Abstract
Background: This study was conducted to determine sex/gender differences in smoke exposure and to quantify the role of potential predictors including puffing behaviors, nicotine dependence, and non-nicotinic factors.
Methods: The Pennsylvania Adult Smoking Study (PASS) of 332 adult cigarette smokers utilized portable handheld topography devices to capture the smokers' profiles in a naturalistic environment. Sex/gender differences in salivary biomarkers were modeled using ANCOVA to account for measures of dependence (Fagerstrom Test for Nicotine Dependence, nicotine metabolite ratio [3-hydroxycotinine/cotinine]), and nondependence covariates including anthropomorphic factors and stress. The Blinder-Oaxaca method was used to decompose the sex/gender differences in nicotine uptake due to covariates.
Results: Men had significantly higher cotinine levels (313.5 ng/mL vs. 255.8 ng/mL, p < 0.01), cotinine +3-hydroxycotinine levels, (0.0787 mol/L vs. 0.0675 mol/L, p = 0.01), puff volumes (52.95 mL vs. 44.77 mL, p < 0.01), and a lower nicotine metabolite ratio (0.396 vs. 0.475, p = 0.01) than women. The mean Fagerström Test for Nicotine Dependence score did not differ between men and women (p = 0.24). Women had a higher mean Hooked on Tobacco Checklist score than men (7.64 vs. 6.87, p < 0.01). In multivariate analysis, nicotine metabolite levels were not significantly different by sex. Decomposition results show that ten predictors can explain 83% of the sex/gender differences in cotinine uptake. Height was the greatest contributor to these differences, followed by average puff volume.
Conclusion and Impact: The higher levels of nicotine metabolites in men, compared to women, can be explained by height, weight, puff volume, and nicotine metabolism.
Keywords: : smoking, sex, gender, disparities, nicotine, tobacco
Introduction
The behavioral, pharmacological, hormonal, neurological, economic, and social predictors of tobacco consumption have been investigated. However, sex/gender differences in smoking behaviors, are not well understood.1,2 Understanding whether sex/gender differences exist in nicotine dependence and characterizing the nature of these differences may be important for optimizing both smoking cessation treatment and for proposed public health strategies to reduce the addictiveness of cigarettes by minimizing nicotine content.
For example, smoking cessation rates, with or without pharmacotherapy, are lower in women.3–8 This could reflect that women might be more responsive to the sensory aspect of smoking and often use smoking as a coping mechanism against stress and negative emotions.9–11 Women also report higher levels of subjective responses to smoking ratings (e.g., cravings, arousal from smoking cues)12 although findings are not consistent and other studies indicate men respond more to smoking cues.13 Fear of weight gain may play a role in women's continued smoking.14,15 Women may be more likely to continue to smoke for reasons of relaxation, social anxiety, or if friends and family members smoke.16–21,22 Race, age, presence of children, and income elasticity also differentially impact women in their use of tobacco.23
In imaging studies involving real-time smoking, the mesolimbic dopamine system is activated in men but not in women.24 This indicates women may smoke more for mood alteration but men experience a greater reinforcing effect of nicotine. Men consume more cigarettes per day24 and smoke more intensely.25 Women metabolize nicotine faster than men, which may affect cigarette uptake.26 Whether the differences in nicotinic and non-nicotinic rewards for smoking between men and women actually affect nicotine exposure and measures of physical or psychological dependence, however, is not well understood. One approach to understanding this issue is by measuring biomarkers of nicotine exposure. There have been mixed findings on sex/gender differences in cotinine. Mean cotinine levels are higher in men than in women in some studies27–29 whereas women have higher levels in others.30–32
With recent advances in portable smoking topography devices, puffing topography of smokers during their daily routines can be accurately captured repeatedly and quantified in a nonlaboratory setting. Puffing behaviors are indicators of nicotine dependence33,34 and measure nicotine exposure (measured by levels of nicotine metabolites either in saliva, blood, or urine).33,35–38 By simultaneously modeling puffing behaviors, behavioral measures of physical dependence, and anthropometric and emotional factors on nicotine metabolites, this study was conducted to determine whether men have a higher degree of nicotine exposure than women, and what factors might explain sex/gender differences. In drug abuse research including nicotine addiction, it is often unknown whether a male-female difference is sex-based or gender-based (e.g., environmental/social) or both or interactions between biology and the environment.39,40 For the purposes of this article and consistency with recent perspectives,41 male-female differences are referred to as sex/gender differences.
Materials and Methods
Study population
The Pennsylvania Adult Smoking Study (PASS) is a cross-sectional, smoking topography and nicotine dependence study of 332 adult cigarette smokers, conducted in 14 contiguous counties of central Pennsylvania. The daily smoker recruitment phase lasted from June 2012 to April 2014, with primary recruiting methods relying on internet and social media, local radio advertisements, posted flyers, and word of mouth. Interested participants were screened for eligibility through a phone interview process to form a convenience sample. Eligible participants gave written consent and were scheduled for two study visits. Upon completion of two study visits, participants were provided with compensation. The study received approval from the Penn State Hershey College of Medicine Institutional Review Board (Hershey, PA). Detailed methods of the study can be found elsewhere.33
Data collection
Trained interviewers administered a multiple-domain, structured questionnaire that contained questions on cigarette-use history (cigarettes per day, menthol vs. non-menthol, commercial vs. make-your-own), sociodemographic and personal measures (age, sex, race, marital status, self-reported height and weight, education, income, occupation, domicile), smoking addiction items (quitting history, Fagerstrom Test for Nicotine Dependence [FTND], adult version of Hooked On Nicotine Checklist), medical history, the 10-item Perceived Stress Scale, and the Kessler 6-item Serious Psychological Stress (K6) scale. The Perceived Stress Scale was developed in 1983 and is one of the most widely used measures to assess stressful events in the last month.42 The K6 obtains information on the frequency of six psychological distress symptoms that are indicative of mental illness.43
The study incorporated items from the PhenX Toolkit version March 23, 2012, Ver 5.1., which are established and validated measures of environmental exposures recommended for use in biomedical research for the purposes of facilitating and harmonizing comparisons across different studies.44 Participants were taught to use the Smoking Puff Analyzer-Mobile (SPA-M) (SODIM SAS, France) and were given the smoking machine on the first study visit to use over a 2-day period in conjunction with all of their cigarettes smoked in that period. The interviewer scheduled a second, follow-up visit to collect the SPA-M machine and obtained saliva samples for laboratory analyses of tobacco nicotine metabolites using Salimetrics® Oral Swabs. Study data were uploaded and managed using REDCap, a secure web-based application that supports data capture and management for research studies.45 The total number of participants with completed nicotine metabolite and questionnaire data was 351, while the total number of participants who had valid and complete smoking topography data was 332.
The SPA-M is a human smoking profile analyzer of cigarettes that allows real-time storage of pressure change profiles, air flow, and atmospheric pressure. The light-weight, portable device operates on batteries and stores the measured data on a memory card. It is equipped with a touch screen that enables users to create a log capturing the smoking profile of each individual cigarette. The participant smokes the cigarette through a mouthpiece that is attached to the SPA-M, while flow and pressure changes are recorded using pressure sensors. Once the memory card is connected to a computer, the recorded data are interpreted by the SodAfc software thus permitting an analysis of the smoking profile. The software determines the puff flow (mL/s), number of puffs, puff duration (s), interval between puffs (s), and puff volume (mL). From the initial smoking parameters, we were able to calculate other related measures of each smoker, such as mean puff volume (MPV), mean puff duration (MPD), mean inter-puff interval (IPI), mean puff flow (MPF), total daily puff volume (TDPV), and total daily puffs throughout the course of a day.
Nicotine exposure
Participants' saliva samples were analyzed using mass-spectrometry for tobacco nicotine metabolites.46,47 Because cotinine may overestimate tobacco exposure somewhat in men compared to women due to the slower CYP2A6-mediated metabolism of cotinine to 3′-hydroxycotinine (3HC),48 we measured both cotinine (COT) and 3HC, the major metabolites of nicotine. The nicotine metabolite ratio (3′-hydroxycotinine/cotinine; NMR) was derived from these measurements. The coefficient of variation for cotinine was 0.53.
Statistical analysis
Differences in baseline characteristics between men and women were calculated for nicotine metabolites and smoking topography data. Questionnaire responses were summarized and were also compared between men and women. We performed t-tests or Wilcoxon rank-sum test (when appropriate) on continuous outcomes. We described categorical and binary frequencies stratified by sex, and tested associations using Chi-squared or Fisher's exact tests.
All variables that had shown value as predictors of COT in previous research from NHANES (sex, age, cigarettes per day, weight, time to first cigarette [TTFC]49,50) or showed significant differences between women and men in the current data (topography, demographics, tobacco addiction measures) were included in a stepwise regression, with bidirectional model selection based upon the Akaike Information Criterion. Since there were a large number of variables, we checked for multi-collinearity and eliminated any measures that had variance inflation factors >3. Following model selection, we analyzed the significance of sex in addition to the measures of the chosen model using ANCOVA modeling.
The Blinder-Oaxaca decomposition technique was used to model sex/gender differences in COT, COT +3HC, and NMR.51 The technique was originally developed to model sex/gender differences in income.52 Its general form is to identify and quantify the separate contributions of measurable characteristics (e.g., education etc.) and unobservable characteristics in explaining differences between two groups. It has been used previously in smoking research, for example, to model sex differences in cigarettes per day.53 We included the measures from the chosen model of the stepwise regression in a two-fold decomposition, investigating the explained versus unexplained effects. The data analysis was conducted using SAS 9.3 (SAS Institute, Cary, NC). The R package oaxaca that incorporates Blinder-Oaxaca method with bootstrapping framework and the STATA module oaxaca, were used in calculating the decomposition effects.
The decomposition is based on modeling separate regression equations for men and women51,54,55:
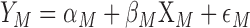 |
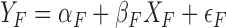 |
The next step is to construct a counterfactual equation, estimating women's nicotine metabolites with the intercept and coefficients from the men's equation:
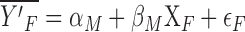 |
To investigate the differences between men and women, we want to model the differences between the expected outcomes between men and women:
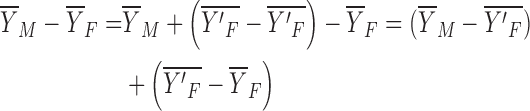 |
The characteristics effect, or the explained component due to the differences in the levels of the predictors between men and women, is as follows:
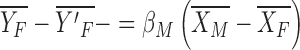 |
The coefficients effect, or the unexplained component due to the differences in the intercepts and coefficients of the measures between men and women, is as follows:
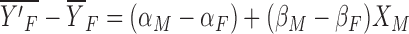 |
We used this technique to explore the causes of sex differences and compared the explained difference attributed to individual measures. As shown above, each of the mean difference in measures multiplied by the coefficient for men, 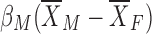 , can contribute positively or negatively to enlarging or abridging the difference in mean response, respectively. The unexplained component is the estimated difference in mean response that is due to unknown sources of variation. The Blinder-Oaxaca is able to detect outcome-explanatory group-level trends based upon differences between two groups that an ANCOVA cannot summarize. All statistical tests were based upon 95% confidence limits. Finally, we modeled these covariates using linear models that included the two-way interaction between sex and the covariate.
, can contribute positively or negatively to enlarging or abridging the difference in mean response, respectively. The unexplained component is the estimated difference in mean response that is due to unknown sources of variation. The Blinder-Oaxaca is able to detect outcome-explanatory group-level trends based upon differences between two groups that an ANCOVA cannot summarize. All statistical tests were based upon 95% confidence limits. Finally, we modeled these covariates using linear models that included the two-way interaction between sex and the covariate.
Results
Table 1 compares the demographic, nicotine metabolite, and smoking topography characteristics between women and men. The number (%) of female smokers was 191 (58%) and the number of male smokers was 141 (42%). There were no significant differences in average age (∼38 in men and women) or annual household income. Men had significantly higher mean height (women: 1.69 m vs. men: 1.71 m, p < 0.01) and body weight (women: 80.37 kg vs. men: 85.02 kg, p < 0.01).
Table 1.
Participant Characteristics and Unadjusted Levels of Biomarkers in Women and Men
| Women | Men | ||
|---|---|---|---|
| Variable | Mean | Mean | p-value |
| Number of subjects | 191 | 141 | |
| Age (SD) | 38.46 (11.77) | 37.13 (11.48) | 0.29 |
| Body mass index (SD) | 27.8 (7.83) | 29.26 (5.86) | 0.06 |
| Height (m) (SD) | 1.69 (0.067) | 1.71 (0.067) | <0.01 |
| Weight (kg) (SD) | 80.37 (22.94) | 85.02 (19.73) | <0.01 |
| Household size (SD) | 3.17 (1.44) | 3.28 (1.56) | 0.52 |
| Total annual income (SD) | 51965.2 (38004.28) | 57189.9 (39663.52) | 0.23 |
| Race | >0.99 | ||
| White (%) | 161 (86.91) | 124 (87.94) | |
| Black (%) | 17 (8.90) | 12 (8.51) | |
| Other (%) | 8 (4.19) | 5 (3.55) | |
| Cotinine (ng/mL) (SD) | 255.8 (138.07) | 313.5 (158.30) | <0.01 |
| 3′-Hydroxycotinine (ng/mL) (SD) | 121.1 (98.22) | 130.8 (114.16) | 0.40 |
| Cotinine +3′-Hydroxycotinine (mol/L) (SD) | 0.0675 (0.038) | 0.0787 (0.041) | 0.01 |
| Nicotine metabolite ratio (SD) | 0.475 (0.31) | 0.396 (0.25) | 0.01 |
| Mean puff volume (mL) (SD) | 44.77 (13.75) | 52.95 (15.11) | <0.01 |
| Mean puff duration (s) (SD) | 1.48 (0.41) | 1.65 (0.46) | <0.01 |
| Mean interpuff interval (s) (SD) | 26.49 (11.13) | 23.54 (9.0) | 0.02 |
| Mean puff flow (mL/s) (SD) | 32.71 (8.55) | 35.88 (9.44) | <0.01 |
| Peak puff flow (mL/s) (SD) | 47.76 (14.61) | 52.38 (16.83) | 0.01 |
| Mean puffs/cigarette (SD) | 11.6 (4.86) | 11.73 (4.13) | 0.83 |
| Total daily puffs (SD) | 110.5 (72.94) | 122.3 (84.03) | 0.19 |
| Puff volume/cigarette (mL) (SD) | 515.3 (232.37) | 609.7 (206.58) | 0.01 |
| Total daily puff volume (mL) (SD) | 4946.9 (3512.39) | 6341.3 (4282.63) | 0.02 |
| Cigarettes per day (SD) | 17.4 (7.91) | 15.92 (8.37) | 0.09 |
| Cigarette butt length (mm) (SD) | 0.795 (1.72) | 0.587 (0.42) | 0.62 |
| Menthol cigarette smokers (%) | 112 (56.28) | 76 (52.05) | 0.44 |
| Make your own cigarette smokers (%) | 34 (17.71) | 32 (22.70) | 0.26 |
| Age at onset of smoking (years) (SD) | 16.6 (4.32) | 17.11 (4.85) | 0.30 |
| Years smoked (SD) | 21.87 (12.20) | 20.02 (11.45) | 0.15 |
| FTND score (SD) | 4.22 (2.39) | 4.51 (2.15) | 0.24 |
| HONC score (SD) | 7.64 (1.92) | 6.87 (2.27) | <0.01 |
| Time to first cigarette (min) (SD) | 37.25 (75.08) | 24.38 (25.88) | 0.02 |
| Home smoking rules | |||
| Smoke usually inside (%) | 77 (40.1) | 53 (37.59) | 0.60 |
| Smoke usually outside (%) | 115 (59.41) | 88 (62.41) | |
| Workplace smoking rules | |||
| Smokes at work (%) | 94 (49.00) | 101 (71.63) | <0.01 |
| Doesn't smoke at work (%) | 30 (15.63) | 4 (2.84) | |
| Not applicable (%) | 69 (35.42) | 36 (25.53) |
FTND, fagerstrom test for nicotine dependence; HONC, hooked on nicotine checklist.
Nicotine metabolites
Nicotine salivary metabolite levels were compared by sex. Unadjusted levels of cotinine were higher in men than in women (men: 313.5 ng/mL vs. women: 255.8 ng/mL, p < 0.01). Similarly, levels of COT +3HC were higher in men (men: 0.0787 mol/L vs. women: 0.0675 mol/L, p = 0.01) whereas the NMR was lower in men (men: 0.396 vs. women: 0.475, p = 0.01). 3HC did not differ by sex (p = 0.40). On a univariate level, sex is significantly associated with COT, COT +3HC, and NMR levels.
Smoking topography
Smoking topography profiles between women and men were tested for differences. Smokers' summarized topography was calculated throughout an entire day's use of a smoking machine. Men had a higher MPV than women (52.95 mL vs. 44.77 mL, p < 0.01). Men also had significantly higher MPD (p < 0.01), inter-puff interval (p = 0.02), MPF (p < 0.01), peak puff flow (p = 0.01), TDPV (p = 0.02), and puff volume per cigarette than women (p = 0.01). Women and men smoked a similar number of cigarettes per day (p = 0.09), took a similar number of total puffs in a day (p = 0.19), and did not differ in number of puffs per cigarette (p = 0.83), as shown in Table 1.
Sex and gender differences
Mean score on the hooked on nicotine checklist (HONC) was higher in women than in men (7.64 vs. 6.87, p < 0.01). The time to first cigarette after waking was also higher in women than in men (37.25 minutes vs. 24.38 minutes, p = 0.02). There were no differences in percentage of menthol smokers, make-your-own smokers, the FTND, age at onset of smoking, cigarettes per day, the number of years of as a smoker, and primary smoking location (outside vs. inside) (Table 1).
We performed AIC-based stepwise regression using covariates that have been shown in previous literature to model COT and COT +3HC. We removed two covariates from the model based on VIF >3 (TDPV was removed due to correlation with TDP and MPV; MD was removed due to correlation with MPV and MPF). The ANCOVA regression coefficients and p-values based upon the chosen stepwise regression models are shown in Table 2. After accounting for several covariates including weight and height, sex was nonsignificant (adjusted mean difference in COT between men and women is 10.95 ng/mL, p = 0.62, and difference for COT +3HC is 0.0003, p = 0.54). An additional model that incorporated a term for body mass index (BMI) showed that BMI was not a significant predictor. Adding BMI to the model did decrease the contribution for weight, but height, MPV, and NMR stayed significant. The only other change in the model was that TTFC became marginally significant.
Table 2.
Multiple ANCOVA Regression of Cotinine and Cotinine +3′-Hydroxycotinine
| Cotinine (ng/mL) | Cotinine +3′-hydroxycotinine (mol/L) | |||
|---|---|---|---|---|
| Measure | Coefficient | p-value | Coefficient | p-value |
| Mean puff volume (mL) | 1.64 | <0.01 | 0.00004 | <0.01 |
| Mean puff flow (mL/s) | −1.90 | 0.03 | −0.00005 | 0.03 |
| Sex | −10.95 | 0.62 | −0.0003 | 0.54 |
| Height (m) | 319.04 | <0.01 | 0.007 | 0.02 |
| Weight (kg) | −1.30 | <0.01 | −0.00003 | <0.01 |
| Total daily puffs | 0.34 | <0.01 | −0.00001 | <0.01 |
| Nicotine metabolite ratio | −112.54 | <0.01 | 0.002 | 0.01 |
| FTND score | 14.94 | <0.01 | 0.0004 | <0.01 |
| Time to first cigarette (min) | −0.29 | 0.03 | −0.000007 | 0.03 |
| Years smoked | 4.07 | <0.01 | 0.0001 | <0.01 |
| Make your own cigarette smoker | −57.70 | <0.01 | −0.002 | <0.01 |
FTND, fagerstrom test for nicotine dependence.
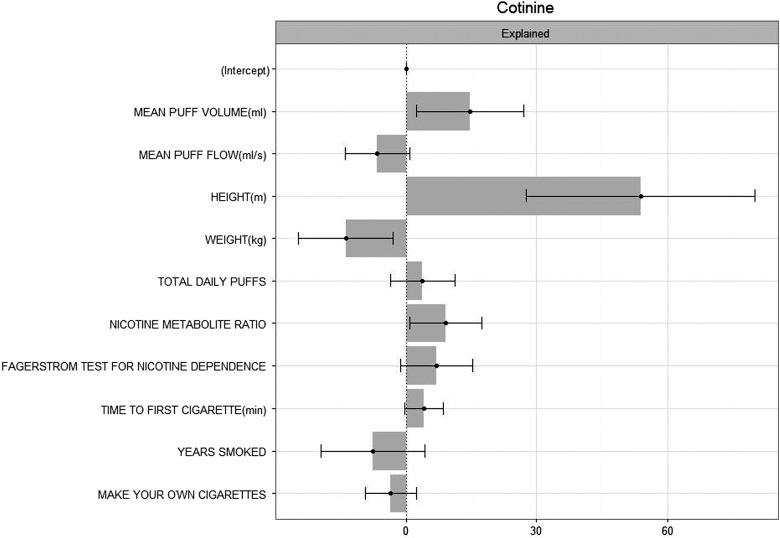
The Blinder-Oaxaca decomposition in Table 3 shows that smokers' MPV, height, weight, and NMR are the driving factors behind the univariate sex differences in COT and COT +3HC. A total of 83.4% of the differences in COT can be attributed to the independent variables, while the remainder is attributed to unknown sources of variation. Likewise, 63.3% of COT +3HC can be explained by the differences in the independent variables between women and men. There was a 65.91 (ng/mL) difference in COT between men and women, 54.97 (ng/mL) can be explained by the chosen model, while 10.94 (ng/mL) remains unexplained. Of the 54.97 (ng/mL), MPV favors men and contributes 14.45 to the gap in COT between men and women; likewise, NMR contributes 9.79 (ng/mL) and height contributes 47.34 (ng/mL) to this gap. Weight is also a significant measure of differences in COT, but contributes by shrinking the difference and decreasing the gap between men and women by 13.29 (ng/mL). The coefficients of the independent variables in Table 3 are the expected difference of the outcome that is explained by the independent variable calculated using the difference between men and women in the expected value of each variable. The sum of the coefficients of the individual independent variables equals the total explained differences. Figure 1 provides a visual comparison of the percent of the sex differences in COT explained by the covariates. The coefficient estimates and confidence limits for the covariates and the overall model explained and unexplained results are also shown in Table 3.
Table 3.
Blinder-Oaxaca Decomposition of Sex Differences in Cotinine and Cotinine +3′-Hydroxycotinine
| Cotinine (ng/mL) | 95% confidence interval | Cotinine +3′-hydroxycotinine (mol/L) | 95% confidence interval | |||||
|---|---|---|---|---|---|---|---|---|
| Measure | Coefficient | p-value | Lower | Upper | Coefficient | p-value | Lower | Upper |
| Difference | 65.91 | <0.01 | 31.53 | 100.29 | 0.001185 | 0.01 | 0.003 | 0.021 |
| Explained | 54.97 | <0.01 | 16.31 | 93.62 | 0.00075 | 0.04 | −0.003 | 0.02 |
| Unexplained | 10.94 | 0.608 | −30.84 | 52.74 | 0.000435 | 0.44 | −0.007 | 0.02 |
| Mean puff volume (mL) | 14.45 | <0.01 | 3.00 | 25.90 | 0.004 | 0.01 | 0.0008 | 0.007 |
| Mean puff flow (mL/s) | −6.69 | 0.06 | −13.36 | 0.52 | −0.0016 | 0.07 | −0.003 | 0.0001 |
| Height (m) | 47.34 | <0.01 | 12.72 | 81.96 | 0.01 | 0.02 | 0.002 | 0.02 |
| Weight (kg) | −13.29 | <0.01 | −22.96 | −3.62 | −0.004 | <0.01 | −0.006 | −0.001 |
| Total daily puffs | 3.57 | 0.31 | −3.26 | 10.40 | 0.001 | 0.34 | −0.001 | 0.003 |
| Nicotine metabolite ratio | 9.79 | 0.02 | 1.65 | 17.93 | 0.001 | 0.04 | 0.0001 | 0.003 |
| FTND score | 6.72 | 0.13 | −1.88 | 15.32 | 0.002 | 0.12 | −0.0005 | 0.005 |
| Time to first cigarette (min) | 4.08 | 0.13 | −1.26 | 9.42 | 0.00006 | 0.73 | −0.0003 | 0.0004 |
| Years smoked | −7.38 | 0.21 | −18.86 | 4.11 | −0.002 | 0.19 | −0.005 | 0.001 |
| Make your own cigarette smoker | −3.64 | 0.22 | −9.50 | 2.22 | −0.001 | 0.21 | −0.003 | 0.0006 |
FIG. 1.
Blinder-Oaxaca Decomposition of Sex Differences in Cotinine.
Discussion
There is relatively little research on sex differences in measures of tobacco dependence. We compared men and women using the FTND56 since its psychometric properties are predictive of cotinine and other tobacco smoke biomarkers.57 FTND scores have been reported to be slightly higher in men although most data were from studies conducted before 1995.58 In this study, men and women had similar FTND scores, which likely reflects the decline in heavy smokers (> 20 cigarettes per day) in men over the past decade.59 A recent web-based survey also found that sex was not associated with the FTND.60 In PASS, men did have a significantly earlier TTFC, the best single predictor of dependence of the FTND items. However, it has been suggested that sex/gender differences in the opportunity to smoke may partially explain these differences.61 Women face more household smoking restrictions than men.62 Although the study did not have a specific question on home smoking bans, there were no differences between men and women in reporting usually smoking inside or smoking outside. In contrast to TTFC, women had a modest but significantly higher HONC score (women: 7.64 vs. men: 6.87). The HONC is a measure of diminished autonomy over tobacco and is not validated against tobacco smoke biomarkers63 whereas TTFC is significantly associated with cotinine levels.64 There is little data on sex differences in HONC, so we cannot make any inferences about the consistency of this finding. In a recent study, no sex differences were found in the Wisconsin Inventory of Smoking Dependence, which assesses the multidimensional motives for smoking including affect, cue response, cravings, and weight control.65 These studies collectively indicate that despite some evidence that women may smoke more for non-nicotinic reasons, there are minor or no differences in behavioral measures of smoking maintenance.
Among the topography parameters, men had higher mean levels of MPV, MPD, TDPV, and MPF, but lower levels of IPI than women. This is interpreted as men taking larger, longer puffs and inhaling more quickly while taking shorter breaks in between puffs than women. Since topography is associated with cotinine levels, it would be expected that puffing patterns would be more frequent or intense in men than in women. In a laboratory-based study of 69 subjects smoking ad lib, women took smaller and shorter puffs than men.4 Another ad lib smoking study also found that women took smaller and shorter puffs but drew more puffs per cigarette. The validity of the FTND when comparing men to women has been further questioned since it does not measure compensatory topography puffing behaviors. Men may be less likely to face smoking restrictions due to higher representation in blue-collar occupations or less time spent for child care, whereas smoking restrictions in women could result in compensatory behaviors like increased MPV.66 Our findings show, however, that the MPV was much greater in men than in women in a naturalistic environment, and the differences in MPV contributed significantly toward the gap in COT and COT +3HC.
In this study, we were able to explain ∼83% of the sex differences in unadjusted COT levels, and ∼63% of the differences in COT +3HC were due to other factors. Figure 1 shows the effect sizes of the variables that impact the difference in nicotine metabolites between men and women. Using statistical deconstruction methods, we showed that observed sex differences in nicotine metabolites can be accounted for primarily by height. The difference in height is the greatest contributor to the differences between sex/gender in COT and COT +3HC in our sample, followed by the differences in MPV and NMR (cotinine). In contrast to height, the difference in weight decreases the differences between sexes in COT and COT +3HC. As the difference in weight between men and women increases, the differences in COT and COT +3HC between men and women decrease. The results were validated in the interaction models. It is uncertain why height and weight explain much of the differences in nicotine metabolite levels between men and women, but in opposite direction. Since the results were consistent after adjustment for BMI, one possible explanation is that taller people have higher lung volume whereas increased weight is associated with smaller lung volume.67,68 Lower body weight was also associated with increased cotinine levels in the 2001–2006 NHANES49 and in the Lung Health Study.69 This could be due to increased distribution volume, which is the amount of a drug in the body relative to the concentration in a biological fluid. Supporting this notion is a finding that female smokers who lost weight had increased salivary cotinine levels.70 Similarly, as noted, MPV was associated with higher nicotine metabolites in men, which is likely due to higher lung volume. Further work in this area could include determining whether height is a mediator of the association between MPV and cotinine.
The limitations of the PASS study include its local population demographics, which may not be generalizable to smokers across the country. We sampled from the population of smokers in Central Pennsylvania, who are predominately white. Blacks smoke fewer cigarettes and are more likely to smoke menthol cigarettes than whites.71,72 In our study, only about 8% (n = 29) of participants were black and 22 of them smoked menthol cigarettes. The findings may also not apply to all age groups. An increasing proportion of young women ages 18–25 are taking up smoking [2], and most smoke less than five cigarettes per day. These light smokers are more likely to be nondaily smokers and less nicotine dependent. The mean age of PASS participants was 38 and few were older than 50 years. Nondaily smokers were not included in the current analysis. The prevalence of smoking is much greater in individuals living at or below the poverty level, whereas the mean household income in the PASS study was over $54,000. Low income smokers are also more likely to be heavily dependent on nicotine and experience higher levels of stress.73 Another possible limitation in our study is that the height and weight measurements were self-reported and recall bias may have affected the findings. Both men and women overestimate their height and underestimate their weight, although the extent of misreporting varies somewhat between sexes.74 We also did not collect information on physical activity, which has a mediating influence in men on acute metabolism of nicotine.75 We accounted for the effects of stress, which is a factor in smoking maintenance in women. However, other aspects of emotion that may affect smoking such as self-esteem were not measured. Another potential limitation may be that men and women use the portable topography device in a different manner, which could have affected the topography measurements. However, we developed a standardized operations manual with graphics that described the correct usage of the device. This was provided to all participants. In addition, the sex differences in portable topography measures in this study were similar to that found in older laboratory-based stationary topography studies.76
Consistent with other studies, men have higher levels of cotinine and women had a higher nicotine metabolite ratio.77,78 By testing models that included and excluded BMI, it can be concluded that the differences in cotinine are primarily due to height, which is possibly attributed to lung volume, MPV, and NMR, while differences in weight tend to extend the differences between men and women. There are mixed findings that men have higher quit rates, which might appear at odds with their higher nicotine levels and earlier time to first cigarette. The higher HONC score in women indicates that loss of autonomy is not a function of nicotine intake, and it may be a factor that can predict sex/gender differences in smoking cessation.
Acknowledgments
We thank the Mass Spectrometry Core Facility at the Penn State University College of Medicine for high-performance liquid chromatography/tandem mass spectrometry services. REDCAP services are supported by the Penn State Clinical and Translational Science Institute, a Pennsylvania State University Clinical and Translational Science Award, and National Institutes of Health/National Center for Advancing Translational Sciences grant UL1 TR000127.
This work was supported by the National Institute of Drug Abuse at the National Institutes of Health (R01DA026815) and P50-DA-036107. The REDCAP project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000127. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Wetherill RR, Jagannathan K, Shin J, Franklin TR. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addict Behav 2014;39:789–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendrek A, Dinh-Williams L, Bourque J, Potvin S. Sex differences and menstrual cycle phase-dependent modulation of craving for cigarette: An FMRI pilot study. Psychiatry J 2014;2014:723632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: Influence of baseline smoking behavior. Nicotine Tob Res 2003;5:111–116 [DOI] [PubMed] [Google Scholar]
- 4.Eissenberg T, Adams C, Riggins EC, 3rd, Likness M. Smokers' sex and the effects of tobacco cigarettes: Subject-rated and physiological measures. Nicotine Tob Res 1999;1:317–324 [DOI] [PubMed] [Google Scholar]
- 5.Potter AS. Smoking cessation in men and women. Am J Psychiatry 2014;171:1148–1150 [DOI] [PubMed] [Google Scholar]
- 6.Smith PH, Kasza KA, Hyland A, et al. Gender differences in medication use and cigarette smoking cessation: Results from the International Tobacco Control Four Country Survey. Nicotine Tob Res 2015;17:463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction 2004;99:1462–1469 [DOI] [PubMed] [Google Scholar]
- 8.Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: Differences between men and women. J Consult Clin Psychol 2004;72:712–722 [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL, Hatsukami D. Gender differences in the pharmacology of nicotine addiction. Addict Biol 1998;3:383–404 [DOI] [PubMed] [Google Scholar]
- 10.Field M, Duka T. Cue reactivity in smokers: The effects of perceived cigarette availability and gender. Pharmacol Biochem Behav 2004;78:647–652 [DOI] [PubMed] [Google Scholar]
- 11.Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict 2012;21:210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology 2008;33:2148–2157 [DOI] [PubMed] [Google Scholar]
- 13.Ferguson SG, Frandsen M, Dunbar MS, Shiffman S. Gender and stimulus control of smoking behavior. Nicotine Tob Res 2015;17:431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pomerleau CS, Kurth CL. Willingness of female smokers to tolerate postcessation weight gain. J Subst Abuse 1996;8:371–378 [DOI] [PubMed] [Google Scholar]
- 15.Abuse NIoD. Are There Gender Differences in Tobacco Smoking? July 2012. Available at: www.drugabuse.gov/publications/research-reports/tobacco/are-there-gender-differences-in-tobacco-smoking Accessed August8, 2017
- 16.Branstetter SA, Blosnich J, Dino G, Nolan J, Horn K. Gender differences in cigarette smoking, social correlates and cessation among adolescents. Addict Behav 2012;37:739–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berlin I, Singleton EG, Pedarriosse AM, et al. The Modified Reasons for Smoking Scale: Factorial structure, gender effects and relationship with nicotine dependence and smoking cessation in French smokers. Addiction 2003;98:1575–1583 [DOI] [PubMed] [Google Scholar]
- 18.Huang B, Inagaki K, Yoshii C, et al. Social nicotine dependence in Australian dental undergraduate students. Int Dent J 2011;61:152–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckner JD, Vinci C. Smoking and social anxiety: The roles of gender and smoking motives. Addict Behav 2013;38:2388–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson NL, VanderVeen JW, Cohen LM, DeMarree KG, Morrell HE. Examining the interrelationships between social anxiety, smoking to cope, and cigarette craving. Addict Behav 2012;37:986–989 [DOI] [PubMed] [Google Scholar]
- 21.Shiffman S, Paton SM. Individual differences in smoking: Gender and nicotine addiction. Nicotine Tob Res 1999;1 Suppl 2:S153–S157; discussion S165–S156 [DOI] [PubMed] [Google Scholar]
- 22.Torres OV, O'Dell LE. Stress is a principal factor that promotes tobacco use in females. Prog Neuropsychopharmacol Biol Psychiatry 2016;65:260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hersch J. Gender, income levels, and the demand for cigarettes. J Risk Uncertainty 2000;263:263–282 [Google Scholar]
- 24.Cosgrove KP, Wang S, Kim SJ, et al. Sex differences in the brain's dopamine signature of cigarette smoking. J Neurosci 2014;34:16851–16855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melikian AA, Djordjevic MV, Hosey J, et al. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob Res 2007;9:377–387 [DOI] [PubMed] [Google Scholar]
- 26.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009;192:29–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etter JF, Vu Duc T, Perneger TV. Saliva cotinine levels in smokers and nonsmokers. Am J Epidemiol 2000;151:251–258 [DOI] [PubMed] [Google Scholar]
- 28.Gan WQ, Cohen SB, Man SF, Sin DD. Sex-related differences in serum cotinine concentrations in daily cigarette smokers. Nicotine Tob Res 2008;10:1293–1300 [DOI] [PubMed] [Google Scholar]
- 29.Assaf AR, Parker D, Lapane KL, McKenney JL, Carleton RA. Are there gender differences in self-reported smoking practices? Correlation with thiocyanate and cotinine levels in smokers and nonsmokers from the Pawtucket Heart Health Program. J Womens Health (Larchmt) 2002;11:899–906 [DOI] [PubMed] [Google Scholar]
- 30.Zeman MV, Hiraki L, Sellers EM. Gender differences in tobacco smoking: Higher relative exposure to smoke than nicotine in women. J Womens Health Gend Based Med 2002;11:147–153 [DOI] [PubMed] [Google Scholar]
- 31.Muscat JE, Djordjevic MV, Colosimo S, Stellman SD, Richie JP., Jr. Racial differences in exposure and glucuronidation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Cancer 2005;103:1420–1426 [DOI] [PubMed] [Google Scholar]
- 32.Wagenknecht LE, Cutter GR, Haley NJ, et al. Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. Am J Public Health 1990;80:1053–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krebs NM, Chen A, Zhu J, et al. Comparison of puff volume with cigarettes per day in predicting nicotine uptake among daily smokers. Am J Epidemiol 2016;184:48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev 2004;13(11 Pt 1):1800–1804 [PubMed] [Google Scholar]
- 35.Hofer I, Nil R, Battig K. Nicotine yield as determinant of smoke exposure indicators and puffing behavior. Pharmacol Biochem Behav 1991;40:139–149 [DOI] [PubMed] [Google Scholar]
- 36.Bridges RB, Combs JG, Humble JW, Turbek JA, Rehm SR, Haley NJ. Puffing topography as a determinant of smoke exposure. Pharmacol Biochem Behav 1990;37:29–39 [DOI] [PubMed] [Google Scholar]
- 37.Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: Reliability and validity in dependent smokers. Nicotine Tob Res 2003;5:673–679 [DOI] [PubMed] [Google Scholar]
- 38.Williams JM, Gandhi KK, Lu SE, Steinberg ML, Benowitz NL. Nicotine intake and smoking topography in smokers with bipolar disorder. Bipolar Disord 2012;14:618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Women & Sex/Gender Differences in Drug and Alcohol Abuse/Dependence (RO1). Department of Health and Human Services website. Accessed April28, 2017
- 40.Pogun S, Yararbas G. Sex differences in nicotine action. Handb Exp Pharmacol 2009:261–291 [DOI] [PubMed] [Google Scholar]
- 41.Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA. Sex/gender differences in smoking cessation: A review. Prev Med 2016;92:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396 [PubMed] [Google Scholar]
- 43.Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002;32:959–976 [DOI] [PubMed] [Google Scholar]
- 44.Hamilton CM, Strader LC, Pratt JG, et al. The PhenX Toolkit: Get the most from your measures. Am J Epidemiol 2011;174:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G, Giambrone NE, Jr., Dluzen DF, et al. Glucuronidation genotypes and nicotine metabolic phenotypes: Importance of functional UGT2B10 and UGT2B17 polymorphisms. Cancer Res 2010;70:7543–7552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krebs NM, Chen A, Zhu J, et al. Comparison of puff volume with cigarettes per day in predicting nicotine uptake among daily smokers. Am J Epidemiol 2016;184:48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu AZ, Renner CC, Hatsukami DK, et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: The influence of genetics, race, and sex. Cancer Epidemiol Biomarkers Prev 2013;22:708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caraballo RS, Holiday DB, Stellman SD, et al. Comparison of serum cotinine concentration within and across smokers of menthol and nonmenthol cigarette brands among non-Hispanic black and non-Hispanic white U.S. adult smokers, 2001–2006. Cancer Epidemiol Biomarkers Prev 2011;20:1329–1340 [DOI] [PubMed] [Google Scholar]
- 50.Branstetter SA, Muscat JE. Time to first cigarette and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) levels in adult smokers; National Health and Nutrition Examination Survey (NHANES), 2007–2010. Cancer Epidemiol Biomarkers Prev 2013;22:615–622 [DOI] [PubMed] [Google Scholar]
- 51.Jann B. The Blinder-Oaxaca decomposition for linear regression models. Stata J 2008;8:453–479 [Google Scholar]
- 52.Oaxaca RL. Male-female wage differentials in urban labor markets. Int Econ Rev 1973;14:693–709 [Google Scholar]
- 53.Bauer T, Göhlmann S, Sinning M. Gender differences in smoking behavior. Health Econ 2007;16:895–909 [DOI] [PubMed] [Google Scholar]
- 54.Oaxaca RL. Another look at tests of equality between sets of coefficients in 2 linear regressions. Am Econ 1974;18:23–32 [Google Scholar]
- 55.Blinder AS. Wage discrimination—reduced form and structural estimates. J Hum Resour 1973;8:436–455 [Google Scholar]
- 56.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991;86:1119–1127 [DOI] [PubMed] [Google Scholar]
- 57.Payne TJ, Smith PO, McCracken LM, McSherry WC, Antony MM. Assessing nicotine dependence: A comparison of the Fagerstrom Tolerance Questionnaire (FTQ) with the Fagerstrom Test for Nicotine Dependence (FTND) in a clinical sample. Addict Behav 1994;19:307–317 [DOI] [PubMed] [Google Scholar]
- 58.Fagerstrom KO, Kunze M, Schoberberger R, et al. Nicotine dependence versus smoking prevalence: Comparisons among countries and categories of smokers. Tob Control 1996;5:52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamal A, Homa DM, O'Connor E, et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep 2015;64:1233–1240 [DOI] [PubMed] [Google Scholar]
- 60.DiFranza JR, Wellman RJ, Savageau JA, Beccia A, Ursprung WW, McMillen R. What aspect of dependence does the fagerstrom test for nicotine dependence measure? ISRN Addict 2013;2013:906276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson L, Graves L. Rethinking an assessment of nicotine dependecne: A sex, gender and diversity analysis of the Fagerstrom Test for Nicotine Dependence. J Smoking Cessation 2007;2:59–67 [Google Scholar]
- 62.Augustson EM, Barzani D, Rutten LJ, Marcus S. Gender differences among hardcore smokers: An analysis of the tobacco use supplement of the current population survey. J Womens Health (Larchmt) 2008;17:1167–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerstrom Test for Nicotine Dependence in adult smokers. Nicotine Tob Res 2006;8:575–580 [DOI] [PubMed] [Google Scholar]
- 64.Muscat JE, Stellman SD, Caraballo RS, Richie JP., Jr. Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiol Biomarkers Prev 2009;18:3415–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allen AM, Scheuermann TS, Nollen N, Hatsukami D, Ahluwalia JS. Gender differences in smoking behavior and dependence motives among daily and nondaily smokers. Nicotine Tob Res 2016;18:1408–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emmons KM, Thompson B, McLerran D, et al. The relationship between organizational characteristics and the adoption of workplace smoking policies. Health Educ Behav 2000;27:483–501 [DOI] [PubMed] [Google Scholar]
- 67.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest 2006;130:827–833 [DOI] [PubMed] [Google Scholar]
- 68.Kiefer EM, Hankinson JL, Barr RG. Similar relation of age and height to lung function among Whites, African Americans, and Hispanics. Am J Epidemiol 2011;173:376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Istvan JA, Nides MA, Buist AS, Greene P, Voelker H. Salivary cotinine, frequency of cigarette smoking, and body mass index: Findings at baseline in the Lung Health Study. Am J Epidemiol 1994;139:628–636 [DOI] [PubMed] [Google Scholar]
- 70.Niaura R, Clark MM, Raciti MA, Pera V, Abrams DB. Increased saliva cotinine concentrations in smokers during rapid weight loss. J Consult Clin Psychol 1992;60:985–987 [DOI] [PubMed] [Google Scholar]
- 71.Muscat JE, Richie JP, Jr., Stellman SD. Mentholated cigarettes and smoking habits in whites and blacks. Tob Control 2002;11:368–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mwenifumbo JC, Sellers EM, Tyndale RF. Nicotine metabolism and CYP2A6 activity in a population of black African descent: Impact of gender and light smoking. Drug Alcohol Depend 2007;89:24–33 [DOI] [PubMed] [Google Scholar]
- 73.Widome R, Joseph AM, Hammett P, et al. Associations between smoking behaviors and financial stress among low-income smokers. Prev Med Rep 2015;2:911–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: A systematic review. Obes Rev 2007;8:307–326 [DOI] [PubMed] [Google Scholar]
- 75.Perkins KA, Epstein LH, Sexton JE, Stiller RL, Jacob RG. Effects of dose, gender, and level of physical activity on acute metabolic response to nicotine. Pharmacol Biochem Behav 1991;40:203–208 [DOI] [PubMed] [Google Scholar]
- 76.Battig K, Buzzi R, Nil R. Smoke yield of cigarettes and puffing behavior in men and women. Psychopharmacology (Berl) 1982;76:139–148 [DOI] [PubMed] [Google Scholar]
- 77.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther 2006;79:480–488 [DOI] [PubMed] [Google Scholar]
- 78.Schnoll RA, George TP, Hawk L, Cinciripini P, Wileyto P, Tyndale RF. The relationship between the nicotine metabolite ratio and three self-report measures of nicotine dependence across sex and race. Psychopharmacology (Berl) 2014;231:2515–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]