Abstract
Tsetse flies (Glossina spp.) transmit parasitic African trypanosomes (Trypanosoma spp.), including Trypanosoma congolense, which causes animal African trypanosomiasis (AAT). AAT detrimentally affects agricultural activities in sub-Saharan Africa and has negative impacts on the livelihood and nutrient availability for the affected communities. After tsetse ingests an infectious blood meal, T. congolense sequentially colonizes the fly’s gut and proboscis (PB) organs before being transmitted to new mammalian hosts during subsequent feedings. Despite the importance of PB in blood feeding and disease transmission, little is known about its molecular composition, function and response to trypanosome infection. To bridge this gap, we used RNA-seq analysis to determine its molecular characteristics and responses to trypanosome infection. By comparing the PB transcriptome to whole head and midgut transcriptomes, we identified 668 PB-enriched transcripts that encoded proteins associated with muscle tissue, organ development, chemosensation and chitin-cuticle structure development. Moreover, transcripts encoding putative mechanoreceptors that monitor blood flow during tsetse feeding and interact with trypanosomes were also expressed in the PB. Microscopic analysis of the PB revealed cellular structures associated with muscles and cells. Infection with T. congolense resulted in increased and decreased expression of 38 and 88 transcripts, respectively. Twelve of these differentially expressed transcripts were PB-enriched. Among the transcripts induced upon infection were those encoding putative proteins associated with cell division function(s), suggesting enhanced tissue renewal, while those suppressed were associated with metabolic processes, extracellular matrix and ATP-binding as well as immunity. These results suggest that PB is a muscular organ with chemosensory and mechanosensory capabilities. The mechanoreceptors may be point of PB-trypanosomes interactions. T. congolense infection resulted in reduced metabolic and immune capacity of the PB. The molecular knowledge on the composition and putative functions of PB forms the foundation to identify new targets to disrupt tsetse’s ability to feed and parasite transmission.
Author summary
Tsetse flies are economically important insects responsible for transmitting African trypanosomes, which cause debilitating and fatal diseases in humans and animals in sub-Saharan Africa. In the tsetse vector, trypanosomes undergo complex developmental processes in the midgut, culminating with the generation of mammalian infective forms in the salivary glands for Trypanosoma brucei spp. and in the proboscis (PB) for Trypanosoma congolense and Trypanosoma vivax. Molecular studies on tsetse’s PB, and its interactions with trypanosomes, are limited. We used RNA-seq analysis to obtain molecular information on the putative products associated with tsetse’s PB and characterized PB responses to infection with T. congolense. Based on the predicted putative protein profile, the PB appears to be a muscular organ with mechanoreceptors and may have the capacity to sense and respond to chemical cues. Parasite infections of the PB lead to decreased expression of genes whose products are associated with metabolic and immune functions. These data provide insights into tsetse-trypanosome interactions in the PB organ and identify potential candidate targets that can be further explored to develop biotechnological strategies to reduce transmission of trypanosomes by tsetse flies.
Introduction
Tsetse flies (Glossina spp.) are vectors of African trypanosomes, which are protozoan parasites that cause human and animal African trypanosomiases (HAT and AAT, respectively) throughout sub-Saharan Africa [1]. AAT caused by Trypanosoma brucei brucei, Trypanosoma vivax and Trypanosoma congolense leads to emaciation and stunted growth of domesticated animals that subsequently produce less meat and milk [2]. These pathologies negatively impact the nutritional well-being of people living in endemic areas and result in a loss of 4.75 billion USD for the African economy each year [3]. Currently, no vaccines exist for either HAT or AAT, and disease control relies mainly on treatment of infected hosts and/or reduction of tsetse populations via trapping and pesticide application [3]. T. congolense is considered to be the most virulent and economically detrimental AAT-causing trypanosome [4, 5] and this is even aggravated by increasing levels of parasite resistance to drugs [6, 7] hindering treatment effectiveness. While vector control can effectively interfere with disease transmission, it experiences sustainability challenges; and over-reliance on insecticide based applications is environmentally undesirable and costly. Consequently, new methods to treat and reduce disease transmission are needed. In-depth molecular knowledge of the biological interactions that shape trypanosome infection dynamics in tsetse can lead to identification of novel disease control methods.
The life cycle of African trypanosomes involves sequential steps of differentiation and proliferation in both mammalian host and tsetse vector [8]. Mammalian stage parasites are designated as bloodstream forms (BSF). Once ingested by tsetse, BSF trypanosomes encounter robust physical and immunological barriers that include the gut peritrophic matrix [9, 10] and a plethora of host immune molecules that are anti-parasitic in nature, including antimicrobial peptides [11–14], reactive oxygen species (ROS) [15], tsetse EP proteins [16], trypanolysin [17–19], peptidoglycan recognition protein-LB [20, 21], lectins and lectin-like molecules [22–24] and other proteolytic enzymes [25–27]. Only in a small percentage of susceptible flies can trypanosomes establish infections and continue their development to colonize the salivary glands (SGs; for T. brucei spp.) or proboscis (PB; for T. congolense) (Fig 1) [28]. In the SG or PB, the parasite population consists of a number of developing epimastigote stages that attach to the luminal walls of the organs prior to undergoing metacyclogenesis [8, 29, 30], suggesting that these organs play key roles in trypanosome development and transmission.
Fig 1. The life cycle of Trypanosoma congolense.
Passage of T. congolense through the tsetse fly host. Colors represent different parasite developmental stages within distinct tsetse tissues. Tsetse ingests bloodstream-form T. congolense (1), which migrate to the fly’s midgut and differentiate into procyclic forms (2). Procyclic parasites then cross tsetse’s peritrophic matrix and move anteriorly through the ectoperitrophic space to the cardia where they again differentiate into long trypomastigotes (3). Finally, trypomastigotes colonize the PB (thecal bulb, labrum and hypopharynx) and differentiate into the epimastigote and then metacyclic forms (4), the latter of which are inoculated into a vertebrate host during a subsequent feed (5).
While a number of molecular studies have addressed tsetse’s SG and its response to infection with T. brucei complex parasites, little is known about the PB and its interaction with T. congolense. The PB is an essential appendage of the head that processes gustatory input to aid in locating and ingesting food [31]. Tsetse has a long piercing PB with a distinct basal bulb, a cuticle-lined tissue that comprises part of the foregut (Fig 1). The PB consists of three parts (labium, hypopharynx and labrum) that are surrounded by a pair of maxillary palps. In tsetse’s PB, only the labrum and hypopharynx are colonized by trypanosomes, while some parasites also attach to the cibarium [29, 30]. Previous scanning and transmission electron microscopic examinations of tsetse’s PB revealed the presence of different types of mechanoreceptors, nerves, neurons [32] and a network of muscles at the thecal bulb [33]. The mechanoreceptors interact with the parasites that formed colonies, or ‘rosettes’, in the proximal third of the labrum where these labral sensory sensilla mechanoreceptors are located [32, 34–36]. Parasites attached to the cibarium [30] also undergo vigorous division [29].
Beyond the predicted role of PB in feeding and an organ critical for trypanosome development and transmission, no information exists on the molecular components and function of the tsetse’s PB or on its responses to infection with T. congolense. Here, we utilized a high throughput RNA-sequencing approach to investigate the putative molecular composition and predicted function(s) of this organ as well as its responses to T. congolense infection. We also performed microscopic analysis of the PB to further understand the cellular structure of this organ.
Materials and methods
Ethical consideration
This work was carried out in strict adherence to the recommendations in the Office of Laboratory Animal Welfare at the National Institutes of Health and the Yale University Institutional Animal Care and Use Committee. The experimental protocol was reviewed and approved by the Yale University Institutional Animal Care and Use Committee (Protocol 2014–07266).
Tsetse flies and trypanosomes
Tsetse flies (Glossina morsitans morsitans) used in this study were reared in the Yale University insectary at 24°C and 50% relative humidity. All flies used in this study were maintained on blood commercially supplied by Hemostat Laboratories (Dixon, CA). All flies were fed at 48 hour intervals using an artificial membrane-based system [37].
Trypanosoma congolense [Trans Mara strain, variant antigenic type (VAT) TC13] [38] was kindly provided by Prof. Utpal Pal of Department of Veterinary Medicine, University of Maryland. Bloodstream form (BSF) parasites were amplified in rats following the strictly approved protocol (Protocol 2014–07266). At peak parasitemia, BSF was harvested from blood, aliquoted and cryopreserved in liquid nitrogen till used.
Tsetse infections and dissections
Teneral (newly eclosed and unfed adults) G. m. morsitans males were provided an infectious blood meal containing 8x106 BSF T. congolense (VAT TC13) per ml of blood in their first blood meal. After the first infectious blood meal, the flies were maintained on normal blood for the duration of the study. Uninfected control flies were maintained on normal blood only. Twenty-eight days post-challenge (dpc), all flies were dissected 72 h after their last blood meal. Infection status of the PB (defined here as labrum, hypopharynx and thecal bulb) was microscopically determined on Zeiss Axiostar Plus Light microscope at 400x. To dissect the PB, mouth parts were detached from the head and two needles (one in each hand) were used to tease apart the labrum and hypopharynx from the labium. The labium was then detached from the labrum and hypopharynx at the junction of the thecal bulb. This left the labrum, hypopharynx and thecal bulb attached together. Infected labrum and hypopharynx were snap frozen in liquid nitrogen and stored at -80°C until use. In the current study, a total of 7.8% (284/3655) of parasite challenged tsetse had T. congolense infections in the PB. All infected PBs, as well as an equal number of PBs dissected from age-matched uninfected control flies, were divided into two independent biological replicates, each of which contained 130 probosces for subsequent analysis.
RNA extraction, cDNA library preparation and sequencing
Total RNA was extracted using TRizol according to the manufacturer’s (Thermo Fisher Scientific Inc. CA, USA) protocol. Total RNA was DNase treated (Thermo Fisher Scientific Inc. CA, USA) and the absence of DNA contamination was confirmed by PCR amplification using primers that target tsetse’s β-tubulin and glyceraldehyde-3-phosphate dehydrogenase (gapdh) genes. RNA quantity and quality were determined using a Bioanalyzer 2100 (Agilent, Palo Alto, CA, USA). For cDNA library preparation, 900 ng of high quality total RNA (RNA integrity number >7.0) was used. The libraries were constructed from the two infected and two uninfected replicates using NEBNext Ultra Directional RNA Library Prep Kit (New England Biolabs, Inc. USA) according to the manufacturer’s protocol. Each replicate of the four libraries was prepared independently. Libraries were barcoded for Illumina HiSeq 2000 sequencing (unpaired 75 bases) at Yale Center for Genome Analysis. The NCBI sequence read archive (SRA) number for the G. m. morsitans PB transcriptomes described herein is SRP093552.
Bioinformatics analysis of tsetse PB datasets
CLC Genomic Workbench (CLC bio, Cambridge, MA) was used for all RNA-seq analyses. The four RNA-seq libraries (two-infected and two-uninfected controls) were assessed to determine read quality, and low quality reads were either trimmed or removed using CLC’s quality check and trimming algorithm, respectively. Subsequently, tsetse ribosomal RNA, symbiont (Sodalis glossinidius) and T. congolense reads were removed by mapping the RNA-seq datasets to tsetse 28S and 18S rRNA sequences [39], Sodalis genome [40] and T. congolense IL 3000 transcripts version 9 obtained from TritrypDB (www.tritrypdb.org; [41]), respectively. The TC13 strain used in this study is different from the strain for which the whole genome data was generated, but both parasite strains had originated from Transmara in Kenya [42]. All the remaining reads were used for downstream analyses. The infected and uninfected PB RNA-seq datasets were mapped to the G. m. morsitans Yale transcripts GmrY version 1.4 obtained from VectorBase (https://www.vectorbase.org/, [43]. Mappings were performed using a CLC-based algorithm that allows for two mismatches per read (with a maximum of 10 hits per read), with at least 80% of each read matching the gene at 95% identity. Reads per kilobase per million mapped (RPKM) was used as a proxy to quantify and compare relative transcript abundance between treatments [44]. The relative number of reads for each transcript in relation to total number of read counts for each RNA-seq dataset was established to calculate p-values based on the Baggeley’s test method following Bonferroni analysis [45]. Relative fold change (FC) between infected and uninfected transcripts was calculated as a ratio of their RPKM values, and normalized based on the number of reads obtained from each library. The normalized values were used in this study. Transcripts that scored p-value ≤ 0.05 (corrected normalized false discovery rate, FDR) were considered differentially expressed (DE). Transcripts that displayed at least 1.5 FC in abundance were considered significantly DE and were used to putatively determine molecular response of tsetse’s PB to T. congolense infection.
Tissue enriched gene expression analysis was performed using the Level Of eXpression (LOX) software [46], with datasets obtained from the uninfected PB (this study) and those previously obtained from uninfected tsetse midgut (NCBI SRA number, PRJNA314786) [47] and whole head (NCBI SRA number, SRP090041). LOX employs a Markov Chain Monte Carlo based method to estimate the level of expression and integrates sequence count tallies that are normalized by total expressed sequence count to provide expression levels for each gene relative to all treatments as well as by Bayesian credible intervals. The LOX estimates across PB, midgut and whole head transcriptomes were assembled to compare transcript expression levels across each tissue. For each tissue, two values were calculated using the upper bound of the 95% confidence interval (CI) or the lower bound of the 95% CI from LOX. To determine if the expression of a transcript in tissue 1 was higher than tissue 2, we calculated the fold difference between the lower bound of expression in tissue 1 and the upper bound of expression in tissue 2. Conversely, to determine if the expression of a transcript in tissue 2 was higher than tissue 1, we calculated the fold difference between the lower bound of tissue 2 and the upper bound of tissue 1. Gene Ontology (GO) terms were assigned to each G. m. morsitans transcript via Blast2GO software version 3.0 [48–50] using the blastx algorithm at a maximum e-value 10−3 to search against NCBI’s non-redundant protein database. The Blast2GO analysis was used to assign GO terms to genes that were preferentially expressed in the PB and GO term enrichment was determined via Fisher’s Exact test at an FDR, p-value ≤ 0.05 [49]. Pathway enrichment in infected and uninfected PB samples was determined using ProfCom [51]. Immunity associated transcripts were identified as previously described [52] based on sequence homology with D. melanogaster immune transcripts (http://flybase.org/); [53] and those sorted from the recently published G. m. morsitans genome [54].
Transcriptome validation using real time quantitative PCR
Total RNA was prepared (and DNase treated) from infected and uninfected PBs (n = 5 biological replicates, each containing 25 PBs) as described above. These biological samples were independent of the ones used for RNA-seq library construction. cDNA was synthesized with oligo-dT primers and random hexamers using the iScript cDNA synthesis reaction kit (Bio-Rad, Catalog No. 170–8891) according to the manufacturer’s protocol. Real time quantitative PCR (RT-qPCR) was performed in technical duplicate (for each biological replicate) on eight selected DE transcripts (S1 Table). In order to validate our transcriptome data, we initially selected three genes; beta-tubulin, GAPDH and 28S ribosomal RNA, for reference gene identification. The expression level of each gene was evaluated between infected and uninfected PB samples by RT-qPCR analysis. Our analysis revealed that the expression of gapdh was the least variable with the standard deviation (SD) of the crossing point (CP) being 0.88 based on BestKeeper analysis [55]. The beta-tubulin was found to be slightly variable with the SD of the CP of 1.08 while 28S rRNA was the most variable. All RT-qPCR results were thus normalized to tsetse gapdh, quantified from each biological replicate. A Pearson’s correlation test was used to validate the transcriptome data.
Light and fluorescent microscopy
Probosces from four weeks-old adult male flies were dissected in PBS and immediately fixed in PBS containing 4% PFA. Tissues were stained as previously described with modifications [56]. The fixed tissues were transferred to 4% PFA, 0.1% Triton-X100 PBS for 24h at 4°C, and then incubated with Alexa Fluor 488 Phalloidin (Life Technologies; 10 units/ml) and DAPI (3μg/ml) in PBS for 6 hours. Tissues were washed (2x 5min) with PBS between all steps. After 6 hour of incubation with Alexa Fluor 488 Phalloidin and DAPI followed by washing, tissues were then mounted on a glass slide and covered with glycerol. The images were observed using Zeiss Axio Imager 2 fluorescence microscope and captured using AxioVision (Zeiss) software. Processing of the images was done using Fiji version of ImageJ software [57].
Results
Description of the PB transcriptomes
To determine the molecular composition and putative function(s) of the PB organ and how it responds to infection with T. congolense, we performed a global gene expression analysis from uninfected and infected-PB. After sequencing, we obtained 19 to 68 million high-quality reads across all four RNA-seq libraries. The variation in the number of reads obtained is due to the different depth we achieved in sequencing of each library. Quality control measures (trimming of low quality reads and removal of tsetse ribosomal RNA and symbiont reads) removed < 3.5% of the total reads generated (S1A Fig). Important to note was detection of reads corresponding to tsetse’s endosymbiont Sodalis, which suggests that this microorganism may be among the constituents transmitted to the mammalian host at the bite site. T. congolense specific reads in infected-PB samples accounted for an average of over 4.0% of the total reads (S1A Fig). To identify tsetse expression profile of the PB, RNA-seq reads that passed quality control were mapped to the G. m. morsitans protein coding transcript from VectorBase (https://www.vectorbase.org/; [43]). Over 50% of the transcripts were categorized as having low relative abundance (≤100 unique reads), while only 1.02% of the transcripts were categorized as having high relative abundance (>10,000 unique reads) (S1B Fig).
We next identified transcripts that were preferentially expressed in the PB organ using LOX (Level Of eXpression) software. Unlike most tools used for gene expression analyses, LOX software can estimate the level of transcript expression from multiple high-throughput expression datasets generated using diverse experimental methodologies [46]. We compared the PB transcriptome to those generated from G. m. morsitans midgut [47] and whole head tissues (containing PB) from uninfected flies. Transcripts were considered to be preferentially expressed in the PB when the expression levels were ≥3-fold higher in the PB relative to the midgut and whole head. Only transcripts with at least an RPKM ≥ 5 and 20 unique reads mapping to it in either of the transcriptomes were considered. Based on these parameters, 668 (5.09%) genes were considered to be preferentially expressed in the PB (hereafter referred to as ‘PB-enriched’) (S1C Fig, S1 Table). Twenty-five genes were expressed at comparable levels in both PB and midgut tissues, while 2859 genes were expressed in both PB and whole head datasets (S1C Fig). PB-enriched transcripts and the complete PB RNA-seq dataset were used for further analyses.
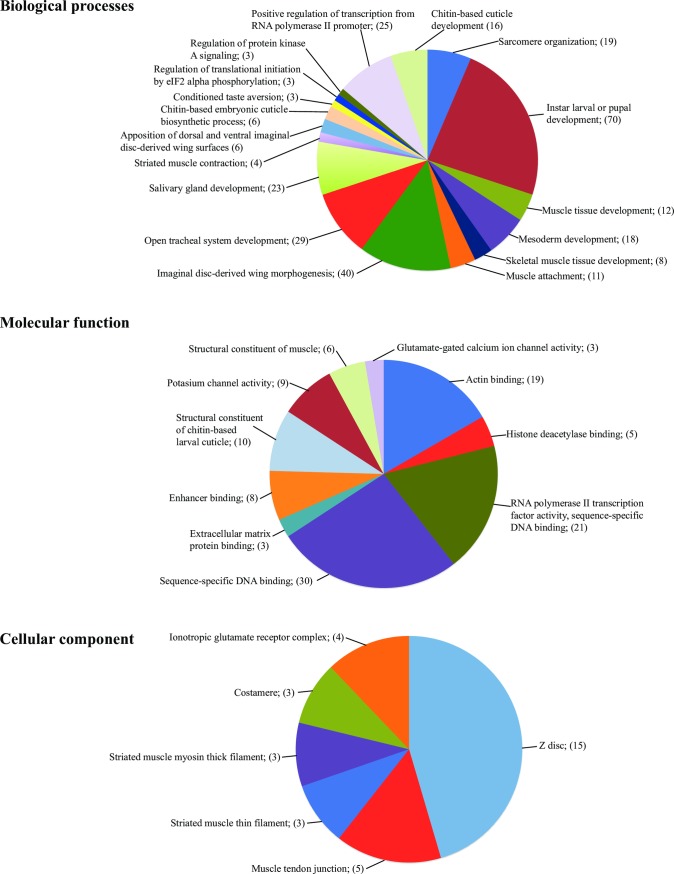
To obtain a global snapshot of the molecular mechanisms that underlie PB functions, the putative PB-enriched gene products were subjected to gene ontology (GO) analysis (Fig 2, S1 Table). With respect to the biological processes analysis, gene products broadly associated with muscle structure and activity, organ development (salivary gland development, mesoderm development, open tracheal system development) and conditioned taste aversion were enriched. For the molecular function category, gene products involved in binding (actin binding, sequence specific DNA-binding, histone deacetylase binding and enhancer binding), structural constituent of muscles and channel activities (potassium channel activity and glutamate calcium ion channel activity) were enriched. In the cellular component analysis, products associated with muscle genes and ionotropic glutamate receptor complexes were enriched. Moreover, transcription factor activity and signaling related gene products were enriched in biological processes and molecular function categories. These GO classifications suggest that the PB is a muscular organ with the capacity to sense and respond to chemical cues from within its internal or external environment.
Fig 2. Functional classification of PB-enriched genes based on gene ontology (GO).
Genes preferentially expressed in the tsetse fly proboscis were analyzed using Blast2GO gene ontology tool. The terms were categorized into biological processes, molecular function and cellular processes. The number of genes assigned to each term in different categories are indicated in brackets.
Using SignalP [58] and TMHMM [59] software packages, we next screened PB-enriched datasets for putative proteins with signal peptides (SP) and/or trans-membrane (TM) domains, respectively. Of the 668 putative PB-enriched transcripts, 148 were predicted to code for proteins with at least one or more TM domains, 62 were predicted to possess a SP domain and 28 were predicted to contain both SP and TM domains (S1 Table). Notable among the genes encoding TM proteins included five of the seventeen G. m. morsitans tetraspanins [60], major facilitator superfamily, ionotropic receptors (IRs), and innexins. Transcripts of two takeout (to) genes, one of which encodes a protein with a TM domain and the other with both TM and SP domains, are also among those that were PB-enriched (S1 Table). The IRs are chemosensory proteins responsive to a variety of odors, acids, amines, aldehydes and humidity [61, 62]. The G. m. morsitans genome encodes 30 chemosensory IR genes [63], four of which were found to be preferentially expressed in the PB suggesting the involvement of PB in chemosensory and olfactory processes.
Tsetse’s PB contains sensory receptors (sensilla), which apart from monitoring rate of blood flow during tsetse feeding, also appear to interact with trypanosomes [32, 35, 64]. Based on microscopy analysis, these sensory hairs were referred to as LCl mechanoreceptors [32]. We searched the PB transcriptome for expression of transcripts that putatively encode mechanoreceptors. To identify these transcripts, we first obtained the Drosophila mechanoreceptor gene sequences by searching the FlyBase database for “mechanoreceptor’ query. This resulted in 44 transcripts. Using the putative protein sequences of the 44 transcripts, we Blastp searched the G. m. morsitans peptide dataset in VectorBase using an E-value 10−10. This query resulted in the identification of 12 putative G. m. morsitans mechanoreceptor proteins (S2 Fig) that were abundantly expressed in the PB relative to the head and midgut tissues. Identification of mechanoreceptor transcripts in PB is in line with the presence of these putative receptors in the labrum.
Microscopic analysis of the tsetse’s PB
Microscopic analysis of tsetse’s PB, using Alexa Fluor 488 Phalloidin staining, demonstrated the presence of muscles at the base of the organ in the thecal bulb and where the PB attaches to the fly’s head (S3A–S3C Fig). DAPI staining revealed the presence of nuclei aligned along the lateral side of the proximal region (closer to the head) of the labrum (S3D Fig), indicating that cells line the organ’s lumen. These cells occupy the region of the organ where the mechanoreceptors interacting with parasites were previously described [32, 34–36]. The microscopy results, in conjunction with the RNA-seq data, support the muscular nature of tsetse’s PB and its potential ability to express receptor targets that may act as docking sites for T. congolense during metacyclogenesis process.
Differential gene expression and enrichment analysis of parasite infected PB
Both PB-enriched and complete PB library datasets were used to characterize the transcriptional response of the PB to infection with T. congolense parasites. Upon infection, 401 (3.06%) transcripts were DE, of which 38 (0.94%) and 88 (2.11%) were significantly (FC≥1.5) up- and down-regulated, respectively (Fig 3A, S2 Table). When the PB-enriched dataset was considered, 43 (6.44%) transcripts were DE with seven and five being significantly up- and down-regulated, respectively (Fig 3A, S1 Table and S2 Table). The transcriptional response of the PB upon T. congolense infection was validated via RT-qPCR on eight DE genes selected from the infected PB dataset (S1 Text). The RT-qPCR data exhibited a high level of correlation with results obtained from the RNA-seq analysis (Pearson correlation = 0.97447216), thus confirming the accuracy of PB infected and uninfected transcriptomes (S2 Text).
Fig 3. Differential expression and gene ontology (GO) analysis of genes exhibiting increased and decreased expression during trypanosome infection.

(A) Differentially expressed genes between T. congolense infected PB and uninfected PB of tsetse fly. (B) Significantly enriched pathways determined through ProfCom [51]. * Differentially expressed dataset ** Entire Drosophila genes in ProfCom database. The ticks in both the Y and X axis are positioned in a Log10 scale.
To gain insight into the nature of the molecular response(s) following infection with T. congolense, we subjected the significantly DE (FC2≥1.5) putative PB gene products to GO enrichment analysis using Profcom [51] (Fig 3B). Our analysis showed that putative proteins associated with protein binding pathway were significantly up-regulated, while putative products associated with metabolic processes, extracellular region and ATP-binding were down-regulated (Fig 3B). These results suggest that T. congolense infection may adversely affects the metabolic processes of the PB organ.
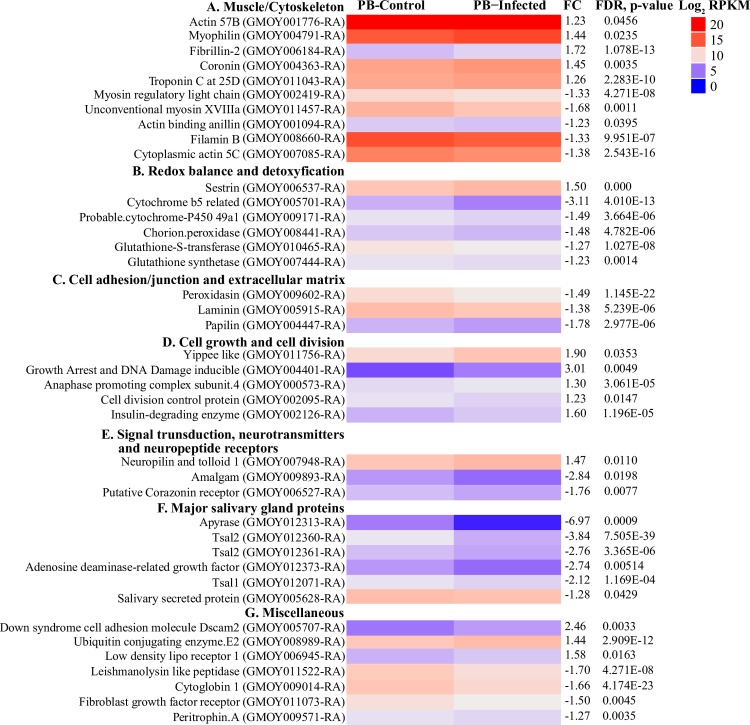
We then analyzed putative functions of the DE gene products to predict processes that may be affected upon trypanosome infection. Our data revealed that two muscle and/or cytoskeleton related proteins, Fibrilin-2 and Unconventional myosin XVIIIa, were up- and down-regulated respectively, while the remaining gene products were only moderately affected (Fig 4A). One cytoplasmic actin-5C up-regulated in the infected PB was also increased in T. brucei infected tsetse SG [65] as well as its orthologue in Plasmodium infected mosquito Anopheles gambiae. In A. gambiae, this protein forms complexes with immune factor AgMDL1, thus enabling it to function as an extracellular pathogen recognition factor in antibacterial defense [66]. The expression of transcripts whose products are associated with oxidoreduction were also affected in infected PB. We observed a general decreased expression of oxidoreduction transcripts, except for sestrin, which was significantly upregulated (Fig 4B). The expression of sestrin is increased in cells exposed to several stress factors, such as DNA-damage, oxidative stress and hypoxia [67–69]. Among those decreased were detoxification genes: cytochromes-P450 (CYPs), cytochrome b5-related and chorion peroxidase. Chorion peroxidase mediates NADH oxidation leading to the formation of hydrogen peroxide (H2O2) [70], thus its reduced expression suggests decreased H2O2 levels in infected-PB. Another group of transcripts reduced in expression upon infection, encoded proteins linked with cell adhesion/junction and extracellular matrix (Fig 4C).
Fig 4.
Heat maps representation of differentially expressed transcripts in different functional categories (A-G). Heat maps obtained by plotting the normalized expression profiles (RPKM, Log2 transformed) of individual transcripts in uninfected and infected conditions in the R-package software. The heat maps (dendrograms) were clustered using euclidean distance calculation and ward.D clustering methods. The clusters were then manually separated to various functional categories.
In addition, we noted that transcript levels for genes encoding proteins linked with cell growth, cell division and survival were increased in expression upon infection (Fig 4D). This expression profile suggests an increased rate of cell division upon infection, likely indicating tissue renewal. Our results also showed differential expression of genes that encode proteins associated with signal transduction and neurotransmission (Fig 4E). Alteration in the expression of such proteins had been documented in the head of T. brucei infected G. palpalis gambiensis, suggesting that the presence of trypanosomes may alter the function(s) of tsetse’s nervous system [71]. Lastly, we also detected decreased levels of transcripts for six major SG proteins in infected PB (Fig 4F). The expression levels of these transcripts are also significantly reduced in T. brucei infected SG [52, 65, 72]. Expression of SG-protein encoding genes in the PB was surprising. We speculate that these transcripts may have originated from tiny pieces of SG tissues (at the SG-hypopharynx junction) that contaminated our PB preparation. Other DE transcripts included peritrophin A, low density lipoprotein receptor and leishmalynosin like peptide protein (Fig 4G).
Expression of immune-associated genes in parasite infected PB
The insect immune system is a critical mediator of vector competence [73]. As such, we interrogated the DE datasets for candidates that may encode proteins with immune related functions. For this analysis, we first extracted immunity-related genes that were previously identified in G. m. morsitans genome project [54]. Secondly, we identified Drosophila immunity genes by combining genes whose GO functions are associated with immunity in FlyBase and Drosophila genes functionally involved in immunity [74, 75]. Using tBLASTx, we compared tsetse PB DE transcripts against the set of Drosophila immune-related genes. We identified 41 immune related transcripts that were affected upon infection of the PB (Table 1, S3 Table). Of these DE genes only four [Tob, Growth-blocking peptide, down syndrome cell adhesion molecule (Dscam) and Secreted Wg-interacting molecule] were up-regulated. All remaining genes were significantly down-regulated in the infected PB dataset (Table 1). Dscam is a gene that undergoes alternative splicing resulting in multiple proteins that function in the nervous systems of both vertebrates and invertebrates [76], and it also play a role in invertebrate immunity [77–80]. The down-regulated transcripts in the infected PB included two prophenoloxidases, hemolectin, C-type lectins, transferrin, eaters, major royal jelly, glucose dehydrogenases and Dscam variant (Table 1, S3 Table). Reduced level of Lectins in the tsetse midgut during the initial stages of parasite infection increases midgut parasite infection rates [81, 82]. The Major royal jelly protein has antimicrobial properties is expressed in response to bacterial infection in honeybees [83, 84], while Transferrin plays an important role in the immune system of insects and vertebrates [85, 86]. Transferrin expression is induced in flies that house bacterial infections but suppressed in the midgut of T. brucei infected tsetse and in baculovirus infected Spodoptera littoralis [86, 87]. The decreased expression of transferrin in infected PB may provide parasites with a more hospitable environment with greater iron availability and lower levels of free radicals [86]. In addition, serine protease inhibitor (Serpin11) and serine proteases, including serine protease immune response integrator and serine protease 7, were also down-regulated in expression (Table 1, S3 Table).
Table 1. Tsetse immunity transcripts differentially expressed between infected-PB compared to uninfected PB.
| Increased expression | |||||
| Gene ID | Gene Description | Fold change | FDR, p-value | Uninfected RPKM | Infected RPKM |
| GMOY005707-RA | Down syndrome cell adhesion molecule | 2.46 | 0.003313035 | 34.05 | 83.9 |
| GMOY010320-RA | Tob (Ecdysone-induced gene 71Ee) | 1.54 | 1.16563E-07 | 13340.1 | 20532.9 |
| GMOY011342-RA | Growth-blocking molecule | 1.43 | 0.006993913 | 811.5 | 1163.25 |
| GMOY006991-RA | Secreted Wg-interacting molecule | 1.21 | 0.003529879 | 713 | 864.35 |
| GMOY001164-RA | GTpase Rab2 | 1.12 | 0.034953043 | 2768.3 | 3121.15 |
| Decreased expression | |||||
| Gene ID | Gene Description | Fold change | FDR, p-value | Uninfected RPKM | Infected RPKM |
| GMOY010972-RA | Larval serum protein-like 3 | -5.43 | 1.00E-06 | 100.5 | 18.5 |
| GMOY010728-RA | Larval serum protein-like 4 | -5.38 | 0.049613273 | 15.6 | 2.9 |
| GMOY000810-RA | Glucose dehydrogenase | -3.18 | 0.015445653 | 17.65 | 5.55 |
| GMOY001557-RA | Major royal jelly 1 | -2.93 | 0.049375898 | 20.05 | 6.85 |
| GMOY003789-RA | Hemolectin | -2.55 | 0.014028789 | 6019.45 | 2364.15 |
| GMOY003159-RA | Eater | -2.52 | 0.026091303 | 527.45 | 209.5 |
| GMOY001221-RA | Glucose dehydrogenase | -1.99 | 2.28673E-07 | 139.6 | 70.2 |
| GMOY011147-RA | CG12213 | -1.96 | 2.18454E-05 | 322.35 | 164.85 |
| GMOY000466-RA | Salivary C-type lectin | -1.93 | 4.78488E-06 | 138.75 | 71.75 |
| GMOY011959-RA | Down syndrome cell adhesion molecule | -1.78 | 0.005805792 | 96.35 | 54.2 |
| GMOY010768-RA | Serine Protease Immune Response Integrator | -1.64 | 0.000833901 | 311.15 | 189.2 |
| GMOY008966-RA | Serine protease 7 | -1.57 | 0.037956883 | 133.55 | 85.3 |
| GMOY010673-RA | Transferrin | -1.53 | 0.001584412 | 1428.25 | 934.8 |
| GMOY002009-RA | Serrate | -1.50 | 0.000261495 | 207.4 | 138.35 |
Transcript levels of genes encoding secreted and transmembrane proteins
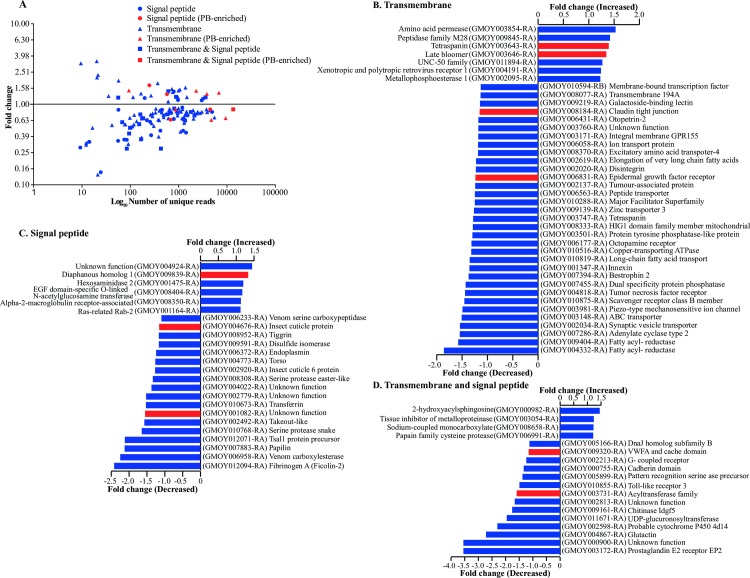
The PB-enriched dataset and the complete PB transcriptome library were used to analyze the expression profile of transcripts encoding proteins with TM and SP domains (Fig 5, S4 Table). Secreted proteins may be injected into the vertebrate host bite site during blood meal acquisition and as such may play critical role(s) in host-parasite interactions. A total of 148 DE transcripts encoded proteins with TM and/or SP domains, of which 12 were preferentially expressed in the PB (Fig 5A, S1 Table). Of the 148 transcripts, 95 encode proteins with TM domains. Trypanosome infection resulted in increased expression of amino acid permease, serotonin receptor, slimfast homolog-2, tetraspanins 42Ei, late bloomer and xenotropic/polytropic receptor genes. Conversely, fatty acyl-reductases, adenylate cyclase type-2 and synaptic vesicle transporter TM encoding transcripts were down-regulated in infected PB (Fig 5B, S4 Table). Serotonin is a neurotransmitter involved in the regulation of feeding and digestion in animals [88]. In insects, Serotonin is involved in post-ingestion examination of food, a process called conditioned taste aversion [89, 90]. We also identified 35 transcripts encoding putative secreted proteins. The expression of seven of these transcripts was induced in infected PB, with the expression of the remaining transcripts being reduced (Fig 5A and 5C, S4 Table). The down-regulated transcripts included fibrinogen A, venom carboxylases, serine protease easter-like, takeout-like and two transcripts coding for hypothetical proteins. Takeout encodes a putative juvenile hormone binding protein linked to circadian rhythm and regulation of feeding behavior in Drosophila [91–93]. The role of takeout in tsetse in modulating feeding is unknown, and whether its decreased expression in the PB upon infection impacts the fly’s feeding biology remains to be determined. These results indicate that trypanosome infection results in decreased expression of most secreted proteins in the PB similar what was observed in T. brucei infected tsetse SG [52, 65, 72] suggesting that T. congolense infection likely influences fly feeding behavior. For transcripts coding for proteins with both TM and SP motifs, 18 were identified (Fig 5A and 5D, S4 Table), four of which were up-regulated upon infection with the remaining 14 being reduced in trypanosome infected PB.
Fig 5. Summary of specific differentially expressed protein encoding genes that contain transmembrane and/or signal-peptide domains in the proboscis.
(A) Read abundance and fold difference in gene expression. Genes in red are PB-enriched while those in blue are from the complete PB transcriptome. (B-D) Fold change (based on RPKM differences) in expression of protein encoding genes that contain transmembrane (TM; B), signal peptide (SP; C) or both TM and SP domains (D) in T. congolense infected proboscis. This analysis is based on RNA-seq data from PB-enriched and complete PB transcriptome datasets and contain only genes whose combined RPKM and number of TM domains is at least 1000 and 3 respectively for TM proteins and a combine RPKM of at least 500 for SP.
Discussion
The proboscis of insect vectors is a component of the mouthparts that is involved in blood meal acquisition and parasite transmission. The present study provides insights into the molecular composition and function of tsetse’s PB as well as its response to T. congolense infection. The enrichment of IRs and glutamate-gated calcium ion channel proteins, normally associated with chemosensation [94], coupled with the expression of proteins functionally linked with conditioned taste aversion (CTA) that enables insects to discriminate between toxic and nutritious foods [89] in our PB-enriched datasets, suggest that tsetse PB have a gustatory function as well. In D. melanogaster, IRs in the gustatory organ [95] are thought to function in detecting tastants [62, 96]. Apart from the gustatory roles, the PB may also assess the feeding environment before taking a blood meal as described in mosquitoes [31, 97, 98] and Drosophila [99]. The CTA response has been demonstrated in several organisms [89, 90] which enable them detect and avoid consuming foods containing virulent pathogens [100, 101]. Collectively, our findings suggest that tsetse’s PB may also function in host selection and gustation choices, including avoidance of toxic foods during feeding. Further functional studies can shed light on how these attributes in tsetse’s PB are linked to the antennae chemosensory apparatus and their potential role in facilitating narrow host selection and exclusive haematophagy in tsetse flies.
The extensive network of muscles at the thecal bulb (visualized via microscopy), and identification of muscle-associated transcripts in PB-enriched dataset, may ensure the structural integrity of the organ [102, 103], enable PB movement [33] and pumping [104–106] processes, all of which are important for blood feeding. Our microscopy results also revealed the presence of cells lining the lateral proximal third of the labrum wall, a region associated with a high density of attached parasites in infected flies [34, 107, 108]. This region also contains a group of sensory receptors (LC1 mechanoreceptors [32]) known to monitor the rate of blood flow during tsetse feeding [35] and interact with T. congolense and T. vivax parasites that firmly attach at their base (of mechanoreceptors) forming rosette structures [34, 64, 107, 109, 110]. We detected the expression of 12 distinct genes encoding putative mechanoreceptors in our PB RNA-seq data. The cells (identified in this study) and expression of mechanoreceptors in the same region of the labrum, suggest that these cells may synthesize the receptors that trypanosomes may attach to during their development. Further functional studies would provide insight into which tsetse receptors are involved in trypanosome-proboscis interactions.
Our analysis of T. congolense infected PB shows that the majority of transcripts were significantly down-regulated, with only a few being up-regulated. The up-regulated transcripts encoded for proteins associated with cell cycle and cell survival processes, which reflect an enhanced cell division and tissue growth and maintenance upon infection. This mirrors the previous findings in G. m. morsitans SG infected with T. brucei [52]. The significantly down-regulated transcripts encoded metabolic, immunity, cell adhesion/junction and extracellular matrix related proteins, and secreted proteins. Among the putative secreted proteins detected in the PB transcriptome, six were major SG-proteins, which were also reduced in T. brucei infected SG [52, 65, 72]. The impact of this reduction, in conjunction with physical interference of parasites with phagoreceptors and reduced labrum diameter by rosette forming parasites, can lead to prolonged tsetse feeding time with multiple feeding attempts before the fly can reach full engorgement [35, 36, 72, 111, 112]. A combination of these phenomenon in parasite transmission and host infection success has been described [34, 35, 72, 110].
Invertebrate immune system can distinguish various pathogens ranging from viral to fungal invaders, and may get triggered when insects get infected with pathogens. In this study, we found several immunity genes that were DE upon T. congolense infection of the PB. Of the DE immune genes, was two variants of Dscam of which one variant was upregulated and the other decreased. Dscam gene is capable of producing many different isoforms [113, 114] and can exhibit pathogen specific immune memory [115]. RNA silencing of Dscam in Drosophila and Anopheles gambiae resulted in an impaired ability to phagocytose bacteria [79] and resist Plasmodium [80], respectively. In mosquitoes, pathogen-specific splice forms of Dscam are expressed upon immune challenge [80, 116]. Future investigations are warranted on the full variants of Dscam encoded in tsetse and on the role of the splice variants expressed in the PB. We also observed decreased expression of transcripts associated with immunity, including lectins, hemolectin (Hml) and transferrin upon infection, suggesting a reduction of tsetse defense systems. Lectins and Hml function by activating the complement system and agglutinating parasite surface carbohydrates [117, 118]. Reduced levels of Lectins in the tsetse midgut during initial stages of trypanosome infection increase infection rates and infection maturation in the fly midgut [81, 82]. Hml is an antimicrobial protein [119] with multiple domains, including von Willebrand factor C and D, and two discoidin domains [120, 121]. In Drosophila, silencing of hml led to bleeding defects upon injury [122]. It remains to be seen if Lectins (some of which have been shown to possess discoidin motifs [119, 123]) and Hml can interfere with establishment of epimastigotes in tsetse’s PB. Taken together, these results suggest that T. congolense infection negatively affects immune function in tsetse’s PB, a situation that can facilitate parasite survival and development in this niche.
We identified several tetraspanin transcripts in the PB-enriched dataset, of which two were up-regulated upon infection. Tetraspanins (Tsps) are molecular facilitators linked with cell adhesion/junction, the extracellular matrix and function in host-pathogen interactions [124–129]. Increased expression of tsps have been reported in T. brucei infected SGs of G. m. morsitans [65] and Dengue virus infected A. aegypti [130]. Although the importance of this induction in the tsetse system is unknown, Tsps are thought to be involved in fly-parasite interactions [60]. On the other hand, the expression of other cell adhesion/junction and extracellular matrix linked transcripts were down-regulated, contrary to results reported from T. brucei infected SGs [65]. The attachment of T. congolense parasites to the PB wall is an important aspect of parasite life cycle, and ensures that the fly remains infected for its entire life span. Attachment of the parasite to the PB via its flagellum results in the formation of a hemi-desmosome-like junctional complex [110], and is mediated by an unidentified ligand receptor interaction. Functional studies can potentially elucidate the direct interactions between putative PB cell surface proteins or TM proteins identified here and T. congolense.
In conclusion, results from this study suggest that tsetse’s PB is a muscular organ that may also exhibit chemosensory functions. Infection with T. congolense led to the reduced expression of gene products associated with metabolic processes and the immune system of the fly. These phenotypes potentially create an environment that facilitates parasite survival and transmission in the insect vector, or may represent vector responses that enable it to survive under stress. Results from this study provide a foundation that will enable functional genomics studies aimed at determining the role(s) of PB proteins in tsetse feeding activities and tsetse-trypanosome interactions.
Supporting information
(A) The total number of PB RNA-seq reads after quality control measures. (B) Proportion of reads that mapped per transcript. (C) Number of transcripts preferentially expressed in the PB (PB-enriched dataset) relative to the whole head and whole midgut transcriptomes. aPB-Proboscis—Trypanosome infection status; bBRep—Biological replicates; cTotal reads—Total number of raw reads obtained after RNA-sequencing; dAfter Trimming—Number of reads after removal of low quality reads; eAfter MR to T. congo—Number of reads that remained after mapping to Trypanosoma congolense parasite transcript version 9.0; fAfter RNA removal—Number of reads that remained after mapping to 18S and 28S rRNA; gAfter Symb removal—Number of reads that remained after mapping to tsetse endosymbiont, Sodalis glossinidius; hMR to Gmm—The number of reads that mapped to Glossina morsitans transcript (assembly GmorY1.4).
(TIF)
The heat map was generated by plotting the normalized RPKM values (Log2 transformed) of individual transcript from uninfected fly tissues, clustered using euclidean distance calculation and ward.D clustering methods. PB proboscis, WH whole head, WMG whole midgut, SG salivary gland.
(TIF)
(A, B and C) Tsetse’s labrum at its site of attachment to the thecal bulb, after removing the labium. The shape and general structure is observed by light microscopy, and muscles are fluorescence green after staining with phalloidin (dyes actin). Shown are the ventral (A), side (B) and dorsal (C) views of the thecal bulb. White arrowheads identify muscles that holds together the entire PB and the thecal bulb. Red arrowheads identify muscles that attach the thecal bulb to the fly’s head. (D) Side view of the labrum and hypophraynx stained with DAPI and observed using fluorescent microscopy. The picture is oriented from head (left) to the tip of the proboscis (right). A chain of nuclei can be observed distributed along the dorsum of the labrum.
(TIF)
Sheet 1. Genes with enriched expression in the proboscis (PB) compared to midgut [47] and whole head compared between uninfected PB and PB-infected with trypanosomes. The genes preferentially expressed in the PB (PB-enriched) was obtained by comparing expression of individual genes from tissues of uninfected flies. Sheet 2. Functional classification of genes preferentially expressed in the PB (PB-enriched) with genes in our datasets and those from the reference. Sheet 3. Organization of data for LOX software analysis.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
Acknowledgments
We are thankful to Dr. Xiaoli Bing for technical assistance during this study.
Data Availability
All relevant data are within the paper and its Supporting Information files. All Proboscis and Whole head data files are available from the NCBI database (accession number(s) PB = SRP093552, Whole Head = SRP090041) and Midgut data from (BioProject ID: PRJNA314786).
Funding Statement
This work was supported by National Institutes of Health (http://www.nih.gov) Fogarty Center grant D43TW007391, RO3TW009444, and a National Institute of Allergy an Infectious Diseases grant RO1AI051584 awarded to SA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Geerts S, Holmes PH, Eisler MC, Diall O. African bovine trypanosomiasis: the problem of drug resistance. Trends in parasitology. 2001;17(1):25–8. Epub 2001/01/04. . [DOI] [PubMed] [Google Scholar]
- 2.Shaw APM, Cecchi G, Wint GRW, Mattioli RC, Robinson TP. Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in Eastern Africa. Preventive veterinary medicine. 2014;113(2):197–210. doi: 10.1016/j.prevetmed.2013.10.024 [DOI] [PubMed] [Google Scholar]
- 3.Holmes P. Tsetse-transmitted trypanosomes—their biology, disease impact and control. J Invertebr Pathol. 2013;112 Suppl:S11–4. Epub 2012/07/31. doi: 10.1016/j.jip.2012.07.014 . [DOI] [PubMed] [Google Scholar]
- 4.Sharpe RT, Langley AM, Mowat GN, Macaskill JA, Holmes PH. Immunosuppression in bovine trypanosomiasis: response of cattle infected with Trypanosoma congolense to foot-and-mouth disease vaccination and subsequent live virus challenge. Research in veterinary science. 1982;32(3):289–93. Epub 1982/05/01. . [PubMed] [Google Scholar]
- 5.Mwangi DM, Munyua WK, Nyaga PN. Immunosuppression in caprine trypanosomiasis: effects of acute Trypanosoma congolense infection on antibody response to anthrax spore vaccine. Tropical animal health and production. 1990;22(2):95–100. Epub 1990/05/01. . [DOI] [PubMed] [Google Scholar]
- 6.Delespaux V, Dinka H, Masumu J, Van den Bossche P, Geerts S. Five-fold increase in Trypanosoma congolense isolates resistant to diminazene aceturate over a seven-year period in Eastern Zambia. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2008;11(6):205–9. Epub 2008/11/11. doi: 10.1016/j.drup.2008.10.002 . [DOI] [PubMed] [Google Scholar]
- 7.Chitanga S, Marcotty T, Namangala B, Van den Bossche P, Van Den Abbeele J, Delespaux V. High Prevalence of Drug Resistance in Animal Trypanosomes without a History of Drug Exposure. PLoS neglected tropical diseases. 2011;5(12):e1454 doi: 10.1371/journal.pntd.0001454 PMC3243716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickerman K, Tetley L, Hendry KA, Turner CM. Biology of African trypanosomes in the tsetse fly. Biology of the cell / under the auspices of the European Cell Biology Organization. 1988;64(2):109–19. Epub 1988/01/01. . [DOI] [PubMed] [Google Scholar]
- 9.Rose C, Belmonte R, Armstrong SD, Molyneux G, Haines LR, Lehane MJ, et al. An investigation into the protein composition of the teneral Glossina morsitans morsitans peritrophic matrix. PLoS neglected tropical diseases. 2014;8(4):e2691 Epub 2014/04/26. doi: 10.1371/journal.pntd.0002691 ; PubMed Central PMCID: PMCPMC3998921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss BL, Savage AF, Griffith BC, Wu Y, Aksoy S. The peritrophic matrix mediates differential infection outcomes in the tsetse fly gut following challenge with commensal, pathogenic, and parasitic microbes. Journal of immunology (Baltimore, Md: 1950). 2014;193(2):773–82. Epub 2014/06/11. doi: 10.4049/jimmunol.1400163 ; PubMed Central PMCID: PMCPMC4107339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Z, Kasumba I, Lehane MJ, Gibson WC, Kwon J, Aksoy S. Tsetse immune responses and trypanosome transmission: implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12648–53. Epub 2001/10/11. doi: 10.1073/pnas.221363798 ; PubMed Central PMCID: PMCPMC60108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Hu C, Wu Y, Stuart A, Amemiya C, Berriman M, et al. Characterization of the antimicrobial peptide attacin loci from Glossina morsitans. Insect molecular biology. 2008;17(3):293–302. Epub 2008/05/15. doi: 10.1111/j.1365-2583.2008.00805.x ; PubMed Central PMCID: PMCPMC2656931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Y, Aksoy S. An antimicrobial peptide with trypanocidal activity characterized from Glossina morsitans morsitans. Insect biochemistry and molecular biology. 2005;35(2):105–15. Epub 2005/02/01. doi: 10.1016/j.ibmb.2004.10.007 . [DOI] [PubMed] [Google Scholar]
- 14.Hu C, Aksoy S. Innate immune responses regulate trypanosome parasite infection of the tsetse fly Glossina morsitans morsitans. Molecular microbiology. 2006;60(5):1194–204. Epub 2006/05/13. doi: 10.1111/j.1365-2958.2006.05180.x . [DOI] [PubMed] [Google Scholar]
- 15.Macleod ET, Darby AC, Maudlin I, Welburn SC. Factors affecting trypanosome maturation in tsetse flies. PloS one. 2007;2(2):e239 Epub 2007/02/24. doi: 10.1371/journal.pone.0000239 ; PubMed Central PMCID: PMCPMC1797825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haines LR, Lehane SM, Pearson TW, Lehane MJ. Tsetse EP protein protects the fly midgut from trypanosome establishment. PLoS pathogens. 2010;6(3):e1000793 Epub 2010/03/12. doi: 10.1371/journal.ppat.1000793 ; PubMed Central PMCID: PMCPMC2832768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiles JK, Ingram GA, Wallbamks KR, Molyneux DH, Maudlin I, Welburn S. Identification of trypanolysin and trypanoagglutinin in Glossina palpalis sspp. (Diptera: Glossinidae). Parasitology. 1990;101:369–76. [DOI] [PubMed] [Google Scholar]
- 18.Nyambega B, Abubakar LU, Imbuga MO, Abakar MH, Osir EO. Lysis of Trypanosoma brucei brucei by Tsetse Trypanolysin. Kenya Journal of Science (B series). 2011;14:26–34. [Google Scholar]
- 19.Osir EO, Abakar M, Abubakar L, editors. The role of trypanolysin in the development of trypanosomes in tsetse. Proceedings of the 25th Meeting of the International Council for Trypanosomiasis Research Control (ISCTRC); 1999; Mombasa, Kenya.
- 20.Wang J, Aksoy S. PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse's offspring. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(26):10552–7. Epub 2012/06/13. doi: 10.1073/pnas.1116431109 ; PubMed Central PMCID: PMCPMC3387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Wu Y, Yang G, Aksoy S. Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proceedings of the National Academy of Sciences. 2009;106(29):12133–8. doi: 10.1073/pnas.0901226106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osir EO, Abubakar L, Imbuga MO. Purification and characterization of a midgut lectin-trypsin complex from the tsetse fly Glossina Iongipennis. Parasitology research. 1995;81:276–81. [DOI] [PubMed] [Google Scholar]
- 23.Abubakar LU, Bulimo WD, Mulaa FJ, Osir EO. Molecular characterization of a tsetse fly midgut proteolytic lectin that mediates differentiation of African trypanosomes. Insect biochemistry and molecular biology. 2006;36(4):344–52. Epub 2006/03/23. doi: 10.1016/j.ibmb.2006.01.010 . [DOI] [PubMed] [Google Scholar]
- 24.Abubakar L, Osir EO, Imbuga MO. Properties of a blood-meal-induced midgut lectin from the tsetse fly Glossina morsitans. Parasitology research. 1995;81(4):271–5. Epub 1995/01/01. . [DOI] [PubMed] [Google Scholar]
- 25.Welburn SC, Maudlin I. Tsetse-trypanosome interactions: rites of passage. Parasitology today (Personal ed). 1999;15(10):399–403. Epub 1999/09/11. . [DOI] [PubMed] [Google Scholar]
- 26.Imbuga MO, Osir EO, Labongo VL, Darji N, Otieno LH. Studies on tsetse midgut factors that induce differentiation of bloodstream Trypanosoma brucei brucei in vitro. Parasitol Res. 1992;78:10–5. [DOI] [PubMed] [Google Scholar]
- 27.Roditi I, Lehane MJ. Interactions between trypanosomes and tsetse flies. Current opinion in microbiology. 2008;11(4):345–51. Epub 2008/07/16. doi: 10.1016/j.mib.2008.06.006 . [DOI] [PubMed] [Google Scholar]
- 28.Rotureau B, Van Den Abbeele J. Through the dark continent: African trypanosome development in the tsetse fly. Frontiers in cellular and infection microbiology. 2013;3:53 Epub 2013/09/26. doi: 10.3389/fcimb.2013.00053 ; PubMed Central PMCID: PMCPMC3776139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peacock L, Cook S, Ferris V, Bailey M, Gibson W. The life cycle of Trypanosoma (Nannomonas) congolense in the tsetse fly. Parasites & vectors. 2012;5:109 Epub 2012/06/09. doi: 10.1186/1756-3305-5-109 ; PubMed Central PMCID: PMCPMC3384477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jefferies D, Helfrich MP, Molyneux DH. Cibarial infections of Trypanosoma vivax and T. congolense in Glossina. Parasitology research. 1987;73(4):289–92. Epub 1987/01/01. . [DOI] [PubMed] [Google Scholar]
- 31.Maekawa E, Aonuma H, Nelson B, Yoshimura A, Tokunaga F, Fukumoto S, et al. The role of proboscis of the malaria vector mosquito Anopheles stephensi in host-seeking behavior. Parasites & vectors. 2011;4:10 Epub 2011/01/29. doi: 10.1186/1756-3305-4-10 ; PubMed Central PMCID: PMCPMC3041766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice MJ, Galun R, Margalit J. Mouthpart sensilla of the tsetse fly and their function. 3. Labrocibarial sensilla. Annals of tropical medicine and parasitology. 1973;67(1):109–16. Epub 1973/03/01. . [DOI] [PubMed] [Google Scholar]
- 33.Jobling B. A Revision of the Structure of the head, mouth-part and salivary glands of Glossina palpalis ROB-DESV. Parasitology. 1933;XXIV(4):449–99. [Google Scholar]
- 34.Molyneux DH, Lavin DR, Elce B. A possible relationship between Salivarian trypanosomes and Glossina labrum mechano-receptors. Annals of tropical medicine and parasitology. 1979;73(3):287–90. Epub 1979/06/01. . [DOI] [PubMed] [Google Scholar]
- 35.Livesey JL, Molyneux DH, Jenni L. Mechanoreceptor—trypanosome interactions in the labrum of Glossina: fluid mechanics. Acta tropica. 1980;37(2):151–61. Epub 1980/06/01. . [PubMed] [Google Scholar]
- 36.Molyneux DH, Jenni L. Mechanoreceptors, feeding behaviour and trypanosome transmission in Glossina. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1981;75(1):160–3. Epub 1981/01/01. . [DOI] [PubMed] [Google Scholar]
- 37.Moloo SK. An artificial feeding technique for Glossina. Parasitology. 1971;63(3):507–12. Epub 1971/12/01. . [DOI] [PubMed] [Google Scholar]
- 38.Tabel H. Activation of the alternative pathway of bovine complement by Trypanosoma congolense. Parasite immunology. 1982;4(5):329–35. Epub 1982/09/01. . [DOI] [PubMed] [Google Scholar]
- 39.Cross NC, Dover GA. Tsetse fly rDNA: an analysis of structure and sequence. Nucleic Acids Res. 1987;15(1):15–30. Epub 1987/01/12. ; PubMed Central PMCID: PMCPMC340395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toh H, Weiss BL, Perkin SA, Yamashita A, Oshima K, Hattori M, et al. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res. 2006;16(2):149–56. Epub 2005/12/21. doi: 10.1101/gr.4106106 ; PubMed Central PMCID: PMCPMC1361709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aslett M, Aurrecoechea C, Berriman M, Brestelli J, Brunk BP, Carrington M, et al. TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Res. 2010;38(Database issue):D457–62. Epub 2009/10/22. doi: 10.1093/nar/gkp851 ; PubMed Central PMCID: PMCPMC2808979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson W. The origins of the trypanosome genome strains Trypanosoma brucei brucei TREU 927, T. b. gambiense DAL 972, T. vivax Y486 and T. congolense IL3000. Parasites & vectors. 2012;5:71 Epub 2012/04/10. doi: 10.1186/1756-3305-5-71 ; PubMed Central PMCID: PMCPMC3361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giraldo-Calderon GI, Emrich SJ, MacCallum RM, Maslen G, Dialynas E, Topalis P, et al. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2015;43(Database issue):D707–13. Epub 2014/12/17. doi: 10.1093/nar/gku1117 ; PubMed Central PMCID: PMCPMC4383932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–8. Epub 2008/06/03. doi: 10.1038/nmeth.1226 . [DOI] [PubMed] [Google Scholar]
- 45.Baggerly KA, Deng L, Morris JS, Aldaz CM. Differential expression in SAGE: accounting for normal between-library variation. Bioinformatics. 2003;19(12):1477–83. Epub 2003/08/13. . [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, López-Giráldez F, Townsend JP. LOX: inferring Level Of eXpression from diverse methods of census sequencing. Bioinformatics. 2010;26(15):1918–9. doi: 10.1093/bioinformatics/btq303 PMC2905554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aksoy E, Vigneron A, Bing X, Zhao X, O'Neill M, Wu YN, et al. Mammalian African trypanosome VSG coat enhances tsetse's vector competence. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(25):6961–6. Epub 2016/05/18. doi: 10.1073/pnas.1600304113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gotz S, Garcia-Gomez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–35. Epub 2008/05/01. doi: 10.1093/nar/gkn176 ; PubMed Central PMCID: PMCPMC2425479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. Epub 2005/08/06. doi: 10.1093/bioinformatics/bti610 . [DOI] [PubMed] [Google Scholar]
- 50.Conesa A, Gotz S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics. 2008;2008:619832 Epub 2008/05/17. doi: 10.1155/2008/619832 ; PubMed Central PMCID: PMCPMC2375974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antonov AV, Schmidt T, Wang Y, Mewes HW. ProfCom: a web tool for profiling the complex functionality of gene groups identified from high-throughput data. Nucleic Acids Res. 2008;36(Web Server issue):W347–51. Epub 2008/05/08. doi: 10.1093/nar/gkn239 ; PubMed Central PMCID: PMCPMC2447768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Telleria EL, Benoit JB, Zhao X, Savage AF, Regmi S, Alves e Silva TL, et al. Insights into the trypanosome-host interactions revealed through transcriptomic analysis of parasitized tsetse fly salivary glands. PLoS neglected tropical diseases. 2014;8(4):e2649 Epub 2014/04/26. doi: 10.1371/journal.pntd.0002649 ; PubMed Central PMCID: PMCPMC3998935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McQuilton P, St Pierre SE, Thurmond J. FlyBase 101—the basics of navigating FlyBase. Nucleic Acids Res. 2012;40(Database issue):D706–14. Epub 2011/12/01. doi: 10.1093/nar/gkr1030 ; PubMed Central PMCID: PMCPMC3245098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.IGGI. Genome sequence of the tsetse fly (Glossina morsitans): vector of African trypanosomiasis. Science. 2014;344(6182):380–6. Epub 2014/04/26. doi: 10.1126/science.1249656 ; PubMed Central PMCID: PMCPMC4077534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnology letters. 2004;26(6):509–15. Epub 2004/05/07. . [DOI] [PubMed] [Google Scholar]
- 56.Vo M, Linser PJ, Bowers DF. Organ-associated muscles in Aedes albopictus (Diptera: Culicidae) respond differentially to Sindbis virus. Journal of medical entomology. 2010;47(2):215–25. Epub 2010/04/13. ; PubMed Central PMCID: PMCPMC2866116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. Epub 2012/06/30. doi: 10.1038/nmeth.2019 ; PubMed Central PMCID: PMCPMC3855844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Meth. 2011;8(10):785–6. http://www.nature.com/nmeth/journal/v8/n10/abs/nmeth.1701.html - supplementary-information. [DOI] [PubMed] [Google Scholar]
- 59.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. Journal of molecular biology. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 60.Murungi EK, Kariithi HM, Adunga V, Obonyo M, Christoffels A. Evolution and Structural Analyses of Glossina morsitans (Diptera; Glossinidae) Tetraspanins. Insects. 2014;5(4):885–908. Epub 2014/01/01. doi: 10.3390/insects5040885 ; PubMed Central PMCID: PMCPMC4592607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao CA, Ignell R, Carlson JR. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25(37):8359–67. Epub 2005/09/16. doi: 10.1523/jneurosci.2432-05.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rytz R, Croset V, Benton R. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect biochemistry and molecular biology. 2013;43(9):888–97. Epub 2013/03/06. doi: 10.1016/j.ibmb.2013.02.007 . [DOI] [PubMed] [Google Scholar]
- 63.Macharia R, Mireji P, Murungi E, Murilla G, Christoffels A, Aksoy S, et al. Genome-Wide Comparative Analysis of Chemosensory Gene Families in Five Tsetse Fly Species. PLoS neglected tropical diseases. 2016;10(2):e0004421 Epub 2016/02/18. doi: 10.1371/journal.pntd.0004421 ; PubMed Central PMCID: PMCPMC4757090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molyneux DH. Host–trypanosome interactions in Glossina. Insect Science and Its Application. 1980;1(1):39–46. doi: 10.1017/S1742758400000114 [Google Scholar]
- 65.Matetovici I, Caljon G, Van Den Abbeele J. Tsetse fly tolerance to T. brucei infection: transcriptome analysis of trypanosome-associated changes in the tsetse fly salivary gland. BMC genomics. 2016;17(1):971 Epub 2016/11/26. doi: 10.1186/s12864-016-3283-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandiford SL, Dong Y, Pike A, Blumberg BJ, Bahia AC, Dimopoulos G. Cytoplasmic actin is an extracellular insect immune factor which is secreted upon immune challenge and mediates phagocytosis and direct killing of bacteria, and is a Plasmodium Antagonist. PLoS pathogens. 2015;11(2):e1004631 Epub 2015/02/07. doi: 10.1371/journal.ppat.1004631 ; PubMed Central PMCID: PMCPMC4450071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327(5970):1223–8. Epub 2010/03/06. doi: 10.1126/science.1182228 ; PubMed Central PMCID: PMCPMC2866632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013;18(6):792–801. Epub 2013/09/24. doi: 10.1016/j.cmet.2013.08.018 ; PubMed Central PMCID: PMCPMC3858445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kodrik D, Bednarova A, Zemanova M, Krishnan N. Hormonal Regulation of Response to Oxidative Stress in Insects-An Update. Int J Mol Sci. 2015;16(10):25788–816. Epub 2015/10/31. doi: 10.3390/ijms161025788 ; PubMed Central PMCID: PMCPMC4632827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han Q, Li G, Li J. Chorion peroxidase-mediated NADH/O(2) oxidoreduction cooperated by chorion malate dehydrogenase-catalyzed NADH production: a feasible pathway leading to H(2)O(2) formation during chorion hardening in Aedes aegypti mosquitoes. Biochimica et biophysica acta. 2000;1523(2–3):246–53. Epub 2000/10/24. ; PubMed Central PMCID: PMCPMC2856698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lefevre T, Thomas F, Ravel S, Patrel D, Renault L, Le Bourligu L, et al. Trypanosoma brucei brucei induces alteration in the head proteome of the tsetse fly vector Glossina palpalis gambiensis. Insect molecular biology. 2007;16(6):651–60. Epub 2007/12/21. doi: 10.1111/j.1365-2583.2007.00761.x . [DOI] [PubMed] [Google Scholar]
- 72.Van Den Abbeele J, Caljon G, De Ridder K, De Baetselier P, Coosemans M. Trypanosoma brucei modifies the tsetse salivary composition, altering the fly feeding behavior that favors parasite transmission. PLoS pathogens. 2010;6(6):e1000926 Epub 2010/06/10. doi: 10.1371/journal.ppat.1000926 ; PubMed Central PMCID: PMCPMC2880569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weiss B, Aksoy S. Microbiome influences on insect host vector competence. Trends in parasitology. 2011;27(11):514–22. Epub 2011/06/24. doi: 10.1016/j.pt.2011.05.001 ; PubMed Central PMCID: PMCPMC3179784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual review of immunology. 2007;25:697–743. Epub 2007/01/05. doi: 10.1146/annurev.immunol.25.022106.141615 . [DOI] [PubMed] [Google Scholar]
- 75.Marygold SJ, Leyland PC, Seal RL, Goodman JL, Thurmond J, Strelets VB, et al. FlyBase: improvements to the bibliography. Nucleic Acids Res. 2013;41(Database issue):D751–7. Epub 2012/11/06. doi: 10.1093/nar/gks1024 ; PubMed Central PMCID: PMCPMC3531214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montesinos ML. Roles for DSCAM and DSCAML1 in central nervous system development and disease. Advances in neurobiology. 2014;8:249–70. Epub 2014/10/11. . [DOI] [PubMed] [Google Scholar]
- 77.Brites D, McTaggart S, Morris K, Anderson J, Thomas K, Colson I, et al. The Dscam homologue of the crustacean Daphnia is diversified by alternative splicing like in insects. Molecular biology and evolution. 2008;25(7):1429–39. Epub 2008/04/12. doi: 10.1093/molbev/msn087 . [DOI] [PubMed] [Google Scholar]
- 78.Du Pasquier L. Immunology. Insects diversify one molecule to serve two systems. Science. 2005;309(5742):1826–7. Epub 2005/09/17. doi: 10.1126/science.1118828 . [DOI] [PubMed] [Google Scholar]
- 79.Watson FL, Puttmann-Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, et al. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309(5742):1874–8. Epub 2005/08/20. doi: 10.1126/science.1116887 . [DOI] [PubMed] [Google Scholar]
- 80.Dong Y, Taylor HE, Dimopoulos G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS biology. 2006;4(7):e229 Epub 2006/06/16. doi: 10.1371/journal.pbio.0040229 ; PubMed Central PMCID: PMCPMC1479700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Welburn SC, Maudlin I. Haemolymph lectin and the maturation of trypanosome infections in tsetse. Medical and veterinary entomology. 1990;4(1):43–8. Epub 1990/01/01. . [DOI] [PubMed] [Google Scholar]
- 82.Maudlin I, Welburn SC. The role of lectins and trypanosome genotype in the maturation of midgut infections in Glossina morsitans. Tropical medicine and parasitology: official organ of Deutsche Tropenmedizinische Gesellschaft and of Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ). 1988;39(1):56–8. Epub 1988/03/01. . [PubMed] [Google Scholar]
- 83.Scharlaken B, De Graaf DC, Memmi S, Devreese B, Van Beeumen J, Jacobs FJ. Differential protein expression in the honey bee head after a bacterial challenge. Archives of insect biochemistry and physiology. 2007;65(4):223–37. Epub 2007/07/17. doi: 10.1002/arch.20179 . [DOI] [PubMed] [Google Scholar]
- 84.Buttstedt A, Moritz RF, Erler S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biological reviews of the Cambridge Philosophical Society. 2014;89(2):255–69. Epub 2013/07/17. doi: 10.1111/brv.12052 . [DOI] [PubMed] [Google Scholar]
- 85.Lehane MJ, Gibson W, Lehane SM. Differential expression of fat body genes in Glossina morsitans morsitans following infection with Trypanosoma brucei brucei. International journal for parasitology. 2008;38(1):93–101. Epub 2007/08/19. doi: 10.1016/j.ijpara.2007.06.004 . [DOI] [PubMed] [Google Scholar]
- 86.Guz N, Attardo GM, Wu Y, Aksoy S. Molecular aspects of transferrin expression in the tsetse fly (Glossina morsitans morsitans). Journal of insect physiology. 2007;53(7):715–23. doi: 10.1016/j.jinsphys.2007.03.013 PMC2065764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guz N, Dageri A, Erdogan T, Mousavi M, Bayram Ş, Gurkan MO. Transcriptional profiling of transferrin gene from Egyptian cotton leaf worm, Spodoptera littoralis*. Turkish Journal of Biology. 2013;37:582–90. Epub 06.09.2013. doi: 10.3906/biy-1303-45 [Google Scholar]
- 88.French AS, Simcock KL, Rolke D, Gartside SE, Blenau W, Wright GA. The role of serotonin in feeding and gut contractions in the honeybee. Journal of insect physiology. 2014;61:8–15. Epub 2014/01/01. doi: 10.1016/j.jinsphys.2013.12.005 ; PubMed Central PMCID: PMCPMC3969292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wright GA, Mustard JA, Simcock NK, Ross-Taylor AA, McNicholas LD, Popescu A, et al. Parallel reinforcement pathways for conditioned food aversions in the honeybee. Current biology: CB. 2010;20(24):2234–40. Epub 2010/12/07. doi: 10.1016/j.cub.2010.11.040 ; PubMed Central PMCID: PMCPMC3011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wright GA. The role of dopamine and serotonin in conditioned food aversion learning in the honeybee. Communicative & integrative biology. 2011;4(3):318–20. Epub 2011/10/08. doi: 10.4161/cib.4.3.14840 ; PubMed Central PMCID: PMCPMC3187896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.So WV, Sarov-Blat L, Kotarski CK, McDonald MJ, Allada R, Rosbash M. takeout, a Novel Drosophila Gene under Circadian Clock Transcriptional Regulation. Molecular and cellular biology. 2000;20(18):6935–44. PMC88769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meunier N, Belgacem YH, Martin JR. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. The Journal of experimental biology. 2007;210(Pt 8):1424–34. Epub 2007/04/03. doi: 10.1242/jeb.02755 . [DOI] [PubMed] [Google Scholar]
- 93.Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PloS one. 2009;4(6):e6063 Epub 2009/06/27. doi: 10.1371/journal.pone.0006063 ; PubMed Central PMCID: PMCPMC2698149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–62. Epub 2009/01/13. doi: 10.1016/j.cell.2008.12.001 ; PubMed Central PMCID: PMCPMC2709536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, et al. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS genetics. 2010;6(8):e1001064 Epub 2010/09/03. doi: 10.1371/journal.pgen.1001064 ; PubMed Central PMCID: PMCPMC2924276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69(1):44–60. doi: 10.1016/j.neuron.2010.11.042 PMC3050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jung JW, Baeck S, Perumalsamy H, Hansson BS, Ahn Y, Kwon HW. A novel olfactory pathway is essential for fast and efficient blood-feeding in mosquitoes. Sci Rep. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matthews BJ, McBride CS, DeGennaro M, Despo O, Vosshall LB. The neurotranscriptome of the Aedes aegypti mosquito. BMC genomics. 2016;17:32 Epub 2016/01/08. doi: 10.1186/s12864-015-2239-0 ; PubMed Central PMCID: PMCPMC4704297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Montell C. A Taste of the Drosophila Gustatory Receptors. Current opinion in neurobiology. 2009;19(4):345–53. doi: 10.1016/j.conb.2009.07.001 PMC2747619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Babin A, Kolly S, Schneider F, Dolivo V, Zini M, Kawecki TJ. Fruit flies learn to avoid odours associated with virulent infection. Biology letters. 2014;10(3):20140048 Epub 2014/03/07. doi: 10.1098/rsbl.2014.0048 ; PubMed Central PMCID: PMCPMC3982440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438(7065):179–84. Epub 2005/11/11. doi: 10.1038/nature04216 . [DOI] [PubMed] [Google Scholar]
- 102.Dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, et al. Actin Binding Proteins: Regulation of Cytoskeletal Microfilaments. Physiological Reviews. 2003;83(2):433–73. doi: 10.1152/physrev.00026.2002 [DOI] [PubMed] [Google Scholar]
- 103.White J, Barro MV, Makarenkova HP, Sanger JW, Sanger JM. Localization of sarcomeric proteins during myofibril assembly in cultured mouse primary skeletal myotubes. Anatomical record (Hoboken, NJ: 2007). 2014;297(9):1571–84. Epub 2014/08/16. doi: 10.1002/ar.22981 ; PubMed Central PMCID: PMCPMC4145531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karolyi F, Colville JF, Handschuh S, Metscher BD, Krenn HW. One proboscis, two tasks: Adaptations to blood-feeding and nectar-extracting in long-proboscid horse flies (Tabanidae, Philoliche). Arthropod structure & development. 2014;43(5):403–13. doi: 10.1016/j.asd.2014.07.003 PMC4175409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bauder JA-S, Handschuh S, Metscher BD, Krenn HW. Functional morphology of the feeding apparatus and evolution of proboscis length in metalmark butterflies (Lepidoptera: Riodinidae). Biological Journal of the Linnean Society Linnean Society of London. 2013;110(2):291–304. doi: 10.1111/bij.12134 PMC4021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rehder V. Sensory pathways and motoneurons of the proboscis reflex in the suboesophageal ganglion of the honey bee. The Journal of comparative neurology. 1989;279(3):499–513. Epub 1989/01/15. doi: 10.1002/cne.902790313 . [DOI] [PubMed] [Google Scholar]
- 107.Vickerman K. The mode of attachment of Trypanosoma vivax in the proboscis of the tsetse fly Glossina fuscipes: an ultrastructural study of the epimastigote stage of the trypanosome. The Journal of protozoology. 1973;20(3):394–404. Epub 1973/08/01. . [DOI] [PubMed] [Google Scholar]
- 108.Thevenaz P, Hecker H. Distribution and attachment of Trypanosoma (Nannomonas) congolense in the proximal part of the proboscis of Glossina morsitans morsitans. Acta tropica. 1980;37(2):163–75. Epub 1980/06/01. . [PubMed] [Google Scholar]
- 109.Clarke JE. TRYPANOSOME INFECTIONS IN THE MOUTHPARTS OF GLOSSINA MORSITANS WESTW.: A CORRELATION BETWEEN EXTENT OF LABRAL INFECTION AND INVASION OF THE HYPOPHARYNX. Annals of tropical medicine and parasitology. 1965;59:235–9. Epub 1965/06/01. . [DOI] [PubMed] [Google Scholar]
- 110.Evans DA, Ellis DS, Stamford S. Ultrastructural studies of certain aspects of the development of Trypanosoma congolense in Glossina morsitans morsitans. The Journal of protozoology. 1979;26(4):557–63. Epub 1979/11/01. . [DOI] [PubMed] [Google Scholar]
- 111.Roberts LW. Probing by Glossina morsitans morsitans and transmission of Trypanosoma (Nannomonas) congolense. The American journal of tropical medicine and hygiene. 1981;30(5):948–51. Epub 1981/09/01. . [DOI] [PubMed] [Google Scholar]
- 112.Moloo SK, Dar F. Probing by Glossina morsitans centralis infected with pathogenic Trypanosoma species. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1985;79(1):119 Epub 1985/01/01. . [DOI] [PubMed] [Google Scholar]
- 113.Yu HH, Yang JS, Wang J, Huang Y, Lee T. Endodomain diversity in the Drosophila Dscam and its roles in neuronal morphogenesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(6):1904–14. Epub 2009/02/13. doi: 10.1523/jneurosci.5743-08.2009 ; PubMed Central PMCID: PMCPMC2671081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith PH, Mwangi JM, Afrane YA, Yan G, Obbard DJ, Ranford-Cartwright LC, et al. Alternative splicing of the Anopheles gambiae Dscam gene in diverse Plasmodium falciparum infections. Malaria journal. 2011;10:156 Epub 2011/06/10. doi: 10.1186/1475-2875-10-156 ; PubMed Central PMCID: PMCPMC3118162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Armitage SA, Peuss R, Kurtz J. Dscam and pancrustacean immune memory—a review of the evidence. Developmental and comparative immunology. 2015;48(2):315–23. Epub 2014/03/25. doi: 10.1016/j.dci.2014.03.004 . [DOI] [PubMed] [Google Scholar]
- 116.Dong Y, Cirimotich CM, Pike A, Chandra R, Dimopoulos G. Anopheles NF-kappaB-regulated splicing factors direct pathogen-specific repertoires of the hypervariable pattern recognition receptor AgDscam. Cell host & microbe. 2012;12(4):521–30. Epub 2012/10/23. doi: 10.1016/j.chom.2012.09.004 ; PubMed Central PMCID: PMCPMC3614911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway—its role in innate immunity and evolution. Immunological reviews. 2004;198:185–202. Epub 2004/06/18. . [DOI] [PubMed] [Google Scholar]
- 118.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2(5):346–53. Epub 2002/05/30. doi: 10.1038/nri800 . [DOI] [PubMed] [Google Scholar]
- 119.Lesch C, Goto A, Lindgren M, Bidla G, Dushay MS, Theopold U. A role for Hemolectin in coagulation and immunity in Drosophila melanogaster. Developmental and comparative immunology. 2007;31(12):1255–63. Epub 2007/05/19. doi: 10.1016/j.dci.2007.03.012 . [DOI] [PubMed] [Google Scholar]
- 120.Kotani E, Yamakawa M, Iwamoto S, Tashiro M, Mori H, Sumida M, et al. Cloning and expression of the gene of hemocytin, an insect humoral lectin which is homologous with the mammalian von Willebrand factor. Biochimica et biophysica acta. 1995;1260(3):245–58. Epub 1995/02/21. . [DOI] [PubMed] [Google Scholar]
- 121.Goto A, Kumagai T, Kumagai C, Hirose J, Narita H, Mori H, et al. A Drosophila haemocyte-specific protein, hemolectin, similar to human von Willebrand factor. The Biochemical journal. 2001;359(Pt 1):99–108. Epub 2001/09/21. ; PubMed Central PMCID: PMCPMC1222125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goto A, Kadowaki T, Kitagawa Y. Drosophila hemolectin gene is expressed in embryonic and larval hemocytes and its knock down causes bleeding defects. Developmental biology. 2003;264(2):582–91. Epub 2003/12/04. . [DOI] [PubMed] [Google Scholar]
- 123.Frazier WA, Rosen SD, Reitherman RW, Barondes SH. Purification and comparison of two developmentally regulated lectins from Dictyostelium discoideum. Discoidin I and II. The Journal of biological chemistry. 1975;250(19):7714–21. Epub 1975/10/10. . [PubMed] [Google Scholar]
- 124.Ley K, Zhang H. Dances with leukocytes: how tetraspanin-enriched microdomains assemble to form endothelial adhesive platforms. The Journal of cell biology. 2008;183(3):375–6. Epub 2008/11/05. doi: 10.1083/jcb.200809173 ; PubMed Central PMCID: PMCPMC2575778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annual review of cell and developmental biology. 2003;19:397–422. Epub 2003/10/23. doi: 10.1146/annurev.cellbio.19.111301.153609 . [DOI] [PubMed] [Google Scholar]
- 126.Adell T, Gamulin V, Perovic-Ottstadt S, Wiens M, Korzhev M, Muller IM, et al. Evolution of metazoan cell junction proteins: the scaffold protein MAGI and the transmembrane receptor tetraspanin in the demosponge Suberites domuncula. Journal of molecular evolution. 2004;59(1):41–50. Epub 2004/09/24. doi: 10.1007/s00239-004-2602-2 . [DOI] [PubMed] [Google Scholar]
- 127.Martin F, Roth DM, Jans DA, Pouton CW, Partridge LJ, Monk PN, et al. Tetraspanins in viral infections: a fundamental role in viral biology? Journal of virology. 2005;79(17):10839–51. Epub 2005/08/17. doi: 10.1128/JVI.79.17.10839-10851.2005 ; PubMed Central PMCID: PMCPMC1193642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.VanCompernolle SE, Wiznycia AV, Rush JR, Dhanasekaran M, Baures PW, Todd SC. Small molecule inhibition of hepatitis C virus E2 binding to CD81. Virology. 2003;314(1):371–80. Epub 2003/10/01. . [DOI] [PubMed] [Google Scholar]
- 129.Spoden G, Freitag K, Husmann M, Boller K, Sapp M, Lambert C, et al. Clathrin- and Caveolin-Independent Entry of Human Papillomavirus Type 16—Involvement of Tetraspanin-Enriched Microdomains (TEMs). PloS one. 2008;3(10):e3313 doi: 10.1371/journal.pone.0003313 PMC2561052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sim S, Ramirez JL, Dimopoulos G. Dengue virus infection of the Aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and blood-feeding behavior. PLoS pathogens. 2012;8(3):e1002631 Epub 2012/04/06. doi: 10.1371/journal.ppat.1002631 ; PubMed Central PMCID: PMCPMC3315490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The total number of PB RNA-seq reads after quality control measures. (B) Proportion of reads that mapped per transcript. (C) Number of transcripts preferentially expressed in the PB (PB-enriched dataset) relative to the whole head and whole midgut transcriptomes. aPB-Proboscis—Trypanosome infection status; bBRep—Biological replicates; cTotal reads—Total number of raw reads obtained after RNA-sequencing; dAfter Trimming—Number of reads after removal of low quality reads; eAfter MR to T. congo—Number of reads that remained after mapping to Trypanosoma congolense parasite transcript version 9.0; fAfter RNA removal—Number of reads that remained after mapping to 18S and 28S rRNA; gAfter Symb removal—Number of reads that remained after mapping to tsetse endosymbiont, Sodalis glossinidius; hMR to Gmm—The number of reads that mapped to Glossina morsitans transcript (assembly GmorY1.4).
(TIF)
The heat map was generated by plotting the normalized RPKM values (Log2 transformed) of individual transcript from uninfected fly tissues, clustered using euclidean distance calculation and ward.D clustering methods. PB proboscis, WH whole head, WMG whole midgut, SG salivary gland.
(TIF)
(A, B and C) Tsetse’s labrum at its site of attachment to the thecal bulb, after removing the labium. The shape and general structure is observed by light microscopy, and muscles are fluorescence green after staining with phalloidin (dyes actin). Shown are the ventral (A), side (B) and dorsal (C) views of the thecal bulb. White arrowheads identify muscles that holds together the entire PB and the thecal bulb. Red arrowheads identify muscles that attach the thecal bulb to the fly’s head. (D) Side view of the labrum and hypophraynx stained with DAPI and observed using fluorescent microscopy. The picture is oriented from head (left) to the tip of the proboscis (right). A chain of nuclei can be observed distributed along the dorsum of the labrum.
(TIF)
Sheet 1. Genes with enriched expression in the proboscis (PB) compared to midgut [47] and whole head compared between uninfected PB and PB-infected with trypanosomes. The genes preferentially expressed in the PB (PB-enriched) was obtained by comparing expression of individual genes from tissues of uninfected flies. Sheet 2. Functional classification of genes preferentially expressed in the PB (PB-enriched) with genes in our datasets and those from the reference. Sheet 3. Organization of data for LOX software analysis.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. All Proboscis and Whole head data files are available from the NCBI database (accession number(s) PB = SRP093552, Whole Head = SRP090041) and Midgut data from (BioProject ID: PRJNA314786).