Abstract
Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are functionally redundant transcriptional regulators that are downstream effectors of the Hippo signaling pathway. They act as major regulators of stem cell maintenance, cell growth, and differentiation. To characterize their roles in the adrenal cortex, we generated a mouse model in which Yap and Taz were conditionally deleted in steroidogenic cells (Yapflox/flox;Tazflox/flox;Nr5a1cre/+). Male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice were characterized by an age-dependent degeneration of the adrenal cortex associated with an increase in apoptosis and a progressive reduction in the expression levels of steroidogenic genes. Evaluation of the expression levels of stem and progenitor cell population markers in the adrenal glands of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice also showed the downregulation of sonic hedgehog (Shh), a marker of the subcapsular progenitor cell population. Gross degenerative changes were not observed in the adrenal glands of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ females, although steroidogenic capacity and Shh expression were reduced, suggesting that mechanisms of adrenocortical maintenance are sex specific. These results define a crucial role for YAP and TAZ in the maintenance of the postnatal adrenal cortex.
In this study, we demonstrated that Hippo signaling effectors YAP and TAZ in the adrenal cortex are necessary for the maintenance of the postnatal adrenal cortex.
In mice, the adrenal cortex is composed of two concentric zones: the zona glomerulosa (zG) and the zona fasciculata (zF). The zG produces the mineralocorticoids that regulate blood volume and pressure, whereas the zF produces glucocorticoids that mediate the response to stress. The adrenal cortex is a dynamic organ in which newly differentiated cells replace senescent cells. The centripetal migration model is the most widely accepted explanation of postnatal adrenocortical zonation. In this model, stem cells in the capsule give rise to mineralocorticoid-producing zG cells, which subsequently migrate centripetally while undergoing lineage conversion into glucocorticoid-producing zF cells. The latter cells eventually reach the cortical-medullary boundary, where they undergo apoptosis [reviewed in (1, 2)]. Although tracing experiments have validated some aspects of this model (3, 4), it was also shown that a functional zF still forms when the conversion of zG to zF cells is abrogated through the deletion of Nr5a1 (3), suggesting that the regulation of zonation is more complex than previously thought. Likewise, it was also shown that capsular stem cells give rise to undifferentiated, nonsteroidogenic adrenocortical progenitor cells that can also differentiate into adrenocortical steroidogenic cells (5, 6). Numerous transcription factors and paracrine signaling molecules, including Wilms tumor 1 (7, 8), nuclear receptor subfamily 5, group A, member 1 (8–11), nuclear receptor subfamily 0, group B, member 1 (9, 12), sonic hedgehog (SHH) (5, 6, 13, 14), β-catenin (CTNNB1) (15, 16), and transcription factor 21 (5), are critical for stem/progenitor cell and adrenal gland maintenance, and their loss in mice leads to adrenal insufficiency−related diseases.
Hippo is an evolutionarily conserved signaling pathway with well-established roles in cell fate determination, differentiation, and proliferation during embryonic and postnatal development, as well as in tissue homeostasis throughout adulthood [reviewed in (17–19)]. The canonical Hippo pathway consists of a kinase cascade that ultimately serves to regulate the functionally redundant transcriptional coregulators Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ). In response to extracellular signals such as the establishment of cell-cell contacts, the mammalian STE20-like protein kinases 1 and 2 are activated and phosphorylate the large tumor suppressor homologue kinases 1 and 2, which in turn phosphorylate YAP and TAZ. Upon phosphorylation, YAP and TAZ are either retained in the cytoplasm via their association with 14-3-3 proteins or degraded by the cellular proteasomal machinery. When the Hippo signaling cascade is inactive, unphosphorylated YAP and TAZ accumulate in the nucleus and form complexes with transcription factors to regulate the transcription of genes involved in cell growth, survival, and proliferation (17–19).
In the adrenal gland, it was shown that YAP expression was associated with poor outcome of adrenocortical tumors in pediatric patients (20). However, potential roles for the Hippo signaling pathway in adrenal gland function and/or homeostasis have never been evaluated. In this study, we aimed to elucidate the role of the key Hippo signaling effectors YAP and TAZ in the adrenal cortex by inactivating their expression in the adrenocortical cells of a transgenic mouse model.
Material and Methods
Ethics
All animal procedures were approved by the Comité d'Éthique de l'Utilisation des Animaux of the Université de Montréal (protocol numbers 14-Rech-1739, 16-Rech-1830, and 16-Rech-1805) and conform to the guidelines of the Canadian Council on Animal Care.
Transgenic mouse strains
Nr5a1cre/+ mice [FVB-Tg-Nr5a1Cre7Lowl/J; Research Resource Identifier (RRID): IMSR_JAX:012462] were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained by crossing Cre-positive males with wild-type females (C57BL/6J; RRID: IMSR_JAX:000664). Yapflox/flox;Tazflox/flox [RRID: MGI:5446510 and RRID: MGI:5544300 (21, 22)] mice were obtained from Dr. Eric N. Olson (Univerisity of Texas Southwestern Medical Center, Dallas, TX) and selectively bred over several generations to obtain the Yapflox/flox;Tazflox/flox;Nr5a1cre/+ genotype. Yapflox/flox;Nr5a1cre/+ mice were generated by breeding Yapflox/+;Tazflox/+;Nr5a1cre/+ mice with Yapflox/flox mice. Genotype analyses were done on tail biopsies by polymerase chain reaction (PCR) as previously described for Yap (21), Taz (22), and Cre (23).
Histopathology and immunohistochemistry
Adrenal glands for light microscopy histopathologic analysis were weighed and fixed in formalin for 24 hours. Tissues were embedded in paraffin, sectioned, and stained with hematoxylin, phloxine, and saffron. Immunohistochemistry was done on formalin-fixed, paraffin-embedded, 4-μm tissue sections using VECTASTAIN Elite avidin–biotin complex method kits (Vector Laboratories, Burlingame, CA) or the mouse on mouse elite peroxidase kit (Vector Laboratories) as directed by the manufacturer. Sections were probed with primary antibodies against YAP, TAZ, CTNNB1, 5-bromo-2′-deoxyuridine (BrdU) or cleaved apoptosis-related cysteine peptidase, D175. Staining was done using the 3,3′-diaminobenzidine peroxidase substrate kit (Vector Laboratories). Negative controls consisted of slides for which the primary antibody was omitted. Antibodies used are listed in Table 1.
Table 1.
List of Antibodies
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., Lot Number | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| BrdU | Monoclonal mouse anti-bromodeoxyuridine, Clone Bu20a | Dako Corp, M0744, lot 31732 | Mouse; monoclonal | 1:100 | AB_10013660 | |
| Cleaved CAS3 | Cleaved caspase-3 (Asp175) antibody #9661 | Cell Signaling, 9661, lot 38 | Rabbit; polyclonal | 1:100 | AB_234188 | |
| TAZ | MNPKPSSWRKKILPESFFKEPDSGSHSRQSSTDSSGGHPGPRLAGGAQHVRSHSSPASLQLGTGAGAAGSPAQQHAHLRQQSYDVTDELPLPPGWEMTFTATGQRYFLNHIEKITTWQDPRKAMNQPLNHMNLHPAVSST | Anti-Wwtr1 | Sigma-Aldrich, HPA007415, lot F104279 | Rabbit; polyclonal | 1:600 | AB_1080602 |
| YAP | YAP antibody #4912 | Cell Signaling, 4912, lot 5 | Rabbit; polyclonal | 1:200 | AB_2218911 | |
| CTNNB1 | CTNNB1 antibody #8480 | Cell Signaling, 8480, lot 5 | Rabbit; polyclonal | 1:100 | AB_11127855 |
Abbreviation: CAS3, caspase-3.
Hormone measurements
Blood samples for serum collection were collected between 13:00 and 14:00 hours by cardiac puncture before euthanasia. Blood was allowed to clot at room temperature for 90 minutes and was centrifuged at 2000g for 15 minutes at room temperature. Serum samples were transferred to polypropylene tubes and stored at −80°C until use. To evaluate the response to acute stimulation with adrenocorticotropic hormone (ACTH), some animals were treated with ACTH fragment 1-24 (Sigma-Aldrich, St. Louis, MO) (500 ng/g of body weight, subcutaneously) 1 hour before euthanasia. Corticosterone levels were then determined by radioimmunoassay (MP Biomedicals, Solon, OH). Follicle-stimulating hormone (FSH)/ luteinizing hormone (LH) levels in the serum were determined using the mouse/rat LH/FSH multiplex assay (EMD Millipore, Billerica, MA). Aldosterone levels were determined using the ab136933-Aldosterone enzyme-linked immunosorbent assay (ELISA) Kit (Abcam, Cambridge, UK). Blood samples for plasma collection were collected between 9:30 and 10:00 hours in 2K-EDTA Microvette tubes (Sarstedt Inc., Saint-Léonard, Quebec, Canada) and centrifuged at 2000g for 15 minutes at 4°C. Plasma samples were transferred to polypropylene tubes and stored at −80°C until use. ACTH levels in the serum were determined by Immulite 2000 (Siemens Healthcare Diagnostics, Tarrytown, NY). Intratesticular testosterone levels were determined in testicular homogenates by enzyme-linked immunosorbent assay (IBL International, Hamburg, Germany). Homogenates were generated by mechanical homogenization of testes in phosphate-buffered saline followed by sonication for 60 seconds. Homogenates were centrifuged at 10,000g for 5 minutes, and the supernatants were stored at −80°C until use. All assays except measurements of circulating aldosterone levels were performed by the Center for Research in Reproduction at the Ligand Assay and Analysis Core Laboratory of the University of Virginia.
Oil Red O staining
Adrenal glands were snap-frozen in OCT compound (Sakura Finetek, Torrance, CA) and stored at −80°C until further processing. Five-micron-thick cryosections were fixed with 10% formalin for 2 minutes. After being washed with running water, slides were transferred to 60% 2-propanol for 1 minute. Lipids were then stained with Oil Red O (Sigma-Aldrich) for 10 minutes followed by immersion in 60% 2-propanol for 30 seconds. After being washed with running water, the tissues were counterstained with hematoxylin for 3 minutes.
Quantitative reverse transcription PCR
Total RNA from adrenal glands of 10- and 20-week-old animals and total RNA from testes, ovaries, and the pituitary of 10-week-old animals were extracted using the Total RNA Mini Kit (FroggaBio) according to the manufacturer’s protocol. Total RNA was reverse-transcribed using 100 ng of RNA and the SuperScript VILO™ complementary DNA (cDNA) synthesis kit (Thermo Fisher Scientific, Ottawa, Ontario, Canada). Real-time PCR reactions were run on a CFX96 Touch instrument (Bio-Rad, Hercules, CA), using SsoAdvanced SYBR Green PCR Master Mix (Bio-Rad). Each PCR reaction consisted of 7.5 μL of Power SYBR Green PCR Master Mix, 2.3 μL of water, 4 μL of cDNA sample, and 0.6 μL (400 nmol) of gene-specific primers. PCR reactions run without cDNA (water blank) served as negative controls. A common thermal cycling program (3 minutes at 95°C, 40 cycles of 15 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C) was used to amplify each transcript. To quantify relative gene expression, the cycle threshold (Ct) of genes of interest was compared with that of Rpl19, according to the ratio R = [ECt Rpl19/ECt target], where E is the amplification efficiency for each primer pair. Rpl19 Ct values did not change significantly between tissues, and Rpl19 was therefore deemed suitable as an internal reference gene. The specific primer sequences used are listed in Supplemental Table 1 (107.9KB, docx) .
BrdU incorporation assay
Ten-week-old mice were injected intraperitoneally with 100 mg/kg body weight BrdU (Sigma-Aldrich) and euthanized 4 hours after the injection. The BrdU-labeled DNA was detected by immunohistochemistry as described previously.
Dexamethasone treatment
Dexamethasone (2 mg/L) or vehicle (ethanol, 0.05% final concentration) was administered in drinking water (replaced every day) to 5-week-old mice for 2 weeks. Some mice were euthanized immediately after the end of the treatment, whereas other mice were euthanized 2 weeks after the end of the treatment to allow for the regeneration of the zF.
Statistical analyses
All statistical analyses were performed with Prism software version 6.0d (GraphPad Software Inc., La Jolla, CA; RRID: SCR_002798). All the data sets were subjected to the F test to determine the equality of variances. The Student t test was used for all comparisons between genotypes except when more than two groups were compared. In the latter cases, analysis of variance (with Tukey multiple comparisons posttest) was used. Means were considered significantly different when P values were<0.05. All data are presented as means ± standard error of the mean.
Results
YAP and TAZ were expressed in the zG and zF of the adrenal cortex
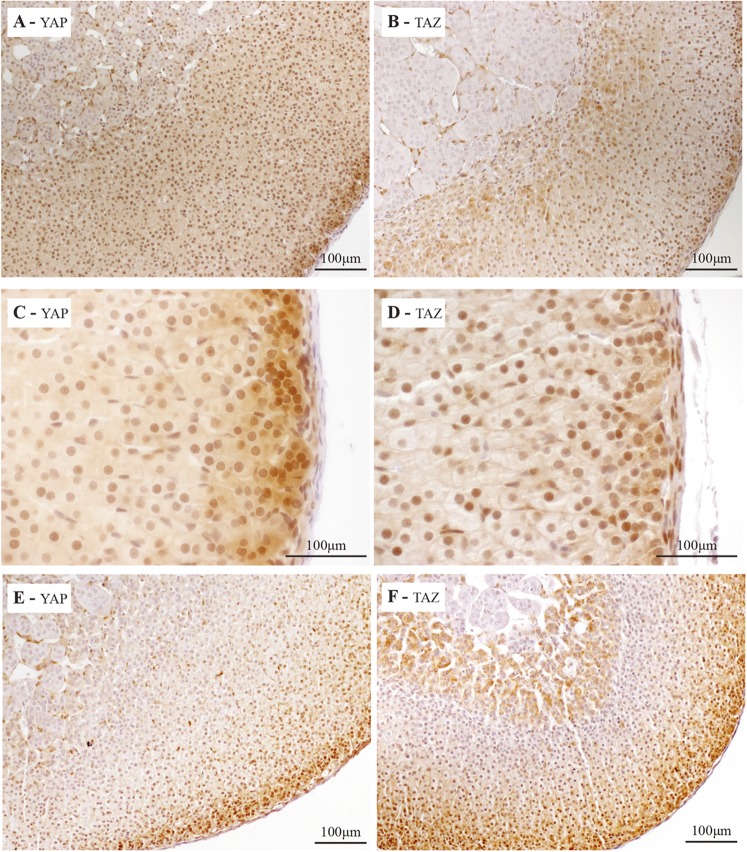
To determine the expression pattern of YAP and TAZ, immunohistochemistry analyses were performed on adrenal glands from mature mice. Abundant nuclear and cytoplasmic expression of YAP (Fig. 1A) was detected in the cells of the zG, and strong nuclear expression of YAP was detected in the zF (Fig. 1A). Nuclear expression of YAP was also detected in the endothelial cells of the adrenal gland (Fig. 1C). Nuclear expression of TAZ was detected in the zG and in the zF (Fig. 1B), with some cytoplasmic expression in a few cells of the adrenal cortex near the medulla (Fig. 1B). Nuclear expression of TAZ was also detected in the endothelial cells and in the adrenal capsule (Fig. 1D). YAP and TAZ expression patterns in the adrenal glands of male and female mice were similar (Fig. 1E and 1F), except that TAZ expression was also strongly detected in the cytoplasm of the adrenocortical cells near the medulla (X-zone) in females (Fig. 1F). The pattern of expression of YAP and TAZ suggests that regulation of transcription by YAP and/or TAZ may be important for the functions and/or the maintenance of the adrenal cortex.
Figure 1.
Localization of YAP and TAZ in the adrenal gland of mice. Immunohistochemical analysis of (A) YAP and (B) TAZ expression in adrenal glands from 10-week-old male mice. Immunohistochemical analysis of (C) YAP and (D) TAZ at higher magnification to illustrate expression in the capsule. Immunohistochemical analysis of (E) YAP and (F) TAZ expression in adrenal glands from a 10-week-old virgin female.
Degeneration of the adrenal cortex in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice
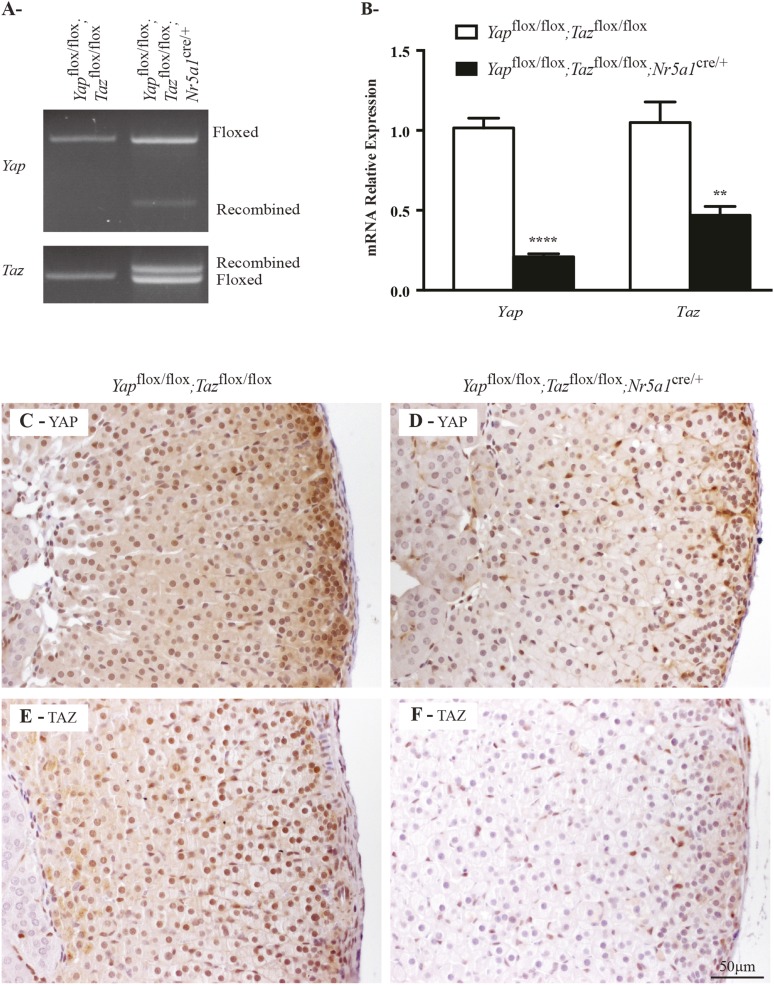
To investigate the role of YAP and TAZ in the adrenal cortex, we generated compound Yap and Taz mutants by using floxed alleles for Yap and Taz (21, 22) and the Nr5acre strain, which was shown to drive Cre expression mainly in steroidogenic cells, including those of the adrenal cortex (23). Because YAP and TAZ are known to have a high degree of functional redundancy, we decided to first evaluate the adrenal glands from mature Yapflox/flox;Tazflox/flox;Nr5a1cre/+ animals. PCR analyses of adrenal gland genomic DNA confirmed that recombination of the floxed alleles occurred in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice (Fig. 2A). Consistent with this result, quantitative reverse transcription PCR (qRT-PCR) analysis showed 79% and 55% decreases in adrenal Yap and Taz messenger RNA (mRNA) levels, respectively (Fig. 2B). Finally, immunohistochemistry analyses of adrenal glands from Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice also confirmed the loss of YAP (Fig. 2C and 2D) and TAZ (Fig. 2E and 2F) in adrenocortical cells.
Figure 2.
Yap and Taz knockdown efficiency in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (A) PCR genotype analyses for Yap and Taz performed on adrenal glands from 10-week-old males of the indicated genotypes. Bands corresponding to the floxed and Cre-recombined alleles are indicated. (B) qRT-PCR analysis of Yap and Taz mRNA levels in the adrenal glands of 10-week-old males of the indicated genotypes (n = 6 animals per genotype). All data were normalized to the housekeeping gene Rpl19 and are expressed as means (columns) ± standard error of the mean (error bars). Asterisks indicate significantly different from control (**P < 0.01; ****P < 0.0001). (C, D) Immunohistochemical analysis of YAP expression in adrenal glands from 10-week-old male (C) Yapflox/flox;Tazflox/flox and (D) Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (E, F) Immunohistochemical analysis of TAZ in adrenal glands from 10-week-old male (E) Yapflox/flox;Tazflox/flox and (F) Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. Scale bar in (F) is valid for all images.
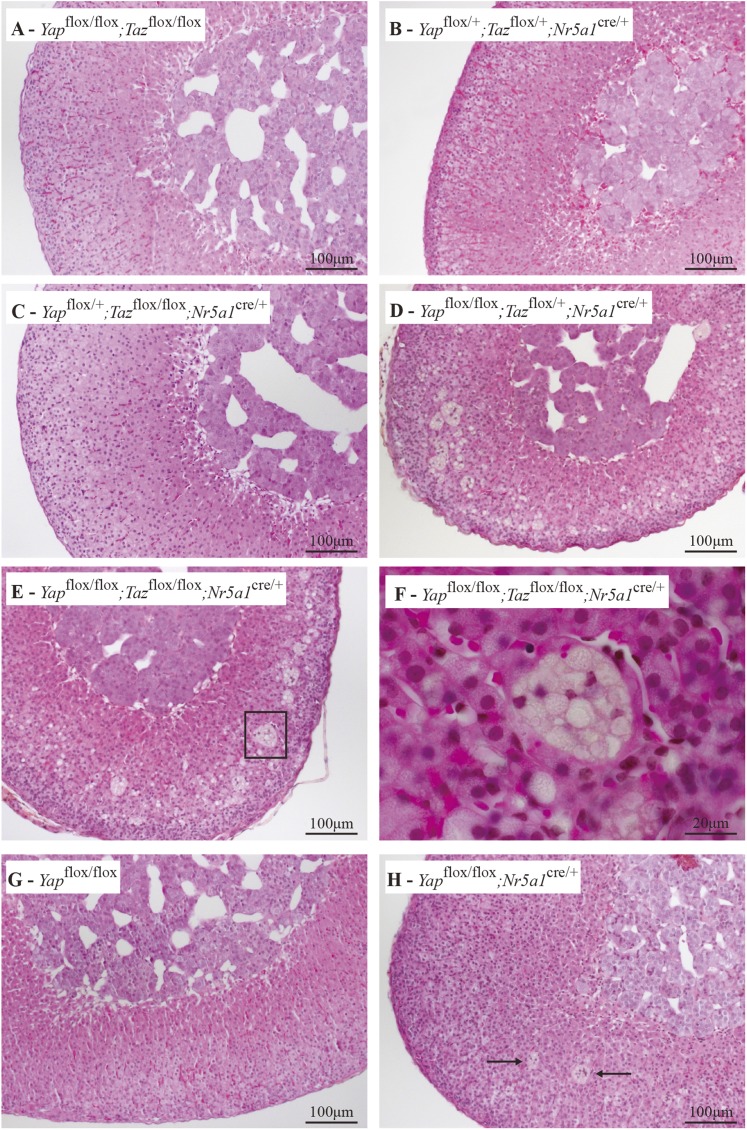
Histopathologic analyses of the adrenal glands of 20-week-old male mice revealed no anomalies in Yapflox/+;Tazflox/+;Nr5a1cre/+, Yapflox/+;Tazflox/flox;Nr5a1cre/+, or Yapflox/flox;Tazflox/flox (i.e., control) animals (Fig. 3A−3C). Conversely, evidence of adrenal failure was observed in Yapflox/flox;Tazflox/+;Nr5a1cre/+ and Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice, as numerous vacuoles and large multinucleated lipoid structures were observed in the adrenal cortex (Fig. 3D−3F). Because Yapflox/flox;Tazflox/+;Nr5a1cre/+ and Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice had similar adrenal phenotypes, we decided to verify whether the loss of Yap alone was sufficient to compromise the maintenance of the adrenal gland. Unlike Yapflox/flox;Tazflox/+;Nr5a1cre/+ or Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice, only rare multinuclear lipoid structures were observed in the adrenal cortices of 20-week-old male Yapflox/flox;Nr5a1cre/+ animals (Fig. 3G and 3H). Contrary to what was observed in the males, no apparent atrophy of the adrenal glands or degeneration of the cortex was observed in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ females as old as 8 months (Supplemental Fig. 1A and 1B (7.2MB, tiff) ) despite decreases in Yap (75%) and Taz (58%) mRNA levels comparable to those observed in males (Supplemental Fig. 2A (17MB, tiff) ). Loss of YAP (Supplemental Fig. 2B and 2C (17MB, tiff) ) and TAZ (Supplemental Fig. 2D and 2E (17MB, tiff) ) expression in the adrenal glands of virgin Yapflox/flox;Tazflox/flox;Nr5a1cre/+ females was confirmed by immunohistochemistry, although TAZ expression remained in a few cells near the medulla (Supplemental Fig. 2E (17MB, tiff) ). These results suggest that mechanisms of adrenocortical maintenance are sex specific.
Figure 3.
YAP and TAZ are required for the maintenance of the adrenal cortex. (A−E) Photomicrographs comparing the adrenal glands of 20-week-old male (A) Yapflox/flox;Tazflox/flox, (B) Yapflox/+;Tazflox/+;Nr5a1cre/+, (C) Yapflox/+;Tazflox/flox;Nr5a1cre/+, (D) Yapflox/flox;Tazflox/+;Nr5a1cre/+, and (E) Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (F) Higher magnification of the boxed area in photomicrograph (E). (G, H) Photomicrographs comparing the adrenal glands of 20-week-old male (G) Yapflox/flox and (H) Yapflox/flox;Nr5a1cre/+ mice (arrows indicate multinuclear lipoid structures). Hematoxylin, phloxine, and saffron stain.
Together, these results indicate that of the two regulators, Yap appears to play a preponderant role, as a single functional Yap (but not Taz) allele was sufficient to ensure normal adrenal function. Nonetheless, the loss of Taz accentuated the phenotype observed with the loss of Yap, indicating that YAP and TAZ play critical, functionally redundant roles in the maintenance of the adrenal cortex in male mice. It was therefore decided to use the Yapflox/flox;Tazflox/flox;Nr5a1cre/+ model for subsequent experiments.
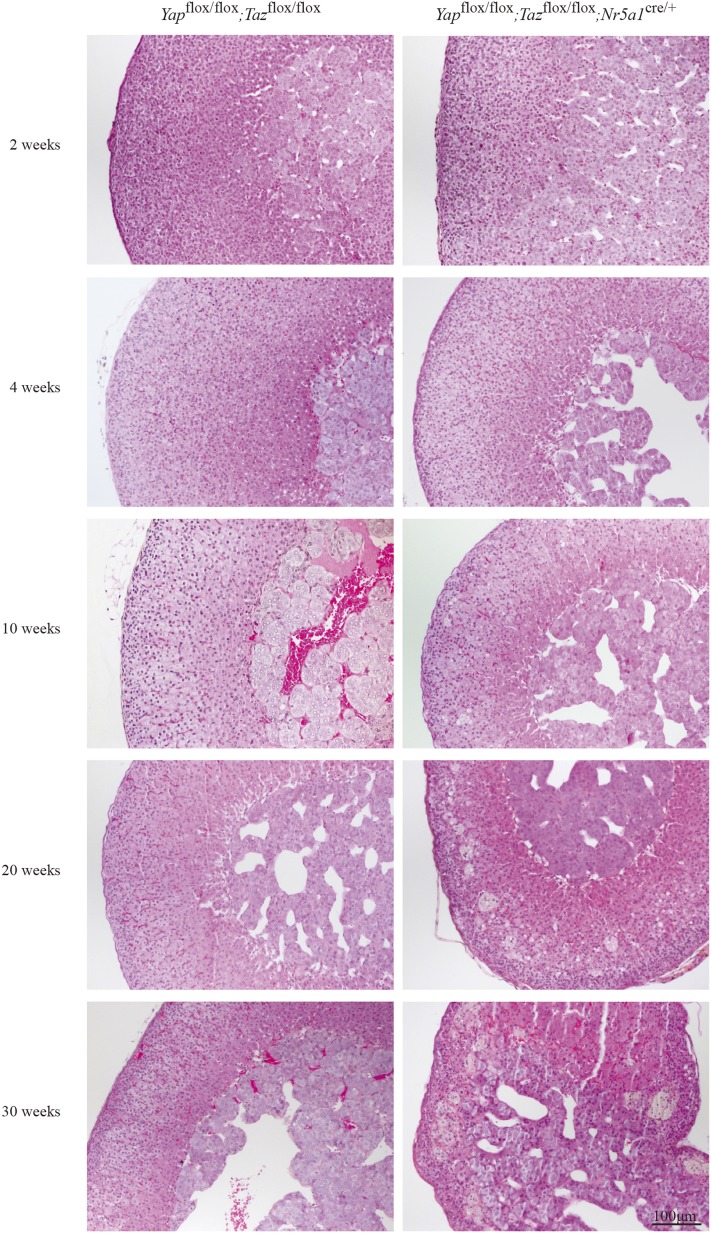
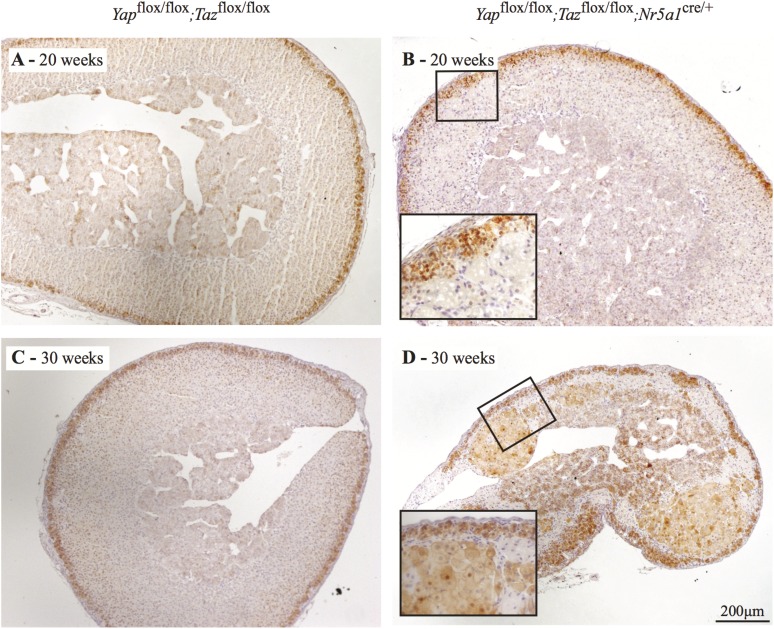
To analyze the onset and evolution of the phenotype observed in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice, adrenal glands were evaluated between 2 and 30 weeks of age. Evaluation of the adrenosomatic index showed progressive atrophy of the adrenal gland starting at 4 weeks of age (Supplemental Fig. 3 (810.5KB, tiff) ). Gross morphologic assessment of the adrenal glands from 2-week-old and 4-week-old Yapflox/flox;Tazflox/flox;Nr5a1cre/+ males showed them to be indistinguishable from those of their Yapflox/flox;Tazflox/flox counterparts (Fig. 4). By 10 weeks of age, a mild hypertrophy of the adrenocortical cells was observed in the adrenal cortex and some vacuoles were present in the zG of the adrenal cortex, with most of these vacuoles located near the junction between the zG and the zF (Fig. 4). At 20 weeks of age, as mentioned previously, numerous vacuolar cells and large multinucleated lipoid structures were observed in the adrenal cortex, and by 30 weeks of age, the adrenal cortex was almost fully destroyed (Fig. 4).
Figure 4.
Progressive adrenal cortex degeneration in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. Photomicrographs comparing adrenal gland histology of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice with that of Yapflox/flox;Tazflox/flox controls at the indicated ages. Scale bar (lower right) is valid for all images. Hematoxylin, phloxine, and saffron stain.
To confirm that the observed vacuoles were present in the zF and not in the zG and to confirm the proper zonation of the adrenal gland, the expression of the zG marker CTNNB1 was analyzed by immunohistochemistry. As expected, CTNNB1 was detected in the zG but not in the region where vacuoles were present in 20-week-old mice (Fig. 5A and 5B). CTNNB1 expression appeared to be absent in some regions of the zG in the adrenal of 30-week-old mutant animals, suggesting that zG might also be affected at this age (Fig. 5C and 5D). Of interest, CTNNB1 expression was also detected in the nucleus of some hypertrophic cells, forming large foci in the zF of 30-week-old mutant animals (Fig. 5D, inset).
Figure 5.
Zonation in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (A−D) Immunohistochemical analysis of CTNNB1 expression in adrenal glands from 20-week-old male (A) Yapflox/flox;Tazflox/flox, (B) Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice, (C) 30-week-old male Yapflox/flox;Tazflox/flox, and (D) Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. Insets in (B) and (D) show the expression of CTNNB1 in the zG at higher magnification. Insert in (D) also shows the expression of CTNNB1 in hypertrophic cells of the foci in the zF of mutant animals. Scale bar in (D) is valid for all images.
To further study the degeneration of the adrenal gland, adrenocortical cell proliferation and apoptosis were evaluated. BrdU incorporation assays showed no difference in the abundance of proliferating cells in the adrenal cortex of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ males relative to controls (Supplemental Fig. 4 (6.8MB, tiff) ). Conversely, cleaved caspase-3 was readily detected in the adrenal cortex of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice and was associated with the multinucleated, vacuolar cells (Fig. 6A and 6B). These results indicate that adrenal gland degeneration in the Yapflox/flox;Tazflox/flox;Nr5a1cre/+ model occurred because of apoptosis of adrenocortical cells and not because of reduced proliferation.
Figure 6.
Adrenal gland degeneration in the Yapflox/flox;Tazflox/flox;Nr5a1cre/+ model is associated with apoptosis. (A, B) Immunohistochemical analysis of cleaved caspase-3 in adrenal glands from 20-week-old male (A) Yapflox/flox;Tazflox/flox and (B) Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. Insets in (B) show that cleaved caspase-3 expression was associated with the multinucleated vacuolar cells. Scale bar in (B) is valid for both images.
Because the Nr5a1cre allele drives Cre expression in Leydig cells and in pituitary gonadotropes (23), abnormal hormonal production in these cell types could have occurred in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice, potentially affecting the adrenal gland and creating a confounding effect. To test this, we performed qRT-PCR analyses of Yap and Taz in the testes and pituitary gland and measured intratesticular testosterone and circulating gonadotropin levels. Although small decreases were observed in testicular Yap (Supplemental Fig. 5A (4.6MB, tif) ) and pituitary Yap and Taz (Supplemental Fig. 5B (4.6MB, tif) ) mRNA levels, testosterone (Supplemental Fig. 5C (4.6MB, tif) ), FSH (Supplemental Fig. 5D (4.6MB, tif) ), and LH (Supplemental Fig. 5E (4.6MB, tif) ) production was similar in control and mutant males. These results suggest that the phenotype observed in the adrenal cortex of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ animals was not indirectly affected by unintended targeting of Yap and Taz in other tissues.
Adrenocortical steroidogenesis was deficient in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice
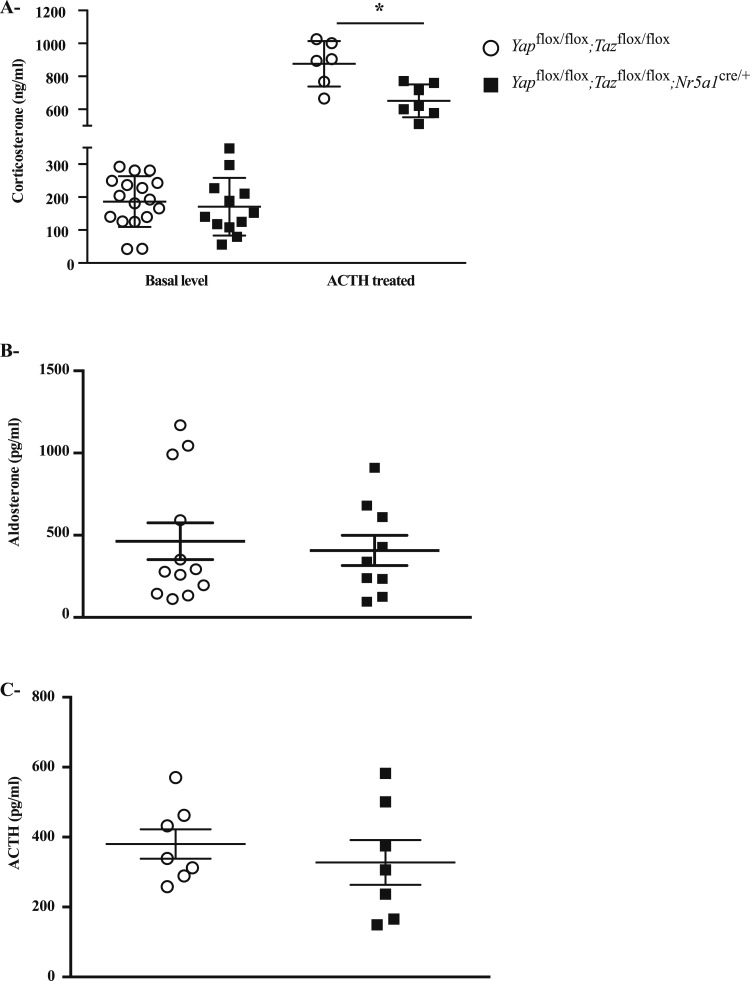
To examine the effects of YAP and TAZ deletion on adrenocortical steroidogenesis, basal and ACTH-induced levels of circulating corticosterone were measured in 10-week-old mutant and control mice. A small but statistically significant reduction in ACTH-induced corticosterone levels was observed in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ males (Fig. 7A), suggesting a minor role for YAP and TAZ in adrenal gland function. Similar results were obtained in mutant females (Supplemental Fig. 6 (470KB, tiff) ). Circulating aldosterone (Fig. 7B) and ACTH (Fig. 7C) levels were measured in 20-week-old and 10-week-old animals, respectively, and no differences were observed between the control and mutant mice.
Figure 7.
Circulating hormone levels in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (A) Serum corticosterone levels from untreated and ACTH-treated 10-week-old male Yapflox/flox;Tazflox/flox and Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (B) Serum aldosterone levels from 20-week-old male Yapflox/flox;Tazflox/flox and Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (C) Plasma ACTH levels in 10-week-old male Yapflox/flox;Tazflox/flox and Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. Sample numbers analyzed varied by hormonal test and genotype. Values for Yapflox/flox;Tazflox/flox mice were basal corticosterone: n = 17; ACTH-induced corticosterone: n = 6; aldosterone: n = 12; and ACTH: n = 7. Values for Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice were basal corticosterone: n = 12; ACTH-induced corticosterone: n = 7; aldosterone: n = 9; and ACTH: n = 7. Data are expressed as mean (columns) ± standard error of the mean (error bars). Asterisks indicate significantly different from control (*P < 0.05).
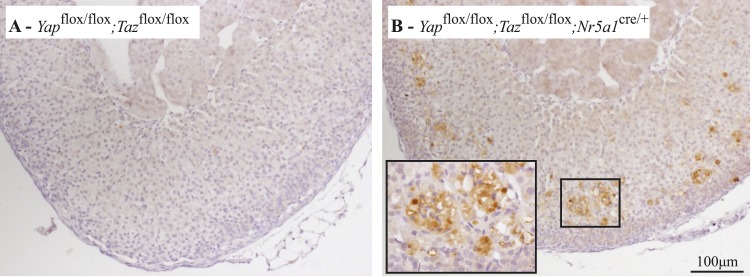
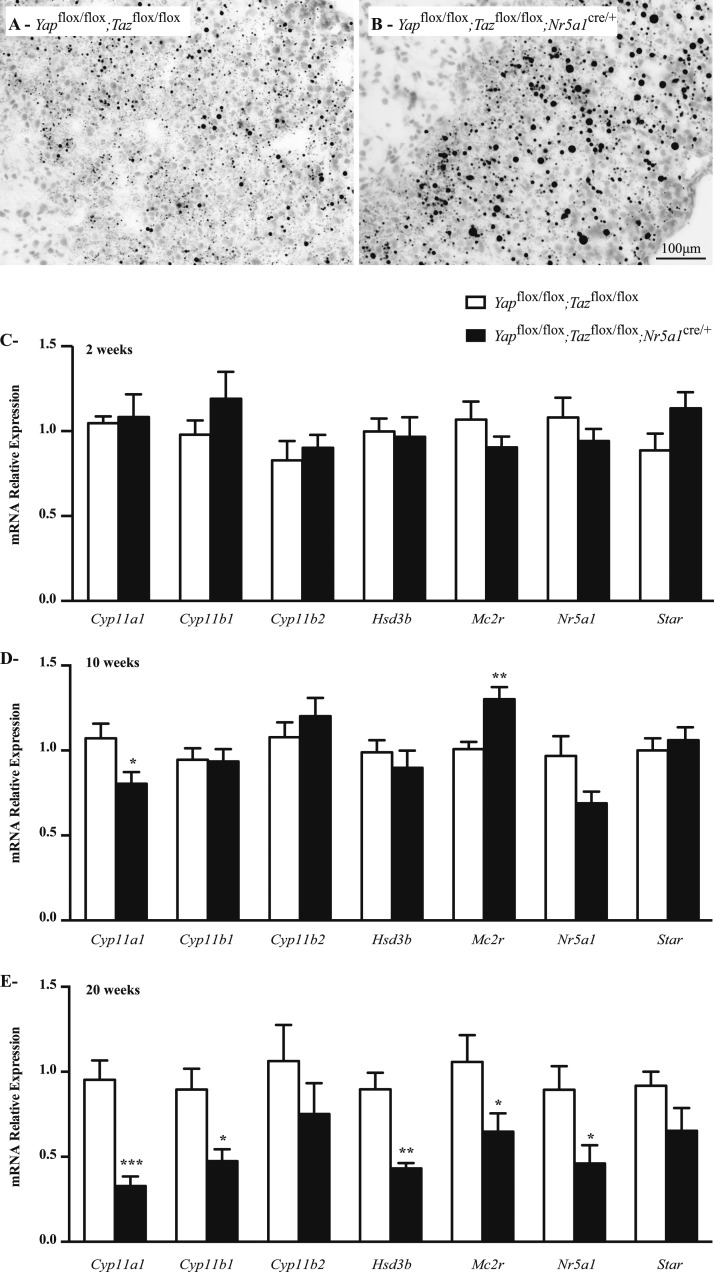
To determine whether decreased adrenal steroidogenesis in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice had an effect on lipid accumulation, Oil Red O staining was performed on adrenal glands from 10-week-old mice. Results showed an increased accumulation of lipid droplets in the adrenal cortex of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ animals (Fig. 8A and 8B), suggesting a decrease in lipid metabolism. To further delineate the changes in adrenal steroid production in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ males, expression of steroidogenic genes was evaluated by qRT-PCR in 2-week-old, 10-week-old, and 20-week-old males. Among the genes tested, no changes in mRNA levels were observed in the adrenal glands of 2-week-old animals (Fig. 8C), and only cytochrome P450, family 11, subfamily a, polypeptide 1 (Cyp11a1) was significantly downregulated in 10-week-old Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice (Fig. 8D). At the latter age, a small increase in melanocortin 2 receptor mRNA levels was also observed. By 20 weeks of age, mRNA levels of Cyp11a1; cytochrome P450, family 11, subfamily b, polypeptide 1 (Cyp11b1); hydroxy-delta-5-steroid dehydrogenase, 3 β- and steroid δ-isomerase; melanocortin 2 receptor; and Nr5a1 were all reduced, whereas the expression of cytochrome P450, family 11, subfamily b, polypeptide 2; and steroidogenic acute regulatory protein were not affected (Fig. 8E). Cyp11a1 mRNA levels were also downregulated in the adrenal glands of 10-week-old mutant females, in addition to hydroxy-delta-5-steroid dehydrogenase, 3 β- and steroid δ-isomerase and Nr5a1 in 20-week-old females (Supplemental Fig. 7A and 7B (1.6MB, tiff) ). Taken together, these results suggest that steroidogenesis is slightly altered in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ animals.
Figure 8.
Lipid accumulation and expression of steroidogenic genes were altered in the adrenal glands of male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (A, B) Oil Red O staining of adrenal glands of 10-week-old male (A) Yapflox/flox;Tazflox/flox and (B) Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (C−E) qRT-PCR analysis of genes involved in steroidogenesis in adrenal gland from (C) 2-week-old, (D) 10-week-old, and (E) 20-week-old male mice of the indicated genotypes (n = 6 animals per genotype). All data were normalized to the housekeeping gene Rpl19 and are expressed as mean (columns) ± standard error of the mean (error bars). Asterisks indicate significantly different from control (*P < 0.05; **P < 0.01; ***P < 0.001).
Adrenal gland progenitor cell defects in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice
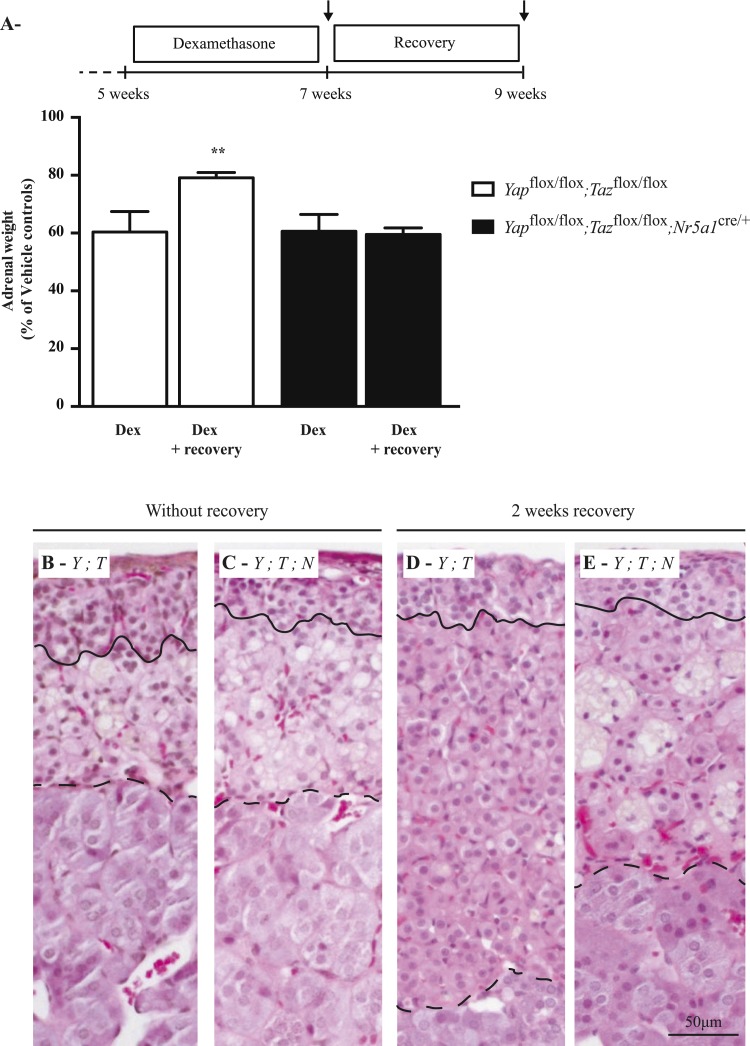
The adrenal glands of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ males showed a progressive degeneration characteristic of adrenal failure, suggesting a potential defect in stem or progenitor cell populations. To determine whether adrenal regeneration was affected in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ males, 5-week-old mutant and control males were treated with dexamethasone for 2 weeks to suppress the zF through negative feedback on the hypothalamus and pituitary gland and then allowed to recover (or not) for 2 weeks. Dexamethasone treatment resulted in an ≈40% reduction in adrenal weight (Fig. 9A) associated with degeneration of the zF (Fig. 9B and 9C) and a reduction in steroidogenic gene expression (Supplemental Fig. 8A (2.4MB, tiff) ) in both mutant and control mice. The adrenal glands from control mice allowed to recover after dexamethasone treatment regained most of their lost weight (Fig. 9A and 9D), and steroidogenic gene expression levels increased (Supplemental Fig. 8B (2.4MB, tiff) ), indicating regeneration of the zF. However, the adrenal glands from the Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice failed to recover any weight (Fig. 9A). Despite the fact that the adrenal glands of the mutant mice failed to recover any weight, expression levels of steroidogenic genes increased after the recovery period (Supplemental Fig. 8B (2.4MB, tiff) ). The zF also appeared slightly larger (Fig. 9E); however, numerous large multinucleated structures reminiscent of the ones present in the adrenal cortices of 20-week-old Yapflox/flox;Tazflox/flox;Nr5a1cre/+ animals were also present in the mutant mice after the recovery period (Fig. 9E). Surprisingly, circulating corticosterone levels did not decrease significantly in the mutant animals compared with the control animals (Supplemental Fig. 8C (2.4MB, tiff) ), and no differences were observed in plasma ACTH levels (Supplemental Fig. 8D (2.4MB, tiff) ). Taken together, these results suggest that some stem/progenitor cells were still present in the adrenal glands of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice, as they were able to partially recover after dexamethasone treatment. However, the acceleration of the degenerative process (Fig. 9E) also suggests that the stem/progenitor cells may have been affected.
Figure 9.
Regeneration of the zF after dexamethasone treatment was impaired in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (A) Comparison of adrenal weights of male Yapflox/flox;Tazflox/flox and Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice 2 weeks after dexamethasone treatment and 2 weeks after dexamethasone treatment with a 2-week recovery period (n = 8 animals per genotype; vehicle = 0.05% ethanol). Data are expressed as means (columns) ± standard error of the mean (error bars) and are presented as percentage of vehicle controls. Asterisks indicate significantly different from control (**P < 0.01). (B−E) Photomicrographs comparing the adrenal glands of (B) Yapflox/flox;Tazflox/flox mice with those of (C) Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice 2 weeks after dexamethasone treatment, and adrenal glands of (D) Yapflox/flox;Tazflox/flox mice with those of (E) Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice 2 weeks after dexamethasone treatment with a 2-week recovery period. The line indicates the border between zG and the zF. The dashed line indicates the border between the zF and the medulla. Scale bar (lower right) is valid for all images. Hematoxylin, phloxine, and saffron stain. Dex, dexamethasone; Y;T, Yapflox/flox;Tazflox/flox; Y;T;N, Yapflox/flox;Tazflox/flox;Nr5a1cre/+.
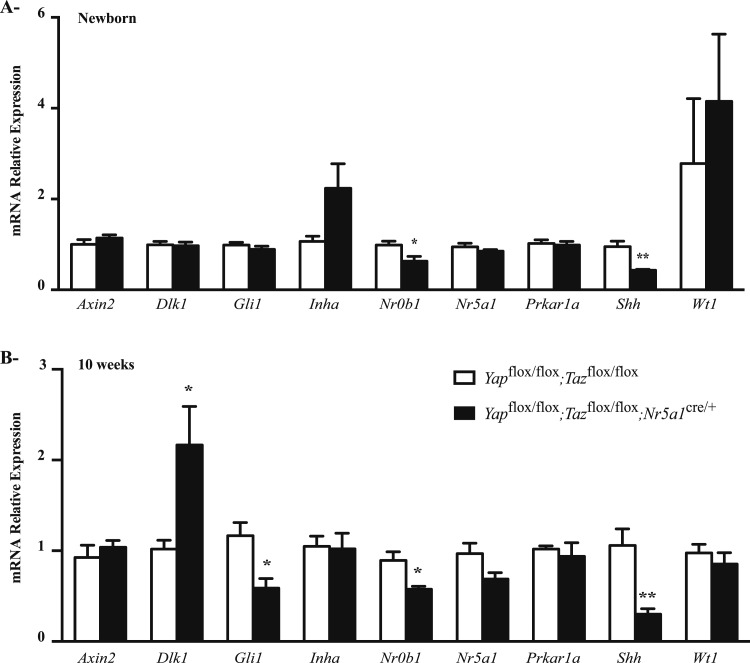
To characterize the adrenal stem and progenitor cell populations in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice, the expression levels of several genes known to be involved (or associated with signaling pathways known to be involved) in adrenocortical development, renewal, or remodeling (5–16, 24–30) were evaluated in newborn and 10-week-old Yapflox/flox;Tazflox/flox;Nr5a1cre/+ males (Fig. 10A and 10B). Shh and Nr0b1 expression was downregulated in mutant animals at both ages. A statistically significant reduction in glioma-associated oncogene homologue (Gli1) expression was also observed in 10-week-old mutant animals, along with a statistically significant increase in deltalike 1 (Dlk1) levels. Shh levels also decreased, whereas Dlk1 levels increased in mutant females (Supplemental Fig. 9A (1.3MB, tiff) ); however, Nr0b1 and Gli1 levels were unchanged (Supplemental Fig. 9A (1.3MB, tiff) ). Interestingly, Nr0b1 levels were also 12 times higher in the adrenal glands of female mice than in those of males (Supplemental Fig. 9B (1.3MB, tiff) ). These results suggest that either the progenitor cell population was reduced or the progenitor cells were among the cells affected in the Yapflox/flox;Tazflox/flox;Nr5a1cre/+ animals.
Figure 10.
Expression of genes involved in stem cell or progenitor cell maintenance in the adrenal glands of male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. (A, B) qRT-PCR analysis of genes involved in stem cell or progenitor cell maintenance in adrenal glands from (A) newborn and (B) 10-week-old male mice of the indicated genotypes (n = 6 animals per genotype). All data were normalized to the housekeeping gene Rpl19 and are expressed as means (columns) ± standard error of the mean (error bars). Asterisks indicate significantly different from control (*P < 0.05; **P < 0.01).
Discussion
In recent years, the Hippo signaling effectors YAP and TAZ have been identified as main contributors to adult stem cell maintenance and tissue homeostasis in several tissues (18); however, no study has evaluated their function(s) in the adrenal glands. Here, we report that the inactivation of Yap and Taz in the adrenocortical cells resulted in adrenal degeneration. This provides functional evidence of the importance of Hippo signaling in adrenocortical cells.
In the postnatal adrenal gland, pools of stem/progenitor cells maintain homeostasis by balancing their proliferation, migration, and differentiation into steroidogenic cells. Differentiation and proliferation of these cells are also essential to repopulate or expand adrenocortical zones after insult (3). Adrenal atrophy and failure have been observed in numerous transgenic mouse models and have been associated with loss of the stem/progenitor cells (5–7, 9, 10, 12, 13, 15). The progressive atrophy of the adrenal gland observed in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice suggests that Hippo signaling is also involved in the maintenance of the adrenal gland. However, unlike most of the previously reported transgenic models (5–7, 9–15), adrenal glands of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ animals appear normal at birth and show proper zonation.
Interestingly, efficient inactivation of Ctnnb1 in adrenocortical cells led to a dramatic decrease in proliferation and the disappearance of the adrenal gland by e18.5 (15), whereas inefficient inactivation of Ctnnb1 led to normal development of the adrenal gland followed by degeneration of the adrenal cortex starting at 15 weeks of age (15). The Nr5a1cre strain used in this study (23) is different from the Nr5a1cre strains used in the Ctnnb1 study (31), but inefficient recombination of the Yap and Taz floxed alleles provides a likely explanation for the absence of an abnormal phenotype in the embryonic adrenal gland of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. If so, transgenic models with more efficient recombination are needed to evaluate the function of YAP and TAZ during adrenal development.
Two main populations of stem/progenitor cells are known to be involved in the homeostasis of the adrenal gland: the stem cells located in the capsule and the subcapsular progenitor cells. Because the Nr5a1cre model does not drive Cre expression in the capsule (https://frama.link/rxwMHTCG), it would seem unlikely that the stem cells were directly affected in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. The subcapsular progenitor cells are known to strongly express SHH (6, 13, 14), which is essential for the development of the adrenal cortex, as Shh-null mice have smaller adrenal primordia and conditional inactivation of Shh in Nr5a1-expressing cells causes hypoplastic adrenal glands (6, 13, 14). The robust downregulation of Shh expression observed in the adrenal glands from Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice suggests that the SHH+ progenitor cells were affected. However, the relationship between the loss of YAP/TAZ expression, the decrease in SHH expression, and the adrenal degeneration phenotype observed in the Yapflox/flox;Tazflox/flox;Nr5a1cre/+ model remains unclear. One possibility is that the deletion of YAP/TAZ, which are known to be involved in cell survival (32–35), simply resulted in the death of adrenal cells, potentially including SHH+ progenitor cells. Because adrenal Shh expression is downregulated in newborn Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice, the pool of progenitor cells may have been partly depleted during embryonic development, leading to inadequate maintenance of homeostasis in the adult. Another possibility is that progenitor cells could be rapidly recruited into the zF to compensate for the loss of adrenocortical cells, perhaps resulting in enhanced differentiation of the progenitor cells. Finally, Hippo signaling may also directly influence the expression of Shh in progenitor cells, as hippo signaling has influenced Shh expression in several tissues. For example, it has been shown that YAP can activate SHH signaling in P19 cells (36). Likewise, the overexpression of YAP inhibited neuronal differentiation in primary mouse cortical progenitors via a mechanism involving SHH (36). Loss of Yap function in the Xenopus tadpole also caused limb bud regeneration defects and the downregulation of Shh expression (37). Reporter gene marking of the progenitor cell population combined with cell lineage tracing experiments in the context of YAP/TAZ genetic manipulation would be required to test these hypotheses.
Aside from Shh, a small decrease in the expression levels of the Shh downstream target Gli1 was observed in 10-week-old mutant animals. No differences in Gli1 expression were observed in younger animals despite the marked decrease in Shh levels. This suggests that either the remaining SHH was sufficient to maintain Gli1 expression or that other mechanisms compensated for the downregulation of Shh to maintain Gli1 expression. One potential factor that could compensate for SHH loss is deltalike 1 homologue (DLK1), as it was shown in the rat that DLK1 can activate Gli1 transcription in the stem cells located in the capsule (38). Compatible with this, we observed an increase in adrenal Dlk1 expression in 10-week-old Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. Aside from genes associated with SHH signaling, Nr0b1 expression was also downregulated in the adrenal glands of male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. It was previously shown that loss-of-function mutations or deletion of Nr0b1 caused the depletion of the subcapsular progenitor cells in both humans and mice (9, 12). As for Shh, whether YAP/TAZ serves to directly regulate Nr0b1 expression in adrenal progenitor cells remains to be determined.
Degeneration of the adrenal cortex was observed in Yapflox/flox;Tazflox/flox;Nr5a1cre/+ males but not in females, despite similar downregulation of Yap, Taz, and Shh expression. Several aspects of adrenal physiology are well known to differ by sex; for instance, the size of the adrenal gland and corticosterone and aldosterone secretion are all higher in females. The basis for these differences is not completely understood, although circulating sex hormone levels are known to play a role (39–41). This study suggests that the signaling mechanisms underlying adrenal regeneration may also vary by sex. Our finding that adrenal degeneration occurred only in male Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice despite a decrease in Shh occurring in both sexes suggests either that SHH plays a more important role in male progenitor cells or that other signaling pathways compensate for the partial loss of Shh expression in the female adrenal gland. If the latter is true, the transcription factor Nr0b1 could be involved because it is known to be important for adrenal gland maintenance (12) and is also known to be expressed more in the female adrenal gland (39, 42).
Another feature of the female adrenal gland is the persistence of the X-zone in virgin animals (43), a zone with poorly defined functions (44). Strong ACTH-independent centrifugal expansion of corticosterone-secreting cells originating from the X-zone were observed in the Prkar1a knockout mouse (27), suggesting that some stem/progenitor cells could arise from the X-zone in some experimental conditions. Despite the persistence of a few TAZ-expressing cells in the X-zone of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice, it seems unlikely that this zone is responsible for the dimorphism observed between males and females, as the X-zone degenerates in parous females and no abnormal phenotype was observed in old parous females.
Features of impaired steroidogenesis, including elevated levels of neutral lipids, decreased expression of steroidogenic genes, and ACTH-stimulated corticosterone production, were all observed in the adrenal glands of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice. The overall decrease in steroidogenic gene expression levels was most likely caused mainly by the loss of adrenocortical cells. However, our analysis of steroidogenic gene expression in 10-week-old Yapflox/flox;Tazflox/flox;Nr5a1cre/+ mice suggests that a specific loss of Cyp11a1 also contributed to the decrease in corticosterone production and that the expression of Cyp11a1 could be regulated by YAP/TAZ. Although it has been reported that YAP can regulate the expression of the steroidogenic enzyme Cyp19a1 in the KGN granulosa tumor cell line (29), whether YAP/TAZ can regulate additional steroidogenic enzymes such as Cyp11a1 is grounds for further study. Interestingly, lipid accumulation was observed in the adrenal cortex of Cyp11a1 knockout mice (45), which die shortly after birth. This suggests that the downregulation of Cyp11a1 could have contributed to the lipid accumulation observed in the adrenal glands of Yapflox/flox;Tazflox/flox;Nr5a1cre/+ males.
In summary, this study reports a role for YAP and TAZ in adrenal gland maintenance and indicates that inactivation of Yap and Taz in the adrenal gland led to adrenal degeneration.
Acknowledgments
The authors thank Dr. Eric N. Olsen (University of Texas Southwestern Medical Center, Dallas, TX) for generously providing the Yapflox/flox;Tazflox/flox mice.
Financial Support: This work was supported by Discovery Grants RGPIN-2014-04358 (to A.B.) and RGPIN-2012-340902 (to D.B.) from the National Sciences and Engineering Research Council of Canada. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD28934.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropic hormone
- BrdU
- 5-bromo-2′-deoxyuridine
- cDNA
- complementary DNA
- Ct
- cycle threshold
- CTNNB1
- β-catenin
- Cyp11a1
- cytochrome P450, family 11, subfamily a, polypeptide 1
- Cyp11b1
- cytochrome P450, family 11, subfamily b, polypeptide 1
- DLK1
- deltalike 1 homologue
- FSH
- follicle-stimulating hormone
- Gli1
- glioma-associated oncogene homologue
- LH
- luteinizing hormone
- mRNA
- messenger RNA
- PCR
- polymerase chain reaction
- qRT-PCR
- quantitative reverse transcription polymerase chain reaction
- RRID
- Research Resource Identifier
- SHH
- sonic hedgehog
- TAZ
- transcriptional coactivator with PDZ-binding motif
- YAP
- Yes-associated protein
- zF
- zona fasciculata
- zG
- zona glomerulosa.
References
- 1.Pihlajoki M, Dörner J, Cochran RS, Heikinheimo M, Wilson DB. Adrenocortical zonation, renewal, and remodeling. Front Endocrinol (Lausanne). 2015;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walczak EM, Hammer GD. Regulation of the adrenocortical stem cell niche: implications for disease. Nat Rev Endocrinol. 2015;11(1):14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BD, Kempna PB, Carlone DL, Shah M, Guagliardo NA, Barrett PQ, Gomez-Sanchez CE, Majzoub JA, Breault DT. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26(6):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morley SD, Viard I, Chung BC, Ikeda Y, Parker KL, Mullins JJ. Variegated expression of a mouse steroid 21-hydroxylase/beta- galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol Endocrinol. 1996;10(5):585–598. [DOI] [PubMed] [Google Scholar]
- 5.Wood MA, Acharya A, Finco I, Swonger JM, Elston MJ, Tallquist MD, Hammer GD. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140(22):4522–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci USA. 2009;106(50):21185–21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandiera R, Vidal VP, Motamedi FJ, Clarkson M, Sahut-Barnola I, von Gise A, Pu WT, Hohenstein P, Martinez A, Schedl A. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev Cell. 2013;27(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134(12):2349–2358. [DOI] [PubMed] [Google Scholar]
- 9.Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell Endocrinol. 2001;185(1-2):17–25. [DOI] [PubMed] [Google Scholar]
- 10.Wong M, Ikeda Y, Luo X, Caron KM, Weber TJ, Swain A, Schimmer BP, Parker KL. Steroidogenic factor 1 plays multiple roles in endocrine development and function. Recent Prog Horm Res. 1997;52:167–182; discussion 182–184. [PubMed] [Google Scholar]
- 11.Zubair M, Oka S, Parker KL, Morohashi K. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol Endocrinol. 2009;23(10):1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheys JO, Heaton JH, Hammer GD. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology. 2011;152(9):3430–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151(3):1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis. 2009;47(9):628–637. [DOI] [PubMed] [Google Scholar]
- 15.Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593–2602. [DOI] [PubMed] [Google Scholar]
- 16.Huang CC, Liu C, Yao HH. Investigating the role of adrenal cortex in organization and differentiation of the adrenal medulla in mice. Mol Cell Endocrinol. 2012;361(1-2):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez M, Gomez V, Hergovich A. The Hippo pathway in disease and therapy: cancer and beyond. Clin Transl Med. 2014;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614–1626. [DOI] [PubMed] [Google Scholar]
- 19.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312. [DOI] [PubMed] [Google Scholar]
- 20.Abduch RH, Carolina Bueno A, Leal LF, Cavalcanti MM, Gomes DC, Brandalise SR, Masterallo MJ, Yunes JA, Martinelli CE Jr, Tone LG, Tucci S, Molina CA, Ramalho FS, Moreira AC, Cardinalli IA, Scrideli CA, Ramalho LN, de Castro M, Antonini SR. Unraveling the expression of the oncogene YAP1, a Wnt/beta-catenin target, in adrenocortical tumors and its association with poor outcome in pediatric patients. Oncotarget. 2016;7(51):84634–84644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4(196):ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA. 2013;110(34):13839–13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. [DOI] [PubMed] [Google Scholar]
- 24.Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci USA. 1994;91(19):8817–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heaton JH, Wood MA, Kim AC, Lima LO, Barlaskar FM, Almeida MQ, Fragoso MC, Kuick R, Lerario AM, Simon DP, Soares IC, Starnes E, Thomas DG, Latronico AC, Giordano TJ, Hammer GD. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am J Pathol. 2012;181(3):1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, Taketo MM, Tissier F, Bertherat J, Lefrançois-Martinez AM, Martinez A, Val P. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19(8):1561–1576. [DOI] [PubMed] [Google Scholar]
- 27.Sahut-Barnola I, de Joussineau C, Val P, Lambert-Langlais S, Damon C, Lefrançois-Martinez AM, Pointud JC, Marceau G, Sapin V, Tissier F, Ragazzon B, Bertherat J, Kirschner LS, Stratakis CA, Martinez A. Cushing’s syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS Genet. 2010;6(6):e1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida MQ, Muchow M, Boikos S, Bauer AJ, Griffin KJ, Tsang KM, Cheadle C, Watkins T, Wen F, Starost MF, Bossis I, Nesterova M, Stratakis CA. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/- or Rb1+/- backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet. 2010;19(8):1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drelon C, Berthon A, Sahut-Barnola I, Mathieu M, Dumontet T, Rodriguez S, Batisse-Lignier M, Tabbal H, Tauveron I, Lefrançois-Martinez AM, Pointud JC, Gomez-Sanchez CE, Vainio S, Shan J, Sacco S, Schedl A, Stratakis CA, Martinez A, Val P. PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nat Commun. 2016;7:12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21(12):2968–2987. [DOI] [PubMed] [Google Scholar]
- 31.Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44(9):419–424. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Ma K, Chen L, Zhu H, Liang S, Liu M, Xu N. TAZ promotes cell growth and inhibits Celastrol-induced cell apoptosis. Biosci Rep. 2016;36(5):e00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigneron AM, Ludwig RL, Vousden KH. Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev. 2010;24(21):2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Y, Matsui Y, Pan W, Li Q, Lai ZC. Yap1 plays a protective role in suppressing free fatty acid-induced apoptosis and promoting beta-cell survival. Protein Cell. 2016;7(5):362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao JJ, Zhao XM, Wang DL, Chen KH, Sheng X, Li WB, Li MC, Liu WJ, He J. YAP is overexpressed in clear cell renal cell carcinoma and its knockdown reduces cell proliferation and induces cell cycle arrest and apoptosis. Oncol Rep. 2014;32(4):1594–1600. [DOI] [PubMed] [Google Scholar]
- 36.Lin YT, Ding JY, Li MY, Yeh TS, Wang TW, Yu JY. YAP regulates neuronal differentiation through Sonic hedgehog signaling pathway. Exp Cell Res. 2012;318(15):1877–1888. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi S, Tamura K, Yokoyama H. Yap1, transcription regulator in the Hippo signaling pathway, is required for Xenopus limb bud regeneration. Dev Biol. 2014;388(1):57–67. [DOI] [PubMed] [Google Scholar]
- 38.Guasti L, Cavlan D, Cogger K, Banu Z, Shakur A, Latif S, King PJ. Dlk1 up-regulates Gli1 expression in male rat adrenal capsule cells through the activation of β1 integrin and ERK1/2. Endocrinology. 2013;154(12):4675–4684. [DOI] [PubMed] [Google Scholar]
- 39.El Wakil A, Mari B, Barhanin J, Lalli E. Genomic analysis of sexual dimorphism of gene expression in the mouse adrenal gland. Horm Metab Res. 2013;45(12):870–873. [DOI] [PubMed] [Google Scholar]
- 40.Bastida CM, Cremades A, Castells MT, López-Contreras AJ, López-García C, Sánchez-Mas J, Peñafiel R. Sexual dimorphism of ornithine decarboxylase in the mouse adrenal: influence of polyamine deprivation on catecholamine and corticoid levels. Am J Physiol Endocrinol Metab. 2007;292(4):E1010–E1017. [DOI] [PubMed] [Google Scholar]
- 41.Bielohuby M, Herbach N, Wanke R, Maser-Gluth C, Beuschlein F, Wolf E, Hoeflich A. Growth analysis of the mouse adrenal gland from weaning to adulthood: time- and gender-dependent alterations of cell size and number in the cortical compartment. Am J Physiol Endocrinol Metab. 2007;293(1):E139–E146. [DOI] [PubMed] [Google Scholar]
- 42.Mukai T, Kusaka M, Kawabe K, Goto K, Nawata H, Fujieda K, Morohashi K. Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells. 2002;7(7):717–729. [DOI] [PubMed] [Google Scholar]
- 43.Sato T. The fine structure of the mouse adrenal X zone. Z Zellforsch Mikrosk Anat. 1968;87(3):315–329. [DOI] [PubMed] [Google Scholar]
- 44.Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148(3):976–988. [DOI] [PubMed] [Google Scholar]
- 45.Hu MC, Hsu NC, El Hadj NB, Pai CI, Chu HP, Wang CK, Chung BC. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol. 2002;16(8):1943–1950. [DOI] [PubMed] [Google Scholar]