Abstract
Defects in the biosynthesis of phospholipids and neutral lipids are associated with cell membrane dysfunction, disrupted energy metabolism, and diseases including lipodystrophy. In these pathways, the 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT) enzymes transfer a fatty acid to the sn-2 carbon of sn-1-acylglycerol-3-phosphate (lysophosphatidic acid) to form sn-1, 2-acylglycerol-3-phosphate [phosphatidic acid (PA)]. PA is a precursor for key phospholipids and diacylglycerol. AGPAT1 and AGPAT2 are highly homologous isoenzymes that are both expressed in adipocytes. Genetic defects in AGPAT2 cause congenital generalized lipodystrophy, indicating that AGPAT1 cannot compensate for loss of AGPAT2 in adipocytes. To further explore the physiology of AGPAT1, we characterized a loss-of-function mouse model (Agpat1−/−). The majority of Agpat1−/− mice died before weaning and had low body weight and low plasma glucose levels, independent of plasma insulin and glucagon levels, with reduced percentage of body fat but not generalized lipodystrophy. These mice also had decreased hepatic messenger RNA expression of Igf-1 and Foxo1, suggesting a decrease in gluconeogenesis. In male mice, sperm development was impaired, with a late meiotic arrest near the onset of round spermatid production, and gonadotropins were elevated. Female mice showed oligoanovulation yet retained responsiveness to gonadotropins. Agpat1−/− mice also demonstrated abnormal hippocampal neuron development and developed audiogenic seizures. In summary, Agpat1−/− mice developed widespread disturbances of metabolism, sperm development, and neurologic function resulting from disrupted phospholipid homeostasis. AGPAT1 appears to serve important functions in the physiology of multiple organ systems. The Agpat1-deficient mouse provides an important model in which to study the contribution of phospholipid and triacylglycerol synthesis to physiology and diseases.
Loss-of-function mouse model for AGPAT1, a phospholipid-synthesizing enzyme, revealed critical roles in glucose homeostasis, spermatogenesis, and audiogenic seizures.
Most of the glycerophospholipids (GPLs) are generated by the pathway first described by Kennedy (1). In this pathway, there is a sequential esterification of glycerol-3-phosphate by glycerol-3-phosphate acyltransferase at the sn-1 position, followed by esterification of 1-acylglycerol-3-phosphate [lysophosphatidic acid (LPA)] at the sn-2 position by 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT). Before the sn-3 position can be esterified by diacylglycerol acyltransferase, the product of the AGPAT enzymatic reaction, phosphatidic acid (PA), is dephosphorylated by lipins (2, 3) [and reviewed in (4)] to produce diacylglycerol (DAG), which is the immediate precursor for triacylglycerol (TAG). Although lipin seems to be the main PA dephosphorylase, another class of lipid phosphate phosphatases can produce DAG (5). PA is also a precursor for several GPLs including phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol (6, 7). These GPLs can also be formed by using DAG as a substrate. In addition to these pathways, several of the GPLs are later remodeled by removal of the sn-2 fatty acid by phospholipases and re-esterified by AGPATs, a phenomenon described by Lands (8).
We and others have now reported 11 isoforms of AGPATs, which are products of different genes and have different tissue expression patterns in humans (9). Some of these AGPATs have additional LPA acyltransferase activities (9–13). Almost all of these AGPATs have been studied to define their substrate specificities and subcellular localizations (9). Although human AGPAT1 and AGPAT2 isoforms are close homologues with similar in vitro substrate specificities for these recombinant proteins (14), their tissue expression patterns are different: AGPAT1 is ubiquitously expressed, whereas AGPAT2 shows more restricted tissue distribution (14). In a mouse tissue survey of all of the AGPAT isoforms, expression of Agpat1 was several-fold lower in liver (∼14-fold), epididymal fat (∼1.5-fold), and brown fat (∼5.8-fold) than expression of Agpat2 (Supplemental Fig. 1 (18.1MB, docx) ).
Despite the presence of AGPAT1 in adipose tissue, genetic loss of AGPAT2 results in congenital generalized lipodystrophy, a syndrome of severe fat loss (15, 16). Given the widespread expression of AGPAT1 and its lack of redundancy with AGPAT2 in adipocyte biology, the functions of AGPAT1 and the most important tissues in which it is active are not known. The association of a single-nucleotide polymorphism (rs 1061808) in the AGPAT1 locus with type 2 diabetes mellitus has been demonstrated in a genome-wide association study (17) and was also observed in the DIAGRAM study (18), suggesting that AGPAT1 may be important for glucose homeostasis. Another single nucleotide polymorphism (rs3130283) in the AGPAT1 locus was associated with Alzheimer’s disease (19), also implicating AGPAT1 in brain function. As an initial step in characterizing the physiologic functions of AGPAT1, we generated Agpat1 knockout (KO) mice by homologous gene deletion and performed a detailed phenotype analysis, particularly on endocrine, metabolic, and neurologic functions.
Materials and Methods
Animals
All animal studies were approved by the Institutional Use and Care of Animals Committee at the University of Texas Southwestern Medical Center. All methods were performed in accordance with the relevant guidelines and regulations.
Agpat1−/− mice and genotyping
Agpat1+/− mice (C57BL/129S6/SvEv) were obtained from Lexicon Inc. (The Woodlands, TX) and were crossed to generate the Agpat1−/− mice. Only wild-type (WT) and Agpat1−/− mice were used in this study. Mice were maintained on Teklad Global 16% Protein Rodent Diet, which contains 16.4% crude protein, 4% fat, 48.5% carbohydrates, and 3.3% crude fiber (Harlan Laboratories, Indianapolis, IN).
For the majority of experiments, genotyping was performed around postnatal day (P)10 to P14 depending on the experimental requirement using WT and KO specific primers. For hippocampal neuron isolation, genotyping was performed at P2 to P3. For the WT allele primer pairs 5′-TCACAGGGCCCAGTCCTCTAGCAGA-3′ (forward) and 5′-ATGGCGTCCCCTGTGCGTTT-3′ (reverse) were used, and for detecting the KO allele, primer pairs 5′-AATGGGCTGACCGCTTCCTCGT-3′ (forward) and 5′-CCATTGTCCAGTGTAGGGCGGA-3′ (reverse) were used employing touchdown polymerase chain reaction (PCR). The PCR products were analyzed on 1.2% agarose gels. Expected size DNA fragments WT 393bp and KO 450bp were observed, confirming the respective genotype.
Immunoblot analysis
Proteins were immunoprobed with specific antibodies. Anti-Stat5b (ab52162; lot #320972; 1:1000 dilution); pStat5b, pS731 (ab52211; lot #GR77090-1; 1:500 dilution) were from Abcam (Cambridge, MA). LC3/MAP1LC3A (NB100-2331; lot #V-1; 1:500 dilution) was from Novus Biologicals (Littleton, CO). Blots were incubated with secondary antibody to immunoglobulin G (IgG) [goat anti-rabbit-IgG conjugated to horseradish peroxidase (HRP)] at a 1:10,000 dilution (SC2004; lot #E2505; Santa Cruz Biotechnology, Dallas, TX) and detected with chemiluminescent HRP substrate (Millipore, Billerica, MA) and exposed onto X-ray films. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (AM4300; 1:5000 dilution) was from Ambion (Foster City, CA). Secondary antibody for GAPDH (goat anti-rabbit-IgG conjugated to HRP; SC2005; lot #E3113; 1:10,000 dilution) was from Santa Cruz Biotechnology. Antibodies used in this study are listed in Table 1. A detailed method is presented in the Supplemental Material/Supporting Information (18.1MB, docx) .
Table 1.
Source of Antibodies Used in This Study
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer (Catalog No.) | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| STAT5b | Synthetic nonphosphopeptide derived from human STAT5b around the phosphorylation site of serine 731(A-P-S-P-A) | Anti-Stat5b | Abcam (ab52162) | Rabbit; polyclonal | 1:1000 | AB_882716 |
| STAT5b (phospho S731) | Synthetic phosphopeptide based on human STAT5B around the phosphorylation site of serine 731 (A-P-SP-P-A) | Anti-pStat5b, pS731 | Abcam (ab52211) | Rabbit; polyclonal | 1:500 | AB_2196931 |
| LC3/MAP1LC3A | A synthetic peptide made to an internal portion of the human LC3 protein sequence (between residues 25 and 121) | Anti-LC3 | Novus Biologicals (NB100-2331) | Rabbit; polyclonal | 1:500 | AB_10001955 |
| GAPDH | Purified rabbit muscle GAPDH (whole molecule) | Anti-GAPDH | Ambion (AM4300) | Mouse; monoclonal | 1:5000 | AB_437392 |
| Goat IgG | Not applicable | Goat anti-rabbit IgG-HRP | Santa Cruz Biotechnology (SC2004) | Goat; polyclonal | 1:10,000 | AB_631746 |
| Goat IgG | Not applicable | Goat anti-mouse IgG-HRP | Santa Cruz Biotechnology (SC2005) | Goat; polyclonal | 1:10,000 | AB_631736 |
Abbreviations: LC3/MAP1LC3A, microtubule-associated proteins 1A/1B light chain 3A; RRID; Research Resource Identifier; Stat5b, signal transducer and activator of transcription 5B.
Audiogenic startle seizure response
P18 to P20 mice (Agpat1−/− mice, n = 11; WT mice, n = 15) were subjected to an audiogenic startle response procedure that consisted of a 1-minute burst of white noise at 100 dB delivered by a speaker (MF-1; Tucker-Davis Technologies, Alachua, FL) mounted to the top of the cage 6 inches above the mouse level. All mice were videoed during the procedure, and behavioral response was noted for the presence of seizure activity and was scored according to a modified Racine scale (20, 21).
Hippocampal neuron culture
Hippocampal neuron culture and ascertainment of neuron purity are described in detail in the Supplemental Material/Supporting Information (18.1MB, docx) . Briefly, the primary hippocampal region from either P4 or P5 mice was isolated from the brain and digested to release the neurons. Neurons were purified by density-gradient centrifugation by layering over a gradient of OptiPrep (Sigma-Aldrich, St. Louis, MO). Cells were seeded on tissue culture dishes or on a 35-mm glass bottom dish (MatTek, Ashland, MA), both previously coated with poly-dl-ornithine and laminin. Cells were allowed to grow in vitro for 4 days before glucose uptake assay was done. To assess the purity of the neuronal preparations on the OptiPrep gradient, cells were stained for neuron-specific markers (NeuroTrace 500; Invitrogen, Inc., Carlsbad, CA) or glial fibrillary acidic protein mouse monoclonal antibody as an astrocyte marker.
Glucose uptake assay in cultured neurons
Glucose uptake in cultured neurons is described in detail in the Supplemental Material/Supporting Information (18.1MB, docx) . Briefly, hippocampal neurons isolated from P4 to P5 Agpat1−/− and WT littermate pups were used for assaying glucose uptake. After neurons were grown for 4 days, cells were starved for 1 hour in sterile Dulbecco’s minimal essential medium containing 5 mM glucose, 44 nM sodium hydrogen carbonate (NaHCO3), and 0.045 mM phenol. Following starvation, the medium was replaced with Dulbecco’s modified Eagle medium containing [3H]-2-deoxyglucose, and radioactive glucose uptake by neurons was allowed to proceed for 1 hour. The incubation was terminated by removing 1 mL of medium, and cells were collected. Cell lysate was added separately to two different vials containing scintillation liquid, and radioactivity was counted using the Beckman LS6500 multipurpose liquid scintillation counter.
Testis cell labeling in histological sections
Testis cell labeling in histological sections is described in detail in the Supplemental Material/Supporting Information (18.1MB, docx) . Briefly, sections were treated for 18 to 24 hours at 22°C to 24°C with respective antibodies diluted in blocking buffer at the following concentrations: [1:400 mouse anti-Sall4 IgG (H00057167-M03; Abnova, Inc., Taipei City, Taiwan); 1:400 rabbit antiphospho-γH2A.X (Ser139) IgG (07-164; Millipore, Inc., Taipei City, Taiwan); 1:400 rabbit anti-CREMƬ IgG (sc-440; Santa Cruz Biotechnology, Inc); 1:2000 rabbit anti-β-galactosidase IgG, Z3783; Promega, Inc., Madison, WI)] diluted into Roche blocking (1% weight-to-volume ratio) reagent.
After treatment with primary antibodies, sections were incubated with respective AlexaFluor594 (Invitrogen, Inc) or AlexaFluor488 (Invitrogen, Inc.) secondary antibodies containing Hoechst 33342 dye (Molecular Probes, Eugene, OR). Sections were cover-slipped for viewing using Fluorogel mounting medium (Electron Microscopy Sciences, Hatfield, PA). Images were acquired using an IX70 Olympus fluorescence microscope (Olympus Inc., Center Valley, PA) equipped with Simple-PCI imaging software (C-Imaging Systems; Compix, Cranberry Township, PA).
Statistical analyses
For statistical analyses of body weight and glucose measurements over time, comparisons were made with mixed-effects repeated-measures analysis to test the effects of genotype, day, and interaction between genotype and day. The animal was modeled as a random effect with an autoregressive covariance pattern. Survival functions were compared with the generalized Wilcoxon test followed by Dunnett test for comparisons to the control group. Pearson correlation analysis was used to evaluate the association between Foxo1, G6Pase, and Pepck expression variables. These statistical analyses were done using SAS version 9.4 software. Statistical significance between two groups was calculated by two-tailed Student t test in Excel. A P value ≤0.05 was considered statistically significant.
All additional methods used in this study are provided in the Supplemental Material/Supporting Information (18.1MB, docx) .
Results
Generation of Agpat1−/− mice, characterization of neonates, efforts to rescue early death, and indirect calorimetry
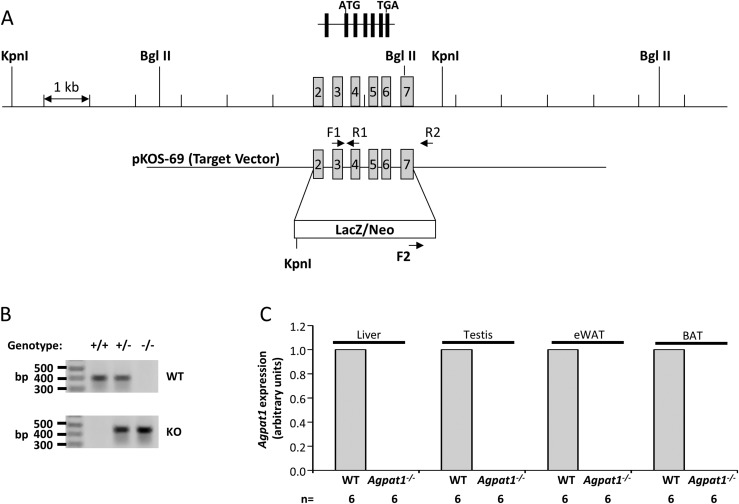
The Agpat1−/− mouse was generated by deleting all the coding exons (exons 2 to 7) of the Agpat1 gene by the homologous gene deletion strategy as shown in Fig. 1A and confirmed by genotyping (Fig. 1B). Mating between Agpat1+/− resulting in the F1 progeny yielded pups of all three genotypes: Agpat1+/+/Agpat1+/−/Agpat1−/− in the ratio of 1:2:0.5 instead of the expected Mendelian ratio of 1:2:1 (P < 0.001), with a sex ratio of 1:1 (M:F). The absence of Agpat1 transcripts in the liver, testis, epididymal white adipose tissue (eWAT), and brown adipose tissue was confirmed by real-time quantitative PCR (Fig. 1C).
Figure 1.
Strategy for deletion of Agpat1 in the mouse and biochemical confirmation. (A) The WT allele marked with exons 2 to 7 is shown; exon 1 is noncoding in the Agpat1 gene. The homologous gene deletion strategy was such that the β-galactosidase (LacZ) gene was inserted in place of exons 2 to 7. The primers used for amplifying the WT (F1 + R1) and KO (F2 + R2) alleles are marked. (B) The expected genotype for WT, heterozygous, and KO alleles by PCR amplification is shown. The image was cropped from the original image shown as Supplemental Fig. 17 (18.1MB, docx) . The location of the primers used for amplification are noted in (A). (C) Agpat1 mRNA expression in the liver, testis, epididymal white adipose tissue (eWAT), and brown adipose tissue (BAT) of Agpat1−/− mice was undetectable. Six individual samples were analyzed for this assay (n = 6). Agpat1 was normalized to cyclophilin. Primers used for amplification are provided in Supplemental Table 3 (18.1MB, docx) .
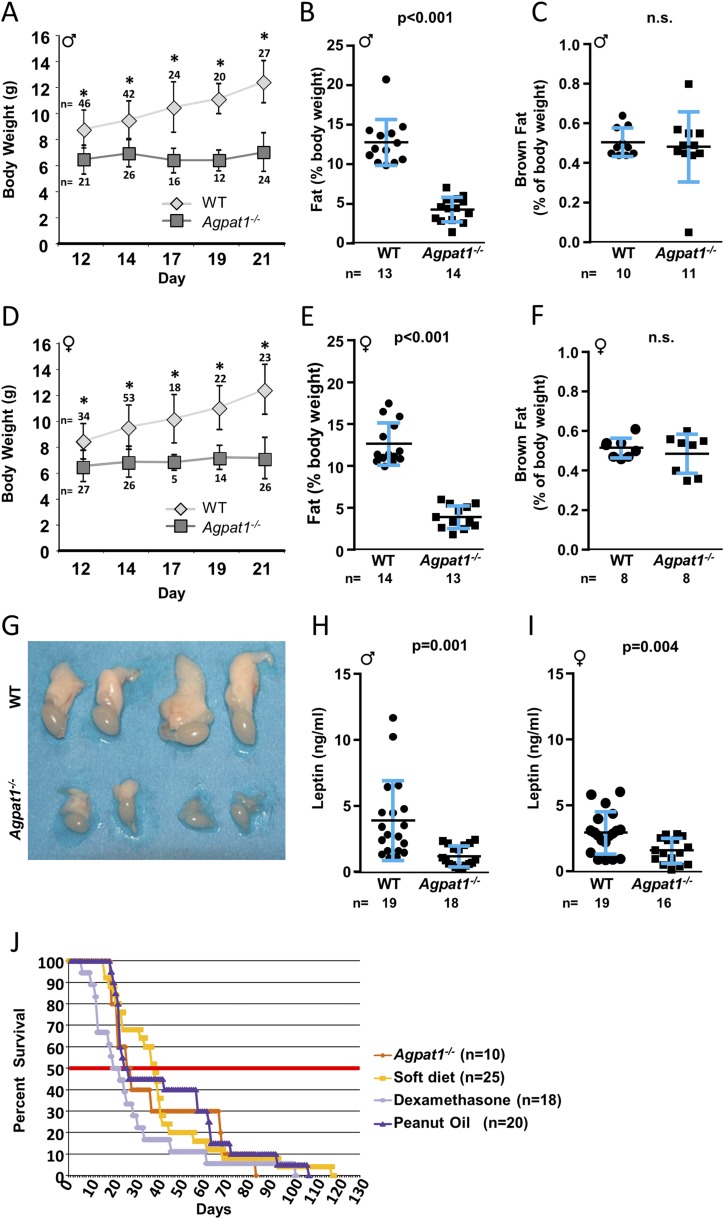
Agpat1−/− neonatal mice of both sexes had considerably reduced body weight by day 12 (Fig. 2A and 2D) and educed but not absent total fat tissues when normalized to total body weight by day 21 (∼66% decrease for males and ∼69% decrease for females) (Fig. 2B and 2E). Neonatal Agpat1−/− mice suckled adequately but then failed to gain weight after weaning. Only two Agpat1−/− mice (one male and one female) reached the appropriate weight to undergo the metabolic cage experiments, which showed no difference in food intake (Supplemental Fig. 2A (18.1MB, docx) ) or water intake (Supplemental Fig. 2B (18.1MB, docx) ) compared with WT mice. In Agpat1−/− mice, there was no change in O2 consumption (Supplemental Fig. 2C (18.1MB, docx) ) and CO2 production (Supplemental Fig. 2D (18.1MB, docx) ), resulting in no difference in the respiratory exchange ratio (Supplemental Fig. 2E (18.1MB, docx) ). Although we did see a small increase (13% to 15%) in energy expenditure (EE) (Supplemental Fig. 2F (18.1MB, docx) ), this difference did not reach statistical significance.
Figure 2.
Agpat1−/− mice had reduced body weight, total body fat, and plasma leptin level. The body weight of Agpat1−/− mice did not increase as the mice aged compared with WT mice [(A) male; (D) female] (numbers of animals are shown above or below the symbols). For statistical analysis, see the Materials and Methods section. The nuclear magnetic resonance spectroscopy measurement shows that Agpat1−/− mice had decreased total body fat [(B) male; (E) female]. There was no significant difference (n.s.) in the brown fat pad between 21-day-old WT and Agpat1−/− (C) male and (F) female mice. (G) We observed reduced epididymal fat pads in all Agpat1−/− male mice. Shown are fat pads attached to testes from 21-day-old WT and Agpat1−/− mice (two each). Reduced leptin levels were observed in 21-day-old (H) male and (I) female mice. (J) Rescue from neonatal death was attempted in Agpat1−/− mice by administration of peanut oil, a soft diet, or dexamethasone (dex). Mixed-sex groups of Agpat1−/− mice were injected intraperitoneally daily with dex (1 μg/g) or peanut oil (100 μL) until the mice succumbed. The soft diet was placed in a petri dish on the cage floor. In the treatment schedule, there was a decrease in the survivability at 50% survival only for the dex-treated group (P = 0.0539). However, the overall survival of treated Agpat1−/− mice was not much different from that of untreated mice. The number of mice tested is shown for each treatment group. Percent survivability was calculated against the total number of mice at the start of the treatment. Such treatment started around day 14, when the genotypes were available. The thick horizontal midline represents 50% survivability. The black line and error bars represent the mean ± standard deviation, with the number of animals used shown beneath graphs (B, C, E, F, H, and I) and beside graph (J). *P < 0.001 by mixed-effects repeated-measures analysis in (A) and (D). Dot plot P value was determined by two-tailed Student t test. Survival curve P value was determined by generalized Wilcoxon test followed by the Dunnett test for comparisons to the control group. ♂, male; ♀, female.
The Agpat1−/− mice did show greater locomotor activity (horizontal: 97% to 131%; vertical: 141% to 392%) (Supplemental Fig. 2G and 2H (18.1MB, docx) ) both during the day and at night than WT mice; however, the increase reached statistical significance only during the nighttime. Given that the food intake was similar between WT and Agpat1−/− mice with no difference in respiratory exchange ratio and EE, it remains unclear how the Agpat1−/− mice were dissipating energy; however, we interpreted these data with caution because only two Agpat1−/− mice were studied. Possible routes of increased EE include increased locomotion during the night (Supplemental Fig. 2G and 2H (18.1MB, docx) ) or futile lipid cycling in pathways not involving Agpat1. Alternatively, these mice might have reduced nutrient absorption and increased fecal excretion (22, 23) in addition to a defect in hepatic gluconeogenesis; however, we did not assess nutrient absorption.
The cervical brown adipose tissue from these mice was reduced, but there was no difference when normalized to body weight (Fig. 2C and 2F). We also observed reduced eWAT by visual inspection (Fig. 2G); however, this tissue was too small to weigh reliably. This decrease in eWAT is consistent with reduced plasma leptin levels (P < 0.001 for both sexes) (Fig. 2H and 2I). Because ∼50% of the Agpat1−/− pups did not survive beyond 3 to 4 weeks, we attempted to rescue this phenotype with daily intraperitoneal injections of dexamethasone, peanut oil, or an accessible soft diet starting at P10. None of these interventions improved survival (Fig. 2J). The early deaths could not be attributed to any specific postmortem histological findings (Supplemental Fig. 3 (18.1MB, docx) ).
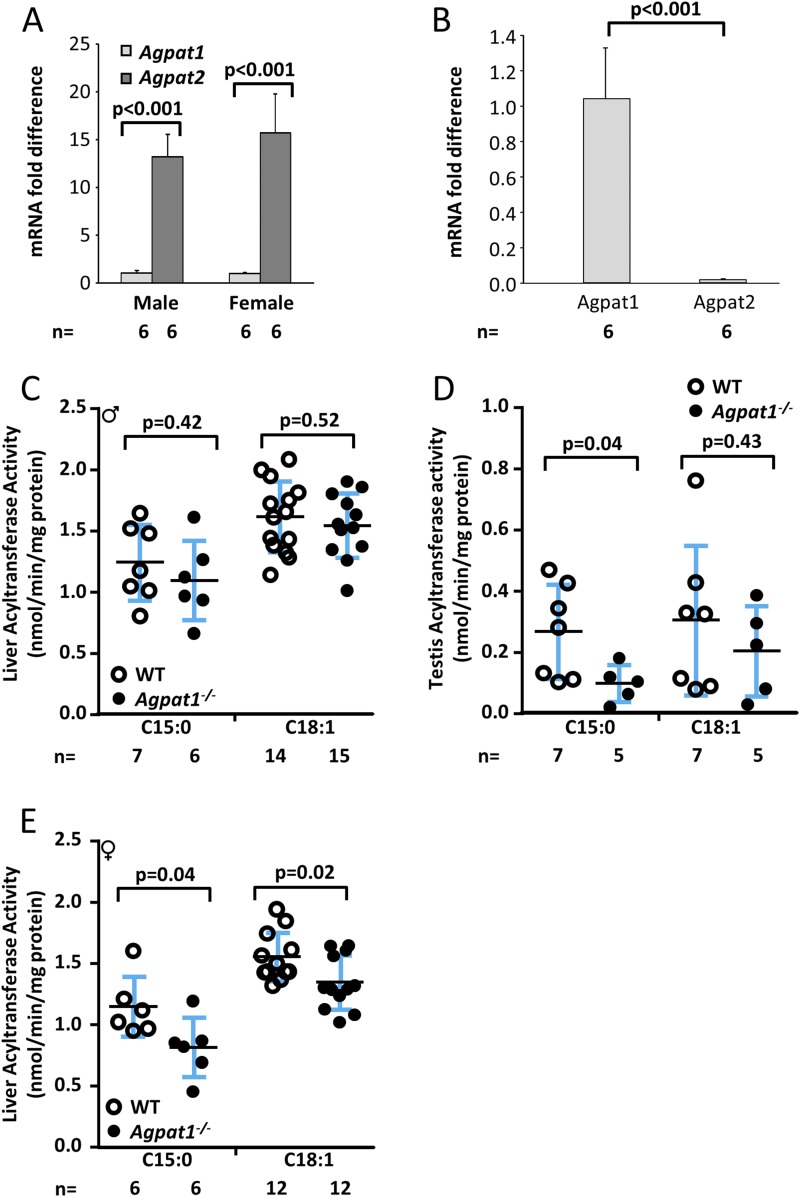
Neonatal Agpat1−/− mice have minor alterations in lipid synthesis
We previously determined that the AGPAT1 enzyme has a slight preference for acyl-CoA C15:0-CoA over C18:1-CoA (14). Therefore, we measured the total AGPAT enzymatic activity in Agpat1−/− mice using both the cosubstrates in homogenates of liver and testis, where AGPAT1 is a minor or major isoform, respectively (Fig. 3A and 3B). For male mice, we observed no significant decrease in the total AGPAT enzymatic activity in the livers of male Agpat1−/− mice using either cosubstrate (Fig. 3C). In contrast, enzymatic activity in the testes of Agpat1−/− mice was significantly decreased compared with that of WT mice when measured with C15:0-CoA (Fig. 3D). Therefore, the residual enzymatic activity in the testes of Agpat1−/− mice seems to be due to the activity of other Agpat isoforms (Supplemental Fig. 4 (18.1MB, docx) ). In addition, a modest but significant decrease in total AGPAT activity was evident in liver homogenates of female Agpat1−/− mice with both the C15:0-CoA and C18:1-CoA substrates (Fig. 3E). These data show that total AGPAT activity was variably reduced but not absent in the liver and testis of Agpat1−/− mice.
Figure 3.
Biochemical analyses in the Agpat1−/− mice. (A) The relative expression of Agpat1 vs Agpat2 in the livers of day 21 male and female WT mice is shown. Expression was analyzed from individual complementary DNA performed once in triplicate, and the data are presented as the mean fold difference (n = 6 each sex). Agpat1 and Agpat2 were normalized to cyclophilin. (B) The relative expression of Agpat1 vs Agpat2 in the testis of day 21 male WT mice is shown. Expression was analyzed from individual complementary DNA performed once in duplicate, and the data are presented as the mean fold difference (n = 6). Agpat1 and Agpat2 were normalized to cyclophilin. (C) Enzymatic measurement of total AGPAT (acyltransferase) activity in the livers of male WT and Agpat1−/− mice is shown. (D) Enzymatic activity of total AGPAT in the testes of WT and Agpat1−/− mice is shown. (E) Enzymatic measurement of total AGPAT (acyltransferase) activity in the livers of female WT and Agpat1−/− mice is shown. The acyl-CoA used was C15:0 = pentadecylic acid and C18:1 = oleic acid. Symbols represent individual data points, and the line represents the mean ± standard deviation (SD) with the n value shown below the graph. Bars represent the mean ± SD. P value was determined by two-tailed Student t test. ♂, male; ♀, female. Primer pairs used are provided in Supplemental Table 3 (18.1MB, docx) .
The expression of three major genes involved in fatty acid synthesis (Acc1, Fas, Scd1), three genes involved in TAG synthesis (Agpat2, Agpat10, Dgat2), and two genes involved in mitochondrial oxidation (Ucp1, Ucp2) was reduced in 3-week-old eWAT (Supplemental Table 1 (18.1MB, docx) ). Most other genes involved in fatty acid oxidation, TAG synthesis, glucose and mitochondrial biogenesis, and transcriptional factors associated with de novo lipogenesis and carbohydrate synthesis were unchanged (Supplemental Table 1 (18.1MB, docx) ).
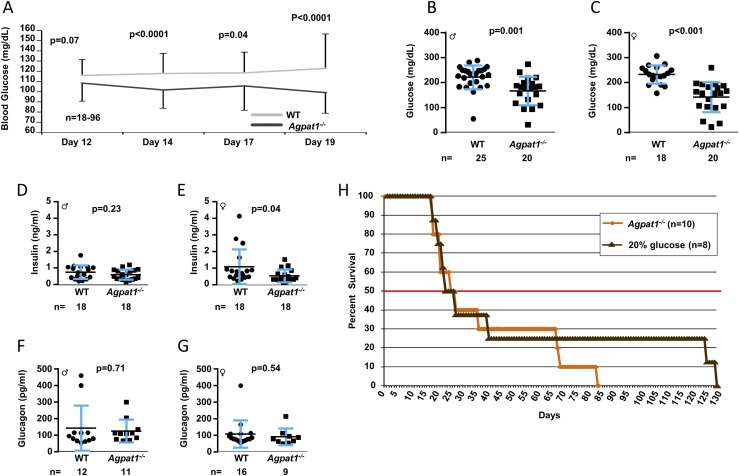
Neonatal Agpat1−/− mice have reduced plasma glucose levels
We observed a significant decrease in plasma glucose levels for the Agpat1−/− mice compared with the WT mice (Fig. 4A). The glucose values were followed in the same mice at P12 to P19 by tail vein puncture. Among animals that survived to day 21, the plasma glucose level in Agpat1−/− mice (obtained by cardiac puncture) was significantly lower than the level in WT mice (Fig. 4B and 4C), but plasma insulin and glucagon levels were similar to those of WT mice (Fig. 4D−G). This result prompted us to attempt to rescue the animals by providing exogenous daily administration of glucose starting at P10; however, similar to administration of dexamethasone or peanut oil treatment, survivability remained unchanged (Fig. 4H).
Figure 4.
Agpat1−/− mice were hypoglycemic. (A) Reduced blood glucose level in Agpat1−/− mice compared with the level in WT mice via tail vein is shown between days 12 and 19. The P value was determined by mixed-effects repeated-measures analysis. The plasma glucose levels remained low even at day 21 as measured in plasma obtained by cardiac puncture [(B) male; (C) female]. The line represents the mean ± standard deviation with the number of animals used underneath the graph. The low plasma glucose levels in Agpat1−/− mice were not due to hyperinsulinemia [(D) male; (E) female]. There was also no difference in glucagon levels in Agpat1−/− mice compared with WT mice [(F) male; (G) female]. (H) A survival curve was generated by administration of glucose (100 μL of 20% glucose per mouse per day) until the death of the mouse, which did not change the survivability of Agpat1−/− mice. The line horizontal graph-width line at 50% represents the %50% survivability. Survival curve P value was determined by the generalized Wilcoxon test followed by the Dunnett test for comparisons to the control group. Dot plot P value was determined by two-tailed Student t test. ♂, male; ♀, female.
Next, we measured the messenger RNA (mRNA) expression of key enzymes and transcription factors involved in gluconeogenesis. As shown in Table 2, we found a decrease in Foxo1 expression by ∼50% in five of 10 livers of male Agpat1−/− mice and five of six livers of female mice compared with WT mice. Those mice in which Foxo1 expression was downregulated compared with WT mice also had a significant decrease in the expression of Pepck (Supplemental Fig. 5 (18.1MB, docx) ; Table 2). Although expression of G6pase was also decreased in this group, it did not reach statistical significance (Supplemental Fig. 5 (18.1MB, docx) ; Table 2). We also measured liver glycogen, triglycerides, cholesterol, and total LPA and PA, as well as genes associated with glycogen synthesis, and found no change in Agpat1−/− mice compared with WT mice (Supplemental Fig. 6 (18.1MB, docx) ). These observations are consistent with a defect in hepatic gluconeogenesis as the cause of reduced plasma glucose level, which is most likely related to reduced Foxo1 expression.
Table 2.
Fold Changes of Key Gluconeogenic Enzymes and Transcription Factor in the Livers of Agpat1−/− Mice
| Animal | Sex | Foxo1 | Pepck | G6pase |
|---|---|---|---|---|
| 1 | Male | 0.41 | 0.47 | 0.91 |
| 2 | Male | 0.48 | 0.42 | 0.17 |
| 3 | Male | 0.79 | 0.76 | 0.60 |
| 4 | Male | 0.68 | 0.37 | 0.73 |
| 5 | Male | 0.83 | 0.69 | 0.41 |
| 6 | Female | 0.31 | 0.27 | 0.35 |
| 7 | Female | 0.14 | 0.18 | 0.33 |
| 8 | Female | 0.36 | 0.40 | 1.23 |
| 9 | Female | 0.53 | 0.54 | 0.33 |
| 10 | Female | 0.61 | 0.65 | 0.43 |
| Mean ± SD | 0.51 ± 0.22 | 0.48 ± 0.19 | 0.55 ± 0.32 | |
| Pearson correlation of Foxo1 vs | r = 0.86 | r = −0.03 | ||
| P = 0.002 | P = 0.93 | |||
| 11 | Male | 1.14 | 0.90 | 0.58 |
| 12 | Male | 1.31 | 0.93 | 0.55 |
| 13 | Male | 1.10 | 0.88 | 1.88 |
| 14 | Male | 1.55 | 1.55 | 1.52 |
| 15 | Male | 1.08 | 0.23 | 0.43 |
| 16 | Female | 1.26 | 0.87 | 0.93 |
| Mean ± SD | 1.24 ± 0.18 | 0.89 ± 0.42 | 0.98 ± 0.59 | |
| Pearson correlation of Foxo1 vs | r = 0.83 | r = 0.23 | ||
| P = 0.04 | P = 0.62 | |||
Gene expression levels of transcription factor Foxo1, which regulates key gluconeogenesis genes Pepck and G6pase, in the livers of 3-week-old Agpat1−/− mice. Shown are the fold changes of each gene in the livers of the same Agpat1−/− mice compared with WT mice = 1. The expression patterns are grouped according to the expression of Foxo1 being less than or more than 1. The mean ± SD of each group is also presented. The correlation coefficient (r) and P value of the expression of Pepck and G6pase compared with that of Foxo1 are shown.
Abbreviations: Foxo1, Forkhead box O1; G6pase, glucose-6-phosphatase; Pepck, phosphoenolpyruvate carboxykinase; SD, standard deviation.
Is low plasma glucose in Agpat1−/− mice unrelated to hepatic gluconeogenesis?
We also measured the expression of key genes associated with fasting in the liver (14), as well as plasma β-hydroxybutyrate and mitochondrial fatty acid oxidation markers, and found these parameters remained unchanged in Agpat1−/− mice compared with WT mice (Supplemental Fig. 7 (18.1MB, docx) ). We measured the additional plasma metabolites TAG, corticosterone, lactate dehydrogenase, alkaline phosphatase, and total bilirubin (Supplemental Fig. 8 (18.1MB, docx) ) and found no difference in the metabolites, which may identify the mechanism of low glucose level. We found slight alterations in aspartate aminotransferase and alanine aminotransferase, measures of liver dysfunction, and in creatinine and creatine kinase, measures of muscle dysfunction. We then measured the expression of genes involved in fatty acid and TAG synthesis, transcription factors that regulate lipid and carbohydrate synthesis, and proximal genes involved in insulin signaling in the liver, and we found no significant differences between Agpat1−/− and WT mice (Supplemental Table 2 (18.1MB, docx) ). These observations suggest that defects in hepatic gluconeogenesis are the main reason for the low plasma glucose level.
Growth hormone–insulinlike growth factor axis in Agpat1−/− mice
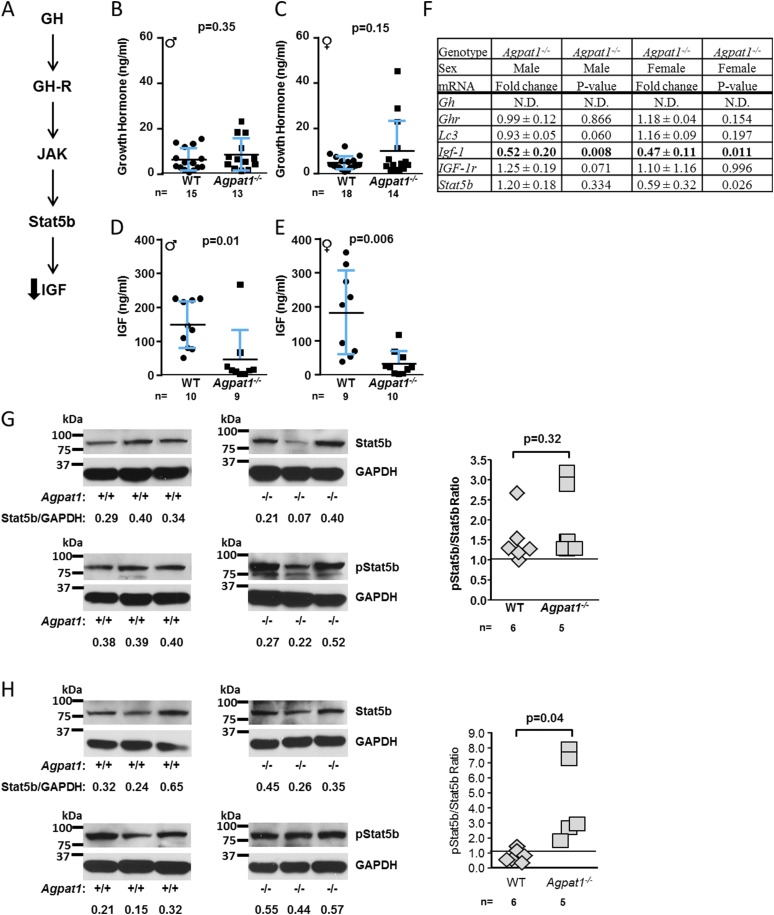
Growth hormone (GH) defends against hypoglycemia during prolonged fasting by stimulating gluconeogenesis (24). Given the likely gluconeogenesis defect in Agpat1−/− mice, we determined whether the GH−insulinlike growth factor (GH-IGF) pathway (Fig. 5A) was dysregulated in the livers of Agpat1−/− mice. Although plasma GH levels were preserved (Fig. 5B and 5C), there was a decrease in plasma IGF-1 levels in Agpat1−/− mice of both sexes (male, P = 0.01; female, P = 0.006) (Fig. 5D and 5E). The decreased plasma IGF-1 level was consistent with the downregulation of liver Igf-1 mRNA expression (Fig. 5F).
Figure 5.
GH signaling pathway in the livers of Agpat1−/− mice. (A) Schematic shows the GH signal transduction in which GH binds its cognate growth hormone receptor (GH-R) and activates Janus kinase (JAK), which then phosphorylates signal transducer and activator of transcription 5b (Stat5b). Phosphorylated Stat5b (p-Stat5b) dimerizes and translocates to the nucleus and activates GH-specific gene expression. (B, C) Plasma GH levels remained unchanged in both sexes. (D, E) However, plasma IGF-1 levels were decreased in Agpat1−/− mice of both sexes. (F) Liver gene expression of Lc3, Gh, Ghr, Igf-1r, and Stat5b remained unaffected in both sexes. Expression of Igf-1 is shown in bold type. N.D., not detectable. Shown are the mean ± standard deviation (SD) fold changes from individual samples (male, n = 5; female, n = 6) compared with WT = 1. (G, H) Immunoblot for Stat5b and p-Stat5b is shown; the representative images of three independent samples were obtained from six WT and five Agpat1−/− mice. The images were used for semiquantification using ImageJ. The Stat5b and p-Stat5b spots were normalized to GAPDH. The ratios of Stat5b to GAPDH are given below the images. The ratio of unphosphorylated to phosphorylated Stat5b reached statistical significance only in females [(G) male; (H) female]. We noticed a faster migrating band also identified with the p-Stat5b antibody used, which is a nonspecific band. The gel blots as shown have been cropped from the X-ray film images provided as Supplemental Fig. 18 (18.1MB, docx) . In (B–E), the line represents the mean ± SD, with the number of animals used underneath the graph. P value was determined by two-tailed Student t test. Gh, growth hormone; Ghr, growth hormone receptor; Lc3, microtubule-associated protein 1A/1B-light chain 3; Igf-1, insulinlike growth factor 1; Igf-1r, insulinlike growth factor 1 receptor; ♂, male; ♀, female.
GH signals through activation of Janus kinase 2, which phosphorylates the signal transducer and activator of transcription 5B (Stat5b) transcription factor. Phosphorylated Stat5b dimers drive expression of GH-specific genes, including Igf-1 (Fig. 5A) (25). We observed a decrease in the expression of Stat5b mRNA but only in female Agpat1−/− livers (P = 0.03) (Fig. 5F). Despite this decrease in mRNA, we observed an increase in phosphorylation in Stat5b protein in the livers of female Agpat1−/− mice (P = 0.04) (Fig. 5H) but not in Stat5b mRNA, protein, or phosphorylation in males (P = 0.32) (Fig. 5G). We found no difference in autophagy-related proteins (see extended results and Supplemental Fig. 9 (18.1MB, docx) ) that are known to generate substrates for energy production and gluconeogenesis in GH-treated livers (26, 27). In summary, alterations in GH-stimulated Stat5b signaling do not explain the reduced Igf-1 expression observed in Agpat1−/− livers.
Agpat1−/− male mice have reproductive abnormalities
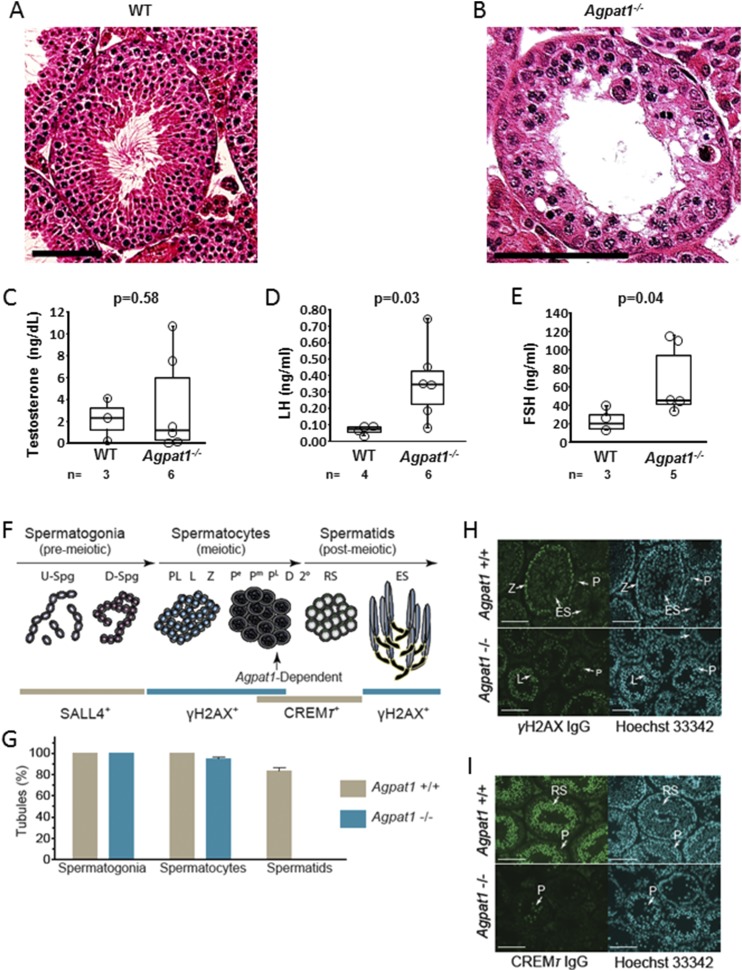
Our initial histological observations of 6-week-old testes suggested that spermatogenesis is disrupted in Agpat1−/− mice (Fig. 6A and 6B). Because luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone are required for spermatogenesis, we measured these hormones in plasma from 6-week-old male mice. Although testosterone was similar, LH and FSH were higher in Agpat1−/− mice than in WT mice (P = 0.03 and P = 0.04, respectively) (Fig. 6C−6E). If the spermatogenesis defect were due to the runted state and leptin deficiency, gonadotropins would be low and not high. These observations, particularly the elevated FSH level, suggest that Agpat1−/− mice have a primary defect in spermatogenesis among their phenotypic abnormalities.
Figure 6.
Absence of elongating spermatids in Agpat1−/− mice. (A) Morphology of representative testicular section from 6-week-old WT mouse, stained with hematoxylin and eosin, is shown. In WT testes, spermatids can be seen. (B) Spermatids are absent in testes of 6-week-old Agpat1−/− mice. Scale, 100 μM. (C−E) At 6 weeks, plasma testosterone (C) is unchanged compared with that of WT mice (n = 3 to 6). (D) LH and (E) FSH levels in Agpat1−/− mice were significantly higher than levels in WT mice, indicating the arrest of spermatogenesis (n = 3 to 6). For the box-and-whisker plots, the boxes represent the 25th and 75th percentiles, the line represents the median, and the whiskers represent the minimum and maximum values. (F) Prominent steps in spermatogenic cell development were monitored in WT and Agpat1−/− mice using antibodies to SALL4, γH2AX, and CREMƬ, which selectively label nuclei in spermatogonia, spermatocytes, and round spermatids, respectively (28–33). γH2AX also selectively labels elongated nuclei in elongating spermatids (31). The vertical arrow notes spermatogenic arrest classified at steps between late-pachytene spermatocyte and round spermatid development. (G) Percentage of seminiferous tubule cross sections containing SALL4+ spermatogonia, γH2AX+ spermatocytes, or CREMƬ+ spermatids in ∼42-day-old WT and Agpat1−/− mice (n = 3 mice per genotype, ± standard error of the mean; 75 to 100 seminiferous tubule cross sections were scored per mouse per genotype). (H) γH2AX (green) and Hoechst 33342 (cyan) colabeling in WT and Agpat1−/− mouse testis sections. Scale, 100 µM. (I) CREMƬ (green) and Hoechst 33342 (cyan) colabeling in WT and Agpat1−/− mouse testis sections. The arrows point to spermatocytes and round spermatids displaying distinct CREMƬ-labeling intensities. Scale, 100 µM. P value was determined by two-tailed Student t test. 2°, secondary spermatocytes; D, diplotene spermatocytes; D-Spg, differentiating spermatogonia; ES, elongating spermatids; L, leptotene spermatocytes; Pe, early pachytene spermatocytes; Pm, middle pachytene spermatocytes; PL, late pachytene spermatocytes; PL, preleptotene spermatocytes; RS, round spermatids; S, Sertoli cells. U-Spg, undifferentiated spermatogonia; Z, zygotene spermatocytes.
Molecular markers for spermatogonia, spermatocytes, or spermatids were applied to analyze spermatogenesis in Agpat1−/− male mice (Fig. 6F). Splat-like 4 (SALL4)/testis expressed gene 20 (TEX20) is a transcription factor that marks type A spermatogonia (28, 29). All seminiferous tubule cross sections analyzed in WT and Agpat1−/− mice contained SALL4+ spermatogonia (Fig. 6G). H2A histone family, member X (γH2AX) is a histone marker for double-stranded DNA breaks that become abundant during early meiotic prophase in leptotene and zygotene spermatocytes before becoming highly localized to the X-Y body in pachytene spermatocytes (30, 31). γH2AX labeling wanes to background levels in round spermatids and then becomes prominent in the nucleus of elongating spermatids (31). All seminiferous tubules in WT testes contained γH2AX+ spermatocytes at respective steps in development (Fig. 6G and 6H). Unlike in WT mice, ∼5% of tubules in Agpat1−/− mice completely lacked γH2AX+ spermatocytes (Fig. 6G).
An antibody to the transcription factor cAMP-responsive element modulator tau (CREMƬ) was used to analyze spermatocyte development during middle to late steps in meiosis and in round spermatids (32). In WT mice, CREMƬ was detected in pachytene, diplotene, and secondary spermatocytes and then increased in relative abundance during steps 1 to 8 of spermiogenesis to yield intense antibody labeling in round spermatids (Fig. 6I). CREMƬ+ middle to late pachytene spermatocytes were detected in testis sections from Agpat1−/− mice on the basis of colabeling nuclei with Hoechst 33342 (Supplemental Fig. 10 (18.1MB, docx) ). Colabeling with Hoechst 33342 nuclear dye and CREMƬ or γH2AX clearly indicated that no round or elongating spermatids were present in Agpat1−/− mouse seminiferous tubules (Fig. 6G−I). Accordingly, all seminiferous tubule cross sections analyzed in WT mice contained round spermatids (CREMƬ+/γH2AX−) and/or elongating spermatids (CREMƬ−/γH2AX+ elongated nucleus) (Fig. 6G−6I). Thus, Agpat1−/− mouse spermatogenic cells developed into pachynema of meiotic prophase I but were not able to develop into round spermatids.
Agpat-family transcript expression profiles generated during postnatal germ cell development in WT mouse testes were mined from microarray data in silico [NCBI GEO Profiles: GSE640 datasets (33)]. Agpat-family transcript profiles were consistent with selective expression in somatic (Agpats 4, 5, 8) and/or various spermatogenic testis cell types (Agpats 1, 2, 3, 4, 5, 6, 8) during postnatal testis development (Supplemental Fig. 11 (18.1MB, docx) ). The relative abundance of Agpat1 transcripts increased sharply between P21 and P29 (Supplemental Fig. 11 (18.1MB, docx) ). The increase in Agpat1 transcripts between P21 and P29 correlated strongly with the accumulation of first-generation round and elongating spermatids during the onset of spermiogenesis (33) and thus was consistent with the lack of spermatid production in Agpat1-null mice (Fig. 6G). Continued accumulation of Agpat1 transcripts up to P45 correlated with peak Agpat1 expression in elongating spermatids (Supplemental Fig. 11 (18.1MB, docx) ) and was consistent with antibody labeling for the β-galactosidase (LacZ) transgene product (not shown) expressed under control of the Agpat1 promoter in Agpat1−/− mice (Fig. 1A).
Agpat1−/− female mice have reproductive abnormalities
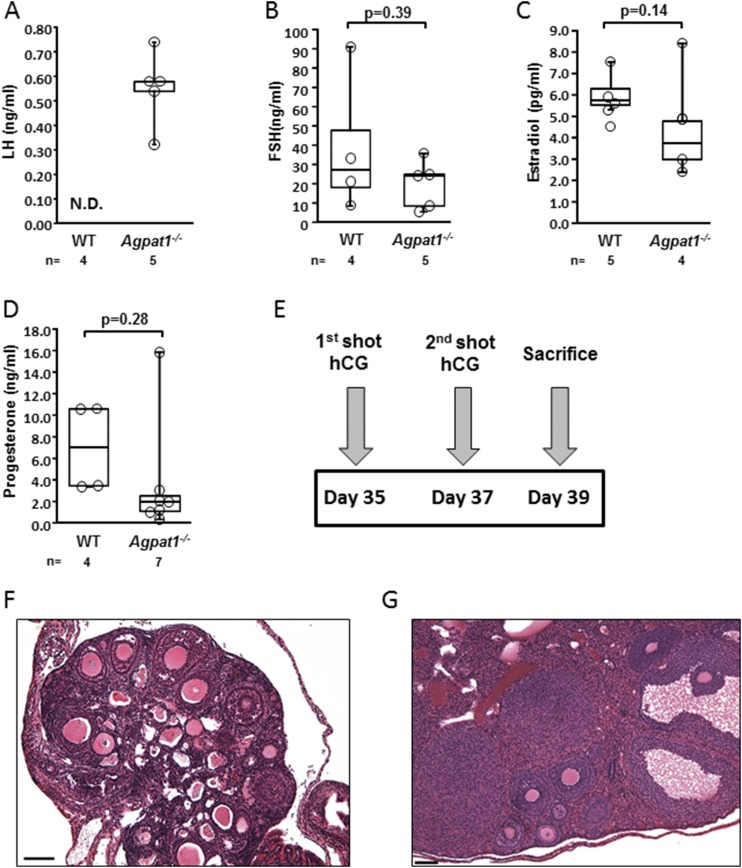
Histological examination of Agpat1−/− ovaries revealed a dramatic increase in the number of antral follicles, consistent with a defect in ovulation ( Supplemental Fig. 12A and 12B (18.1MB, docx) ). LH levels seemed to be higher in the Agpat1−/− mice, although LH levels in the plasma of WT mice were below the detectable range of the assay, so no statistical conclusion could be made (Fig. 7A). Plasma FSH levels, estradiol, and progesterone levels were unchanged in Agpat1−/− mice compared with WT mice (Fig, 7B−7D).
Figure 7.
The ovaries of Agpat1−/− mice were responsive to human chorionic gonadotropin (hCG) stimulation. The levels of (A) LH, (B) FSH, (C) estradiol, and (D) progesterone in WT and Agpat1−/− plasma at 6 weeks are shown. (E) The treatment schedule of mice injected with hCG is shown. WT and Agpat1−/− mice were given hCG injections (5 IU) as shown in the schematic and were euthanized 2 days later. (F, G) The morphology of hematoxylin and eosin−stained ovarian sections from (F) untreated and (G) treated Agpat1−/− mice show an increase in distinct regions of the corpora lutea in treated female mice. Images are representative of three independent images. Scale, 100 μM. For the box-and-whisker plots, the boxes represent the 25th and 75th percentiles, the line represents the median, and the whiskers represent the minimum and maximum values. The number of animals used is shown underneath the graph. The P value was determined by two-tailed Student t test. N.D., not detectable.
To determine whether the ovulatory defect was primarily central or ovarian, Agpat1−/− female mice were administered two injections of human chorionic gonadotropin 2 days apart and were euthanized 48 hours after the second injection (Fig. 7E). Compared with untreated Agpat1−/− mice (Fig. 7F), the treated animals showed development of corpora lutea, indicating responsiveness to gonadotropins and ovulation (Fig. 7G). These experiments show that the ovaries of Agpat1−/− mice were responsive to gonadotropins and suggest that the ovulation defect was primarily central, most likely related to low body fat and leptin deficiency.
Agpat1−/− mice show features of epilepsy
During routine colony maintenance, we noticed that some of the Agpat1−/− mice displayed features of epilepsy, such as tonic-clonic seizures, which were brought on by changes in sound, light, and sudden movements as in handling, transport of mice, or routine cage changes. In most cases, these mice recovered from the seizures, but occasionally the seizures resulted in death.
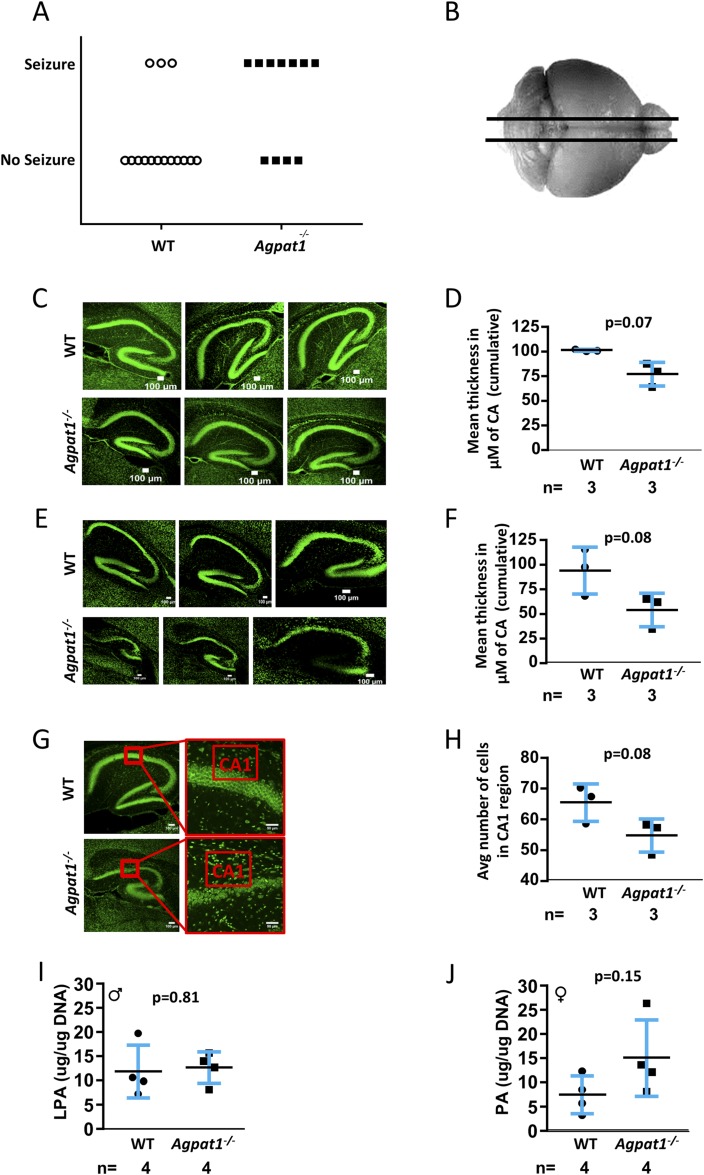
To further explore this phenotype, we subjected the mice to white noise pulses at 100 dB (db) for 1 minute to determine whether the seizures were indeed audiogenic inducible seizures. We observed that 7 of 11 Agpat1−/− mice had behavioral seizure activity when subjected to the audiogenic startle response, whereas only 3 of 15 WT mice responded with behavioral seizure activity during audiogenic stimulus (Mann-Whitney U test, P = 0.0426) (Fig. 8A). In audiogenic responders, behavioral responses were typical for the audiogenic crisis response with wild running and jumping followed by loss of balance and tonic-clonic activity of the forelimbs and hind limbs (see Supplemental Video 1 (6.1MB, avi) ).
Figure 8.
Agpat1−/− mice showed audiogenic seizure response and abnormal hippocampus structure. (A) A significant number of Agpat1−/− mice showed audiogenic seizure response compared with WT mice under similar conditions. Seizure was ranked by the Racine scale (20, 21). Seven of 11 Agpat1−/− mice had behavioral seizure activity when subjected to a 100-dB audiogenic startle stimulus for 1 minute, whereas only 3 of 15 WT mice showed the same response (P = 0.0426, Mann-Whitney U test) (see also Supplemental Video 1) (6.1MB, avi) . (B) Schematic of the brain region used for sectioning is shown. (C−E) Sagittal sections of WT and Agpat1−/− brains (C) stained with NeuroD to mark the neurons at day 14 and (D) the mean thickness of the CA region are shown. Sagittal sections of WT and Agpat1−/− brains (E) stained with NeuroD to mark the neurons at day 21 and (F) the mean thickness of the CA region are shown. The independent biological replicates are depicted. Each sample represents the mean of three measurements. (G) Neurons were counted in three different mouse hippocampal CA1 regions. Shown are representative images from WT and Agpat1−/− sections used for counting neurons and (H) the quantification of neuron counts. There was no change in the LPA or PA level in the hippocampus of Agpat1−/− mice compared with levels in WT mice. The line represents the mean ± standard deviation with the number of animals used underneath the graph. The P value was determined by two-tailed Student t test. ♂, male; ♀, female.
To explore the link between seizures and Agpat1 expression in the brain, we took advantage of the Agpat1 gene-KO strategy, in which LacZ is expressed under the Agpat1 promoter. We sectioned the entire brain from anterior to posterior in 33 to 35 sections of ∼400-μm thickness and stained for the expression of LacZ. Intense staining was observed from approximately 2400 to 9200 μm (forebrain to hindbrain) (Supplemental Fig. 13 (18.1MB, docx) , images 8 to 22). Additional LacZ staining (marking the expression pattern of Agpat1) was noted in the prelimbic cortex, medial orbital cortex, anterior olfactory nucleus, hippocampal region, cornu ammonis (CA) 1, CA2, CA3, dentate gyrus, auditory cortex, medial dorsal thalamic nucleus, and Purkinje neurons found in the cerebellum (Supplemental Fig. 14A−C (18.1MB, docx) ). Some LacZ staining was also noticed in the solitary tract of the brain (Supplemental Fig. 14A−C (18.1MB, docx) ).
To measure whether the brains of Agpat1−/− mice have any abnormality in neuron migration and localization, we chose representative sections from the forebrain, midbrain, and hindbrain and further stained them with nuclear fast red (Supplemental Fig. 14A−C (18.1MB, docx) ). These images did not reveal any defect in neuron localization or migration.
The hippocampus is a hub of information processing and can be affected during epileptic seizures (34), although it is unclear whether seizures originate in the hippocampus (35, 36). We analyzed the anatomical features of hippocampi from the Agpat1−/− mice (Fig. 8B). We observed that the CA region was slightly deformed in Agpat1−/− mice compared with WT mice in neonatal (day 14) (Fig, 8C) and was more pronounced at day 21 (Fig, 8E); however, the trend toward a decreased CA-region thickness in Agpat1−/− mice compared with WT mice did not reach statistical significance on day 14 (Fig. 8D) or day 21 (Fig. 8F). Furthermore, when we counted the number of neurons in a selected area of the CA region at day 21, we again noted a trend toward fewer neurons in Agpat1−/− mice than in WT mice, but it did not reach statistical significance (Fig. 8G and 8H). We also measured the LPA and PA levels in the whole hippocampi of 4-day-old Agpat1−/− and WT mice but did not find any differences (Fig. 8I and 8J), with the caveat that these samples were mixtures of neurons, glial cells, and other cell types. These observations suggest that these mice have some anatomical abnormalities in the hippocampal region, including a trend toward a reduced number of neurons.
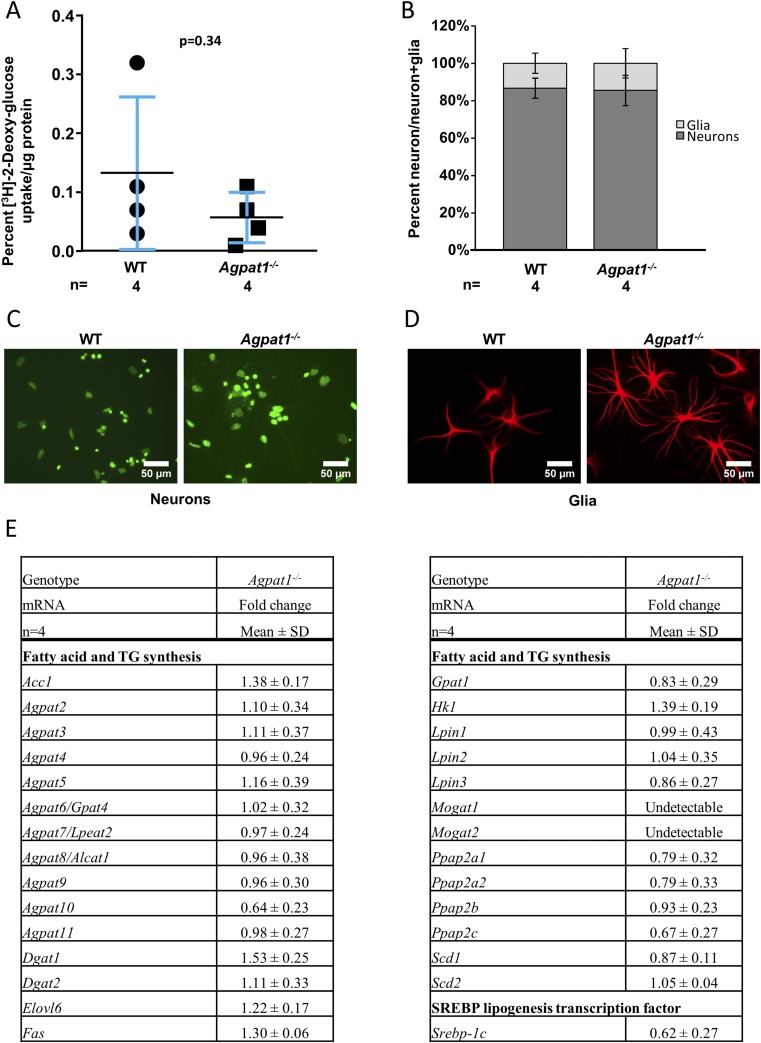
Because neurons use only glucose as their energy source, we next questioned whether hippocampal neurons in these mice have abnormal glucose uptake, which might affect their function. To accomplish this goal, we used primary hippocampal neuronal cultures obtained from days 3 to 5 in WT and Agpat1−/− mice to assess their glucose uptake efficiency. When such in vitro cultured neurons were incubated with [3H]-2-deoxyglucose, we observed no statistically significant difference in glucose uptake between the WT and Agpat1−/− mice (Fig. 9A). In these experiments, the majority of cells were neurons (∼85% of the total number of cells), with glial cells corresponding to ∼15% as measured by counting for neuron- and glial cell−specific markers (Fig. 9B−9D). We also measured the expression of genes of the lipid synthesis pathway, including sterol regulatory element-binding transcription factor 1c, a transcription factor regulating lipogenesis, in the hippocampus. None of the genes examined changed more than 1.5-fold up or down in Agpat1−/− mice compared with WT mice (Fig. 9E).
Figure 9.
Neurons of the hippocampal region of Agpat1−/− mice showed no reduced ability in [3H]-2-deoxyglucose uptake. (A) Cultured neurons exposed to [3H]-2-deoxyglucose showed no difference in glucose uptake. Symbols represent the individual data points. The line represents the mean ± standard deviation (SD), with the number of animals used underneath the graph. The P value was determined by two-tailed Student t test. (B) The neuron and glia populations in cultured neurons are shown. In our culture, the neuron population was ∼85% for both WT and Agpat1−/− mice. (C, D) Isolated neurons from day 4 to 5 hippocampi were cultured and stained (C) with NeuroD to identify neurons and (D) with glial fibrillary acidic protein, a glia-specific antibody. (E) There was no change in the expression pattern of genes related to fatty acid and triglyceride (TG) synthesis in the neurons of the Agpat1−/− hippocampus compared with the WT hippocampus. Fold change was compared with WT = 1. Shown are the mean ± SD fold changes from individual samples (n = 4). Acc1, acetyl–coenzyme A carboxylase; Agpat, acylglycerol-3-phosphate O-acyltransferase; Dgat, diglyceride acyltransferase; Elovl6, fatty acid elongase 6; Fas, fatty acid synthase; Gpat1, glycerol-3-phosphate acyltransferase, mitochondrial; Hk1, hexokinase 1; Lpin, lipin; Mogat, monoacylglycerol acyltransferase; Ppap, phosphatidic acid phosphatase; Scd, stearoyl–coenzyme A desaturase; Srebp-1c, sterol regulatory element-binding protein 1c.
Discussion
The human AGPAT1 and AGPAT2 proteins are highly homologous (14). Likewise, mouse Agpat1 and Agpat2 proteins are highly homologous [47.0% identity, 74.6% similar as analyzed using reference (37)]. In addition, the substrate-binding sites and catalytic sites of the two isoforms are predicted to be identical. Because of the substantial homology between mouse and human AGPAT1 and AGPAT2 proteins, mouse Agpat1 and Agpat2 proteins are predicted to localize in the endoplasmic reticulum like the human isoforms (14).
Despite the strong homology between the proteins, the phenotypes of the Agpat1−/− and Agpat2−/− mice were very different. When we generated the Agpat2−/−mouse (38), which was based on the human congenital generalized lipodystrophy phenotype (15), the mouse replicated the features of human lipodystrophy. However, the Agpat1−/− mouse revealed entirely different characteristics. Compared with the Agpat2−/− mice, the Agpat1−/− mice had (1) much higher mortality after weaning, (2) hypoglycemia vs the hyperglycemia of Agpat2−/− mice, (3) a lack of hepatic steatosis, (4) the presence of adipose tissue depots, (5) higher plasma leptin levels, and (6) the presence of audiogenic seizures.
In addition, the only tissue in Agpat1−/− mice in which the expression of Agpat2 was changed was the eWAT at day 21. In this tissue, Agpat2 expression was reduced 0.35-fold (or 1/0.35 = 2.9-fold decrease) in Agpat1−/− mice compared with WT mice (Supplemental Table 1 (18.1MB, docx) ); however, this defect did not cause complete loss of adipose tissue. There was no change in the expression of Agpat2 in the liver or testes of Agpat1−/− mice compared with WT mice at day 21. Furthermore, we did not observe any changes in the expression of Agpat2 in 4-day-old hippocampal cultured neurons obtained from Agpat1−/− mice compared with those from WT mice.
Global deletion of the Agpat1 gene in mice resulted in multiple severe abnormalities and major neonatal death between P0 and P21. Approximately half of Agpat1−/− mice succumbed very early in life, and of those who survived past day 21, only ∼10% reached sexual maturity at 6 weeks of age. Few animals lived beyond 10 weeks despite efforts to rescue them with food or glucocorticoids. This high early mortality rate seriously challenged our characterization of these mice. Nevertheless, we showed that these mice had reduced body fat and plasma glucose level, infertility in both males and females, and audiogenic seizures. These findings indicate the importance of Agpat1 in several organs, primarily the testis and brain.
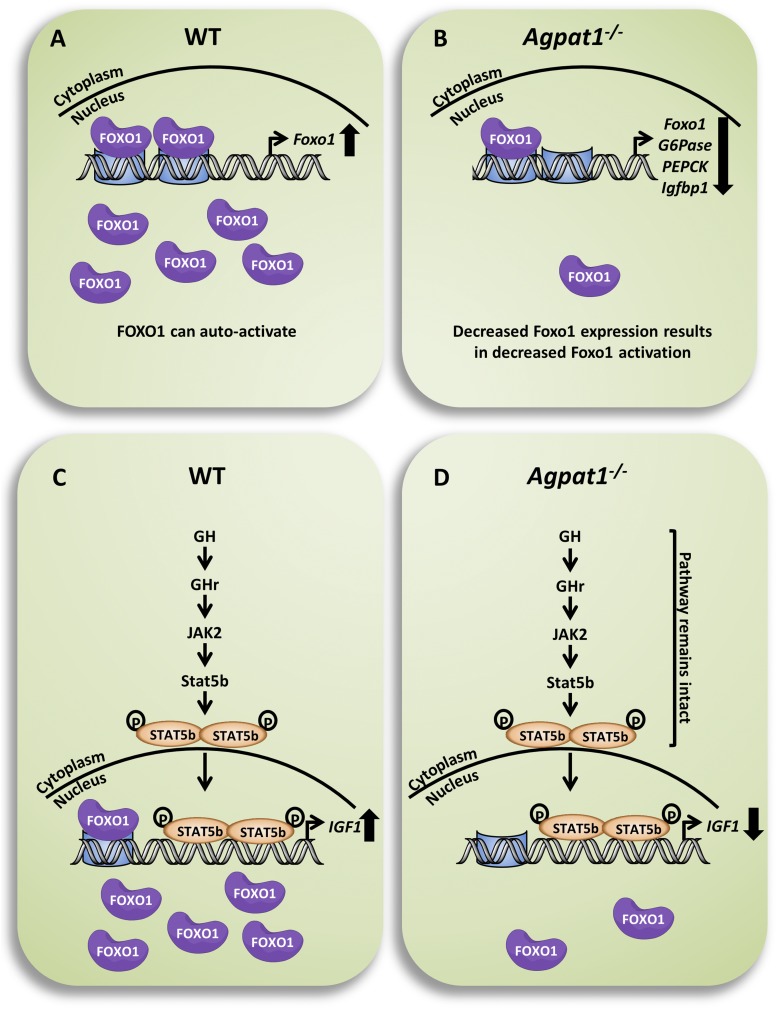
The Agpat1−/− mice had reduced fat mass and plasma glucose levels at 3 weeks of age. Hypoglycemia in newborn mice is not well studied, given the paucity of methods for interrogation and intervention in suckling mice; furthermore, no consensus definition for hypoglycemia in mice has been established. Nevertheless, we explored likely causes for reduced plasma glucose levels in Agpat1−/− mice. We noted a decrease in the expression of enzymes involved in liver gluconeogenesis, as well as the transcriptional suppression of Foxo1. Foxo1 has been shown to transcriptionally regulate key gluconeogenesis enzymes (39), and low expression of Foxo1 reduced the upregulation of G6pase and Pepck in response to low glucose levels. We also observed a downregulation of Igf1 in the livers of Agpat1−/− mice, despite preservation of the GH-Stat5b signaling pathway (Fig. 10). These findings are consistent with GH insensitivity, as occurs with malnourishment, but we cannot exclude a contribution from reduced Foxo1 activity or even coordinate dysregulation of both the GH−IGF-1 and gluconeogenesis pathways through an unidentified third factor, which may be primarily related to Agpat1 deficiency.
Figure 10.
Schematic of pathways for transcriptional activation of Forkhead box protein O1 (FOXO1) and IGF-1 in the mouse liver. (A) FOXO1 activates its own transcription, which leads to activation of genes such as glucose-6-phosphatase (G6pase), phosphoenolpyruvate kinase (Pepck), and insulinlike growth factor binding protein 1 (Igfbp1). (B) In the livers of Agpat1−/− mice, decreased expression of Foxo1 resulted in decreased expression of Foxo1, G6Pase, Pepck, and Igfbp1. (C) The GH signaling pathway, which results in the phosphorylation of Stat5b, is shown. Upon dimerization, phosphorylated Stat5b moves into the nucleus and activates transcription of Igf-1. The Igf-1 promoter also contains FOXO1 binding sites, which could also activate Igf-1; however, this has not been shown experimentally. (D) In the livers of Agpat1−/− mice, the GH signaling pathway remains intact. However, a decrease in Igf-1 expression, which could result from the decreased Foxo1 levels, is shown. This event is not related to the phosphorylation of FOXO1. The thick downward arrows on far right represent decreased transcription, whereas the thick upward arrows represent increased transcription.
In addition to metabolic defects, the Agpat1−/− mice displayed infertility, but the mechanisms of infertility appeared different in male and female animals. Advances in sperm developmental studies have identified numerous stage- and developmental step−specific transcript and protein markers that helped us examine spermatogenic arrest (28–33, 40, 41). In Agpat1−/− mice, spermatogenesis arrested at steps between mid- to late-pachytene spermatocyte and round spermatid development. Molecular mechanisms by which testicular Agpat1 deficiency disrupts spermatid development remain unclear, but Agpat1 is a dominant isoform in the testis. The AGPAT enzyme produces PA, which acts as both a signaling molecule and a precursor for various other phospholipids essential for cellular membrane biosynthesis. Membrane biosynthetic roles for another acyltransferase, Agpat3, were recently implicated in the TM4 Sertoli-like cell line (42). It was proposed that storing polyunsaturated fatty acids in phosphatidylcholine within Sertoli cells would provide polyunsaturated fatty acids to associated spermatogenic cells via a controlled paracrine release to support spermatozoon development (42). Immunohistochemical studies of mouse testes also suggested the presence of AGPAT3 in spermatocytes and spermatids (43, 44). AGPAT3 colocalized with a marker for cis-Golgi (GM130) in spermatogenic cells and correlated with increased levels of unsaturated fatty acids in phospholipids obtained from the maturating mouse germ cells (43, 44). Like Agpat1, Agpat 2, 3, and 6 displayed expression profiles consistent with robust expression in spermatids, whereas other Agpat isoenzymes displayed transcript profiles consistent with peak expression in earlier premeiotic, meiotic (e.g., Agpat 4 and 5), and/or somatic testis cells (e.g., Agpat 4 and 8) (see Supplemental Fig. 11 (18.1MB, docx) ).
Unlike the testes, the ovaries of Agpat1−/− mice showed apparently normal germline development at histology, although with significant defects in late follicular maturation. Although female Agpat1−/− mice were responsive to exogenous gonadotropin (see Fig. 7), they had erratic estrus cycles compared with those of WT mice. The estrus cycles of most female Agpat1−/− mice tended to have prolonged diestrus stages (Supplemental Fig. 15 (18.1MB, docx) ).
Both male and female Agpat1−/− mice had low plasma leptin levels because of decreased adipose tissue, but gonadotrpins were elevated only in the males. Given the well-established influence of leptin on the hypothalamic-pituitary-gonadal axis (45), we attribute the oligoanovulation in the female animals to functional hypothalamic infertility without a major primary ovarian defect.
The final major phenotype of Agpat1−/− mice was seizures/epilepsy (46, 47), most commonly that due to sound (audiogenic) (48, 49). The fact that this type of seizure originates in the CA region in the hippocampus [(50, 51), and reviewed by Wolfart and Laker (52)] and that AGPAT1 is highly expressed in the hippocampal region of the brain, shown both by our LacZ staining and in the Allen Brain Atlas (www.brain-map.org) (Supplemental Fig. 16 (18.1MB, docx) ), prompted us to search for abnormalities in this region of the brain. We found subtle abnormalities of hippocampal development and phospholipid content that were not statistically significant and do not appear to account for the predisposition to seizures. Although we did not see any changes in the ability of hippocampal neurons from Agpat1−/− mice to use glucose, we have not examined whether these neurons have the ability to use other substrates such as lactate, which is provided mainly by astrocytes (53).
There is scant literature exploring energy balance (fuel utilization) and brain biochemistry in animals with audiogenic seizures (54, 55). In these studies, the relationship between audiogenic seizures and energy was studied using the whole brain, in contrast to the current study, in which we assessed energy in isolated neurons. In another study using Wistar Audiogenic Rats, Pereira et al. (56) studied the relationship of energy and audiogenic seizures in peripheral tissues. They assessed the metabolism of glucose in the jejunum and skeletal muscle and found no correlation with seizures; however, they did notice a decrease in muscle glycogen content. Interestingly, these studies were conducted in adult WT mice, and no specific genetic alterations were ascertained. Thus, there seems to be no animal model for studying neonatal audiogenic seizures in a well-defined monogenic background.
Although it is still possible that peripheral tissues such as muscle or intestine participated in initiating audiogenic seizures in our Agpat1−/− mice, this will require additional Agpat1−/− mice for further experiments. On the other hand, generating a hippocampal-specific Agpat1−/− mouse model could further explain this phenotype and avoid the difficulties in husbandry with the global Agpat1−/− animals.
The lack of compelling alterations in brain metabolism for Agpat1−/− mice suggests that the biochemical defects leading to seizures are found in other pathways. Various receptors and channels are implicated for the early onset of seizures (57), and these proteins are embedded in the plasma membrane. Recently, mutations in genes encoding potassium channels—KCNQ2 and KCNQ3 (58, 59), which encode voltage-gated potassium channels Kv7.2 and Kv7.3—have been implicated in early seizure disorders of infants and young children. Mice with these mutant potassium channel genes expressed in the brain also showed early life seizures (60). We speculate that abnormal generation of PA species from LPA because of the Agpat1 deficiency might influence membrane composition and therefore ion-channel function in these membranes. In support of this model, PA has been shown to regulate potassium channel function, primarily through the regulation of voltage gating (61). Consequently, the Agpat1−/− mouse model might serve as a useful model of epilepsy, which affects ∼1% of the population (62, 63), both for elucidating the pathogenesis and for screening of new therapies.
In summary, the Agpat1−/− mice revealed multiple defects, including hypoglycemia, reproductive abnormalities, and audiogenic seizures, which is consistent with the ubiquitous tissue expression pattern of Agpat1. These results illustrate how each member of the sn-2 acyltransferase family seems to serve specific biological function(s) with limited or no redundancy from other isoenzymes. Furthermore, the regulation of AGPAT enzymatic activity includes not only expression of its cognate genes but also tissue distribution, subcellular localization, and access to substrate(s). Nevertheless, our data support indispensable functions for Agpat1 in the testis and brain, as well as the liver and other tissues.
Acknowledgments
We thank Jaideep Chaudhary for his help with testicular experiments; Vinay Parameswara for fatty acid oxidation; Victor Cortes for a few of the quantitative reverse transcription PCRs; Giselle Huet for enzyme assay; Roger Unger for glucagon assay; John Shelton and James Richardson, pathology core, for all the histological work; Levi Good and the University of Texas Southwestern Medical Center Neuro-Models Facility (LBG) for audiogenic seizure data collection (supported by the Haggerty Center for Brain Injury and Repair); Diego Castrillon for reviewing the manuscript; and Beverley Adams-Huet for statistical analysis.
Financial Support: This work was supported in part by National Institutes of Health grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD053889) and the Office of the Director (R24OD011108 and DK54387). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: A.K.A. conceived, supervised, assisted with all data collection and analyses, and wrote the manuscript. K.T. assisted with data collection, prepared figures, and assisted with revision and copyediting of the manuscript. J.S.D. collected and helped analyze the data for neuron studies. S.S.N. collected and helped analyze the data for gonadal studies. F.K.H. performed experiments and helped write the male spermatogenesis section. S.S. performed all the phospholipid experiments. X.S. assisted in initial genotyping and histological experiments. R.J.A. reviewed and edited the manuscript. A.G. reviewed all aspects of the work and copyedited the manuscript. All authors have read, edited, and consented to this work.
Current Affiliation: J. S. Dalal’s current affiliation is the Department of Neurology, Children’s Hospital Boston, Harvard Medical School, Boston, Massachusetts 02115. X. Shao’s current affiliation is Dallas International Health Care Services, PLLC, Richardson, Texas 75082. R. J. Auchus’s current affiliation is the Departments of Pharmacology and Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, Michigan 48109.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AGPAT
- 1-acylglycerol-3-phosphate O-acyltransferase
- CA
- cornu ammonis
- CREMƬ
- cyclic adenosine monophosphate–responsive element modulator tau
- DAG
- diacylglycerol
- EE
- energy expenditure
- eWAT
- epididymal white adipose tissue
- FSH
- follicle-stimulating hormone
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GH
- growth hormone
- GPL
- glycerophospholipid
- HRP
- horseradish peroxidase
- IGF
- insulinlike growth factor
- IgG
- immunoglobulin G
- KO
- knockout
- LacZ
- β-galactosidase transgene
- LH
- luteinizing hormone
- LPA
- lysophosphatidic acid
- mRNA
- messenger RNA
- P
- postnatal day
- PA
- phosphatidic acid
- PCR
- polymerase chain reaction
- SALL4
- splat-like 4
- Stat5b
- signal transducer and activator of transcription 5B
- TAG
- triacylglycerol
- WT
- wild-type
- γH2AX
- γH2A histone family, member X.
References
- 1.Kennedy EP. The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. J Biol Chem. 1956;222(1):185–191. [PubMed] [Google Scholar]
- 2.Han GS, Wu WI, Carman GM. The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J Biol Chem. 2006;281(14):9210–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem. 2007;282(6):3450–3457. [DOI] [PubMed] [Google Scholar]
- 4.Tang X, Benesch MG, Brindley DN. Lipid phosphate phosphatases and their roles in mammalian physiology and pathology. J Lipid Res. 2015;56(11):2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temprano A, Sembongi H, Han GS, Sebastián D, Capellades J, Moreno C, Guardiola J, Wabitsch M, Richart C, Yanes O, Zorzano A, Carman GM, Siniossoglou S, Miranda M. Redundant roles of the phosphatidate phosphatase family in triacylglycerol synthesis in human adipocytes. Diabetologia. 2016;59(9):1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49(11):2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43(2):134–176. [DOI] [PubMed] [Google Scholar]
- 8.Lands WE. Metabolism of glycerolipides: a comparison of lecithin and triglyceride synthesis. J Biol Chem. 1958;231(2):883–888. [PubMed] [Google Scholar]
- 9.Agarwal AK. Lysophospholipid acyltransferases: 1-acylglycerol-3-phosphate O-acyltransferases. From discovery to disease. Curr Opin Lipidol. 2012;23(4):290–302. [DOI] [PubMed] [Google Scholar]
- 10.Sukumaran S, Barnes RI, Garg A, Agarwal AK. Functional characterization of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 10/glycerol-3-phosphate acyltransferase isoform 3. J Mol Endocrinol. 2009;42(6):469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao J, Liu Y, Lockwood J, Burn P, Shi Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J Biol Chem. 2004;279(30):31727–31734. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Chen YQ, Li S, Konrad RJ, Cao G. The microsomal cardiolipin remodeling enzyme acyl-CoA lysocardiolipin acyltransferase is an acyltransferase of multiple anionic lysophospholipids. J Lipid Res. 2009;50(5):945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harayama T, Shindou H, Ogasawara R, Suwabe A, Shimizu T. Identification of a novel noninflammatory biosynthetic pathway of platelet-activating factor. J Biol Chem. 2008;283(17):11097–11106. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal AK, Sukumaran S, Cortés VA, Tunison K, Mizrachi D, Sankella S, Gerard RD, Horton JD, Garg A. Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice. J Biol Chem. 2011;286(43):37676–37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31(1):21–23. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal AK, Simha V, Oral EA, Moran SA, Gorden P, O’Rahilly S, Zaidi Z, Gurakan F, Arslanian SA, Klar A, Ricker A, White NH, Bindl L, Herbst K, Kennel K, Patel SB, Al-Gazali L, Garg A. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88(10):4840–4847. [DOI] [PubMed] [Google Scholar]
- 17.Demirkan A, van Duijn CM, Ugocsai P, Isaacs A, Pramstaller PP, Liebisch G, Wilson JF, Johansson Å, Rudan I, Aulchenko YS, Kirichenko AV, Janssens AC, Jansen RC, Gnewuch C, Domingues FS, Pattaro C, Wild SH, Jonasson I, Polasek O, Zorkoltseva IV, Hofman A, Karssen LC, Struchalin M, Floyd J, Igl W, Biloglav Z, Broer L, Pfeufer A, Pichler I, Campbell S, Zaboli G, Kolcic I, Rivadeneira F, Huffman J, Hastie ND, Uitterlinden A, Franke L, Franklin CS, Vitart V, Nelson CP, Preuss M, Bis JC, O’Donnell CJ, Franceschini N, Witteman JC, Axenovich T, Oostra BA, Meitinger T, Hicks AA, Hayward C, Wright AF, Gyllensten U, Campbell H, Schmitz G; DIAGRAM Consortium; CARDIoGRAM Consortium; CHARGE Consortium; EUROSPAN consortium . Genome-wide association study identifies novel loci associated with circulating phospho- and sphingolipid concentrations. PLoS Genet. 2012;8(2):e1002490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Langenberg C, Hofmann OM, Dupuis J, Qi L, Segrè AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Bengtsson Boström K, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Couper DJ, Crawford G, Doney AS, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PR, Jørgensen T, Kao WH, Klopp N, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Perry JR, Petersen AK, Platou C, Proença C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparsø T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, Tichet J, Tuomi T, van Dam RM, van Haeften TW, van Herpt T, van Vliet-Ostaptchouk JV, Walters GB, Weedon MN, Wijmenga C, Witteman J, Bergman RN, Cauchi S, Collins FS, Gloyn AL, Gyllensten U, Hansen T, Hide WA, Hitman GA, Hofman A, Hunter DJ, Hveem K, Laakso M, Mohlke KL, Morris AD, Palmer CN, Pramstaller PP, Rudan I, Sijbrands E, Stein LD, Tuomilehto J, Uitterlinden A, Walker M, Wareham NJ, Watanabe RM, Abecasis GR, Boehm BO, Campbell H, Daly MJ, Hattersley AT, Hu FB, Meigs JB, Pankow JS, Pedersen O, Wichmann HE, Barroso I, Florez JC, Frayling TM, Groop L, Sladek R, Thorsteinsdottir U, Wilson JF, Illig T, Froguel P, van Duijn CM, Stefansson K, Altshuler D, Boehnke M, McCarthy MI; MAGIC investigators; GIANT Consortium . Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42(7):579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherva R, Baldwin CT, Inzelberg R, Vardarajan B, Cupples LA, Lunetta K, Bowirrat A, Naj A, Pericak-Vance M, Friedland RP, Farrer LA. Identification of novel candidate genes for Alzheimer’s disease by autozygosity mapping using genome wide SNP data. J Alzheimers Dis. 2011;23(2):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racine RJ. Modification of seizure activity by electrical stimulation, II: motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. [DOI] [PubMed] [Google Scholar]
- 21.Racine RJ. Modification of seizure activity by electrical stimulation, I: after-discharge threshold. Electroencephalogr Clin Neurophysiol. 1972;32(3):269–279. [DOI] [PubMed] [Google Scholar]
- 22.Tschöp MH, Speakman JR, Arch JR, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speakman JR. Measuring energy metabolism in the mouse - theoretical, practical, and analytical considerations. Front Physiol. 2013;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Fang F, Goldstein JL, Brown MS, Zhao TJ. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: reversal by growth hormone. Proc Natl Acad Sci USA. 2015;112(4):1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chia DJ, Varco-Merth B, Rotwein P. Dispersed chromosomal Stat5b-binding elements mediate growth hormone-activated insulin-like growth factor-I gene transcription. J Biol Chem. 2010;285(23):17636–17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. [DOI] [PubMed] [Google Scholar]
- 27.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. [DOI] [PubMed] [Google Scholar]
- 28.Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27(4):422–426. [DOI] [PubMed] [Google Scholar]
- 29.Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365(1546):1663–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodríguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27(3):271–276. [DOI] [PubMed] [Google Scholar]
- 31.Blanco-Rodríguez J. gammaH2AX marks the main events of the spermatogenic process. Microsc Res Tech. 2009;72(11):823–832. [DOI] [PubMed] [Google Scholar]
- 32.Foulkes NS, Mellström B, Benusiglio E, Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992;355(6355):80–84. [DOI] [PubMed] [Google Scholar]
- 33.Schultz N, Hamra FK, Garbers DL. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA. 2003;100(21):12201–12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–120. [DOI] [PubMed] [Google Scholar]
- 35.Devinsky O, Gazzola D, LaFrance WC Jr. Differentiating between nonepileptic and epileptic seizures. Nat Rev Neurol. 2011;7(4):210–220. [DOI] [PubMed] [Google Scholar]
- 36.Rao VR, Lowenstein DH. Epilepsy. Curr Biol. 2015;25(17):R742–R746. [DOI] [PubMed] [Google Scholar]
- 37.Notredame C, Higgins DG, Heringa J. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302(1):205–217. [DOI] [PubMed] [Google Scholar]
- 38.Cortés VA, Curtis DE, Sukumaran S, Shao X, Parameswara V, Rashid S, Smith AR, Ren J, Esser V, Hammer RE, Agarwal AK, Horton JD, Garg A. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 2009;9(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6(3):208–216. [DOI] [PubMed] [Google Scholar]
- 40.Johnston DS, Wright WW, Dicandeloro P, Wilson E, Kopf GS, Jelinsky SA. Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc Natl Acad Sci USA. 2008;105(24):8315–8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koeberle A, Shindou H, Harayama T, Shimizu T. Role of lysophosphatidic acid acyltransferase 3 for the supply of highly polyunsaturated fatty acids in TM4 Sertoli cells. FASEB J. 2010;24(12):4929–4938. [DOI] [PubMed] [Google Scholar]
- 43.Koeberle A, Shindou H, Harayama T, Yuki K, Shimizu T. Polyunsaturated fatty acids are incorporated into maturating male mouse germ cells by lysophosphatidic acid acyltransferase 3. FASEB J. 2012;26(1):169–180. [DOI] [PubMed] [Google Scholar]
- 44.Yuki K, Shindou H, Hishikawa D, Shimizu T. Characterization of mouse lysophosphatidic acid acyltransferase 3: an enzyme with dual functions in the testis. J Lipid Res. 2009;50(5):860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou SH, Mantzoros C. 20 years of leptin: role of leptin in human reproductive disorders. J Endocrinol. 2014;223(1):T49–T62. [DOI] [PubMed] [Google Scholar]
- 46.Ledri M, Sørensen AT, Madsen MG, Christiansen SH, Ledri LN, Cifra A, Bengzon J, Lindberg E, Pinborg LH, Jespersen B, Gøtzsche CR, Woldbye DP, Andersson M, Kokaia M. Differential effect of neuropeptides on excitatory synaptic transmission in human epileptic hippocampus. J Neurosci. 2015;35(26):9622–9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinkelacker V, Valabregue R, Thivard L, Lehéricy S, Baulac M, Samson S, Dupont S. Hippocampal-thalamic wiring in medial temporal lobe epilepsy: enhanced connectivity per hippocampal voxel. Epilepsia. 2015;56(8):1217–1226. [DOI] [PubMed] [Google Scholar]
- 48.Servít Z, Sterc J. Audiogenic epileptic seizures evoked in rats by artificial epileptogenic foci. Nature. 1958;181(4621):1475–1476. [DOI] [PubMed] [Google Scholar]
- 49.Charizopoulou N, Lelli A, Schraders M, Ray K, Hildebrand MS, Ramesh A, Srisailapathy CR, Oostrik J, Admiraal RJ, Neely HR, Latoche JR, Smith RJ, Northup JK, Kremer H, Holt JR, Noben-Trauth K. Gipc3 mutations associated with audiogenic seizures and sensorineural hearing loss in mouse and human. Nat Commun. 2011;2:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ketelaars SO, Gorter JA, van Vliet EA, Lopes da Silva FH, Wadman WJ. Sodium currents in isolated rat CA1 pyramidal and dentate granule neurones in the post-status epilepticus model of epilepsy. Neuroscience. 2001;105(1):109–120. [DOI] [PubMed] [Google Scholar]
- 51.Khirug S, Ahmad F, Puskarjov M, Afzalov R, Kaila K, Blaesse P. A single seizure episode leads to rapid functional activation of KCC2 in the neonatal rat hippocampus. J Neurosci. 2010;30(36):12028–12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfart J, Laker D. Homeostasis or channelopathy? Acquired cell type-specific ion channel changes in temporal lobe epilepsy and their antiepileptic potential. Front Physiol. 2015;6:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allaman I, Bélanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34(2):76–87. [DOI] [PubMed] [Google Scholar]
- 54.Schreiber RA. Sources of energy for the brain and susceptibility to audiogenic seizures. Med Hypotheses. 1979;5(4):487–492. [DOI] [PubMed] [Google Scholar]
- 55.Ferrendelli JA, McDougal DB Jr. The effect of audiogenic seizures on regional CNS energy reserves, glycolysis and citric acid cycle flux. J Neurochem. 1971;18(7):1207–1220. [DOI] [PubMed] [Google Scholar]
- 56.Pereira FK, Neves MJ, Lima MP, Braga AA, Pesquero JL, Doretto MC, Borges EL. Peripheral glucose metabolism is altered by epileptic seizures. Metab Brain Dis. 2008;23(1):105–114. [DOI] [PubMed] [Google Scholar]
- 57.Scott RC, Holmes GL. Before epilepsy unfolds: opening up the potassium door in neonatal seizures. Nat Med. 2012;18(11):1624–1625. [DOI] [PubMed] [Google Scholar]
- 58.Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, Deprez L, Smets K, Hristova D, Yordanova I, Jordanova A, Ceulemans B, Jansen A, Hasaerts D, Roelens F, Lagae L, Yendle S, Stanley T, Heron SE, Mulley JC, Berkovic SF, Scheffer IE, de Jonghe P. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71(1):15–25. [DOI] [PubMed] [Google Scholar]
- 59.Miceli F, Soldovieri MV, Ambrosino P, De Maria M, Migliore M, Migliore R, Taglialatela M. Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits. J Neurosci. 2015;35(9):3782–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh NA, Otto JF, Dahle EJ, Pappas C, Leslie JD, Vilaythong A, Noebels JL, White HS, Wilcox KS, Leppert MF. Mouse models of human KCNQ2 and KCNQ3 mutations for benign familial neonatal convulsions show seizures and neuronal plasticity without synaptic reorganization. J Physiol. 2008;586(14):3405–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hite RK, Butterwick JA, MacKinnon R. Phosphatidic acid modulation of Kv channel voltage sensor function. eLife. 2014;3:e04366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cowan LD. The epidemiology of the epilepsies in children. Ment Retard Dev Disabil Res Rev. 2002;8(3):171–181. [DOI] [PubMed] [Google Scholar]
- 63.Steinlein OK. Genes and mutations in human idiopathic epilepsy. Brain Dev. 2004;26(4):213–218. [DOI] [PubMed] [Google Scholar]