Abstract
Parathyroid hormone (PTH) regulates the transcription of many genes in the osteoblast. One of these genes is Mmp13, which is involved in bone remodeling and early stages of endochondral bone formation. Previously, we reported that PTH induces Mmp13 transcription by regulating the dissociation of histone deacetylase 4 (HDAC4) from runt-related transcription factor 2 (Runx2), and the association of the HATs, p300, and p300/CREB binding protein (CBP)–associated factor. It is known that, in addition to Runx2, HDAC4 binds to the transcription factor, myocyte-specific enhancer factor 2c (MEF2C), and represses its activity. In this work, we investigated whether MEF2C participates in PTH-stimulated Mmp13 gene expression in osteoblastic cells and how it does so. Knockdown of Mef2c in UMR 106-01 cells repressed Mmp13 messenger RNA expression and promoter activity with or without PTH treatment. Chromatin immunoprecipitation (ChIP) assays showed that MEF2C associated with the Mmp13 promoter; this increased after 4 hours of PTH treatment. ChIP-reChIP results indicate that endogenous MEF2C associates with HDAC4 on the Mmp13 promoter; after PTH treatment, this association decreased. From gel shift, ChIP, and promoter-reporter assays, MEF2C was found to associate with the activator protein-1 (AP-1) site without directly binding to DNA and had its stimulatory effect through interaction with c-FOS. In conclusion, MEF2C is necessary for Mmp13 gene expression at the transcriptional level and participates in PTH-stimulated Mmp13 gene expression by increased binding to c-FOS at the AP-1 site in the Mmp13 promoter. The observation of MEF2C interacting with a member of the AP-1 transcription factor family provides knowledge of the functions of HDAC4, c-FOS, and MEF2C.
MEF2C is necessary for Mmp13 gene expression at the transcriptional level and participates in PTH-stimulated Mmp13 gene expression by increased binding to c-FOS at the AP-1 site in the Mmp13 promoter.
Parathyroid hormone (PTH) is an essential regulator of calcium homeostasis. It has multiple actions, including indirect activation of the osteoclast through osteoblastic production of receptor activator of nuclear factor-κB ligand (1, 2), resulting in increased bone resorption as well as many direct changes in the functions of the osteoblast. Besides the stimulation of the production of receptor activator of nuclear factor-κB ligand, PTH increases the production of proteases such as matrix metalloproteinase-13 (MMP13, also called collagenase-3). MMP13 is a member of the large family of matrix metalloproteinases, is expressed in terminal hypertrophic chondrocytes and trabecular osteoblasts, and is thought to be involved in endochondral ossification and bone remodeling (3, 4). Mmp13 gene expression is highly stimulated by PTH in osteoblastic cells (5, 6) as well as in vivo (7). In fact, Zhao et al. (8) have shown that PTH-elicited bone resorption and calcemic responses are diminished in mice whose cleavage site of type I collagen was mutated. Previous work from our laboratory has shown that the runt domain (RD) and activator protein-1 (AP-1) sites in the proximal promoter region are essential for PTH stimulation of Mmp13 promoter activity (9–11). At the Mmp13 promoter in osteoblastic cells, runt-related transcription factor 2 (Runx2) binds histone deacetylase 4 (HDAC4) at the RD binding site, resulting in a repression of transcription under basal conditions (12). After PTH treatment, HDAC4 dissociates from Runx2,which is then free to recruit HATs, especially p300 and p300/CBP-associated factor (P/CAF) to activate transcription (13, 14). However, transcriptional regulation of Mmp13 expression by PTH in osteoblasts is not completely understood. Although myocyte enhancer factor 2C (MEF2C) proteins were originally studied in their role as transcriptional regulators of myogenic cells that interact with other transcriptional factors, it has been reported that MEF2C proteins control bone development and chondrocyte hypertrophy (15) as well as bone mass by regulation of osteoclastic bone resorption (16). MEF2C is expressed in osteoblastic cells and is a key osteoblast transcription factor, upstream of Runx2 and osterix (17). MEF2 proteins belong to the evolutionarily ancient MADS (MCM1, agamous, deficients, SRF) family of transcription factors. There are four MEF2 members: MEF2A, MEF2B, MEF2C, and MEF2D. The N-terminal MADS box is flanked by an MEF2 domain, both of which together mediate DNA binding, dimerization, and interaction with transcriptional cofactors. HDAC4 has been shown to associate with MEF2C as well as Runx2 and represses MEF2C activity in cardiomyocytes (18). The C-terminus regions of MEF2 proteins function as transcriptional activation domains (19, 20). In the rat osteoblastic UMR 106-01 cells, Mef2c is the most highly expressed of the four Mef2 genes (21). In the present work, we investigated whether the transcription factor, MEF2C, participates in PTH-stimulated Mmp13 gene expression in osteoblastic cells and how it does so.
Materials and Methods
Cell culture and transient transfection
Rat osteoblastic UMR 106-01 cells were maintained in modified Eagle medium (MEM) supplemented with nonessential amino acids, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2 and 95% air. For transfection, cells were seeded at a density of 106 cells/100 mm dish or 2 × 105 cells/well of a six-well plate 24 hours before transfection. Plasmids were transfected using GeneJammer transfection reagent (Stratagene, San Diego, CA). For small interfering RNA (siRNA) transfection, X-tremeGENE siRNA transfection reagent (Roche Applied Science) was used according to the manufacturer’s protocol. siRNA oligos were purchased from EZBioLab (Carmel, IN). Cells were cultured at 37°C in a CO2 incubator for 48 to 72 hours before harvest. Cells were treated with 10−8 M PTH-(1-34) for 4 hours and collected for RNA analysis.
Mouse primary calvarial cell isolation and transient transfection
Mouse primary osteoblasts were derived from postnatal day 2 wild-type mouse calvariae by digestion for 30 minutes at 37°C in 1 mg/mL collagenase A in MEM. Cells from the first and second digests were discarded. Cells from the final digest were collected and plated at 0.4 × 105 cells/well in 24-well plates. The following day, cells were transfected with the siRNA for Mef2c using X-tremeGENE siRNA transfection reagent. Cells were maintained in MEM supplemented with 10% fetal bovine serum, 1% nonessential amino acids, and 1% penicillin-streptomycin for 72 hours after transfection. Cells were treated with 10−8 M PTH (1-34) for 4 hours and collected for RNA analysis.
Immunoprecipitation
UMR cells were lysed with radioimmunoprecipitation assay buffer [50 mM Tris-HCl, pH 7.4, 10 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM EDTA, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitors]. Lysates were precleared with protein A/G-agarose beads (Santa Cruz Biotechnology, Dallas, TX) for 1 hour at 4°C and then 0.3 mg of total protein lysate was immunoprecipitated overnight with specific antibodies, or with control immunoglobulin G (IgG; Santa Cruz Biotechnology).
Western blot analyses
UMR cells were treated with 10−8 M PTH-(1-34) for 30 minutes and lysed with radioimmunoprecipitation assay buffer. Amounts of total protein were determined by the Bradford dye binding (Bio-Rad, Hercules, CA) method. The proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred electrophoretically to polyvinylidene difluoride membranes (Bio-Rad). After blocking with 5% (w/v) nonfat dry milk in Tris-buffered saline buffer containing 0.1% Tween 20, the membranes were exposed to primary antibody (Abcam Inc., Cambridge, United Kingdom, for MEF2C) overnight at 4°C. The following day, after washing, the membranes were exposed to horseradish peroxidase–conjugated secondary antibodies. The immunoreactive signals were visualized using an enhanced chemiluminescence detection kit (Amersham Bioscience, Piscataway, NJ).
Quantitative real-time polymerase chain reaction
Total RNA was extracted using TRIzol (Sigma, St. Louis, MO). Complementary DNA (cDNA) was synthesized from 0.25 μg of total RNA using a TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA) with hexamer primers following the protocol described by the manufacturer. Gene expression levels were measured using SYBR Green polymerase chain reaction (PCR) Reagents (Applied Biosystems). Primer pairs used for quantitative detection of gene expression are listed in Table 1. The quantity of messenger RNA (mRNA) was calculated by normalizing the threshold cycle value (Ct) of specific genes to the Ct of the housekeeping gene β-actin for mouse primary cells or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for UMR 106-01 cells.
Table 1.
Primer Sequences Used in QuantitativeRT-PCR
| Primer Sequence | |
|---|---|
| rMef2A | |
| Forward | CAAGCAAGGGCATGATGC |
| Reverse | GCGCTGGTCAATGAGTAATCAG |
| rMef2B | |
| Forward | CTCGCCTCACTTTCGACCC |
| Reverse | AGCTCCTCGATGTGCCCAGT |
| rMef2C | |
| Forward | GCAAGAACACAATGCCATCA |
| Reverse | ACTGGGGTAGCCAATGACTG |
| rMef2D | |
| Forward | CAACGGCCTAAACAAGGTCA |
| Reverse | GTGGTGAGCGAGTGGGTAGA |
| rMmp13 | |
| Forward | GCCCTATCCCTTGATGCCATT |
| Reverse | ACAGTTCAGGCTCAACCTGCTG |
| rGAPDH | |
| Forward | AACCCATCACCATCTTCCAGG |
| Reverse | GCCTTCTCCATGGTGGTGAA |
| mMef2C | |
| Forward | CTTCTGCCACTGGCCCAC |
| Reverse | GGGGTTGCCGTATCCATTC |
| mMmp13 | |
| Forward | GCCCTGATGTTTCCCATCTA |
| Reverse | TTTTGGGATGCTTAGGGTTG |
| mβ-Actin | |
| Forward | TCCTCCTGAGCGCAAGTACTCT |
| Reverse | CGGACTCATCGTACTCCTGC |
Site-directed mutagenesis
Mutations were synthesized using a double-stranded site-directed mutagenesis kit (Stratagene).
Luciferase assay
UMR 106-01 cells were seeded at a concentration of 1 × 105 cells/well in 12-well plates and transfected with 100 ng of Mmp13-Luc reporter promoter construct, 1 ng pRL-TK (for some experiments), and 50 ng pcDNA-Myc-Mef2c. The latter plasmid was kindly provided by Eric Olson (University of Texas Southwestern Medical Center). After 48 to 72 hours, the cells were treated with PTH for 6 hours. The lysates were analyzed for luciferase activity using the luciferase assay reagent (Promega). Luciferase activity was measured using an Optocomp II luminometer (MGM Instruments) or GloMax-multidetection System (Promega).
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were carried out using a ChIP assay kit (Upstate Biotechnology, Lake Placid, NY), following the supplier's instructions. UMR 106-01 cells were treated with 10−8 M rat PTH(1-34) for 4 hours and subsequently incubated in 1% formaldehyde for 10 minutes. The cells were then washed in ice-cold phosphate-buffered saline containing protease inhibitors and 1 mM PMSF and suspended in SDS-lysis buffer containing protease inhibitors and PMSF for 10 minutes on ice. The samples were sonicated on ice with four 10-second pulses with 20-second intervals. Following centrifugation for 10 minutes at 14,000g, the supernatants were collected and diluted with ChIP dilution buffer containing protease inhibitors and PMSF. For PCR analysis, aliquots (1:100) of total chromatin DNA before immunoprecipitation were saved (input). After preclearing with protein A/G-agarose beads, the supernatants were collected for overnight incubation at 4°C with the antibodies of interest. The beads were collected and sequentially washed with the following buffers: low-salt wash buffer, high-salt wash buffer, LiCl wash buffer, and twice with Tris-EDTA buffer. To elute DNA, samples were incubated in 200 μL of elution buffer (1% SDS and 0.1 M NaHCO3) for 1 hour at room temperature. The supernatant from each elution was collected and incubated at 65°C for at least 5 hours to reverse cross-links after the addition of 8 μL of 5 M NaCl. The next day, the samples were digested with RNase A and proteinase K at 45°C for 2 hours, and DNA was purified by phenol-chloroform extraction. The DNA was precipitated with 2.5 volumes of ethanol using 1 μL of 20 mg/mL glycogen as carrier. The input lysates were also processed as described previously. The DNA then was amplified using real-time PCR. The data obtained were analyzed using the equation, 2[(ΔCtIgG − CtInput) − (CtAb − CtInput)], where IgG is the normal IgG, Ab is the specific antibody, and Input is the input genomic DNA.
Gel shift assay
The DNA sequences of the oligonucleotides used for gel shift assays were as follows: RD site, GTTCTGCCACAAACCACACGTACGA; and AP-1 site, CCAAGTGGTGACTCATCACTAT.
The DNA oligonucleotides were labeled using a Biotin 3′ end DNA Labeling Kit (Pierce Biotechnology Inc.). The nuclear extracts from pcDNA-Myc-Mef2c-transfected UMR 106-01 cells (NE-PER nuclear and cytoplasmic extraction reagents; Pierce) and biotin-labeled DNA probe were incubated in binding buffer for 20 minutes at room temperature using a LightShift Chemiluminescent EMSA kit (Pierce Biotechnology Inc.). Protein-DNA complexes were separated on 6% polyacrylamide gels in 0.5× TBE buffer, and transferred onto Biodyne B Nylon membranes (GE Healthcare, Bronx, NY). The membrane was blocked in blocking buffer, washed five times with wash buffer, and visualized by a Chemiluminescent Nuclear Acid Detection Module (Pierce Biotechnology Inc.). Two hundred–fold molar excess of unlabeled Mmp13 promoter oligonucleotides were used as competitor DNA.
Statistical analysis
Statistical differences between means were calculated using Student t test or two-way analysis of variance using IBM SPSS (version 22, Armonk, NY). Results are expressed as mean ± standard error (SE) and P < 0.05 was considered significant.
Results
MEF2C is required for Mmp13 gene expression
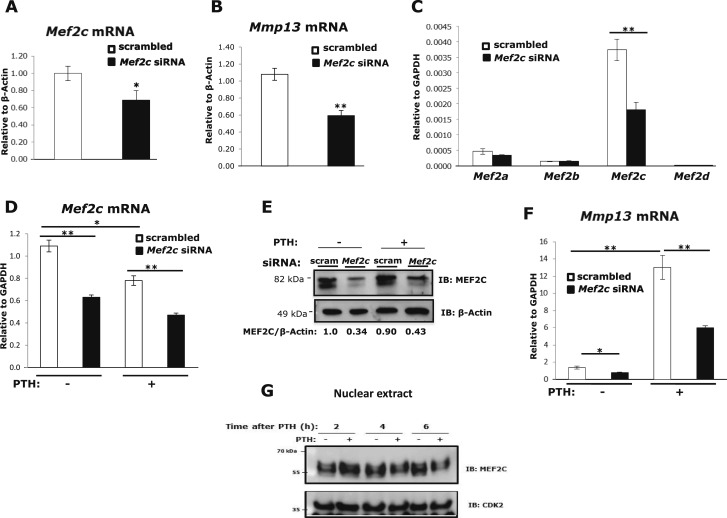
To examine whether MEF2C is required for Mmp13 gene expression, we performed knockdown by siRNA transfection in mouse primary calvarial osteoblasts. The 30% repression of Mef2c mRNA expression by siRNA (Fig. 1A) resulted in a 45% reduction in Mmp13 mRNA (Fig. 1B). These data suggest that MEF2C is necessary for basal Mmp13 mRNA expression in primary mouse osteoblasts. To investigate whether MEF2C participates in PTH-stimulated Mmp13 gene expression in osteoblastic cells, Mef2c expression was transiently knocked down in the rat osteoblastic osteosarcoma cell line, UMR 106-01, by siRNA transfection. First, experiments tested the efficiency of Mef2c siRNA to knock down Mef2c in these cells. Transfection of Mef2c siRNA significantly reduced Mef2c gene expression by approximately 50% but did not influence the amount of Mef2a, Mef2b, or Mef2d gene expression (Fig. 1C). Mef2c is the most highly expressed MEF2 member in these osteoblastic cells. Repression of Mef2c expression by siRNA was confirmed at both the RNA and the protein level; an average of 40% knockdown in Mef2c RNA (Fig. 1D) and more than 65% knockdown in protein expression (Fig. 1E) was observed. It is notable that PTH causes a decrease in Mef2c mRNA levels. In UMR cells transfected with Mef2c siRNA, the increase in Mmp13 mRNA in response to PTH was diminished by 55% compared with cells with control scrambled siRNA (Fig. 1F), but even the basal expression was decreased by Mef2c knockdown; thus, MEF2C appears to be necessary for basal and PTH-regulated Mmp13 mRNA expression and PTH regulates Mef2c expression. PTH treatment not only downregulated Mef2c mRNA (Fig. 1D), but also caused MEF2C proteins to migrate more slowly at 2 to 6 hours, likely indicating structural modification, and abundance decreased in the nucleus after 6 hours of PTH treatment (Fig. 1G).
Figure 1.
MEF2C is required for Mmp13 gene expression and PTH regulates Mef2c. Primary mouse osteoblastic cells were transfected with 60 nM Mef2c siRNA or scrambled siRNA. Total RNA was isolated and amplified via real-time reverse transcription PCR. Relative levels of (A) Mef2c or (B) Mmp13 mRNAs (mean ± SE) were normalized to β-actin. *P < 0.05, **P < 0.01 vs scrambled control, n = 3. (C) Expression of Mef2 RNAs after Mef2c knockdown. UMR 106-01 cells were transfected with 60 nM Mef2c siRNA or scrambled siRNA. Seventy-two hours after transfection, relative levels of the mRNAs (mean ± SE) were normalized to Gapdh. **P < 0.01 vs scrambled control, n = 3. (D) UMR 106-01 cells were transfected with Mef2c siRNA or scrambled siRNA. Cells were treated with 10−8 M PTH-(1-34) for 4 hours and collected for RNA analysis. Relative levels of Mef2c mRNA (mean ± SE) were normalized to Gapdh. *P < 0.05, **P < 0.01 vs control or PTH-treated scrambled set, n = 3. (E) Western blot of MEF2C. Cells were treated with PTH for 30 minutes and whole cell lysates were collected for protein expression analysis. Protein samples were separated using 8% SDS-PAGE and immunoblotted with antibodies against MEF2C and β-actin as indicated. (F) After transfection, cells were treated with PTH for 4 hours and collected for RNA analysis. Relative levels of Mmp13 mRNA were normalized to Gapdh. Data shown are mean ± SE. *P < 0.05, **P < 0.01 vs control or PTH-treated scrambled set, n = 3. (G) UMR 106-01 cells were treated with or without PTH at indicated times and nuclear extracts were collected for protein expression. Protein samples were separated using 8% SDS-PAGE and immunoblotted with antibodies against MEF2C and CDK2 as indicated.
MEF2C is necessary for Mmp13 promoter activity; binding of MEF2C to the Mmp13 promoter was transiently increased by PTH treatment
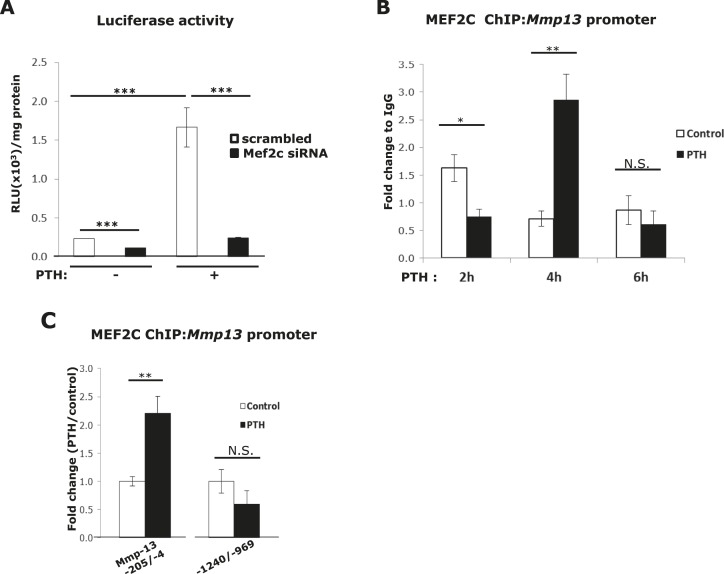
Mmp13 promoter reporter analysis was performed to determine if MEF2C would directly regulate Mmp13 promoter activity. In UMR 106-01 cells in which Mef2c expression was reduced by siRNA transfection, a substantial decrease was found in the promoter activity of Mmp13 (rat Mmp13; −148/+14) with or without treatment with PTH (Fig. 2A). Thus, MEF2C is necessary for both basal and PTH stimulation of Mmp13 gene transcription.
Figure 2.
MEF2C is necessary for Mmp13 basal and PTH-stimulated promoter activity and associates with the Mmp13 promoter. (A) UMR 106-01 cells were plated in 12-well plates and transfected with 100 ng of pGL2-Mmp13 (−148 rat Mmp13 promoter) and 60 nM of Mef2c siRNA or scrambled siRNA as indicated. Cells were treated with or without 10−8 M PTH-(1-34) for 6 hours. Firefly luciferase activity was measured, and relative light units (RLU) were normalized to the amount of total protein. Data shown are mean ± SE. ***P < 0.001 vs control or PTH-treated scrambled set, n = 3. (B) UMR 106-01 cells were treated with or without PTH for the indicated time, and collected for ChIP assays with antibodies against MEF2C, or IgG as a negative control. DNA was amplified by real-time PCR using primers for the rat Mmp13 proximal promoter region (−205/−4). Data shown are mean ± SE. *P < 0.05, **P < 0.01 vs control at each time point, n = 3. N.S., not significant. (C) The cells were treated with or without PTH for 4 hours and collected for ChIP assays with antibodies against MEF2C, or IgG as a negative control. The primers used for real-time PCR were from the Mmp13 proximal (−205/−4), or the distal (−1240/−969) promoter regions. Data shown are mean ± SE. **P < 0.01 vs control, n = 3.
To determine whether MEF2C could physically bind to the Mmp13 proximal promoter (−205/−4), we performed ChIP assays after 2, 4, and 6 hours of PTH treatment. We found that there was a cycle of initial decrease in binding at 2 hours followed by an increase at 4 hours, and then a return to lower basal levels at 6 hours (Fig. 2B). Figure 2C shows that increased binding of MEF2C was specific at the proximal promoter and was not observed at a distal (−1240/−969) region of MEF2C 969) region, which has no elements involved in PTH regulation and has been previously used by us as a negative control region for ChIP assays (13). In fact, Ct values at this latter site were in the 34 to 35 range, indicating minimal binding of MEF2C at this site.
MEF2C associates with the AP-1 site of the Mmp13 promoter
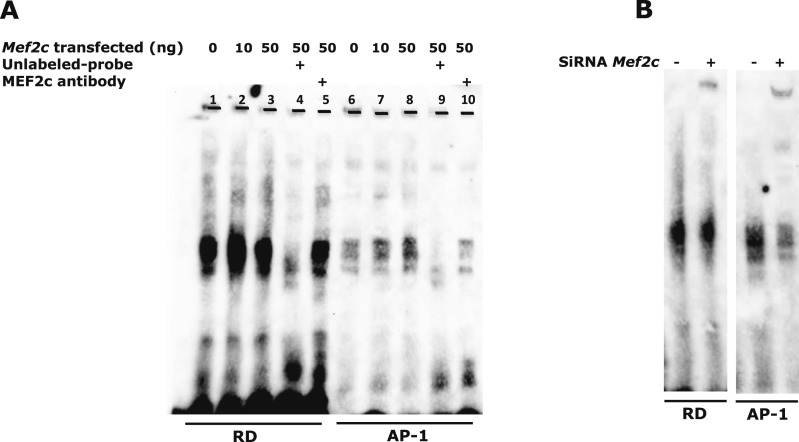
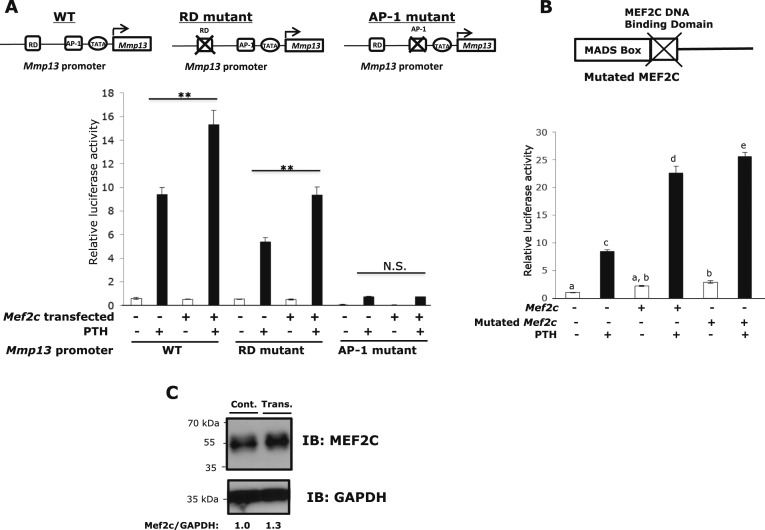
MEF2 factors activate transcription via binding to a consensus A/T-rich cis-element. Within the Mmp13 proximal promoter (−148/+14), the only A+T rich element is the TATA box (CTATAAAATAGA), but if we mutate this region, it is likely that all transcriptional activity will be abolished. Because the RD (−132/−128) and AP-1 (−48/−42) sites are essential elements in PTH-induced transcriptional activation of Mmp13 (9–11) and HDAC4 associates with this region, we determined whether MEF2C associates with the RD or AP-1 sites. To identify the MEF2C response element in the Mmp13 proximal promoter, nuclear extracts were prepared from PTH-treated and Mef2c-transfected UMR 106-01 cells, and electrophoretic gel mobility shift assays were performed with Biotin-labeled RD or AP-1 site probes. Figure 3A shows that the binding of MEF2C to the AP-1 site increased dose-dependently with amount of Mef2c transfected into UMR cells. The binding specificity of MEF2C to each site was examined by competing with excess unlabeled RD or AP-1 site oligonucleotide to the MEF2C-DNA complex. The antibody to MEF2C caused a substantial reduction of protein binding to the AP-1 site. On the other hand, it did not affect binding at the RD site (Fig. 3A). Ablation of Mef2c decreased association of factors with the AP-1 site (Fig. 3B). These results suggest that MEF2C associates with the AP-1 site and /or with other factors binding to this site and does not bind to the RD site or directly to Runx2 bound to the RD site, as far as can be assessed by electrophoretic gel mobility shift assay. We further tested the MEF2C response element by performing promoter-reporter assays and mutating the elements. UMR cells were transfected with or without Myc-Mef2c in the presence or absence of PTH. The promoter activity was enhanced in response to PTH and was even further activated with added MEF2C (160%). Two nucleotides of the consensus RD or AP-1 sequences were mutated (Table 2). In comparison with the wild-type, however, the RD mutant had reduced promoter activity (approximately 43% with PTH treatment). With added MEF2C, the activity was enhanced by the same amount as the wild-type promoter (170%). On the other hand, the AP-1 mutant had almost entirely abolished basal and PTH-stimulated activity and the activity was unable to be stimulated in the presence of MEF2C (Fig. 4A). To further examine whether MEF2C directly interacts with the Mmp13 promoter, the reporter assay was performed using MEF2C with the DNA binding domain mutated. Promoter activity continued to be enhanced, even with the mutation (Fig. 4B). This result suggests that MEF2C binds indirectly to the Mmp13 promoter and acts through the AP-1 site. Figure 4C shows MEF2C protein levels with and without transfection in UMR 106-01 cells.
Figure 3.
MEF2C associates with the AP-1 site on the Mmp13 promoter. (A) The nuclear extracts from Mef2c-transfected UMR 106-01 cells treated with 10−8 M PTH (1-34) for 2 hours were subjected to gel mobility shift analyses using biotin-labeled oligomers corresponding to RD or AP-1 sequences of the rat Mmp13 promoter as shown in Table 1. Binding specificity of the complex was verified in competition experiments performed with a 200-fold molar excess of unlabeled oligomers (lanes 4 and 9). An antibody against MEF2C was added to the incubations in lanes 5 and 10. (B) UMR 106-01 cells were transfected with 60 nM Mef2c siRNA or scrambled siRNA. The nuclear extracts from the cells treated with PTH for 2 hours were subjected to gel mobility shift analysis.
Table 2.
Primer Sequences Used in Luciferase Assay
| Site | Primer Sequences |
|---|---|
| Wild-type RD | GTTCTGCCACAAACCACACGTACGA |
| CAAGACGGTGTTTGGTGTGCATGCT | |
| Mutant RD | GTTCTGCCACAACTAACACGTACGA |
| CAAGACGGTGTTGATTGTGCATGCT | |
| Wild-type AP-1 | CCAAGTGGTGACTCATCACTAT |
| GGTTCACCACTGAGTAGTGATA | |
| Mutant AP-1 | CCAAGTGGACTCTCATCACTAT |
| GGTTCACCTGAGAGTAGTGATA | |
| Mutated MEF2C | GTACACCGAGTACAATAGGCCGCACGAGAG |
| CTCTCGTGCGGCCTATTGTACTCGGTGTAC |
Figure 4.
MEF2C stimulates the Mmp13 promoter through the AP-1 site. (A) UMR 106-01 cells were plated in 12-well plates and transfected with 100 ng of pGL2-Mmp13 (wild-type) or RD, or AP-1 mutant promoters and 50 ng of Mef2c constructs as indicated. Firefly luciferase activity was measured after 6 hours of PTH treatment, and relative light units (RLU) were normalized with Renilla luciferase activity. Primer sequences used are shown in Table 2. Data shown are mean ± SE. **P < 0.01 vs PTH treated control in each set, n = 3. (B) UMR 106-01 cells were plated in 12-well plates and transfected with 100 ng of pGL2-Mmp13 (wild-type) promoters and Mef2c or Mef2c with a mutated DNA binding domain construct as indicated. The cells were treated with or without PTH for 6 hours. Data shown are mean ± SE. Different letters indicate P < 0.05 vs one another. (C) UMR 106-01 cells were transfected with or without Mef2c constructs. Protein samples (whole cell lysates) were separated using 8% SDS-PAGE and immunoblotted with antibodies against MEF2C and GAPDH as indicated.
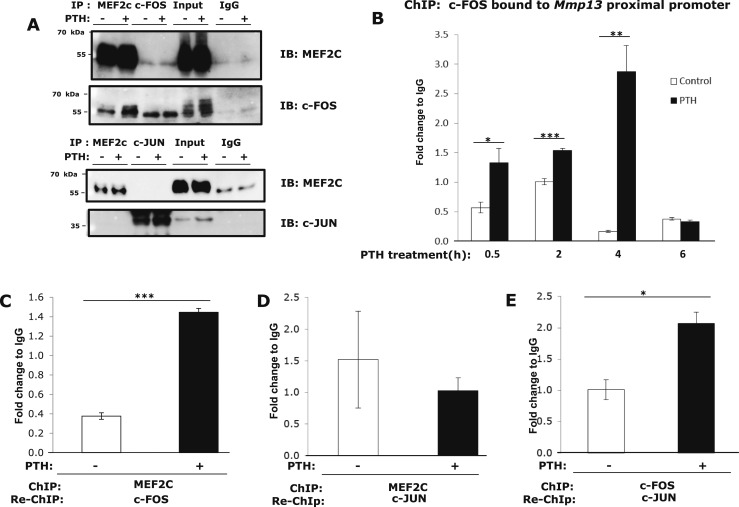
MEF2C binds c-FOS on the Mmp13 promoter after4 hours of PTH treatment
The AP-1 site binds dimers of the FOS and JUN families. Previously, our laboratory showed that PTH induced c-fos and c-jun transcription and increased binding of these at the AP-1 site, and association with Runx2 already bound to the RD (9, 11). Here, we found that MEF2C coimmunoprecipitated with c-FOS, but not c-JUN (Fig. 5A). Overall, our results indicate that MEF2C associates with the AP-1 site of the Mmp13 promoter; this effect appears to be through interaction with c-FOS. To determine alterations of c-FOS interaction with the Mmp13 promoter region after PTH treatment, we performed ChIP assays after 0.5, 2, 4, and 6 hours of hormone treatment. We found that binding of c-FOS to the Mmp13 promoter increased and reached a peak at 4 hours and returned to basal lower levels by 6 hours (Fig. 5B). We next performed ChIP-reChIP experiments to determine if MEF2C associates with c-FOS on the Mmp13 promoter region and if the interactions are influenced by PTH treatment. The reChIP result showed that PTH treatment for 4 hours increased the association of endogenous MEF2C and c-FOS at the proximal Mmp13 promoter region (Fig. 5C). On the other hand, ChIP-reChIP showed that PTH treatment did not change the association of MEF2C and c-JUN (Fig. 5D), whereas association of c-FOS and c-JUN was increased (Fig. 5E), again suggesting that association of MEF2C and c-JUN is indirect and showing that c-FOS and c-JUN proteins form a complex at the Mmp13 promoter. These results indicate that, after PTH treatment, MEF2C binds to c-FOS at the AP-1 site of the Mmp13 promoter and this is substantially increased over basal.
Figure 5.
MEF2C associates with c-FOS on the Mmp13 promoter after PTH treatment in osteoblastic UMR 106-01 cells. (A) UMR 106-01 cells were treated with or without 10−8 M PTH-(1-34) for 1 hour, and lysates were immunoprecipitated (IP) with anti-MEF2C, c-FOS, c-JUN, or normal IgG (Santa Cruz), then immunoblotted with anti-MEF2C-FOS, c-JUN. (B) UMR 106-01 cells were treated with or without PTH for the indicated times and collected for ChIP assays with antibodies against c-FOS, or IgG as a negative control. DNA was amplified by real-time PCR using primers for the rat Mmp13 proximal promoter region. Data shown are mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs control in each set, n = 3. (C) UMR 106-01 cells were treated with or without PTH for 4 hours. Cell lysates were collected for ChIP-reChIP assays with antibodies against MEF2C or IgG as a negative control for the first ChIP, and antibodies to c-FOS or IgG were used for the second immunoprecipitation. DNA was amplified by real-time PCR using primers for the rat Mmp13 proximal promoter region. Data shown are mean ± SE. ***P < 0.001 vs control, n = 3. (D) Cell lysates were collected for ChIP-reChIP assays with antibodies against MEF2C or IgG as a negative control for the first ChIP, and antibodies to c-JUN or IgG were used for the second immunoprecipitation. (E) Cell lysates were collected for ChIP-reChIP assays with antibodies against c-FOS or IgG as a negative control for the first ChIP, and antibodies to c-JUN or IgG were used for the second immunoprecipitation. Data shown are mean ± SE. *P < 0.05 vs control, n = 3.
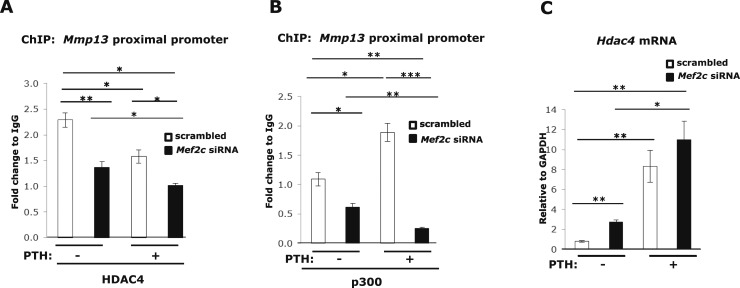
MEF2C is involved with binding of HDAC4 and p300 at the Mmp13 promoter
Because cofactors HDAC4 and p300, which act as transcriptional repressor and activator, respectively, have been reported to interact with the MADS/MEF2 domain of MEF2, we investigated whether HDAC4 or p300 associates with MEF2C at the Mmp13 promoter and are affected by PTH treatment. Repression of Mef2c expression by siRNA decreased HDAC4 (Fig. 6A) or p300 (Fig. 6B) association with the Mmp13 promoter using ChIP assays. These results demonstrate that HDAC4 or p300 bind to the Mmp13 promoter and MEF2C is involved with this association. Because MEF2C does not appear to bind to the RD site of the Mmp13 promoter by gel shift and promoter analyses (Figs. 3 and 4) and yet its knockdown affects HDAC4 association, we conclude that MEF2C stabilizes the complex of HDAC4 or p300 association with RUNX2. Interestingly, MEF2C seems to inhibit Hdac4 gene expression (Fig. 6C), because this is increased with Mef2c knockdown.
Figure 6.
MEF2C is necessary for HDAC4 and p300 binding to the Mmp13 promoter and negatively regulates HDAC4 expression. UMR 106-01 cells were transfected with 60 nM Mef2c siRNA or scrambled siRNA. Cells were treated with 10−8 M PTH-(1-34) for 4 hours and collected for ChIP assays or RNA analysis. ChIP assays were performed with antibodies against (A) HDAC4, (B) p300, or IgG as a negative control. DNA was amplified by real-time PCR using primers for the rat Mmp13 proximal promoter region. Data shown are mean ± SE. * P < 0.05, ** P < 0.01, *** P < 0.001 between the indicated set, n = 3. (C) Total RNA was isolated and amplified via real-time reverse transcription PCR. Relative levels of Hdac4 mRNA were normalized to Gapdh. Data shown are mean ± SE. *P < 0.05, **P < 0.01 between the indicated set, n = 3.
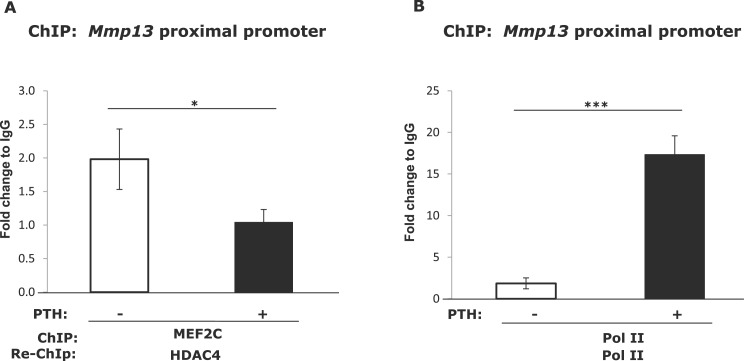
MEF2C and HDAC4 exist as a complex on the Mmp13 promoter, which interaction was decreased after PTH treatment
Because MEF2C has been shown to be repressed by its association with HDAC4 in cardiomyocytes (17) and chondrocytes (21) and HDAC4 represses Mmp13 promoter activity (12), we investigated if this association also occurred on the Mmp13 promoter in osteoblastic cells. We performed ChIP-reChIP experiments to determine if MEF2C associates with HDAC4 on the Mmp13 promoter region and if the interactions are influenced by PTH treatment. The cross-linked chromatin from untreated or PTH-treated cells was immunoprecipitated with the first antibody (MEF2C); the resulting immunocomplex was then eluted and further subjected to immunoprecipitation with the second antibody (HDAC4). The reChIP result indicates that endogenous MEF2C associates with HDAC4 at the proximal Mmp13 promoter region under basal conditions and after PTH treatment this association was decreased (Fig. 7A). These results showed the simultaneous recruitment of MEF2C and HDAC4 to the Mmp13 proximal promoter region and after PTH treatment, HDAC4 dissociates from MEF2C. The binding of RNA pol II, a positive control for active promoters, was increased after PTH treatment (Fig. 7B). These results are similar to our previous observations that HDAC4 also associates with Runx2 on the Mmp13 proximal promoter and this association declines with PTH treatment (12).
Figure 7.
After PTH treatment (4 hours), the binding between MEF2C and HDAC4 to the Mmp13 promoter is decreased. UMR 106-01 cells were treated with or without 10−8 M PTH for 4 hours. (A) Cell lysates were collected for ChIP-reChIP assays with antibody against MEF2C, or IgG as a negative control for the first ChIP, and antibody to HDAC4 or IgG was used for the second immunoprecipitation. DNA was amplified by real-time PCR using primers for the rat Mmp13 proximal promoter region. Data shown are mean ± SE. *P < 0.05 vs control, n = 3. (B) ChIP-reChIP assays with antibody against Pol II, or IgG as a negative control for the first and second ChIPs. DNA was amplified by real-time PCR using primers for rat Mmp13 proximal promoter region. Data shown are mean ± SE. ***P < 0.001 vs control, n = 3.
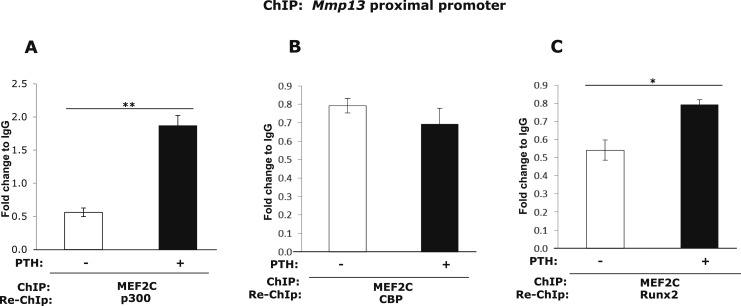
The interaction of MEF2C and p300 on the Mmp13 promoter was increased after PTH treatment
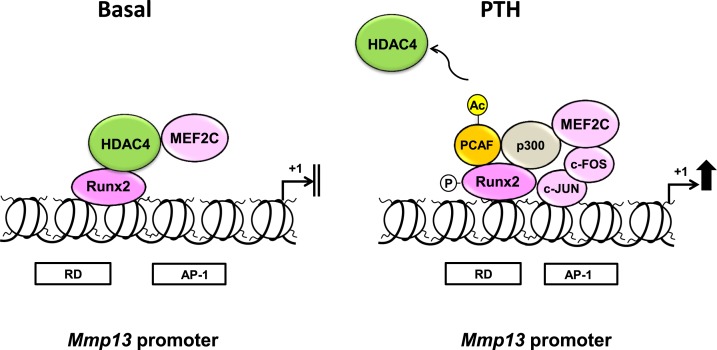
It is known that once released from HDACs, MEF2C is then able to associate with the coactivator, p300, which stimulates MEF2-dependent genes (20). To investigate if the interaction between MEF2C and p300 are changed by PTH treatment on the Mmp13 promoter in osteoblastic cells, we performed ChIP-reChIP experiments. The reChIP result shows that the association between MEF2C and p300 on the Mmp13 promoter was strongly increased after PTH treatment (Fig. 8A). However, the association of MEF2C with another histone acetyltransferase that is closely related to p300, CBP, was not changed after PTH treatment (Fig. 8B). Last, the association with Runx2 was poor and slightly increased by PTH treatment (Fig. 8C). Thus, PTH causes release of HDAC4 from both Runx2 and MEF2C and p300 is recruited to bind both transcription factors on the Mmp13 proximal promoter (Fig. 9).
Figure 8.
After PTH treatment (4 hours), the binding between MEF2C and p300 and Runx2 to the Mmp13 promoter is increased. UMR 106-01 cells were treated with or without 10−8 M PTH for 4 hours. Cell lysates were collected for ChIP-reChIP assays with antibody against MEF2C, or IgG as a negative control for the first ChIP, and antibody to (A) p300, (B) CBP, (C) Runx2, or IgG was used for the second immunoprecipitation. DNA was amplified by real-time PCR using primers for the rat Mmp13 proximal promoter region. Data shown are mean ± SE. *P < 0.05, **P < 0.01 vs control, n = 3.
Figure 9.
Proposed model depicting the mechanism of activation of Mmp13 promoter after PTH treatment. Under basal conditions, Runx2 is bound to the RD of the Mmp13 promoter and associated with HDAC4, resulting in a repression of transcription in osteoblastic cells. MEF2C is also associated with HDAC4 at the Mmp13 promoter. After PTH treatment, HDAC4 dissociates from Runx2 and MEF2C and the latter associates with c-FOS at the AP-1 site, the complex is then free to recruit HATs, especially p300 and P/CAF to activate transcription. MEF2C also associates with p300.
Discussion
In this study, we have shown that MEF2C participates in PTH-stimulated Mmp13 gene expression in osteoblastic cells by increased binding to the Mmp13 promoter. The region of MEF2C association is the AP-1 site of the Mmp13 promoter and this is mediated through binding c-FOS.
MMP13 is expressed by both terminal hypertrophic chondrocytes and osteoblasts and is necessary for bone remodeling (23). Mmp-13 gene expression is highly stimulated by PTH in osteoblastic UMR 106-01 cells and primary osteoblasts (5, 6), as well as in vivo (7). Previously, our laboratory has shown that there are PTH-response elements, RD and AP-1 sites (the binding sites for Runx2 and c-FOS/c-JUN, respectively), in the Mmp13 promoter. Under basal conditions, HDAC4 binds Runx2 at the RD site, resulting in a repression of transcription. After PTH treatment, HDAC4 is phosphorylated and dissociates from Runx2 (12, 24), which is then free to recruit HATs, especially p300, to activate transcription (13). In addition to p300 and Runx2, another HAT, p300/P/CAF associates with p300 and Runx2 is required for PTH activation of Mmp13 transcription (25). Many factors are involved in this “system,” but the detailed mechanism is still not clear.
MEF2 proteins were originally studied in their role as transcription regulators of myogenic cells that interact with other transcriptional factors. MEF2C is one of a family of transcription factors important in muscle and cardiovascular development. However, it was reported that MEF2C controls bone development and chondrocyte hypertrophy (14), adult bone mass by regulation of osteoclastic bone resorption (16), and MEF2C is expressed in osteoblastic cells (UMR 106-01) and is a key osteoblast transcription factor, upstream of Runx2 and osterix (17).
To determine whether MEF2C participates in PTH-stimulated Mmp13 gene expression in osteoblastic cells, we performed inhibition of endogenous MEF2C activity by use of siRNA molecules. We found that decreased endogenous MEF2C leads to a decrease in both basal and PTH-stimulated Mmp13 gene expression (Fig. 1B and 1F) and, in particular, appears to be required for the PTH regulation of the Mmp13 promoter (Fig. 2A). ChIP assays showed that MEF2C binding to the Mmp13 promoter is increased after 4 hours of PTH treatment. In addition, ChIP-reChIP assays demonstrated that the interaction between MEF2C and HDAC4 at the Mmp13 promoter is decreased after PTH treatment (4 hours), whereas the association with p300 is increased.
MEF2 proteins contain C-terminus transcriptional activation domains. MEF2C associates with a variety of transcriptional cofactors. Some cofactors stimulate MEF2 activity, whereas some factors repress MEF2 function. Class II HDACs bind the C-terminus domain and function as repressors (22). The N-terminal extension of class II HDACs (HDAC4, 5, 7, and 9) contains binding sites for MEF2 and the binding motif is conserved from Caenorhabditis elegans to mammals (20). It is known that HDAC4 associates with MEF2C and represses its activity in cardiomyocytes. Calcium/calmodulin-dependent protein kinase forces HDAC4 to translocate to the cytosol, thereby activating MEF2C, whereas protein kinase A (PKA) triggers proteolytic cleavage of HDAC4, generating a repressor of MEF2C (18). It is known that, similar to HDAC4, the transcription activator and HAT, p300, associates with the MADS/MEF2 domain of MEF2 (25). After release of HDACs, MEF2 associates with the p300 coactivator and then stimulates MEF2-dependent genes. Indeed, we showed that, after PTH treatment, the association of p300 with MEF2C was increased on the Mmp13 promoter. MEF2C has been demonstrated to have a role in regulating other PTH-regulated genes, in particular sclerostin (encoded by the gene SOST). MEF2C controls SOST gene expression via binding to the SOST distal enhancer in UMR 106-01 cells (20). PTH signaling appears to also influence another class IIa HDAC, HDAC5. Baertschi et al. (26) report that PTH stimulates HDAC5 dephosphorylation, nuclear translocation, and inhibition of SOST bone enhancer activity by masking MEF2C in UMR 106-01 cells. Also, Wein et al. (27) report that HDAC5 deficiency increases SOST enhancer MEF2C chromatin association using the osteocytic cell line, Ocy454.
To determine the mechanism of MEF2C action, we investigated the MEF2 response element on the Mmp13 promoter after PTH treatment. Previous work from this laboratory has shown that the PTH-responsive region is within the first 148 base pairs upstream of the transcriptional start site (9). In the present paper, the reporter assay using the Mmp13 promoter construct (−148/+14) shows that siRNA for MEF2C suppressed Mmp13 promoter activity in UMR 106-01 cells. Thus, the MEF2C association site in response to PTH should also be within this region. It is known that MEF2 proteins bind to a consensus DNA-binding element [A/T-rich DNA sequence; CTA (A/T)4 TAG/A] as homo- and heterodimers and interact with other transcription factors to function as both positive and negative regulators of gene expression, in part through their association with class II HDACs (25, 28). Within the Mmp13 promoter reporter (−148/+14), the only A+T rich element is the TATA box (CTATAAAATAGA). Several studies have shown that overlapping sites for MEF2 and the TATA-binding site are contained in the mouse MRF4 promoter (29, 30), the Xenopus MyoDa promoter (31), and MEF2C and MEF2D can activate transcription of the XMyoDa promoter by binding to the TATA motif (32). We examined whether MEF2C would bind to the TATA-binding site of the Mmp13 promoter but could not confirm it using gel shifts (data not shown). We observed that the association of MEF2C was increased dose-dependently at the AP-1 site using AP-1 site oligo nucleotides (Fig. 4A). Further, the introduction of a mutation in the AP-1 site abolished Mmp13 promoter activity stimulation by MEF2C (Fig. 5). These results indicate that MEF2C associates with factors binding to the AP-1 site and/or MEF2C binds to the AP-1 site for PTH stimulation of the rat Mmp13 promoter. It is notable that MEF2C does not appear to bind or act through RUNX2 bound to the RD. However, mutation of the DNA-binding domain of MEF2C did not abolish its enhancement of promoter activity, indicating it is acting through binding to another protein at the AP-1 site. UMR 106-01 cells contain a number of factors to activate the Mmp13 promoter in response to PTH (Figs. 2 and 6). There is a likelihood that MEF2C associates at the AP-1 site with some of these. The AP-1 site [TGA(C/G)/TCA] in the human, mouse, and rat Mmp13 promoters is a target for AP-1–mediated activation of transcription (33–35). The AP-1 site binds dimers of the FOS and JUN families. We and others have also shown that c-Fos is a primary gene for transcriptional activation of matrix metalloproteinases (34, 36). From the results of the current study, there is a likelihood that MEF2C binds to the AP-1 site of the Mmp13 promoter through binding c-FOS. In fact, we have demonstrated that MEF2C coimmunoprecipitates with c-FOS but not with c-JUN. The amount of MEF2C that coimmunoprecipitates with c-FOS is small. In UMR 106-01 cells, MEF2C is abundant but the ratio of association between MEF2C and c-FOS of the total MEF2C might be low. On the other hand, we could clearly see the relationship between MEF2C and c-FOS from immunoprecipitation of MEF2C and immunoblot of c-FOS. Enhanced Mmp13 transcriptional activity was maintained using MEF2C with a DNA binding domain mutation in the reporter assay. Together, these data suggest that MEF2C binds indirectly to the Mmp13 promoter; this result is consistent with our hypothesis that MEF2C binds to the promoter through c-FOS. We further showed that the association of MEF2C and c-FOS but not with c-JUN with the Mmp13 promoter was increased in response to PTH in the reChIP assays. There are several instances in which a protein interacts with one of these two proteins and not the other. Recently, we have reported that SIRT1 specifically associates with c-JUN on the Mmp13 promoter, but not with c-FOS (37). Similar to our observations with MEF2C, MafB has been shown to interact with c-FOS and not c-JUN (38).
Previous work in our laboratory has shown increased binding of c-FOS and c-JUN at the AP-1 site in response to PTH, but no substantial change in the amount of Runx2 binding to the RD site (9). It has been demonstrated that Cbfa/Runt proteins interact directly with c-JUN and c-FOS (39). PTH induces c-fos and c-jun transcription, newly synthesized FOS and JUN then occupy the AP-1 site of the Mmp13 promoter (9) and associate with Runx2 already bound to the RD (9, 11). PTH also induces p300 HAT activity and recruits this enzyme plus P/CAF to Mmp13 promoter-bound Runx2 (13). The distance between the RD and AP-1 sites is only 76 base pairs, so it is possible that MEF2C attaches to the Runx2/p300/PCAF complex; this might increase after PTH treatment because of increasing MEF2C binding to c-FOS at the AP-1 site. Here, we showed that HDAC4 interacts with MEF2C on the Mmp13 promoter. Although it indicates the possibility that HDAC4 is bound to both MEF2C and Runx2, the mechanism remains to be determined. We conclude that, in response to PTH, MEF2C increased binding of c-FOS and p300 directly or indirectly with Runx2 on the Mmp13 promoter. We speculate that MEF2C stabilizes the association of HDAC4 or p300 with Runx2. Many factors seem to be needed in the complex to regulate Mmp13 gene expression and deletion of one may destabilize the complex.
Our data also showed that PTH regulated Mef2c mRNA and protein levels. MEF2C protein migrated more slowly at 2 to 6 hours after PTH treatment and showed some degradation at 6 hours. Others have shown that Western blot for MEF2C yields two bands (40), possibly reflecting alternative spliced variants of MEF2C (41), phosphorylation (20), or sumoylation (42). Several papers have shown MEF2C phosphorylation results in positive or negative regulation. MEF2C is activated by extracellular signal-regulated kinase 5 and p38 mitogen-activated protein kinases via phosphorylation (Thr293, Thr300, and Ser387 in MEF2C) (43–45). On the other hand, MEF2C is degraded after phosphorylation (Ser98 and Ser110) during cell-cycle progression (46). PKA directly phosphorylates MEF2 (Thr20) to increase MEF2 DNA binding activity and support neuronal survival (47). PKA also induces the generation of an N-terminal HDAC4 cleavage product (∼28 kDa), which selectively inhibits MEF2 activity (18). Our laboratory showed that PTH induces the PKA-dependent phosphorylation of HDAC4 in the nucleus of UMR 106-01 cells. This leads to its release from Runx2 bound to the Mmp13 promoter, and HDAC4 migrates from the nucleus to cytoplasm and is partially degraded (24). There is a possibility that PTH regulates MEF2C through PKA, but this will require further investigation.
We have been investigating the mechanism of Mmp13 activation by PTH and found that PTH induces Fos and Jun synthesis, which then occupy the AP-1 site of the Mmp13 promoter (9) and associate with Runx2, which is already bound to the RD (11). Runx2 recruits the HAT, p300, which then acetylates histone H4 and H3 (13). Another HAT, P/CAF cooperates with p300 (its acetylation is increased by PTH treatment) and Runx2 to mediate PTH activation of Mmp13 transcription (14). Many factors are involved in this system for regulation of Mmp13 gene expression by PTH. In the current paper, we investigated whether MEF2C also participates in this process. Here, we showed that MEF2C is required for Mmp13 gene expression in response to PTH in osteoblastic cells and associates with c-FOS, activating transcription through the AP-1 site of the Mmp13 promoter. In addition, PTH causes increased association of MEF2C with p300 while decreasing association with HDAC4 on the Mmp13 promoter. This complex then elicits transcription of the gene. Based on this work, we propose a model for Mmp13 upregulation by PTH in osteoblastic cells (UMR 106-01) (Fig. 9). Because of the transient increase in MEF2C and c-FOS binding to the Mmp13 promoter and the evidence that MEF2C seems to be degraded in response to PTH, we hypothesize that the complex is transient and upon completion of its function, the complex quickly dissociates and returns to basal conditions.
Acknowledgments
We thank Dr. Eric Olson for kindly providing pcDNA-Myc-Mef2c, Dr. Malvin Janal for advice on statistical analyses, and Florante Ricarte for advice.
Financial Support: This work was funded by National Institutes of Health Grant DK047420 (to N.C.P.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| RRID | Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | Purpose |
|---|---|---|---|---|---|---|---|
| AB_2650492 | Synthetic peptide within Human MEF2C aa 400 to the C-terminus. | MEF2C | Abcam, ab191428 | Rabbit; monoclonal | ×2000 | WB | |
| AB_213451 | Synthetic peptide corresponding to amino acids 4-17 of human c-Fos | c-FOS | Calbiochem (Millipore) PC05L | Rabbit; polyclonal | ×500 | WB | |
| AB_397716 | Mouse c-Jun aa. 26-175 | c-JUN | BD transduction, 610326 | Mouse;monoclonal | ×2000 | WB | |
| AB_6131215 | C-terminus of Cdk2 of human origin | Cdk2 | Santa Cruz, sc-163 | Rabbit;polyclonal | ×2000 | WB | |
| AB_330288 | Synthetic peptide corresponding to amino-terminal residues of human β-actin | b-Actin | Cell Signaling, 4967 | Rabbit; polyclonal | ×3000 | WB | |
| AB_10622025 | Synthetic peptide near the C-terminus of human GAPDH | GAPDH | Cell Signaling, 5174 | Rabbit; monoclonal | ×2000 | WB | |
| AB_2250567 | An internal region of MEF2C of human origin | MEF2C | Santa Cruz, sc-13266 | Goat; polyclonal | 10 μL for 700 μg protein | IP | |
| AB_2106765 | C-terminus of c-FOS of human origin | c-FOS | Santa Cruz, sc-7202 | Rabbit; polyclonal | IP | ||
| AB_397716 | Mouse c-Jun aa. 26-175 | c-JUN | BD transduction, 610327 | Mouse; monoclonal | IP | ||
| AB_737197 | Donkey antigoat IgG-HRP | Santa Cruz, sc-2027 | IP | ||||
| AB_2250567 | Internal region of MEF2C of human origin | MEF2C | Santa Cruz, sc-13266 | Goat; polyclonal | ×80 | ChIP assay | |
| AB_2106765 | C-terminus of c-FOS of human origin | c-FOS | Santa Cruz, sc-7202 | Rabbit; polyclonal | ×80 | ChIP assay | |
| AB_2118872 | Amino acids 530-631 of HDAC4 of human origin | HDAC4 | Santa Cruz, sc-11418 | Rabbit; polyclonal | ×80 | ChIP assay | |
| AB_631006 | Peptide mapping at the N-terminus of CBP of human origin | CBP | Santa Cruz, sc-369 | Rabbit; polyclonal | ×80 | ChIP assay | |
| AB_2184247 | Amino acids 294-363 of RUNX2 of mouse origin | Runx2 | Santa Cruz, sc-10758 | Rabbit; polyclonal | ×80 | ChIP assay | |
| AB_309852 | Linear peptide corresponding to human RNA polymerase II | Pol II | Millipore, 05-623 | Mouse; monoclonal | ×250 | ChIP assay | |
| AB_309670 | GST fusion to the C-terminus of human p300 | p300 | Millipore, 05-257 | Mouse; monoclonal | ×250 | ChIP assay | |
| AB_397716 | Mouse c-Jun aa. 26-175 | c-JUN | BD transduction, 610327 | Mouse; monoclonal | ×80 | ChIP assay |
Abbreviations: HRP, horseradish peroxidase; IP, immunoprecipitation; RRID, Research Resource Identifier; WB, western blotting.
Footnotes
- AP-1
- activator protein 1
- CBP
- p300/CREB binding protein
- cDNA
- complementary DNA
- ChIP
- chromatin immunoprecipitation
- Ct
- threshold cycle
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- HAT
- histone acetyltransferase
- HDAC4
- histone deacetylase 4
- IgG
- immunoglobulin G
- MEF2C
- myocyte-specific enhancer factor 2c
- MEM
- modified Eagle medium
- MMP
- matrix metalloproteinase
- mRNA
- messenger RNA
- PAGE
- polyacrylamide gel electrophoresis
- P/CAF
- p300/CBP-associated factor
- PCR
- polymerase chain reaction
- PKA
- protein kinase A
- PMSF
- phenylmethylsulfonyl fluoride
- PTH
- parathyroid hormone
- RANKL
- receptor activator of NF-κB ligand
- RD
- runt domain
- Runx2
- runt-related transcription factor 2
- SDS
- sodium dodecyl sulfate
- SE
- standard error
- siRNA
- small interfering RNA.
References
- 1.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. [DOI] [PubMed] [Google Scholar]
- 2.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95(7):3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ståhle-Bäckdahl M, Sandstedt B, Bruce K, Lindahl A, Jiménez MG, Vega JA, López-Otín C. Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab Invest. 1997;76(5):717–728. [PubMed] [Google Scholar]
- 4.Johansson N, Saarialho-Kere U, Airola K, Herva R, Nissinen L, Westermarck J, Vuorio E, Heino J, Kähäri VM. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev Dyn. 1997;208(3):387–397. [DOI] [PubMed] [Google Scholar]
- 5.Scott DK, Brakenhoff KD, Clohisy JC, Quinn CO, Partridge NC. Parathyroid hormone induces transcription of collagenase in rat osteoblastic cells by a mechanism using cyclic adenosine 3′,5′-monophosphate and requiring protein synthesis. Mol Endocrinol. 1992;6(12):2153–2159. [DOI] [PubMed] [Google Scholar]
- 6.Winchester SK, Bloch SR, Fiacco GJ, Partridge NC. Regulation of expression of collagenase-3 in normal, differentiating rat osteoblasts. J Cell Physiol. 1999;181(3):479–488. [DOI] [PubMed] [Google Scholar]
- 7.Porte D, Tuckermann J, Becker M, Baumann B, Teurich S, Higgins T, Owen MJ, Schorpp-Kistner M, Angel P. Both AP-1 and Cbfa1-like factors are required for the induction of interstitial collagenase by parathyroid hormone. Oncogene. 1999;18(3):667–678. [DOI] [PubMed] [Google Scholar]
- 8.Zhao W, Byrne MH, Boyce BF, Krane SM. Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest. 1999;103(4):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvamurugan N, Chou WY, Pearman AT, Pulumati MR, Partridge NC. Parathyroid hormone regulates the rat collagenase-3 promoter in osteoblastic cells through the cooperative interaction of the activator protein-1 site and the runt domain binding sequence. J Biol Chem. 1998;273(17):10647–10657. [DOI] [PubMed] [Google Scholar]
- 10.Winchester SK, Selvamurugan N, D’Alonzo RC, Partridge NC. Developmental regulation of collagenase-3 mRNA in normal, differentiating osteoblasts through the activator protein-1 and the runt domain binding sites. J Biol Chem. 2000;275(30):23310–23318. [DOI] [PubMed] [Google Scholar]
- 11.D’Alonzo RC, Selvamurugan N, Karsenty G, Partridge NC. Physical interaction of the activator protein-1 factors c-Fos and c-Jun with Cbfa1 for collagenase-3 promoter activation. J Biol Chem. 2002;277(1):816–822. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu E, Selvamurugan N, Westendorf JJ, Olson EN, Partridge NC. HDAC4 represses matrix metalloproteinase-13 transcription in osteoblastic cells, and parathyroid hormone controls this repression. J Biol Chem. 2010;285(13):9616–9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boumah CE, Lee M, Selvamurugan N, Shimizu E, Partridge NC. Runx2 recruits p300 to mediate parathyroid hormone’s effects on histone acetylation and transcriptional activation of the matrix metalloproteinase-13 gene. Mol Endocrinol. 2009;23(8):1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Partridge NC. Parathyroid hormone activation of matrix metalloproteinase-13 transcription requires the histone acetyltransferase activity of p300 and PCAF and p300-dependent acetylation of PCAF. J Biol Chem. 2010;285(49):38014–38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12(3):377–389. [DOI] [PubMed] [Google Scholar]
- 16.Kramer I, Baertschi S, Halleux C, Keller H, Kneissel M. Mef2c deletion in osteocytes results in increased bone mass. J Bone Miner Res. 2012;27(2):360–373. [DOI] [PubMed] [Google Scholar]
- 17.Stephens AS, Stephens SR, Hobbs C, Hutmacher DW, Bacic-Welsh D, Woodruff MA, Morrison NA. Myocyte enhancer factor 2c, an osteoblast transcription factor identified by dimethyl sulfoxide (DMSO)-enhanced mineralization. J Biol Chem. 2011;286(34):30071–30086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Backs J, Worst BC, Lehmann LH, Patrick DM, Jebessa Z, Kreusser MM, Sun Q, Chen L, Heft C, Katus HA, Olson EN. Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J Cell Biol. 2011;195(3):403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134(23):4131–4140. [DOI] [PubMed] [Google Scholar]
- 20.McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci. 2002;27(1):40–47. [DOI] [PubMed] [Google Scholar]
- 21.Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res. 2007;22(12):1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9(3):206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131(23):5883–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu E, Nakatani T, He Z, Partridge NC. Parathyroid hormone regulates histone deacetylase (HDAC) 4 through protein kinase A-mediated phosphorylation and dephosphorylation in osteoblastic cells. J Biol Chem. 2014;289(31):21340–21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinsey TA, Zhang CL, Olson EN. Identification of a signal-responsive nuclear export sequence in class II histone deacetylases. Mol Cell Biol. 2001;21(18):6312–6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baertschi S, Baur N, Lueders-Lefevre V, Voshol J, Keller H. Class I and IIa histone deacetylases have opposite effects on sclerostin gene regulation. J Biol Chem. 2014;289(36):24995–25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wein MN, Spatz J, Nishimori S, Doench J, Root D, Babij P, Nagano K, Baron R, Brooks D, Bouxsein M, Pajevic PD, Kronenberg HM. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J Bone Miner Res. 2015;30(3):400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang CL, McKinsey TA, Olson EN. The transcriptional corepressor MITR is a signal-responsive inhibitor of myogenesis. Proc Natl Acad Sci USA. 2001;98(13):7354–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black BL, Martin JF, Olson EN. The mouse MRF4 promoter is trans-activated directly and indirectly by muscle-specific transcription factors. J Biol Chem. 1995;270(7):2889–2892. [DOI] [PubMed] [Google Scholar]
- 30.Naidu PS, Ludolph DC, To RQ, Hinterberger TJ, Konieczny SF. Myogenin and MEF2 function synergistically to activate the MRF4 promoter during myogenesis. Mol Cell Biol. 1995;15(5):2707–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leibham D, Wong MW, Cheng TC, Schroeder S, Weil PA, Olson EN, Perry M. Binding of TFIID and MEF2 to the TATA element activates transcription of the Xenopus MyoDa promoter. Mol Cell Biol. 1994;14(1):686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong MW, Pisegna M, Lu MF, Leibham D, Perry M. Activation of Xenopus MyoD transcription by members of the MEF2 protein family. Dev Biol. 1994;166(2):683–695. [DOI] [PubMed] [Google Scholar]
- 33.Pendás AM, Balbín M, Llano E, Jiménez MG, López-Otín C. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13). Genomics. 1997;40(2):222–233. [DOI] [PubMed] [Google Scholar]
- 34.Angel P, Baumann I, Stein B, Delius H, Rahmsdorf HJ, Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5′-flanking region. Mol Cell Biol. 1987;7(6):2256–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schönthal A, Herrlich P, Rahmsdorf HJ, Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988;54(3):325–334. [DOI] [PubMed] [Google Scholar]
- 36.Clohisy JC, Scott DK, Brakenhoff KD, Quinn CO, Partridge NC. Parathyroid hormone induces c-fos and c-jun messenger RNA in rat osteoblastic cells. Mol Endocrinol. 1992;6(11):1834–1842. [DOI] [PubMed] [Google Scholar]
- 37.Fei Y, Shimizu E, McBurney MW, Partridge NC. Sirtuin 1 is a negative regulator of parathyroid hormone stimulation of matrix metalloproteinase 13 expression in osteoblastic cells: role of sirtuin 1 in the action of PTH on osteoblasts. J Biol Chem. 2015;290(13):8373–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kataoka K, Fujiwara KT, Noda M, Nishizawa M. MafB, a new Maf family transcription activator that can associate with Maf and Fos but not with Jun. Mol Cell Biol. 1994;14(11):7581–7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hess J, Porte D, Munz C, Angel P. AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J Biol Chem. 2001;276(23):20029–20038. [DOI] [PubMed] [Google Scholar]
- 40.Maiti D, Xu Z, Duh EJ. Vascular endothelial growth factor induces MEF2C and MEF2-dependent activity in endothelial cells. Invest Ophthalmol Vis Sci. 2008;49(8):3640–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu B, Gulick T. Phosphorylation and alternative pre-mRNA splicing converge to regulate myocyte enhancer factor 2C activity. Mol Cell Biol. 2004;24(18):8264–8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang J, Gocke CB, and Yu H. Phosphorylation-facilitated sumoylation of MEF2C negatively regulates its transcriptional activity. 2006; 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386(6622):296–299. [DOI] [PubMed] [Google Scholar]
- 44.Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16(23):7054–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, Olson EN, Ulevitch RJ, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badodi S, Baruffaldi F, Ganassi M, Battini R, Molinari S. Phosphorylation-dependent degradation of MEF2C contributes to regulate G2/M transition. Cell Cycle. 2015;14(10):1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Tang X, Li M, Marshall J, Mao Z. Regulation of neuroprotective activity of myocyte-enhancer factor 2 by cAMP-protein kinase A signaling pathway in neuronal survival. J Biol Chem. 2005;280(17):16705–16713. [DOI] [PubMed] [Google Scholar]