Abstract
Psychosocial stress, such as isolation and restraint, disrupts reproductive neuroendocrine activity. Here we investigate the impact of psychosocial stress on luteinizing hormone (LH) pulses and gene expression and neuronal activation within Rfrp and Kiss1 cells in female mice. Mice were ovariectomized (OVX) and handled daily to habituate to the tail-tip blood collection procedure. Blood was collected every 5 minutes for 180 minutes for measurement of LH. After 90 minutes, stress animals were placed into restraint devices and isolated to new cages. No-stress control animals remained in their home cages. LH pulses occurred at regular intervals during the entire 180-minute sampling period in controls. In contrast, stress induced a rapid and robust suppression of pulsatile LH secretion. Stress reduced the frequency of pulses by 60% and diminished basal LH levels by 40%; pulse amplitude was unaffected. In a separate cohort of OVX females, brains were collected after 45, 90, or 180 minutes of stress or in no-stress controls. At all time points, stress induced a potent decrease in arcuate Kiss1 neuronal activation, using cfos induction as a marker, with a 50% to 60% suppression vs control levels, whereas Rfrp and cfos coexpression in the dorsal-medial nucleus was elevated after 45 minutes of stress. Although arcuate Kiss1 gene expression remained stable, Rfrp expression was elevated 20% after 180 minutes of stress. These findings demonstrate rapid suppression of LH pulsatile secretion by psychosocial stress, associated with reduced cfos induction in Kiss1 neurons and time-dependent increases in Rfrp neuronal activation and messenger RNA.
Stress inhibits LH pulses in mice.
Psychological stress can negatively impact reproduction in many mammalian species, including humans (1–5). In females, stress can disrupt ovarian cyclicity, as well as upstream gonadotropin synthesis and secretion (5–8). For example, restraint and/or isolation, commonly used models of psychosocial stress, reduces luteinizing hormone (LH) and follicle stimulating hormone (8–15). In some studies, stress-induced suppression of gonadotropins has been measured as “one-off” single measures, either for simplicity or for technical reasons. However, in species allowing repeated serial measures, psychosocial stress exposure or corticosterone treatment was shown to inhibit the pulsatile release of LH (8, 16–19). In mice, measurement of LH pulses has been technically challenging, due to the difficulty in obtaining enough blood volume for repeated LH measurements and the absence of an assay validated for measuring LH in low blood volumes. Recently, technical advances in assay sensitivity and blood collection paradigms have allowed researchers to begin accurately measuring LH pulses in mice (20, 21).
To date, little is known about underlying mechanisms for stress suppression of gonadotropin-releasing hormone (GnRH) and downstream LH secretion, especially at the level of the brain. Emerging evidence suggests that kisspeptin neurons in the arcuate nucleus are key components for generating and stimulating GnRH pulse secretion. Thus, it is possible that stress—through factors and pathways yet to be determined—impacts arcuate kisspeptin neurons, thereby altering downstream GnRH and LH pulsatility. In addition, recent evidence suggests that the peptide RFamide-related peptide 3 (RFRP-3) can inhibit GnRH and LH output and may do so under stressful conditions. In rats, 5 hours of restraint stress increased hypothalamic Rfrp messenger RNA (mRNA) expression and decreased mean LH via a pathway dependent on elevated corticosterone (9). By contrast, acute 1-hour restraint in female rats caused decreased Kiss1 levels in the arcuate when measured 5 hours later, as did metabolic (insulin-induced hypoglycemia) or immunological lipopolysaccharide stress (17, 22). Whether such alterations in Rfrp or Kiss1 occur similarly in mice under stress paradigms has not yet been studied.
In this communication, we (1) took advantage of the recent breakthrough in mouse LH pulsatility measurement to analyze how acute (90 minutes) restraint stress alters several different LH pulsatility parameters in awake female mice and (2) determined how acute restraint stress influences gene expression and cfos induction, a common marker of neuronal activation, in Rfrp and Kiss1 neurons. We report a significant stress-induced inhibition of LH pulsatile secretion, which occurs in correlation with decreased Kiss1 and cfos coexpression and elevated Rfrp and cfos coexpression.
Materials and Methods
Animals
Adult female C57Bl/6 mice (>2 months old) were housed in groups of two to four under a 12-hour light/dark cycle (lights off at 1800) and provided food and water ad libitum. Mice were bilaterally ovariectomized (OVX) under isoflurane anesthesia to promote elevated circulating LH levels due to lack of sex steroid feedback. All experiments were approved by the University of California, San Diego, Institutional Animal Care and Use Committee.
Experiment 1: LH pulse measures before and during acute stress in female mice
OVX mice were handled daily for 28 days to allow for habituation to the tail-tip bleeding procedure. On the day of the experiment (between 0800 hours and 1200 hours), serial tail bleeds were performed, with 3 µL whole blood collected from the tail tip every 5 minutes for 180 minutes. These whole blood samples were immediately diluted into 57 µL of assay buffer, mixed, and placed on ice until storage at −20°C. The 180-minute sampling period was divided into two consecutive pre/post periods: for the first 90 minutes, all animals remained unstressed. For the second 90 minutes, control animals remained in their home cages and stress animals were placed into Broome rodent restraint devices (Harvard Apparatus, Holliston, MA) and isolated to new cages.
Experiment 2: Effect of acute stress on Kiss1 and Rfrp neurons in female mice
Two weeks following OVX, mice were restraint stressed for either 45, 90, or 180 minutes and then immediately euthanized and their blood and brains collected. All stress and control (0 minutes; not exposed to restraint) mice were euthanized between 0930 and 1200 hours. At the time of euthanasia, mice were quickly anesthetized with isoflurane and blood collected via retro-orbital sampling just before rapid decapitation. Blood serum was isolated and stored at −20°C. Brains were immediately frozen on dry ice before being stored at −80°C until use in in situ hybridization (ISH) assays.
Hormone assays
Ultrasensitive mouse LH enzyme-linked immunosorbent assay
For LH pulse detection in experiment 1, LH was measured in 3 µL of whole blood by in-house enzyme-linked immunosorbent assay at the University of Virginia Ligand Assay Core, based on a method and reagents published in Steyn et al. (21). The limit of quantitation (functional sensitivity) is defined as the lowest concentration that demonstrates accuracy within 20% of expected values and intraassay coefficient of variation (%CV) <20%, and was determined by serial dilutions of a defined sample pool. Intra- and interassay %CVs were <2.3% and <7%, respectively. Functional sensitivity was 0.320 ng/mL.
LH radioimmunoassay
For experiment 2, serum LH was measured by two-site sandwich immunoassay, as described previously (23–25). The limit of detectability was 0.04 ng/mL.
Corticosterone enzyme-linked immunosorbent assay
For experiment 2, blood serum from stress (45, 90, or 180 minutes) and no-stress controls was assayed using DetectX Corticosterone EIA kit (Cat. K014; Arbor Assays), per the manufacturer’s instructions. Assay sensitivity was 18.6 pg/mL.
Single- and double-label ISH
Frozen brains were sectioned on a cryostat into 5 sets of 20-μm sections and thaw mounted on Superfrost-plus slides that were stored at −80°C until assay. Single-label ISH for arcuate Kiss1 or dorsal-medial nucleus (DMN) Rfrp, and double-label ISH for cfos+Kiss1 or cfos+Rfrp (as proxy measure of neuronal activation) were performed as previously described using previously validated riboprobes (26, 27). All ISH slides were analyzed under microscopy by a person blinded to treatment groups using an automated image processing and quantification software program (Dr. Don Clifton, University of Washington), as previously described (26–28).
LH pulse analysis
LH pulses were identified using the DynPeak pulse detection algorithm previously described (29). Default values were used for all parameters, except that (1) the global relative threshold was increased to 35% to account for the high basal LH levels in OVX animals, (2) the nominal peak threshold was reduced to a value of 20 minutes due to the high frequency of LH pulses in OVX animals (20), and (3) the three-point peak threshold was removed due to the limitations in 5-minute sampling frequency of OVX mice. Uncertainty of the assay was based on assay %CVs. Average values for each LH parameter (frequency, interpulse interval, amplitude, mean LH, and basal LH) were calculated across the pre and post periods (first 90-minute and second 90-minute periods, respectively). Frequency was defined as the number of pulses per 90-minute period. Interpulse interval was defined at the period of time between pulse peaks. When only one peak occurred within the 90-minute sampling period, the interpulse interval was calculated from the time of the peak to the end of the sampling period; if no peaks occurred, the interpulse interval was calculated as 90 minutes. Pulse amplitude was defined as the difference between peak value and preceding nadir and calculated for each period in which pulses were detected. Two animals did not show pulses during the stress period and were removed from the assessment of pulse amplitude. Mean LH was calculated by averaging all LH values within the sampling period. Basal LH was determined by averaging all nadirs within the 90-minute sampling period, or for the two animals that did not show pulses, averaging the post values at 30, 60, and 90 minutes.
Statistics
LH pulse parameters were analyzed by two-way repeated measures analysis of variance (ANOVA) with Tukey post hoc testing to identify differences between pre- and post-sampling periods of the stress and no-stress groups. For the ISH data, values are expressed as the mean ± standard error of the mean (SEM), and group differences were identified by one-way ANOVA with Fisher least significant difference post hoc testing. All statistics were performed using JMP 10.0 (SAS, Cary, NC) and significance set at P < 0.05.
Results
Acute restraint stress suppresses LH pulses in female mice
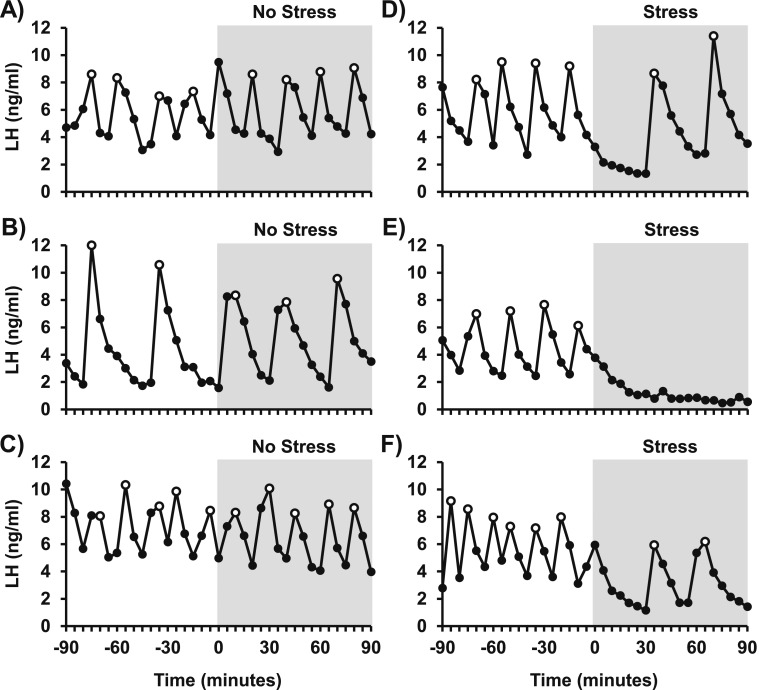
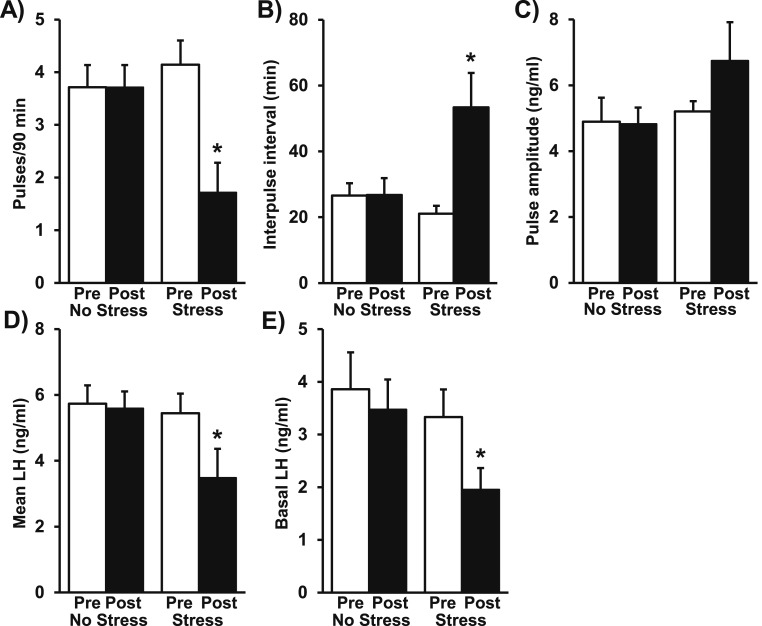
Figure 1 illustrates profiles of LH in three representative OVX no-stress control mice [Fig. 1(A–C)] and three representative OVX mice in response to restraint stress [Fig. 1(D–F)]. The pattern of LH pulses in control females was robust, rapid, and regular during the entire 180-minute sampling period. Pulse frequency averaged 3.8 pulses/90 minutes, with an interpulse interval of 26 minutes. LH pulse amplitude averaged 4.8 ng/mL during the first 90-minute period, and values did not significantly differ between the pre and post periods in controls (Fig. 2; control, pre vs post). In contrast, restraint stress induced a rapid and unambiguous suppression of pulsatile LH secretion [Fig. 1(D–F)] that was not observed in controls. Quantitative analysis determined that stress suppressed LH pulse frequency by 60% and prolonged the interpulse interval from the prestress baseline of 20 minutes to 58 minutes during the stress period [P < 0.05; Fig. 2(A) and 2(B); stress, pre vs post]. Although LH pulse amplitude was unchanged [Fig. 2(C)], stress decreased mean LH by approximately 36% and basal LH by 41% [P < 0.05; Fig. 2(D) and 2(E); stress, pre vs post].
Figure 1.
Restraint stress inhibits pulsatile LH in OVX mice. Profiles of circulating LH in (A–C) three representative OVX no-stress control mice and (D–F) three OVX mice exposed to restraint stress for 90 minutes beginning at time 0. Pulse peaks are indicated by open symbols. The period of treatment is depicted by gray shading. Tail-tip blood samples (3 μL) were collected every 5 minutes by pipette, and LH pulses were detected by DynPeak pulse algorithm.
Figure 2.
LH pulse parameters in stress and no-stress female mice. Quantitative summary of the LH responses during the first 90-minute (Pre) or second 90-minute (Post) sampling period in control OVX mice or mice in response to stress (n = 7 mice/group). Mean (± SEM) values for each LH parameter—(A), frequency; (B), interpulse interval; (C), pulse amplitude; (D), mean LH; (E), basal LH—were calculated across the pre and post periods and analyzed by two-way repeated measures ANOVA with Tukey post hoc testing to determine stress effects. Note: Two stress animals did not show pulses during the stress period and were not included in the assessment of LH pulse amplitude. None of the LH parameters differed significantly during the pre period between control and stress groups. *P < 0.05, pre vs post period.
Acute restraint stress does not alter Kiss1 expression, but increases Rfrp levels at 180 minutes
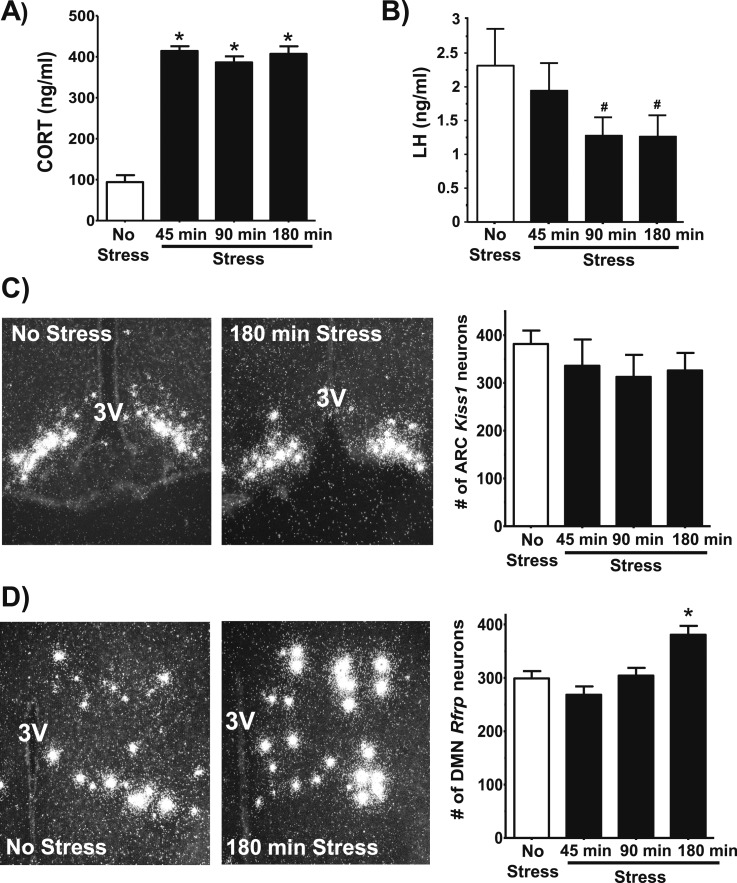
In experiment 2, OVX female mice were subjected to restraint stress for 45, 90, or 180 minutes, and compared with no-stress controls. Serum corticosterone levels were low in controls, as expected, and dramatically elevated in the stress groups [P < 0.05; Fig. 3(A)]. Serum LH at 90 and 180 minutes, measured as single one-off values, was trending lower [P = 0.09; Fig. 3(B)]. ISH analysis revealed that arcuate Kiss1 expression, determined by the number of Kiss1-expressing cells, was not significantly different between the no-stress controls and stress groups [Fig. 3(C)]. In contrast, Rfrp cell numbers in the DMN remained unchanged after 45 or 90 minutes of stress, yet were significantly elevated by 20% after 180 minutes of stress relative to controls [P < 0.05; Fig. 3(D)].
Figure 3.
Neural gene expression in stress and no-stress female mice. (A) Mean circulating corticosterone levels in stress and no-stress control female mice. (B) Mean serum LH levels in stress and no-stress control female mice. (C) Representative images of Kiss1 mRNA expression in the arcuate nucleus of a no-stress control (left) or after 180 minutes of stress (middle). Mean (± SEM) Kiss1 cell numbers in no-stress controls and 45, 90, and 180-minute stress groups (right). (D) Representative images of Rfrp mRNA expression in the DMN of a no-stress control and after 180 minutes of stress, and mean (± SEM) Rfrp cell numbers in each group. *P < 0.05 vs no-stress controls. #P = 0.09. 3V, third ventricle; ARC, arcuate; CORT, corticosterone.
Acute restraint stress decreases Kiss1 neuronal activation and increases Rfrp neuronal activation
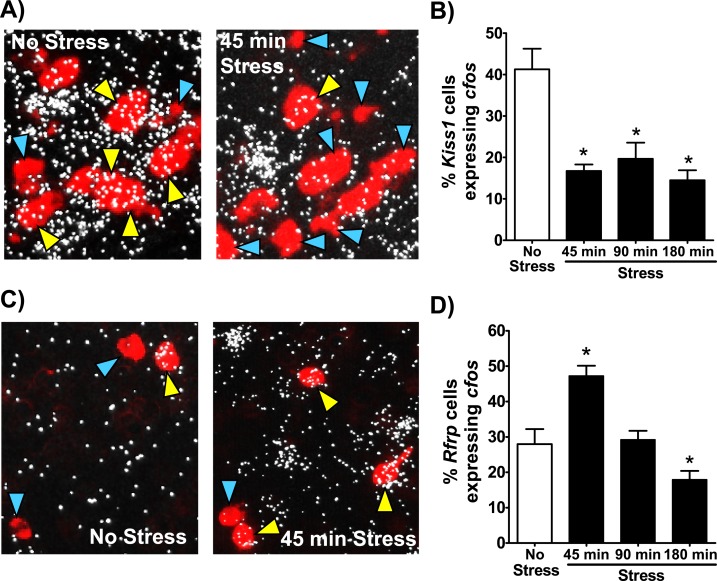
Brains of stress and no-stress control mice were also analyzed for neuronal activation of arcuate Kiss1 or Rfrp neurons, using cfos induction as a marker. All three stress groups showed a significant reduction in arcuate Kiss1 and cfos coexpression relative to controls [P < 0.05; Fig. 4(A) and 4(B)]. The magnitude of decrease in Kiss1 neuronal activation did not differ between stress groups, ranging from 51% to 63% lower than control levels. In contrast, Rfrp neuronal activation, based on cfos induction, was increased after 45 minutes of stress (P < 0.05) but not significantly elevated at later times [Fig. 4(C) and 4(D)].
Figure 4.
Kiss1 and Rfrp neuronal activation levels in stress and no-stress female mice. (A) Representative high-magnification images of Kiss1 (red fluorescence) and cfos (silver grains) gene expression in the arcuate nucleus of a no-stress control female (left) or female after 45 minutes of stress (right). Yellow arrowheads denote example Kiss1 cells with cfos induction. Blue arrowheads denote example Kiss1 cells not coexpressing cfos. (B) Quantification of the percent of Kiss1 neurons coexpressing cfos in each group. (C) Representative lower-magnification images of Rfrp (red fluorescence) and cfos (silver grains) expression in the DMN of a no-stress control female (left) or female after 45 minutes of stress (right). Yellow arrowheads denote example Rfrp cells with cfos induction. Blue arrowheads denote example Rfrp cells not coexpressing cfos. (D) Quantification of the percent of Rfrp neurons coexpressing cfos in each group. *P < 0.05 vs no-stress controls.
Discussion
The present rapid communication examines stress effects on LH pulse output and neural gene expression of key reproductive regulators. We determined that acute restraint stress in female mice rapidly diminishes pulsatile LH secretion, within 30 minutes, and alters neuronal activation of arcuate Kiss1 and DMN Rfrp cells at 45 minutes, the first poststress time point evaluated. Despite that, Kiss1 gene expression was unchanged after acute stress, and Rfrp gene expression increased only after 180 minutes of stress exposure. Collectively, these findings highlight the rapidity of stress-induced changes in LH pulsatility relative to the changes in gene expression of Kiss1 and Rfrp, and underscore the need for future studies to evaluate mechanisms underlying the initiation and maintenance of reproductive impairment associated with stress.
Our findings demonstrate that psychosocial stress can robustly inhibit LH pulses in OVX female mice. Using an experimental design in which each animal serves as its own control, we demonstrate that the GnRH pulse generator is exquisitely responsive to acute psychosocial stress. Restraint stress reduces LH pulse frequency within minutes with no detectable reduction in LH pulse amplitude observed during 90 minutes of stress. The reduction in pulse frequency, in the face of steady pulse amplitude, strongly suggests central impairment by stress. A major qualifier to this conclusion is that the females were devoid of ovarian steroids. Considering that the magnitude of the response to stress, including restraint stress, can differ between sexes or with gonadal steroid milieu (10, 30–36), future studies are required to evaluate the impact of these factors on the reproductive neuroendocrine response to acute stress. It is also notable that the stress suppression of LH was statistically significant only when LH was measured in repeated serial sampling that allowed for the analysis of pulse frequency. Because stressed females still showed the occurrence of LH pulses of normal amplitude, just at a reduced frequency, it is likely that single “one-off” measures occasionally captured, by chance, LH during a pulse, thereby obscuring the overall reduction in LH that serial sampling demonstrated. This underscores the importance of pulse sampling and highlights the difficulty to interpret stress-induced changes in LH from single measures.
The current study quantified stress-induced changes in reproductive gene expression (based on the number of identified mRNA-expressing cells) and neuronal activation (based on cfos mRNA induction) in key populations either involved in stimulating GnRH, arcuate Kiss1 neurons, or postulated to be inhibitory to the reproductive axis, Rfrp neurons. Although we observed no notable change in arcuate Kiss1 levels (based on Kiss1 neuron number) up to 3 hours following acute restraint stress, the percent of arcuate Kiss1 cells expressing cfos is reduced at 45 minutes and this suppression persists for 180 minutes. This indicates that changes in kisspeptin synthesis do not underlie the rapid suppression of LH pulses observed within 3 hours of stress exposure, but raises the possibility that stress-induced suppression of Kiss1 neuronal signaling underlies the rapid suppression in GnRH/LH pulsatility associated with stress. We also observed a rapid upregulation of Rfrp neuronal activation at 45 minutes after stress induction, suggesting that acute stress exposure may concurrently decrease kisspeptin neuron signaling and activate the RFRP-3 system, thereby providing increased restraint on the reproductive axis. In contrast to rapid changes in neuronal activation, changes in neuron gene expression take longer to manifest and may therefore underlie later decreases in LH at 180 minutes or longer. Indeed, we observed a significant increase in Rfrp levels (i.e., more detectable Rfrp cells) 180 minutes following the initiation of restraint stress, a response previously observed in male rats 5 hours after stress exposure (9). This later increase in Rfrp gene expression may be related to the earlier increase in activation of Rfrp neurons, perhaps as a mechanism to replenish RFRP-3 stores. Furthermore, prior evidence that restraint can suppress Kiss1 mRNA in female rats 5 hours following restraint stress suggests that Kiss1 gene expression may be reduced at later time points beyond 180 minutes (17).
In summary, we show that acute stress in mice elicits rapid and robust suppression of LH pulse frequency and alterations in kisspeptin and RFRP-3 neuron activation. Given the cutting-edge genetic tools currently available in mice, the advancement in our ability to study repeated measurements of LH in this species is critical to our examination of how stress pathways influence gonadotropin secretion. Future studies will evaluate the mechanisms by which Kiss1 neurons respond to stress and determine how Rfrp cells are involved in this suppression.
Acknowledgments
The authors thank Dr. Erica Schoeller and Dr. Karen Tonsfeldt for technical assistance and members of the Mellon, Kauffman, Lawson, Natale, and Thackray laboratories for helpful discussions throughout this work. We thank Dr. Al Parlow of the National Hormone and Peptide Program for providing reagents.
Financial Support: This work was supported by National Institutes of Health (NIH) Grants R00 HD060947 and R01 HD086100 (to K.M.B.) and National Science Foundation Grant IOS-1457226 (to A.S.K.). Serum hormone assays were performed by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by the Eunice Kennedy Shriver National Institute of Child Health and Development [NICHD/NIH (National Centers for Translational Research in Reproduction and Infertility)] Grant P50-HD28934. Additional support was provided by NIH P50 HD012303 and NIH T32 HD007203.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| LH | Antibovine LH | 581B7, Dr. Janet Roser | Monoclonal | UVA Ligand Core | AB_2665514 | |
| LH | Antihuman LH | 5303, Medix Kauniainen | Monoclonal | UVA Ligand Core | AB_2665513 | |
| LH | Rabbit LH antiserum | AFP240580Rb, NHPP | Polyclonal | UVA Ligand Core | AB_2665533 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- %CV
- coefficient of variation
- ANOVA
- analysis of variance
- DMN
- dorsal-medial nucleus
- GnRH
- gonadotropin-releasing hormone
- ISH
- in situ hybridization
- LH
- luteinizing hormone
- mRNA
- messenger RNA
- OVX
- ovariectomized
- RFRP-3
- RFamide-related peptide 3
- SEM
- standard error of the mean.
References
- 1.Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod. 1991;45(4):523–532. [DOI] [PubMed] [Google Scholar]
- 2.Fenster L, Katz DF, Wyrobek AJ, Pieper C, Rempel DM, Oman D, Swan SH. Effects of psychological stress on human semen quality. J Androl. 1997;18(2):194–202. [PubMed] [Google Scholar]
- 3.Mann DR, Orr TE. Effect of restraint stress on gonadal proopiomelanocortin peptides and the pituitary-testicular axis in rats. Life Sci. 1990;46(22):1601–1609. [DOI] [PubMed] [Google Scholar]
- 4.Fourman LT, Fazeli PK. Neuroendocrine causes of amenorrhea--an update. J Clin Endocrinol Metab. 2015;100(3):812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293(1):E270–E276. [DOI] [PubMed] [Google Scholar]
- 6.Breen KM, Thackray VG, Hsu T, Mak-McCully RA, Coss D, Mellon PL. Stress levels of glucocorticoids inhibit LHβ-subunit gene expression in gonadotrope cells. Mol Endocrinol. 2012;26(10):1716–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagenmaker ER, Moenter SM. Exposure to acute psychosocial stress disrupts the luteinizing hormone surge independent of estrous cycle alterations in female mice. Endocrinology. 2017;158(8):2593–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. Psychosocial stress inhibits amplitude of gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology. 2009;150(2):762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci USA. 2009;106(27):11324–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilbrook AJ, Turner AI, Clarke IJ. Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress. 2002;5(2):83–100. [DOI] [PubMed] [Google Scholar]
- 11.Ciechanowska M, Łapot M, Antkowiak B, Mateusiak K, Paruszewska E, Malewski T, Paluch M, Przekop F. Effect of short-term and prolonged stress on the biosynthesis of gonadotropin-releasing hormone (GnRH) and GnRH receptor (GnRHR) in the hypothalamus and GnRHR in the pituitary of ewes during various physiological states. Anim Reprod Sci. 2016;174:65–72. [DOI] [PubMed] [Google Scholar]
- 12.Łapot M, Ciechanowska M, Malewski T, Misztal T, Mateusiak K, Przekop F. The effect of stress on the expression of GnRH and GnRH receptor genes in the discrete regions of the hypothalamus and pituitary of anestrous ewes. Reprod Biol. 2007;7(1):55–71. [PubMed] [Google Scholar]
- 13.Chen MD, O’Byrne KT, Chiappini SE, Hotchkiss J, Knobil E. Hypoglycemic ‘stress’ and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology. 1992;56(5):666–673. [DOI] [PubMed] [Google Scholar]
- 14.Orr TE, Mann DR. Effects of restraint stress on plasma LH and testosterone concentrations, Leydig cell LH/hCG receptors, and in vitro testicular steroidogenesis in adult rats. Horm Behav. 1990;24(3):324–341. [DOI] [PubMed] [Google Scholar]
- 15.López-Calderón A, Gonzaléz-Quijano MI, Tresguerres JA, Ariznavarreta C. Role of LHRH in the gonadotrophin response to restraint stress in intact male rats. J Endocrinol. 1990;124(2):241–246. [DOI] [PubMed] [Google Scholar]
- 16.Saketos M, Sharma N, Santoro NF. Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod. 1993;49(6):1270–1276. [DOI] [PubMed] [Google Scholar]
- 17.Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Milligan SR, Lightman SL, O’Byrne KT. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21(1):20–29. [DOI] [PubMed] [Google Scholar]
- 18.Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ. Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology. 2005;146(4):2107–2115. [DOI] [PubMed] [Google Scholar]
- 19.Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab. 2011;300(1):E19–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czieselsky K, Prescott M, Porteous R, Campos P, Clarkson J, Steyn FJ, Campbell RE, Herbison AE. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794–4802. [DOI] [PubMed] [Google Scholar]
- 21.Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kawami T, Niki H, Kato T, Kuwahara A, Uemura H, Yasui T, Irahara M. Hypothalamic Kiss1 and RFRP gene expressions are changed by a high dose of lipopolysaccharide in female rats. Horm Behav. 2014;66(2):309–316. [DOI] [PubMed] [Google Scholar]
- 23.Fallest PC, Trader GL, Darrow JM, Shupnik MA. Regulation of rat luteinizing hormone beta gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod. 1995;53(1):103–109. [DOI] [PubMed] [Google Scholar]
- 24.Matteri RL, Roser JF, Baldwin DM, Lipovetsky V, Papkoff H. Characterization of a monoclonal antibody which detects luteinizing hormone from diverse mammalian species. Domest Anim Endocrinol. 1987;4(3):157–165. [DOI] [PubMed] [Google Scholar]
- 25.Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132(4):1687–1691. [DOI] [PubMed] [Google Scholar]
- 26.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poling MC, Kim J, Dhamija S, Kauffman AS. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology. 2012;153(4):1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo E, Stephens SB, Chaing S, Munaganuru N, Kauffman AS, Breen KM. Corticosterone blocks ovarian cyclicity and the LH surge via decreased kisspeptin neuron activation in female mice. Endocrinology. 2016;157(3):1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal A, Zhang Q, Médigue C, Fabre S, Clément F. DynPeak: an algorithm for pulse detection and frequency analysis in hormonal time series. PLoS One. 2012;7(7):e39001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elbassuoni EA. Gender differences in ghrelin response to chronic immobilization stress in rats: possible role of estrogen. Gen Physiol Biophys. 2014;33(1):111–120. [DOI] [PubMed] [Google Scholar]
- 31.Gallucci WT, Baum A, Laue L, Rabin DS, Chrousos GP, Gold PW, Kling MA. Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health Psychol. 1993;12(5):420–425. [DOI] [PubMed] [Google Scholar]
- 32.Goel N, Bale TL. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 2010;151(4):1784–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21(4):415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilbrook AJ, Turner AI, Clarke IJ. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod. 2000;5(2):105–113. [DOI] [PubMed] [Google Scholar]
- 35.Weathington JM, Cooke BM. Corticotropin-releasing factor receptor binding in the amygdala changes across puberty in a sex-specific manner. Endocrinology. 2012;153(12):5701–5705. [DOI] [PubMed] [Google Scholar]
- 36.Yamaura K, Bi Y, Ishiwatari M, Oishi N, Fukata H, Ueno K. Sex differences in stress reactivity of hippocampal BDNF in mice are associated with the female preponderance of decreased locomotor activity in response to restraint stress. Zoolog Sci. 2013;30(12):1019–1024. [DOI] [PubMed] [Google Scholar]