Infect Dis. 2011b;17:668–675. doi: 10.3201/eid1704.101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset E. Durand B. Berri M. Dufour P, et al. Comparative diagnostic potential of three serological tests for abortive Q fever in goat herds. Vet Microbiol. 2007;124:286–297. doi: 10.1016/j.vetmic.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Ruppanner R. Riemann HP. Farver TB. West G, et al. Prevalence of Coxiella burnetii (Q fever) and Toxoplasma gondii among dairy goats in California. Am J Vet Res. 1978;39:867–870. [PubMed] [Google Scholar]
- Thrusfield M. Veterinary Epidemiology. 3rd. Blackwell Publishing; 2005. [Google Scholar]
- Tissot-Dupont H. Torres S. Nezri M. Raoult D. Hyperendemic focus of Q fever related to sheep and wind. Am J Epidemiol. 1999;150:67–74. doi: 10.1093/oxfordjournals.aje.a009920. [DOI] [PubMed] [Google Scholar]
- Woldehiwet Z. Q fever (coxiellosis): Epidemiology and pathogenesis. Res Vet Sci. 2004;77:93–100. doi: 10.1016/j.rvsc.2003.09.001. [DOI] [PubMed] [Google Scholar]