Abstract
Steady state bone marrow (BM) is the preferred hematopoietic stem cell (HSC) source for gene therapy in sickle cell disease (SCD) due to the recognized risk of vaso-occlusive crisis during granulocyte colony–stimulating factor mobilization. We previously established clinically relevant HSC gene transfer in the rhesus model following transplantation of mobilized peripheral blood (PB) CD34+ cells transduced with lentiviral vectors. In this study, we examined steady state bone marrow (BM) in the rhesus competitive repopulation model and demonstrate similar gene marking in vitro and in vivo, as compared with mobilized PB CD34+ cells. We then evaluated PB and steady state BM in subjects with SCD and observed a higher frequency of CD34+ cells when compared with controls, likely due to enhanced hematopoiesis. However, CD34+ cell counts were reduced in both the PB and BM in patients treated with hydroxyurea, and hydroxyurea treatment strongly inhibited iPS cell generation from SCD subjects. Our data support that steady state BM is a useful HSC source for SCD gene therapy with similar transduction. The lower CD34+ percentages observed with hydroxyurea treatment warrants withholding hydroxyurea temporarily prior to harvesting HSCs. Our results are important for the design of gene targeting strategies for SCD.
Keywords: : gene therapy, hematopoietic stem cells, sickle cell disease
Introduction
Sickle cell disease (SCD) is characterized by a pathologic hemoglobin that is prone to polymerization, producing poorly deformable red blood cells and resulting in sickling, vaso-occlusion, hemolysis, and severe anemia. The downstream consequences include frequent painful crises, stroke, and end-organ damage, which ultimately culminate in early mortality. Currently, hydroxyurea (HU) is the only Food and Drug Administration (FDA)-approved treatment in the United States and acts principally through the induction of fetal hemoglobin thereby reducing sickling.1,2 However, responses to HU are variable, with most patients experiencing only modest improvements in their health, and HU must be continuously administered to maintain therapeutic benefit.
In contrast, a number of single interventional strategies targeting hematopoietic stem cells (HSCs) are actively being investigated based upon the curative results of allogeneic HSC transplantation. Indeed, allogeneic HSC transplantation using a matched sibling donor can now reliably be applied to both children and adult SCD patients; however, suitable sibling donors are found for only around 10% of patients.3–5 Alternative strategies with curative intent include HSC-targeted gene transfer, HSC-targeted genome editing, and induced pluripotent stem (iPS) cell–based regenerative therapy, the former two requiring autologous HSCs for manipulation and transplantation.3,6,7 HSC-targeted gene therapy is now established for the immunodeficiencies, and application to SCD remains an enduring goal.6,8–12 Furthermore, rapid progress in the design and deployment of endonucleases along with the continued discovery of genetic elements that control fetal hemoglobin expression or correct the sickle mutation have elevated the prospects of HSC-targeted genome editing for SCD.13–16 iPS cell–based regenerative therapy is also very attractive, since iPS cells can be generated from virtually any cell type in SCD patients. These iPS cells allow ease of genetic modification to correct the SCD mutation, should robust differentiation to HSCs be achieved, and could allow another means for correction of the disease.17 However, human iPS cell research is still limited to in vitro cell culture and animal models as differentiation to engrafting HSCs remains elusive.7,17
For HSC transplantation in adults, the most common HSC source is granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood (PB) progenitor cells.3,4 However, G-CSF mobilization has a known risk of provoking vaso-occlusive crisis in SCD patients.18 Thus steady state bone marrow (BM) remains the preferred HSC source for gene therapy for SCD patients, as BM harvesting does not require a mobilization step. We previously established efficient, clinically relevant lentiviral transduction for hematopoietic repopulating cells in a rhesus HSC gene therapy model using mobilized CD34+ stem/progenitor cells.19–21 Therefore, in this study, we sought to compare steady state BM cells to mobilized PB as a HSC source for genetic manipulation in the rhesus competitive repopulation model. In addition, we also sought to evaluate the frequency of both steady state BM and PB CD34+ cells in SCD patients to determine the feasibility of collecting sufficient CD34+ HSCs for gene therapy applications in this patient population.
Methods
Rhesus HSC-targeted gene therapy model with mobilized CD34+ cells and steady state BM CD34+ cells
We performed animal research following the guidelines set out by the Public Health Services Policy on Humane Care and Use of Laboratory Animals under a protocol approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute (NHLBI). We previously demonstrated efficient transduction for hematopoietic repopulating cells in a rhesus HSC gene therapy model, when using mobilized CD34+ cells.19–21 In this study, we evaluated transduction efficiency for steady state BM CD34+ cells in the rhesus HSC gene therapy model. We immunologically selected CD34+ cells using either G-CSF (Amgen, Thousand Oaks, CA) and stem cell factor (SCF; Amgen)-mobilized cells or steady state BM cells from the same rhesus macaque.19,20,22 Equal numbers of frozen CD34+ cells from each source were transduced with enhanced green fluorescent protein (GFP) or enhanced yellow fluorescent protein (YFP)-expressing chimeric human immunodeficiency virus type 1 (HIV-1) vector (χHIV vector) on identical conditions at multiplicity of infection 50 in X-VIVO10 media (Lonza, Allendale, NJ) containing each 100ng/mL of cytokines (SCF, fms-like tyrosine kinase 3 ligand [FLT3L], and thrombopoietin [TPO]; R&D Systems, Minneapolis, MN), and these autologous cells were infused after 10 Gy total body irradiation. We evaluated %GFP or YFP in PB cells by flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ). The average vector copy number per cell (VCN) was evaluated with GFP or YFP specific probe and primers by real time polymerase chain reaction (PCR; QuantStudio™ 6 Flex Real-Time PCR System; Life Technologies, Grand Island, NY).20,23
CD34+ cell counts in PB and BM cells in SCD patients
Human PB cells and BM cells were collected from healthy donors and SCD patients under studies (08-H-0156 and 03-H-0015) that were approved by the Institutional Review Board of NHLBI and the National Institute of Diabetes, Digestive, and Kidney diseases. We used the BD™ Stem Cell Enumeration Kit (BD Biosciences) to more accurately calculate very low amounts of CD34+ cells in PB cells in healthy donors and SCD patients. The BM CD34+ cells in SCD patients were detected with anti-human CD34 antibody (clone 563; BD Biosciences) using flow cytometry.
The colony forming unit (CFU) assay was performed as previously described.4 The 2.0 × 105 peripheral blood mononuclear cells (PBMCs) were cultured in semi-solid media (MethoCult H4434 Classic; STEMCELL Technologies, Vancouver, BC), and after a 14-day culture, we counted the CFUs by microscope. The cell differentiation in aspirated BM cells was evaluated by microscope after Wright-Giemsa stain.24
iPS cell generation with lentiviral transduction from PBMCs and BM stromal cells in SCD patients
We generated iPS cell lines using PBMCs and BM stromal cells in SCD patients, as previously described.25,26 All human subject materials were collected under protocols approved by the Institutional Review Board of NHLBI (07-H-0113, 08-H-0156, and 03-H-0015). The PBMCs and BM stromal cells were transduced with an Oct4, Klf4, Sox2, and c-Myc encoding lentiviral vector (hSTEMCCA-loxP) and the transduced cells were cultured on irradiated mouse embryonic fibroblast feeder cells (CF1-MEF; GlobalStem, Gaithersburg, MD) in Dulbecco's modified Eagle medium/Nutrient Mixture F-12 (Life Technologies) containing 20% KnockOut Serum Replacement (Life Technologies), 10 ng/mL basic fibroblast growth factor (PeproTech, Rocky Hill, NJ), 0.1 mM nonessential amino acids (Life Technologies), 1mM l-glutamine (Life Technologies), and 0.1 mM 2-mercaptoethanol (Life Technologies). At 2–5 weeks later, we picked iPS cell–like colonies, and the reprogramming cassette was later excised by Cre recombinase. The iPS cells were evaluated by immunostaining (Nanog, Oct4, SSEA4, Tra1-60, and Tra1-81), alkaline phosphatase stain, karyotyping, and teratoma assay.
Statistical analysis
Statistical analyses were performed using the JMP 11 software (SAS Institute Inc., Cary, NC). The averages in various conditions were evaluated by Tukey's test (one-way ANOVA among all groups). Two averages were evaluated by the Student's t-test. Correlations were evaluated by R-squared in linear regression and p values in correlation coefficient. A p value of <0.01 or <0.05 was deemed significant. Standard deviations were shown as error bars in all figures.
Results
Rhesus steady state BM CD34+ cells as an alternative to mobilized CD34+ cells
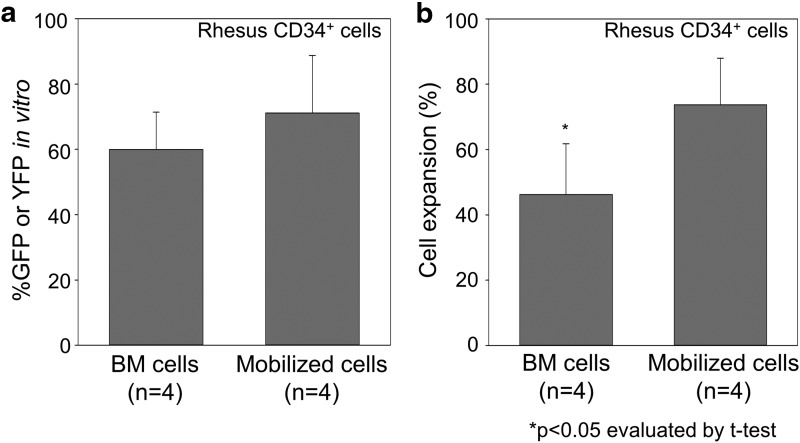
We immunologically selected CD34+ cells derived from both steady state BM cells and G-CSF and SCF-mobilized cells from rhesus macaques (n = 4; RQ6802, RQ6815, ZG06, and ZH21), and these cells were transduced with GFP or YFP-expressing lentiviral vectors at multiplicity of infection of 50 followed by cryopreservation. A fraction was continued in culture for an additional 2–3 days for analysis of transduction efficiency. The percentage of GFP or YFP positive (%GFP or YFP) among BM CD34+ cells was similar (n.s.) (Fig. 1a), while less cell expansion was observed in the BM CD34+ cells in 2-to 3- day culture, as compared with mobilized CD34+ cells (p < 0.05) (Fig. 1b).
Figure 1.
Similar transduction efficiency in rhesus steady state bone marrow (BM) CD34+ cells in vitro, as compared with mobilized CD34+ cells. (a) We immunologically selected CD34+ cells using either granulocyte-colony stimulating factor and stem cell factor–mobilized cells or steady state BM cells from same rhesus macaques (n = 4; RQ6802, RQ6815, ZG06, and ZH21), and these cells were transduced with enhanced green fluorescent protein (GFP) or enhanced yellow fluorescent protein (YFP)–expressing lentiviral vector at multiplicity of infection 50. After additional 2- to 3- day culture, similar GFP or YFP-positive rates (%GFP or YFP) in BM CD34+ cells were detected by flow cytometry, as compared with mobilized CD34+ cells (n.s.). (b) Less cell expansion was observed in the BM CD34+ cells in 2- to 3-day culture, as compared with mobilized CD34+ cells (p < 0.05).
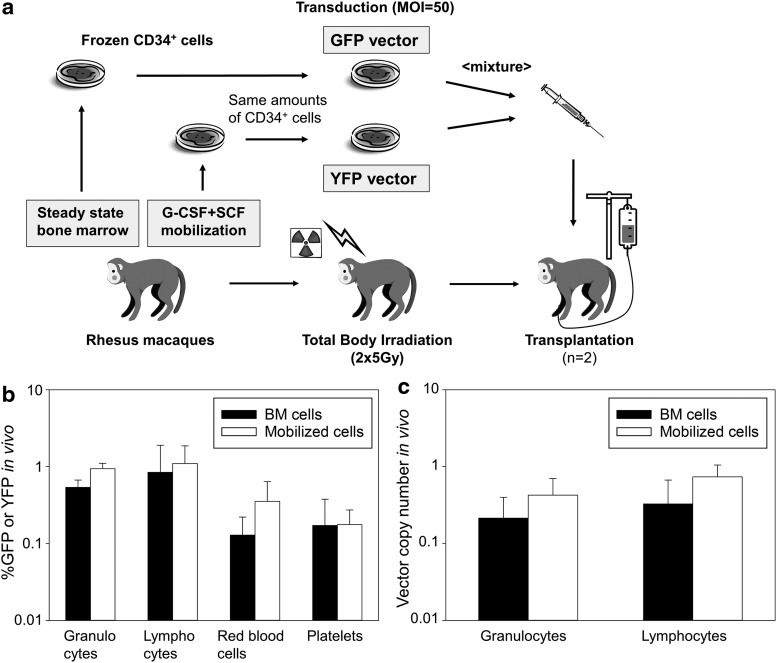
To compare transduction efficiency in hematopoietic repopulating cells between BM CD34+ cells and mobilized PB CD34+ cells (n = 2, ZG06 and ZH21), equal numbers of CD34+ cells from each source were transduced with GFP or YFP-expressing lentiviral vectors on identical conditions (Fig. 2a). These cells were transplanted into autologous animals following 10 Gy total body irradiation. On average during ∼3 years after transplantation in both animals, we observed similar %GFP or YFP in all lineage cells (granulocytes, lymphocytes, red blood cells, and platelets) derived from BM CD34+ cells, as compared with that from mobilized CD34+ cells (n.s. in all lineage cells) (Fig. 2b). In addition, we evaluated VCNs in PB cells during ∼3 years by real-time PCR. We observed similar VCNs in both granulocytes and lymphocytes derived from BM CD34+ cells, as compared with that from mobilized CD34+ cells (n.s. in both cells) (Fig. 2c). These data suggest that rhesus steady state BM CD34+ cells have less expansion ability in in vitro culture, compared with G-CSF and SCF-mobilized CD34+ cells. However, transduction efficiency in steady state BM CD34+ cells is similar to mobilized CD34+ cells, based on in vitro culture and PB cell expression after HSC transplantation. Our data support that steady state BM CD34+ cells are a useful source for HSC gene therapy.
Figure 2.
Similar gene marking levels in rhesus peripheral blood (PB) cells derived from steady state BM CD34+ cells, as compared with mobilized CD34+ cells. (a) To compare transduction efficiency in hematopoietic repopulating cells between BM CD34+ cells and mobilized CD34+ cells (n = 2, ZG06 and ZH21), equal numbers of CD34+ cells from each source were transduced with GFP or YFP-expressing lentiviral vectors on identical conditions. These cells were transplanted into autologous animals following 10Gy total body irradiation. (b) On average during ∼3 years after transplantation in both animals, we observed similar %GFP or YFP in all lineage cells derived from BM cells, as compared with that from mobilized cells (n.s. in all lineage cells). (c) We observed similar vector copy numbers in both granulocytes and lymphocytes derived from BM cells, as compared with that from mobilized cells (n.s. in both cells).
Increased CD34+ cells in steady state SCD without HU treatment
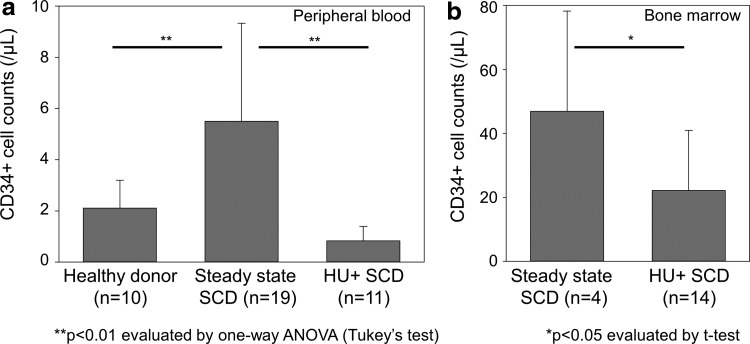
We then sought to investigate both PB and BM samples in SCD patients as an HSC source; however, the majority of our SCD subjects were treated with HU, which may influence HSC number or behavior. Therefore, we evaluated CD34+ cell counts in PBMCs among healthy donors, steady state SCD patients without HU treatment, and SCD patients under HU treatment (Fig. 3a). We observed higher CD34+ cell counts in SCD patients without HU treatment (n = 19), as compared with healthy donors (n = 10) and SCD patients under HU treatment (n = 11) (p < 0.01). We also evaluated CD34+ cell counts in BM cells among SCD patients, and our results demonstrated higher CD34+ cell counts in SCD patients without HU treatment (n = 4), as compared with SCD patients under HU treatment (n = 14) (p < 0.05) (Fig. 3b). In addition, we evaluated effects of length and dose of HU treatment on CD34+ cell counts in SCD patients; however, in both PBMCs and BM cells, no significant correlation was observed between CD34+ cell counts and treatment length (R2 = 0.001–0.06) or dose (R2 = 0.02–0.12) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/humc). These data show that circulating CD34+ cell counts are elevated in SCD patients at steady state, as compared with healthy donors, while HU treatment reduces the number of CD34+ cells in SCD patients.
Figure 3.
Higher CD34+ cell counts in steady state sickle cell disease (SCD) patients without hydroxyurea (HU) treatment compared with healthy donors and SCD patients under HU treatment. (a) We evaluated CD34+ cell counts in peripheral blood mononuclear cells (PBMCs) among healthy donors, steady state SCD patients without HU treatment, and SCD patients under HU treatment (HU+). We observed higher CD34+ cell counts in steady state SCD patients without HU treatment, as compared with healthy donors and SCD patients under HU treatment (p < 0.01), while there was no significant difference between healthy donors and SCD patients under HU treatment. (b) We also evaluated CD34+ cell counts in BM cells among SCD patients, which resulted in higher CD34+ cell counts in steady state SCD patients without HU treatment, as compared with SCD patients under HU treatment (p < 0.05).
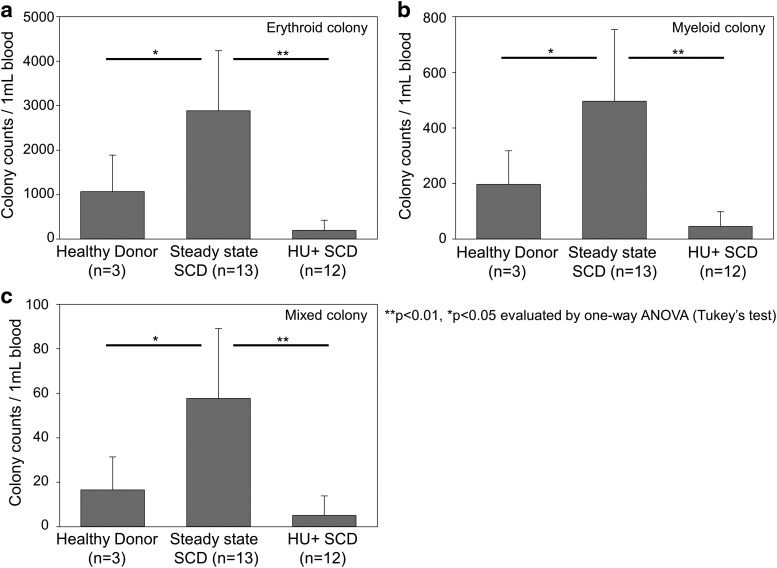
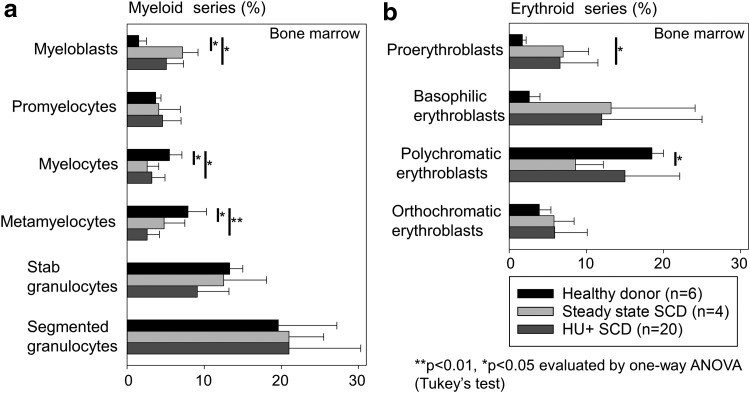
To evaluate proliferative potential from myeloid and erythroid cells, we performed CFU assays using PBMCs among healthy donors, SCD patients without HU treatment, and SCD patients under HU treatment (Fig. 4). Among all erythroid (Fig. 4a), myeloid (Fig. 4b), and mixed (Fig. 4c) colonies, we observed greater amounts of CFUs in SCD patients without HU treatment (including myeloid colonies), as compared with healthy donors (p < 0.05) and SCD patients under HU treatment (p < 0.01). In addition, we evaluated differentiation status from bone marrow aspirate smears by Wright–Giemsa stain among the same three groups (Fig. 5). We observed greater percentages of immature myeloid cells (myeloblasts) in SCD patients without or under HU treatment, as compared with healthy donors (p < 0.05) (Fig. 5a). We also observed relatively greater percentages of immature erythroid cells (proerythroblasts and basophilic erythroblasts) in both groups of SCD patients, as compared with healthy donors (p < 0.05 in proerythroblasts between SCD patients under HU treatment and healthy donors) (Fig. 5b). These data suggest that hematopoiesis is enhanced in SCD for not only erythroid progenitors but also myeloid progenitors, probably resulting in an increase of CD34+ cells.
Figure 4.
Greater amounts of colony forming units (CFUs) in PBMCs in steady state SCD patients without HU treatment, as compared with healthy donors and SCD patients under HU treatment. We performed CFU assays using PBMCs among healthy donors, steady state SCD patients without HU treatment, and SCD patients under HU treatment. Among all erythroid (a), myeloid (b), and mixed (c) colonies, we observed greater amounts of CFUs in steady state SCD patients without HU treatment, as compared with healthy donors (p < 0.05) and SCD patients under HU treatment (p < 0.01), while there was no significant difference between healthy donors and SCD patients under HU treatment.
Figure 5.
Greater percentages of immature BM cells in SCD patients, as compared with healthy donors. (a) We evaluated cell differentiation in aspirated BM cells by Wright-Giemsa stain among healthy donors, steady state SCD patients without HU treatment, and SCD patients under HU treatment. We observed greater percentages of immature myeloid cells (myeloblasts) in SCD patients with or under HU treatment, as compared with healthy donors (p < 0.05). (b) We also observed relatively greater percentages of immature erythroid cells (proerythroblasts and basophilic erythroblasts) in SCD patients for both groups, as compared with healthy donors (p < 0.05 in proerythroblasts between SCD patients under HU treatment and healthy donors).
To investigate the potential for pluripotent stem cell generation from SCD patients, we performed iPS cell generation using BM stromal cells and PBMCs derived from steady state SCD patients without HU treatment (n = 2) and under HU treatment (n = 5). The cells were transduced with a reprogramming lentiviral vector, and these cells were cultured on an iPS cell culture condition.25,26 Reprogramming was only successful from cells without HU treatment (2 of 2), whereas iPS colonies were not observed in cells under HU treatment (0 of 5). These data suggest that HU treatment not only reduces cell amounts but also functionally inhibits cell proliferation in SCD patients.
Discussion
We previously demonstrated in the rhesus competitive repopulation model that steady state bone marrow is a viable source for genetic modification using oncoretroviral vectors,27 yet these vectors are unsuitable for constructs including complex payloads such as those required for achieving erythroid specific β-globin28,29 and have been associated with insertional mutagenesis.30 The introduction of lentiviral vectors based upon HIV-1 has largely circumvented these problems, but a species specific block to HIV-1 in old world monkeys prevented their testing in this large animal model. We then developed a chimeric HIV-1 vector in which the HIV-1 capsid was replaced by that of simian immunodeficiency virus to avoid degradation by TRIM5α, allowing high-level gene transfer to rhesus repopulating HSCs.19,20 In the current work, we have utilized our model to again examine steady state BM as a CD34+ cell source for genetic modification using HIV-1-based lentiviral vectors, given both the improvements associated with this vector system and that steady state BM as the default source of HSCs for gene therapy in SCD.
Here we demonstrate similar transduction efficiency of steady state BM both in vitro and in vivo after HSC transplantation, as compared with mobilized CD34+ cells, although a trend of less efficient transduction was observed in steady state BM CD34+ cells. We obtained less expansion during transduction in rhesus steady state BM CD34+ cells in vitro than in mobilized CD34+ cells. The observation that steady state BM CD34+ cells in serum-free media, including cytokines (SCF, FLT3L, and TPO), expanded less efficiently could explain small differences in in vivo marking, as we previously demonstrated that lentiviral transduction efficiency in human CD34+ cells is positively correlated with cell expansion during in vitro culture.31 The greater expansion of mobilized CD34+ cells may be caused by higher cytokine sensitivity, since only activated CD34+ cells could be mobilized to PB by cytokine treatment in donors. Interestingly, while higher transduction efficiency in vitro was observed in mobilized CD34+ cells than steady state BM CD34+ cells in ZH21 (85% vs. 57%, respectively), comparable gene marking levels in vivo were obtained between the two sources of CD34+ cells after transplantation of these transduced cells (VCNs 0.35 vs. 0.23 in granulocytes and 0.57 vs. 0.51 in lymphocytes, respectively). Overall, our data suggest that steady state BM CD34+ cells are an alternative cell source for efficient transduction with lentiviral vectors.
We then examined stem cell sources in SCD. Circulating CD34+ cells are elevated in SCD patients at steady state, probably due to, as previously noted,32 increased hematopoiesis. CD34+ cell percentage among steady state BM from SCD patients were also higher as compared with that previously reported in healthy donors (∼40/μL).33 In CFU assays from PBMCs, a greater number of erythroid colonies were observed from SCD patients without HU treatment as compared with healthy donors, likely due to enhanced erythropoiesis.
Interestingly, myeloid and mixed colonies were also increased at steady state in subjects with SCD. In addition, we observed higher percentages of not only proerythroblasts and basophilic erythroblasts but also myeloblasts among BM cells in SCD patients, compared with healthy donors. Similar myeloid:erythroid ratios were observed among healthy donors, steady state SCD patients without HU treatment, and SCD patients under HU treatment in both CFU assays (0.19–0.27) and BM aspirate smears (1.3–2.0). These data suggest that hematopoiesis overall in SCD is enhanced, resulting in an elevation of CD34+ hematopoietic stem/progenitor cells in the BM and PB in SCD subjects, leading to higher peripheral leukocyte and platelet counts.
Many patients with SCD eligible for gene therapy may be on HU treatment, thus it is important to describe the effects of HU in the context of gene therapy. HU is the only drug currently FDA-approved for the treatment of SCD and acts predominantly through induction of fetal hemoglobin production; however, the treatment dose is limited by reduction of blood counts due to the cytotoxicity.1,2 In this work, HU treatment significantly reduced CD34+ cell counts in SCD patients in both PB and BM compartments. Expectedly, HU treatment decreased CD34+ cell counts in both PB and BM; HU also decreased CFUs from SCD PBMCs. We were surprised that iPS cells could not be generated from primary cells of SCD patients under HU treatment, likely caused by reduced sensitivities to cell growth conditions in in vitro culture. This finding is critically important for gene therapy, since autologous CD34+ cells are cultured in vitro during lentiviral transduction, and cell expansion is necessary for efficient transduction in human CD34+ cells.31 We recommend holding HU treatment for 2–4 weeks prior to collection of CD34+ cells (and stromal cells) from SCD patients for gene therapy and regenerative therapy applications. Though we have not done time course studies, this schedule has proven effective in our current gene therapy trial.34 This suppressive effect of HU in SCD is consistent with another report showing similarly myelosuppressive effect of HU treatment in β-thalassemia patients, also suggesting that HU treatment should be stopped ∼2 weeks before HSC collection.35
Based on the current data, steady state BM is the default for the collection of autologous HSCs for gene therapy in SCD. Indeed, steady state BM CD34+ cells in an SCD patient transduced with a lentiviral vector encoding a β-globin gene proved capable of therapeutic adult hemoglobin production up to 15 months after transplantation, suggesting that steady state BM CD34+ cells are useful as target cells in SCD gene therapy.36 In addition, circulating CD34+ cells are elevated in SCD patients at steady state; therefore, mobilized peripheral blood is still an interesting alternative source for HSCs in SCD. Mobilization can be achieved by G-CSF administration, allowing the collection of HSCs from the peripheral blood by apheresis.37,38 However, G-CSF leads to intolerable adverse effects in SCD patients, including vaso-occlusive crisis, multiorgan failure, and even death, probably due to granulocyte stimulation associated with G-CSF.18,39,40 An alternative agent for HSC mobilization is plerixafor, which is an inhibitor of the CXCR4 chemokine receptor and allows for an increase of circulating HSCs.41–43 Theoretically, plerixafor can be applied safely to SCD patients, since it mobilizes HSCs into the peripheral blood without direct stimulation of granulocytes.41,44 We are now initiating a clinical trial testing mobilization in SCD with plerixafor.
In conclusion, rhesus steady state BM CD34+ cells are a viable alternative to mobilized CD34+ cells, and these results support the use of steady state BM CD34+ cells in gene therapy applications targeting SCD given the disastrous consequences previously described after mobilization with G-CSF in SCD.18,39,40 Our data further suggest that PB is a rich source of CD34+ cells in SCD, potentially amenable to mobilization with agents such as plerixafor, which mobilize through a mechanism distinct from G-CSF. Importantly, as HU treatment reduces the frequency of cell expansion and efficiency of transduction, withholding HU temporarily is advisable prior to collecting PB or harvesting BM for gene therapy and regenerative medicine applications. Our results are important for the design of gene targeting strategies for SCD.
Supplementary Material
Acknowledgments
This work was supported by the intramural research program of NHLBI and National Institute of Diabetes, Digestive, and Kidney diseases at the National Institutes of Health. We thank Barrington E. Thompson, Sandra D. Price, and the National Institutes of Health animal care and veterinary staff for animal care and handling. We thank R. Patrick Weitzel, Anna Shvygina, Luke P. Skala, and Lydia Raines for helping with experiments.
Author Disclosure
No competing financial interests exist.
References
- 1.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317–1322 [DOI] [PubMed] [Google Scholar]
- 2.Fitzhugh CD, Hsieh MM, Allen D, et al. Hydroxyurea-increased fetal hemoglobin is associated with less organ damage and longer survival in adults with sickle cell anemia. PLoS One 2015;10:e0141706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 2009;361:2309–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh MM, Fitzhugh CD, Weitzel RP, et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA 2014;312:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med 1996;335:369–376 [DOI] [PubMed] [Google Scholar]
- 6.Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010;467:318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou J, Mali P, Huang X, Dowey SN, Cheng L. Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease. Blood 2011;118:4599–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 2009;360:447–458 [DOI] [PubMed] [Google Scholar]
- 9.Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002;296:2410–2413 [DOI] [PubMed] [Google Scholar]
- 10.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2003;348:255–256 [DOI] [PubMed] [Google Scholar]
- 11.Ott MG, Schmidt M, Schwarzwaelder K, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006;12:401–409 [DOI] [PubMed] [Google Scholar]
- 12.Schwarzwaelder K, Howe SJ, Schmidt M, et al. Gammaretrovirus-mediated correction of SCID-X1 is associated with skewed vector integration site distribution in vivo. J Clin Invest 2007;117:2241–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith EC, Orkin SH. Hemoglobin genetics: recent contributions of GWAS and gene editing. Human molecular genetics 2016;25:R99–r105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urbinati F, Hargrove PW, Geiger S, et al. Potentially therapeutic levels of anti-sickling globin gene expression following lentivirus-mediated gene transfer in sickle cell disease bone marrow CD34+ cells. Exp Hematol 2015;43:346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoban MD, Cost GJ, Mendel MC, et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood 2015;125:2597–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoban MD, Lumaquin D, Kuo CY, et al. CRISPR/Cas9-Mediated Correction of the Sickle Mutation in Human CD34+ cells. Mol Ther 2016;24:1561–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 2007;318:1920–1923 [DOI] [PubMed] [Google Scholar]
- 18.Fitzhugh CD, Hsieh MM, Bolan CD, Saenz C, Tisdale JF. Granulocyte colony-stimulating factor (G-CSF) administration in individuals with sickle cell disease: time for a moratorium? Cytotherapy 2009;11:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida N, Hargrove PW, Lap CJ, et al. High-efficiency transduction of rhesus hematopoietic repopulating cells by a modified HIV1-based lentiviral vector. Mol Ther 2012;20:1882–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida N, Washington KN, Hayakawa J, et al. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J Virol 2009;83:9854–9862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida N, Bonifacino A, Krouse AE, et al. Accelerated lymphocyte reconstitution and long-term recovery after transplantation of lentiviral-transduced rhesus CD34+ cells mobilized by G-CSF and plerixafor. Exp Hematol 2011;39:795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida N, Hsieh MM, Washington KN, Tisdale JF. Efficient transduction of human hematopoietic repopulating cells with a chimeric HIV1-based vector including SIV capsid. Exp Hematol 2013;41:779–788 e771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida N, Evans ME, Hsieh MM, et al. Integration-specific in vitro evaluation of lentivirally transduced rhesus CD34(+) cells correlates with in vivo vector copy number. Mol Ther Nucleic Acids 2013;2:e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida N, Hanawa H, Dan K, Inokuchi K, Shimada T. Leukemogenesis of b2a2-type p210 BCR/ABL in a bone marrow transplantation mouse model using a lentiviral vector. J Nippon Med Sch 2009;76:134–147 [DOI] [PubMed] [Google Scholar]
- 25.Fujita A, Uchida N, Haro-Mora JJ, Winkler T, Tisdale J. beta-globin-expressing definitive erythroid progenitor cells generated from embryonic and induced pluripotent stem cell-derived sacs. Stem Cells 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchida N, Haro-Mora JJ, Fujita A, et al. Efficient generation of β-globin-expressing erythroid cells using stromal cell-derived induced pluripotent stem cells from patients with sickle cell disease. Stem Cells 2016:n/a-n/a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hematti P, Tuchman S, Larochelle A, Metzger ME, Donahue RE, Tisdale JF. Comparison of retroviral transduction efficiency in CD34+ cells derived from bone marrow versus G-CSF-mobilized or G-CSF plus stem cell factor-mobilized peripheral blood in nonhuman primates. Stem Cells 2004;22:1062–1069 [DOI] [PubMed] [Google Scholar]
- 28.May C, Rivella S, Callegari J, et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature 2000;406:82–86 [DOI] [PubMed] [Google Scholar]
- 29.Pawliuk R, Westerman KA, Fabry ME, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science 2001;294:2368–2371 [DOI] [PubMed] [Google Scholar]
- 30.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003;302:415–419 [DOI] [PubMed] [Google Scholar]
- 31.Evans ME, Kumkhaek C, Hsieh MM, Donahue RE, Tisdale JF, Uchida N. TRIM5alpha variations influence transduction efficiency with lentiviral vectors in both human and rhesus CD34(+) cells in vitro and in vivo. Mol Ther 2014;22:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luck L, Zeng L, Hiti AL, Weinberg KI, Malik P. Human CD34(+) and CD34(+)CD38(-) hematopoietic progenitors in sickle cell disease differ phenotypically and functionally from normal and suggest distinct subpopulations that generate F cells. Exp Hematol 2004;32:483–493 [DOI] [PubMed] [Google Scholar]
- 33.Dedeepiya VD, Rao YY, Jayakrishnan GA, et al. Index of CD34+ Cells and Mononuclear cells in the bone marrow of spinal cord injury patients of different age groups: a comparative analysis. Bone Marrow Res 2012;2012:787414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanter J, Walters MC, Hsieh MM, et al. Interim results from a Phase 1/2 clinical study of lentiglobin gene therapy for severe sickle cell disease. Blood 2016;128:1176–1176 [Google Scholar]
- 35.Yannaki E, Papayannopoulou T, Jonlin E, et al. Hematopoietic stem cell mobilization for gene therapy of adult patients with severe beta-thalassemia: results of clinical trials using G-CSF or plerixafor in splenectomized and nonsplenectomized subjects. Mol Ther 2012;20:230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribeil JA, Hacein-Bey-Abina S, Payen E, et al. Gene Therapy in a patient with sickle cell disease. N Engl J Med 2017;376:848–855 [DOI] [PubMed] [Google Scholar]
- 37.Anderlini P, Rizzo JD, Nugent ML, Schmitz N, Champlin RE, Horowitz MM. Peripheral blood stem cell donation: an analysis from the International Bone Marrow Transplant Registry (IBMTR) and European Group for Blood and Marrow Transplant (EBMT) databases. Bone Marrow Transplant 2001;27:689–692 [DOI] [PubMed] [Google Scholar]
- 38.Lane TA. Allogeneic marrow reconstitution using peripheral blood stem cells: the dawn of a new era. Transfusion 1996;36:585–589 [DOI] [PubMed] [Google Scholar]
- 39.Abboud M, Laver J, Blau CA. Granulocytosis causing sickle-cell crisis. Lancet 1998;351:959. [DOI] [PubMed] [Google Scholar]
- 40.Adler BK, Salzman DE, Carabasi MH, Vaughan WP, Reddy VV, Prchal JT. Fatal sickle cell crisis after granulocyte colony-stimulating factor administration. Blood 2001;97:3313–3314 [DOI] [PubMed] [Google Scholar]
- 41.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 2003;102:2728–2730 [DOI] [PubMed] [Google Scholar]
- 42.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 2005;201:1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood 2008;112:990–998 [DOI] [PubMed] [Google Scholar]
- 44.Hendrix CW, Collier AC, Lederman MM, et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr 2004;37:1253–1262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.