Abstract
Purpose: Greater visual contrast between calculi and tissue would improve ultrasound (US) imaging of urolithiasis and potentially expand clinical use. The color Doppler twinkling artifact has been suggested to provide enhanced contrast of stones compared with brightness mode (B-mode) imaging, but results are variable. This work provides the first quantitative measure of stone contrast in humans for B-mode and color Doppler mode, forming the basis to improve US for the detection of stones.
Materials and Methods: Using a research ultrasound system, B-mode imaging was tuned for detecting stones by applying a single transmit angle and reduced signal compression. Stone twinkling with color Doppler was tuned by using low-frequency transmit pulses, longer pulse durations, and a high-pulse repetition frequency. Data were captured from 32 subjects, with 297 B-mode and Doppler images analyzed from 21 subjects exhibiting twinkling signals. The signal to clutter ratio (i.e., stone to background tissue) (SCR) was used to compare the contrast of a stone on B-mode with color Doppler, and the contrast between stone twinkling and blood-flow signals within the kidney.
Results: The stone was the brightest object in only 54% of B-mode images and 100% of Doppler images containing stone twinkling. On average, stones were isoechoic with the tissue clutter on B-mode (SCR = 0 dB). Stone twinkling averaged 37 times greater contrast than B-mode (16 dB, p < 0.0001) and 3.5 times greater contrast than blood-flow signals (5.5 dB, p = 0.088).
Conclusions: This study provides the first quantitative measure of US stone to tissue contrast in humans. Stone twinkling contrast is significantly greater than the contrast of a stone on B-mode. There was also a trend of stone twinkling signals having greater contrast than blood-flow signals in the kidney. Dedicated optimization of B-mode and color Doppler stone imaging could improve US detection of stones.
Keywords: : ultrasound, urolithiasis, imaging, detection, twinkling artifact
Introduction
There is incentive to use ultrasound (US) for imaging urinary stones, because of its low cost, availability, and nonionizing nature. Smith-Bindman and colleagues showed that US could be used in place of CT for the initial diagnosis of acute kidney stone events.1 However, reported sensitivity and specificity of US for stone detection is lower than that of CT,2–5 and potentially insufficient for clinical treatment planning.6 B-mode imaging is the most common modality used for detecting kidney stones with US. B-mode imaging is optimized to assess subtle contrast differences in soft tissues. As such, stones can appear with similar grayscale intensity to the surrounding tissue. In addition, other bright objects that are not stones can be observed within the kidney.
The twinkling artifact (TA) has been suggested to improve kidney stone detection.7–15 The signal consists of rapidly changing colors observed in the vicinity of a stone (stone twinkling) when operating in color Doppler mode.16 The presence of twinkling is used to confirm that a hyperechoic region on B-mode is a kidney stone. Because the modality is not designed for stone detection, it lacks clinical sensitivity and specificity as a stand-alone diagnostic tool, and is referred to as an artifact. Furthermore, color Doppler signals not associated with a stone, (e.g., blood flow or tissue motion), can resemble stone twinkling, leading to false positives.17,18 The presence of stone twinkling is also considered dependent on the operator, US system, and system settings.19,20 Since the goal of this work was to enhance stone twinkling, it is considered a true signal and not an artifact.
Shabana and colleagues compared the sensitivity of the stone twinkling signal in detecting stones to the acoustic shadow in an in vitro model, and found stone twinkling to significantly increase the contrast of stones to the background.21 We report in this study the first quantitative measure of stone B-mode and stone twinkling signals and their contrast in humans. The power of the color Doppler signal was used to represent stone twinkling, enabling direct comparison with that of the surrounding tissue and blood-flow signals.
Materials and Methods
This was a prospective study of kidney stone patients presenting to the University of Washington, Department of Urology, from January 2015 through March of 2016. Institutional Review Board approval and written consent by each patient was obtained.
Patient population
Inclusion criterion was at least one renal calculus visible on a CT scan performed within 100 days of the US visit. Individuals with ureteral stents or who were younger than 18 years of age were excluded from this study.
Ultrasound system
US imaging was conducted using a research instrument (V-1; Verasonics, Inc., Redmond, WA) and C5-2 curvilinear imaging probe (Philips Ultrasound, Bothell, WA). The research system allowed for the collection of unprocessed frames of data for offline analysis. A custom software interface was implemented, with B-mode and color Doppler settings adjusted for the detection of renal calculi.
Scanning protocol
An experienced sonographer used CT images and interpretation from the urologist to confirm US stone detection. With the stone located anywhere within the image frame, the subject was asked to perform a brief breath hold. The sonographer would adjust the probe to optimize the image and then six consecutive B-mode grayscale frames and six color Doppler frames were captured simultaneously. This process was repeated for each stone.
Postprocessing
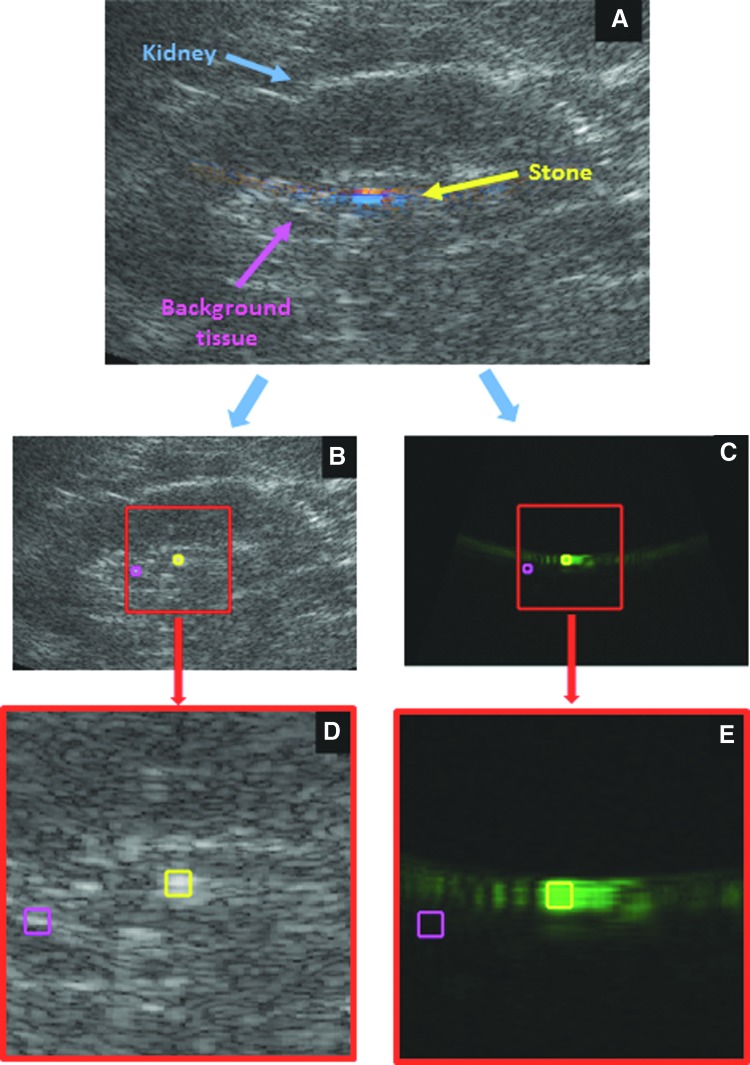
An illustrative flowchart of the postprocessing steps is presented in Figure 1. For each frame, in-phase and quadrature-phase data were processed for B-mode and color Doppler and displayed in separate images. The power of the color Doppler signal was calculated, not the directional color Doppler velocity estimate commonly used. A square region of interest (ROI) (2.5 × 2.5 mm, or 15 pixels by 15 pixels) was positioned around the brightest region on the Doppler image (signal). The same ROI was placed in the corresponding location in the B-mode image. A second ROI of the same size was positioned around the brightest nonstone region in the B-mode image (background clutter) and in the same location in the Doppler image. The mean Doppler power and B-mode intensity within each ROI were then calculated for every frame.
FIG. 1.
Illustrative flowchart for processing the US data. (A) Directional Doppler is overlaid on B-mode in the duplex image. The duplex data are separated and presented as B-mode (B, D) and power Doppler (vs directional Doppler) (C, E). The contrast is then measured for the B-mode and Doppler portions separately using the raw uncompressed data. Signal power within the stone (yellow ROI) is divided by the clutter power in the background tissue (magenta ROI) to calculate the SCR. ROI = region of interest; SCR = signal to clutter ratio; US = ultrasound.
Signal to clutter ratio
Contrast was measured as the ratio of the signal (stone or blood flow) to the clutter (background tissue) (SCR).22–25 The results are presented in decibels (dB) to compare data of significantly different magnitudes. For example, 3 dB means the signal has twice the power of the clutter. Zero decibel indicates the signal and clutter are equal. A negative dB value means the signal is weaker than the clutter or background reference signal.
Three SCRs were calculated based upon the signal type. Specifically, the SCR for a stone in B-mode is defined as:
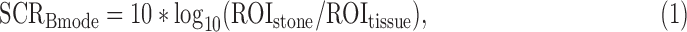 |
where ROIstone and ROItissue are the mean of the precompressed B-mode intensity (which is derived from the square of the B-mode amplitude and is directly proportional to power) within the ROI of the stone and nearby bright tissue. The SCR for the stone twinkling signal is defined as
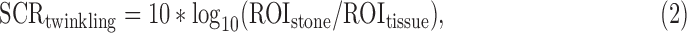 |
where ROIstone and ROItissue are the mean of the color Doppler power within the ROI of the stone and nearby bright tissue. The SCR calculated for the blood flow signal of the peak renal flow is defined as:
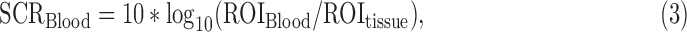 |
where ROIBlood and ROItissue are the mean of the color Doppler power within the ROI of the blood flow and nearby bright tissue.
Statistics
Stone twinkling and blood flow SCR results were skewed, and therefore, the median (rather than mean) was used to represent the average summary statistic. p-Values for the comparison between SCR medians were calculated using a mixed effects model that accounted for within-patient and within-stone correlations.
Imaging for stone detection
B-mode imaging
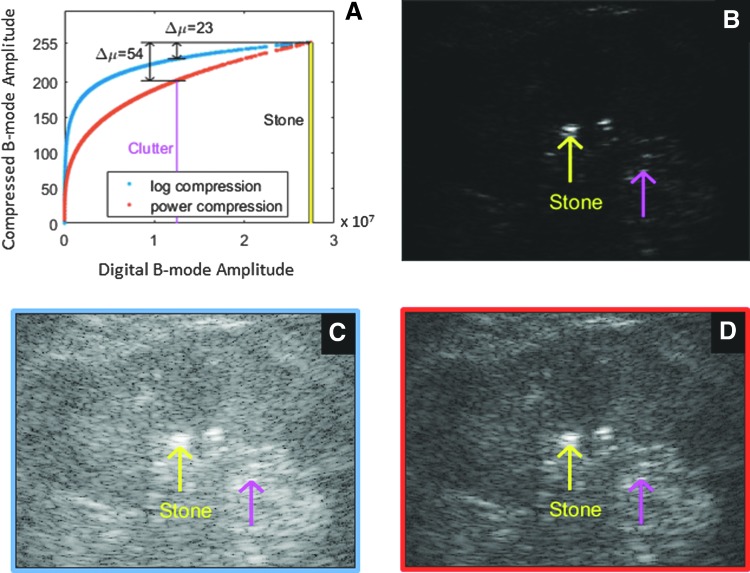
The algorithm for generating the B-mode image was similar to that of a commercial ultrasound system with three main differences.26 First, the upper part of the transducer's frequency response (4.5 MHz) was used in a fundamental mode, not harmonic mode. Second, images were reconstructed with minimal image processing (e.g., no speckle reduction or edge enhancement) commonly used on commercial systems. In addition, a single transmit angle was utilized. Multiple transmit angles (spatial compound imaging), often used to reduce speckle noise and improve soft tissue contrast, can blur bright objects and hinder visualization of the posterior acoustic shadow.21 Third, the backscattered US signal was converted to a grayscale intensity map using power compression rather than traditional logarithmic (log) compression. Figure 2 illustrates how the power compression maintains more contrast between a stone and background tissue.
FIG. 2.
Example of how visualization of the stone is impacted by compression in B-mode processing. In each image, the yellow arrow indicates the stone and the magenta arrow indicates the clutter (brightest nonstone tissue signal in the kidney). (A) The B-mode values with higher amplitude must be compressed to be displayed in the 256 brightness levels of the monitor. (B) Linear mapping has significant contrast between the stone and tissue, but the tissue signals are lost. Traditionally, a logarithmic compression is used (C), whose curve is shown in blue of (A). In this case, there are 23 grayscale levels between the stone brightness and the clutter brightness. For our optimized B-mode imaging, we use a power compression (D), indicated by the orange line of (A). The power compression has more grayscale levels (54 levels) between the stone brightness and the clutter brightness than log compression. This allows greater separation of bright objects over more brightness levels. Power compression improves the stone to tissue contrast from 0.8 dB (C) to 2.1 dB (D).
Doppler imaging
Doppler transmit pulse parameters were based on previous studies for enhancing stone detection.27,28 These enhancements included reducing the transmit frequency to 2.3 MHz and increasing burst length to 7.5 cycles per pulse. The ensemble length, or number of Doppler pulses used, was 9 for each frame. Plane wave Doppler was used instead of conventional focused ray lines to allow for a broad imaging field without compromising frame rate.29 In this study, the Doppler “box” extended over the entire depth along a centered sector overlapping 50% of the B-mode image. The pulse repetition frequency (PRF) was set to 4 kHz to minimize motion artifact and lower velocity blood-flow signals, while still allowing for a maximum imaging depth of 15 cm. A quadratic regression filter was used to further remove motion artifact and low-velocity blood-flow signals.
Results
A total of 32 subjects and 744 frames of US data (124 sets × 6 frames each) were captured for this study. The patient demographics are listed in Table 1. Each Doppler frame was visually inspected for evidence of twinkling. Since the focus of this article was quantifying the Doppler twinkle power, frames were excluded that did not show twinkling or had poor image quality resulting from patient movement or a difficult acoustic window. Finally, CT images and US videos were used to identify frames having twinkling signals associated with a stone (166 frames) and twinkling signals not associated with a stone (131 frames).
Table 1.
Subject Demographics
| Mean ± SD (n = 32) | |
|---|---|
| Age | 52 ± 17 years |
| Sex, n (%) | |
| Male | 24 (75) |
| Female | 8 (25) |
| BMI | 28.8 ± 6.1 kg/m2 |
| Days between CT-US | 37.6 ± 32.1 |
| Stone size | 6.3 ± 3.5 mm (range 2–19 mm) |
BMI = body mass index; SD = standard deviation; US = ultrasound.
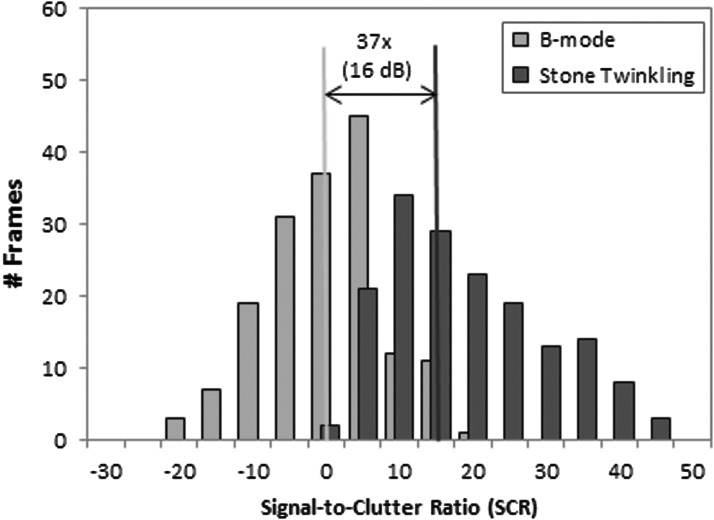
The distribution of the SCR for B-mode intensity (SCRBmode) and the Doppler twinkling power associated with a stone (SCRtwinkling) is presented in Figure 3. The median SCR for B-mode was 0.8 dB. This means that, on average, the stone was equal in B-mode intensity to the nearby bright nonstone object. In 46% of the cases, the stone B-mode intensity was less than the intensity of the background region (SCRBmode <0). The median SCR for stone twinkling was 37 times (16 dB) more intense than for B-mode (p < 0.0001). In 95% of the cases, SCRtwinkling was greater than its paired SCRBmode. Figure 4 shows how the minimum, mean, and maximum values in Figure 3 for B-mode intensity and Doppler power would appear on an US system.
FIG. 3.
Histogram of the SCR calculated from each frame of B-mode (pink) and Doppler stone twinkling (blue). The median SCR (stone to background tissue ratio) for stone twinkle power is 37 times (or 16 dB) greater than the mean SCR for the B-mode intensity (p < 0.0001). Post hoc power analysis is 99%.
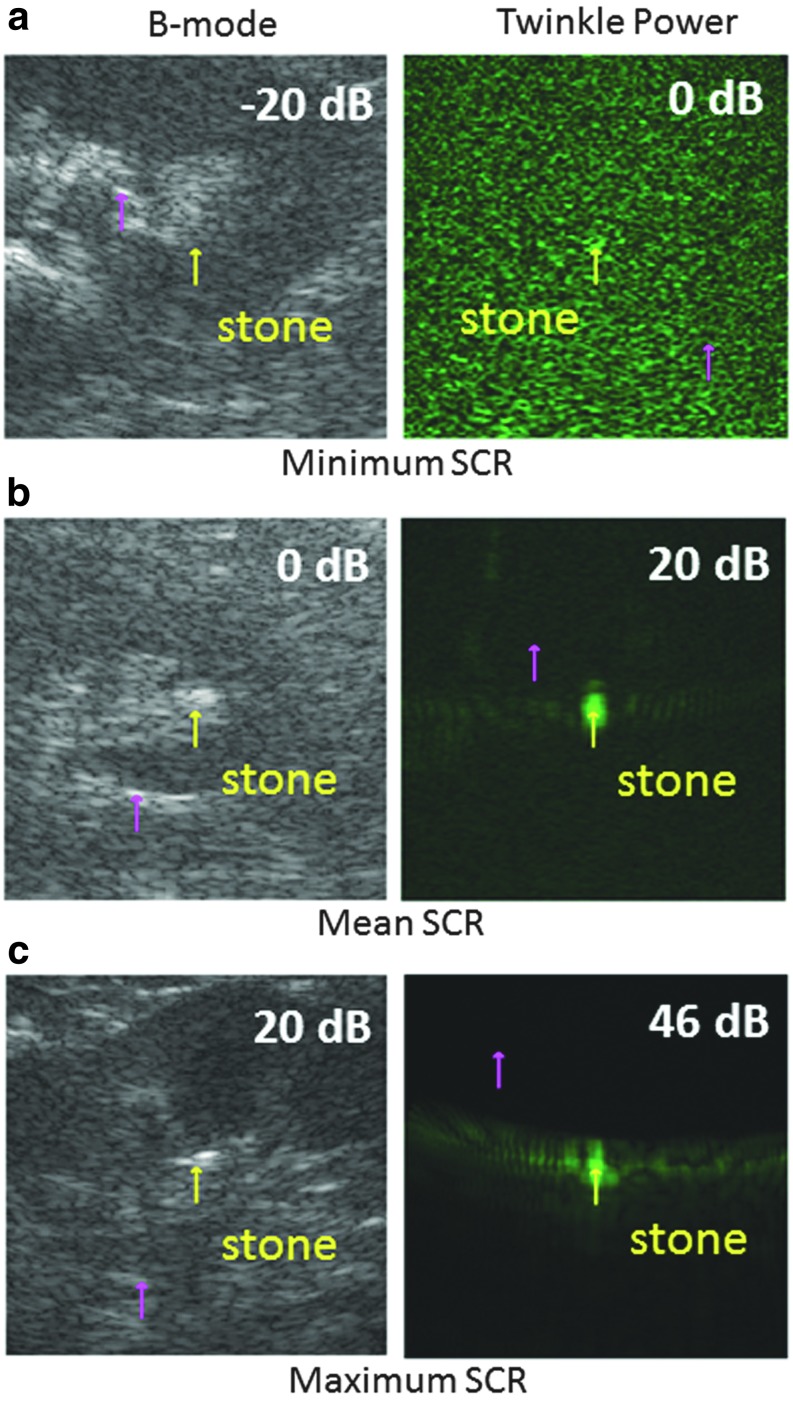
FIG. 4.
Pairs of B-mode intensity (left column) and stone twinkling power (right column) images to illustrate how the minimum, mean, and maximum SCRs would appear on an ultrasound image. The yellow arrow shows the location of the stone and the magenta arrow indicates the clutter region. Each of the six images is a representative sample taken from the data for that dB ratio, and as such, do not correspond to the same stone. For negative SCRBmode (a) the stone is darker than the clutter. B-mode (b) and stone twinkling (a) signals are equal (isoechoic) when dB = 0. When twinkling is present, the stone is easily distinguished as seen in the color Doppler images (b) and (c).
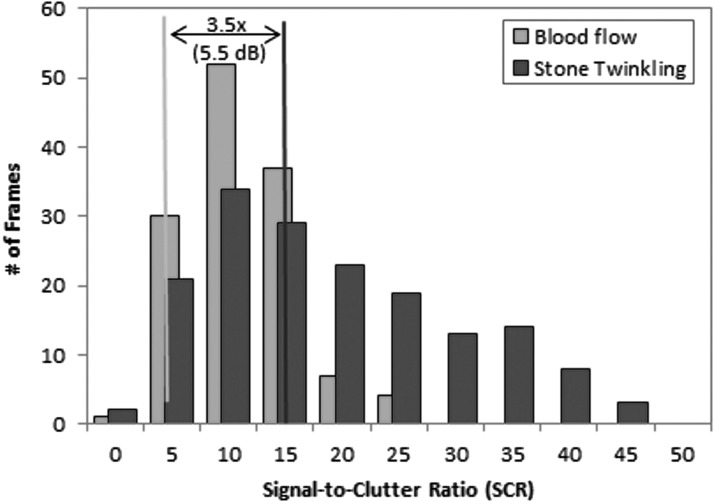
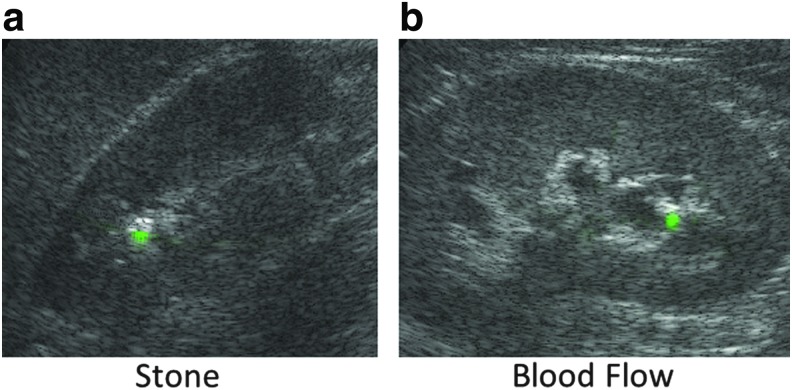
Visual inspection of the US data and comparison to CT images revealed 131 frames having color Doppler signals originating from blood flow versus a stone. A comparison of SCR between stone twinkle and blood flow is shown in Figure 5. The median stone twinkle ratio (SCRtwinkling) was 3.5 times (5.5 dB) greater than blood flow (SCRBlood), (p = 0.088), with a 73% overlap in the distributions of SCRtwinkling and SCRBlood. For these data, the Doppler signals for kidney stones and renal blood flow could appear similar, as illustrated in Figure 6. In this example, the Doppler power is overlaid as green on the B-mode image.
FIG. 5.
Histogram of the SCR calculated from the color Doppler data for stone twinkling power (blue) and maximum renal blood flow (pink). The median Doppler power for stone is 3.5 times (or 5.5 dB) greater than the signal from blood flow (p = 0.088). Post hoc power analysis is 40%.
FIG. 6.
(a) Example of kidney stone twinkling with the Doppler power shown in green overlaid on the B-mode image. (b) Example of blood flow with Doppler power shown in green overlaid on the B-mode image. The blood flow could be misinterpreted as a kidney stone signal.
Discussion
This research provides the first quantitative measure of contrast between kidney stones and background tissue on US B-mode and color Doppler mode in humans. We demonstrated that stone twinkling provided greater contrast than B-mode in 95% of the cases, and the median SCR for stone twinkling was nearly 40 times greater than the median SCR for B-mode. Our results also showed that the contrast of the stone twinkling signal was, on average, greater than the contrast of the Doppler signal from blood flow. This could be important for reducing potential false positives in stone identification. Color Doppler processing is designed to detect signal changes, usually due to motion, and to suppress signals from slower moving tissue. Our group has found growing evidence that a major component of stone twinkling is the interaction of US with submicron bubbles on or within the stone.27 The interaction results in bubble oscillation, which is interpreted as motion by the Doppler processing. These signals appear as higher velocity blood flow and are preserved in this processing, resulting in an increase in stone contrast (conspicuity) (Fig. 3). This study shows that the power in the stone twinkling signal can be larger than that of blood flow. In practice, the conspicuity of stone twinkling could be further enhanced using differences, such as the temporal fluctuations of the directional color Doppler signals, to further highlight the presence of a stone and prevent misinterpretation.
This work used a research US system that allowed control of both transmit and receive parameters and processing. As such, B-mode was adjusted for stone detection. Conventional US systems are optimized to enhance subtle contrast differences of soft tissue. As a result, high-amplitude reflections from kidney stones are often compressed into the same grayscale range as that of lower amplitude reflections from surrounding tissue, resulting in a reduction of stone contrast. We have demonstrated that less compression can improve the B-mode contrast of stones to surrounding tissue while still enabling visualization of renal tissue and the collecting system (Fig. 2). Prior studies have demonstrated that this approach, along with omitting averaging processes such as speckle reduction and spatial compounding, potentially improves the B-mode detection and sizing of stones.26 Adjustments made with the research system to improve stone contrast on B-mode can be approximated with user controls on commercial machines. This includes reducing speckle averaging, reducing spatial compounding, reducing gain, and adjusting the dynamic range. Some units permit the user to select among a choice of compression maps as well. Even with our enhancements to improve B-mode contrast, we found that in approximately half the cases, the kidney stones appeared with similar contrast to or darker than surrounding tissue.
Stone twinkling has demonstrated significant and consistent contrast of the stone to the background tissue, but a broad range of specificities and sensitivities have been reported over the years for the use of the TA to detect kidney stones. This has limited the use of stone twinkling as a diagnostic tool.10–17 The broad range of results is potentially due to variations in equipment performance, color Doppler implementations, radiologist interpretation, and system settings. One adjustment that can be made to reduce variability and improve stone contrast is operating the probe at the lowest Doppler frequency available. As stated above, the interaction of US with submicron bubbles on or within the stone is a potential source of stone twinkling.27 Bubble motion is excited more strongly by lower frequency. The use of lower color Doppler transmit frequencies (<2 MHz) is supported by the latest published TA results, and has become an option only on the newest generation probes and systems.18,30 To reduce confounding Doppler signals from blood flow, the highest depth-allowed PRF should also be used.
This study has limitations. The low number of patients and use of a research system may limit the generalizability of our findings. Stone twinkling was observed in only 54% of our subjects, in comparison to 68% to 98% of subjects reported with commercial systems.18,30 As this was a pilot study establishing baseline parameters, the study was not statistically powered a priori. Post hoc analysis showed the statistical comparison between SCRTwinkling and SCRBlood was underpowered (40%). Color Doppler was implemented with plane wave transmits. This reduces the incident pressure and likelihood of generating Doppler twinkling signals. In addition, an older generation curvilinear transducer, with limitations on the lowest Doppler frequency available, was used, again reducing our ability to generate twinkling signals. Finally, this is not a study of detection; subjects were known to have stones.
Despite these limitations, we demonstrate an ability to quantify the signal contrast between stones and background tissue with Doppler twinkling. This signal contrast was exceptionally strong, and consistent. Our research-based US system also allows the unique opportunity to deconstruct the transmit and receive signals to optimize the contrast between stones and tissue. By optimizing the inherent capabilities of US and understanding contrast differences between stones and other bright regions, US could be a much more reliable imaging modality for stone management.
Conclusions
We demonstrated that the contrast of stones to the background tissue clutter was 40 times greater for the Doppler twinkling signal than B-mode imaging. On average, stone contrast in B-mode was zero, or isoechoic with the surrounding tissue, whereas all stones displaying a twinkling signal had a positive contrast. Likewise, the stone twinkling contrast tended to be greater than the contrast from non-stone twinkling signals. This work provides the quantitative basis to improve the sensitivity and specificity of US to detect kidney stones, which may enhance the role of US in stone management.
Abbreviations Used
- BMI
body mass index
- B-mode
ultrasound brightness mode (grayscale) imaging
- CT
computed tomography
- PRF
pulse repetition frequency
- ROI
region of interest
- SCR
signal to clutter ratio
- SD
standard deviation
- TA
twinkling artifact
- US
ultrasound
Acknowledgments
This work is part of a large collaborative effort, and we appreciate the help of our many collaborators at the University of Washington (UW) Center for Industrial and Medical Ultrasound, the UW Department of Urology, and within NIDDK Program Project DK043881. Funding was provided by the National Space Biomedical Research Institute through NASA NCC 9–58, grants from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (DK043881 and DK092197), the University of Washington Applied Physics Laboratory, the University of Washington Department of Urology, CoMotion at the University of Washington, and the University of Washington Institute of Translational Health Sciences (ITHS). Some of this material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, WA. We particularly appreciate the help of Adam D. Maxwell, Oleg A. Sapozhnikov, and Julianna C. Simon.
Author Disclosure Statement
M.R.B., B.W.C., B.D., and M.D.S. have a financial interest in SonoMotion, Inc. For the remaining authors, no competing financial interests exist.
References
- 1.Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med 2014;371:1100–1110 [DOI] [PubMed] [Google Scholar]
- 2.Ray AA, Ghiculete D, Pace KT, Honey RJ. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology 2010;76:295–300 [DOI] [PubMed] [Google Scholar]
- 3.Fowler KAB, Locken JA, Duchesne JH, et al. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology 2002;222:109–113 [DOI] [PubMed] [Google Scholar]
- 4.Kanno T, Kubota M, Sakamoto H, et al. The efficacy of ultrasonography for the detection of renal stone. Urology 2014;84:285–288 [DOI] [PubMed] [Google Scholar]
- 5.Ulusan S, Koc Z, Tokmak N. Accuracy of sonography for detecting renal stone: Comparision with CT. J Clin Ultrasound 2007;35:256–261 [DOI] [PubMed] [Google Scholar]
- 6.Ganesan V, De S, Greene D, Torricelli FCM, Monga M. Accuracy of ultrasonography for renal stone detection and size determination: Is it good enough for management decisions?. BJU Int 2017;119:464–469 [DOI] [PubMed] [Google Scholar]
- 7.Aytac SK, Ozcan H. Effect of color Doppler system on the twinkling sign associated with urinary tract calculi. J Clin Ultrasound 1999;27:433–439 [DOI] [PubMed] [Google Scholar]
- 8.Chelfouh N, Grenier N, Higueret D, Trillaud H, Levantal O, Pariente J-L, Ballanger P. Characteriation of urinary calculi: In vitro study of “Twinkling Artifact” revealed by color-flow sonography. AJR 1998;171:1055–1060 [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Kim SH, Cho JY, Han D. Color and power Doppler twinkling artifacts from urinary stones: Clinical observations and phantom studies. AJR 2001;176:1441–1445 [DOI] [PubMed] [Google Scholar]
- 10.Turrin A, Minola P, Costa F, Cerati L, Andrulli S, Trinchieri A. Diagnostic value of colour Doppler twinkling artefact in sites negative for stones on B mode renal sonography. Urol Res 2007;35:313–317 [DOI] [PubMed] [Google Scholar]
- 11.Davran R. The usefullness of color Doppler twinkling artifact in the diagnosis of urinary calculi. Eur J Radiol 2009;71:378. [DOI] [PubMed] [Google Scholar]
- 12.Winkel RR, Kalhauge A, Fredfeldt K-E. The usefullness of ultrasound color-Doppler twinkling artefact for detecting urolithiasis compared with low dose nonenhanced computerized tomography. Ultrasound Med Biol 2012;38:1180–1187 [DOI] [PubMed] [Google Scholar]
- 13.Kieler AZ, Shabana W, Vakili M, Rubin J. Prospective evaluation of Dopper sonography to detect the twinkling artifact versus unenhanced computed tomography for identifying urinary tract calculi. J Ultrasound Med 2012;31:1619–1625 [DOI] [PubMed] [Google Scholar]
- 14.Sorensen MD, Harper JD, Hsi RS, et al. B-mode ultrasound versus color Doppler twinkling artifact in detecting kidney stones. J Endourol 2013;27:149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korkmaz M, Aras B, Sanal B, Yucel M, Guneyli S, Kocak A, Uruc F. Investigating the clinical significance of twinkling artifacts in patients with urolithiasis smaller than 5 mm. Jpn J Radiol 2014;32:482–486 [DOI] [PubMed] [Google Scholar]
- 16.Rahmouni A, Bargoin R, Herment A, Bargion N, Vasile N. Color Doppler twinkling artifact in hyperechoic regions. Radiology 1996;199:269–271 [DOI] [PubMed] [Google Scholar]
- 17.Dillman JR, Kappil M, Weadock WJ, Rubin JM, Platt JF, DiPietro MA, Bude RO. Sonographic twinkling artifact for renal calculus detection: Correlation with CT. Radiology 2011;259:911–916 [DOI] [PubMed] [Google Scholar]
- 18.Masch WR, Cohan RH, Ellis JH, Dillman JR, Rubin JM, Davenport MS. Clinical effectiveness of propsectively reported sonographic twinkling artifact for the diagnosis of renal calculus in patients without known urolithiasis. AJR 2016;206:326–331 [DOI] [PubMed] [Google Scholar]
- 19.Kamaya A, Tuthill T, Rubin JM. Twinkling artifact on color Doppler sonography: Dependence on machine parameters and underlying cause. AJR 2003;180:215–222 [DOI] [PubMed] [Google Scholar]
- 20.Gao J, Hentel K, Rubin JM. Correlation between twinkling artifact and color Doppler carrier frequency: Preliminary observations in renal calculi. Ultrasound Med Biol 2012;38:1534–1539 [DOI] [PubMed] [Google Scholar]
- 21.Shabana W, Bude RO, Rubin JM. Comparison between color Doppler twinkling artifact and acoustic shadowing for renal calculus detection: An in vitro study. Ultrasound Med Biol 2009;35:335–350 [DOI] [PubMed] [Google Scholar]
- 22.Par G, Yeo S, Lee JJ, et al. New Adaptive clutter rejection based on spectral analysis for ultrasound color Doppler imaging: Phantom and in vivo abdominal study. IEEE Trans Biomed Eng 2014;61:55–63 [DOI] [PubMed] [Google Scholar]
- 23.Kruse DE, Ferrarra KW. A new high resolution color flow system using an eigendecomposition-based adaptive filter for clutter rejection. IEEE Trans Ultrason Ferroelectr Freq Control 2002;49:1739–1754 [DOI] [PubMed] [Google Scholar]
- 24.Bjaerum S, Torp H, Kristoffersen K. Clutter filters adapted to tissue motion in ultrasound color flow imaging. IEEE Trans Ultrason Ferroelectr Freq Control 2002;49:693–704 [DOI] [PubMed] [Google Scholar]
- 25.Torp H. Clutter rejection filters in color flow imaging: A theoretical approach. IEEE Trans Ultrason Ferroelectr Freq Control 1997;44:417–424 [DOI] [PubMed] [Google Scholar]
- 26.May PC, Haider Y, Dunmire B, et al. Stone-mode ultrasound for determining renal stone size. J Endourology 2016;30:958–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W, Sapozhnikov OA, Bailey MR, Kaczkowski PJ, Crum LA. Evidence for trapped surface bubbles as the cause for the twinkling artifact in ultrasound imaging. Ultrasound Med Biol 2013;39:1026–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunitz B, Dunmire B, Paun M, et al. Improved detection of kidney stones using an optimized Doppler imaging sequence. IEEE Int Ultrason Symp 2014;2014:452–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bercoff J, Montaldo G, Loupas T, Savery D, Mézière F, Fink M, Tanter M. Ultrafast compound Doppler imaging: Providing full blood flow characterization. IEEE Trans Ultrason Ferroelectr Freq Control 2011;58:134–147 [DOI] [PubMed] [Google Scholar]
- 30.Shivaprasad V, Govardhanan S, Reddy VV, Katankot NE, Raju V, Basha SU. Doppler parameters in elicitation of twinkling artefacts in suspected cases of renal calculi. J Evid Based Med Healthc 2016;3:2958–2961 [Google Scholar]