Abstract
Estrogen sulfotransferase catalyzes the sulfoconjugation and deactivation of estrogens. Previously, we showed that loss of Est in male ob/ob mice, but not in female ob/ob mice, exacerbated the diabetic phenotype, but the underlying mechanism was unclear. In this study, we show that transgenic reconstitution of Est in the adipose tissue, but not in the liver, attenuated diabetic phenotype in Est-deficient ob/ob mice (obe mice). Mechanistically, adipose reconstitution of Est in obe mice (oae mice) resulted in reduced local and systemic inflammation, improved insulin sensitivity, and increased energy expenditure. At the molecular level, adipose induction of lipocalin-2 (Lcn2) in oae males may have contributed to the inhibition of inflammation because the level of Lcn2 was negatively associated with tumor necrosis factor (Tnf) α expression, and treatment of differentiated adipocytes with Lcn2 antagonized Tnfα-responsive inhibition of insulin signaling. The metabolic benefit of adipose reconstitution of Est was sex specific, because adipose reconstitution of Est in obe females had little effect. Interestingly, despite their improved metabolic functions, obe male mice with reconstituted Est in their adipose tissue failed to ameliorate the impairment of the structure and function of the pancreatic islets. In summary, our study uncovers a crucial adipose- and male-specific role of Est in maintaining the whole-body energy homeostasis.
This estrogen sulfotransferase has a crucial adipose- and male-specific role in maintaining the whole-body energy homeostasis and insulin sensitivity.
Obesity is often associated with a low-grade inflammation in obese subjects or animals (1, 2). It has been suggested that this low-grade inflammation contributes to the development of peripheral insulin resistance (3), as well as damage to the pancreatic β cells (4). The white adipose tissue is a major source of proinflammatory cytokines in obesity (5).
The adipose tissue is also a key action site of estrogens, which have important roles in the control of energy balance and glucose homeostasis through multiple mechanisms (6). Although inactivation of estrogen receptor α in mice was associated with obesity and metabolic dysfunction in both sexes (7), the estrogen action in energy metabolism is better understood in women and female rodents (8–11). Men and male mice have lower levels of circulating estrogens than premenopausal women and cycling female mice, respectively. It has been reported that treatment of male mice with estrogens improves metabolic functions under obesity or aging conditions (12, 13). It is believed that the estrogen effect on metabolic function largely depends on the magnitude of estrogen stimulation. For example, high levels of estrogens in pregnant women may trigger the repression of muscular GLUT4, an insulin-responsive glucose uptake transporter, therefore contributing to insulin resistance in pregnancy (14). The tissue specificity of estrogen action is also important. For example, treatment of high-fat diet (HFD)–fed female mice with estradiol increased the expression of proinflammatory cytokines, such as interleukin 6 (Il-6) and tumor necrosis factor (Tnf) α, in the liver and visceral fat (15).
The estrogen sulfotransferase (Est or Sult1e1) plays an important role in regulating the tissue and systemic estrogen activity. Sulfoconjugation inactivates estrogens because the sulfonated estrogens cannot bind to the estrogen receptors and they are more susceptible to urinary excretion (16). The expression of Est exhibits both tissue and sex specificity. Liver has a low basal expression of Est, but the hepatic expression of Est is highly induced in the ob/ob, db/db, and HFD-induced obese mice (17). In male mice, the expression of Est is high in the white adipose tissue (WAT) and testis. The WAT expression of Est ensures a sufficient deactivation of estrogens in males, whereas the testicular expression of Est protects the male reproductive system from estrogen toxicity (18).
We have previously explored the role of Est in obesity and type 2 diabetes. Est oblation in ob/ob mice produced a sex-specific metabolic effect. Specifically, female Est-deficient ob/ob mice (obe mice) exhibited improved metabolic function, most likely due to their increased hepatic estrogen activity as a result of decreased estrogen deactivation. The improved metabolic function in obe females was not unexpected because increased hepatic estrogen activity is known to be protective by suppressing hepatic gluconeogenesis and lipogenesis and by increasing hepatic insulin sensitivity (6). In contrast, the male obe mice showed an exacerbated diabetic phenotype, which was reasoned to be due to the loss of the pancreatic β cell mass and adipose inflammation (19). However, the mechanism by which obe males have worsened metabolic phenotype remains to be better defined. Specifically, because the male mice have a high level of Est expression in the WAT, but a low basal and high inducible expression of Est in the liver, we want to know whether it is the loss of Est in WAT or liver that is responsible for the worsened metabolic function in obe males.
In this study, by using transgenic reconstitution of Est in the adipose tissue or liver of the obe mice, we demonstrated that the Est expression in the adipose tissue, but not in the liver, is essential to protect mice from systemic and local inflammation and metabolic syndrome in a male-specific manner.
Research Design and Methods
Mice
Mice with adipose reconstitution of Est in Est-deficient ob/ob mice (oae mice) were generated by crossing the obe mice (19) with the aP2-Est transgenic mice that express Est in the adipose tissue under the control of the aP2 gene promoter (20). The resulting oae mice bear the expression of Est in the adipose tissue in the background of obe. Est-deficient ob/ob mice with transgenic reconstitution of Est in liver (ole mice) were generated by crossing the obe mice with the Lap-Est transgenic mice that express Est in the liver under the control of the liver-enriched activator protein (Lap) gene promoter (21). The resulting ole mice bear the expression of Est in the liver in the background of obe. All animals were maintained on the C57BL/6J background. Mice were maintained on normal chow diet. The animal body composition was analyzed by using a magnetic resonance imaging system (EchoMRI, Houston, TX).
Study approval
The Central Animal Facility of the University of Pittsburgh is fully accredited by American Association of Laboratory Animal Care. All procedures were performed in accordance with relevant federal guidelines and with the approval of the University of Pittsburgh Institutional Animal Care and Use Committee.
Glucose tolerance test, insulin tolerance test, glucose-stimulated insulin secretion test, and homeostatic model assessment
Glucose tolerance test (GTT) was performed on 14- to 16-week-old mice after an overnight fasting. Animals received an intraperitoneal injection of d-glucose at the dose of 1 g/kg body weight. Insulin tolerance test (ITT) was performed on 14- to 16-week-old mice after a 6-hour fasting. Animals received an intraperitoneal injection of insulin at the dose of 0.75 U/kg body weight, as we have previously described (22). For glucose-stimulated insulin secretion (GSIS) tests, an additional 20 µL blood was collected by tail bleeding at 0, 30, 60, and 90 minutes during GTT for the measurement of plasma insulin levels. Homeostatic model assessment for insulin resistance (HOMA-IR) and for β cell function were calculated using the following formulas: insulin resistance = [fasting insulin (µU/mL)] × [fasting glucose level (mg/dL)]/405; and β cell function (%) = [360 × fasting insulin (µU/mL)]/[fasting glucose level (mg/dL)] − 63], as previously described (23).
Acute insulin sensitivity test
Overnight fasted 16-week-old male mice received an intraperitoneal injection of insulin (0.75 U/kg; Novolin) from Novo Nordisk (Princeton, NJ). Mice were euthanized and tissues were collected 17 minutes after the insulin injection.
Isolation of mouse pancreatic islets
Mice were euthanized and pancreatic intraductal perfusion was performed by using 3 mL cold Hank’s buffer containing 1.95 mg/mL fresh collagenase (Sigma-Aldrich, St. Louis, MO). The perfused pancreas was then used for islet isolation, as we have previously described (24).
Serum and liver biochemistry
Serum levels of estradiol were measured by Ligand Assay and Analysis Core Laboratory, Center for Research in Reproduction, University of Virginia (Charlottesville, VA). The serum levels of total triglyceride cholesterol, alanine transaminase, aspartate transaminase (Stanbio Laboratory, Boerne, TX), and insulin (Crystal Chem, Downers Grove, IL) were measured by using commercial assay kits. Liver lipids were extracted using the chloroform/methanol Folch method (25) before being measured for the triglyceride levels.
Histology and Oil Red O staining
The general histology was evaluated by hematoxylin and eosin (H&E) staining. Immunostaining for Cd68 and insulin was performed on paraffin sections. For the Oil Red O staining, liver tissues were dehydrated in 30% sucrose solution, embedded in optimal cutting temperature compound, sectioned using cryostat, and stained with Oil Red O (0.5% in isopropanol) from Sigma-Aldrich.
Real-time polymerase chain reaction, Northern blot, and Western blot analysis
Total RNA was extracted using the TRIzol reagent from Invitrogen (Carlsbad, CA). Real-time polymerase chain reaction (PCR) was performed using SYBR Green reagent from Applied Biosystems (Foster City, CA) with the ABI 7300 Real-Time PCR System. Cyclophilin was used as the internal control. Northern blotting and hybridization using 32P-labeled cDNA probe was performed, as previously described (26). For Western blot analysis, 30 μg protein from tissue lysates was separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred onto polyvinylidene difluoride membrane. The primary antibodies used include the following: anti-Sult1e1 (Est) (catalogue 12522-1-AP) from Proteintech (Rosemont, IL); anti-Cd68 (catalogue sc7084) from Santa Cruz (Dallas, TX); anti–lipocalin-2 (Lcn2) (catalogue MAB1857-SP) from R&D Systems (Minneapolis, MN); anti-total Akt (catalogue 9727), anti–phospho-Akt (serine 473) (catalogue 9271), anti-insulin (catalogue C27C9), and anti–α-tubulin (catalogue 2144) from Cell Signaling (Beverly, MA); and anti–β-actin (catalogue A1978) from Sigma-Aldrich.
3T3-L1 cell culture, differentiation, and treatment
The 3T3-L1 cells were grown in Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, Cleveland, OH) with 10% fetal bovine serum (FBS) and penicillin/streptomycin until confluency. The cells were then treated with DMEM containing 10% FBS, 1 µM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, and 1.6 µM insulin. After 48 hours of incubation, the medium was replaced with DMEM supplemented with 1.6 µM insulin. On day 6 of the differentiation, the medium was replaced with DMEM with 1% FBS and vehicle, or 500 ng/mL recombinant mouse Lcn2 (R&D Systems; catalogue 1857-LC-050). After overnight incubation, cells were treated in four ways: (1) vehicle; (2) recombinant mouse 1 ng/mL Tnfα (Abcam, Cambridge, MA; catalogue ab9740); (3) 450 ng/mL Lcn2 (previously pretreated with Lcn2); and (4) 1 ng/mL Tnfα + 450 ng/mL Lcn2 (previously pretreated with Lcn2) for 24 hours. After this time, 1 µM insulin was added to the medium, and cells were harvested within 10 minutes. These experiments were repeated three times for reproducibility.
Statistics
GraphPad Prism software (GraphPad, Inc., San Diego, CA) was used for statistical analysis. The unpaired Student t test was performed. Statistical significance threshold was set at P < 0.05. Data represent mean ± standard deviation. The islet, immunostaining for insulin and Cd68, and the crownlike structure areas were quantified by using the ImageJ software from National Institutes of Health (Bethesda, MD).
Results
Transgenic reconstitution of Est in the adipose tissue and liver of the obe mice
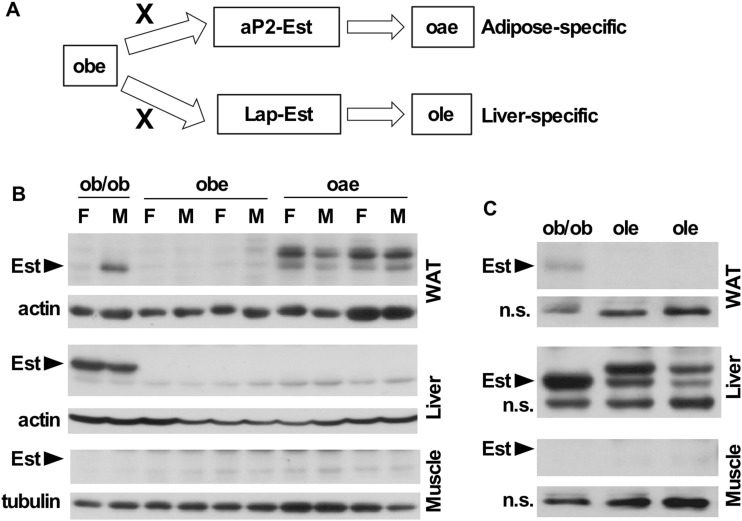
We have previously reported that ob/ob mice show a liver-specific induction of Est in both sexes. Interestingly, Est oblation in ob/ob mice produced a metabolic effect in a sex-specific manner. Specifically, male ob/ob mice deficient of Est (i.e., the obe mice), but not their female counterparts, exhibited exacerbated diabetic phenotype, including the adipose inflammation and loss of pancreatic β cell mass (19). Est is expressed in both the adipose tissue and liver. We have previously reported the creation and characterization of tetracycline-inducible transgenic mice overexpressing Est in the adipose tissue under the control of the aP2 gene promoter (20), or in the liver under the control of the liver-enriched activator protein (Lap) gene promoter (21). To define the tissue-specific role of Est in energy homeostasis and as outlined in Fig. 1A, we cross the obe mice with the aP2-Est or Lap-Est transgenic mice to generate the oae and ole mice that bear the reconstitution of Est in the adipose tissue and liver in the background of obe, respectively. The expression of the Est transgene in WAT of oae mice was confirmed at messenger RNA (mRNA) level by real-time PCR (data not shown) and Western blotting (Fig. 1B), respectively. In the oae mice, the Est transgene was similarly expressed in the WAT in both male and female mice (Fig. 1B). The protein expression of the Est transgene in the liver of ole mice was also confirmed by Western blotting (Fig. 1C). At the protein level, the WAT expression of the transgenic Est in the oae mice (Fig. 1B) and liver expression of the transgenic Est in the ole mice (Fig. 1C) were comparable to that observed in their counterpart tissues in the ob/ob mice. The tissue specificity of the transgene expression was also verified by Western blotting, as the protein expression of Est was not detectable in the liver and skeletal muscle of the oae mice (Fig. 1B), or in the WAT and skeletal muscle of the ole mice (Fig. 1C).
Figure 1.
Transgenic reconstitution of Est in the adipose tissue and liver of the obe mice. (A) The oae mice that bear the expression of Est in the adipose tissue in the background of obe were created by crossing the obe mice with the adipose-specific aP2-Est transgenic mice. The ole mice that bear the expression of Est in the liver in the background of obe were created by crossing the obe mice with the liver-specific Lap-Est transgenic mice. (B and C) The endogenous and transgenic Est protein expression in the WAT, liver, and skeletal muscle of (B) ob/ob, obe, and oae female and male mice and (C) ob/ob and ole male mice was measured by Western blot analysis. n.s., nonspecific bands.
Adipose reconstitution of Est improves the metabolic function of obe mice in a male-specific manner
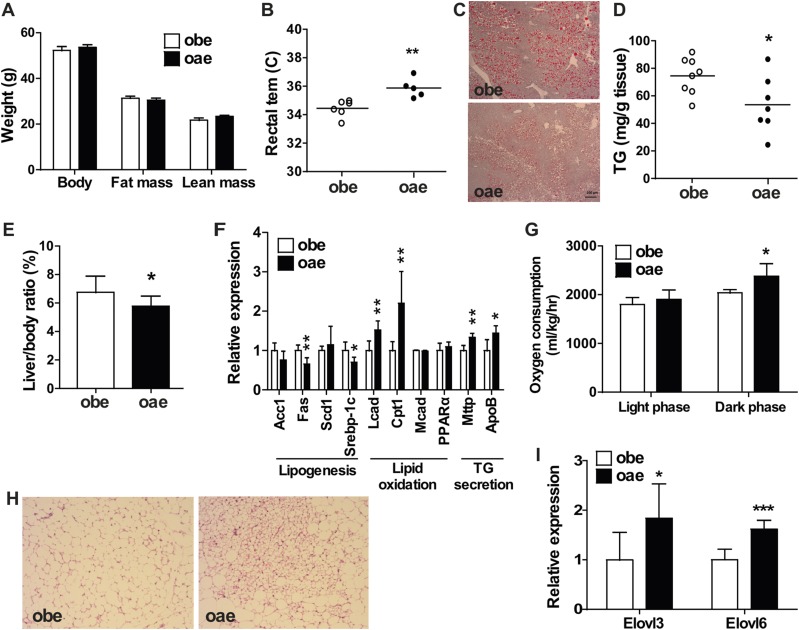
Compared with the obe males, although the aP2-Est transgene had little effect on the body weight and body composition (Fig. 2A), the oae male mice showed improved metabolic function, including increased/normalized rectal temperature (Fig. 2B), decreased hepatic steatosis as shown by Oil Red O staining (Fig. 2C), measurement of liver triglyceride level (Fig. 2D), and liver to body weight ratio (Fig. 2E). The alleviation of hepatic steatosis in oae males was consistent with the pattern of hepatic gene expression that included the suppression of lipogenic genes and induction of genes involved in fatty acid oxidation and triglyceride secretion (Fig. 2F). Metabolic cage analysis showed that the oae male mice had a modest, but significant increase of oxygen consumption during the dark phase (Fig. 2G). Additionally, the brown adipose tissue in oae males showed less whitening of the brown fat (Fig. 2H) and higher expression of Elovl3 and Elovl6, two probrowning transcription factors (27) (Fig. 2I). The serum biochemistry profile was also improved in the oae males, including the decreased fasting serum levels of glucose and insulin (Table 1). However, the serum levels of triglycerides, cholesterol, alanine transaminase, aspartate transaminase, and estradiol were not significantly affected by the transgene (Table 1). Interestingly, the metabolic benefit seen in the oae mice was male specific, because the aP2-Est transgene had little effect on the body weight and composition (Supplemental Fig. 1A (1.4MB, tif) ), rectal temperature (Supplemental Fig. 1B (1.4MB, tif) ), liver to body weight ratio (Supplemental Fig. 1C (1.4MB, tif) ), and oxygen consumption (Supplemental Fig. 1D (1.4MB, tif) ) in female mice.
Figure 2.
Adipose reconstitution of Est improves the metabolic function of obe mice in a male-specific manner. (A) Body weight and body composition analysis by magnetic resonance imaging in male mice. n = 6 to 8. (B) Rectal temperature (tem) in adult obe and oae male mice. (C) Oil Red O staining of liver sections from 17-week-old male obe and oae mice. (D) Hepatic triglyceride (TG) levels in male obe and oae mice. (E) Liver to body ratio in male obe and oae mice. n = 5 to 8. (F) The expression of genes involved in lipogenesis, fatty acid oxidation, and triglyceride export in the liver was measured by real-time PCR. n = 6. (G) Oxygen consumption was measured by metabolic cages. n = 4. (H) H&E staining of brown adipose tissue of male obe and oae mice. (I) Expression of transcription factors involved in the browning of brown adipose tissue, as measured by real-time PCR. n = 6. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 1.
Serum Biochemistry of obe and oae Male Mice
| obe | oae | |
|---|---|---|
| Total cholesterol (mg/dL) | 163.1 ± 17.1 | 160.5 ± 14.5 |
| Triglyceride (mg/dL) | 144.3 ± 34 | 158.8 ± 36 |
| ALT (U/L) | 170.1 ± 26.8 | 165.2 ± 43.1 |
| AST (U/L) | 211 ± 22.1 | 263.3 ± 66.36 |
| Adiponectin (ng/mL) | 1.827 ± 0.43 | 1.823 ± 0.53 |
| Fasting blood glucose (mg/dL) | 202.4 ± 44.8 | 137.2 ± 16.6a |
| Fasting insulin (ng/mL) | 7.8 ± 2.9 | 4.8 ± 1.7b |
| Estradiol (17β-E2) (pg/mL) | 2.25 ± 0.64 | 1.68 ± 0.46 |
Results are presented as mean ± standard deviation. obe, n = 7 to 8; oae, n = 6 to 8.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase.
P < 0.01; comparisons are between the two genotypes.
P < 0.05; comparisons are between the two genotypes.
Adipose reconstitution of Est improves the glucose tolerance and insulin sensitivity of obe mice in a male-specific manner
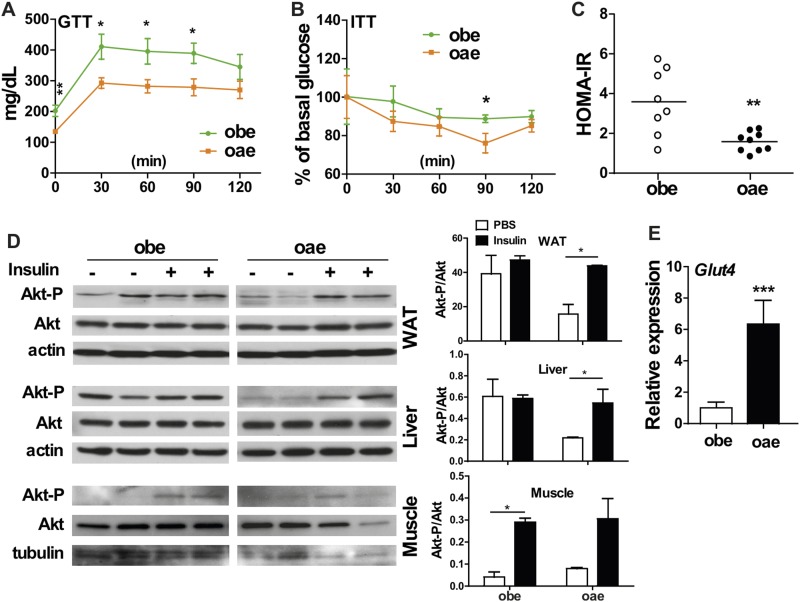
In addition to their decreased fasting serum insulin level (Table 1), the oae males also showed improved performance in the GTT (Fig. 3A) and ITT (Fig. 3B) compared with the obe males. The HOMA-IR, an index to assess insulin resistance, was also decreased in oae males (Fig. 3C). To directly assess the insulin sensitivity in peripheral tissues, obe and oae males were injected with insulin or phosphate-buffered saline. Tissues were harvested and assessed for the phosphorylation of Akt (protein kinase B) by Western blotting. The WAT and liver of obe males showed a high basal phosphorylation of Akt, but they lacked the insulin response, indicating insulin resistance (Fig. 3D). In contrast, the WAT and liver of oae males showed low basal, but higher insulin-stimulated Akt phosphorylation, which was suggestive of a higher insulin sensitivity in these two tissues (Fig. 3D). The pattern of Akt phosphorylation in the skeletal muscle showed no difference between these two genotypes, but the expression of muscular glucose uptake transporter Glut4 was highly upregulated in oae males (Fig. 3E). Again, the metabolic benefit seen in the oae mice was male specific. The aP2-Est transgene had little effect on GTT (Supplemental Fig. 2A (3.7MB, tif) ), ITT (Supplemental Fig. 2B (3.7MB, tif) ), and HOMA-IR (Supplemental Fig. 2C (3.7MB, tif) ) in oae female mice.
Figure 3.
Adipose reconstitution of Est improves the glucose tolerance and insulin sensitivity of obe mice in a male-specific manner. (A) GTT and (B) ITT in male obe and oae mice. n = 4 to 6. (C) Insulin resistance calculated and expressed as HOMA-IR score in male obe and oae mice. (D) Western blotting measurement of Akt phosphorylation in the WAT, liver, and skeletal muscle of male mice that were treated with phosphate-buffered saline or insulin. Shown on the right are densitometric quantifications of the blots. (E) The expression of Glut4 in the skeletal muscle of male obe and oae mice was measured by real-time PCR. n = 6. *P < 0.05; **P < 0.01; ***P < 0.001.
Adipose reconstitution of Est in obe mice attenuates adipose and systemic inflammation in a male-specific manner
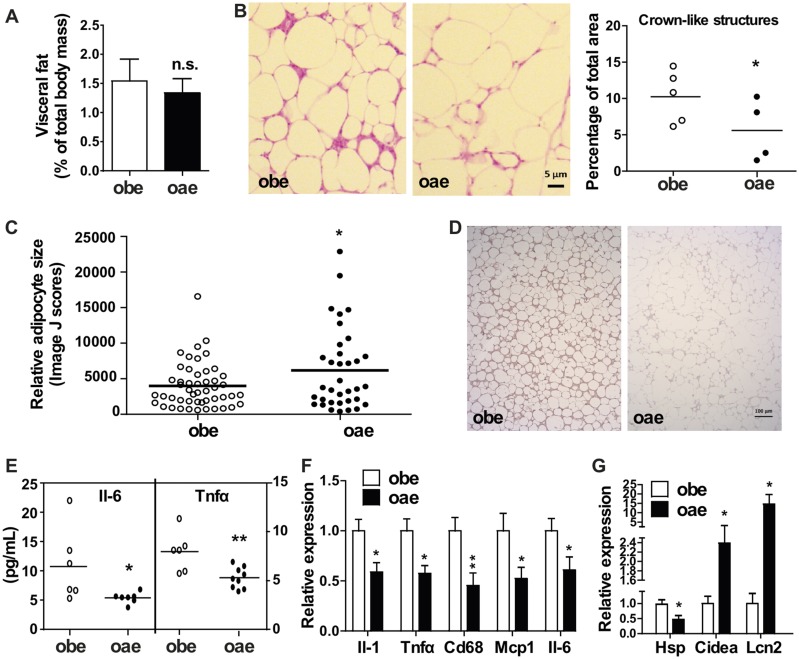
Obesity is commonly associated with chronic and low-grade inflammation primarily originated from the excess of adipose tissue, which often leads to elevated circulating levels of proinflammatory cytokines such as Il-6 and Tnfα (28). In our previous report, we attributed the worsened metabolic function of obe males to increased WAT inflammation (19). We then analyzed the WAT local and systemic inflammation to determine whether the attenuation of inflammation can explain the improved metabolic function in oae male mice. The weight of the visceral fat was not affected by the aP2-Est transgene (Fig. 4A), which was consistent with the unchanged serum level of adiponectin (Table 1) and WAT mRNA expression of adiponectin (data not shown). Histological analysis by H&E staining showed that the oae male mice showed a substantially reduced number and size of the crownlike structures compared with the obe males (Fig. 4B). Interestingly, the size of adipocytes in the visceral fat of oae mice appeared to be larger than that of the obe mice (Fig. 4C), the mechanism of which remains to be understood. Reduced infiltration of macrophages in the WAT of oae males was confirmed by immunostaining for Cd68 (Fig. 4D). The serum levels of Il-6 and Tnfα were also significantly reduced in the oae males (Fig. 4E). The attenuation of WAT and systemic inflammation was consistent with the reduced expression of several macrophage marker genes (F4/80 and Cd68) and inflammatory marker genes (Il-6, Tnfα, Il-1b, and Mcp1) in the visceral fat (Fig. 4F). The attenuation of WAT local and systemic inflammation in oae mice was again male specific, because the aP2-Est transgene had little effect on the crownlike structure (Supplemental Fig. 3A (4.5MB, tif) ), or the circulating levels of Il-6 and Tnfα (Supplemental Fig. 3B (4.5MB, tif) ) in female mice.
Figure 4.
Adipose reconstitution of Est in obe mice attenuates adipose and systemic inflammation in a male-specific manner. (A) The weight of visceral fat shown as a percentage of body mass in male obe and oae mice. n = 4 to 6. (B) H&E staining of sections of visceral fat in male mice. Bar = 5 μm. Shown on the right are Image J quantifications of the crownlike structures. (C) The quantifications of adipocyte sizes are shown as relative sizes. (D) Immunohistochemical staining of Cd68 in visceral fat sections of male mice. Bar = 100 μm. (E) The serum levels of Il-6 and Tnfα in male obe and oae mice were measured by ELISA. (F and G) The expression of (F) inflammatory marker genes and (G) estrogen-responsive genes in the visceral fat of male mice was measured by real-time PCR. n = 7. *P < 0.05; **P < 0.01.
Because a primary function of Est is to sulfonate and deactivate estrogens, and estrogens are generally known to have anti-inflammatory activity (29, 30), we went on to determine whether the aP2-Est transgene affected systemic or WAT local estrogen activities. The serum level of estradiol (17β-E2) in oae males tended to be lower, but the difference did not reach a statistical significance (Table 1). When the expression of estrogen-responsive genes in the visceral fat was measured, we found the expression of genes that are positively regulated by estrogens, such as the heat shock protein, was decreased in oae mice compared with obe mice. In contrast, the expression of genes known to be suppressed by estrogens, such as the cell death activator CIDE-A and Lcn2, was significantly increased in oae mice (Fig. 4G). These results suggested that the aP2-Est transgene inhibited estrogen activity in the adipose tissue.
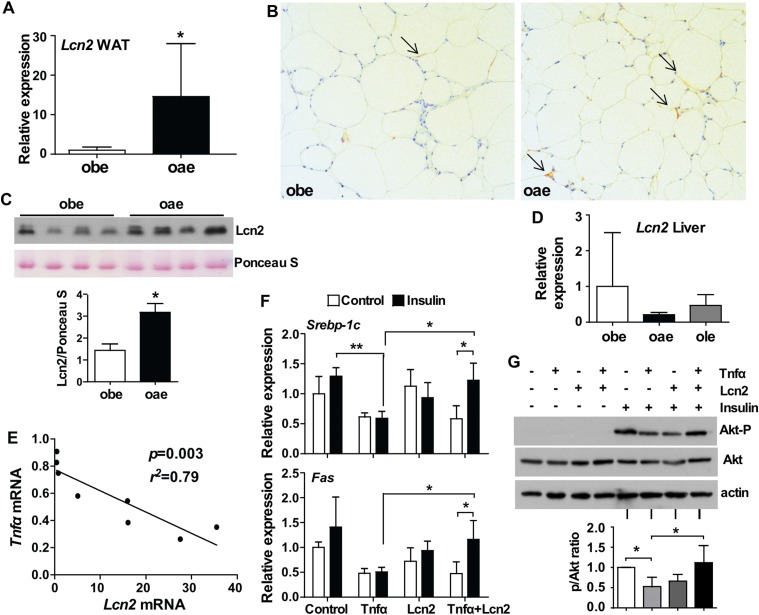
The reduced WAT local and systemic inflammation in oae males may have been accounted for by the upregulation of Lcn2
Lcn2, a glycoprotein mainly secreted by the adipose tissue and bone (31), has been assigned to multiple pathophysiological roles (32). Lcn2 was proposed as an inflammatory marker induced primarily by Tnfα and other proinflammatory cytokines (33). However, to our surprise, the visceral WAT of oae males was found to express a higher mRNA level of Lcn2 compared with the obe mice (Figs. 4F and 5A). The increased expression of Lcn2 at the protein level was verified by immunohistochemistry on visceral WAT (Fig. 5B) and by Western blotting on the serum (Fig. 5C). The induction of Lcn2 was consistent with the notion that the expression of Lcn2 can be suppressed by estrogens (34), so the increased deactivation of estrogen in oae mice caused a desuppression of Lcn2 in the adipose tissue. The induction of Lcn2 was both WAT specific and male specific because the liver expression of Lcn2 was comparable between the obe and oae males (Fig. 5D), and the aP2-Est transgene had little effect on the adipose expression of Lcn2 in oae females (Supplemental Fig. 4 (336.2KB, tif) ). In contrast to the notion that Lcn2 might be an inflammatory marker, we found a striking negative association between the WAT expression of Lcn2 and Tnfα (Fig. 5E), suggesting that Lcn2 might actually antagonize Tnfα as part of a negative feedback loop. Indeed, treatment of differentiated 3T3-L1 cells with Lcn2 attenuated the Tnfα-responsive suppression of insulin signaling, including the expression of insulin-responsive genes such as Srbep-1c and Fas (Fig. 5F) and phosphorylation of Akt (Fig. 5G). We speculate that the induction of Lcn2 in oae males may have contributed to the reduced levels of systemic Tnfα and Il-6 and subsequently improved insulin sensitivity in peripheral tissues.
Figure 5.
The reduced WAT local and systemic inflammation in oae males may have been accounted for by the upregulation of Lcn2. (A) The expression of Lcn2 in the visceral WAT of obe and oae male mice was measured by real-time PCR. n = 7. (B) Immunostaining of Lcn2 in visceral fat of male obe and oae mice. Arrows indicate the positive staining. (C) The serum level of Lcn2 protein in male obe and oae mice was measured by Western blotting. Shown at the bottom is the densitometric quantification of the blots. (D) The hepatic mRNA expression of Lcn2 was measured by real-time PCR. n = 4 to 7. (E) Association between the mRNA expression of Lcn2 and Tnfα in the visceral fat of male oae mice. (F) The mRNA expression of insulin-responsive genes in differentiated 3T3-L1 cells was measured by real-time PCR. (G) Insulin-induced Akt phosphorylation in differentiated 3T3-L1 cells was measured by Western blotting. Shown are the representative blot and densitometric quantification of the results from three independent experiments. *P < 0.05; **P < 0.01.
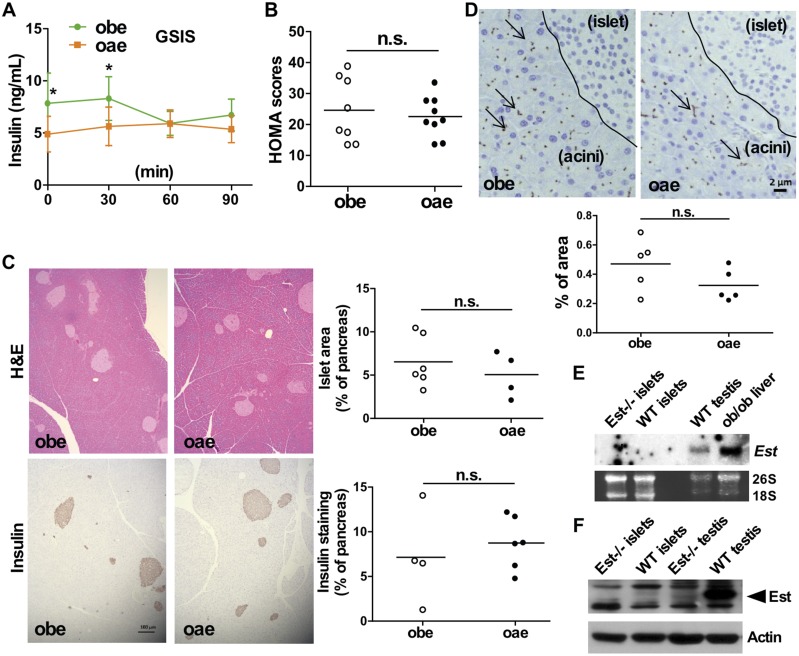
Adipose reconstitution of Est fails to rescue the pancreatic β cell damage in obe males
Having shown the improved metabolic function in oae males, we were surprised to find that the compromised structure and function of pancreatic islets were not rescued in oae mice. Compared with the obe mice, the oae mice showed little improvement in the GSIS test (Fig. 6A), the calculated index of β cell function (Fig. 6B), and the histology of the pancreatic islets as evaluated by H&E staining and immunostaining of insulin (Fig. 6C). The pancreatic immunostaining of Cd68 showed a trend of decreased Cd68 immunostaining in oae mice, but the difference was not statistically significant (Fig. 6D). It was noted that the Cd68 immunostaining was mostly present in the pancreatic acinar cells, but not within the islets regardless of the mouse genotype. The pancreatic islets isolated from wild-type mice showed no detectable expression of Est at the mRNA level, as shown by Northern blotting (Fig. 6E) and real-time PCR (data not shown), or at the protein level, as shown by Western blotting (Fig. 6F). Adipose reconstitution of Est also had little effect on the structure and function of pancreatic islets in oae female mice (data not shown).
Figure 6.
Adipose reconstitution of Est fails to rescue the pancreatic β cell damage in obe males. (A) GSIS in male obe and oae mice. n = 7 to 8. (B) Homeostatic model assessment (HOMA) of β cell function in male mice. (C) H&E staining (upper two panels) and immunostaining of insulin (lower two panels) of pancreatic sections from male obe and oae mice. Shown on the right are Image J quantifications of pancreatic islets and insulin-positive areas. Bar = 100 μm. (D) Immunostaining of Cd68 in pancreatic sections of male obe and oae mice. Dashed line borders pancreatic islet area from the acini area. Shown at the bottom are Image J quantifications of the Cd68 staining. Bar = 2 μm. (E and F) The mRNA and protein expression of mouse Est in isolated pancreatic islets was measured by (E) Northern blot analysis and (F) Western blot analysis, respectively. *P < 0.05. n.s., statistically not significant; WT, wild-type.
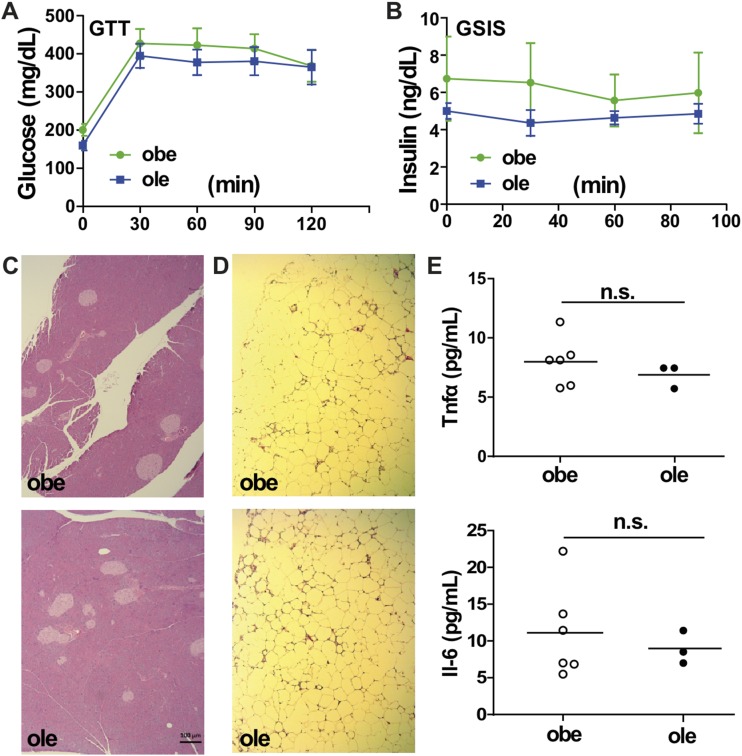
Liver reconstitution of Est fails to ameliorate inflammation and diabetic phenotype in obe male mice
Although the expression of Est was markedly induced in ob/ob mice (19), reconstitution of Est in the liver of obe mice by the Lap-Est transgene (ole mice) failed to improve the metabolic function. Specifically, the ole mice did not show improvement in GTT (Fig. 7A) and GSIS (Fig. 7B) tests. The lack of effect of the Lap-Est transgene on GSIS was supported by the unchanged histology of the pancreatic islets (Fig. 7C). When inflammation was analyzed, we observed no attenuation of inflammation in the visceral WAT, as indicated by the density of the crownlike structures (Fig. 7D). The serum levels of Il-6 and Tnfα were also not affected by the Lap-Est transgene (Fig. 7E). Liver reconstitution of Est in ole females also had little effect on the metabolic function (data not shown).
Figure 7.
Liver reconstitution of Est fails to ameliorate inflammation and diabetic phenotype in obe male mice. (A) GTT and (B) GSIS in male obe and ole mice. n = 6 to 7. (C and D) H&E staining of (C) pancreatic and (D) visceral fat sections in male obe and ole mice. Bar = 100 μm. (E) The serum levels of Tnfα and Il-6 in male obe and ole mice were measured by enzyme-linked immunosorbent assay. n.s., not significant.
Discussion
Estrogens exert their biological effects on human or animal bodies far beyond serving as sex hormones (21, 35, 36). The roles of estrogens in maintaining metabolic homeostasis are significant, but not fully understood (6). One of the challenges in deciphering the role of estrogens in energy homeostasis is the sex-specific effect of estrogens on metabolism, obesity, and immune responses (29, 37).
In this study, we discovered that the expression and activity of Est specifically in the male white adipose tissue are essential for metabolic homeostasis under the obese condition. Reconstitution of Est in the adipose tissue of Est-deficient ob/ob (obe) male mice improved metabolic function, most likely as a result of attenuated WAT local and systemic inflammation, as well as improved insulin sensitivity in peripheral tissue. The reduced insulin resistance in oae mice was consistent with the attenuated fasting hyperinsulinemia in this genotype (Table 1). This is a surprising result considering that Est is an estrogen-deactivating enzyme, and estrogens have been suggested to protect humans and animals from insulin resistance, elevated inflammation, and other metabolic complications associated with obesity (12, 13, 35, 38). This surprising result may be explained by the tissue-specific expression of Est as well as the tissue-specific action of estrogens. The basal expression of Est in the adipose tissue of male mice is markedly higher than the female mice, which turns out to be essential in maintaining energy homeostasis, because Est ablation in ob/ob males worsened the metabolic phenotype. In contrast, the basal adipose expression of Est is low in female mice, and, as a result, Est ablation in this tissue did not produce the same metabolic effect as in males. The perceived metabolic benefit of estrogens is also not without controversy. In humans, the high levels of estrogens in pregnant women are believed to have contributed to insulin resistance and gestational diabetes through estrogen’s suppression of GLUT4 in the skeletal muscle (14). In addition, despite their attenuated insulin resistance, the E2-treated HFD-fed female mice showed an increased expression of proinflammatory cytokines Il-6 and Tnfα in their visceral fat (15). Although these reports were somewhat contradictory to the notion that estrogens have an overall anti-inflammatory effect (30), they were consistent with our observation that the WAT of oae males showed decreased estrogen activity and attenuated inflammation.
We cannot exclude the possibility that reconstitution of Est in the adipose tissue of oae males attenuated WAT inflammation in an estrogen-independent manner. Based on our results, another possible mechanism to explain the improved metabolic function in oae males is Lcn2-induced attenuation of proinflammatory signaling in WAT. The role of Lcn2 in diabetes and inflammation has been controversial (32, 33, 39). When the Lcn2−/− mice were challenged with lipopolysaccharide or concavalin A, they showed more injury than the wild-type mice, including elevated levels of Il-β, Tnfα, and Il-6 (40). These results suggested that Lcn2 may have an anti-inflammatory role in vivo, instead of being an inflammatory marker as suggested in an earlier study (33). The notion that Lcn2 may have an anti-inflammatory activity was consistent with our observation that the reduced WAT local and systemic inflammation in oae mice was associated with the upregulation of Lcn2 in a male-specific manner. The involvement of Lcn2 in thermoregulation (41) is also consistent with the phenotype observed in our oae male mice.
Another intriguing finding is the lack of metabolic effect when the expression of Est was reconstituted to the liver. The expression of Est in the liver is low, but the hepatic expression of Est was highly induced in ob/ob mice and in several other mouse models of obesity and type 2 diabetes (17). The lack of metabolic phenotype change in ole mice of either sex suggested that it was the loss of Est in extrahepatic tissues that was responsible for the metabolic phenotype of obe mice. Based on our current results, this extrahepatic tissue is the white adipose tissue in male mice because reconstitution of Est in the adipose tissue improved the metabolic function of obe males. The extrahepatic tissue responsible for the improved metabolic function in obe females remains to be defined.
The third surprising finding is that, despite their improved overall metabolic function, the oae males were not rescued from the loss of β cell mass. Chronic inflammation is known to play a pathological role in metabolic disease, including its role in compromising the structure and function of β cells (4). Meanwhile, activation of the estrogen receptor has been reported to reduce lipid synthesis and prevent lipotoxicity, oxidative stress, and apoptosis in pancreatic islets and prevent β cell failure in rodent models of type 2 diabetes (38, 42). Apparently, attenuation of WAT local and systemic inflammation in our oae males was not sufficient to recover the β cell mass. Adipose reconstitution of Est did not affect the circulating level of estrogen in a significant manner, so the lack of rescue cannot be explained by the estrogens either. The lack of detectable expression of Est in pancreatic islets excluded the possibility that the loss of β cell mass in obe and oae mice was due to the loss of Est in situ. We conclude that the attenuation of local and systemic inflammation improved the metabolic function of oae males in a β cell–independent manner. Indeed, the improved insulin sensitivity in peripheral tissues may have benefited from the attenuation of inflammation, as suggested by the literature (43).
In summary, our current study has uncovered a function of adipose Est in preventing WAT inflammation and maintaining whole-body energy homeostasis in a male-specific manner. Our results also suggested that the expression of Est in the adipose tissue or liver is not responsible for the structural and functional integrity of the pancreatic islets under metabolic stress.
Acknowledgments
Financial Support: This work was supported in part by National Institutes of Health Grants DK083952 and ES023438 (to W.X.). W.X. is also supported in part by the Joseph Koslow Endowed Chair Professorship from the University of Pittsburgh School of Pharmacy.
Acknowledgments
Author Contributions: W.G.G., M.J., and W.X. participated in research design. W.G.G., M.J., M.X., and J.Y. conducted experiments. W.G.G., M.J., and W.X. performed data analysis. W.G.G., H.H.D., and W.X. wrote or contributed to the writing of the manuscript. W.X. acquired funding. The guarantors are W.G.G. and W.X. who, as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Antibody | Dilution Used | RRID |
|---|---|---|
| Anti-Sult1e1 (Est) (WB) | 1:1000 | AB_2302772 |
| Anti-Cd68 (IHC) | 1:100 | AB_2074854 |
| Anti-Lcn2 (IHC) | 1:100 | AB_2136584 |
| Anti-Lcn2 (WB) | 1:1000 | AB_2136584 |
| Anti-total Akt | 1:2000 | AB_329827 |
| Anti–phospho-Akt (serine 473) | 1:2000 | AB_329825 |
| Anti-insulin (IHC) | 1:100 | AB_2126503 |
| Anti–α-tubulin (WB) | 1:5000 | AB_2210548 |
| Anti–β-actin (WB) | 1:10000 | AB_476692 |
Abbreviations: IHC, immunohistochemistry; RRID, research resource identifier; WB, Western blotting.
Footnotes
- DMEM
- Dulbecco’s modified Eagle medium
- FBS
- fetal bovine serum
- GSIS
- glucose-stimulated insulin secretion
- GTT
- glucose tolerance test
- H&E
- hematoxylin and eosin
- HFD
- high-fat diet
- HOMA-IR
- homeostatic model assessment for insulin resistance
- Il-6
- interleukin 6
- ITT
- insulin tolerance test
- Lcn2
- lipocalin-2
- mRNA
- messenger RNA
- oae mice
- adipose reconstitution of Est in Est-deficient ob/ob mice
- obe mice
- Est-deficient ob/ob mice
- ole mice
- liver reconstitution of Est in Est-deficient ob/ob mice
- PCR
- polymerase chain reaction
- Tnf
- tumor necrosis factor
- WAT
- white adipose tissue.
References
- 1.Blüher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci (Lond). 2016;130(18):1603–1614. [DOI] [PubMed] [Google Scholar]
- 2.Sargent J. Obesity: rethinking inflammation and adipocyte homeostasis. Nat Rev Endocrinol. 2014;10(8):446. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116(7):1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. [DOI] [PubMed] [Google Scholar]
- 6.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97(23):12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27(9):1020–1027. [DOI] [PubMed] [Google Scholar]
- 9.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142(11):4751–4757. [DOI] [PubMed] [Google Scholar]
- 10.Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13(4):220–231. [DOI] [PubMed] [Google Scholar]
- 11.Pettersson US, Waldén TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7(9):e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dakin RS, Walker BR, Seckl JR, Hadoke PW, Drake AJ. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int J Obes. 2015;39(10):1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stout MB, Steyn FJ, Jurczak MJ, Camporez JG, Zhu Y, Hawse JR, Jurk D, Palmer AK, Xu M, Pirtskhalava T, Evans GL, de Souza Santos R, Frank AP, White TA, Monroe DG, Singh RJ, Casaclang-Verzosa G, Miller JD, Clegg DJ, LeBrasseur NK, von Zglinicki T, Shulman GI, Tchkonia T, Kirkland JL. 17α-Estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J Gerontol A Biol Sci Med Sci. 2017;72(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barros RP, Morani A, Moriscot A, Machado UF. Insulin resistance of pregnancy involves estrogen-induced repression of muscle GLUT4. Mol Cell Endocrinol. 2008;295(1-2):24–31. [DOI] [PubMed] [Google Scholar]
- 15.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–2117. [DOI] [PubMed] [Google Scholar]
- 16.Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23(5):703–732. [DOI] [PubMed] [Google Scholar]
- 17.Leiter EH, Chapman HD. Obesity-induced diabetes (diabesity) in C57BL/KsJ mice produces aberrant trans-regulation of sex steroid sulfotransferase genes. J Clin Invest. 1994;93(5):2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong MH, Christenson LK, Song WC. Aberrant cholesterol transport and impaired steroidogenesis in Leydig cells lacking estrogen sulfotransferase. Endocrinology. 2004;145(5):2487–2497. [DOI] [PubMed] [Google Scholar]
- 19.Gao J, He J, Shi X, Stefanovic-Racic M, Xu M, O’Doherty RM, Garcia-Ocana A, Xie W. Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes. Diabetes. 2012;61(6):1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada T, Ihunnah CA, Gao J, Chai X, Zeng S, Philips BJ, Rubin JP, Marra KG, Xie W. Estrogen sulfotransferase inhibits adipocyte differentiation. Mol Endocrinol. 2011;25(9):1612–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chai X, Guo Y, Jiang M, Hu B, Li Z, Fan J, Deng M, Billiar TR, Kucera HR, Gaikwad NW, Xu M, Lu P, Yan J, Fu H, Liu Y, Yu L, Huang M, Zeng S, Xie W. Oestrogen sulfotransferase ablation sensitizes mice to sepsis. Nat Commun. 2015;6:7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garbacz WG, Lu P, Miller TM, Poloyac SM, Eyre NS, Mayrhofer G, Xu M, Ren S, Xie W. Hepatic overexpression of CD36 improves glycogen homeostasis and attenuates high-fat diet-induced hepatic steatosis and insulin resistance. Mol Cell Biol. 2016;36(21):2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 24.Liu YZ, Cheng X, Zhang T, Lee S, Yamauchi J, Xiao X, Gittes G, Qu S, Jiang CL, Dong HH. Effect of hypertriglyceridemia on beta cell mass and function in ApoC3 transgenic mice. J Biol Chem. 2016;291(28):14695–14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 26.Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem. 2006;281(21):15013–15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan CY, Virtue S, Bidault G, Dale M, Hagen R, Griffin JL, Vidal-Puig A. Brown adipose tissue thermogenic capacity is regulated by Elovl6. Cell Reports. 2015;13(10):2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 30.Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015;5:15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, Huang Y, Zong H, Friedman RA, Barasch J, Lanzano P, Deng L, Leibel RL, Rubin M, Nicholas T, Chung W, Zeltser LM, Williams KW, Pessin JE, Kousteni S. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543(7645):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asimakopoulou A, Weiskirchen S, Weiskirchen R. Lipocalin 2 (LCN2) expression in hepatic malfunction and therapy. Front Physiol. 2016;7:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abella V, Scotece M, Conde J, Gómez R, Lois A, Pino J, Gómez-Reino JJ, Lago F, Mobasheri A, Gualillo O. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers. 2015;20(8):565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drew BG, Hamidi H, Zhou Z, Villanueva CJ, Krum SA, Calkin AC, Parks BW, Ribas V, Kalajian NY, Phun J, Daraei P, Christofk HR, Hewitt SC, Korach KS, Tontonoz P, Lusis AJ, Slamon DJ, Hurvitz SA, Hevener AL. Estrogen receptor (ER)α-regulated lipocalin 2 expression in adipose tissue links obesity with breast cancer progression. J Biol Chem. 2015;290(9):5566–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ihunnah CA, Wada T, Philips BJ, Ravuri SK, Gibbs RB, Kirisci L, Rubin JP, Marra KG, Xie W. Estrogen sulfotransferase/SULT1E1 promotes human adipogenesis. Mol Cell Biol. 2014;34(9):1682–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97(23):12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis KE, Neinast MD, Sun K, Skiles WM, Bills JD, Zehr JA, Zeve D, Hahner LD, Cox DW, Gent LM, Xu Y, Wang ZV, Khan SA, Clegg DJ The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2(3):227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol. 2012;8(6):342–351. [DOI] [PubMed] [Google Scholar]
- 39.Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B, Wang Y. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59(4):872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borkham-Kamphorst E, Drews F, Weiskirchen R. Induction of lipocalin-2 expression in acute and chronic experimental liver injury moderated by pro-inflammatory cytokines interleukin-1β through nuclear factor-κB activation. Liver Int. 2011;31(5):656–665. [DOI] [PubMed] [Google Scholar]
- 41.Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA, Chen X. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59(6):1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, Mauvais-Jarvis F. Minireview: estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151(3):859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]