Abstract
Sulfur dioxide (SO2), a gaseous signaling molecule in animal cells, has recently been found to play a physiological role in plants. Here we studied the role of SO2 in gibberellic acid (GA3)-induced programmed cell death (PCD) in barley (Hordeum vulgare L.) aleurone layers. The application of the SO2 donor (NaHSO3/Na2SO3, 1:3 M/M) effectively alleviated PCD in barley aleurone layers in a dose-dependent manner with an optimal concentration of 50 μM. Further investigations showed that SO2 reduced the accumulation of hydrogen peroxide (H2O2), superoxide anion (⋅O2−) and malondialdehyde (MDA) in aleurone layers. Moreover, the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR) and guaiacol peroxidase (POD) were enhanced by SO2 donor treatment. Meanwhile, lipoxygenase (LOX) activity was attenuated by SO2 donor treatment. Furthermore, an induction of endogenous H2S and NO were also observed in SO2-treated aleurone layers, suggesting interactions of SO2 with other well-known signaling molecules. Taken together, we show that SO2 negatively regulated PCD by acting as an antioxidant to scavenge excessive reactive oxygen species (ROS) generated during PCD.
Introduction
Programmed cell death (PCD), a form of cell death initiated and regulated by genes, is a common physiological process during plant development [1]. The cereal aleurone layer is a specialized tissue whose function is to synthesize and secrete hydrolytic enzymes that break down reserves in the starchy endosperm. The process of aleurone cell death is a form of PCD. PCD in cereal aleurone cells occurs after germination, a process that is tightly regulated by gibberellic acid (GA3) and abscisic acid (ABA) [2]. Reactive oxygen species (ROS) such as superoxide anion (⋅O2−), hydrogen peroxide (H2O2) and hydroxyl radicals are key players in the PCD process in both plant and animal cells [3]. In aleurone cells, ROS, especially hydrogen peroxide, are key players in the hormone-induced PCD [4]. GA3 treatment initiates a decrease in the activities of ROS metabolizing enzymes catalase (CAT), ascorbate peroxidase (APX) and superoxide dismutase (SOD) and increases the susceptibility of aleurone cells to oxidative stress [5], suggesting that the reduced ability to scavenge ROS in GA3-treated cells may contribute to PCD in aleurone layers.
The gaseous pollutant sulfur dioxide (SO2) can be emitted from natural sources, such as microbial and volcanic activities, and by anthropogenic combustion of sulfur-containing fossil fuels. SO2 readily hydrates in water to form the sulfite ions, (HSO31− and SO32−), strong nucleophiles that can cause damage to a wide variety of cellular components [6]. However, recent findings suggest that endogenous SO2 was a novel gasotransmitter in the cardiovascular system and exhibited multiple pathophysiological effects [7]. In plants, exposure to high doses of SO2 can cause visible effects including chlorophyll destruction, tissue death and long-term yield reduction [8, 9]. At below toxic levels, plants are able to utilize SO2 to satisfy the requirement of sulfur for growth. Indeed, sulfur assimilation and biomass production are correlated with atmospheric SO2 which can be reduced to sulfide and further assimilated into cysteine [10, 11]. A recent study found that low concentrations of SO2 are able to induce transcriptome reprogramming associated with oxidative signaling and biotic defense responses in plants, suggesting a physiological role for SO2 in plants [12]. More evidence indicates that SO2/sulfite can induce stomatal closure and promote tolerance to aluminum stress in wheat, and it has been proposed hydrogen sulfide (H2S) might mediate the effects of SO2 [13, 14].
Like nitric oxide (NO) and H2S, SO2 can also be produced endogenously from sulfur containing amino acids or sulfate, suggesting that SO2 might be a genuine signal in plants [11]. Given the functional similarity between SO2, H2S and NO in animal cells, we hypothesize that rather than just acting as a harmful gas, SO2 might also function as a signaling molecule in delaying GA3-induced PCD of barley aleurone layers. Therefore we studied the effect of SO2 on antioxidant system to understand the mechanism of the role of SO2.
Materials and methods
Plant material and treatments
Seeds of barley (Hordeum vulgare L.) were kindly supplied by Jiangsu Academy of Agricultural Sciences, Jiangsu Province, China. SO2 donor (NaHSO3:Na2SO3, 1:3 M/M) and gibberellic acid (GA3) were purchased from Sigma. Seeds were surface-sterilized as described by Chrispeels and Varner [15]. Then the embryo end of seed was removed, and the embryoless half seed imbibed in water at 25°C for 3 days on Petri dishes and culture solutions were renewed every 24 hours. Aleurone layers were gently isolated by scraping away the starchy endosperm with metal spatulas and incubated in a medium containing 10 mM CaCl2 and 20 μM GA3 with various concentrations of SO2 donor (0, 5, 50, 100, 250 or 500 μM) for indicated time. 50 μM SO2 donor (12.5 μM NaHSO3: 37.5 μM Na2SO3) was used for parameters determination.
Cell viability assay in barley aleurone layers
To determine the effect of SO2 on cell viability of barley aleurone layers, isolated layers were stained with 0.4% trypan blue [16] for 10 min and photographed with Nikon Eclipse 80i fluorescence microscope (Nikon, Tokyo, Japan). The percentage of dead cells was determined by calculating the percentage of blue or purple cells which indicating dead cells in randomly selected fields from three different aleurone layers per treatment.
Determination of the contents of superoxide anion, hydrogen peroxide and malondialdehyde
Embryoless half-grains were pretreated with sterile water for 3 d and then the isolated aleurone layers incubated in GA3 alone or GA3 with 50 μM SO2 donor. Contents of ⋅O2–, H2O2 and MDA were measured according to the methods in [17]. Three independent experiments with three replicates of 15 half-aleurone layers (0.45 ± 0.001 g) were sampled every 12 h for each treatment.
Assays of the activities of antioxidant enzymes and lipoxygenase
Activities of superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), ascorbate peroxidase (APX, EC 1.11.1.11), glutathione reductase (GR, EC 1.6.4.2) and guaiacol peroxidase (POD, EC 1.11.1.7) were determined according to García-Limones et al. [18]. Embryoless half seeds were pretreated with sterile water for 3 days and then incubated in GA3 alone or GA3 plus 50 μM SO2 donor. Frozen aleurone layers (0.45 ± 0.001 g) were homogenized with 1 mL of 200 mM ice-cold phosphate buffer (pH7.8) containing 1.0 mM ethylenediaminetetraacetic acid (EDTA). The homogenate was centrifuged at 12,000 g at 4°C for 20 min, and the supernatant was used for antioxidant enzyme activity assay.
Lipoxygenase (LOX, EC 1.13.11.12) activity was determined following the description by Surrey [19]. Samples (0.45 ± 0.001 g) were homogenized with 1 mL of 200 mM phosphate buffer (pH6.0). The homogenate was centrifuged at 15,000 g at 4°C for 10 min, and the supernatant was used for activity assay. The assay mixture in a total volume of 3 mL contained 200 mM borate buffer (pH6.0), 0.25% linoleic acid, 0.25% Tween-20, and 50 μL of enzyme extract. The reaction was carried out at 25°C for 5 min, and the activity of LOX was monitored by the changes in absorbance at 234 nm.
Effect of SO2 on the activities of α-/β-amylase
Crude extracts of free and bound β-amylase were prepared according to the method of Guerin et al. [20]. Twenty embryoless half grains (0.7 ± 0.001 g) were homogenized with 10 mL Tris–HCl (50 mM, pH7.5). Then, the homogenate passed through three layers of cheesecloth, and was centrifuged at 10,000 g for 30 min. This extraction was repeated three times. The re-suspension of the residue in Tris–HCl buffer was regarded as the bound β-amylase crude enzyme preparation, and the supernatant was collected as free β-amylase crude enzyme. Free form β-amylase was treated with SO2 donor at different concentrations (0, 0.01, 0.02, 0.03, 0.04, 0.05, 1.0, 2.0 mM) for 9 h at 4°C. Meanwhile, bound form β-amylase was incubated in 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 or 0.8 mM SO2 donor for 9 h at 4°C. To study the effect of SO2 to bound β-amylase along with time, 0.8 mM SO2 donor was applied to bound form β-amylase for 0, 3, 6, 9 or 12 h at 4°C.
Twenty embryoless half-grains were imbibed in distilled water at 25°C for 3 days on Petri dishes and incubated in Erlenmeyer flasks which contained different concentrations of SO2 donor in 20 μM GA3 and 10 mM CaCl2. Incubation medium was sampled after 24 h and heated at 70°C for 15 min to eliminate β-amylase activity. The activities of β-amylase and α-amylase secreted to the medium were visualized in 10% native PAGE gels by the starch-iodine method according to Collins et al. [21]. To visualize the bands of α-/β-amylase activity, the gel was incubated at 25°C for 30 min in 50 mM PBS (pH7.0) containing 1% boiled soluble starch. After being washed three times with distilled water, the gel was stained with 0.6% I2 and 6% KI solution. The experiment was repeated three times and similar results were obtained.
Embryoless half seeds were treated with 20 μM GA3 + H2O or 20 μM GA3 + 1 mM SO2 donor and the secreted α-amylase in incubation medium surrounding the half seeds was determined at 0, 12, 24, 36, 48 and 60 h. The DNS method for the determination of secreted α-amylase activity in medium was performed in 0.01 M sodium acetate buffer, pH5.4. The reaction mixture containing 1% soluble starch was incubated at 25°C for 5 min without substrate. Then, the reaction was initiated by adding the substrate and was continued for an additional 10 min at 37°C. The reaction was terminated and hydrolysis was determined with 3,5-dinitrosalicylic acid reagent as modified by Noelting and Bernfeld [22].
Detection of ROS, H2S and NO in aleurone layers by fluorescent probes
Embryoless half seeds were pretreated with sterile water for 3 days. Then aleurone layers were isolated from the embryoless half seeds and were incubated in GA3 alone or GA3 plus 50 μM SO2 donor for 24 and 48 h. Isolated aleurone layers were incubated with the ROS fluorescent probe 2', 7'-dichlorodihydrofluorescein diacetate (DCHF-DA) in 5 μM [23], H2S fluorescent probe 3'-methoxy-3-oxo-3H-spiro [isobenzofuran-1, 9'-xanthen]-6'-yl 2-(pyridin-2-yldisulfanyl) benzoate (WSP-1) in 10 μM [24] or NO fluorescent probe 4-amino-5-methylamino-2',7'-difluorofluorescein diacetate (DAF-FMDA) in 10 μM [25] for 20 min at 37°C in the dark according to manufacturer’s instructions. After that, the aleurone layers were washed with distilled water for three times. The fluorescence of DCHF-DA (excitation at 488 nm, emission at 525 nm), WSP-1 (excitation at 465 nm, emission at 515 nm) or DAF-FMDA (excitation at 495 nm, emission at 515 nm) was observed in aleurone layers using a Nikon Eclipse 80i fluorescence microscope (Nikon, Tokyo, Japan). Non-stained aleurone layers were used as negative control. To quantify the intensity of florescence, three different images were analyzed by ImageJ (NIH, Bethesda, Maryland) software, with higher value representing lower intensity of florescence, and vice versa.
Statistical analysis
Statistical significance was tested by one-way analysis of variance (ANOVA) using IBM SPSS Statistics (SPSS version 20.0; Armonk, NY), and the results were expressed as the means ± SD (standard deviation).
Results
SO2 donor delays programmed cell death of GA3-treated barley aleurone layers
Programmed cell death in aleurone layers is stimulated by GA3. To study whether SO2 is involved in hormonally regulated PCD in aleurone layers, SO2 concentrations ranging from 0 to 500 μM was applied to barley aleurone layers subjected to 20 μM GA3, and the viability of aleurone cells was monitored at 24 and 48 h (Fig 1). As shown in Fig 1, SO2 delayed PCD in GA3-treated barley aleurone layers in a dose-dependent manner, with a maximal biological response at 50 μM. After 24 h incubation, 8% of cells were found dead in aleurone layers treated with 50 μM SO2 donor, while approximately 38% of cells in control layers underwent PCD (Fig 1A and 1C). After 48 h incubation, the percentage of dead cells increased to 87% in GA3-treated aleurone layers in comparison with only a percentage of 21% dead cells in SO2 treatment (Fig 1B and 1C).
Fig 1. Effect of SO2 donor on cell viability in barley aleurone layers.
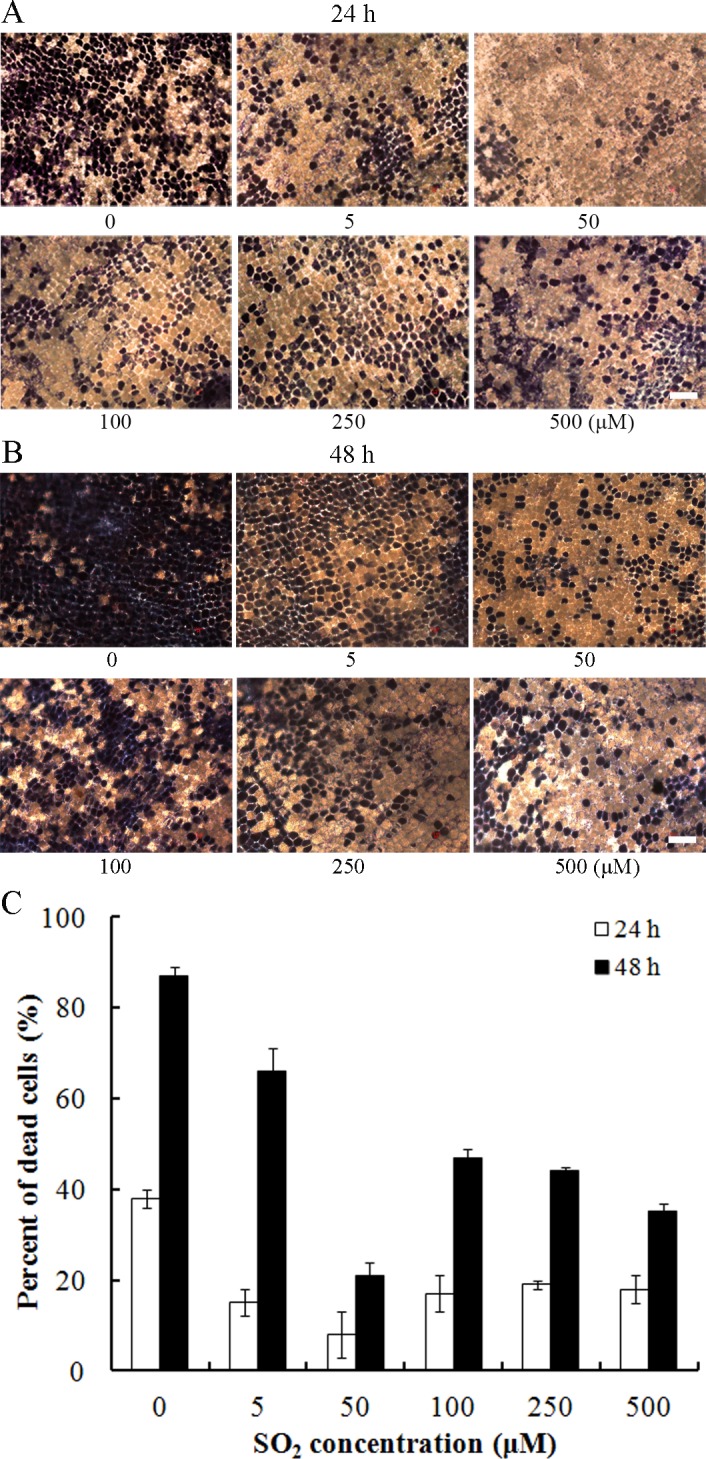
Aleurone layers are incubated in different concentrations of SO2 donor (0, 5, 50, 100, 250, and 500 μM) for 24 h (A) and 48 h (B) in presence of 20 μM GA3 at 25°C. After staining with trypan blue, barley aleurone layers were viewed by light microscopy. The percentages of dead cells (blue or purple staining) are shown in (C). Bar, 100 μm. Data are expressed as means ± SD of three different aleurone layers per treatment.
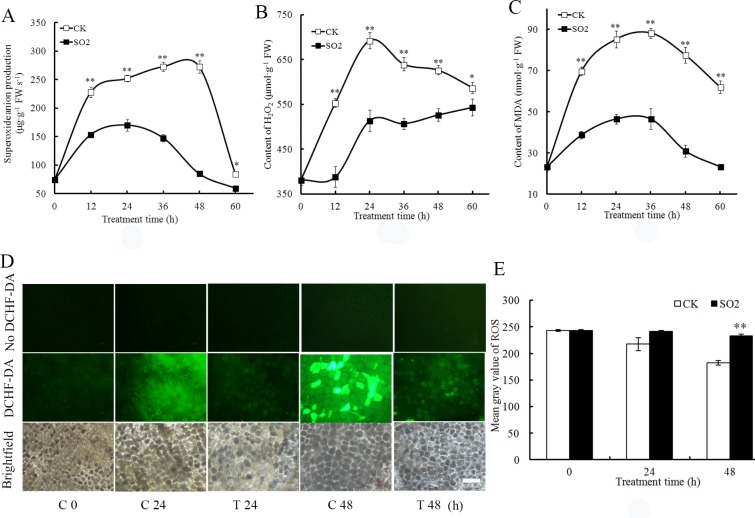
Effects of SO2 donor on the contents of reactive oxygen species and malondialdehyde in GA3-treated barley aleurone layers
It has been reported that ROS are tightly associated with the promotion of PCD in barley aleurone cells [4]. The alleviating role of SO2 in PCD of barley aleurone layers led us to examine whether ROS accumulation was attenuated by SO2 treatment. As shown in Fig 2A, the SO2 donor significantly alleviated the accumulation of ⋅O2– in GA3-treated barley aleurone layers. The production of ⋅O2– in control aleurone layers increased dramatically during the first 48 h of incubation followed by a decline at 60 h. However the addition of SO2 prevented ⋅O2– accumulation in aleurone layers. For instance, the production of ⋅O2– in SO2 treatment at 36 h was about half of that in control layers. Fig 2B illustrates that H2O2 content in GA3-treated layers increased rapidly and peaked at 24 h followed by a gradual decrease. In contrast, the content of H2O2 in SO2-treated layers was maintained at a significantly lower level than that of control as an attenuated accumulation of H2O2 was observed in SO2 treatment (Fig 2B).
Fig 2.
Effects of SO2 donor on the contents of superoxide anion (⋅O2–) (A), hydrogen peroxide (H2O2) (B) and malondialdehyde (MDA) (C) in GA3-treated barley aleurone layers. Aleurone layers are treated with 20 μM GA3 + H2O (CK) or 20 μM GA3 + 50 μM SO2 donor (SO2) for 60 h. Aleurone layers treated for 24 and 48 h are incubated with DCHF-DA and observed by fluorescence microscopy (D). The relative fluorescence intensity of images analyzed by ImageJ is shown in (E). Data are expressed as means ± SD of three independent experiments with three replicates of 15 aleurone layers per treatment. The symbols * and ** in this figure and following ones stand for significant difference at P < 0.05 and P < 0.01 between the control and SO2 treatment, respectively. Bar, 100 μm.
MDA was determined as an index of lipid peroxidation. MDA content increased rapidly in control aleurone layers and peaked at 36 h followed by a decrease. In contrast, SO2 treatment significantly lowered the level of MDA (Fig 2C).
ROS-sensitive fluorescent probe DCHF-DA was applied to aleurone layers to observe ROS production (Fig 2D and 2E). Fluorescence from layers incubated in SO2 plus GA3 was much less intense than GA3 controls at 24 and 48 h. More weak fluorescence was detected in tissue treated with SO2.
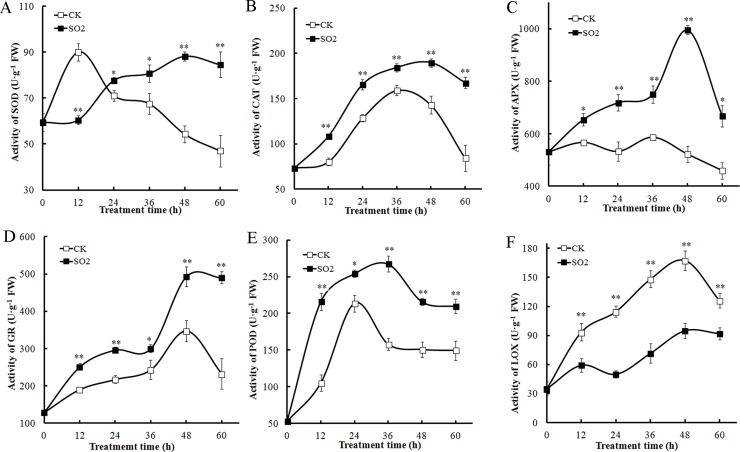
Effects of SO2 on the activities of SOD, CAT, APX, GR, POD and LOX in GA3-treated barley aleurone layers
To study the antioxidant role of SO2 donor in GA3-treated barley aleurone layer, we determined the activities of antioxidant enzymes, such as SOD, CAT, APX, GR and POD, and lipid peroxidation-related enzyme LOX (Fig 3). The activity of SOD increased rapidly in GA3-treated aleurone layers in the first 12 h and decreased gradually. However, SOD activity in SO2 treatment kept stable during the first 12 h of incubation followed by a steady increase which led to a significantly higher level compared with that of control after 24 h (Fig 3A). As shown in Fig 3B, CAT activity in control increased dramatically till 36 h of incubation followed by a drop. Similar change pattern of CAT activity was observed in SO2 treatment, but SO2 treatment significantly enhanced CAT activity compared with water control. SO2 treatment also significantly improved APX activity in GA3-treated aleurone layers during the whole treatment period (Fig 3C). The activity of APX in SO2 treatment dramatically increased and peaked at 48 h followed by a sharp decrease. However, APX activity in water control fluctuated and a gradual decrease was observed after 36 h of incubation. GR activity in SO2-treated layers increased rapidly during the first 48 h of incubation followed by a plateau, while GR activity in control increased slightly and peaked at 48 h followed by a drop (Fig 3D). During the whole treatment time, SO2 significantly enhanced GR activity in barley aleurone layers in comparison to control. Fig 3E shows that the SO2 donor maintained a higher level of POD activity in barley aleurone layers compared with control during the entire incubation. POD activity in GA3 controls increased and peaked at 24 h followed by a decrease. Compared with GA3-treated samples, GA3 plus SO2 treatment induced a more rapid increase in POD activity until 36 h followed by a decrease (Fig 3E).
Fig 3.
Effects of SO2 donor on the activities of SOD (A), CAT (B), APX (C), GR (D), POD (E), and LOX (F) in GA3-treated barley aleurone layers. The aleurone layers were treated with 20 μM GA3 + H2O (CK) or 20 μM GA3 + 50 μM SO2 donor (SO2) for 60 h. Data are expressed as means ± SD of three independent experiments.
LOX activities are responsible for lipid peroxidation. LOX activity increased greatly in GA3-treated aleurone layers during the first 48 h followed by a decrease (Fig 3F). By contrast, GA3 plus SO2 treatment dramatically attenuated the increase in LOX activity though an increasing trend of LOX activity was observed (Fig 3F).
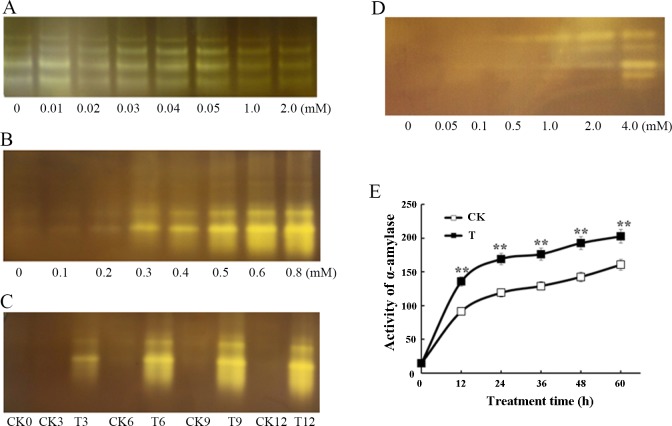
SO2 donor promotes β-amylase activity and α-amylase secretion in GA3-treated barley aleurone layers
β-Amylase is an exoamylase that hydrolyzes α-1, 4 glycosidic linkages of polyglucan chains at the non-reducing end to produce maltose. Activation of β-amylase by NO was regarded as an early event in seed germination [26]. Thus we examined whether SO2 also has a role in β-amylase activation. After treatment of free β-amylase with different concentrations of SO2 donor, 0.01 mM SO2 donor exhibited a maximal biological response as shown in native PAGE (Fig 4A). Fig 4B shows native PAGE analysis of the activity of bound form β-amylase treated with different concentrations of SO2 donor and 0.8 mM SO2 donor was found to maximally activate bound β-amylase compared with samples not treated with SO2. SO2 donor at 0.8 mM was applied to bound form β-amylase for 0, 3, 6, 9 and 12 h to study the time changes of SO2 effect (Fig 4C). As shown in native PAGE, treatment of SO2 for 9 h effectively activated bound form β-amylase.
Fig 4. SO2 enhances the activities of free and bound forms of β-amylase, and of α-amylase in embryoless barley grains.
(A) shows native PAGE analysis of the activity of free form β-amylase in embryoless barley grains treated with different concentrations of SO2 donor for 9 h and (B) native PAGE analysis of bound form β-amylase activity. SO2 donor at 0.8 mM is applied to bound form β-amylase for 0, 3, 6, 9 and 12 h and the activity is shown on native PAGE (C). Embryoless barley grains are incubated with different concentrations of SO2 donor plus 20 μM GA3 for 48 h and α-amylase activity in incubation medium surrounding the aleurone layers is visualized by native PAGE (D). (E) shows secreted α-amylase activity in incubation medium surrounding embryoless half seeds treated with 20 μM GA3 + H2O (CK) or 20 μM GA3 + 1 mM SO2 donor (T) at different times of incubation. Data in (E) are expressed as means ± SD of three independent experiments with three replicates of 20 embryoless half grains per treatment.
In response to GA3, α-amylase is secreted from aleurone layers to degrade the starch granules in the non-living endosperm [1]. We therefore tested whether the alleviating effect of SO2 on PCD affected the release of α-amylase. As shown in native PAGE of Fig 4D, SO2 concentrations above 0.5 mM in the presence of GA3 promoted the secretion of α-amylase at 48 h of treatment. Fig 4E shows the time changes in α-amylase secretion from GA3-treated aleurone layers and GA3 plus 1 mM SO2 treated layers. The activity of α-amylase in incubation medium in GA3-treated layers increased with time, whereas addition of 1 mM SO2 brought about a more rapid increase till 60 h (Fig 4E). The activity of α-amylase released following GA3 plus SO2 treatment was significantly higher than that of layers incubated only in GA3 during the whole treatment time.
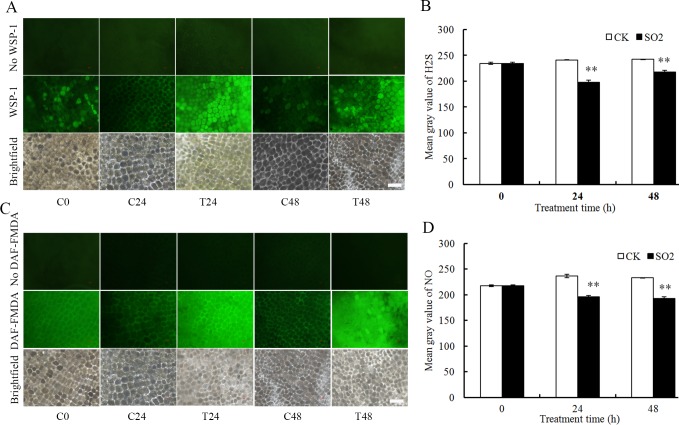
Endogenous H2S and NO in SO2-treated barley aleurone layers
Sulfite can be reduced by sulfite reductase to H2S and NO has been found to mediate H2S’s function in guard cell movement [11, 27]. To investigate whether exogenous SO2 application can induce endogenous H2S and NO production, we examined contents of endogenous H2S and NO in SO2-treated barley aleurone layers. H2S in barley aleurone layers was indicated by fluorescent probe WSP-1. As shown in Fig 5A and 5B, SO2 treatment significantly enhanced H2S fluorescence intensity at 24 and 48 h compared with the weak fluorescence in control. Fig 5C and 5D showed that endogenous NO content in control layers decreased significantly at 24 and 48 h, whereas SO2 sustained endogenous NO production.
Fig 5.
Effects of SO2 donor on endogenous contents of H2S (A, B) and NO (C, D) in GA3-treated barley aleurone layers. Microscopy images of barley aleurone layers (A, C) and relative fluorescence intensity of H2S (B) and NO (D) analyzed by ImageJ are shown. Barley aleurone layers incubated with the probe WSP-1 (A, B) or DAF-FM DA (C, D) or no probe. CK: 20 μM GA3 + H2O treatment; T or SO2: 20 μM GA3 + 50 μM SO2 donor treatment. Bar, 100 μm. Data are means ± SD of three independent experiments.
Discussion
SO2 was regarded as a toxic gas and environmental pollutant. However, SO2 can be endogenously generated from metabolism of the sulfur-containing amino acid L-cysteine and from sulfate in plants and its physiological role was recently found in plants [11, 13, 14]. In neural fluid or mammalian plasma, SO2 was broken down to its derivatives, bisulfite and sulfite, as the physiological active form of SO2 in vivo [28]. Thus NaHSO3/Na2SO3 (1:3 M/M) was chosen as an SO2 donor in our study. GA3 treatment induced cell death in about 87% aleurone cells at 48 h, while the addition of the SO2 donor effectively alleviated PCD process in barley aleurone layers (Fig 1).
ROS, such as ⋅O2− and H2O2, can promote PCD in plant and animal cells [3]. In aleurone cells, GA3-stimulated cell death is possibly mediated by ROS while ABA inhibits it [4]. The mechanism of ROS burst is due to GA3-caused decrease in the activities of antioxidant enzymes CAT, APX and SOD [1], suggesting that the reduced ability to scavenge ROS in GA3-treated cells contribute to PCD. Consistently, the increase of ⋅O2− and H2O2 and the accumulation of MDA were also accompanied by PCD in barley aleurone layers (Fig 2), confirming the vital role ROS played in PCD. However SO2 treatment effectively reduced the accumulation of ROS in barley aleurone layers in the presence of GA3 (Fig 2), thereby delaying PCD process in these cells. Further investigation indicates that SO2 treatment enhanced the activities of ROS-scavenging enzymes SOD, CAT, APX, GR and POD (Fig 3A–3E). The increased activities of ROS-scavenging enzymes in SO2+GA3 treatment might promote the cell’s ability to scavenge excessive ROS. In addition, LOX activities which are responsible for lipid peroxidation were down-regulated in SO2 and GA3-treated aleurone layers (Fig 3F).
The role of ROS in GA3 and ABA signaling in barley aleurone cells was recently clarified [29]. Furthermore, exogenous H2O2 could promote the induction of α-amylase by promoting the expression of GAMyb and α-amylase genes, whereas antioxidants suppressed the induction of α-amylase. Unexpectedly, we found that SO2 +GA3 reduced ROS accumulation and delayed PCD process in barley aleurone layers and meanwhile promoted the secretion of α-amylase (Fig 4D and 4E), suggesting that antioxidants do not always suppress the induction of α-amylase. The effect of SO2 on isolated free and bound β-amylase was also researched. SO2 treatment significantly enhanced the activity of bound β-amylase and only weakly activated free β-amylase (Fig 4A–4D). The underlying mechanism of SO2 in the activation of β-amylase is unknown and still needs further investigation, but the works on NO may shed light on it. As previously reported, NO donor, was able to induce a rapid increase in β-amylase activity by directly releasing β-amylase from its bound form [26].
The sulfite can be reduced by sulfite reductase (SiR; EC 1.8.7.1) by a process that transfers six electrons from ferredoxin to produce the fully reduced sulfide form [11]. H2S is already known to function in multiple processes in plants and in some cases, NO mediates the physiological role of H2S [27]. Thus the contents of endogenous H2S and NO were determined in SO2-treated aleurone layers. The increased contents of H2S and NO in SO2-treated aleurone layers suggest the interplay between sulfite and the two already confirmed signals H2S and NO.
Conclusions
In summary, this data show that the SO2 donor alleviates PCD of GA3-treated barley aleurone cells by reducing ROS accumulation through enhancing the activities of antioxidant enzymes. Besides, SO2+GA3 treatment also decreases the activity of LOX, an important indicator of lipid peroxidation. Increased levels of endogenous H2S and NO further add more evidence that SO2 acts as a novel antioxidant gasotransmitter in PCD of aleurone layers and H2S and NO may mediate SO2’s role in alleviating PCD.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by National Natural Science Foundation of China (31271803, 31470013, 31670278 to Dr. Hua Zhang; 31300133 to Dr. Kang-Di Hu), Scientific Research Foundation for Returned Overseas Chinese Scholars (SRF for ROCS, MOE to Dr. Hua Zhang), Natural Science Foundations of Anhui Province (11040606M85 to Dr. Hua Zhang), Anhui Provincial Education Department (2012AJZR0028, ZD200910 to Dr. Hua Zhang), the earmarked fund for China Agriculture Research System (CARS-10-B1 to Jun Tang) and Anhui Provincial Science and Technology Major Project (6030701073 to Dr. Hua Zhang). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fath A, Bethke P, Lonsdale J, Meza-Romero R, Jones R. Programmed cell death in cereal aleurone. Plant Mol Biol. 2000; 44(3): 255–266. [DOI] [PubMed] [Google Scholar]
- 2.Bethke PC, Lonsdale JE, Fath A, Jones RL. Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell. 1999; 11(6): 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 1999; 57(3): 231–245. [DOI] [PubMed] [Google Scholar]
- 4.Bethke PC, Jones RL. Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J. 2001; 25(1): 19–29. [DOI] [PubMed] [Google Scholar]
- 5.Fath A, Bethke PC, Jones RL. Enzymes that scavenge reactive oxygen species are down-regulated prior to gibberellic acid-induced programmed cell death in barley aleurone. Plant Physiol. 2001; 126(1): 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro R. Genetic effects of bisulfite (sulfur dioxide). Mutat Res. 1977; 39(2): 149–175. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Tang C, Du J, Jin H. Endogenous sulfur dioxide: a new member of gasotransmitter family in the cardiovascular system. Oxid Med Cell Longev. 2016; 2016: 8961951 doi: 10.1155/2016/8961951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Kooij TAW, De Kok LJ, Haneklaus S, Schnug E. Uptake and metabolism of sulphur dioxide by Arabidopsis thaliana. New Phytol. 1997; 135(1): 101–107. [DOI] [PubMed] [Google Scholar]
- 9.Noji M, Saito M, Nakamura M, Aono M, Saji H, Saito K. Cysteine synthase overexpression in tobacco confers tolerance to sulfur-containing environmental pollutants. Plant Physiol. 2001; 126(3): 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rennenberg H. The fate of excess sulfur in higher plants. Annu Rev Plant Biol. 1984; 35(1): 121–153. [Google Scholar]
- 11.Rausch T, Wachter A. Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci. 2005; 10(10): 503–509. doi: 10.1016/j.tplants.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 12.Giraud E, Ivanova A, Gordon CS, Whelan J, Considine MJ. Sulphur dioxide evokes a large scale reprogramming of the grape berry transcriptome associated with oxidative signalling and biotic defence responses. Plant Cell Environ. 2012; 35(2): 405–417. doi: 10.1111/j.1365-3040.2011.02379.x [DOI] [PubMed] [Google Scholar]
- 13.Hu KD, Tang J, Zhao DL, Hu LY, Li YH, Liu YS, et al. Stomatal closure in sweet potato leaves induced by sulfur dioxide involves H2S and NO signaling pathways. Biol Plant. 2014; 58(4): 676–680. [Google Scholar]
- 14.Zhu DB, Hu KD, Guo XK, Liu Y, Hu LY, Li YH, et al. Sulfur dioxide enhances endogenous hydrogen sulfide accumulation and alleviates oxidative stress induced by aluminum stress in germinating wheat seeds. Oxid Med Cell Longev. 2015; 2015: 612363 doi: 10.1155/2015/612363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrispeels MJ, Varner JE. Gibberellic acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967; 42(3): 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tennant JR. Evaluation of the trypan blue technique for determination of cell viability. Transplantation. 1964; 2: 685–694. [DOI] [PubMed] [Google Scholar]
- 17.Maestro RFD, Björk J, Arfors KE. Increase in microvascular permeability induced by enzymatically generated free radicals. I. In vivo study. Microvasc Res. 1981; 22(3): 239–254. [DOI] [PubMed] [Google Scholar]
- 18.García-Limones C, Hervás A, Navas-Cortés JA, Jiménez-Díaz RM, Tena M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris ☆. Physiol Mol Plant Pathol. 2002; 61(6): 325–337. [Google Scholar]
- 19.Surrey K. Spectrophotometric method for determination of lipoxidase activity. Plant Physiol. 1964; 39(1): 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerin JR, Lance RCM, Wallace W. Release and activation of barley β-amylase, by malt endopeptidases. J Cereal Sci. 1992; 15(1): 5–14. [Google Scholar]
- 21.Collins GG, Paleg LG. The metabolism of soluble nucleotides in wheat aleurone layers treated with gibberellic acid. Plant Physiol. 1972; 49(3): 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noelting G, Bernfeld P. Sur les enzymes amylolytiqucs III. La β-amylase: dosage d'activité et contrôle de l'absence d'α-amylase. Helv Chim Acta. 1948; 31(1): 286–290. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchiya M, Suematsu M, Suzuki H. In vivo visualization of oxygen radical-dependent photoemission. Methods Enzymol. 1994; 233: 128–140. [DOI] [PubMed] [Google Scholar]
- 24.Xue YF, Zhang M, Qi ZQ, Li YQ, Shi Z, Chen J. Cinnamaldehyde promotes root branching by regulating endogenous hydrogen sulfide. J Sci Food Agric. 2016; 96(3): 909–914. doi: 10.1002/jsfa.7164 [DOI] [PubMed] [Google Scholar]
- 25.Mur LAJ, Mandon J, Cristescu SM, Harren FJM, Prats E. Methods of nitric oxide detection in plants: a commentary. Plant Sci. 2011; 181(5): 509–519. doi: 10.1016/j.plantsci.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Shen WB, Zhang W, Xu LL. A rapid response of β-amylase to nitric oxide but not gibberellin in wheat seeds during the early stage of germination. Planta. 2005; 220(5): 708–716. doi: 10.1007/s00425-004-1390-7 [DOI] [PubMed] [Google Scholar]
- 27.Scuffi D, Alvarez C, Laspina N, Gotor C, Lamattina L, Garcia-Mata C. Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol. 2014; 166(4): 2065–2076. doi: 10.1104/pp.114.245373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed GA, Ryan MJ, Adams KS. Sulfite enhancement of diolepoxide mutagenicity: the role of altered glutathione metabolism. Carcinogenesis. 1990; 11(9): 1635–1639. [DOI] [PubMed] [Google Scholar]
- 29.Ishibashi Y, Tawaratsumida T, Kondo K, Kasa S, Sakamoto M, Aoki N, et al. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol. 2012; 158(4): 1705–1714. doi: 10.1104/pp.111.192740 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.