Abstract
Selenium (Se) is a critical element in thyroid function, and variable dietary Se intake influences immunity. Consequently, dietary Se could influence development of thyroid autoimmunity and provide an adjunct to treat autoimmune thyroid dysfunction. Nonobese diabetic (NOD).H2h4 mice spontaneously develop autoantibodies to thyroglobulin (Tg) and thyroid peroxidase (TPO). This mouse strain expressing a human thyroid-stimulating hormone receptor (TSHR) A-subunit transgene in the thyroid also develops pathogenic TSHR autoantibodies. In this report, we investigated whether dietary Se influences these immune processes. Male and female wild-type and transgenic NOD.H2h4 mice were maintained on normal-, low-, or high-Se (0.1, 0, or 1.0 mg/kg) rodent diets. After 4 months, Se serum levels were extremely low or significantly increased on 0 or 1.0 mg/kg Se, respectively. Varying Se intake affected Tg antibody (TgAb) levels after 2 (but not 4) months; conversely, TPO antibody (TPOAb) levels were altered by dietary Se after 4 (but not 2) months. These data correspond to the earlier development of TgAb than TPOAb in NOD.H2h4 mice. In males, TgAb levels were enhanced by high Se and in females by low Se intake. Se intake had no effect on pathogenic TSHR autoantibodies in TSHR transgenic NOD.H2h4 females. In conclusion, in susceptible NOD.H2h4 mice, we found no evidence that a higher dietary Se intake ameliorates thyroid autoimmunity by reducing autoantibodies to Tg, TPO, or the TSHR. Instead, our finding that low dietary Se potentiates the development of autoantibodies to Tg and TPO in females is consistent with reports in humans of an increased prevalence of autoimmune thyroiditis in low-Se regions.
In NOD.H2h4 mice, low dietary Se potentiates the development of thyroid autoantibodies in females, consistent with an increased prevalence of autoimmune thyroiditis in low selenium regions.
Selenium (Se) is a critical element for normal thyroid function, and variability in dietary Se influences immune responses [reviewed in (1–5)]. Consequently, Se intake has the potential to affect thyroid autoimmunity in humans both before disease manifestation and as a possible adjunct to therapy. Serum levels of Se are low in some newly diagnosed patients who have Graves disease (6). Similarly, low Se intake was associated with an increased prevalence of thyroiditis in a large group of Chinese patients (7). In the reverse direction, increased dietary Se was associated with decreased thyroid autoantibody levels in some investigations but was without effect in other studies (8). However, in a recent meta-analysis, increased Se intake reduced autoantibodies to thyroid peroxidase (TPO) for up to 12 months when combined with l-thyroxine (T4) but for only 3 months without l-T4 (9).
In mice, numerous studies have investigated the outcome of variable Se dietary intake on immune responses. For example, nonautoimmune-prone mice (C57BL/6 strain) infected with Listeria and maintained on a Se-deficient diet produced less interferon-γ; tumor necrosis factor-α; and interleukins 6, 10, and 18 than mice of the same strain on a control-Se diet (10). Similarly, production of interferon-γ and interleukin 6 was defective in FVB/N mice on a Se-deficient diet (11). In the nonobese diabetic (NOD).H2h4 strain in which spontaneous thyroiditis is enhanced by dietary iodine (12–14), Se supplementation increased regulatory T cells and caused a small (but significant) decrease in autoantibodies to thyroglobulin (Tg) (15, 16).
Recently, we developed a mouse strain that spontaneously develops pathogenic antibodies to the thyrotropin receptor (TSHR) (17). This novel TSHR/NOD.H2h4 strain was generated by transferring the transgene for the human thyroid-stimulating hormone receptor (TSHR) A-subunit targeted to the thyroid from BALB/c mice (18, 19) to nontransgenic NOD.H2h4 recipients. As we and others have shown, the TSHR A-subunit shed after cleavage of the membrane bound TSHR is the target of the autoimmune response in Graves disease (20–22). Unlike nontransgenic NOD.H2h4 mice, which require immunization to develop TSHR antibody (TSHRAb), mice of the TSHR/NOD.H2h4 strain develop pathogenic TSHRAbs spontaneously (17). In addition, transgenic TSHR/NOD.H2h4 mice develop Tg antibodies (TgAbs) and TPO antibodies (TPOAbs), like their nontransgenic littermates (12–14).
In the current study, we used NOD.H2h4 mice with and without the TSHR A-subunit transgene to address the question of whether long-term dietary intake of Se influences, on the one hand, the spontaneous development of autoantibodies to Tg and TPO and, on the other hand, pathogenic autoantibodies to the TSHR.
Methods
Mice studied
NOD.H2h4 mice (originally from The Jackson Laboratory, Bar Harbor, ME) and transgenic TSHR/NOD.H2h4 mice (17) (which express low levels of the human TSHR A-subunit in the thyroid and thymus) were bred at Cedars-Sinai Medical Center. Mice of the TSHR/NOD.H2h4 strain have been cryopreserved by the Mutant Mouse Regional Resource Center under the designation NOD.Cg-Tg(TG-TSHR)51.9Smcl/Mmmh (MMRRC:037586-MU). Beginning at 8 weeks of age, all mice were provided with drinking water containing 0.05% sodium iodide (NaI). At the same time and continuing until the end of the study, different groups of NOD.H2h4 and TSHR/NOD.H2h4 mice (similar numbers of males and females) were fed custom diets containing various amounts of Se (see later). Blood was drawn 2 months after starting the Se diets together with NaI water. After 4 months on the diets and NaI water, mice were euthanized to harvest blood and thyroid glands. All mouse studies were approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center and conducted in accordance with mandated standards of humane animal care.
Custom Se diets
The following custom diets were obtained from Envigo (Madison WI): Se deficient (Lo; 0 mg/kg TD.92163), Se control (Con; 0.1 mg/kg TD.96363), and Se enriched (Hi; 1.0 mg/kg TD.120305). These diets had previously been used to investigate the role of Se in preeclampsia in rats and rhabdomyolysis in rabbits (23, 24).
Se content of custom diets and mouse sera
Sera from mice exposed for 4 months to diets with varying Se content were pooled (four to 10 mice) to provide duplicate aliquots of 220 μL per mouse group. We also analyzed ∼100 mg of each diet for Se content. After digestion with concentrated acid (see Supplemental Methods), samples were analyzed by inductively coupled plasma mass spectrometry (Agilent 9000c; Agilent Technologies, Wilmington, DE) with argon plasma. Se data are presented for sera as μg/L and for custom diets as mg/g dry weight.
Autoantibodies to Tg and TPO (TgAb and TPOAb)
Mouse Tg was isolated from murine thyroid glands as previously described (25). Enzyme-linked immunosorbent assay (ELISA) wells (Immulon 4HBX; Thermo Scientific, Rochester, NY) were coated with mouse Tg (1.5 μg/ml) and incubated with test sera (1:100 dilution) in duplicate. Antibody binding was detected with horseradish peroxidase–conjugated goat anti-mouse immunoglobulin G (IgG; catalog no. A3673; Sigma Chemical Co., St. Louis, MO; Table 1), the signal developed with o-phenylenediamine (Sigma Chemical Co.) and the reaction stopped with 20% (volume-to-volume ratio) H2SO4. The negative control was serum from 8-week-old NOD.H2h4 mice on regular water; the positive control was serum from BALB/c mice immunized with mouse Tg and complete Freund adjuvant (26). TgAb values are presented as the optical density (OD) at 490 nm.
Table 1.
Antibodies Used to Detect Mouse IgG Class (or IgG Subclass) Antibodies Binding in ELISA or in Flow Cytometry
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Catalog No., Manufacturer | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Mouse IgG | Horseradish peroxidase-conjugated anti-mouse IgG | A3673, Sigma Chemical Co. | Polyclonal; goat | 1:1000 | AB_258099 | |
| Mouse IgG | Goat anti-mouse IgG (H+L) secondary antibody FITC | A16073, Thermo Fisher | Polyclonal; goat | 10 μg/mL | AB_253476 | |
| Mouse T reg | Mouse regulatory T cell staining kit #1 | 88-8111, Affymetrix | Monoclonal; rat | As per kit | AB_2575187 | |
| Mouse IgG1 | Anti-mouse IgG1 polyclonal antibody, biotin conjugated | 10708, Southern Biotech | Polyclonal; goat | 1:1000 | AB_609714 | |
| Mouse IgG2a | Anti-mouse IgG2b: biotin conjugated | 10908, Southern Biotech | Polyclonal; goat | 1:1000 | AB_609729 |
Abbreviation: RRID, Research Resource Identifier.
TPOAb were measured using Chinese hamster ovary (CHO) cells stably expressing murine TPO (25). Sera (1:50 dilution) were incubated with mouse TPO-CHO cells and binding was detected with goat anti-mouse IgG conjugated to fluoroscein-isothiocyanate (catalog no. A16073; Thermo Fisher, Waltham, MA; Table 1). Cells staining with propidium iodide (1 μg/mL) were excluded from analysis. The negative control for IgG class antibody binding to mouse TPO-CHO cells was serum from young NOD.H2h4 mice (8 weeks old) on control water. Positive controls were mouse monoclonal antibodies 15 and 64 to human TPO (27), provided to us by Dr. J. Ruf (Marseille, France), which recognize mouse TPO (25, 27). Flow cytometry was performed (10,000 events) using a BD FACScanto II with FACSDiva Software (Becton Dickinson, San Jose, CA) for collection and FloJo v.X.07 software (FloJo, Ashland, OR) for analysis. Data are reported as the geometric mean.
IgG subclasses of TgAb
The distribution of TgAb between subclass IgG2b and IgG1 was measured using a modification of the TgAb ELISA described previously. One set of duplicate mouse Tg coated wells was exposed to biotinylated anti-IgG2b and another set to biotinylated anti-IgG1 (both antibodies from Southern Biotech, Birmingham AL; Table 1). After washing, wells were incubated with streptavidin peroxidase (BD Biosciences, San Jose, CA) and washed again, and the signal developed with o-phenylenediamine and the reaction stopped with 20% (volume-to-volume ratio) H2SO4. IgG subclass data are reported for individual mice as the ratio of OD values obtained using anti-IgG2b to the OD values obtained using anti-IgG1. Sera were studied after 4 months on the diet (insufficient serum was available for separate analysis of males and females after 2 months).
Assay for pathogenic TSHR antibodies
TSHR antibodies were measured by thyroid-stimulating hormone (TSH)–binding inhibition (TBI) in 25μL mouse serum using a clinical assay kit (Kronus Inc, Star ID). The data are reported as the percent inhibition of 125I-TSH binding to the TSH holoreceptor.
Regulatory T cells in splenocytes
At euthanasia, spleens were aseptically collected, washed and disrupted manually to release mononuclear cells. Red blood cells were lysed with ammonium chloride potassium lysing buffer. Viability determined with Trypan blue (Sigma Chemical Co.) was >90%. Splenocytes (107 cells) were stained with mouse regulatory T cell staining kit 1 (Cleveland, OH) following the manufacturer’s instructions: splenocytes were first incubated with anti-mouse CD4 and anti-mouse CD25; subsequently, the cells were permeabilized and incubated with anti-rat Foxp3 (or isotype control antibody). Stained cells were analyzed with a BD FACScanto II and FACSDiva Software (Becton Dickinson). Cells in the lymphocyte gate were used for analysis. Data are reported as percentage of FoxP3 positive (+ve) CD25 +ve T cells.
Serum T4 and TSH and thyroid histology
T4 levels were measured (10 μL aliquots) by ELISA (mouse/rat T4 ELISA; Calbiotech, El Cajon, CA); data are reported as μg T4/dL. TSH was measured by radioimmunoassay (28) (Dr. S. Refetoff; University of Chicago; fee for service) and reported as mU/L. Thyroid glands were preserved in zinc fixative (BD Pharmingen, San Diego, CA) and paraffin-embedded, and serial sections were stained with hematoxylin and eosin (IDEXX BioResearch Laboratory Animal and Biological Materials Diagnostic Testing, Columbia, MO). The percentage of the thyroid gland infiltrated with lymphocytes (the extent of thyroiditis) was scored “blind” by two researchers (B.B. and S.M.M.) and used to calculate the mean values.
Statistics
Significant differences between responses in different groups were determined by the Mann–Whitney rank sum test or, when normally distributed, by Student t test. Multiple comparisons were made using analysis of variance (ANOVA). Tests were performed using SigmaStat (Jandel Scientific Software, San Rafael, CA).
Results
In NOD.H2h4 mice, beginning at 8 weeks of age, we assessed the effect of varying Se dietary intake (Con, Lo, and Hi), provided for up to 4 months, on the spontaneous development of autoimmune thyroiditis (TgAb, TPOAb, and lymphocytic infiltration). Also, we examined NOD.H2h4 mice transgenic for the human TSHR A-subunit that, in addition to TgAb and TPOAb, spontaneously develops TSHR antibodies (17). Dietary iodine supplementation, the standard protocol used in the NOD.H2h4 mouse model to accelerate the autoimmune process (12–14), remained constant throughout the period of varying dietary Se intake. We studied males and females separately in both wild-type and transgenic NOD.H2h4 mice because sexual dimorphism is well recognized in autoimmunity [reviewed in (29)], and serum TSH levels are significantly higher in male than in female NOD.H2h4 (17).
Two critical time points were selected to study the effects of varying Se intake: (1) after 2 months of exposure, when TgAb are detectable in most mice, and (2) after 4 months, when TPOAb are detectable (25) and TgAb levels are significantly higher than after 2 months (30).
Thyroglobulin antibodies
As occurs in NOD.H2h4 mice fed regular chow, TgAb levels generally increased from the 2-month to the 4-month time point independent of the dietary Se content or the presence or absence of the human TSHR A-subunit transgene (Fig. 1). However, there were significant differences between subgroups and at different times.
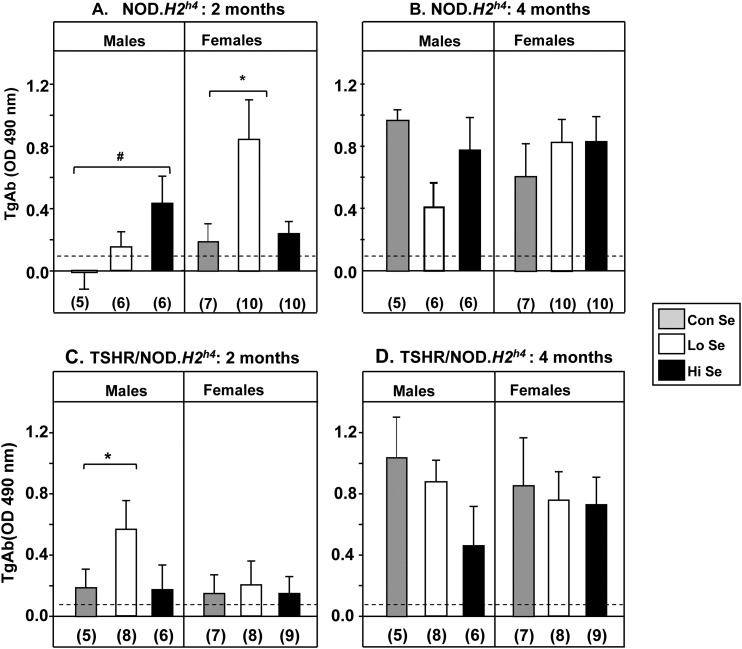
Figure 1.
TgAb levels in NOD.H2h4 mice are affected after 2 (but not 4) months’ exposure to variable Se dietary intake. Males and females respond differently. Rodent diets indicated as Lo, Con, or Hi contained 0.0, 0.1, or 1.0 mg/kg Se, respectively, together with iodide-supplemented water. Nontransgenic NOD.H2h4 males and females after (A) 2 months and (B) 4 months on the diets Transgenic TSHR/NOD.H2h4 males and females after (C) 2 months and (D) 4 months on the diets. Data are shown as the mean + standard error of the mean values for TgAb (OD 490 nm in ELISA); the number of mice in each group is given in parentheses. Statistically significant differences: (A) males #P = 0.036 (ANOVA); females *P = 0.045 (rank sum test); (C) males: #P = 0.029 (rank sum test). The dashed lines indicate the mean + 2 standard deviations of TgAb observed in negative controls (see Methods).
After 2 months on varying Se dietary intake, TgAb levels in NOD.H2h4 mice were higher in males on Hi-Se vs Con-Se diet (P = 0.036) as opposed to females in which the Lo-Se diet significantly increased TgAb levels (P = 0.045; Fig. 1A). Remarkably, we observed the reverse outcome with the male transgenic TSHR/NOD.H2h4 mice in that the Lo-Se (not the Hi-Se) diet increased TgAb levels (P = 0.029). Varying Se intake was without effect on the female transgenics (Fig. 1C).
After 4 months on diets with different Se contents, at which time point TgAb levels were generally much higher, no significant differences were observed for any group of NOD.H2h4 mice, male or female, wild-type (Fig. 1B), or TSHR A-subunit transgenics (Fig. 1D).
Thyroid peroxidase antibodies
In agreement with our previous study (25), TPOAb in nontransgenic and transgenic hTSHR/NOD.H2h4 mice developed later than TgAb, being very low or absent at the 2-month time point and increasing substantially after 4 months (Fig. 2). Given the lack of TPOAb levels at the 2-month time point, different Se intakes were without effect on this parameter in all four groups studied, namely, male and female nontransgenic (Fig. 2A) and transgenic (Fig. 2C) NOD.H2h4 mice.
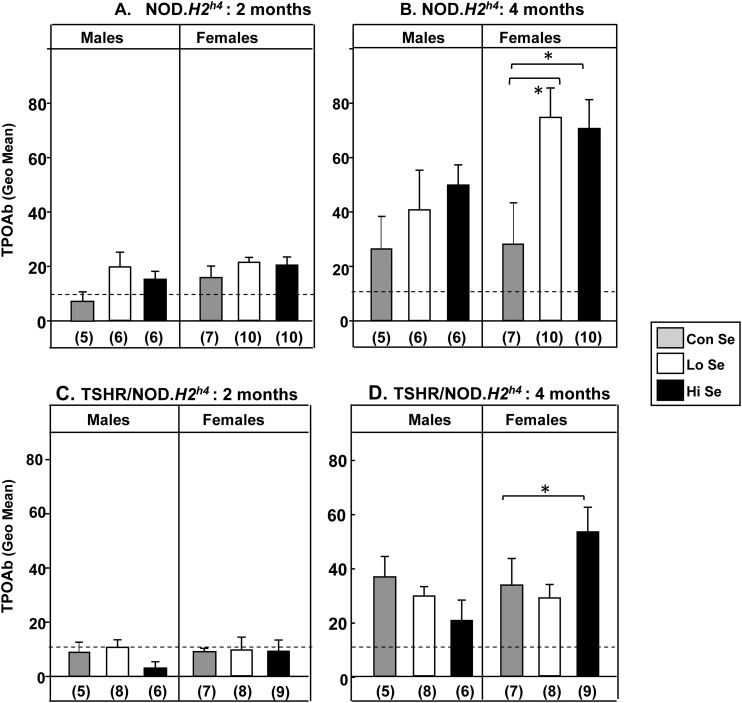
Figure 2.
TPO levels in NOD.H2h4 female mice are affected after 4 months (but not 2 months) exposure to variable Se diets. Rodent diets indicated as Lo, Con, or Hi contained 0.0, 0.1, or 1.0 mg/kg Se, respectively, together with iodide supplemented water. Nontransgenic NOD.H2h4 males and females after (A) 2 months and (B) 4 months. Transgenic TSHR/NOD.H2h4 males and females after (C) 2 months and (D) 4 months. Dashed line: Mean + 2 standard deviation for TPOAb in BALB/c mice after 16 weeks. Data are shown as the mean + standard error of the geometric mean values for TPOAb; the number of mice in each group is given in parentheses. Statistically significant differences: (B) females *P < 0.05 (ANOVA); (D) females *P = 0.035 (t test).
When TPOAb levels were clearly evident at the 4-month time point, different Se intakes had an effect on this parameter, but only in female mice. Wild-type NOD.H2h4 females fed either a Hi- or Lo-Se diet developed higher TPOAb levels than animals maintained on a Con-Se diet (Fig. 2B). In transgenic hTSHR/NOD.H2h4 females, only a Hi-Se intake enhanced TPOAb levels (Fig. 2D).
Histological evidence of thyroiditis, the extent of lymphocytic infiltration, studied at euthanasia (the 4-month time point) was variable and was not significantly different between groups (Supplemental Fig. 1 (34.4KB, pdf) ).
TSHR antibodies
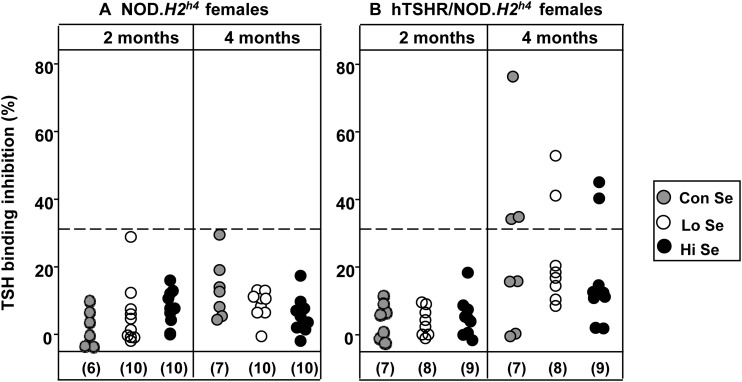
Unlike TgAb and TPOAb, pathogenic TSHRAb do not develop spontaneously in wild-type NOD.H2h4 mice, but only in this strain with the human TSHR A-subunit transgene expressed in the thyroid. Because TSHRAb detected by ELISA are nonfunctional, we explored the effect of different dietary Se intake on these antibodies using a clinically relevant TBI assay. Functional pathogenic TSHRAb can only be assayed in sera from female NOD.H2h4 mice because high TSH levels in males produce spurious values in TBI or bioassays (17). After 2 months, TSHRAb were not evident in female wild-type or transgenic NOD.H2h4 mice (Fig. 3A). At the 4-month time point, TBI positivity was detected in three of seven female TSHR A-subunit transgenics on the Con-Se diet, with similar small numbers positive on the Lo-Se (two of eight) and Hi-Se (two of nine) diets (Fig. 3B).
Figure 3.
(A) TBI in female nontransgenic NOD.H2h4 and (B) transgenic TSHR/NOD.H2h4 mice exposed to rodent diet containing Lo, Con, or Hi levels of Se for 2 or 4 months together with iodide-supplemented water. Rodent diets indicated as Lo, Con, or Hi contained 0.0, 0.1, or 1.0 mg/kg Se, respectively. Data are shown for individual mice after exposure for 2 months and 4 months. Dashed line: mean + 2 standard deviation values in nontransgenic mice. The number of mice in each group is given in parentheses.
T4 and body weight
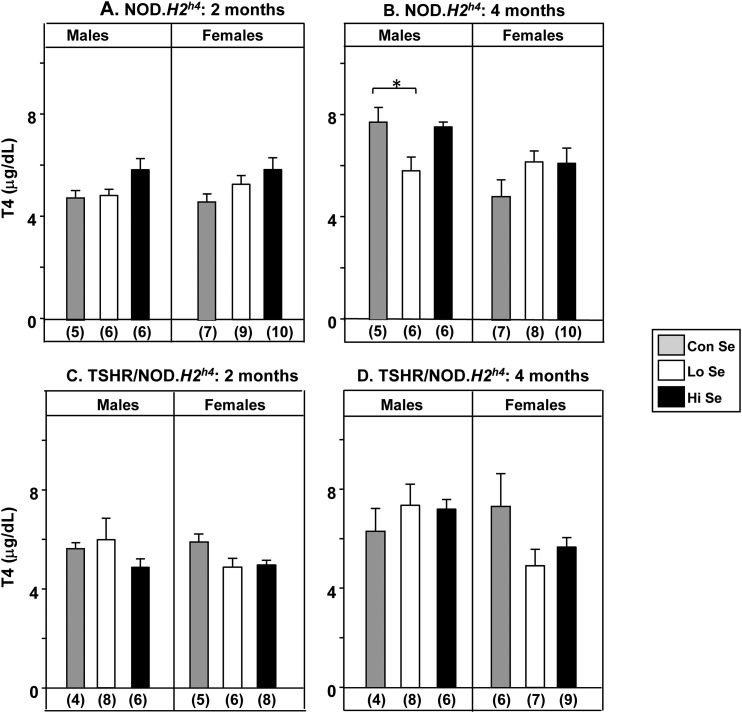
Variation in dietary Se intake had little effect on serum T4 levels, with a small but significant difference only evident after 4 months in male nontransgenic NOD.H2h4 mice on a Lo-Se diet (P < 0.05; Fig. 4B). Body weights were also lower after 2 months on the Lo-Se diet in male nontransgenics (P < 0.05) and transgenics (P < 0.05), but only in female transgenics (P < 0.001; Supplemental Fig. 2A and 2B (34.4KB, pdf) ).
Figure 4.
Serum T4 levels after exposure for 2 or 4 months in NOD.H2h4 mice to rodent diets containing Lo, Con, or Hi Se (0.0, 0.1, or 1.0 mg/kg Se, respectively) together with iodide-supplemented water. Nontransgenic NOD.H2h4 males and females after (A) 2 months and (B) 4 months. Transgenic TSHR/NOD.H2h4 males and females after (C) 2 months and (D) 4 months. Data are shown as the mean + standard error of the mean values for T4 (mg/dL); the number of mice in each group is given in parentheses. Statistically significant differences: (B) males *P < 0.05 (ANOVA).
Relationship between thyroid autoantibodies and TSH
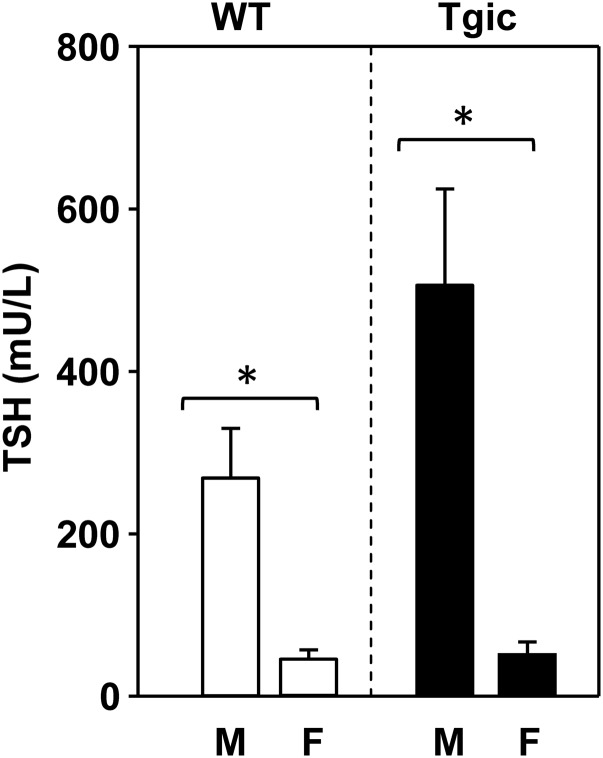
We addressed the issue of whether serum TSH levels at an early stage in the development of thyroiditis (2-month exposure) could influence the subsequent autoantibody response at a later stage (4-month exposure). At the 2-month time point, we measured serum TSH levels in the four groups of mice (male and female wild-type and transgenic NOD.H2h4 strains), each group fed Con-, Lo-, or Hi-Se diets. The high cost (fee for service) limited our sampling to three mice in each category (36 samples). Because varying dietary Se intake revealed no suggestive effect on TSH (not shown), we pooled the data for mice on all three diets. TSH levels in males, whether wild-type or transgenic, were significantly higher than in females (Fig. 5).
Figure 5.
Serum TSH levels in NOD.H2h4 and TSHR/NOD.H2h4 transgenic males and females mice. For each group, the data are pooled for three mice each on Con-, Lo-, and Hi-Se diets (n = 9) for 4 months. Statistically significant differences *P < 0.05 (ANOVA).
Regulatory T cells and TgAb IgG subclasses
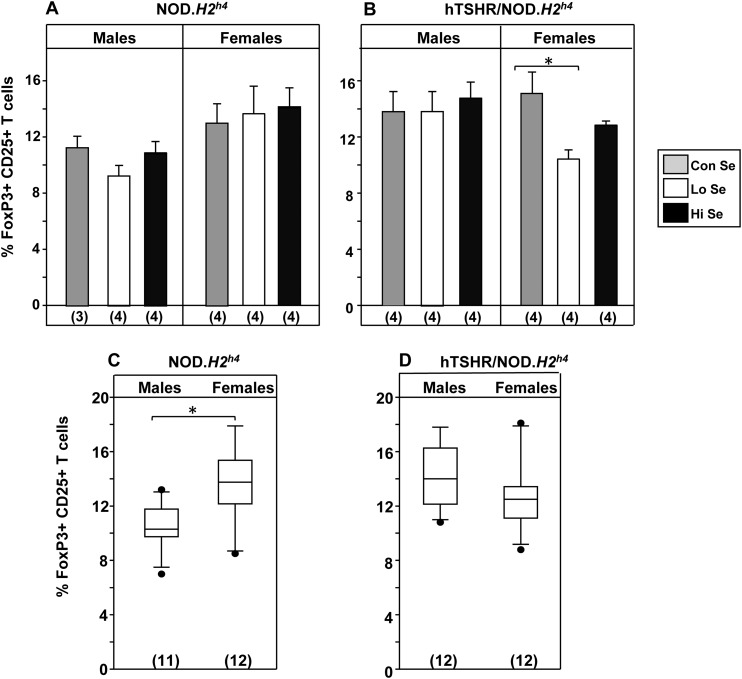
To provide potential insight into mechanisms associated with the effects of varying Se intake on thyroid autoimmunity, we examined two other parameters in wild-type and transgenic NOD.H2h4 strains mice after 4 months on different Se dietary exposure. First, regulatory T cells were quantified by flow cytometry as splenocytes positive for the cell surface markers CD4 and CD25 (31) and subsequently for the intracellular marker FoxP3 (32). No differences were observed in the percentage of FoxP3+ CD25+ T cells in nontransgenic NOD.H2h4 males or females on varying Se intake (Fig. 6A) or in male TSHR/NOD.H2h4 mice (Fig. 6B, left panel). However, in female TSHR/NOD.H2h4 mice, the percentage of FoxP3+ CD25+ T cells was significantly lower on a Lo-Se diet relative to the Con-Se diet (Fig. 6B, right panel; P < 0.05, ANOVA). Of interest, regulatory T cells levels were significantly higher in female than male wild-type, but not transgenic, NOD.H2h4 mice when data were pooled for animals on Con-, Lo-, and Hi-Se diets (Fig. 6C vs 6D).
Figure 6.
Regulatory T cells in (A) nontransgenic NOD.H2h4 and (B) transgenic TSHR/NOD.H2h4 mice on rodent diet containing Lo, Con, or Hi levels of Se (together with iodide-supplemented water) for 4 months. Regulatory T cells were quantified by flow cytometry to identify FoxP3 +ve CD25 +ve splenic T cells. Total numbers of regulatory T cells in (C) nontransgenic NOD.H2h4 vs (D) transgenic TSHR/NOD.H2h4 mice [data pooled from mice on different diets in panels (A) and (B)]. Statistically significant differences: (B) *P < 0.05 (ANOVA); (C) *P = 0.004 (t test).
Second, we examined the distribution of TgAb among the IgG subclasses. In mice, antibodies of subclass IgG2b reflect T-helper type 1 cells and TgAb of subclass IgG1 reflect cytokines from T-helper type 2 cells [e.g., (33, 34)]. No significant differences were observed for the TgAb IgG2a:IgG1 ratio in nontransgenic or transgenic TSHR/NOD.H2h4 mice on varying Se intake (Supplemental Fig. 3 (34.4KB, pdf) ). These data do not indicate a switch between cytokines from T-helper type 1 or type 2 cells.
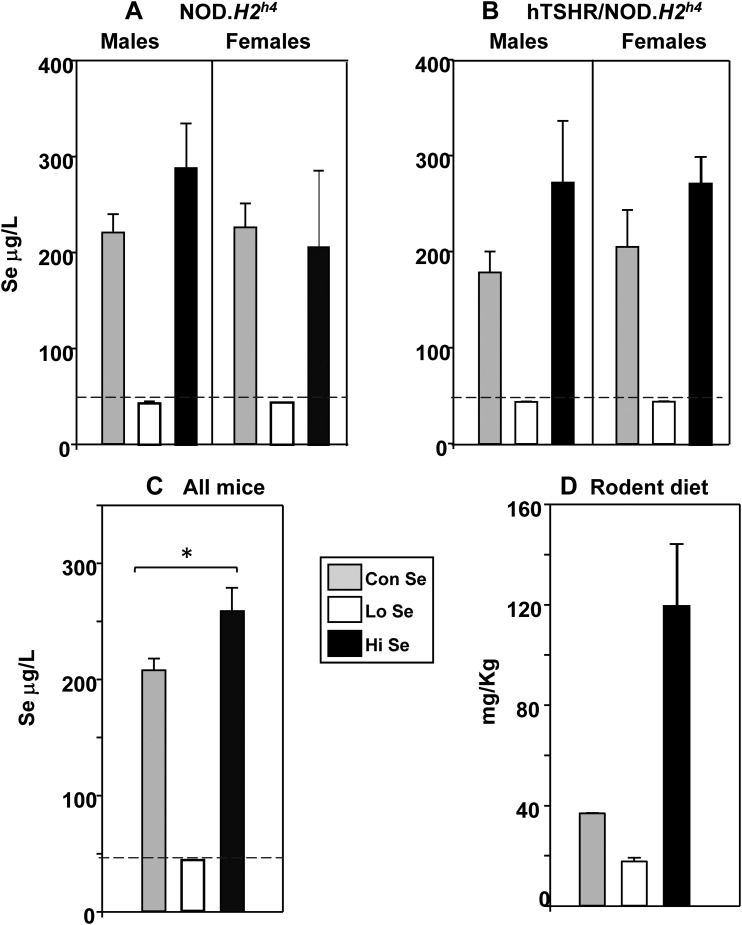
Se in mouse sera and custom diets
Se was measured in nontransgenic and transgenic NOD.H2h4 mice exposed for 4 months to Lo-, Con-, and Hi-Se diets. In males and females of both strains on the Lo-Se diet, serum Se levels were extremely low (at the limits of assay detection) and substantially higher in mice on either Con- or Hi-Se diet (Fig. 7A and 7B). Combining the values for all mice in each dietary group, serum Se levels were slightly but significantly higher in mice on the Hi-Se diet than on the Con-Se diet (Fig. 7C). The Se contents of the custom diets were also tested (Fig. 7D). It is notable that the magnitude of the Se increase in sera from mice on the Hi diet relative to the Con diet was disproportionately lower (nearly 10-fold) than the Se content of the Hi vs the Con diet (25% in serum vs 224% in the diet).
Figure 7.
Se content of mouse sera and custom diets. (A–C) Se was analyzed (by inductively coupled plasma mass spectrometry) in sera from mice exposed for 4 months to custom Se diets containing Lo, Con, or Hi levels (together with iodide supplemented water). (A) Nontransgenic NOD.H2h4 males and females and (B) transgenic TSHR/NOD.H2h4 males and females; Se data are shown as μg/L [mean + standard deviation (SD) for duplicate aliquots]. (C) Combined Se values for all mice on the three custom diets (mg/L, mean + standard error of the mean, n = 8 measurements). *P = 0.029, Rank sum tests. (D) Se content of custom diets (ng/g diet sample, mean + SD of duplicates). The dashed lines in (A–C) represent the mean + 2 SDs of Se values in all mice on Lo Se.
Discussion
NOD.H2h4 mice spontaneously develop autoantibodies to Tg and TPO (12–14), whereas the same strain transgenic for the human TSHR A-subunit targeted to the thyroid, in addition, develop pathogenic autoantibodies to the TSHR (17). We asked the question whether long-term dietary Se intake influences the development of these autoantibodies in both mouse strains. Male and female mice, 8 weeks old, were maintained for 4 months on rodent pellets with a Con-, Lo-, or Hi-Se content (0.1, 0.0, or 1.0 ppm Se). As noted previously (17) and confirmed in this study, serum TSH levels were far higher in male than in female NOD.H2h4 and TSHR transgenic NOD.H2h4 mice, independent of dietary Se intake. Sexual dimorphism is well recognized in autoimmunity, due (for example) to the effects of sex hormones and sex specific gut microbiota [reviewed in (29)]. Therefore, we were not surprised to observe some differences between the responses of male and female NOD.H2h4 mice on variable Se intake.
We observed three major effects of dietary Se. First, changes occurred earlier for TgAb (after 2 months) than for TPOAb (4-month exposure), consistent with the much earlier spontaneous appearance of TgAb than of TPOAb in NOD.H2h4 mice (25). Second, the number of TSHR A-subunit transgenic female mice that developed pathogenic TSHR antibodies (measured by TBI) was unchanged by variable Se dietary intake. Third, there was no effect of dietary Se intake on the proportion of regulatory T cells (FoxP3+, CD25+) in wild-type NOD.H2h4 and male transgenic mice. Only in transgenic female NOD.H2h4 mice did Lo-Se diets reduce the proportion of regulatory T cells cells.
Another point to be noted is a difference between the current study and previous reports from our laboratory on TgAb levels. After the 2-month exposure, TgAb levels were comparable in wild-type NOD.H2h4 and transgenic TSHR/NOD.H2h4 mice maintained on regular chow (17, 35) and the Con-Se diet (present study). In contrast, we now find that variable dietary Se exposes significant differences in TgAb levels between wild-type and transgenic strains at this early time point. These differences are no longer evident later (4-month time point) when the TgAb response is more robust (30). The data suggest that human TSHR A-subunit protein expression in the mouse thyroid influences the effect of dietary Se on TgAb generation.
Is there any evidence for an effect of the TSHR A-subunit transgene on the immune response? The low-expressor human TSHR A-subunit transgene in NOD.H2h4 mice used in this study is located on chromosome 1 (approximately 33 Mb) (36). This location excludes genes that control self-tolerance by their effects on thymic expression of tissue restricted antigens (37–40), genes encoding the TSHR and Tg (chromosomes 12 and 15), and genes encoding immune molecules associated with susceptibility to thyroid autoimmunity in humans (41–44). Overall, this information does not suggest any effect of the transgene on the development of thyroid autoantibodies in transgenic NOD.H2h4 mice.
Turning to other parameters studied, serum T4 was significantly reduced (unrelated to TSH levels) in male wild-type NOD.H2h4 mice on Lo-Se diet. Varying Se intake had no effect in TSHR A-subunit NOD.H2h4 mice. Body weight was significantly reduced in some NOD.H2h4 mice, both wild-type and transgenic, particularly in males on the Lo-Se diet. The latter findings parallel observations in humans of weight loss in men with a Lo-Se dietary intake (45).
Levels of serum Se in wild-type NOD.H2h4 and transgenic TSHR/NOD.H2h4 mice, exposed for 4 months to custom diets, provide insight into some of our findings. The most marked changes were the reduced serum Se levels in all mice on Lo-Se diet. To our knowledge, the effects of Lo Se on thyroid autoimmunity in mice have not previously been studied. We observed that Lo-Se intake was associated with increased TgAb and TPOAb in female NOD.H2h4 mice and with low body weight in both nontransgenic and transgenic TSHR/NOD.H2h4 mice.
Turning to Hi-Se diet, in both sexes, serum Se levels were raised by about 25%, comparable with the increases previously observed in male wild-type NOD.H2h4 exposed to sodium-selenite-supplemented drinking water (15, 16). The increased magnitude of these serum Se levels is less than the much higher Se levels in the diet or sodium-selenate water. The Hi-Se diet (1 mg/kg) did not exceed the recommended maximum for animal experimentation (2 mg/kg) (46). However, on Hi-Se intake, the Se content of red blood cells is a better index of Se status of an animal than plasma (or serum) (46).
How do our immunologic data compare with other findings in mice? We did not observe the small decrease in TgAb levels reported by Wang and colleagues (16) in male wild-type NOD.H2h4 mice whose drinking water was supplemented with sodium selenite for similar time intervals as in our study. In addition, contrary to a previous report, and despite a comparable increase in serum Se, we did not observe that regulatory T cells were increased by Hi-Se intake in NOD.H2h4 mice (15). However, the experimental protocol used in the earlier studies differed from that in our study: mice were younger than ours at the start (4 weeks vs 8 weeks), the iodide concentration was lower than in our study (0.005% vs 0.05%), and Se supplementation was added after 8 weeks on iodide (15, 16). Consequently, in the earlier studies, the effects of Se were tested in established and ongoing thyroid autoimmunity. In contrast, we investigated the effects of variable Se intake on the development of thyroid autoantibodies and other parameters in NOD.H2h4 mice.
Compared with studies in humans, our data show similarities and differences which may be related (at least in part) to gender differences as well as the time of appearance of thyroid autoantibodies. The increase in TgAb at the early time interval in NOD.H2h4 females on a Lo diet is consistent with data from a large study of Chinese patients (>6000 individuals) that showed increased thyroiditis in patients in from an area with a Lo- vs Con-Se intake (7). However, our observation that the spontaneous development of pathogenic TSHR antibodies in transgenic female mice is unaffected by variable Se dietary intake differs from the report of low serum Se levels in some (but not all) newly diagnosed patients who have Graves disease (6). Finally, the absence of an effect of high Se levels on thyroid autoantibodies differs from studies in humans showing a reduction in thyroid autoantibodies (particularly TPOAb) in individuals on diets supplemented with Se [e.g., (8)].
In conclusion, using autoimmune-prone NOD.H2h4 mice exposed to variable Se dietary intake, we found no evidence that higher Se intake ameliorates thyroid autoimmunity. On the other hand, our data support the findings in humans that Lo-Se intake may potentiate the development of thyroid autoimmunity, at least in females.
Acknowledgments
We thank Dr. Jean Ruf (INSERM_URA, Faculté de Médecine, Marseille, France) for generously providing us with mouse monoclonal antibodies to human thyroid peroxidase.
Financial Support: This work was supported by National Institutes of Health Grants from the National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) 54684 (to S.M.M.) and NIDDK 19289 (to B.R.). The inductively coupled plasma mass spectrometry was purchased from a Shared Instrumentation Grant, National Institutes of Environmental Health Sciences 1S10 RR017770 (to S.S.Q.H.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- +ve
- positive
- ANOVA
- analysis of variance
- CHO
- Chinese hamster ovary
- Con
- selenium control
- ELISA
- enzyme-linked immunosorbent assay
- Hi
- selenium enriched
- IgG
- immunoglobulin G
- Lo
- selenium deficient
- NaI
- sodium iodide
- NOD
- nonobese diabetic
- OD
- optical density
- Se
- selenium
- T4
- thyroxine
- TBI
- thyroid-stimulating hormone–binding inhibition
- Tg
- thyroglobulin
- TgAb
- thyroglobulin antibody
- TPO
- thyroid peroxidase
- TPOAb
- thyroid peroxidase antibody
- TSH
- thyroid-stimulating hormone
- TSHR
- thyroid-stimulating hormone receptor
- TSHRAb
- thyroid-stimulating hormone receptor antibody.
References
- 1.Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52(11):1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16(7):705–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duntas LH. The role of iodine and selenium in autoimmune thyroiditis. Horm Metab Res. 2015;47(10):721–726. [DOI] [PubMed] [Google Scholar]
- 4.Köhrle J. Selenium and the thyroid. Curr Opin Endocrinol Diabetes Obes. 2015;22(5):392–401. [DOI] [PubMed] [Google Scholar]
- 5.Hu S, Rayman MP. Multiple nutritional factors and the risk of Hashimoto’s thyroiditis. Thyroid. 2017;27(5):597–610. [DOI] [PubMed] [Google Scholar]
- 6.Bülow Pedersen I, Knudsen N, Carlé A, Schomburg L, Köhrle J, Jørgensen T, Rasmussen LB, Ovesen L, Laurberg P. Serum selenium is low in newly diagnosed Graves’ disease: a population-based study. Clin Endocrinol (Oxf). 2013;79(4):584–590. [DOI] [PubMed] [Google Scholar]
- 7.Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, Gao C, Chen P, Zhuang G, Zhang Z, Peng X, Li H, Zhao Y, He X, Zeng G, Qin F, Hou P, Shi B. Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab. 2015;100(11):4037–4047. [DOI] [PubMed] [Google Scholar]
- 8.Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95(12):5180–5188. [DOI] [PubMed] [Google Scholar]
- 9.Wichman J, Winther KH, Bonnema SJ, Hegedüs L. Selenium supplementation significantly reduces thyroid autoantibody levels in patients with chronic autoimmune thyroiditis: A systematic review and meta-analysis. Thyroid. 2016;26(12):1681–1692. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Wang H, Luo J, Hu Y, Wei L, Duan M, He H. Selenium deficiency impairs host innate immune response and induces susceptibility to Listeria monocytogenes infection. BMC Immunol. 2009;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuji PA, Carlson BA, Anderson CB, Seifried HE, Hatfield DL, Howard MT. Dietary selenium levels affect selenoprotein expression and support the interferon-γ and IL-6 immune response pathways in mice. Nutrients. 2015;7(8):6529–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81(3):287–292. [DOI] [PubMed] [Google Scholar]
- 13.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun. 1999;12(3):157–165. [DOI] [PubMed] [Google Scholar]
- 14.Hutchings PR, Verma S, Phillips JM, Harach SZ, Howlett S, Cooke A. Both CD4(+) T cells and CD8(+) T cells are required for iodine accelerated thyroiditis in NOD mice. Cell Immunol. 1999;192(2):113–121. [DOI] [PubMed] [Google Scholar]
- 15.Xue H, Wang W, Li Y, Shan Z, Li Y, Teng X, Gao Y, Fan C, Teng W. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD.H-2(h4) mice. Endocr J. 2010;57(7):595–601. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Xue H, Li Y, Hou X, Fan C, Wang H, Zhang H, Shan Z, Teng W. Effects of selenium supplementation on spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. Thyroid. 2015;25(10):1137–1144. [DOI] [PubMed] [Google Scholar]
- 17.Rapoport B, Aliesky HA, Banuelos B, Chen CR, McLachlan SM. A unique mouse strain that develops spontaneous, iodine-accelerated, pathogenic antibodies to the human thyrotrophin receptor. J Immunol. 2015;194(9):4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichurin PN, Chen C-R, Chazenbalk GD, Aliesky H, Pham N, Rapoport B, McLachlan SM. Targeted expression of the human thyrotropin receptor A-subunit to the mouse thyroid: insight into overcoming the lack of response to A-subunit adenovirus immunization. J Immunol. 2006;176(1):668–676. [DOI] [PubMed] [Google Scholar]
- 19.McLachlan SM, Nagayama Y, Pichurin PN, Mizutori Y, Chen CR, Misharin A, Aliesky HA, Rapoport B. The link between Graves’ disease and Hashimoto’s thyroiditis: a role for regulatory T cells. Endocrinology. 2007;148(12):5724–5733. [DOI] [PubMed] [Google Scholar]
- 20.Chazenbalk GD, Pichurin P, Chen CR, Latrofa F, Johnstone AP, McLachlan SM, Rapoport B. Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest. 2002;110(2):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C-R, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM. The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. J Clin Invest. 2003;111(12):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneda T, Honda A, Hakozaki A, Fuse T, Muto A, Yoshida T. An improved Graves’ disease model established by using in vivo electroporation exhibited long-term immunity to hyperthyroidism in BALB/c mice. Endocrinology. 2007;148(5):2335–2344. [DOI] [PubMed] [Google Scholar]
- 23.Vanderlelie J, Venardos K, Perkins AV. Selenium deficiency as a model of experimental pre-eclampsia in rats. Reproduction. 2004;128(5):635–641. [DOI] [PubMed] [Google Scholar]
- 24.Shanu A, Groebler L, Kim HB, Wood S, Weekley CM, Aitken JB, Harris HH, Witting PK. Selenium inhibits renal oxidation and inflammation but not acute kidney injury in an animal model of rhabdomyolysis. Antioxid Redox Signal. 2013;18(7):756–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CR, Hamidi S, Braley-Mullen H, Nagayama Y, Bresee C, Aliesky HA, Rapoport B, McLachlan SM. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology. 2010;151(9):4583–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLachlan SM, Aliesky HA, Chen CR, Chong G, Rapoport B. Breaking tolerance in transgenic mice expressing the human TSH receptor A-subunit: thyroiditis, epitope spreading and adjuvant as a “double edged sword.” PLoS One. 2012;7(9):e43517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruf J, Toubert ME, Czarnocka B, Durand-Gorde JM, Ferrand M, Carayon P. Relationship between immunological structure and biochemical properties of human thyroid peroxidase. Endocrinology. 1989;125(3):1211–1218. [DOI] [PubMed] [Google Scholar]
- 28.Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9(12):1265–1271. [DOI] [PubMed] [Google Scholar]
- 29.Rubtsova K, Marrack P, Rubtsov AV. Sexual dimorphism in autoimmunity. J Clin Invest. 2015;125(6):2187–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLachlan SM, Lesage S, Collin R, Banuelos B, Aliesky HA, Rapoport B. Genes outside the major histocompatibility complex locus are linked to the development of thyroid autoantibodies and thyroiditis in NOD.H2h4 mice. Endocrinology. 2017;158(4):702–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13(3):108–116. [DOI] [PubMed] [Google Scholar]
- 32.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241(1):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. [DOI] [PubMed] [Google Scholar]
- 34.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140(4):1022–1027. [PubMed] [Google Scholar]
- 35.Rapoport B, Banuelos B, Aliesky HA, Hartwig Trier N, McLachlan SM. Critical differences between induced and spontaneous mouse models of Graves’ disease with implications for antigen-specific immunotherapy in humans. J Immunol. 2016;197(12):4560–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLachlan SM, Aliesky HA, Banuelos B, Lesage S, Collin R, Rapoport B. High-level intrathymic thyrotrophin receptor expression in thyroiditis-prone mice protects against the spontaneous generation of pathogenic thyrotrophin receptor autoantibodies. Clin Exp Immunol. 2017;188(2):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–1401. [DOI] [PubMed] [Google Scholar]
- 38.Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kämpe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11(4):397–409. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202(6):805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4(4):350–354. [DOI] [PubMed] [Google Scholar]
- 41.Brand OJ, Gough SC. Immunogenetic mechanisms leading to thyroid autoimmunity: recent advances in identifying susceptibility genes and regions. Curr Genomics. 2011;12(8):526–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. 2014;9:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teumer A, Rawal R, Homuth G, Ernst F, Heier M, Evert M, Dombrowski F, Völker U, Nauck M, Radke D, Ittermann T, Biffar R, Döring A, Gieger C, Klopp N, Wichmann HE, Wallaschofski H, Meisinger C, Völzke H. Genome-wide association study identifies four genetic loci associated with thyroid volume and goiter risk. Am J Hum Genet. 2011;88(5):664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Płoski R, Szymański K, Bednarczuk T. The genetic basis of Graves’ disease. Curr Genomics. 2011;12(8):542–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawkes WC, Keim NL. Dietary selenium intake modulates thyroid hormone and energy metabolism in men. J Nutr. 2003;133(11):3443–3448. [DOI] [PubMed] [Google Scholar]
- 46.Coudray C, Hida H, Boucher F, Tirard V, de Leiris J, Favier A. Effect of selenium supplementation on biological constants and antioxidant status in rats. J Trace Elem Med Biol. 1996;10(1):12–19. [DOI] [PubMed] [Google Scholar]