Abstract
We have analyzed reverse transcriptase (RT) region of HIV-1 pol gene from 97 HIV-infected children who were identified as failing first-line therapy that included first-generation non-nucleoside RT inhibitors (Nevirapine and Efavirenz) for at least 6 months. We found that 54% and 65% of the children had genotypically predicted resistance to second-generation non-nucleoside RT inhibitors drugs Etravirine (ETR) and Rilpivirine, respectively. These cross-resistance mutations may compromise future NNRTI-based regimens, especially in resource-limited settings. To complement these investigations, we also analyzed the sequences in Stanford database, Monogram weighted score, and DUET weighted score algorithms for ETR susceptibility and found almost perfect agreement between the three algorithms in predicting ETR susceptibility from genotypic data.
Keywords: : HIV drug resistance, etravirine resistance in children, HIV drug resistance in South India, etravirine, rilpivirine, HIV in children, subtype C resistance
Introduction
It was estimated that about 115,000 children were living with HIV in India during 2013 according to NACO survey. Access to HIV testing and counseling is available at 5,069 ICTCs (Integrated Counseling and Testing Centers) nationwide, enabling more and more children to be diagnosed and included into care, support, and treatment services. As on March 2013, 35,345 children less than 15 years were provided with antiretroviral therapy (ART) by the national program at 400 ART centers and 810 Link ART centers. Most of these are older children who are above 5 years of age.1 Asymptomatic children under 18 months were not getting diagnosed earlier and were missing out on prevention, care, support, and treatment. However, with early infant diagnosis by DNA PCR becoming available in the national program, more infants and children are now being brought into the fold of care, support, and treatment.2 The extent of the HIV-1 epidemic in children reflects the risk factors for HIV-1 infection in the adult population and the frequency with which maternal HIV-1 infection is not detected and mother-to-child transmission not prevented.3
Successful clinical management of HIV-1 infection in children is difficult because of poor adherence to ART drugs and therapy schedules, drug toxicity, and inadequate metabolism of drugs and unpredictable pharmacokinetics3 leading to the incomplete suppression of viral replication. In addition, the pediatric-formulated ART options are limited with complicated dosing.4 Finally, as a group, children living with HIV infection are growing older, bringing new challenges related to drug resistance, reproductive health planning, transition to adult medical care, and potential for long-term complications from HIV and its treatments.2
The routine viral load monitoring in patients on ART is not the standard of care in resource-limited settings.5 Hence, the patients diagnosed to have immunological failure based on their CD4 counts or clinical failure were in fact harboring viral replication long time before the treatment failure would be identified. Consequently, resistance-associated mutations (RAMs) would have occurred under continuous drug pressure.5
The normal choice of treatment in children in India consists of two nucleoside reverse transcriptase inhibitors (NRTIs) plus one active drug from the following classes: non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI) generally Atazanavir (ATV) boosted with low-dose Ritonavir (RTV). The preferred regimen being Stavudine (D4T)/Zidovudine (AZT) + Lamivudine (3TC) + NNRTI/RTV boosted Lopinavir (LPV).6 The use of Tenofovir (TDF) in combination with 3TC or Emtricitabine (FTC) is preferred only in adolescent children due to its known effect on reduction in bone mineral density.
The available choice of first-generation NNRTIs is Nevirapine (NVP) and Efavirenz (EFV) and their use is limited due to low genetic barrier to resistance and marked cross resistance between the two drugs.7 The use of EFV is preferred only for children >3 years of age, and the use of NVP only as alternate therapy in infants and children >3 years of age due to peripartum exposure in mothers and known hepatotoxicity.
Due to the massive selective advantage of NNRTI mutations, resistance to NNRTI arises rapidly after viral escape during NNRTI treatment.8 Etravirine (ETR) and Rilpivirine (RPV) are second-generation NNRTI drugs with higher genetic barriers to drug resistance than first-generation NNRTI.9 They exhibit activity against many HIV-1 variants resistant to first-generation NNRTI.10 At least two mutations are required to reduce susceptibility to ETR, and multiple mutations are necessary for the resistant variant to be selected.8 This allows them to be potential third-line options for patients who have already been treated with NVP or EFV. They are approved in NNRTI-resistant patients if adequately supported by other active drugs. ETR is approved by FDA in pediatric formulation for children aged >6 years, although there are currently insufficient data for ETR and RPV to be used as initial therapy and makes them potential alternate therapy options for the future.6
However, previous reports have identified presence of ETR resistance in NVP- and EFV-exposed nonsubtype-B-infected patients. Specifically, Kiertiburanakul et al. in 2008 reported that 33% of 184 Thai HIV-infected adults who had mainly used D4T/3TC/NVP and who had experienced virological failure (VF) had a reduced response to ETR.11 Similarly, it was reported by Taiwo et al. that half of their study population (47.3%) consisting of Nigerian adults infected with non-B subtype HIV-1 and failing NVP-based therapy had high levels of ETR resistance with Y181C being the most frequent ETR RAM.12
In light of the previous reports on presence of ETR resistance in adults exposed to first-generation NNRTIs, we aimed to analyze the frequency of predicted genotypic cross-resistance to ETR and RPV in first-line NNRTI-treated children unexposed to ETR and RPV who were born to HIV-1 infected mothers to identify the effectiveness of ETR as a potential third-line regimen. We also evaluated the concordance of three publicly available algorithms in predicting ETR resistance; Stanford HIV drug resistance database, DUET-weighted score (DWS) based on the 17 mutations identified to reduce ETR susceptibility in DUET 1 and DUET 2 trials,13 and Monogram-weighted score (MWS; Monogram Biosciences, San Francisco, CA) based on the phenotypic data for ETR susceptibility of 30 mutations by site-directed mutagenesis.14
Methodology
Study setting
The study was conducted at the YRG Centre for AIDS Research and Education (CARE), a medical and research institution that is providing medical and psychosocial care to >20,000 HIV infected individuals in Chennai, southern India. All the patients were treated and cared according to NACO guidelines.15 CD4 cell count monitoring is done every 6 months, but routine viral load monitoring is not the standard of care. At the time of analysis, ∼300 children were registered, of which 70% were on ART and attended our clinic for routine patient care.
Study participants
We retrospectively analyzed the sequences of children visiting YRG CARE and were referred for resistance testing during the period from 2011 to 2014 due to suspected treatment failure. The study protocol was submitted to and approved by the Institutional Review Board, YRG CARE. The study protocol was explained to parents/guardians of the children whose sequences have been analyzed in the study, and informed consent was obtained from the parents/guardians. Since viral load testing is not the standard-of-care in resource-limited settings, treatment failure was monitored by routine CD4 count for every 6 months. Plasma HIV RNA measurement was performed only when treatment failure was suspected and was considered to have occurred when a child showed clinical disease progression, incomplete immunologic response to therapy, and failure to maintain or achieve a CD4 T lymphocyte (CD4) cell count/percentage that is at least above the age-specific range for severe immunodeficiency.7 The targeted viral load testing was performed on children suspected of treatment failure with resistance testing if the viral load is >1,000 copies/ml and were included in the analysis.
Specimen collection
Blood was collected from these children for CD4 count followed by separation of plasma for viral load and resistance testing and stored at −700 degrees until testing.
Laboratory testing
CD4 count and viral load testing
CD4 cell count was done as a part of routine clinical care using 2-color single-platform flow cytometer, FACS Count (Becton Dickson Immunocytometry Systems, San Jose, CA). The viral load testing for the children suspected of virologic failure was done by Abbott m2000rt (Abbott Molecular, Inc., Des Plaines, IL) viral load assay, which has a lower detection limit of 40 copies/ml.
Resistance testing
Reverse transcriptase (RT) genotyping for resistance identification was performed for children having viral load of >1,000 copies/ml. Briefly, blood was collected from these children and viral RNA was extracted from blood plasma using Qiagen RNA MiniAmp kit (Qiagen, Valencia, CA). The HIV-1 genotyping assay, which sequences the HIV-1 pol gene (630 base pairs covering from RT 18th amino acid to 220th amino acid), was performed with the in-house method, as previously described.15 The primers used are described in Table 1 (NA and NNE for first round amplification followed by nested amplification with NANO2 and EO3). Sequences were aligned (ClustalX)16 to an Indian subtype C reference (C.IN.AF067155) and examined for HIV-1 subtype in REGA v2.17 Phylogenetic analysis and quality control were performed with MEGA software 4,15 as previously described, and Sequence Quality Analysis Tool (SQUAT).18 Sequences were then analyzed for resistance by Stanford HIV drug resistance database V 7.0.19
Table 1.
Primers Used for Reverse Transcriptase Gene Amplification
| S. no. | Name | Primer sequence | HXB2 position |
|---|---|---|---|
| 1 | NA | 5′-CCT ATT GAA ACT GTA CCA GT-3′ | 2558–2578 |
| 2 | NNE | 5′-ACT GTC CAT TTA TCA GGA TG-3′ | 3251–3271 |
| 3 | NANO2 | 5′-AAG CCA GGA ATG GAT GGA CCA-3′ | 2585–2606 |
| 4 | EO3 | 5′-CCA TTT ATC AGG ATG GAG TTC-3′ | 3245–3266 |
ETR susceptibility scoring by DWS and MWS
For MWS, a total of 30 mutations were identified to cause reduced susceptibility to ETR. Four mutations merited a weighting factor of 5: L100I, K101P, and Y181C/I/V. Mutations with a weighting factor of 3 were E138A/G, V179E, G190Q, M230L, and K238N. Weighting scores of 2 were assigned to K101E, V106A/I, E138K, V179L, Y188L, and G190S, while mutations at 12 sites had a score of 1: V90I, A98G, K101H, V106M, E138Q, V179D/F/I/M/T, Y181F, V189I, G190A/E/T, H221Y, P225H, and K238T. A weight mutation score of ≥4 was interpreted as being associated with a significant reduction in ETR efficacy.
For DWS, 17 NNRTI mutations with scores between 0 and 3 for each mutation identified previously16,17,20 were considered for calculating additive DWS scores. The mutations were weighed as follows: Score 3.0–Y181I/V; Score 2.5–L100I, K101P, Y181C, and M230L; Score 1.5–V106I, V179F, E138A, and G190S; Score 1.0–V90I, A98G, K101E/H, V179D/T, and G190A.
Statistical analysis
Descriptive statistics (medians, interquartile ranges [IQRs], means, and percentages) were used to summarize demographic and clinical characteristics of the study children. The agreement of Stanford HIV database with DWS and MWS was calculated by kappa statistic.21 A kappa score of 0.61–0.80 represented substantial agreement and 0.81–0.99 represented almost perfect agreement. For kappa statistic calculation, the level of agreement between two prediction algorithms was calculated based on samples being susceptible or with reduced susceptibility to ETR. For the Stanford database, a score of 0 to 29 was considered susceptible since ETR is known to show reduced phenotypic resistance only in the presence of two or more drug resistance mutations (DRMs), whereas a score of above 30 was considered to have reduced susceptibility. For prediction by DWS and MWS, scores of 0–2 and 0–3 were considered susceptible for DWS and MWS, respectively, and a score of ≥2.5 in DWS and ≥4 in MWS was considered to have reduced susceptibility to ETR.
Concordance between the ETR susceptibility and resistance scores from Stanford HIV database, DWS and MWS were assessed using Kendall's tau. The calculations of Kendall's tau between Stanford database and DWS were made based on three categories: ETR susceptibility, possible ETR resistance, and probable ETR resistance. A score of 0 to 29 and 0 to 2 were considered ETR susceptible in Stanford database and DWS, respectively. Sequences with a score of 30 to 59 in Stanford database and 2.5 to 3.5 in DWS were considered possible ETR resistance followed by scores ≥60 in Stanford database and ≥4 in DWS to be considered as probable ETR resistance. Predictions by MWS were considered to be ETR susceptible if the scores fell between 0 and 3 and scores ≥4 were considered to have either probable or possible ETR resistance depending on the presence of single mutation such as Y181C/I/V with a score of 4 or due to the presence of combination of RAMs adding up to a score ≥4. Any patterns of sequencing results deviating from these correlations in the three algorithms were considered discordant with each other.
Results
Demographic characteristics of the study children
A total of 149 children visited our clinic for routine patient care during the period of 2011 to 2014 who were on ART for >6 months and diagnosed to have treatment failure with a viral load of >1,000 copies/ml. Out of the 149 children, complete treatment history was available for only 97 children, and hence, they were included in this analysis. The demographic characteristics of the children included in the analysis are shown in Table 2. The mean age of the children was 9.1 ± 4.2 with a median viral load and CD4 count of 30,200 (IQR: 2080–444131) copies/ml and 200 (IQR: 34–2094) cells/μl, respectively. Seventy-six percent of children were on first-line treatment with AZT/D4T+3TC+NVP being the most frequent regimen followed by 10% of children on AZT/D4T+3TC+EFV and three children each on TDF-based and ABC-based first-line treatment. Five children were on TDF-based second-line regimen with ATV/r with one child on IDV +3TC+NVP.
Table 2.
Demographics of the Study Population
| Characteristics | Children failing therapy (n = 97) |
|---|---|
| Mean age in years (SD) | 9.1 ± 4.2 |
| Gender (M/F) | Male = 62 (64%), female = 35 (36%) |
| Mode of HIV transmission | Vertical Transmission |
| Median CD4 cells/μl at the time of failure | 200 (IQR; 34–2094) |
| Median PVL copies/ml at the time of failure | 30,200 (IQR; 2080–444131) |
| Median time to fail therapy (Months) | 32.9 (IQR: 6–96) |
| Treatment history | d4T + 3TC + NVP (43) |
| d4T + 3TC + EFV (7) | |
| AZT +3TC + NVP (31) | |
| AZT +3TC + EFV (3) | |
| IDV +3TC + NVP (1) | |
| ABC +3TC + NVP (4) | |
| TDF +3TC + EFV/NVP (3) | |
| TDF/3TC/RTV + ATV (5) |
3TC, Lamivudine; ABC, Abacavir; ATV, Atazanavir; AZT, Zidovudine; D4T, Stavudine; EFV, Efavirenz; IDV, Indinavir; IQR, interquartile range; NVP, Nevirapine; PVL, plasma viral load; RTV, Ritonavir; TDF, Tenofovir.
RT mutations
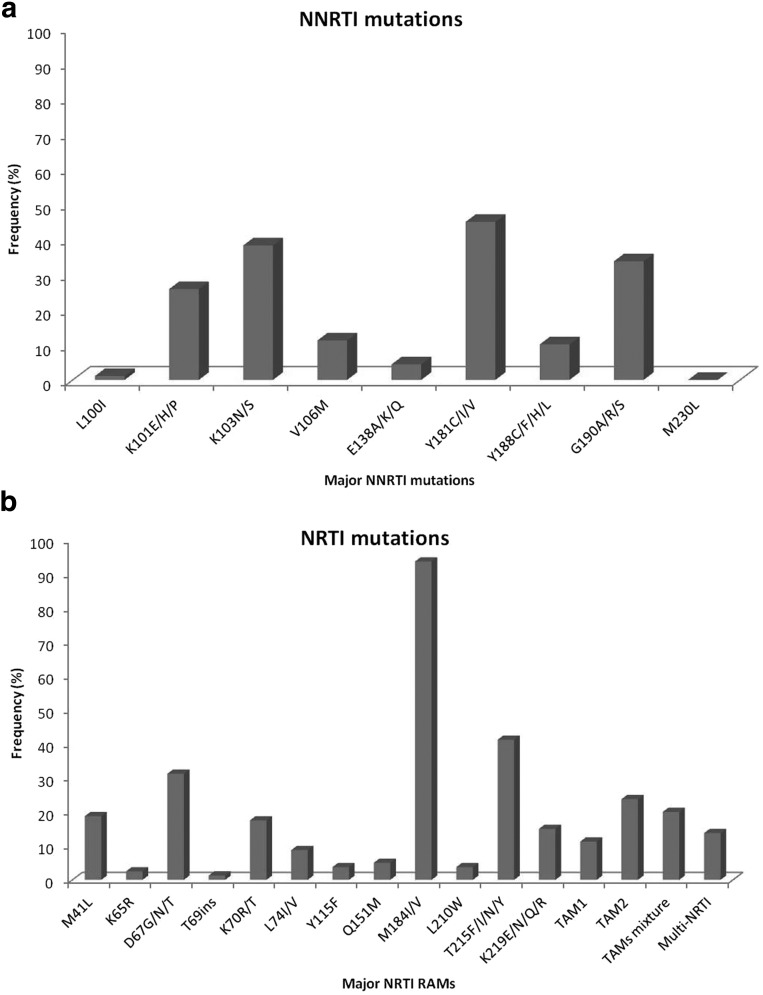
Of the 97 children tested, 91 children had at least one RT DRM with 88% (80/91) harboring at least one NRTI mutation and 98% (89/91) harboring at least one NNRTI mutation. Dual-class mutations were present in 85.7% (78/91) of the children. Six children showed no resistance mutations in RT and harbored wild-type viruses. The most frequent NRTI DRM was M184 V/I with 94% (75/80) followed by T215F/Y/I/N with a frequency of 41% (33/80) (Fig. 1a). Mixture of thymidine analogue mutations (TAMs) 1 and 2 was found in 20% (16/80) of children with TAM 2 being more frequent (19/80) than TAM 1 (9/80) mutations. Q151M complex was present in four children; K65R in two children and T69i in one child. Of the 89 children with at least one NNRTI mutation, Y181C/I/V was the most prevalent with a frequency of 45% (40/89) followed closely by K103N/S with a prevalence of 37% (33/89) (Fig. 1b). G190A/S, a mutation in combination with Y181C/V known to cause high-level ETR resistance, was present in 32.5% (29/89) of children. K101E/H/P had a prevalence of 26% (23/89), and V106M, a mutation common for subtype C, was present in 9 (10%) patients. The prevalence of multi-NRTI-associated mutations was 7.2% for at least four TAMs, 4.1% for the Q151M complex, and 1% for the 69 insertion.
FIG. 1.
(a) Frequency of major NNRTI mutations. (b) Frequency of major NRTI mutations. NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors.
As expected, Y181C/I/V was the most prevalent (59%) mutation seen in children on NVP-based regimen with a 38% prevalence of K103N due to previous exposures to EFV-based regimen. G190A/S was more prevalent in EFV group than in NVP group (37.5% vs. 17.2%). TAMs 1 and 2 were more prevalent in children on NVP regimen than on EFV regimen with highest prevalence of T215Y/F in NVP group than in EFV group (38% vs. 12.5%). M184 V was the highest prevalent NRTI in both regimens.
Predicted drug susceptibilities according to the Stanford database are reported in Figure 2. Of NRTIs, intermediate or high resistance was found to 3TC in 80%, ABC in 43%, AZT in 39%, D4T in 37%, DDI in 33%, FTC in 80%, and TDF in 20%. Of NNRTIs, intermediate or high resistance was found to EFV in 89%, NVP in 91%, ETR in 50%, and RPV in 61%. Half of participants (50%) had high or intermediate resistance to both ETR and RPV resistance.
FIG. 2.
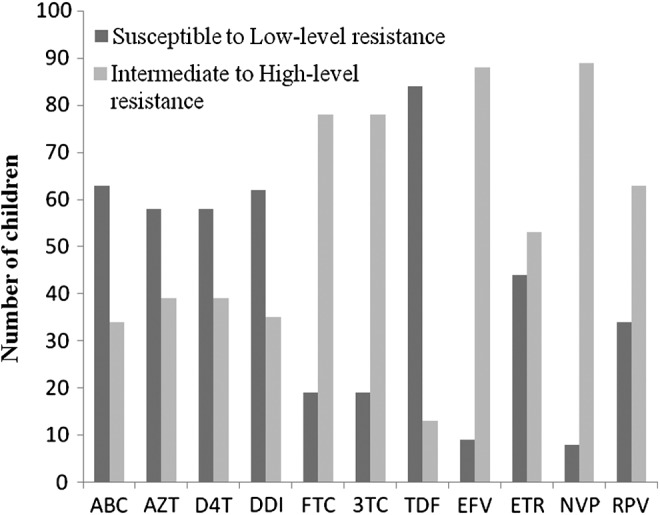
Predicted Stanford database drug susceptibilities. ABC, Abacavir; AZT, Zidovudine; D4T, Stavudine; DDI, Didanosine; FTC, Emtricitabine; 3TC, Lamivudine; TDF, Tenofovir; EFV, Efavirenz; ETR, Etravirine; NVP, Nevirapine; RPV, Rilpivirine.
ETR resistance
According to Stanford database, the frequency of children with 0 ETR mutations was 23% (22/97); with 1 ETR mutation was 41%; with 2 ETR mutations was 22%; and with ≥3 ETR mutations was 14.5%. A total of 53 (54.5%) children were identified to have intermediate to high-level ETR resistance by Stanford scoring, remaining being susceptible to ETR. According to Stanford database, 22, 22, 39, and 14 children were scored as 0, 10–29, 30–59, and ≥60, respectively.
The MWS algorithm predicted a total of 51.5% children being resistant to ETR with a score of ≥4. Children—17.5%, 34%, and 29%—were identified to have 0, 1, and 2 ETR mutations in accordance with MWS, while 19.5% children had ≥3 ETR mutations. The range of monogram score was from 0 to 10 in our study subjects, and 17, 30, 49 children were scored 0, 1–3, and ≥4, respectively.
A total of 47.4% children were predicted to have ETR resistance by DWS and 23.7%, 43.2%, 19.5% of children having 0, 1, and 2 ETR mutations, respectively. Thirteen (13.4%) children harbored ≥3 ETR mutations. According to DWS, 28, 23, 34, and 12 children were scored 0, 1–2, 2.5–3.5, and ≥4, respectively.
Apart from Y181C and G190A, other ETR RAMs present were A98G (11%), V90I (13%), K101E (24%), Y188L (7%), and H221Y (17%). Changes such as K101P, Y181I, Y181 V, V179D, and G190S were less frequently found, with a rate <3% in all instances. Mutations L100I and V179F were not found, and the last of these seems to have the most pronounced impact on ETR susceptibility.22 The combinations of mutations identified in our study population that reduce ETR susceptibility to ≥10-folds are Y181C-K101E (10.3%), Y181C-A98G (4.1%), and Y181C-H221Y (11.3%). The other combinations that reduce ETR susceptibility to ≤5-folds are K101E-G190A/S (15.4%), Y181C-G190A (11.3%), and A98G-G190A (4.1%).23
RPV resistance-associated mutations
The frequency of children with mutations associated to RPV resistance was 65% with Y181C, G190A, and K101E being the most frequent mutations. Y188L, a change that is given a score of 60 in Stanford database for RPV resistance, was present in six children. A98G and H221Y were present in 10 and 15 children, respectively. Other mutations such as Y181 V/I, L100I, and K101P, which are given a score of 60, were present in ≤3% of children. All children harboring ETR resistance were also resistant to RPV. All the combinations of mutations identified to have reduced ETR susceptibility in our study population also reduced RPV susceptibility to ≤8-folds.
Tables 3 and 4 give the comparison of ETR susceptibility in Stanford, DWS, and Monogram WS by a group of scores. The overall concordance between DWS with Stanford database was good with 0.778 and between MWS and Stanford database was 0.855, which represents an almost perfect agreement. The agreement between MWS and DWS appeared highest of all with a kappa statistic of 0.918. Kendall's tau rank correlation test between all three comparisons appeared to be same with highest correlation between Stanford database and MWS (Kendall's tau = 0.868) followed by correlation between DWS and MWS (Kendall's tau = 0.866). The correlation between DWS and Stanford database appeared considerably lesser (Kendall's tau = 0.832).
Table 3.
Etravirine Susceptibility Profile in Stanford Database, DUET-Weighted Score, and Monogram-Weighted Score Algorithms
| Stanford database | DWS | MWS | ||||
|---|---|---|---|---|---|---|
| Susceptible | 0–29 | 44/97 (45.3%) | 0–2 | 51/97 (52.5%) | 0–3 | 47/97 (48.4%) |
| Possible | 30–59 | 39/97 (40.2%) | 2.5–3.5 | 34/97 (35%) | >4 | 50/97 (51.6%) |
| Probable | >60 | 14/97 (14.4%) | >4 | 12/97 (12.3%) | ||
DWS, DUET-weighted score; ETR, Etravirine; MWS, monogram-weighted score.
Table 4.
ETR Resistance Concordance between Stanford Database, Duet-Weighted Score and Monogram-Weighted Score Algorithms
| (A) DWS vs. Stanford database | ||||
|---|---|---|---|---|
| DWS | ||||
| Stanford database | 0 | 1–2 | 2.5–3.5 | ≥4 |
| 0 | 20 | 2 | 0 | 0 |
| 10–29 | 8 | 14 | 0 | 0 |
| 30–59 | 0 | 7 | 30 | 2 |
| ≥60 | 0 | 0 | 4 | 10 |
| (B) MWS vs. Stanford database | |||
|---|---|---|---|
| MWS | |||
| Stanford database | 0 | 1–3 | ≥4 |
| 0 | 17 | 5 | 0 |
| 10–29 | 0 | 20 | 2 |
| 30–59 | 0 | 5 | 34 |
| ≥60 | 0 | 0 | 14 |
| (C) MWS vs. DWS | ||||
|---|---|---|---|---|
| DWS | ||||
| MWS | 0 | 1–2 | 2.5–3.5 | ≥4 |
| 0 | 17 | 0 | 0 | 0 |
| 1–3 | 11 | 19 | 0 | 0 |
| ≥4 | 0 | 4 | 34 | 12 |
Shaded region shows concordance.
Discussion
In this study, we report >50% children harboring NNRTI mutations leading to intermediate- to high-level resistance to ETR and 65% children showing resistance to RPV by Stanford drug resistance database. In a previous study by Cotte et al., only 21% and 19.3% of NNRTI—experienced patients in their study population were seen to have probable to possible ETR resistance as per DWS and MWS, respectively,14 while we found a 47.4% and 50.5% of children with a predicted probable to possible ETR resistance by DWS and MWS, respectively. A study by Bunupuradah et al. in 2011 conducted in Thailand adults with first-line NNRTI failure showed an ∼60% of ETR resistance with more prevalence in patients exposed to NVP than EFV.10
In a similar study by Puthanakit et al., in the Thailand pediatric population, ETR resistance was identified in 48% of children using a DUET-weighted scoring system for assessing ETR resistance, which was higher than the proportion found in other reports in children5 and in adults.24 ETR has been used successfully in adults with multiclass failure as an alternative to PI-based salvage regimens,25 it is not yet approved in children, but studies are ongoing to evaluate the efficacy of this drug in the setting of triple class failure. Our data show that the opportunity to use ETR in late NNRTI failure is limited because of the substantial rates of high-grade ETR resistance.
K103N and Y181C are the mutations that frequently emerge in patients failing first-generation NNRTIs, with K103N tending to emerge more in patients failing EFV and Y181C in patients failing NVP.26 Although K103N was the most prevalent NNRTI RAM noted at baseline in the DUET studies, overall, it had no effect on the virologic response to ETR and most frequently emerged RAMs were V179F and V179I followed by Y181C.13 However, phenotypic data derived from patients failing NVP- and EFV-based regimens outside of clinical trials showed that specific ETR RAMs (i.e., Y181C or K101H) might display a greater impact on ETR resistance than others, of which Y181C was found in ≥40% of or study population.27
Progressive loss of susceptibility is seen as mutations accumulate and certain double mutations such as Y181C+ V179F, which was not present in our study children, reduce ETR susceptibility by >100-fold.28 Similarly, E138A/K/G, K101E/P, Y181I/C/V, along with H221Y are mutations that confer RPV resistance, which was present in considerable frequencies in our patients with exception to E138A/K/G, which was present only in two children.15,29 Furthermore, previous studies have noted that failing a HAART regimen containing D4T/3TC/NVP is associated with development of ETR RAMs,12 which was the most frequent treatment regimen in our pediatric population. Since use of NVP rather than EFV was associated with 2.7-fold increased odds of a higher number of ETR RAMs30 and the fact that higher frequency of our study subjects was on NVP-based first-line regimen, this could be a probable reason for the high estimate of ETR resistance in our study population.
We found good agreement between the three algorithms to analyze ETR resistance in terms of kappa statistic, and the best correlation was between Stanford database and MWS by kendall's tau.
In almost all cases, discordance between high MWS and low DWS was due to combinations of two RAMs with a high weight in MWS, which was either not considered in DWS (V179E) or had a lower weight in DWS than in MWS (E138A). The discordance between DWS and MWS was mainly due to the mutations Y188L, E138K, and V179L, where a score of 2 was assigned in MWS for each mutation but not considered in DWS. Similarly, the mutations V106M, V179L, and H221Y were assigned a score of 1 in MWS and were not considered in DWS. V106M, which is a subtype C specific mutation,31,32 was 11.1% prevalent in our study population.
The reverse situation (high DWS and null MWS) was not found in any patient. Most discordant cases were attributable to high MWS and intermediary DWS. Most of these sequences harbored the RAMs Y181C or L100I, either alone or in various combinations. Since these two RAMs were assigned unequal high weights in both scores, the discrepancy between the interpretations of these two algorithms was mainly due to the relative contribution of these RAMs to the total score in each algorithm. Since Y181C and L100I were in most cases associated with other RAMs with a lower weight in both algorithms, and in view of the decreased virological response observed for sequences with medium DWS (2.5–3.5) in the DUET studies, this discrepancy might only be apparent, and such RAM patterns should probably be considered as being associated with at least partial resistance to ETR.
The 12.9% prevalence of V90I led to the major discordance observed between the DWS versus Stanford database and MWS versus Stanford database. Specifically, V90I was assigned a score of one in both MWS and DWS while it was not considered significant in Stanford database. Other mutations leading to minor inconsistency between Stanford database with DWS were H221Y, Y188L, E138K, and V179L. The minor discrepancy observed between Stanford database and MWS was due to the V106M mutation, which had a prevalence of 11.1% in our study population.
First-line NNRTI-based treatment failure is a major public health problem, especially in children, because of the limited availability of approved second-line antiretroviral drugs and access to new drugs. Moreover, the lack of routine viral load monitoring in many resource-limited countries leads to delay in early detection of children who have VF. This causes accumulation of mutations within the NRTI and NNRTI drug classes until treatment failure is diagnosed on the basis of clinical or immunological criteria.
Conclusion
In summary, in children who did not have access to routine viral load monitoring and who experienced failure of WHO-recommended first-line NNRTI therapy, there were high rates of 3TC, NVP, and EFV resistance. Multi-NRTI resistance was also found in few children and almost half had high-grade ETR and RPV resistance. Therefore, the appropriate second-line regimen is a boosted PI-based regimen, with a limited role for ETR.
Acknowledgments
The authors would like to acknowledge and thank the staff of YRG CARE for providing support in completing this analysis. They profusely thank the children whose samples were used in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection
- 2.NACO paediatric guidelines. 2013.
- 3.Gifford RJ, et al. : The calibrated population resistance tool: Standardized genotypic estimation of transmitted HIV-1 drug resistance. Bioinformatics 2009;25:1197–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kebe K, et al. : High rate of antiretroviral drug resistance mutations in HIV type 1-infected Senegalese children in virological failure on first-line treatment according to the World Health Organization guidelines. AIDS Res Hum Retroviruses 2013;29:242–249 [DOI] [PubMed] [Google Scholar]
- 5.Puthanakit T, et al. : HIV-1 drug resistance mutations in children after failure of first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. HIV Med 2010;11:565–572 [DOI] [PubMed] [Google Scholar]
- 6.NACO paediatric guidelines. 2014.
- 7.Usach I, Melis V, Peris J-E: Non-nucleoside reverse transcriptase inhibitors: A review on pharmacokinetics, pharmacodynamics, safety and tolerability. J Int AIDS Soc 2013;16:18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vingerhoets J, et al. : TMC125 displays a high genetic barrier to the development of resistance: Evidence from in vitro selection experiments. J Virol 2005;79:12773–12782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das K, et al. : Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J Med Chem 2004;47:2550–2560 [DOI] [PubMed] [Google Scholar]
- 10.Bunupuradah T, Ananworanich J, Chetchotisakd P, Kantipong P, Jirajariyavej S, Sirivichayakul S, Munsakul M, et al. : Etravirine and rilpivirine resistance in HIV-1 subtype CRF01-AE-infected adults failing non-nucleoside reverse transcriptase inhibitor-based regimens. Antiviral therapy 2011;16:1113–1121 [DOI] [PubMed] [Google Scholar]
- 11.Kiertiburanakul S, Wiboonchutikul S, Sukasem C, Chantratita W, Sungkanuparph S: Using of Nevirapine is associated with intermediate and reduced response to Etravirine among HIV-infected patients who experienced virologic failure in a resource-limited setting. Curr HIV Res 2008;6:474–476 [DOI] [PubMed] [Google Scholar]
- 12.Taiwo B, Chaplin B, Penugonda S, et al. : Suboptimal etravirine activity is common during failure of nevirapine-based combination antiretroviral therapy in a cohort infected with non-B subtype HIV-1. Curr HIV Res 2010;8:194–198 [DOI] [PubMed] [Google Scholar]
- 13.Katlama C, et al. : Short communication Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir Ther 2010;15:1045–1052 [DOI] [PubMed] [Google Scholar]
- 14.Cotte L, Trabaud MA, Tardy JC, et al. : Prediction of the virological response to etravirine in clinical practice: Comparison of three genotype algorithms. J Med Virol 2009;81:672–677 [DOI] [PubMed] [Google Scholar]
- 15.Saravanan S, Vidya M, Balakrishanan P, et al. : Evaluation of two human immunodeficiency virus-1 genotyping systems: ViroSeq 2.0 and an in-house method. J Virol Methods 2009;159:211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG: ClustalW and ClustalX version 2.0. Bioinformatics 2007;23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira T, Deforche K, Cassol S, Salminem M, Paraskevis D, Seebregts C, Snoeck J, van Rensburg EJ, Wensing AMJ, van de Vijver DA, Boucher CA, Camacho R, Vandamme A-M: An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinfomatics 2005;21:3797–3800 [DOI] [PubMed] [Google Scholar]
- 18.DeLong AK, et al. : Sequence quality analysis tool for HIV type 1 protease and reverse transcriptase. AIDS Res Hum Retroviruses 2012;28:894–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.http://sierra2.stanford.edu/sierra/servlet/JSierra, accessed February27, 2014
- 20.Vingerhoets J, Peeters M, Azijn H, Tambuyzer L, Hoogstoel A, Nijs S, De Bethune MP, Picchio G: An update of the list of NNRTI mutations associated with decreases virologic response to etravirine (ETR): Multivariate analyses on the pooled DUT-1 and DUET-2 clinical trial data. Antivir Ther 2008;13:S3 (Abstract 24). [Google Scholar]
- 21.Viera AJ, Garrett JM: Understanding interobserver agreement: The kappa statistic. Fam Med 2005;37:360–363 [PubMed] [Google Scholar]
- 22.Vingerhoets J, Buelens A, Peeters M, Picchio G, Tambuyzer L, Van Marck H, et al. : Impact of baseline NNRTI mutations on the virological response to TMC-125 in the phase III clinical trials DUET-1 and DUET-2. Antivir Ther 2007;12(Suppl):34 [Google Scholar]
- 23.Basson AE, et al. : Impact of drug resistance-associated amino acid changes in HIV-1 subtype C on susceptibility to newer nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 2015;59:960–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sungkanuparph S, Manosuthi W, Kiertiburanakul S, Piyavong B, Chantratita W: Evaluating the role of etravirine in the second-line antiretroviral therapy after failing an initial nonnucleoside reverse transcriptase inhibitor-based regimen in a resource-limited setting. Curr HIV Res 2008;6:474–476 [DOI] [PubMed] [Google Scholar]
- 25.Madruga JV, et al. : Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007;370:29–38 [DOI] [PubMed] [Google Scholar]
- 26.Bannister WP, et al. : Comparison of genotypic resistance profiles and virological response between patients starting nevirapine and efavirenz in EuroSIDA. AIDS 2008;22:367–376 [DOI] [PubMed] [Google Scholar]
- 27.Poveda E, et al. : Phenotypic impact of resistance mutations on etravirine susceptibility in HIV patients with prior failure to nonnucleoside analogues. AIDS 2008;22:2395–2398 [DOI] [PubMed] [Google Scholar]
- 28.Hirsch M, Günthard H, Schapiro J, Brun-Vézinet F, Clotet B, Hammer S, et al. : Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis 2008;47:266–285 [DOI] [PubMed] [Google Scholar]
- 29.Melikian GL, Rhee S-Y, Varghese V, Porter D, White K, Taylor J, Towner W, et al. : Non-nucleoside reverse transcriptase inhibitor (NNRTI) cross-resistance: Implications for preclinical evaluation of novel NNRTIs and clinical genotypic resistance testing. J Antimicrob Chemother 2014;69:12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapadula G, Calabresi A, Castelnuovo F, Costarelli S, Quiros-Roldan E, Paraninfo G, Ceresoli F, et al. : Prevalence and risk factors for etravirine resistance among patients failing on non-nucleoside reverse transcriptase inhibitors. Antivir Ther 2008;13:601–605 [PubMed] [Google Scholar]
- 31.Brenner B, Turner D, Oliveira M, Moisi D, Detorio M, Carobene M, Marlink RG, Schapiro J, Roger M, Wainberg MA: A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS 2003;17:F1–F5 [DOI] [PubMed] [Google Scholar]
- 32.Grossman Z, Istomin V, Averbuch D, Lorber M, Risenberg K, Levi I, Chowers M, Burke M, Bar Yaacov N, Schapiro JM: Genetic variation at NNRTI resistance-associated positions in patients infected with HIV-1 subtype C. AIDS 2004;18:909–915 [DOI] [PubMed] [Google Scholar]