Abstract
Aims: The manner in which hydrogen sulfide (H2S) suppresses neuroinflammation is poorly understood. We investigated whether H2S polarized microglia to an anti-inflammatory (M2) phenotype by activating AMP-activated protein kinase (AMPK). Results: Three structurally unrelated H2S donors (5-(4-hydroxyphenyl)-3H-1,2-dithiocyclopentene-3-thione [ADT-OH], (p-methoxyphenyl) morpholino-phosphinodithioic acid [GYY4137], and sodium hydrosulfide [NaHS]) enhanced AMPK activation in BV2 microglial cells in the presence and absence of lipopolysaccharide (LPS). The overexpression of the H2S synthase cystathionine β-synthase (CBS) in BV2 cells enhanced endogenous H2S production and AMPK activation regardless of LPS stimulation. On LPS stimulation, overexpression of both ADT-OH and CBS promoted M2 polarization of BV2 cells, as evidenced by suppressed M1 and elevated M2 signature gene expression. The promoting effects of ADT-OH on M2 polarization were attenuated by an AMPK inhibitor or AMPK knockdown. Liver kinase B1 (LKB1) and calmodulin-dependent protein kinase kinase β (CaMKKβ) are upstream kinases that activate AMPK. ADT-OH activated AMPK in Hela cells lacking LKB1. In contrast, both the CaMKKβ inhibitor and siRNA abolished ADT-OH activation of AMPK in LPS-stimulated BV2 cells. Moreover, the CaMKKβ inhibitor and siRNA blunted ADT-OH suppression on M1 gene expression and enhancement of M2 gene expression in LPS-stimulated BV2 cells. Moreover, ADT-OH promoted M2 polarization of primary microglia in an AMPK activation- and CaMKKβ-dependent manner. Finally, in an LPS-induced in vivo neuroinflammation model, both ADT-OH and NaHS enhanced AMPK activation in the brain area where microglia were over-activated on LPS stimulation. Furthermore, ADT-OH suppressed M1 and promoted M2 gene expression in this in vivo model. Innovation and Conclusion: CaMKKβ-dependent AMPK activation is an unrecognized mechanism underlying H2S suppression on neuroinflammation. Antioxid. Redox Signal. 21, 1741–1758.
Introduction
Hydrogen sulfide (H2S), an endogenous gasotransmitter, is increasingly recognized to be actively involved in the pathogenesis of various central nervous system (CNS) diseases (46). H2S is highly produced in the brain, and cystathionine β-synthase (CBS) has been identified as the major H2S synthase that is responsible for the great bulk of H2S production in the brain (1, 32). Furthermore, endogenous H2S as well as exogenous H2S donors have been shown to confer neuroprotection in various neurodegeneration disease models, at least in part, by suppressing neuroinflammation that is mediated by microglia (16, 24, 26). Microglia are resident macrophage lineage cells in the brain. Similar to macrophages, microglia are highly plastic cells, assuming diverse functional phenotypes in response to specific stimulations (8, 29, 35). “Classic M1 polarization” of microglia/macrophages is characterized by pronounced production of pro-inflammatory mediators. In contrast, alternatively polarized M2 microglia/macrophages limit inflammation and are typically characterized by pronounced production of anti-inflammatory mediators. Functionally, M1 microglia/macrophages exacerbate neuronal damage and impede neural repair after CNS injury, whereas M2 microglia/macrophages confer neuroprotection and promote CNS repair and remodeling in several CNS injury models (8, 20, 35).
Innovation.
The mechanisms underlying hydrogen sulfide (H2S) suppression on inflammation are poorly understood. This study provides new evidence that calmodulin-dependent protein kinase kinase β (CaMKKβ)-dependent activation of AMP-activated protein kinase (AMPK) is an unrecognized mechanism underlying H2S suppression on neuroinflammation. In contrast to published evidence that CaMKKβ is pro-inflammatory in macrophages, we found that CaMKKβ functionally mediated H2S suppression on neuroinflammation. Our results also convincingly show that H2S donors are a novel class of AMPK activators. Especially, the slow-releasing H2S donor 5-(4-hydroxyphenyl)-3H-1,2-dithiocyclopentene-3-thione (ADT-OH) is a more potent AMPK activator than the clinical drug metformin. As AMPK activators, H2S donors hold promise as novel therapeutic agents for various diseases, including neuroinflammation-related diseases.
H2S is well recognized to suppress microglia-mediated neuroinflammation. However, the underlying mechanisms are poorly understood, although nuclear factor-kappa B (NF-κB) and MAPK signaling cascades have been implicated in H2S inhibition on neuroinflammation (17, 48). One emerging concept regarding inflammation is that metabolic changes not only participate in, but also critically impact functional phenotypes of immune cells (35). Particularly, AMP-activated protein kinase (AMPK), a highly conserved protein kinase that regulates mammalian metabolism, is increasingly recognized as playing a central role in inflammation (35). AMPK activation is emerging as a master molecular switch that promotes M2 polarization of macrophages/microglia, thereby inhibiting inflammation (27, 40). In fact, AMPK activators have been shown to inhibit inflammation in various model systems (4, 22, 33, 34). On the other hand, some of the well-known anti-inflammatory drugs have also been reported to activate AMPK (5, 15).
H2S is involved in metabolism (50). Moreover, we and others report that exogenous H2S donors activate AMPK in some cell types (19, 23, 28). However, whether AMPK activation is an important mechanism underlying H2S suppressive effects on inflammation has not been investigated. We tested the hypothesis in the study. We found that both exogenous H2S donors and endogenously generated H2S activated AMPK in microglia. Furthermore, we demonstrated that calmodulin-dependent protein kinase kinase β (CaMKKβ)-dependent AMPK activation is a currently unrecognized important mechanism underlying H2S suppression on neuroinflammation.
Results
Three structurally unrelated H2S donors activated AMPK in BV2 microglial cells
We used three structurally unrelated H2S donors to comprehensively characterize H2S effects on AMPK activation: sodium hydrosulfide (NaHS), the most widely used inorganic H2S donor; GYY4137, the only water-soluble organic H2S donor identified so far (48); and 5-(4-hydroxyphenyl)-3H-1,2-dithiocyclopentene-3-thione (ADT-OH), the most widely used moiety for synthesizing slow-releasing organic H2S donors (30). Microglia/macrophage-mediated inflammation is characterized by the pronounced production of the pro-inflammatory mediator nitric oxide (NO) (17). To investigate whether H2S donors activate microglial AMPK at the concentrations that suppress neuroinflammation, we first examined the dose-dependent effects of these H2S donors on neuroinflammation by assessing lipopolysaccharide (LPS)-evoked NO production in BV2 cells. NaHS at 50 to 1000 μM (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/ars) and GYY4137 (Supplementary Fig. S1B) at 50 to 500 μM inhibited LPS-evoked NO production in BV2 cells in a concentration-dependent manner at 24 h after LPS stimulation. Both NaHS and GYY4137 exhibited maximum inhibition starting at the concentration of 500 μM. Treatment with ADT-OH (1–100 μM) also resulted in concentration-dependent inhibition on LPS-stimulated NO formation in BV2 microglia, with maximum inhibition observed at 50 μM (Supplementary Fig. S1C). We could not test the inhibitory effects of ADT-OH at the concentrations beyond 100 μM due to the poor solubility of ADT-OH in DMEM media. We also assessed the effects of the H2S donors on cell viability using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays. As indicated by MTT results, all donors did not display obvious cytotoxicity to BV2 cells regardless of LPS treatment at all tested concentrations (Data not shown). Thus, for the next few studies, H2S donors were used at the concentrations at which they displayed maximum inhibition on LPS-evoked NO production; that is, ADT-OH at 50 μM, GYY4137 and NaHS at 500 μM.
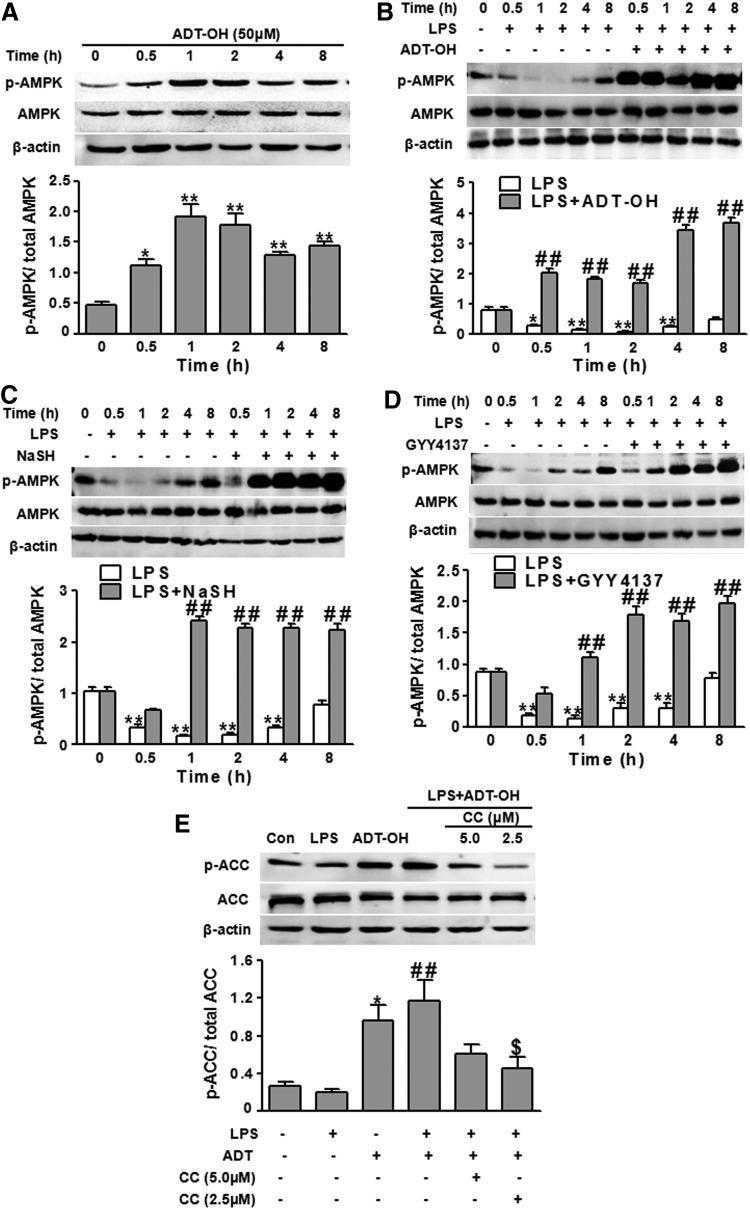
AMPK activation is directly correlated to the phosphorylation state at threonine (Thr)-172 on the AMPK α subunit. To evaluate H2S effects on microglial AMPK activation, we examined whether ADT-OH enhanced AMPK phosphorylation (Thr-172) levels in BV2 cells. In the absence of LPS, ADT-OH elevated AMPK phosphorylation within 30 min, and the elevation lasted at least for approximately 8 h (Fig. 1A). Similarly, GYY4137 and NaHS increased AMPK activation (phosphorylation) in the absence of LPS stimulation (Supplementary Fig. S2). LPS is a potent inducer of M1 polarization of macrophages/microglia. On LPS stimulation, macrophages exhibit a decrease in AMPK activation (40). Consistently, we found that LPS significantly decreased AMPK activation (phosphorylation) in BV2 cells over 4 h after LPS stimulation (Fig. 1B). In the presence of LPS stimulation, ADT-OH remarkably enhanced AMPK activation within 30 min and at least for approximately 8 h after ADT-OH treatment (Fig. 1B). Consistently, BV2 cells treated with NaHS or GYY4137 also displayed increased AMPK phosphorylation in the presence of LPS stimulation compared with control cells treated with vehicle (Fig. 1C, D).
FIG. 1.
H2S donors robustly enhanced AMPK activation in BV2 microglial cells. (A) Western blot analysis of ADT-OH effects on AMPK activation (phosphorylation) in BV2 cells without LPS stimulation at the indicated time points post ADT-OH treatment (n=3). Compared with nontreated control cells, ADT-OH-treated BV2 cells displayed significantly enhanced AMPK activation, as indicated by increased ratios of phosphorylated AMPK (p-AMPK)/AMPK (*p<0.05; **p<0.01). (B–D) Western blot analysis of effects of H2S donors ADT-OH (B), NaHS (C), and GYY4137 (D) on AMPK activation (phosphorylation) in BV2 cells in the presence of LPS stimulation (n=3). Compared with control cells without LPS and H2S donor treatment, LPS-treated cells displayed a significant decrease in AMPK activation for approximately 4 h post LPS stimulation (*p<0.05 or **p<0.01). ADT-OH (B), NaHS (C), and GYY4137 (D) significantly enhanced AMPK phosphorylation in LPS-stimulated BV2 cells. ##p<0.01, compared with cells treated with LPS alone (LPS) at the indicated time points. (E) Western blot analysis of ADT-OH effects on phosphorylation of ACC, a substrate of activated AMPK, in BV2 cells (n=4). Phosphorylation of ACC (p-ACC) was assessed to indicate AMPK activity. In the absence of LPS, compared with vehicle (Con), ADT-OH enhanced the ratios of p-ACC/ACC (*p<0.05). ADT-OH also remarkably enhanced p-ACC/ACC ratios in LPS-stimulated cells. ##p<0.01, compared with cells treated with LPS alone (LPS). ADT-OH enhancement of p-ACC/ACC ratios was significantly attenuated by the AMPK inhibitor compound C (CC) at 2.5 μM. $p<0.05, compared with cells treated with LPS and ADT-OH (LPS+ADT-OH). ACC, acetyl-CoA carboxylase; ADT-OH, 5-(4-hydroxyphenyl)-3H-1,2-dithiocyclopentene-3-thione; AMPK, AMP-activated protein kinase; GYY4137, (p-methoxyphenyl) morpholino-phosphinodithioic acid; H2S, hydrogen sulfide; LPS, lipopolysaccharide; NaHS, sodium hydrosulfide.
Of note, we found that ADT-OH at 50 μM robustly activated AMPK in both the presence and absence of LPS (Fig. 1A, B). In contrast, metformin, a well-established AMPK activator and a clinically used drug for treating diabetes, did not activate AMPK at 500 μM in either the presence or absence of LPS stimulation (Supplementary Fig. S3). Metformin activated AMPK at 1000 μM in the absence of LPS (Supplementary Fig. S3) and the presence of LPS (Manuscript in revision). These results showed that the H2S donor ADT-OH was a more potent AMPK activator than metformin.
To further validate that H2S enhanced AMPK activity, we evaluated ADT-OH effects on phosphorylation (Ser-79) of acetyl-CoA carboxylase (ACC), a well-established substrate of activated AMPK. Regardless of LPS stimulation, ADT-OH significantly increased Ser-79 phosphorylation of ACC (Fig. 1E), indicating that ADT-OH enhanced AMPK activity. More relevantly, ADT-OH-enhanced AMPK activity, as indicated by ACC phosphorylation, was remarkably blunted by compound C, a well-established AMPK inhibitor (Fig. 1E).
CBS overexpression increased AMPK activation in BV2 microglial cells
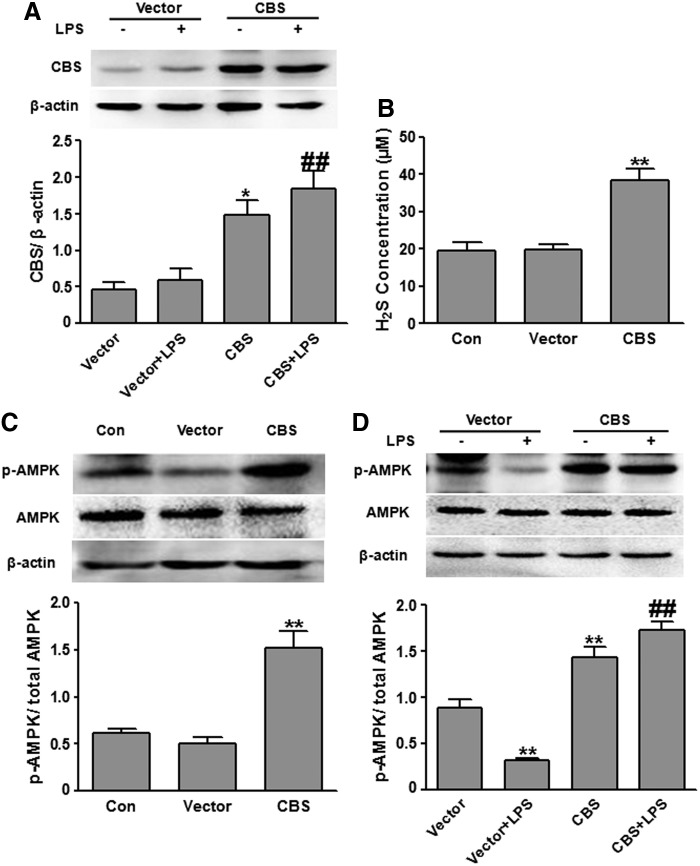
After confirming that exogenously applied H2S donors activated microglial AMPK, we further examined whether increasing endogenous H2S production also enhanced AMPK activation. To this end, we transfected BV2 cells with the plasmids overexpressing CBS, the major H2S synthase present in the brain. Compared with vector transfection, CBS plasmid transfection resulted in significantly enhanced CBS expression in BV2 cells regardless of LPS stimulation (Fig. 2A). Consequently, CBS overexpression markedly elevated H2S concentrations in media of BV2 cell cultures (Fig. 2B). In both the presence and absence of LPS treatment, CBS overexpression significantly increased AMPK activation in BV2 cells compared with vector transfection (Fig. 2C, D). The results suggested that enhancing endogenous H2S generation by CBS overexpression elevated AMPK activation in microglial cells.
FIG. 2.
Overexpressing the H2S synthase CBS-enhanced AMPK activation in BV2 microglial cells. (A) Western blot analysis of CBS in BV2 cells. Regardless of LPS stimulation, CBS protein levels were significantly elevated in BV2 cells that were transfected with the plasmids expressing CBS (n=3). *p<0.05, compared with vector-transfected cells without LPS stimulation (Vector); ##p<0.01, compared with vector-transfected cells with LPS stimulation (vector+LPS). (B) CBS overexpression significantly increased H2S concentration in the media of BV2 cell cultures (n=5). **p<0.01 compared with nontransfected control cells (Con) or cells transfected with the vector plasmids (Vector). (C) CBS overexpression significantly increased AMPK activation in BV2 cells without LPS stimulation (n=3). **p<0.01, compared with control cells (Con) or cells transfected with the vector plasmids (Vector). (D) CBS overexpression significantly increased AMPK activation in BV2 cells in the presence of LPS stimulation (n=3). **p<0.01, compared with vector-transfected cells without LPS stimulation (Vector); ##p<0.01, compared with vector-transfected cells with LPS stimulation (Vector+LPS). CBS, cystathionine β-synthase.
H2S promoted M2 polarization of BV2 microglial cells
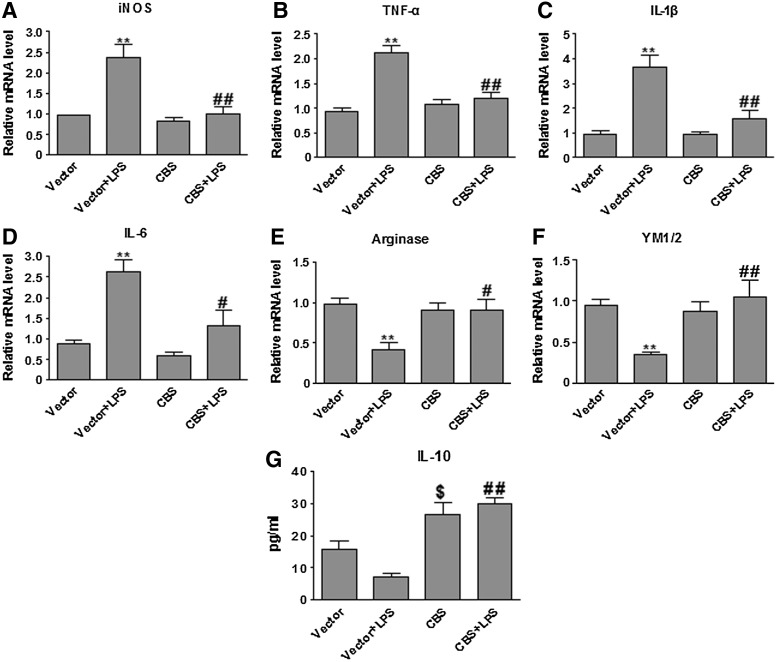
Macrophage/microglia polarization is commonly characterized by the expression of signature genes that are associated with the M1 or M2 phenotype. We overexpressed CBS in BV2 cells to investigate whether enhancing endogenous generation of H2S promoted M2 polarization of BV2 cells on LPS stimulation. As shown in Figure 3, LPS induced M1 polarization of BV2 cells transfected with the vector plasmids, as evidenced by the induction of mRNA expression of various pro-inflammatory M1 genes iNOS, TNFα, IL-1β, and IL-6 (18, 20). After LPS stimulation, compared with vector-transfected cells, BV2 cells overexpressing CBS not only displayed a significant reduction in LPS-evoked expression of M1 genes (iNOS, TNFα, IL-1β, and IL-6), but also exhibited a remarkable elevation in the production of the M2 mediators (arginase 1, YM1/2, and IL-10). These results suggested that enhancing endogenous H2S production suppressed LPS-evoked microglial inflammation by polarizing microglia to the anti-inflammatory (M2) phenotype.
FIG. 3.
Overexpression of the H2S synthase CBS suppressed LPS-evoked M1 signature gene expression, while it increased M2 signature gene expression in LPS-stimulated BV2 cells. (A–D) qPCR measurement of mRNA expression of M1 genes (iNOS, TNF-α, IL-1β, and IL-6) in BV2 cells at 6 h after LPS stimulation. ADT-OH suppressed LPS-evoked iNOS, TNF-α, IL-1β, and IL-6 expression in BV2 cells (n=3). **p<0.01, compared with vector-transfected cells without LPS stimulation (Vector); ##p<0.01, compared with vector-transfected cells with LPS stimulation (Vector+LPS). (E, F) qPCR measurement of mRNA expression of M2 genes arginase 1 and YM1/2 in BV2 cells at 6 h after LPS stimulation. CBS overexpression enhanced mRNA levels of arginase 1 (n=4) and YM1/2 (n=5) in LPS-stimulated BV2 cells. *p<0.05 or **p<0.01, compared with vector-transfected cells without LPS stimulation (Vector); #p<0.05 or ##p<0.01, compared with vector-transfected cells with LPS stimulation (Vector+LPS). (G) ELISA measurement of protein concentrations of the M2 mediator IL-10 in culture media of BV2 cells at 24 h after LPS stimulation. ADT-OH enhanced IL-10 production in LPS-stimulated BV2 cells (n=5). $p<0.05, compared with vector-transfected cells without LPS stimulation (Vector); ##p<0.01, compared with vector-transfected cells with LPS stimulation (Vector+LPS). ELISA, enzyme-linked immunosorbent assay; qPCR, quantitative polymerase chain reaction.
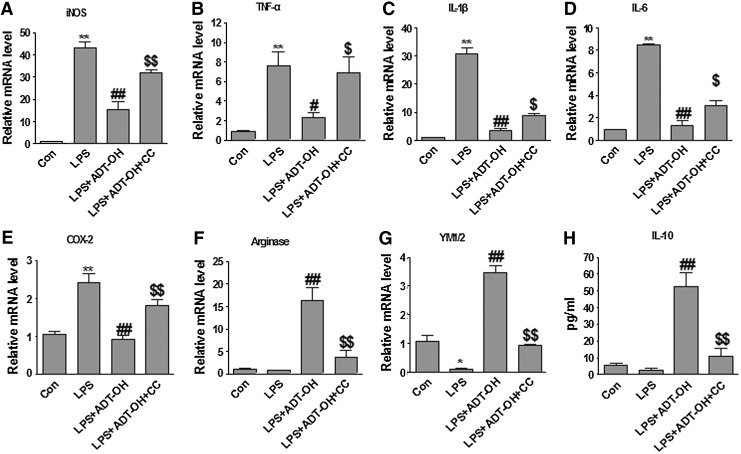
We further investigated whether exogenous H2S donors also promoted M2 polarization of BV2 cells. As shown in Figure 4, LPS induced mRNA expression of various pro-inflammatory M1 genes (iNOS, TNFα, IL-1β, IL-6, and COX-2) in BV2 cells. ADT-OH not only blunted LPS-induced expression of M1 genes (iNOS, TNFα, IL-1β, IL-6, and COX-2), but also significantly increased mRNA expression of the M2 signature genes (arginase 1 and YM1/2) in LPS-treated BV2 cells. Enzyme-linked immunosorbent assay (ELISA) results further revealed that ADT-OH increased protein production of the anti-inflammatory cytokine IL-10 from LPS-stimulated BV2 cells (Fig. 4). To conclude, both exogenous application of H2S donors and enhancement of endogenous H2S production promoted microglial polarization to the M2 anti-inflammatory phenotype.
FIG. 4.
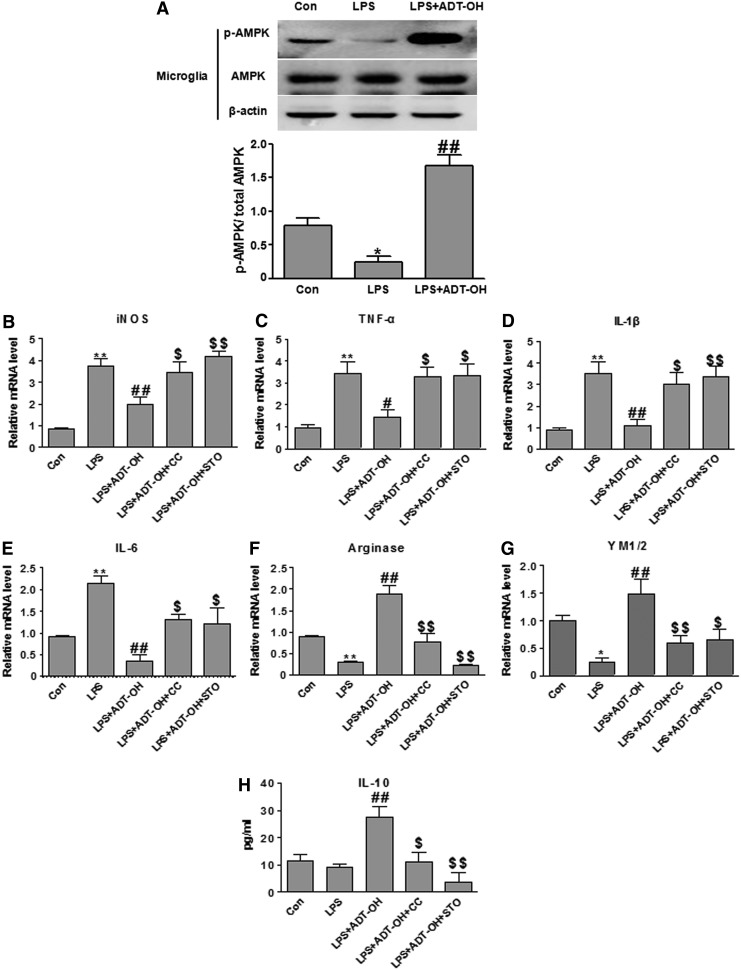
ADT-OH suppressed M1 signature gene expression while it increased M2 signature gene expression in LPS-stimulated BV2 microglial cells, and both effects were attenuated by the AMPK inhibitor CC. (A–E) qPCR measurement of mRNA expression of M1 genes iNOS (n=3), TNF-α (n=6), IL-1β (n=4), IL-6 (n=3), and COX-2 (n=4) in BV2 cells at 6 h after LPS stimulation. ADT-OH suppressed LPS-evoked mRNA expression of iNOS, TNF-α, IL-1β, IL-6, and COX-2 in BV2 cells. ADT-OH suppression of LPS-evoked M1 gene expression was markedly attenuated by the AMPK inhibitor CC. (F, G) qPCR measurement of mRNA expression of the M2 genes arginase 1 and YM1/2 in BV2 cells at 6 h after LPS stimulation (n=3). ADT-OH enhanced mRNA expression of arginase 1 and YM1/2 in BV2 cells in the presence of LPS stimulation, which was markedly attenuated by the AMPK inhibitor CC. (H) ELISA measurement of IL-10 protein levels in media of BV2 cell cultures at 24 h after LPS stimulation (n=7). ADT-OH enhanced IL-10 production in the presence of LPS stimulation. ADT-OH-elevated IL-10 production was markedly attenuated by the AMPK inhibitor CC. *p<0.05 or **p<0.01, compared with control cells treated with vehicle (Con); #p<0.05 or ##p<0.01, compared with cells treated with LPS alone (LPS); and $p<0.05 or $$p<0.01, compared with BV2 cells treated with LPS plus ADT-OH (LPS+ADT-OH).
H2S promoted M2 polarization of BV2 cells in an AMPK activation-dependent manner
To investigate whether H2S promoted M2 polarization of microglia via activating AMPK, we investigated whether AMPK inhibition blocked ADT-OH-induced M2 polarization of BV2 cells. As shown in Figure 4, ADT-OH promoting effects on M2 polarization of BV2 cells were, in part, abolished by the AMPK inhibitor compound C (CC), as evidenced by the fact that CC significantly attenuated ADT-OH suppression on M1 gene expression (iNOS, TNFα, IL-1β, IL-6, and COX-2) and ADT-OH elevation of M2 gene expression (arginase 1, YM1/2, and IL-10) in the LPS-treated BV2 cells (Fig. 4).
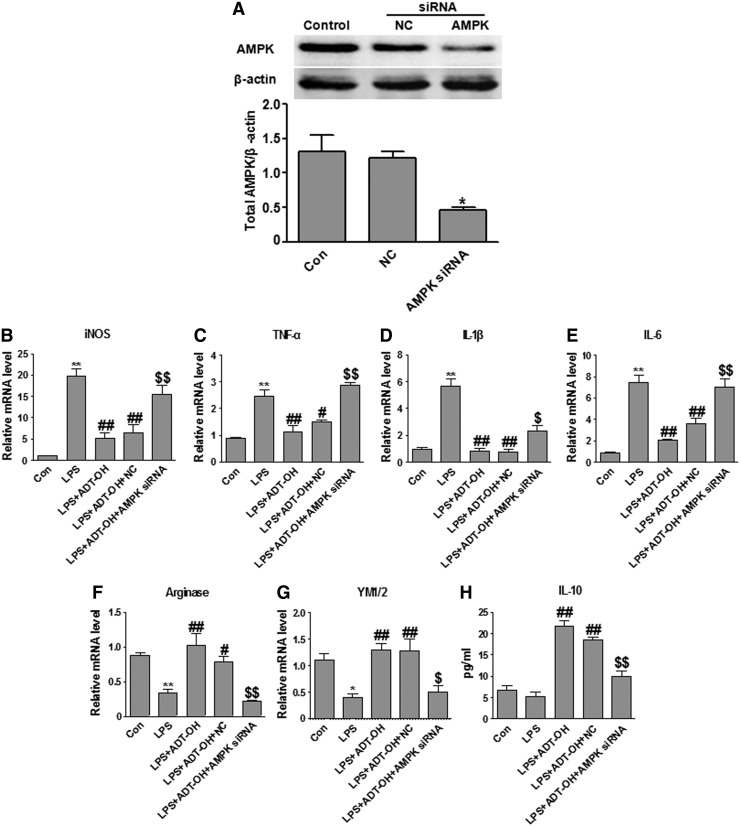
We further used the siRNA approach to investigate whether AMPK knockdown blocked ADT-OH-induced M2 polarization of BV2 cells. AMPK siRNA transfection significantly decreased AMPK expression as compared with nontransfection or nonsense siRNA (NC) transfection (Fig. 5A). Consistent with results obtained using the AMPK inhibitor, AMPK knockdown also attenuated ADT-OH suppressive effects on M1 gene expression and enhancing effects on M2 gene expression (Fig. 5). Collectively, by using both the pharmacologic and siRNA approaches, we showed that AMPK activation was indispensible to H2S promoting effects on M2 polarization of microglia.
FIG. 5.
Knockdown of AMPK by siRNA attenuated ADT-OH promoting effects on M2 polarization of BV2 microglial cells. (A) AMPK siRNA significantly reduced AMPK protein expression in BV2 cells (n=3). * p<0.05, compared with nontransfected control cells (Con) or cells transfected with nonsense siRNA (NC). (B–E) qPCR measurement of mRNA expression of M1 genes iNOS (n=4), TNF-α (n=3), IL-1β (n=4), and IL-6 (n=3) in BV2 cells at 6 h after LPS stimulation. ADT-OH suppression of LPS-evoked M1 gene expression (iNOS, TNF-α, IL-1β, and IL-6) was markedly attenuated by the AMPK siRNA. (F, G) qPCR measurement of mRNA expression of M2 genes arginase 1 (n=5) and YM1/2 (n=4) in BV2 cells at 6 h after LPS stimulation. ADT-OH-enhanced mRNA expression of arginase 1 and YM1/2 was markedly attenuated by the AMPK siRNA. (H) ELISA measurement of IL-10 protein levels in media of BV2 cell cultures at 24 h after LPS stimulation (n=4). ADT-OH elevated IL-10 production was markedly attenuated by the AMPK siRNA. *p<0.05 or **p<0.01, compared with control cells treated with vehicle (Con); #p<0.05 or ##p<0.01, compared with cells treated with LPS alone (LPS); and $p<0.05 or $$p<0.01, compared with nontransfected cells treated with LPS plus ADT-OH (LPS+ADT-OH) or NC-transfected cells treated with LPS plus ADT-OH (LPS+ADT-OH+NC).
CaMKKβ, but not LKB1, was the upstream kinase through which H2S activated AMPK
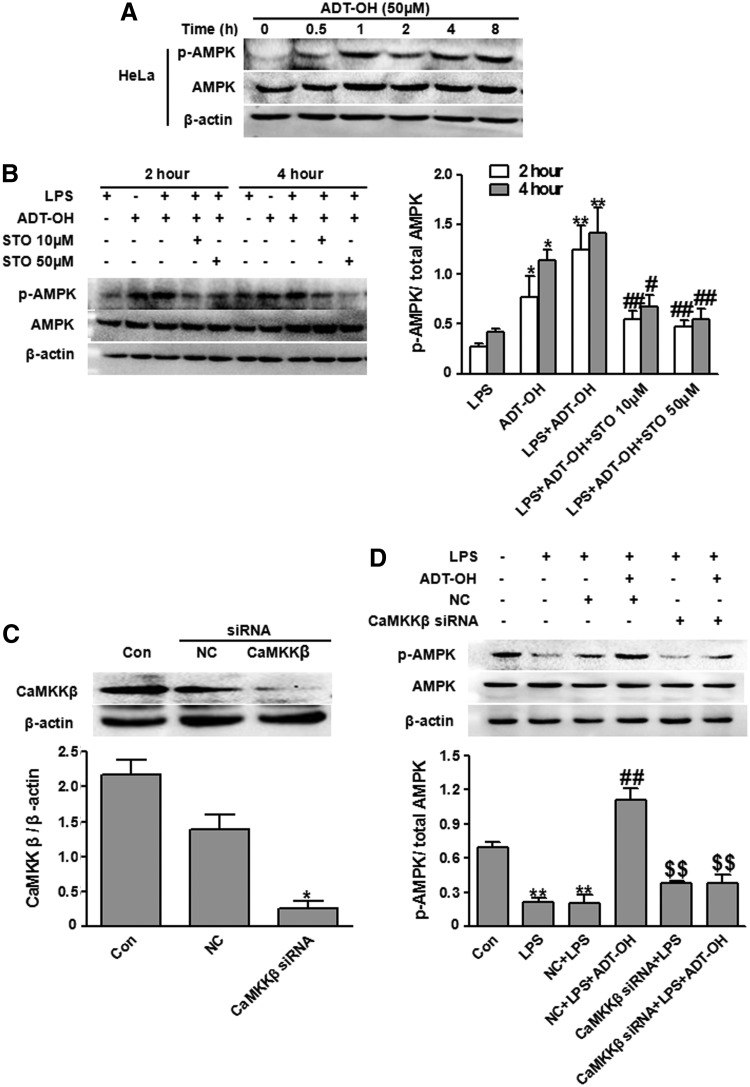
CaMKKβ and liver kinase B1 (LKB1) are two major kinases that phosphorylate and activate AMPK (12, 14, 41). To identify the kinases responsible for ADT-OH activation of AMPK, we examined whether ADT-OH activated AMPK in the cells that do not express LKB1 (14, 45). LKB1 is a tumor suppressive gene product that is down-regulated in several tumor cell lines. Hela is one of the tumor cell lines that does not express LKB1 due to promoter hypermethylation (45). Metformin is an LKB1-dependent AMPK activator. As expected, metformin failed to induce AMPK activation in LKB1-deficient Hela cells at 1000 μM (Supplementary Fig. S4). In contrast, ADT-OH at 50 μM remarkably stimulated AMPK activation (phosphorylation) in Hela cells (Fig. 6A), suggesting that ADT-OH activated AMPK independent of LKB1.
FIG. 6.
ADT-OH activated AMPK in a CaMKKβ-dependent manner. (A) Western blot analysis of ADT-OH effects on AMPK activation (phosphorylation) in Hela cells lacking LKB1. Compared with control cells (Con), ADT-OH-treated Hela cells displayed significantly enhanced AMPK phosphorylation at the indicated time points post ADT-OH treatment. (B) The CaMKKβ inhibitor STO-609 at 10 or 50 μM significantly attenuated ADT-OH-enhanced AMPK activation (phosphorylation) in LPS-stimulated BV2 cells at 2 and 4 h after ADT-OH plus LPS treatment. Left panel: representative images; right panel: the bar graph (n=3). *p<0.05 or **p<0.01, compared with BV2 cells-treated with LPS (LPS); #p<0.05, ##p<0.01, compared with BV2 cells treated with LPS and ADT-OH (LPS+ADT-OH). (C) CaMKKβ siRNA significantly reduced CaMKKβ protein expression in BV2 cells (n=3). *p<0.05, compared with nontransfected control cells (Con) or cells transfected with nonsense siRNA (NC). (D) CaMKKβ siRNA significantly attenuated ADT-OH-enhanced AMPK activation (phosphorylation) in BV2 cells in the presence of LPS stimulation (n=3). **p<0.01, compared with control cells treated with vehicle (Con); ##p<0.01, compared with LPS-treated BV2 cells without transfection (LPS) or transfected with NC (LPS+NC); and $$p<0.01, compared with NC-transfected BV2 cells treated with LPS plus ADT-OH (NC+LPS+ADT-OH). CaMKKβ, calmodulin-dependent protein kinase kinase β; LKB1, liver kinase B1.
Next, we investigated whether CaMKKβ was required for ADT-OH activation of AMPK. We observed that AMPK phosphorylation enhanced by ADT-OH was significantly attenuated by the CaMKKβ inhibitor STO-609 in LPS-treated BV2 cells (Fig. 6B). We further used the siRNA approach to knock down endogenous CaMKKβ expression in BV2 cells (Fig. 6C). Consistent with results obtained using the CaMKKβ inhibitor, CaMKKβ knockdown by siRNA markedly attenuated ADT-OH-enhanced AMPK activation in LPS-treated BV2 cells (Fig. 6D). Collectively, by using both pharmacologic and siRNA approaches, we showed that ADT-OH activated microglial AMPK in a CaMKKβ-dependent manner.
CaMKKβ functionally mediated ADT-OH promoting effects on M2 polarization of microglia
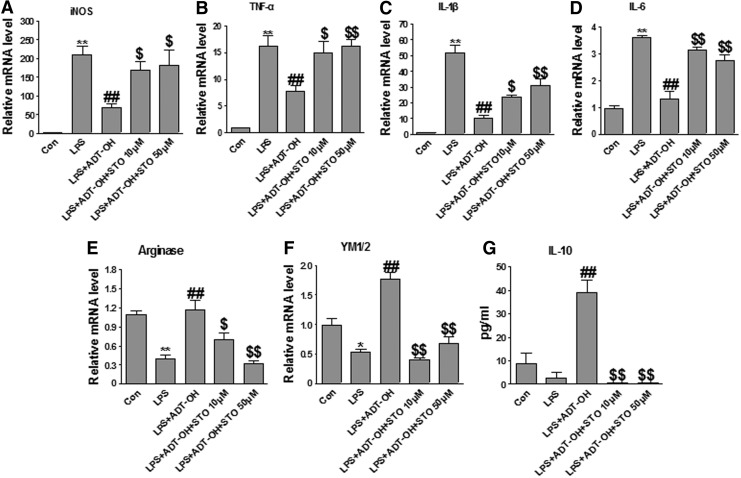
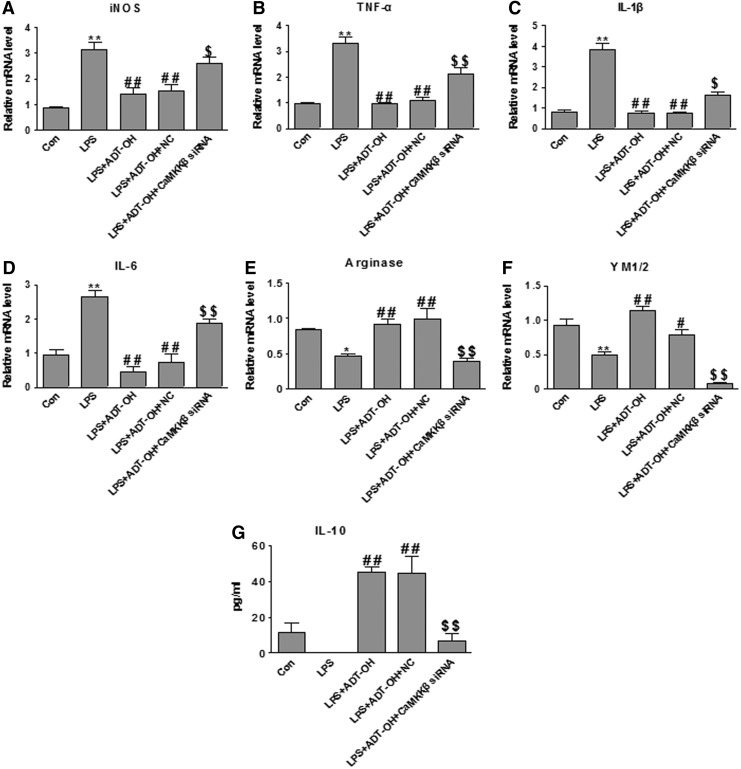
We further investigated whether CaMKKβ was functionally required for ADT-OH promoting effects on M2 polarization of microglia. As shown in Figure 7, the CaMKKβ inhibitor STO-609 significantly attenuated ADT-OH suppressive effects on LPS-evoked expression of M1 gene expression (iNOS, TNF-a, IL-1β, and IL-6) and ADT-OH enhancing effects on M2 gene expression (arginase 1, YM1/2, and IL-10) in LPS-treated BV2 cells. Consistently, CaMKKβ siRNA also displayed similar effects on M1 and M2 gene expression in ADT-OH-treated BV2 cells after LPS stimulation (Fig. 8). Collectively, our results revealed that CaMKKβ was not only required for ADT-OH activation of AMPK, but also functionally mediated the promoting effects of ADT-OH on M2 polarization of microglia.
FIG. 7.
The CaMKKβ inhibitor STO-609 significantly attenuated ADT-OH-promoted M2 polarization of BV2 microglial cells. (A–D) qPCR measurement of mRNA expression of M1 genes iNOS (n=5), TNF-α (n=4), IL-1β (n=3), and IL-6 (n=6) in BV2 cells at 6 h after LPS stimulation. ADT-OH suppression of LPS-evoked M1 gene expression (iNOS, TNF-α, IL-1β, and IL-6) was markedly attenuated by STO-609 at 10 or 50 μM. (E, F) qPCR measurement of mRNA expression of the M2 genes arginase 1 (n=4) and YM1/2 (n=3) in BV2 cells at 6 h after LPS stimulation. ADT-OH-elevated expression of arginase 1 and YM1/2 was markedly attenuated by STO-609 at both concentrations. (G) ELISA measurement of protein levels of the M2 mediator IL-10 in media of BV2 cell cultures at 24 h after LPS stimulation. ADT-OH-elevated IL-10 production was markedly attenuated by STO-609 at both concentrations (n=3). *p<0.05 or **p<0.01, compared with control cells treated with vehicle (Con); ##p<0.01, compared with cells treated with LPS alone (LPS); and $p<0.05 or $$p<0.01, compared with BV2 cells treated with LPS plus ADT-OH (LPS+ADT-OH).
FIG. 8.
CaMKKβ siRNA significantly attenuated ADT-OH-promoted M2 polarization of BV2 microglial cells. (A–D) qPCR measurement of mRNA expression of M1 genes iNOS (n=6), TNF-α (n=4), IL-1β (n=5), and IL-6 (n=3) in BV2 cells at 6 h after LPS stimulation. ADT-OH suppression of LPS-evoked M1 gene expression (iNOS, TNF-α, IL-1β, and IL-6) was markedly attenuated by CaMKKβ siRNA. (E, F) qPCR measurement of mRNA expression of M2 genes arginase 1 (n=5) and YM1/2 (n=4) in BV2 cells at 6 h after LPS stimulation. ADT-OH-enhanced mRNA expression of arginase 1 and YM1/2 was markedly attenuated by CaMKKβ siRNA in LPS-stimulated BV2 cells. (G) ELISA measurement of IL-10 protein levels in media of BV2 cell cultures at 24 h after LPS stimulation. ADT-OH-elevated IL-10 production was markedly attenuated by CaMKKβ siRNA (n=4). *p<0.05 or **p<0.01, compared with control cells treated with vehicle (Con); #p<0.05 or ##p<0.01, compared with BV2 cells treated with LPS alone (LPS); and $p<0.05 or $$p<0.01, compared with nontransfected BV2 cells treated with LPS plus ADT-OH (LPS+ADT-OH) or NC-transfected cells treated with LPS plus ADT-OH (NC+LPS+ADT-OH).
ADT-OH promoted M2 polarization of primary microglia in an AMPK activation- and CaMKKβ-dependent manner
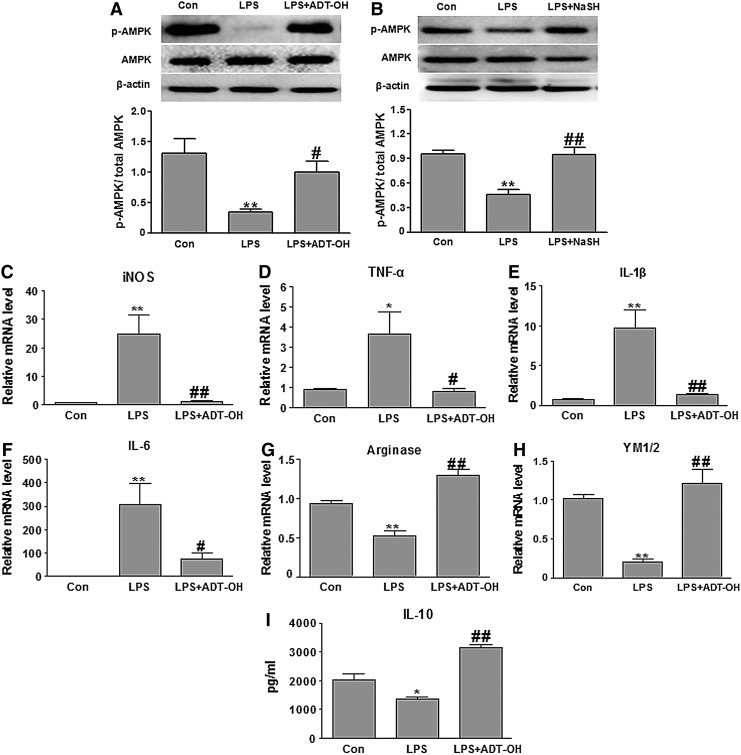
To validate the results obtained from the BV2 cell line, we further investigated whether the H2S donor ADT-OH promoted M2 polarization of primary microglia in an AMPK activation- and CaMKKβ-dependent manner. In the presence of LPS, ADT-OH significantly enhanced AMPK activation (phosphorylation) in primarily cultured murine microglia (Fig. 9A). Moreover, in primary microglia, ADT-OH remarkably attenuated LPS-induced expression of M1 signature genes (iNOS, TNFα, IL-1β, and IL-6) but enhanced M2 signature gene expression (arginase 1, YM1/2, and IL-10) after LPS stimulation. Both effects of ADT-OH, that is, attenuation of M1 gene expression and enhancement of M2 gene expression in LPS-stimulated primary microglia, were remarkably attenuated by the AMPK inhibitor CC and the CaMKKβ inhibitor STO-609 (Fig. 9). In conclusion, ADT-OH promoted M2 polarization of primary microglia, which also depended on AMPK activation and CaMKKβ.
FIG. 9.
ADT-OH activated AMPK and promoted M2 polarization of primarily cultured murine microglia. (A) Western blot analysis of the effects of ADT-OH on AMPK activation (phosphorylation) in primary microglia at 4 h after LPS stimulation (n=4). Compared with control cells without LPS and ADT-OH treatment, LPS-treated cells displayed a significant decrease in AMPK activation. ADT-OH significantly enhanced AMPK phosphorylation in LPS-stimulated primary microglia. *p<0.05, compared with control cells treated with vehicle (Con); ##p<0.01, compared with cells treated with LPS alone (LPS). (B–E) qPCR measurement of mRNA expression of M1 genes iNOS, TNF-α, IL-1β, and IL-6 in primary microglia at 6 h after LPS stimulation (n=4 for each gene). ADT-OH suppressed LPS-evoked mRNA expression of iNOS, TNF-α, IL-1β, and IL-6 in primary microglia. ADT-OH suppression of LPS-evoked M1 gene expression was markedly attenuated by the AMPK inhibitor CC and the CaMKKβ inhibitor STO-609. (F, G) qPCR measurement of mRNA expression of the M2 genes arginase 1 and YM1/2 in primary microglia at 6 h after LPS stimulation (n=5 for each gene). ADT-OH enhanced mRNA expression of arginase 1 and YM1/2 in primary microglia in the presence of LPS stimulation, which was markedly attenuated by the AMPK inhibitor CC and the CaMKKβ inhibitor STO-609. (H) ELISA measurement of IL-10 protein levels in media of primary microglia at 24 h after LPS stimulation (n=4). ADT-OH enhanced IL-10 production in the presence of LPS stimulation. ADT-OH elevated IL-10 production was markedly attenuated by the AMPK inhibitor CC and the CaMKKβ inhibitor STO-609 (CC). **p<0.01 or *p<0.05 compared with control cells treated with vehicle (Con); ##p<0.01 or #p<0.05, compared with cells treated with LPS alone (LPS); and $p<0.05 or $$p<0.01, compared with cells treated with LPS plus ADT-OH (LPS+ADT-OH).
ADT-OH and NaHS activated AMPK and suppressed neuroinflammation in vivo
To investigate whether the earlier in vitro findings could be translated into an in vivo neuroinflammation model, LPS was injected via the intracerebroventricular (ICV) route to induce microglial activation and neuroinflammation in the brain lateral septal complex area (13). It is reported that an intraperitoneal injection of an ADT-OH-derived H2S donor increases plasma and brain sulfide levels (31). Moreover, an intraperitoneal injection of NaHS at the dose of 5 mg/kg reduces proinflammation gene (M1) expression in the brains of rats receiving an ICV injection of LPS (10). However, it is not clear whether NaHS or ADT-OH enhances AMPK activation in the brain area where microglia are over-activated after an ICV injection of LPS (13). We administered ADT-OH or NaHS intraperitoneally 30 min before LPS ICV injection. Consistent with in vitro results, AMPK phosphorylation (activation) in the lateral septal complex area was remarkably decreased by an LPS ICV injection compared with a vehicle injection (Con). Both ADT-OH and NaHS significantly increased AMPK activation in this area at 4 h after an ICV injection of LPS (Fig. 10A, B). Furthermore, we used immunohistochemistry to examine the co-localization of phosphorylated AMPK (p-AMPK) and the microglia/macrophage marker Iba1. In the lateral septal complex area of ADT-OH plus LPS-treated mice, cells positive for Iba1 were also co-stained with p-AMPK at 4 h after an LPS injection, although a lot of non-Iba1+ cells (probably neurons) also expressed p-AMPK (Supplementary Fig. S5).
FIG. 10.
H2S donors ADT-OH and NaHS activated AMPK and suppressed M1 polarization in an in vivo microglia-mediated neuroinflammation model. (A, B) Western blotting of p-AMPK and total AMPK expression levels in the lateral septal complex area at 4 h after an ICV injection of LPS. Compared with control mice receiving an ICV injection of saline (Con), AMPK activation (phosphorylation) was markedly reduced in the lateral septal complex area of LPS-injected mice (LPS). ADT-OH (A) or NaHS (B) significantly enhanced AMPK activation in the lateral septal complex area of LPS-injected mice (n=6). **p<0.01, compared with control mice; #p<0.05 or ##p<0.01, compared with LPS-injected mice (LPS). (C–F) qPCR measurement of mRNA expression of M1 genes (iNOS, TNF-α, IL-1β, and IL-6) in the lateral septal complex area at 6 h after LPS injection. ADT-OH suppressed LPS-evoked mRNA expression of iNOS (n=6), TNF-α (n=8), IL-1β (n=7), and IL-6 (n=6) in the lateral septal complex area of LPS-injected mice. (G, H) qPCR measurement of mRNA expression of the M2 gene arginase 1 (n=6) and YM1/2 (n=7) in the lateral septal complex area at 6 h after LPS injection. ADT-OH enhanced arginase 1 and YM1/2 mRNA in the lateral septal complex area of LPS-injected mice. (I) ELISA measurement of IL-10 protein levels in the lateral septal complex area at 12 h after LPS injection (n=7). ADT-OH enhanced IL-10 production in the LPS-induced mice. *p<0.05 or **p<0.01, compared with control mice (Con); #p<0.05 or ##p<0 .01, compared with LPS-injected mice (LPS). ICV, intracerebroventricular.
Moreover, in the lateral septal complex area, LPS-induced expression of M1 signature genes (iNOS, IL-1β, TNFα, and IL-6) was attenuated by ADT-OH (Fig. 10). On the other hand, ADT-OH also enhanced expression of the M2 signature genes (arginase 1, YM1/2, and IL-10) in the LPS-stimulated lateral septal complex area (Fig. 10). Taken together, these results suggested H2S-enhanced AMPK activation and suppressed neuroinflammation in vivo.
Discussion
This study presented three major findings. First, H2S inhibited microglia-mediated neuroinflammation by acting through AMPK activation to polarize microglia to the M2 anti-inflammatory phenotype. Second, CaMKKβ, but not LKB1, was the upstream kinase through which H2S activated AMPK. Furthermore, CaMKKβ functionally mediated H2S suppressive effects on neuroinflammation. Third, H2S donors were a novel class of AMPK activators. Particularly, ADT-OH, the most widely used moiety for synthesizing slow-releasing H2S donors, was a more potent AMPK activator than metformin. Since AMPK activation has been documented as a therapeutic intervention for several diseases (9, 39), H2S donors hold promise for a wide clinical application.
Microglia-mediated neuroinflammation is involved in multiple neurological diseases (7, 8). Similar to macrophages, microglia are highly plastic and assume various functional phenotypes with M1 and M2 polarization as two extremes (8, 18, 20). Although H2S donors inhibit neuroinflammation in both primarily cultured microglia and immortalized microglial cell lines (16, 17, 24), their underlying mechanisms are poorly understood. Currently, NF-κB and MAPK cascades are implicated to mediate H2S suppressive effects on inflammation (17, 48). AMPK activation is a master molecular switch that promotes M2 polarization of macrophages/microglia (35, 40). We and others report that H2S donors activate AMPK in some cell types (19, 23, 28). However, whether H2S acts through AMPK activation to polarize microglia to the M2 phenotype remains unclear.
This study showed that exogenous H2S donors or enhancing endogenous H2S production elevated AMPK activation in microglia. At the concentrations that inhibited microglial inflammation, three structurally unrelated H2S donors activated AMPK in BV2 microglial cells in both the presence and absence of LPS, suggesting that H2S activation of AMPK was donor structure independent. We further validated that the H2S donor ADT-OH enhanced AMPK activation in LPS-stimulated primary microglia. CBS and cystathionine γ-lyase (CSE) are two major synthases that are responsible for endogenous H2S production. In addition, 3-mercaptopyruvate sulfurtransferase (3-MST), a kinase mainly expressed in neurons and vascular endothelium, produces H2S from 3-mercaptopyruvate that is converted from cysteine and α-ketoglutarate by cysteine aminotransferase (CAT) (42). Thus, 3-MST/CAT serves as a new pathway for H2S generation in the brain. Among three H2S synthases, CBS has been suggested to be a functional subtype in microglia (17). We overexpressed CBS in BV2 microglial cells, and observed that CBS overexpression enhanced endogenous H2S production and AMPK activation with or without LPS stimulation. In conclusion, H2S activated AMPK in microglia.
Consistent with the previous publication (40), we observed that LPS, as a potent M1 inducer, decreased AMPK activation in microglia. H2S donors robustly increased baseline AMPK activation in the absence of LPS. Remarkably, in the presence of LPS, H2S donors not only blocked LPS-induced reduction in AMPK activation, but also elevated AMPK activation to the levels beyond baseline AMPK activation. These results suggested that H2S donors exerted robust effects on AMPK activation even in the presence of LPS stimulation.
We further showed that H2S promoted microglial polarization to the M2 anti-inflammatory phenotype. M1 and M2 polarization of macrophages/microglia is characterized by the expression of M1 and M2 signature genes (10, 26). ADT-OH suppressed LPS-evoked M1 gene expression, while it enhanced M2 gene expression in the BV2 microglial cell line as well as in primary microglia. Moreover, enhancing endogenous H2S production by overexpressing CBS in BV2 cells also promoted M2 polarization of BV2 cells. Finally, in the in vivo microglia-mediated neuroinflammation model, ADT-OH inhibited LPS-evoked M1 signature gene expression but increased M2 gene expression. Taken together, H2S suppressed microglia-mediated neuroinflammation by polarizing microglia to the M2 phenotype.
We further investigated the mechanisms underlying H2S-induced M2 polarization of microglia. ADT-OH suppressive effects on LPS-evoked M1 gene expression and enhancing effects on M2 gene expression were blunted by the AMPK inhibitor or siRNA in LPS-stimulated BV2 cells or primary microglia. Furthermore, in the in vivo microglia-mediated neuroinflammation model (13), ADT-OH enhanced AMPK activation, suppressed LPS-evoked M1 gene expression, and increased M2 gene expression. Collectively, the results supported the fact that H2S acted through AMPK activation to promote M2 polarization of microglia in vitro and probably also in vivo.
AMPK is activated through multiple mechanisms. For instance, metformin acts through respiratory chain inhibition and indirectly activates AMPK, and LKB1 is indispensable for metformin activation of AMPK (36, 41). The AMPK activator AICAR riboside is metabolized to a ribotide (ZMP) that functions as an AMP mimic to activate AMPK (6). A novel class of AMPK activators directly binds to and activates AMPK (11). In addition to LKB1, CaMKKβ is another upstream kinase that activates AMPK (12). ADT-OH activated AMPK in Hela cells lacking LKB1, suggesting LKB1 was not the upstream kinase through which H2S activated AMPK. In contrast, CaMKKβ was indispensable for ADT-OH activation of AMPK, as both the CaMKKβ inhibitor and siRNA blocked ADT-OH activation of AMPK. Of note, the role of CaMKKβ in macrophage-mediated inflammation currently remains elusive. It is assumed that CaMKKβ, as an AMPK-activating kinase, may exert anti-inflammatory effects (37). However, CaMKKβ null macrophages display reduced inflammatory responses in response to LPS stimulation (38), suggesting that CaMKKβ promotes inflammation in macrophages. In contrast, we observed that CaMKKβ was not only required for ADT-OH activation of AMPK, but also functionally mediated the suppressive effects of ADT-OH on microglial inflammation. The discrepancy about the role of CaMKKβ in macrophage/microglia-mediated inflammation deserves further investigation.
H2S is an endogenous gasotransmitter. H2S donors exert robust therapeutic effects in a wide variety of disease models, including the models of inflammation, myocardial ischemia-reperfusion, lethal hemorrhage, and neurodegeneration diseases (44). In addition, it is generally accepted that H2S donors are safer than the donors releasing another important gasotransmitter NO. NO donors have been clinically used. However, NO donors can be very toxic due to its reactivity with superoxide anion (2). The toxicity of NO is clinically evidenced by tachyphylaxie reactions that are associated with patients receiving long-tem treatment of NO donors. In contrast, H2S inactivates superoxide anion (2). Due to the therapeutic effects and high safety profile, we expect that H2S donors can be developed into novel therapeutic agents.
In this study, we found that ADT-OH was a more potent AMPK activator than metformin, the clinically used AMPK activator. AMPK activation is a therapeutic intervention for multiple diseases, including diabetes, cancer, myocardial ischemia-reperfusion injury, and neuroinflammation-associated diseases (39). Thus, H2S donors can be exploited as novel therapeutic agents to treat these diseases. Interestingly, H2S used to be viewed as a deleterious mediator in diabetes mellitus, as it impairs glucose metabolism and inhibits insulin release. However, some recent studies challenged this traditional view. For instance, NaHS exerts insulin-sensitizing effects and alleviates insulin resistance (50). Moreover, metformin, the well-established AMPK activator and a favored first-line anti-diabetes drug, increases H2S tissue concentrations in various organs (49). These published results as well as our finding presented in this study suggest that H2S donors can be exploited to treat diabetes.
Interestingly, some anti-inflammation drugs, such as salicylate, are reported to exert AMPK-activating effects. However, salicylate is a much less potent AMPK activator than ADT-OH (10 mM vs. 50 μM) (15). Thus, whether salicylate activates AMPK under currently used pharmacological doses is questionable. Moreover, currently only the drugs that act on mitochondrial inhibition to increase AMP/ATP ratios in the process of AMPK activation are in clinical use. In contrast, the chemicals that directly activate AMPK or indirectly activate upstream kinases other than LKB1 have not been proved in human (51). Our observation suggested a CaMKKβ-dependent mechanism for AMPK activation by H2S. Whether H2S activation of AMPK is also dependent on the increase in AMP/ATP ratios or CaMKKβ activation alone is sufficient for H2S to activate AMPK remains to be investigated. Nevertheless, our results clearly showed that, unlike metformin, H2S donors acted through the upstream kinase CaMKKβ rather than LKB1 to activate AMPK. Thus, H2S donors can be exploited as a novel class of therapeutic AMPK activators that act through an alternative upstream kinase to activate AMPK.
Experimental Procedures
BV-2 cell cultures
BV2 is an immortalized mouse microglial cell line. BV2 cells were cultured in DMEM containing 10% fetal bovine serum (FBS) at 37°C in an atmosphere of 95% oxygen and 5% CO2, as previously described (17). Confluent cells were trypsinized and passaged twice a week. For experiments, confluent cells cultured in 100-mm dishes were trypsinized and seeded onto 96-well plates for NO assay, 24-well plates for quantitative polymerase chain reaction (PCR) assay, or 6-well plates for western blot. Cells cultured in dishes were used when they reached 80% confluence.
Primary microglial cultures
Primary microglia were prepared from newborn mice (C57BL/6J) at postnatal day 1–2. Briefly, brain cortices were harvested and minced in Hank's solution. Tissue was dissociated in trypsin-EDTA at 37°C for 4 min. Trypsin was neutralized with DMEM/F12 media that was supplemented with 15% FBS. The cells were then strained through 200 μm mesh filters, plated in a culture flask that was precoated with poly-ornithine, and incubated at 37°C and 5% CO2. Half of the culture media were replaced every 3 days. Once cultures reached lower astrocytic confluence, primary microglia were harvested by mechanical agitation (180 rpm) for 90 min. Isolated microglia were plated in DMEM/F12 that was supplemented with 15% FBS at a desired density.
NO assay
Microglial production of NO was assessed by measuring accumulated nitrite released into culture media. Briefly, BV2 cells were stimulated with LPS (100 ng/ml; Sigma, St Louis, MO). Culture media were harvested at 24 h after stimulation (17). The culture supernatant (50 μl) was mixed with 50 μl of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloridein, and 2.5% H3PO4) in 96-well plates. The absorbance was measured at 540 nm with a microplate reader (Infinite M200 PRO; Tecan, Männedorf, Switzerland). Nitrite concentrations were calculated from a standard curve. Cell viabilities were measured at 24 h after LPS treatment using MTT assay as previously described (21).
Quantitative PCR
Total RNA was isolated from BV2 cells, primary microglia, or brain tissue using Trizol reagent (Invitrogen, Camarillo, CA). cDNA was reverse transcribed from 1 μg total RNA using a high-capacity cDNA synthesis kit (Applied Biosystem, Foster City, CA). Quantitative PCR (qPCR) reactions were performed using a real-time SYBR technique and an ABI Prism 7000 DNA Detection System. qPCR primers were as follows: mouse iNOS (forward: 5′-CAGGAGGAGAGAGATCCGATTTA-3′, reverse: 5′-GCATTAGCATGGAAGCAAAGA-3′), mouse TNF-α (forward: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′, reverse: 5′-TGGGAGTAGACAAGGTACAACCC-3′), mouse IL-1β (forward: 5′-TGGAAAAGCGGTTTGTCTTC-3′, reverse: 5′-TACCAGTTGGGGAACTCTGC-3′), mouse IL-6 (forward: 5′-GAGGATACCACTCCCAACAGACC-3′, reverse: 5′-AAGTGCATCATCGTTGTTCATACA-3′), mouse COX-2 (forward: 5′-CGAGTCGTTCTGCCAATA-3′, reverse: 5′-CTGGTCGGTTTGATGCTA-3′); mouse arginase 1 (forward: 5′-GAACACGGCAGTGGCTTTAAC-3′, reverse: 5′-TGCTTAGCTCTGTCTGCTTTGC-3′), mouse YM1/2 (forward: 5′-CAGGGTAATGAGTGGGTTGG-3′, reverse: 5′-CACGGCACCTCCTAAATTGT-3′), 18S RNA (forward: 5′-GTAACCCGTTGAACCCCATT-3′, reverse: 5′-CCATCCAATCGGTAGTAGCG-3′). For each sample, 18S RNA was also assayed as an internal control. Final results were normalized to and expressed as ratios of the target gene/18S.
Western blot
BV2 cells, primary microglia, or brain tissue samples were lysed using RIPA lysis buffer (Beyotime, Shanghai, China). Homogenates were centrifuged at 13,200 rpm for 20 min. Supernatants were collected, and protein concentrations were measured with a commercial BCA kit (Thermo Scientific Pierce, Rockford, IL). The extracted proteins (30 μg) were denatured, separated on 10% SDS-PAGE gels, and then transferred to PVDF membranes. Membranes were blocked with 5% nonfat milk and probed with the following primary antibodies that were purchased from Cell Signaling (Beverly, MA): AMPK (1:1000), p-AMPK (1:1000), ACC (1:1000), phosphorylated-ACC (p-ACC, 1:1000), and CaMMKβ (1:300). After overnight incubation at 4°C, membranes were washed and probed with appropriate secondary antibodies (Cell Signaling) for 2 h at room temperature. β-actin was measured as a loading control using antibodies against β-actin (1:1000; Beyotime, Shanghai, China). Protein bands were visualized with a Kodak imaging system using a SuperSignal West Pico chemiluminescence kit (Pierce, Rockford, IL). The final results were expressed as the ratios of phosphorylated proteins to total proteins or normalized to β-actin.
Measurement of IL-10 with ELISA
Media were collected from cell cultures at 24 h after treatment with LPS (100 ng/ml) and/or ADT-OH. Tissue samples were lysed with RIPA lysis buffer. IL-10 was measured using a commercial ELISA kit (Boster Company, WuHan, China) as per the manual. Measurement was performed using Tecan Infinite M1000 Pro Reader.
CBS overexpression
The plasmids overexpressing CBS were kindly provided by Hideo Kimura (National Institute of Neuroscience, Japan). BV2 cells were transiently transfected with the plasmids expressing the CBS gene using PEI purchased from Sunma Biotechnology (Xiamen, China) as per the manufacturer's manual. Cells transfected with the vector plasmids served as controls. At 6 h after transfection, BV2 cells were treated with LPS and/or ADT-OH. BV2 cells were harvested at 4 h after LPS treatment, and total proteins were extracted for western blot analysis of CBS, p-AMPK, or total AMPK. For qPCR quantification of M1 or M2 signature genes, BV2 cells were harvested at 6 h after LPS/ADT-OH treatment, and total RNA was isolated. Culture media were collected at 24 h after LPS/ADT-OH treatment for ELISA analysis of IL-10.
Measurement of H2S concentrations in media collected from BV2 cell cultures after CBS transfection
H2S concentrations in media were measured as previously reported (47). Briefly, BV2 cells were cultured in phenol red-free media after transfection. Five hundred microliters of medium was collected at 12 h after transfection and mixed with 250 μl of zinc acetate (1% w/v) in duplicate. Subsequently, NNDPD (20 μM; 133 μl) in 7.2 M HCL was added, followed by the addition of FeCL3 (30 μM; 133 μl in 1.2 M HCL). Next, TCA (10% w/v; 250 μl) was added to precipitate proteins followed by centrifugation at 24,000 g for 5 min at 4°C. The absorbance of supernatants was measured at 670 nm using a 96-well microplate reader (Tecan System, Inc.). H2S concentration for each sample was calculated against a calibration curve made using an NaHS standard. Results were expressed in μM.
Knockdown of AMPK and CaMKKβ in BV2 cells by siRNA
siRNAs against mouse AMPK and CaMKKβ were previously reported (3, 25): AMPK sense strand (5′-GAGAAGCAGAAGCACGACGTT-3′) and CaMKKβ sense strand (5′-CAGGAGAUUGCUAUCCUCAAATT-3′). The siRNA duplexes were synthesized by Genepharm (Suzhou, China). BV2 cells seeded on 24-well or 6-well plates were transiently transfected with siRNA duplexes using the siRNA transfection reagent (Engreen Biosystem, Beijing, China) as per the manufacturer's instructions. The cells transfected with nonsense siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) served as controls.
BV2 cells transfected with AMPK siRNA were treated with LPS and/or ADT-OH at 48 h after transfection. BV2 cells transfected with CaMKKβ siRNA were treated with LPS and/or ADT-OH at 24 h after transfection. For western blot analysis of AMPK activation, cells were harvested at 4 h after LPS treatment, and total proteins were extracted. For qPCR quantification of M1 or M2 signature genes, cells were harvested at 6 h after LPS treatment, and total RNA was isolated. In another set of experiments, culture media were collected from BV2 cells at 24 h after LPS/ADT-OH treatment for ELISA measurement of IL-10.
Assessment of AMPK activation and the expression of M1 and M2 signature genes in a mouse neuroinflammation model
An ICV injection of LPS leads to microglial activation and neuroinflammation in the brain lateral septal complex region (13). We investigated whether ADT-OH/NaHS enhanced AMPK activation in the lateral septal complex region in this model. Briefly, CD-1 male mice were intraperitoneally injected with ADT-OH (50 mg/kg) or NaHS (5 mg/kg) (32) or an equal volume of vehicle or saline. At 30 min after ADT-OH/NaHS injection, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in a stereotaxic apparatus. A skull incision was made to expose the bregma, and a small hole was drilled through the skull at the location of 1.0 mm anterior to the bregma, 2.0 mm lateral to the midline, and 2 mm below the pia. LPS (1.0 mg/kg) or saline was administered at the speed of 0.2 ml/min and completed within 10 min. Mice were decapitated at 4 h after stereotaxic injection. Brain tissues were harvested from the ipsilateral lateral septal complex region for western blot analysis of AMPK activation, as described earlier.
In another set of experiments, tissue samples were harvested from the lateral septal complex region of ADT-OH-injected or vehicle-treated mice at 6 h after an ICV injection of LPS. Total RNA was isolated using Trizol reagent (Invitrogen, Camarillo, CA). qPCR or ELISA was performed to assess mRNA expression of M1 or M2 signature genes or protein levels of IL-10, as described earlier.
Statistical analysis
Data were presented as mean±SEM. Statistical analysis was performed using a software package (SPSS 17.0). A comparison with three or more groups was performed using one-way analysis of variance models, followed by Tukey–Kramer post-hoc tests. For all analyses, the null hypothesis was rejected at the 0.05 level.
Supplementary Material
Abbreviations Used
- ACC
acetyl-CoA carboxylase
- ADT-OH
5-(4-hydroxyphenyl)-3H-1,2-dithiocyclopentene-3-thione
- AMP
adenosine 5′-monophosphate
- AMPK
AMP-activated protein kinase
- CaMKKβ
calmodulin-dependent protein kinase kinase β
- CBS
cystathionine β-synthase
- CNS
central nervous system
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- GYY4137
(p-methoxyphenyl) morpholino-phosphinodithioic acid
- H2S
hydrogen sulfide
- ICV
intracerebroventricular
- IL-10
interleukin-10
- LKB1
liver kinase B1
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinases
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- NaHS
sodium hydrosulfide
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide
- p-AMPK
phosphorylated AMPK
Acknowledgments
The project was supported by grants from the National Science Foundation of China (81371278, 81171246, 81130023) National Basic Research Plan (2011CB504403), the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (PAPD), the Science & Technology Program from Suzhou City (SYS201317), and funding from BM2013003.
Author Disclosure Statement
A patent application on the therapeutic application of ADT-OH and its derivatives has been filed with the China Intellectual Property Office.
References
- 1.Abe K. and Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou-Mohamed G, Johnson JA, Jin L, El-Remessy AB, Do K, Kaesemeyer WH, Caldwell RB, and Caldwell RW. Roles of superoxide, peroxynitrite, and protein kinase C in the development of tolerance to nitroglycerin. J Pharmacol Exp Ther 308: 289–299, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, and Pende M. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating amp kinase. Cell Metab 5: 476–487, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan Q, Bernstein CN, and Peng Z. Ampk agonist downregulates innate and adaptive immune responses in tnbs-induced murine acute and relapsing colitis. Biochem Pharmacol 80: 1708–1717, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Chi Y, Li K, Yan Q, Koizumi S, Shi L, Takahashi S, Zhu Y, Matsue H, Takeda M, Kitamura M, and Yao J. Nonsteroidal anti-inflammatory drug flufenamic acid is a potent activator of amp-activated protein kinase. J Pharmacol Exp Ther 339: 257–266, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Corton JM, Gillespie JG, Hawley SA, and Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating amp-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Craft JM, Watterson DM, and Van Eldik LJ. Neuroinflammation: a potential therapeutic target. Expert Opin Ther Targets 9: 887–900, 2005 [DOI] [PubMed] [Google Scholar]
- 8.David S. and Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12: 388–399, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Giordanetto F. and Karis D. Direct amp-activated protein kinase activators: a review of evidence from the patent literature. Expert Opin Ther Pat 22: 1467–1477, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Gong QH, Wang Q, Pan LL, Liu XH, Huang H, and Zhu YZ. Hydrogen sulfide attenuates lipopolysaccharide-induced cognitive impairment: a pro-inflammatory pathway in rats. Pharmacol Biochem Behav 96: 52–58, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, and Sakamoto K. Mechanism of action of a-769662, a valuable tool for activation of amp-activated protein kinase. J Biol Chem 282: 32549–32560, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green MF, Anderson KA, and Means AR. Characterization of the camkkbeta-ampk signaling complex. Cell Signal 23: 2005–2012, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grin'kina NM, Karnabi EE, Damania D, Wadgaonkar S, Muslimov IA, and Wadgaonkar R. Sphingosine kinase 1 deficiency exacerbates lps-induced neuroinflammation. PLoS One 7: e36475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, and Hardie DG. Complexes between the lkb1 tumor suppressor, strad alpha/beta and mo25 alpha/beta are upstream kinases in the amp-activated protein kinase cascade. J Biol 2: 28, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR, and Hardie DG. The ancient drug salicylate directly activates amp-activated protein kinase. Science 336: 918–922, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu LF, Lu M, Hon Wong PT, and Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal 15: 405–419, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Hu LF, Wong PT, Moore PK, and Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem 100: 1121–1128, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, and Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43: 3063–3070, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Jia J, Xiao Y, Wang W, Qing L, Xu Y, Song H, Zhen X, Ao G, Alkayed NJ, and Cheng J. Differential mechanisms underlying neuroprotection of hydrogen sulfide donors against oxidative stress. Neurochem Int 62: 1072–1078, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, and Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29: 13435–13444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura Y, Dargusch R, Schubert D, and Kimura H. Hydrogen sulfide protects ht22 neuronal cells from oxidative stress. Antioxid Redox Signal 8: 661–670, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Labuzek K, Liber S, Gabryel B, Adamczyk J, and Okopien B. Metformin increases phagocytosis and acidifies lysosomal/endosomal compartments in ampk-dependent manner in rat primary microglia. Naunyn Schmiedebergs Arch Pharmacol 381: 171–186, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Mariappan MM, Feliers D, Cavaglieri RC, Sataranatarajan K, Abboud HE, Choudhury GG, and Kasinath BS. Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating amp-activated protein kinase in renal epithelial cells. J Biol Chem 287: 4451–4461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M, Sparatore A, Del Soldato P, McGeer E, and McGeer PL. Hydrogen sulfide-releasing nsaids attenuate neuroinflammation induced by microglial and astrocytic activation. Glia 58: 103–113, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Lee SK, Lee JO, Kim JH, Kim N, You GY, Moon JW, Sha J, Kim SJ, Lee YW, Kang HJ, Park SH, and Kim HS. Coenzyme q10 increases the fatty acid oxidation through ampk-mediated pparalpha induction in 3t3-l1 preadipocytes. Cell Signal 24: 2329–2336, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Liu YY, Sparatore A, Del Soldato P, and Bian JS. Acs84, a novel hydrogen sulfide-releasing compound, protects against amyloid beta-induced cell cytotoxicity. Neurochem Int 58: 591–598, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Lu DY, Tang CH, Chen YH, and Wei IH. Berberine suppresses neuroinflammatory responses through amp-activated protein kinase activation in bv-2 microglia. J Cell Biochem 110: 697–705, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Manna P. and Jain SK. L-cysteine and hydrogen sulfide increase pip3 and ampk/ppargamma expression and decrease ros and vascular inflammation markers in high glucose treated human u937 monocytes. J Cell Biochem 114: 2334–2345, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Biswas SK, Galdiero MR, Sica A, and Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229: 176–185, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Martelli A, Testai L, Breschi MC, Blandizzi C, Virdis A, Taddei S, and Calderone V. Hydrogen sulphide: novel opportunity for drug discovery. Med Res Rev 32: 1093–1130, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Marutani E, Kosugi S, Tokuda K, Khatri A, Nguyen R, Atochin DN, Kida K, Van Leyen K, Arai K, and Ichinose F. A novel hydrogen sulfide-releasing n-methyl-d-aspartate receptor antagonist prevents ischemic neuronal death. J Biol Chem 287: 32124–32135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, Yukutake Y, Nagahata Y, Ishikawa M, Hattori K, Takenouchi T, Takahashi T, Ishii I, Matsubara K, Kabe Y, Uchiyama S, Nagata E, Gadalla MM, Snyder SH, and Suematsu M. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci U S A 109: 1293–1298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nath N, Giri S, Prasad R, Salem ML, Singh AK, and Singh I. 5-aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J Immunol 175: 566–574, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, and Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol 182: 8005–8014, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Neill LA. and Hardie DG. Metabolism of inflammation limited by ampk and pseudo-starvation. Nature 493: 346–355, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Owen MR, Doran E, and Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348 Pt 3: 607–614, 2000 [PMC free article] [PubMed] [Google Scholar]
- 37.Racioppi L. and Means AR. Calcium/calmodulin-dependent protein kinase kinase 2: roles in signaling and pathophysiology. J Biol Chem 287: 31658–31665, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Racioppi L, Noeldner PK, Lin F, Arvai S, and Means AR. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J Biol Chem 287: 11579–11591, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo GL, Russo M, and Ungaro P. Amp-activated protein kinase: a target for old drugs against diabetes and cancer. Biochem Pharmacol 86: 339–350, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Sag D, Carling D, Stout RD, and Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol 181: 8633–8641, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, and Cantley LC. The kinase lkb1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, and Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714, 2009b [DOI] [PubMed] [Google Scholar]
- 43. This reference has been deleted.
- 44.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov 6: 917–935, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Tiainen M, Ylikorkala A, and Mäkelä TP. Growth suppression by Lkb1 is mediated by a G1 cell cycle arrest. PNAS 96: 9248–9251, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Wen YD, Wang H, Kho SH, Rinkiko S, Sheng X, Shen HM, and Zhu YZ. Hydrogen sulfide protects huvecs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One 8: e53147, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, and Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal 12: 1147–1154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiliñski B, Wiliñski J, Somogyi E, Piotrowska J, and Opoka W. Metformin raises hydrogen sulfide tissue concentrations in various mouse organs. Pharmacol Rep 65: 737–742, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Xue R, Hao DD, Sun JP, Li WW, Zhao MM, Li XH, Chen Y, Zhu JH, Ding YJ, Jun Liu, and Zhu YC. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid Redox Signal 19: 5–23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y. and Ye J. Mitochondrial inhibitor as a new class of insulin sensitizer. Acta Pharm Sin B 2: 341–349, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.