Abstract
Although the intestine plays the major role in 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] action on calcium homeostasis, the mechanisms involved remain incompletely understood. The established model of 1,25(OH)2D3-regulated intestinal calcium absorption postulates a critical role for the duodenum. However, the distal intestine is where 70% to 80% of ingested calcium is absorbed. To test directly the role of 1,25(OH)2D3 and the vitamin D receptor (VDR) in the distal intestine, three independent knockout (KO)/transgenic (TG) lines expressing VDR exclusively in the ileum, cecum, and colon were generated by breeding VDR KO mice with TG mice expressing human VDR (hVDR) under the control of the 9.5-kb caudal type homeobox 2 promoter. Mice from one TG line (KO/TG3) showed low VDR expression in the distal intestine (<50% of the levels observed in KO/TG1, KO/TG2, and wild-type mice). In the KO/TG mice, hVDR was not expressed in the duodenum, jejunum, kidney, or other tissues. Growth arrest, elevated parathyroid hormone level, and hypocalcemia of the VDR KO mice were prevented in mice from KO/TG lines 1 and 2. Microcomputed tomography analysis revealed that the expression of hVDR in the distal intestine of KO/TG1 and KO/TG2 mice rescued the bone defects associated with systemic VDR deficiency, including growth plate abnormalities and altered trabecular and cortical parameters. KO/TG3 mice showed rickets, but less severely than VDR KO mice. These findings show that expression of VDR exclusively in the distal intestine can prevent abnormalities in calcium homeostasis and bone mineralization associated with systemic VDR deficiency.
Here we show, using transgenic mice with VDR restricted to the ileum, cecum, and colon, the importance of the distal intestine in proper bone mineralization.
The principal action of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] and the vitamin D receptor (VDR) is intestinal calcium absorption (1–5). The proposed model indicates that calcium enters the enterocyte through the epithelial calcium channel transient receptor potential vanilloid type 6 (TRPV6) and then binds to the calcium-binding protein calbindin (1, 2). At the basolateral membrane, calcium is transported by the plasma membrane calcium pump into the extracellular space (1, 2). The duodenum has been a focus of research related to this 1,25(OH)2D3-regulated calcium absorptive process (6). However, the distal intestinal is where 70% to 80% of ingested calcium is absorbed (7). VDR, calbindin-D9k, and TRPV6 are expressed in all segments of the small and large intestine (5, 8–12). The highest levels of TRPV6 are in the cecum and colon (12). 1,25(OH)2D3 regulation of active calcium absorption has been reported in the ileum, cecum, and colon (13–16). In addition, the distal intestine as well as the duodenum are responsive to 1,25(OH)2D3, and early studies suggested that the distal intestine plays a major role in adaptation to low dietary calcium (10, 15, 17). Furthermore, studies in patients with extensive resection of the small intestine reported that calcium absorption was higher when the colon was preserved (18).
Although these findings indicate that the distal segments of the intestine in addition to the duodenum play an important role in 1,25(OH)2D3-mediated calcium homeostasis, very little is known about 1,25(OH)2D3 action in the distal intestine. Although it has been a matter of debate, in the current study we provide evidence that transgenic (TG) expression of VDR exclusively in the distal intestine can prevent abnormalities in calcium homeostasis and bone mineralization associated with systemic VDR deficiency. These findings have important implications regarding how regional bowel disease may disrupt calcium homeostasis and the impact of gastric bypass surgery on distal intestinal calcium absorption.
Materials and Methods
Materials
Polyvinylidene difluoride membranes and prestained markers were obtained from Bio-Rad Laboratories (Hercules, CA). The enhanced chemiluminescent detection system was purchased from Denville Scientific (Holliston, MA). Anti-VDR (D-6) [Research Resource Identifier (RRID): AB_628040] and anti−β-actin (RRID: AB_626632) as well as secondary antibodies against mouse and rabbit anti-sera (RRIDs: AB_631737 and AB_631746, respectively) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX). Calbindin-D9k antibody was procured from Swant Antibodies (RRID: AB_10000348; Bellinzona, Switzerland). 1,25(OH)2D3 was purchased from Cayman Chemical Company (Ann Arbor, MI). Lipofectamine 2000 was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA). NEB5-α competent cells were purchased from New England BioLabs Inc. (Ipswich, MA). DNA sequencing to confirm the clones and synthesis of the primers used in this study were done at the Molecular Resource Facility at the Rutgers New Jersey Medical School. Caco-2 cells were purchased from ATCC (Manassas, VA).
Plasmids
pBSKS-CDX2 (caudal type homeobox 2) 9.5-kb promoter region (CDX2P 9.5) plasmid was a kind gift from Dr. E. Fearon (University of Michigan Medical School). The pcDNA3.1-hVDR plasmid encoding amino acid residues 4 to 427 of the human vitamin D receptor (hVDR) was provided by Dr. P. McDonald (Case Western Reserve University). A poly(A) region containing pL3 plasmid was purchased from Addgene (Cambridge, MA). All restriction and modification enzymes were obtained from New England BioLabs, Inc.
Generation of distal intestine−specific hVDR-expressing TG mice
The transgene was constructed in the pBSKS-9.5kb CDX2 plasmid. The 9.5-kb fragment from the CDX2 proximal promoter region was shown to direct transgene expression specifically in the distal ileum, the cecum, and the colon (19). The hVDR fragment from the pcDNA3.1 hVDR plasmid was subcloned together with a polyadenylation cassette into the EcoRI and HindIII sites, respectively, of the pBSKS-9.5kb CDX2 plasmid to generate the pBSKS-9.5kb CDX2-hVDR poly(A) plasmid. Restriction digestion analysis and sequencing confirmed the presence and correct orientation of hVDR. The pBSKS-9.5kb CDX2-hVDR poly(A) plasmid was tested in vitro for VDR protein expression by transfection (using lipofectamine 2000) in Caco-2 cells grown in medium and conditions as previously described (20), followed by hVDR Western blot analysis (21). The 9.5-kb CDX2-hVDR poly(A) transgene was isolated from vector sequences and injected into pronuclei of fertilized FVB/N mouse oocytes at the Rutgers New Jersey Medical School Genome Editing Core Facility. Founders were identified using DNA extracted from tail biopsies by polymerase chain reaction (PCR) with hVDR-specific primers that do not recognize mouse VDR (5′ 613 - 635; 3′ 1020 - 999).
TG mice were backcrossed to the C56BL/6J background for multiple generations (eight or greater). TG mice with high or low hVDR expression were mated to VDR knockout (KO) mice [male VDR KO mice fed a calcium/lactose rescue diet (3) were used for breeding; VDR KO mice are on the C57BL/6 background and were obtained from The Jackson Laboratory (originally from M. Demay, Harvard Medical School). Offspring heterozygous for transgene integration and for ablation of the endogenous receptor (TG+/−, KO+/−) were bred to obtain mice with intestine-specific TG expression (TG/KO mice). Genotyping was done using mouse- and human-specific VDR primers. Mice were maintained in a virus- and parasite-free barrier facility and exposed to a 12-hour light, 12-hour dark cycle. Food and water were given ad libitum. All animal experiments were approved by the Rutgers New Jersey Medical School Animal Care and Use Committee.
Characterization of TG mice expressing hVDR in the distal intestine
Mice were fed a standard rodent chow diet (Rodent Laboratory Chow 5001; Ralston Purina Co.) ad libitum from birth. Eight- to 9-week-old mice were used for initial characterization studies. In other studies, including those related to the skeletal phenotype, 10- to 12-week-old mice were used. Both male and female mice were studied. No sexual dimorphism was observed in the vitamin D target genes or proteins examined at these ages. Blood was collected, and serum was prepared for analysis of calcium using atomic absorption spectrometry (22) with a standard calcium chloride solution (Thermo Fisher Scientific) and parathyroid hormone using the two-site immunoradiometric assay (Immutopics, Clemente, CA). Levels of osteocalcin were measured by radioimmunoassay as previously described (23). Tissues were harvested, and RNA and protein were isolated. Tibiae were harvested for histology and for the assessment of microarchitecture using microcomputed tomography (micro-CT) and histomorphometry. In studies examining the response to 1,25(OH)2D3 treatment, mice were injected intraperitoneally with either vehicle or 1,25(OH)2D3 three times over 48 hours (48, 24, and 6 hours before being euthanized: 100 ng/100 g of body weight in 0.1 mL of a 9:1 mix of propylene glycol and ethanol) before termination. The three-dose protocol was used to study both short-term and long-term effects of 1,25(OH)2D3 administration.
RNA isolation and analysis
Total RNA was isolated after disruption of the tissues using RiboZol RNA extraction reagent from Amresco, Inc. (Solon, OH). Reverse transcription PCR (RT-PCR) was performed using 2 µg of total RNA, the SuperscriptTM One-Step RT-PCR System with Platinum Taq DNA polymerase (Thermo Fisher Scientific, Inc.), and specific human or mouse VDR primers (Supplemental Table 1 (12.4KB, docx) ). The resulting PCR products were subjected to electrophoresis on a 1% agarose gel containing ethidium bromide, and bands were visualized under UV light. Gel data were recorded using the Gene Genius bioImaging System (Syngene, Frederick, MD), and relative densities of the bands were determined using Gene Tool Software (Syngene). β-Actin messenger RNA (mRNA) was used as a control. For each primer set, PCR cycle numbers were chosen so that the amplification was in the linear range of amplification efficiency. In addition, quantitative real-time PCR (qRT-PCR) was used to quantify hVDR mRNA and Cyp24a1 and Trpv6 mRNAs in the mouse intestine using Taqman analyses. TaqMan Gene Expression probes (Applied Biosystems, Foster City, CA) were used for qRT-PCR and are found in Supplemental Table 1 (12.4KB, docx) . Expression of the gene of interest was normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase, and the 2-ΔΔCt method was used to calculate relative gene expression. All qRT-PCR reactions were performed in triplicate.
Western blot analysis
Tissue harvested from the animals was rinsed in ice-cold phosphate-buffered saline containing a protease inhibitor cocktail, frozen in liquid nitrogen, and stored at −80°C or used immediately. For Western blot analysis of calbindin-D9k, postmitochondrial supernatants were prepared. For Western blot analysis of VDR, total cellular protein was prepared using a lysis buffer containing 50 mM of Tris-HCl (pH, 7.5), 150 mM of NaCl, 0.1% sodium dodecyl sulfate, 1.0% NP-40, and protease inhibitors. After centrifugation, the protein content of the supernatants was measured by the Bradford method (24) or by using the Reducing Agent and Detergent Compatible Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA), divided into 50-µg aliquots, and stored at −80°C until used. Using an enhanced chemiluminescent detection system (Denville Scientific, Inc., Holliston, MA), 50 µg of denatured protein was subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes for Western blot analysis. Ponceau S staining (intestine) or β-actin (kidney) immunoblotting was used to normalize for sample variation.
Bone analysis
Changes in bone mineralization were examined by micro-CT and histomorphometry as previously described (25). Micro-CT analysis of the left tibiae was performed ex vivo using a high-resolution SkyScan 1172 system to examine trabecular and cortical bone volume and parameters (trabecular volume, trabecular number, trabecular thickness, and cortical porosity). For histology, sections were stained according to von Kossa or Goldner to assess mineralized bone and unmineralized bone matrix, respectively. Histomorphometric analysis was performed on (un)decalcified tibiae sections as described (26). Appearance of the growth plate, trabecular mineralized bone volume, osteoid abundance, osteoblast and osteoclast numbers, and dynamic bone parameters were determined using a Zeiss Axiovert microscope and AxioVision imaging analyzing system and was expressed according to the American Society for Bone and Mineral Research standardized histomorphometry nomenclature (27).
Statistical analysis
Results are expressed as mean ± standard error (standard error of the mean). Normal distribution of the data was confirmed with the Shapiro-Wilk normality test. To test differences between different genotypes, we applied one- or two-way analysis of variance or the Student t test for two-group comparison, as indicated in the figure legends. Differences were considered significant at P < 0.05.
Results
Generation and characterization of distal intestine−specific hVDR TG mice
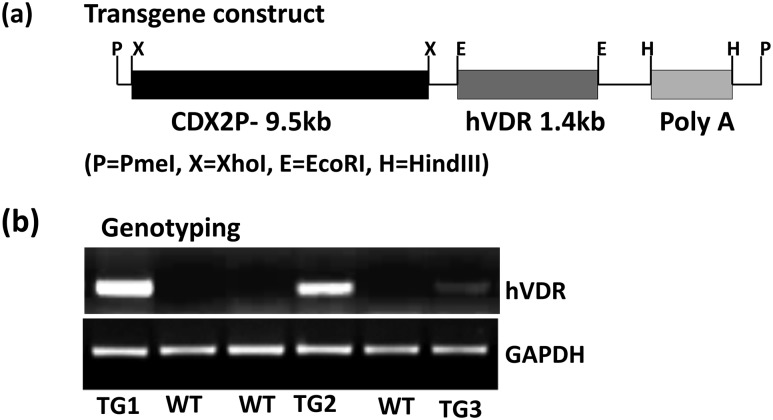
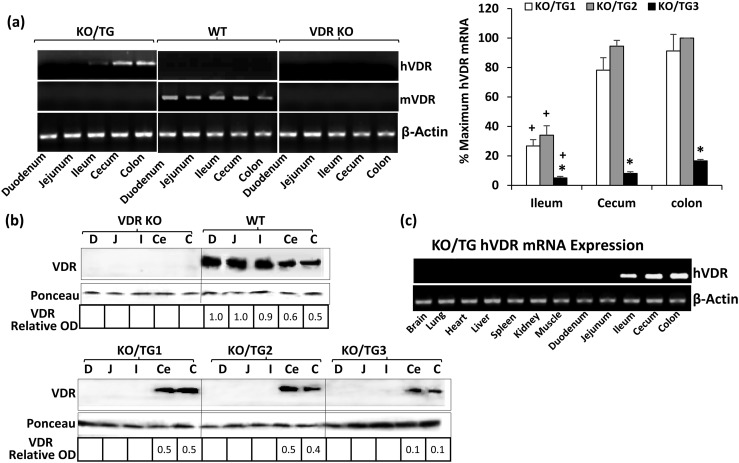
To test directly in vivo the role of distal intestinal segments in the biological actions of 1,25(OH)2D3, TG mice expressing hVDR exclusively in the ileum, cecum, and colon were generated. Full-length hVDR complementary DNA was subcloned together with a polyadenylation cassette into EcoRI and HindIII sites, respectively, of the pBSKS-9.5kb CDX2 plasmid [Fig. 1(a)]; the 9.5-kb fragment of the CDX2 promoter was previously reported to direct transgene expression specifically in the distal ileum, the cecum, and the colon (20). The resulting pBSKS-9.5kb CDX2-hVDR poly(A) plasmid was first tested in vitro for VDR protein expression by transfection in Caco-2 cells. Western blot analysis indicated strong expression of the VDR protein in Caco-2 cells using the transgene construct (not shown). This construct was injected into the pronucleus of wild-type (WT) mouse oocytes, and three founders were identified [Fig. 1(b)]. The founders were mated to VDR KO mice, and VDR KO mice expressing hVDR specifically in the distal intestine were generated. Figure 2(a) (left panel) shows the expression of hVDR mRNA in KO/TG mice (low levels in the ileum, higher levels in the cecum and colon). hVDR mRNA was not observed in WT or VDR KO mice. Quantitative analysis of hVDR mRNA expression indicated that the highest expression of the transgene was observed in the cecum and colon (compared with the ileum) in all three TG lines [Fig. 2(a), right panel]. Mice from KO/TG line 3 showed low hVDR mRNA levels in the distal intestine compared with levels in KO/TG1 and KO/TG2 mice [Fig. 2(a), right panel]. Western blot analysis indicated that VDR protein was undetectable in the duodenum and jejunum in all three TG lines [Fig. 2(b)]. Levels of VDR in the cecum and colon of KO/TG1 and KO/TG2 mice and WT mice were equivalent [Fig. 2(b)]. VDR protein could be detected in the ileum of the KO/TG mice when the protein concentration was increased (not shown). KO/TG3 mice expressed <50% of the levels of VDR protein observed in KO/TG1 and KO/TG2 mice and WT mice [Fig. 2(b)]. Note that in Fig. 2(c) hVDR mRNA was expressed only in the ileum, cecum, and colon. hVDR mRNA was not expressed in other parts of the intestine and in other organs and tissues in KO/TG mice (in the 2- and 3-month-old mice examined). Similar results were observed for all three KO/TG lines.
Figure 1.
Generation of distal intestine−specific hVDR-expressing transgenic mice. (a) Transgene construct. A 9.5-kb fragment of the CDX2 promoter [CDX2P 9.5; (19)] was used to drive expression of the human VDR complementary DNA transgene in the distal intestine. (b) Identification of hVDR-Tg founders. WT, wild-type.
Figure 2.
Characterization of transgene mRNA and protein. (a) PCR analysis of mRNA expression in the mouse intestine. Left panel: Representative RT-PCR analysis. Expression of hVDR mRNA is restricted to the ileum, cecum, and colon. Mouse VDR (mVDR) was present in all segments of the intestine in WT mice but not in KO/TG mice or VDR KO mice. Right panel: Quantitative analysis of hVDR mRNA expression. Results are reported as percentage of maximum, which is KO/TG2 colon (100%), to which other samples are compared. hVDR mRNA in the ileum, cecum, and colon of KO/TG3 mice was significantly reduced compared with levels in KO/TG1 and KO/TG2 mice (*P < 0.05). For all three KO/TG lines, decreased levels of hVDR mRNA were observed in the ileum compared with the cecum and colon (+P < 0.05) (by two-way analysis of variance with multiple-comparison Tukey posttest). Data are mean ± standard error of the mean; n = 4 to 5 per group. (b) Western blot analysis of VDR protein. Levels of VDR in the cecum and colon of KO/TG1 and KO/TG2 mice were equivalent but were reduced in KO/TG3 mice. The relative optical density (OD) obtained using the VDR antibody was divided by the OD obtained after Ponceau staining to produce VDR-relative OD. VDR is recognized as a single protein band at approximately 48 kDa. Ponceau S normalization used the single protein band at approximately 15 kDa. VDR protein was detected in the ileum of KO/TG mice when the protein concentration was increased (not shown). (c) Transgene mRNA expression in mouse tissues. Note: hVDR was specifically expressed in the distal intestine. C, colon; Ce, cecum; D, duodenum; I, ileum; J, jejunum.
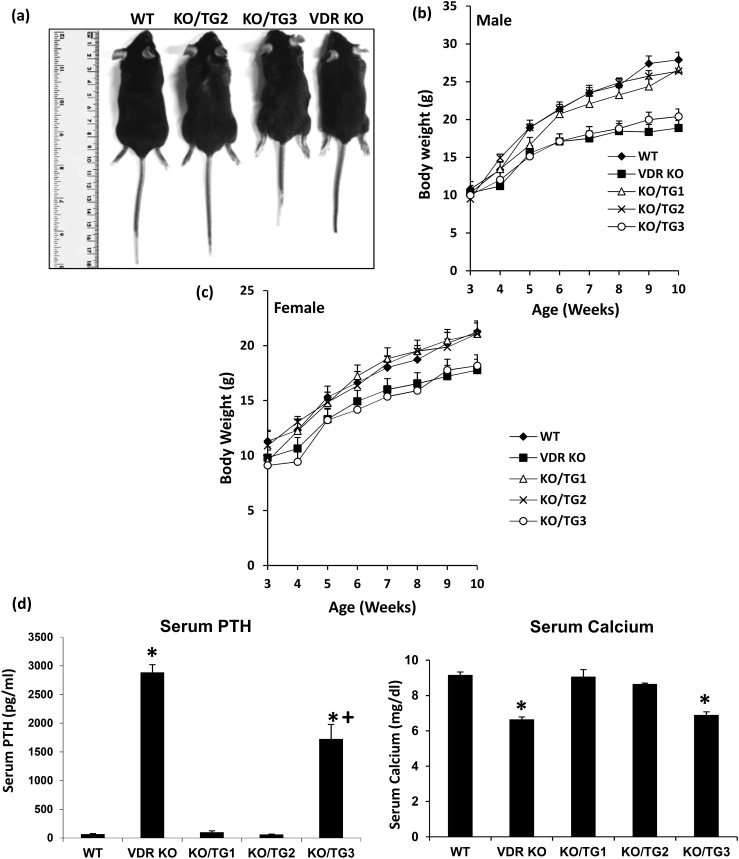
The growth arrest and decreased body weight of the VDR KO mice were prevented in mice from KO/TG lines 1 and 2 but not in mice from KO/TG line 3 [Fig. 3(a)−3(c)]. Progressive hair loss was observed in all three KO/TG lines, similar to loss in VDR KO mice. Alopecia was progressive and was more pronounced after 2 months of age (not shown). Elevated serum parathyroid hormone (PTH) levels observed in the VDR KO mice were reduced to values seen in WT mice in KO/TG1 and KO/TG2 mice [Fig. 3(d), left panel]. Serum PTH levels remained elevated in KO/TG3 mice but were significantly lower than the levels observed in the VDR KO mice [Fig. 3(d), left panel]. The reduced serum calcium levels observed in VDR KO mice were restored to levels observed in WT mice in KO/TG lines 1 and 2 but not in KO/TG line 3 [Fig. 3(d), right panel].
Figure 3.
Body weights and concentration of PTH and calcium in the serum of WT, VDR KO, KO/TG1, KO/TG2, and KO/TG3 mice. (a) Representative WT, KO/TG2, KO/TG3, and VDR KO male mice. (b and c) Body weights of KO/TG1, KO/TG2, and KO/TG3 male and female mice compared with WT and VDR KO mice. Each point is the average of five to eight mice. Data are represented as the mean ± the standard error of the mean (SEM). KO/TG3 mice weighed significantly less than WT or KO/TG1 and KO/TG2 mice (P < 0.05 for males from 6 to 10 weeks of age and for females from 7 to 10 weeks of age). The body weights of WT, KO/TG1, and KO/TG2 mice were not significantly different (P > 0.1). The body weights of VDR KO and KO/TG3 mice were not significantly different (P > 0.1). Analyzed by one-way analysis of variance (ANOVA) with multiple-comparison Tukey posttest. (d) Serum PTH (left panel) and serum calcium (right panel) levels. Each value is the mean ± SEM (n = 5 to 10 per group) and represents data from both male and female mice. The number of male and female mice was balanced. Analysis by one-way ANOVA with multiple-comparison Tukey posttest. *P < 0.05 compared with WT; +P < 0.05 compared with VDR KO.
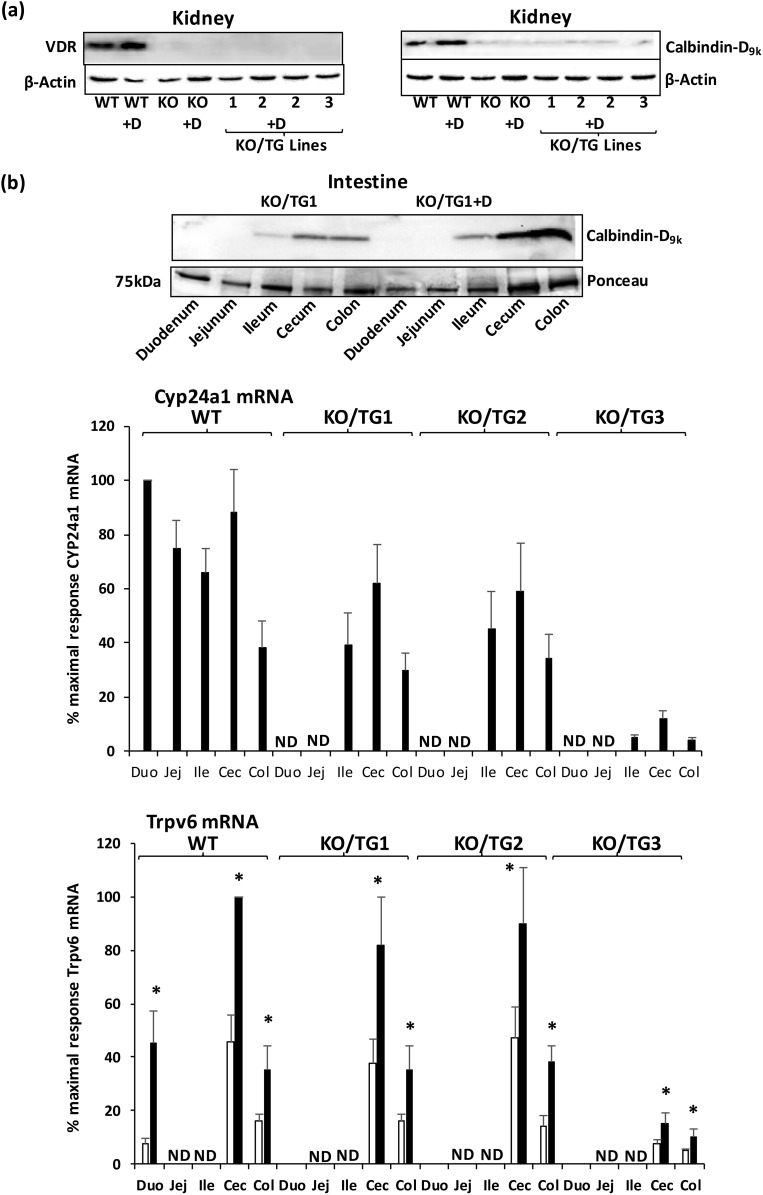
Similar to findings in VDR KO mice, VDR protein was absent in the kidney in all three KO/TG lines [Fig. 4(a), left panel]. In addition, compared with WT mice, levels of calbindin-D9k were markedly decreased in the kidneys of mice from the three KO/TG lines in the presence or absence of 1,25(OH)2D3 injection [similar to VDR KO mice; Fig. 4(a), right panel]. In addition, Cyp24a1 mRNA was undetectable in the kidney of VDR KO mice and KO/TG1, KO/TG2, and KO/TG3 mice in the presence or absence of 1,25(OH)2D3 injection (not shown). In the intestines of KO/TG mice, injection of 1,25(OH)2D3 resulted in inductions of calbindin-D9k protein and Cyp24a1 mRNA in the ileum, cecum, and colon and in Trpv6 mRNA in the cecum and colon [Fig. 4(b), top, middle, and bottom panels, respectively].
Figure 4.
Response to 1,25(OH)2D3 treatment in KO/TG mice. (a) Kidney. Representative Western blot analysis of renal VDR and calbindin-D9k. Left panel: VDR protein is absent in all three KO/TG lines. Right panel: Compared with WT levels of calbindin-D9k, levels are markedly decreased in VDR KO mice as well as in mice from KO/TG lines 1, 2, and 3. Similar to VDR KO mice, mice from KO/TG line 2 were unresponsive to 1,25(OH)2D3 treatment (induction of calbindin-D9k was not observed). No induction of renal calbindin-D9k was observed with 1,25(OH)2D3 treatment in KO/TG lines 1 and 3 (not shown). (b) Intestine. Top panel: Representative Western blot analysis of calbindin-D9k in the intestine. Calbindin-D9k is recognized as a single protein band at 9 kDa. Ponceau S normalization used the single protein band at approximately 75 kDa. Note that in response to 1,25(OH)2D3, calbindin-D9k was induced in the ileum, cecum, and colon (KO/TG1; 1.5- to 3.5-fold). Similar results were observed in KO/TG2 mice. In KO/TG3 mice, calbindin-D9k was induced by 1,25(OH)2D3 in the cecum and colon but not in the ileum (not shown). Middle panel: Cyp24a1 mRNA levels. Bottom panel: Trpv6 mRNA levels in KO/TG mice in the presence (black bar) or absence (open bar) of 1,25(OH)2D3 treatment. CYP24a1 mRNA was not detectable in untreated mice. Trpv6 mRNA was detected in low levels in KO/TG mice in the duodenum but was not detected in the jejunum and ileum in WT or KO/TG mice. Results are reported as percentage of maximum, which is duodenum from WT 1,25(OH)2D3–treated mice for Cyp24a1 mRNA (100%), to which other samples were compared. For Trpv6 mRNA maximum (100%) is cecum from WT 1,25(OH)2D3 treated mice, to which other samples were compared. VDR KO mice were unresponsive to 1,25(OH)2D3 (induction of Cyp24a1 mRNA or Trpv6 mRNA was not observed in any intestinal segment; not shown). Student t test; * significantly different from the nontreated group at P < 0.05. Bars, mean ± standard error of the mean; n = 4 to 6 per group. 1,25(OH)2D3 treatment resulted in an increase in serum calcium level (>12 mg/dL) in all three KO/TG lines. Cec, cecum; Col, colon; Duo, duodenum; E, Ile, ileum; Jej, jejunum; ND, not detectable.
Skeletal phenotype
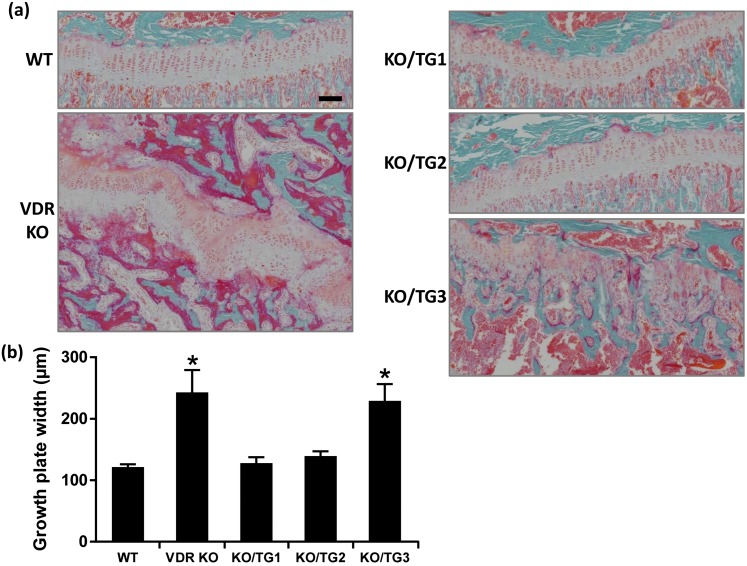
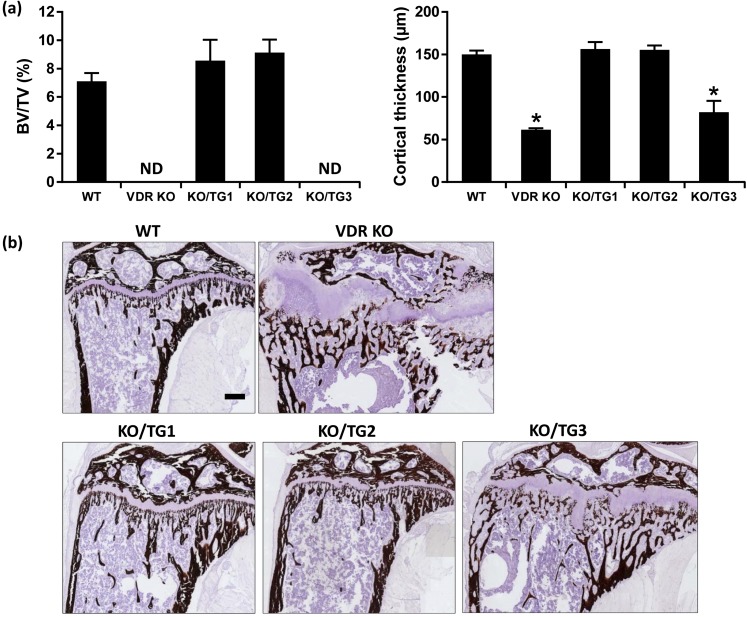
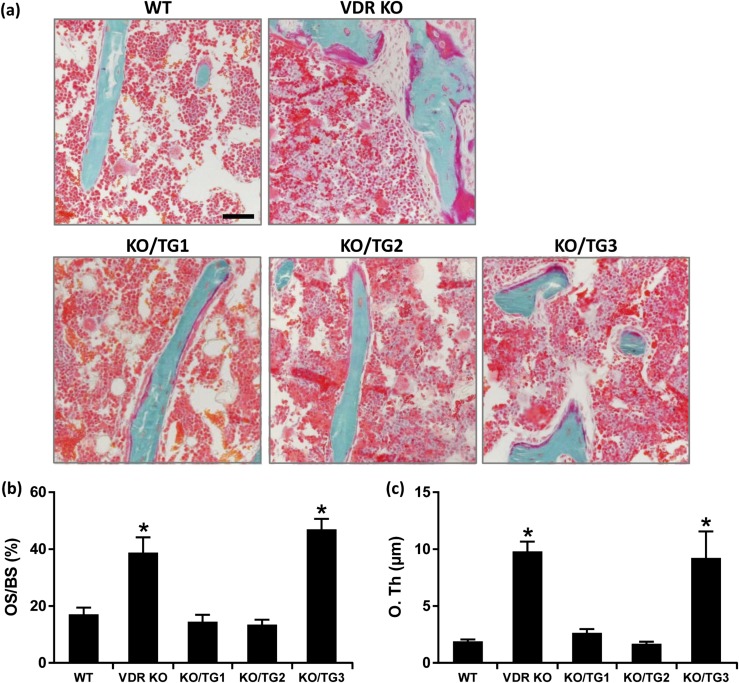
Histological analysis of the growth plate showed that the rachitic phenotype of VDR KO mice was prevented in mice from KO/TG lines 1 and 2 but not in mice from KO/TG line 3 [Fig. 5(a)]. Growth plate width was increased compared with that of WT in VDR KO mice and in mice from KO/TG line 3 but not in mice from KO/TG lines 1 and 2 [Fig. 5(a) and 5(b)]. Micro-CT analysis of the mineralized bone revealed that the abnormal trabecular bone volume and decreased cortical thickness associated with systemic VDR deficiency were rescued in mice from KO/TG lines 1 and 2 [Fig. 6(a)]. The trabecular and cortical abnormalities observed in the VDR KO mice were not rescued in mice from KO/TG line 3 [Fig. 6(a)]. Prevention of the altered trabecular and cortical parameters was further confirmed by von Kossa staining of histological sections of the tibia [Fig. 6(b)]. Goldner staining done to assess unmineralized bone matrix also indicated that mice from KO/TG lines 1 and 2 had amounts of unmineralized bone matrix similar to that of WT mice and that mice from KO/TG line 3 still showed signs of osteomalacia (but less severe than that of VDR KO mice) [Fig. 7(a)]. As shown in Fig. 7(b) and 7(c), the increases in osteoid surface and osteoid thickness observed in VDR KO mice were restored to the levels of WT mice in KO/TG lines 1 and 2. Serum osteocalcin levels were increased in VDR KO and KO/TG3 mice (174.4 ± 10.8 and 176.2 ± 24.9 ng/mL, respectively) compared with the level in WT mice (87.6 ± 19.9 ng/mL; P < 0.05) in accordance with the more pronounced presence of unmineralized bone matrix in VDR KO and KO/TG3 mice. Levels of serum osteocalcin in KO/TG1 and KO/TG2 mice (73.1 ± 8 and 69.0 ± 7.4 ng/mL, respectively) were not significantly different from that of WT mice (P > 0.1) (n = five mice per genotype).
Figure 5.
Rickets was prevented in mice from KO/TG lines 1 and 2 mice. (a) Representative Goldner-stained tibia sections; scale bar: 100 µm. (b) Quantification of growth plate width. Data are expressed as mean ± standard error of the mean. *P < 0.05 analyzed by one-way analysis of variance followed by Holm-Sidak multiple-comparisons test; n = 6 to 8 female mice of the same genotype. Similar results were observed in male mice (enhanced growth plate width in VDR KO and KO/TG3 mice compared with WT mice; n = 3 male mice of the same genotype; not shown).
Figure 6.
Expression of hVDR in the distal intestine of mice from KO/TG lines 1 and 2 rescued the bone defects associated with systemic VDR deficiency. (a) Quantification of trabecular bone volume (left panel) and cortical thickness (right panel) by micro-CT. ND indicates not determined because of osteomalacia in VDR KO and KO/TG3 strains; trabecular volume could not be correctly quantified. Data are expressed as mean ± standard error of the mean. *P < 0.05 analyzed by one-way analysis of variance followed by Holm-Sidak’s multiple-comparisons test; n = 7 to 9 female mice of the same genotype. Similar results were observed in male mice (KO/TG1 and KO/TG2 lines had similar amounts of unmineralized bone matrix as WT mice; n = 3 to 6 male mice of the same genotype; not shown). (b) Representative von Kossa staining of the tibia showing the disorganized growth plate and abnormal trabecular organization that precluded correct quantification in the VDR KO and KOTG3 mice, whereas these parameters were normal in KO/TG1 and KO/TG2 mice. Scale bar: 200 µm. BV/TV, bone volume/total volume.
Figure 7.
Rescue of osteomalacia in mice from KO/TG lines 1 and 2. (a) Representative Goldner-stained tibia sections showing osteoid matrix in dark pink. Scale bar: 50 µm. (b) Quantification of osteoid surface (OS/bone surface) and (c) quantification of osteoid thickness (O. Th). Data are expressed as mean ± standard error of the mean. *P < 0.05 analyzed by one-way analysis of variance followed by Holm-Sidak’s multiple comparisons test; n = 5 to 7 female mice of the same genotype. Similar results were observed in male mice (increased osteoid surface and thickness in VDR KO and KO/TG3 mice; n = 3 male mice of the same genotype; not shown).
Discussion
Although vitamin D is the major factor controlling intestinal calcium absorption and the duodenum has been the major focus of research, the mechanisms involved and the relative contribution of different segments of the intestinal tract to 1,25(OH)2D3−mediated calcium absorption remain incompletely understood. In this study, we showed that TG expression of VDR exclusively in the distal intestine can prevent abnormalities in calcium homeostasis and bone mineralization associated with systemic VDR deficiency.
Previous studies noted that the principal function of vitamin D/VDR in maintaining calcium homeostasis is to increase calcium absorption from the intestine. This conclusion was made from studies in VDR-null mice that showed that rickets, osteomalacia, and hypocalcemia were prevented when the VDR-null mice were fed a rescue diet that included high calcium (2%) (3, 4). Subsequent findings from Xue and Fleet (5) showed that TG expression of VDR in the entire intestine of VDR-null mice was sufficient to prevent rickets and normalize serum calcium levels, providing direct evidence of the importance of VDR in intestinal calcium absorption.
An important consideration that has been a matter of debate is the relative contribution of the proximal vs the distal intestine to VDR-mediated intestinal calcium absorption. Early studies by Pansu et al. (28) reported that active transcellular calcium transport was completely dependent on vitamin D and was localized to the duodenum. This is in contrast to studies that demonstrated that a low calcium diet [which results in increased synthesis of 1,25(OH)2D3] caused an increase in saturable mucosal to serosal Ca2+ influx in the ileum with little effect on the paracellular pathway (29, 30). Studies using everted sacs also noted a significant increase in active transport in the ileum in response to 1,25(OH)2D3 in 1,25(OH)2D3-deficient young rats (31). Calbindin-D9k and the calcium pump are induced by 1,25(OH)2D3 in the ileum (10, 17, 32). The capacity of the ileum (as well as the cecum) in deficient animals to respond to 1,25(OH)2D3 as measured by an increase in calbindin-D9k was reported to be greater than that of the duodenum (10, 17). We also noted 1,25(OH)2D3-mediated induction of calbindin-D9k in the ileum (as well as in the cecum and colon), with TG expression of VDR in the distal intestine of VDR KO mice. However, similar to our findings, previous studies reported that TRPV6 mRNA was not detected in the ileum and that TRPV6 protein was expressed at very low levels in the ileum and the proximal jejunum (12, 33). It has been suggested that low levels of TRPV6 in the ileum contribute to the slow rate of calcium transport in this intestinal segment compared with other segments (7).
Unlike the ileum, which transports calcium at a relatively slow rate, the cecum has been reported to have the highest rate of calcium transport compared with other intestinal segments (34). Nevertheless, the contribution of the cecum to total calcium absorption has been a matter of debate (35, 36). The physiological significance of the cecum for body calcium homeostasis was recently noted in studies in cecectomized rats, which showed significant cortical and trabecular bone loss (37). Active calcium transport and calbindin-D9k and TRPV6 mRNAs were enhanced in the colon but not in the duodenum, jejunum, or ileum of the cecectomized rats, suggesting compensation in the lower bowel but not the proximal intestine (37).
In studies using isolated rat intestinal segments, 1,25(OH)2D3 was reported to stimulate active calcium transport in the cecum and colon (14, 15). 1,25(OH)2D3 has also been reported to enhance calcium absorption in the colon of healthy humans (38). In our study, both calbindin-D9k and TRPV6 mRNA were present in the cecum as well as in the colon and were induced by 1,25(OH)2D3. A limitation of our study at this time is a lack of direct measurement of calcium absorption in the individual intestinal segments. Nevertheless, our findings show that TG expression of VDR restricted to the distal ileum, cecum, and colon of VDR KO mice rescued VDR-dependent rickets and normalized serum calcium levels and that the distal intestine was sensitive to 1,25(OH)2D3 as indicated by induction of vitamin D target genes. These findings are consistent with the major role of 1,25(OH)2D3−mediated calcium absorption in the distal intestine for the maintenance of calcium homeostasis.
In our study, we found that the degree of rescue of the bone phenotype was dependent on the level of VDR expressed in the distal intestine. Previous in vivo and in vitro studies noted that changes in VDR levels in intestinal cells altered their sensitivity to 1,25(OH)2D3 (39–41). Deletion of VDR specifically from all segments of the intestine (Vdrint− mice) resulted in a marked decrease in bone mass, with spontaneous bone fractures frequently observed (26). However, in the Vdrint− mice, the serum calcium level was normal because of the presence of VDR in other tissues and therefore increased renal calcium reabsorption, increased bone resorption, and inhibition of calcium incorporation into bone mediated by 1,25(OH)2D3 (26). When VDR is deleted specifically from the caudal region of the mouse intestine, altered calcium metabolism is also observed. In mice that lack VDR in the large intestine, there is a modest decrease in femoral bone density, a decrease in TRPV6 and calbindin-D9k mRNAs in the distal intestine, a compensatory increase in the expression of these genes in the duodenum, and normal serum calcium levels (42). Together, these findings and our results indicate that intestinal VDR, including VDR in the caudal region of the intestine, is essential for normal bone homeostasis. Further studies using our mouse model are needed to identify the contribution of individual segments of the distal intestine to VDR-mediated calcium homeostasis and to determine whether different as well as similar targets are contributing to calcium absorption in proximal vs distal intestinal segments.
In addition to using an active transcellular process, intestinal calcium absorption also occurs by a passive nonsaturable process. Absorption by passive diffusion has been reported to occur through tight junctions and intercellular spaces and to predominate in the distal region of the intestine when dietary calcium is adequate or high (7). There are conflicting data, however, related to the vitamin D dependency of paracellular intestinal calcium transport. Early in vivo studies showed that both active and passive nonsaturable processes were enhanced by vitamin D (43, 44). However, studies by Pansu et al. (28, 45) reported that the paracellular process was independent of vitamin D. More recent studies have shown that 1,25(OH)2D3 can regulate proteins involved in tight junction complexes (claudin-2 and -12) and cadherin-17 (a cell adhesion protein important for cell-to-cell contact) in the intestine (46–50). Thus, it is possible that normalization of serum calcium due to TG expression of VDR in the distal intestine may involve in part VDR regulation of the paracellular as well as the transcellular pathway. However, further studies are needed to determine the role of these intercellular adhesion molecules in intestinal physiology and whether their regulation by 1,25(OH)2D3 is related to intestinal calcium absorption. In a recent report, it was noted that under conditions of dietary calcium restriction, intestinal calcium absorption correlated to TRPV6, calbindin-D9k, and PMCA1b mRNAs (the traditional transcellular mediators) but not to the expression of claudin-2 or claudin-12 mRNA (51). Thus, as previously suggested, multiple factors yet to be identified are involved in VDR-mediated intestinal calcium absorption (52).
Although studies examining both active and passive transport in individual intestinal segments are needed, our findings showing a correlation between increased serum calcium in the TG/KO mice and induction of TRPV6 mRNA and calbindin-D9k suggest that active transport is involved in the rescue of VDR-dependent rickets by VDR expression in the distal intestine.
In summary, our data show the importance of the distal intestinal segments in vitamin D−mediated calcium homeostasis and bone mineralization. Gastric bypass surgery has resulted in a number of complications in patients, including malabsorption of calcium and decreased bone mineral density (53–55). Animal studies have also noted gastric bypass−associated bone resorption (56, 57). Future studies related to mechanisms involved in VDR-mediated activation of calcium absorption in the distal intestine may suggest new strategies involving the distal intestine that can compensate for calcium malabsorption and increase the efficiency of intestinal calcium uptake to minimize bone loss due to bariatric surgery or small bowel resection. Our findings also have implications for enhancement of calcium absorption in other individuals at risk for bone loss, including patients with inflammatory bowel disease and those with reduced calcium absorption after menopause or due to aging.
Acknowledgments
We acknowledge Dr. Roman Wernyj for help with statistical analyses. We also gratefully acknowledge the assistance of Leyla Oz in certain aspects of this investigation.
Financial Support: This work was supported by National Institutes of Health Grants DK38961, AG-044552, and DK112365 (to S.C.). L.L, L.V., and G.C. were supported by Fund for Scientific Research Flanders Grants G.0573.13N and G0A2416N.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer; Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution | RRID |
|---|---|---|---|---|---|---|
| Vitamin D receptor | aa 344-424 | Anti-human VDR | Santa Cruz Biotechnology, D6, sc13133 | Mouse; monoclonal | 1:1000 | AB_628040 |
| Calbindin-D9k | Anti-rat calbindin-D9k | Swant, CB9 | Rabbit; polyclonal | 1:1000 | AB_10000348 | |
| β-Actin | Anti-β actin | Santa Cruz Biotechnology, C4, sc47778 | Mouse; monoclonal | 1:1000 | AB_626632 | |
| IgG | Goat anti-rabbit, IgG: HRP conjugate | Santa Cruz Biotechnology, sc-2004 | Goat; polyclonal | 1:1500 | AB_631746 | |
| IgG | Goat anti-mouse, IgG: HRP conjugate | Santa Cruz Biotechnology, sc-2005 | Goat; polyclonal | 1:2500 | AB_631736 |
Abbreviation: IgG, immunoglobulin G.
Footnotes
- 1,25(OH)2D3
- 1,25-dihydroxyvitamin D3
- CDX2
- caudal type homeobox 2
- hVDR
- human vitamin D receptor
- KO
- knockout
- micro-CT
- microcomputed tomography
- mRNA
- messenger RNA
- PCR
- polymerase chain reaction
- PTH
- parathyroid hormone
- qRT-PCR
- quantitative real-time polymerase chain reaction
- RRID
- Research Resource Identifier
- RT-PCR
- reverse transcription polymerase chain reaction
- TG
- transgenic
- TRPV6
- transient receptor potential vanilloid type 6
- VDR
- vitamin D receptor
- WT
- wild-type.
References
- 1.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christakos S. Recent advances in our understanding of 1,25-dihydroxyvitamin D(3) regulation of intestinal calcium absorption. Arch Biochem Biophys. 2012;523(1):73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140(11):4982–4987. [DOI] [PubMed] [Google Scholar]
- 4.Masuyama R, Nakaya Y, Katsumata S, Kajita Y, Uehara M, Tanaka S, Sakai A, Kato S, Nakamura T, Suzuki K. Dietary calcium and phosphorus ratio regulates bone mineralization and turnover in vitamin D receptor knockout mice by affecting intestinal calcium and phosphorus absorption. J Bone Miner Res. 2003;18(7):1217–1226. [DOI] [PubMed] [Google Scholar]
- 5.Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136(4):1317–1327, e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronner F. Mechanisms of intestinal calcium absorption. J Cell Biochem. 2003;88(2):387–393. [DOI] [PubMed] [Google Scholar]
- 7.Wasserman RH. Vitamin D and the dual processes of intestinal calcium absorption. J Nutr. 2004;134(11):3137–3139. [DOI] [PubMed] [Google Scholar]
- 8.Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206(4423):1188–1190. [DOI] [PubMed] [Google Scholar]
- 9.Hirst MA, Feldman D. 1,25-Dihydroxyvitamin D3 receptors in mouse colon. J Steroid Biochem. 1981;14(4):315–319. [DOI] [PubMed] [Google Scholar]
- 10.Perret C, Desplan C, Thomasset M. Cholecalcin (a 9-kDa cholecalciferol-induced calcium-binding protein) messenger RNA: distribution and induction by calcitriol in the rat digestive tract. Eur J Biochem. 1985;150(1):211–217. [DOI] [PubMed] [Google Scholar]
- 11.Teerapornpuntakit J, Dorkkam N, Wongdee K, Krishnamra N, Charoenphandhu N. Endurance swimming stimulates transepithelial calcium transport and alters the expression of genes related to calcium absorption in the intestine of rats. Am J Physiol Endocrinol Metab. 2009;296(4):E775–E786. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Na T, Wu G, Jing H, Peng JB. Down-regulation of intestinal apical calcium entry channel TRPV6 by ubiquitin E3 ligase Nedd4-2. J Biol Chem. 2010;285(47):36586– 36596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DB, Walling MM, Levine BS, Gafter U, Silis V, Hodsman A, Coburn JW. Intestinal and metabolic effect of 1,25-dihydroxyvitamin D3 in normal adult rat. Am J Physiol. 1981;240(1):G90–G96. [DOI] [PubMed] [Google Scholar]
- 14.Favus MJ, Angeid-Backman E. Effects of 1,25(OH)2D3 and calcium channel blockers on cecal calcium transport in the rat. Am J Physiol. 1985;248(6 Pt 1):G676–G681. [DOI] [PubMed] [Google Scholar]
- 15.Favus MJ, Kathpalia SC, Coe FL, Mond AE. Effects of diet calcium and 1,25-dihydroxyvitamin D3 on colon calcium active transport. Am J Physiol. 1980;238(2):G75–G78. [DOI] [PubMed] [Google Scholar]
- 16.Vergne-Marini P, Parker TF, Pak CY, Hull AR, DeLuca HF, Fordtran JS. Jejunal and ileal absorption in patients with chronic renal disease: effect of 1alpha-hydroxycholecalciferol. J Clin Invest. 1976;57(4):861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armbrecht HJ, Boltz MA, Christakos S, Bruns ME. Capacity of 1,25-dihydroxyvitamin D to stimulate expression of calbindin D changes with age in the rat. Arch Biochem Biophys. 1998;352(2):159–164. [DOI] [PubMed] [Google Scholar]
- 18.Hylander E, Ladefoged K, Jarnum S. Calcium absorption after intestinal resection: the importance of a preserved colon. Scand J Gastroenterol. 1990;25(7):705–710. [DOI] [PubMed] [Google Scholar]
- 19.Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, Cho KR, Fearon ER. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007;67(20):9721–9730. [DOI] [PubMed] [Google Scholar]
- 20.Ajibade DV, Dhawan P, Fechner AJ, Meyer MB, Pike JW, Christakos S. Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin. Endocrinology. 2010;151(7):2974–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhawan P, Wieder R, Christakos S. CCAAT enhancer-binding protein alpha is a molecular target of 1,25-dihydroxyvitamin D3 in MCF-7 breast cancer cells. J Biol Chem. 2009;284(5):3086–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trudeau DL, Freier EF. Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS). Clin Chem. 1967;13(2):101–114. [PubMed] [Google Scholar]
- 23.Verhaeghe J, Van Herck E, Van Bree R, Van Assche FA, Bouillon R. Osteocalcin during the reproductive cycle in normal and diabetic rats. J Endocrinol. 1989;120(1):143–151. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 25.Lieben L, Benn BS, Ajibade D, Stockmans I, Moermans K, Hediger MA, Peng JB, Christakos S, Bouillon R, Carmeliet G. Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis. Bone. 2010;47(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieben L, Masuyama R, Torrekens S, Van Looveren R, Schrooten J, Baatsen P, Lafage-Proust MH, Dresselaers T, Feng JQ, Bonewald LF, Meyer MB, Pike JW, Bouillon R, Carmeliet G. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122(5):1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units: report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610. [DOI] [PubMed] [Google Scholar]
- 28.Pansu D, Bellaton C, Roche C, Bronner F. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am J Physiol. 1983;244(6):G695–G700. [DOI] [PubMed] [Google Scholar]
- 29.Nellans HN, Kimberg DV. Cellular and paracellular calcium transport in rat ileum: effects of dietary calcium. Am J Physiol. 1978;235(6):E726–E737. [DOI] [PubMed] [Google Scholar]
- 30.Auchère D, Tardivel S, Gounelle JC, Drüeke T, Lacour B. Role of transcellular pathway in ileal Ca2+ absorption: stimulation by low-Ca2+ diet. Am J Physiol. 1998;275(5 Pt 1):G951–G956. [DOI] [PubMed] [Google Scholar]
- 31.Armbrecht HJ. Age-related changes in calcium and phosphorus uptake by rat small intestine. Biochim Biophys Acta. 1986;882(3):281–286. [DOI] [PubMed] [Google Scholar]
- 32.Armbrecht HJ, Boltz MA, Kumar VB. Intestinal plasma membrane calcium pump protein and its induction by 1,25(OH)(2)D(3) decrease with age. Am J Physiol. 1999;277(1 Pt 1):G41–G47. [DOI] [PubMed] [Google Scholar]
- 33.Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem. 1999;274(32):22739–22746. [DOI] [PubMed] [Google Scholar]
- 34.Karbach U, Feldmeier H. The cecum is the site with the highest calcium absorption in rat intestine. Dig Dis Sci. 1993;38(10):1815–1824. [DOI] [PubMed] [Google Scholar]
- 35.Ammann P, Rizzoli R, Fleisch H. Calcium absorption in rat large intestine in vivo: availability of dietary calcium. Am J Physiol. 1986;251(1 Pt 1):G14–G18. [DOI] [PubMed] [Google Scholar]
- 36.Nellans HN, Goldsmith RS. Transepithelial calcium transport by rat cecum: high-efficiency absorptive site. Am J Physiol. 1981;240(6):G424–G431. [DOI] [PubMed] [Google Scholar]
- 37.Jongwattanapisan P, Suntornsaratoon P, Wongdee K, Dorkkam N, Krishnamra N, Charoenphandhu N.. Impaired body calcium metabolism with low bone density and compensatory colonic calcium absorption in cecectomized rats. Am J Physiol Endocrinol Metab. 2012;302(7):E852–E863. [DOI] [PubMed] [Google Scholar]
- 38.Grinstead WC, Pak CY, Krejs GJ. Effect of 1,25-dihydroxyvitamin D3 on calcium absorption in the colon of healthy humans. Am J Physiol. 1984;247(2 Pt 1):G189–G192. [DOI] [PubMed] [Google Scholar]
- 39.Song Y, Fleet JC. Intestinal resistance to 1,25 dihydroxyvitamin D in mice heterozygous for the vitamin D receptor knockout allele. Endocrinology. 2007;148(3):1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao A, Wood RJ, Fleet JC. Increased vitamin D receptor level enhances 1,25-dihydroxyvitamin D3-mediated gene expression and calcium transport in Caco-2 cells. J Bone Miner Res. 2001;16(4):615–624. [DOI] [PubMed] [Google Scholar]
- 41.Lieben L, Verlinden L, Masuyama R, Torrekens S, Moermans K, Schoonjans L, Carmeliet P, Carmeliet G. Extra-intestinal calcium handling contributes to normal serum calcium levels when intestinal calcium absorption is suboptimal. Bone. 2015;81:502–512. [DOI] [PubMed] [Google Scholar]
- 42.Reyes-Fernandez PC, Fleet JC. Compensatory changes in calcium metabolism accompany the loss of vitamin D receptor (VDR) from the distal intestine and kidney of mice. J Bone Miner Res. 2016;31(1):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dostal LA, Toverud SU. Effect of vitamin D3 on duodenal calcium absorption in vivo during early development. Am J Physiol. 1984;246(5 Pt 1):G528–G534. [DOI] [PubMed] [Google Scholar]
- 44.Wasserman RH, Kallfelz FA. Vitamin D3 and unidirectional calcium fluxes across the rachitic chick duodenum. Am J Physiol. 1962;203:221–224. [DOI] [PubMed] [Google Scholar]
- 45.Pansu D, Bellaton C, Bronner F. Effect of Ca intake on saturable and nonsaturable components of duodenal Ca transport. Am J Physiol. 1981;240(1):G32–G37. [DOI] [PubMed] [Google Scholar]
- 46.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6(6):581–588. [DOI] [PubMed] [Google Scholar]
- 47.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19(5):1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zella LA, Meyer MB, Nerenz RD, Pike JW. The enhanced hypercalcemic response to 20-epi-1,25-dihydroxyvitamin D3 results from a selective and prolonged induction of intestinal calcium-regulating genes. Endocrinology. 2009;150(8):3448–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SM, Riley EM, Meyer MB, Benkusky NA, Plum LA, DeLuca HF, Pike JW. 1,25-Dihydroxyvitamin D3 controls a cohort of vitamin D receptor target genes in the proximal intestine that is enriched for calcium-regulating components. J Biol Chem. 2015;290(29):18199–18215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kutuzova GD, Deluca HF. Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys. 2004;432(2):152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Replogle RA, Li Q, Wang L, Zhang M, Fleet JC. Gene-by-diet interactions influence calcium absorption and bone density in mice. J Bone Miner Res. 2014;29(3):657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149(6):3196–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casagrande DS, Repetto G, Mottin CC, Shah J, Pietrobon R, Worni M, Schaan BD. Changes in bone mineral density in women following 1-year gastric bypass surgery [published correction appears in Obes Surg. 2015;25(9):1763]. Obes Surg. 2012;22(8):1287–1292. [DOI] [PubMed] [Google Scholar]
- 54.Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schafer AL, Weaver CM, Black DM, Wheeler AL, Chang H, Szefc GV, Stewart L, Rogers SJ, Carter JT, Posselt AM, Shoback DM, Sellmeyer DE. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015;30(8):1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abegg K, Gehring N, Wagner CA, Liesegang A, Schiesser M, Bueter M, Lutz TA. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. Am J Physiol Regul Integr Comp Physiol. 2013;305(9):R999–R1009. [DOI] [PubMed] [Google Scholar]
- 57.Canales BK, Schafer AL, Shoback DM, Carpenter TO. Gastric bypass in obese rats causes bone loss, vitamin D deficiency, metabolic acidosis, and elevated peptide YY. Surg Obes Relat Dis. 2014;10(5):878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]