Abstract
Gonadotropin-releasing hormone (GnRH) neurons regulate reproduction though pulsatile hormone release. Disruption of GnRH release as measured via luteinizing hormone (LH) pulses occurs in polycystic ovary syndrome (PCOS), and in young hyperandrogenemic girls. In adult prenatally androgenized (PNA) mice, which exhibit many aspects of PCOS, increased LH is associated with increased GnRH neuron action potential firing. How GnRH neuron activity develops over the prepubertal period and whether this is altered by sex or prenatal androgen treatment are unknown. We hypothesized GnRH neurons are active before puberty and that this activity is sexually differentiated and altered by PNA. Dams were injected with dihydrotestosterone (DHT) on days 16 to 18 post copulation to generate PNA mice. Action potential firing of GFP-identified GnRH neurons in brain slices from 1-, 2-, 3-, and 4-week-old and adult mice was monitored. GnRH neurons were active at all ages tested. In control females, activity increased with age through 3 weeks, then decreased to adult levels. In contrast, activity did not change in PNA females and was reduced at 3 weeks. Activity was higher in control females than males from 2 to 3 weeks. PNA did not affect GnRH neuron firing rate in males at any age. Short-term action potential patterns were also affected by age and PNA treatment. GnRH neurons are thus typically more active during the prepubertal period than adulthood, and PNA reduces prepubertal activity in females. Prepubertal activity may play a role in establishing sexually differentiated neuronal networks upstream of GnRH neurons; androgen-induced changes during this time may contribute to the adult PNA, and possibly PCOS, phenotype.
GnRH neurons control fertility but are surprisingly most active before puberty. This activity is reduced in females exposed in utero to androgens, potentially altering adult function of GnRH neurons.
The ability to reproduce is typically associated with maturation into adulthood. The mechanisms underlying the establishment of the reproductive axis prior to and during puberty—and how disruptions of such mechanisms may be linked to various types of infertility—are not fully understood, particularly at the neural level. Gonadotropin-releasing hormone (GnRH) neurons link the central nervous system to the peripheral reproductive system through the release of GnRH. GnRH stimulates anterior pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone, which activate gonadal functions; gonadal hormones exert feedback to control GnRH release and pituitary response. GnRH is released in pulses (1, 2) with higher frequency favoring LH synthesis and release and lower frequency favoring follicle-stimulating hormone (3, 4). Disruption of pulsatile GnRH release or interfering with the typical modulation of pulse frequency during the reproductive cycle can lead to infertility (5). An example of the latter is polycystic ovary syndrome (PCOS), the leading cause of infertility in women (5). Many women with PCOS exhibit persistently high GnRH-pulse frequency as reflected by LH pulses in the peripheral circulation (6–8). This exacerbates hyperandrogenemia and produces a gonadotropin milieu that does not promote effective follicle maturation and ovulation.
To study neurobiological mechanisms potentially contributing to PCOS, prenatally androgenized (PNA) animal models are commonly used; women with PCOS who do achieve pregnancy exhibit elevated androgen levels in late gestation, potentially exposing their offspring to androgen excess during development (9, 10). In several species, PNA females exhibit many neuroendocrine aspects of PCOS, including disrupted cycles and elevated LH pulse frequency, as well as mildly increased testosterone levels (11–16). In adult female PNA mice, GnRH neuron action potential firing activity is increased (17), as is GABAergic synaptic transmission to these cells; GABA can excite GnRH neurons to fire action potentials (16, 18). These studies in PNA mice have revealed possible mechanisms contributing to the neuroendocrine phenotype in adults, but have not addressed when programming-induced changes become evident during development. These early changes are potentially relevant to the human condition because clinical studies suggest altered LH release manifests before the pubertal transition is complete in hyperandrogenemic girls (19). Additionally, late pubertal (Tanner stages 4 to 5) girls with hyperandrogenemia exhibit increased LH pulse frequency (20, 21), and girls diagnosed with PCOS have increased LH pulse frequency as young as 12 years of age (21). A role for androgens in this increased pulse frequency is suggested by studies in rhesus macaques, in which mild elevation of testosterone, characteristic of PCOS, during pubertal development increases LH pulse frequency during the early follicular phase (22). In prepubertal control mice, gonadotropin levels are lower than adults (23–25). In contrast, studies measuring GnRH release in brain slices from male mice indicate GnRH release frequency is highest during the first week of postnatal life (23). This discrepancy is potentially explained by the well-established downregulation of pituitary response to GnRH when exposed to high frequency pulses or continuously high levels of this hormone (3, 26). These observations suggest GnRH neurons are functional before puberty; that is, before downstream activation of the reproductive system is evident.
In these studies, we characterized GnRH neuron activity during prepubertal development in control and PNA mice. We hypothesized that GnRH neurons are active before puberty and that PNA increases this activity. To examine sex differences in typical development as well as to determine if effects of PNA are similar to those of typical masculinization, both females and males were studied.
Methods
Chemicals were purchased from Sigma Chemical Company (St. Louis, MO) unless noted.
Animals
GnRH-GFP mice on a C57Bl6/J background were held on a 14-hour light/10-hour dark light cycle with lights on at 0300 Eastern Standard Time. Mice had ad libitum access to water and chow (Teklad 2916) except breeders, which received higher protein 2919 chow (both from Envigo, Madison, WI). To generate PNA mice, a GnRH-GFP and a CD1 female were paired for 1 to 3 weeks and then a stud male introduced. Males were removed after pregnancy was established. PNA mice were generated by injecting pregnant GnRH-GFP dams with dihydrotestosterone (225 µg subcutaneously in sesame oil vehicle) on days 16 to 18 of gestation (day 1, copulatory plug observed). Controls included mice from vehicle-treated or uninjected GnRH-GFP dams; no differences were observed between control types and they were combined. The CD1 mouse was included for maternal and nutritional support to increase survival of PNA pups. Litter sizes were adjusted to <15 pups per cage (two lactating females; CD1 and GnRH-GFP dam in each cage) by culling CD1 pups to normalize nutrition. Pups not used for postnatal recordings were weaned at 3 weeks of age. Androgenization of PNA females was examined by measuring time of vaginal opening, anogenital distance, and estrous cycles by vaginal lavage for 15 consecutive days in remaining female littermates in adulthood; preputial separation was monitored in males. Because of the strict age requirements for recording pups, pups from four to nine litters were used. For adult recordings, two to five litters were used as these were recapitulating previous observations (16, 27, 28). The Institutional Animal Care and Use Committee of the University of Michigan (PRO00006816) approved all animal procedures preformed in this study.
Slice preparation
All solutions were bubbled with 95% O2/5% CO2 throughout the experiments and for at least 30 minutes before exposure to tissue. Between 0550 and 1530 Eastern Standard Time, the brain was rapidly removed and placed in ice-cold sucrose saline solution containing (in mM): 250 sucrose, 3.5 KCl, 26 NaHCO3, 10 d-glucose, 1.25 NaH2PO4, 1.2 MgSO4, and 3.8 MgCl2. Coronal (300 µm) slices were cut with a Leica VT1200S (Leica Biosystems, Buffalo Grove, IL). Slices were incubated in a 1:1 mixture of sucrose saline and artificial cerebrospinal fluid (ACSF) containing (in mM): 135 NaCl, 3.5 KCl, 26 NaHCO3, 10 d-glucose, 1.25 Na2HPO4, 1.2 MgSO4, 2.5 CaCl2 (pH 7.4) for 30 minutes at room temperature (21 to 23°C) and then transferred to 100% ACSF for additional 30 minutes at room temperature before recording. Recordings were performed 1 to 5 hours after brain slice preparation; no difference in firing patterns were evident based on time after brain slice preparation or time of day.
Extracellular recording
For recording, slices were placed into a chamber continuously perfused with ACSF at a rate of 2 to 3 mL/min with oxygenated ACSF heated to 30 to 32°C with an inline-heating unit (Warner Instruments, Hamden, CT). GFP-positive GnRH neurons were identified by brief illumination at 488 nm on an Olympus (Center Valley, PA) BX51WI microscope. Recording micropipettes were pulled from borosilicate capillary glass (type 7052, 1.65 mm outer diameter; 1.12 mm inner diameter; World Precision Instruments, Inc., Sarasota, FL) using a Flaming/Brown P-97 puller (Sutter Instruments, Novato, CA) to obtain pipettes with a resistance of 2 to 3 MΩ when filled with HEPES-buffered solution containing (in mM): 150 NaCl, 10 HEPES, 10 glucose, 2.5 CaCl2, 1.3 MgCl2, and 3.5 KCl. Recordings were made with an EPC-8 with ITC-18 interface or one channel of an EPC-10 dual patch clamp amplifier using Patchmaster software (HEKA Elektronik, Pfalz, Germany) running on a Macintosh computer. Low-resistance (<50 MΩ) seals were formed between the pipette and neuron after first exposing the pipette to the slice tissue in the absence of positive pressure. Recordings were made in voltage-clamp mode with a 0-mV pipette holding potential. Signals were acquired at 10 kHz and filtered at 5 kHz. Resistance of the loose seal and baseline was checked during an initial 5- to 15-minute stability period, and also at 10-minute intervals during recording; data were not used if seal resistance increased above 50 MΩ.
Experimental design
Brain slices were prepared from control and PNA female and male mice at 1, 2, 3, and 4 weeks of age and adults (17 to 38 weeks of age). Three-week-old mice were used before weaning to avoid changes subsequent to abrupt social and nutritional changes. Targeted extracellular recordings were used to record long-term (20 to 60 minutes) patterns of firing activity. This method maintains internal milieu and has minimal impact on the firing rate of neurons (29, 30). Recorded cells were mapped to an atlas (31) to determine if any trends based on anatomical location emerged; no such trends were apparent in these data sets. No more than three cells per animal and two cells per slice were included for analysis, and at least four mice were tested per group. Variation within an animal was not less than among animals.
Analysis
Action currents (events) were detected off-line using custom programs in Igor Pro 6.31 (Wavemetrics, Lake Oswego, OR). Data were binned at 60-second intervals and were transferred to Excel. Mean firing rate (Hz) was calculated by dividing the total number of events by the duration of recording. Further pattern analyses were done for the 3-week-old and adult groups, in which the most striking differences in mean firing rate were observed. The distribution of interspike intervals (ISIs) for each group was examined by comparing the probability of occurrence of ISIs between 0.01 and 100 seconds in increments of 0.5 seconds. A histogram of ISIs for all cells within a group was constructed and these distributions normalizing by dividing each interval by the total number of ISIs for the group. Normalized ISI distributions were compared using a Kolmogorov-Smirnov (KS) test.
Short-term burst-firing patterns are typically associated with neurosecretion (32). Burst parameters (duration, spikes/burst, frequency, intraburst and interevent intervals) were compared among groups. Bursts have been characterized in adult GnRH neurons (33–35), but given the difference in firing rate and patterns observed in the current study between prepubertal and adult mice, previously described burst parameters may not be optimal to analyze bursting activity at all ages. Bursts were thus first detected using software that systematically adjusted the maximum time between events (burst window) for inclusion in a burst (10-ms intervals up to 2 seconds total duration). The number of bursts was graphed as function of burst window. In most groups, a distinct peak was observed revealing a burst window that yielded the maximum number of bursts. This burst window was used for further analysis. Two groups (adult PNA females and males) did not exhibit a distinct peak, rather the number of bursts detected increased gradually as the burst window was lengthened; these groups were included in the analysis of burst parameters, but because they lacked a clear peak, were not used to select the burst window for further analysis. The peak of the burst window (range, 0.36 to 0.78 seconds) for each group exhibiting a peak was used to calculate burst parameters for all groups. One-way analysis of variance (ANOVA) revealed that the same statistical differences were found for burst frequency among groups regardless of the burst window chosen within this range (P > 0.99). We therefore chose a burst window of 0.36 (maximum burst number for 3-week-old control females) to compare burst parameters among age, and treatment groups by sex.
Statistics
Statistical analyses were performed using Prism 7 (GraphPad Software, La Jolla, CA) and IBM SPSS version 22 (IBM Corp., Armonk, NY). Data are reported as individual values with mean ± standard error of the mean (SEM). Data distributions were tested using Shapiro-Wilk normality test and used along with experimental design to select appropriate statistical comparisons. For within-sex comparisons, two-way ANOVA was used to compare all groups with Fisher least significant difference (LSD) post hoc test; this choice was justified by the large number of comparisons (i.e., 45 unique comparisons within each sex between both treatments at five different ages). When comparisons were restricted to between groups at the same age, Sidak was used a post hoc. Burst parameters for each sex were compared for effects of age and treatment with two-way ANOVAs with Tukey honest significant difference test; Tukey was chosen for this analysis to permit comparisons of the mean of each group to all others. Further tests are specified in the figure legends and results. The null hypothesis was rejected if P < 0.05, but all P values < 0.1 are reported.
Results
Prenatal androgenization alters timing of pubertal indicators in both sexes and reproductive parameters in adult female mice
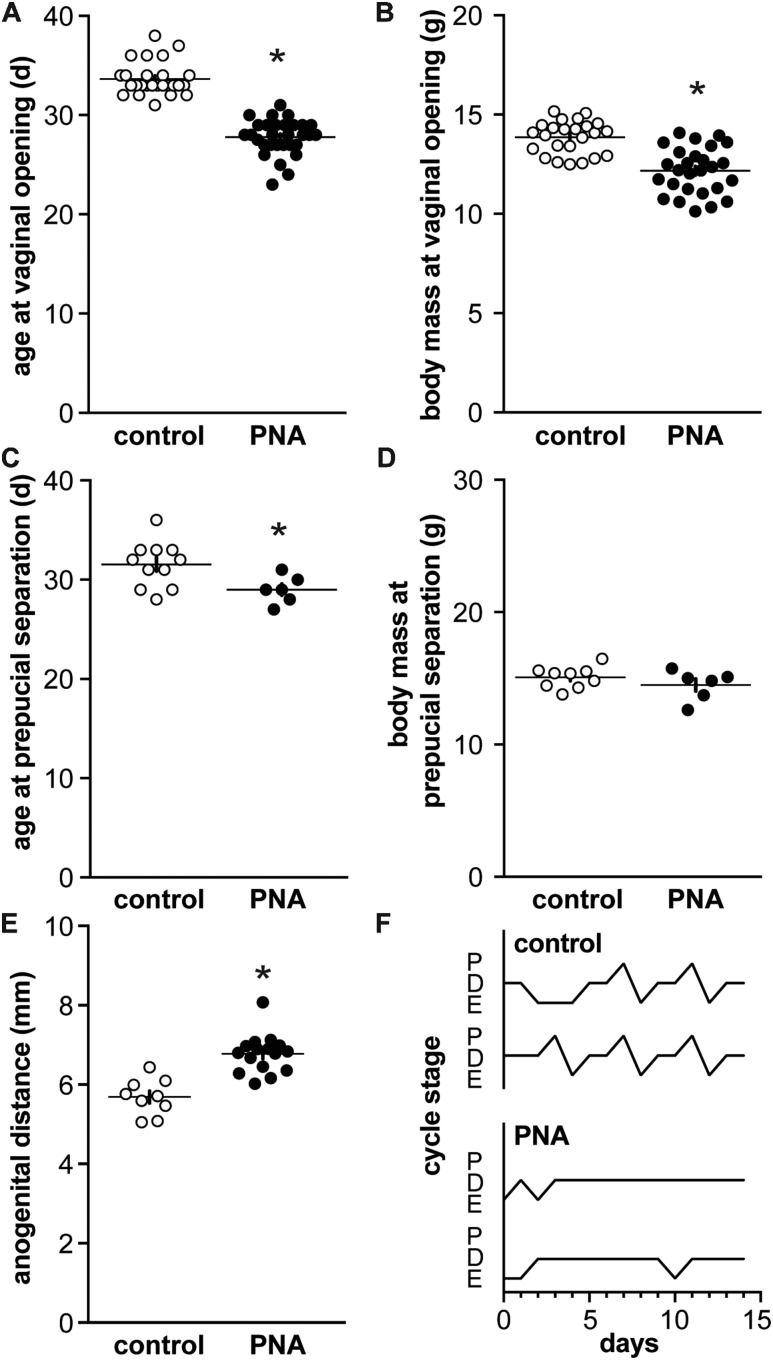
PNA females exhibit early vaginal opening, and altered hormone levels and disrupted cycles as adults (17, 36, 37). Here, we studied when PNA-induced differences emerge with postnatal development, often precluding evaluation of these parameters in the study subjects. These aspects were thus verified in littermates that were allowed to survive to adulthood; in our experience, the PNA phenotype is consistent among littermates. To verify the prenatal androgenization phenotype, vaginal opening in females and preputial separation in males were monitored from weaning. As reported (14), vaginal opening occurred at a younger age in PNA females despite a lower body mass, which was attributable to younger age (Fig. 1A and 1B; n = 26 control and 30 PNA, age at vaginal opening P < 0.0001 two-tailed Mann-Whitney U test, body mass at vaginal opening P < 0.0001 two-tailed unpaired Student t test for samples with similar standard deviations). In males, preputial separation was advanced in PNA mice but no difference in body mass was observed, likely because the absolute shift in age of preputial separation for males was only ∼2 days (Fig. 1C and 1D; n = 11 control and 6 PNA, both P < 0.05 two-tailed unpaired Student t test for samples with similar standard deviations). PNA treatment increased anogenital distance in females (Fig. 1E, unpaired Student t test), and also disrupted cyclicity in adult littermates of PNA mice used for recordings (Fig. 1F). Specifically, PNA decreased the percent of days in estrus and proestrus (n = 15 both control and PNA; estrus control 29% ± 1.6%, PNA 7% ± 1.8%, proestrus control 15% ± 1.4%, PNA 1% ± 0.75%, both P < 0.0001, χ2 test). The outward effects of PNA are thus present by puberty in both sexes and persist in females into adulthood.
Figure 1.
Characterization of PNA animals. (A) Age and (B) body mass at vaginal opening in control (open symbols) and PNA (filled symbols) females. (C) Age and (D) body mass at preputial separation in control and PNA males. (E) Anogenital distance in adult female littermates of mice that were used for recording before puberty. (F) Representative estrous cycles in adult female littermates: *P < 0.05 control vs PNA, unpaired Student t test in all but (A) in which a Mann-Whitney U test was used. D, diestrus; E, estrus; P, proestrus.
Prepubertal development of GnRH neuron activity is sexually differentiated
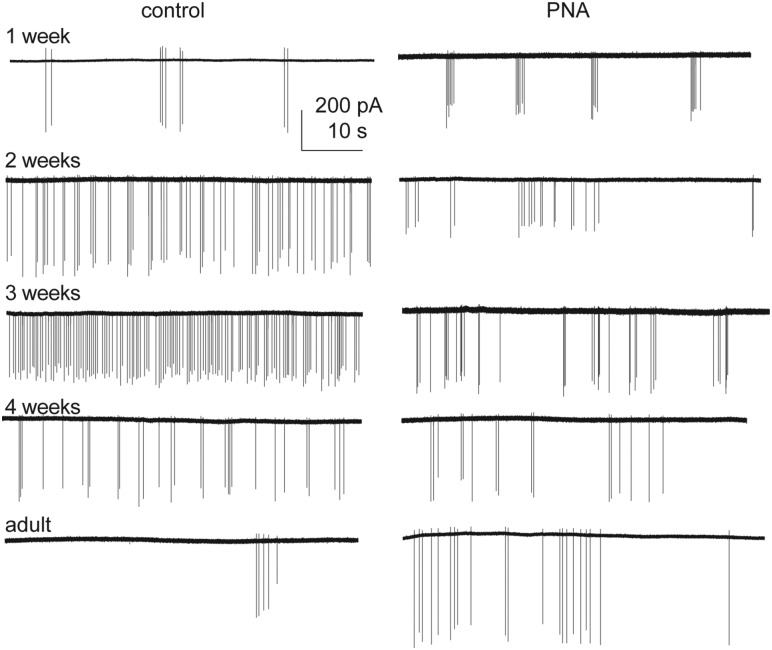
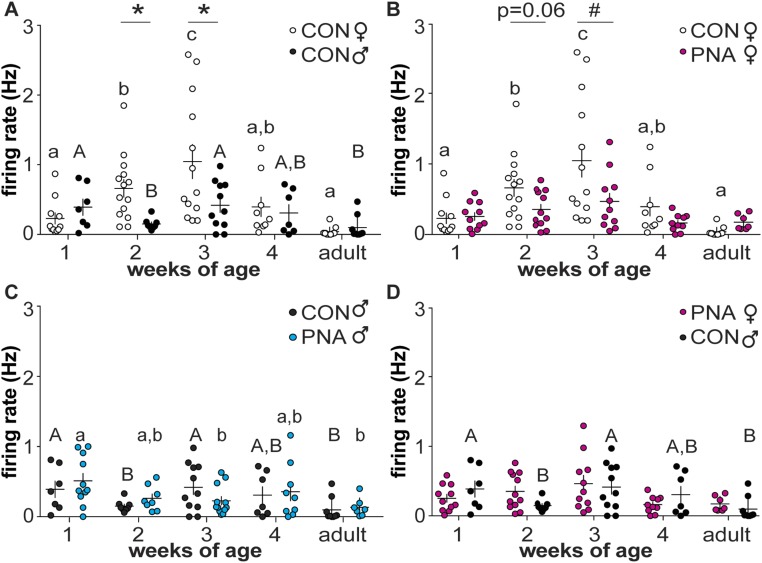
To test the hypotheses that GnRH neurons are dynamically active through prepubertal development, extracellular recordings were made of GFP-identified GnRH neurons. Representative traces from female mice are shown in Fig. 2, summary data in Fig. 3, and statistical parameters in Tables 1 and 2. In cells from control females, firing activity was low during 1 week of age, and increased each week to a peak at 3 weeks of age, before declining to adult levels. In contrast to females, GnRH neuron activity in control males decreased from the first to second postnatal week of age, then rose again at week 3 before returning to lower levels during adulthood (Fig. 3A). As a result of these different developmental patterns, activity was greater in control females than control males at 2 and 3 weeks of age. GnRH neuron firing activity during prepubertal development is thus both notably higher than adults and is sexually differentiated in control mice.
Figure 2.
Activity of GnRH neurons from female mice changes with age and prenatal androgenization. One-minute representative raw recordings of GnRH neurons from control (left) and PNA (right) females.
Figure 3.
GnRH neuron activity changes throughout the prepubertal period in both sexes and is altered by PNA. (A–D) Individual values and mean ± SEM of firing rate at 1, 2, 3, and 4 weeks of age and adults. (A) Control females (open circles) and males (black circles); (B) control and PNA (magenta circles) females; (C) control and PNA (blue circles) males; (D) PNA females and control males. Different letters of the same case indicate differences with age within a group; there were no changes with age in female PNA mice (P ≥ 0.0978). *P < 0.05, #P < 0.01 between treatment groups at each age. Two-way ANOVA/Fisher LSD for comparisons among all groups within sex; two-way ANOVA/Sidak for comparisons at the same age between sexes (control female vs control male; PNA female vs control male). CON, control.
Table 1.
Cells/Group for Activity Recordings
| Age, wk | Control ♀ | PNA ♀ | Control ♂ | PNA ♂ |
|---|---|---|---|---|
| 1 | 11 | 11 | 7 | 11 |
| 2 | 14 | 12 | 8 | 8 |
| 3 | 13 | 11 | 11 | 12 |
| 4 | 9 | 10 | 7 | 9 |
| Adult | 11 | 7 | 9 | 7 |
Table 2.
Two-Way ANOVA Parameters for Comparison Among Firing Rates Within Groups
| Comparison (Figure) | Age | Sex | Interaction |
|---|---|---|---|
| ♀ Control vs ♂control (3A) | F(4, 90) = 6.613a | F(1, 90) = 5.091b | F(4, 90) = 3.304b |
| Age | Treatment | Interaction | |
| ♀ Control vs ♀PNA (3B) | F(4, 99) = 8.074a | F(1, 99) = 5.464b | F(4, 99) = 2.405 |
| ♂ Control vs ♂ PNA (3C) | F(4, 79 ) = 3.712c | F(1, 79) = 0.1815 | F(4, 79) = 1.139 |
| Age | Sex and Treatment | Interaction | |
| ♀ PNA vs ♂ control (3D) | F(4, 83) = 3.724c | F(1, 83) = 0.0388 | F(4, 83) = 1.504 |
P < 0.001.
P < 0.05.
P < 0.01.
PNA alters the development of GnRH neuron activity in both sexes
Based on observations of increased reproductive neuroendocrine activity in both women with PCOS and PNA animal models, we hypothesized that PNA increases GnRH neuron firing in female mice during the prepubertal period. Contrary to our hypothesis, firing rate was lower in PNA mice at 3 weeks of age and the P value approached the level set for significance at 2 weeks of age. Activity did not change with age in cells from PNA females over the period examined (Fig. 3B; P ≥ 0.0978). In utero exposure to androgens thus alters the normal development of GnRH neurons in females, reducing firing activity during the prepubertal period rather than increasing firing as postulated.
In contrast to cells from PNA females, activity of cells from PNA males varied with age. Specifically, activity of GnRH neurons from male PNA mice was decreased at 3 weeks of age and in adults compared with 1-week-old mice (Fig. 3C). Of note, PNA treatment partially ameliorated the decline of activity from 1 to 2 weeks of age that was observed in control males. Also in contrast to females, there was no difference in GnRH neuron activity between control and PNA males of the same age, suggesting that effects of PNA treatment in male offspring may be milder than in female offspring.
Prepubertal development of GnRH neuron activity in PNA females is similar to that of control males
To examine if PNA treatment in females results in a GnRH neuron activity development pattern that is similar to males, we compared recordings between PNA female and control male mice at all ages tested. There was no difference in GnRH neuron activity between PNA female and control males at any age (P > 0.3, two-way ANOVA/Sidak, Fig. 3D; P > 0.08 two-way ANOVA/Fisher LSD, not shown). These data suggest PNA treatment in females, at least in part, masculinizes development of GnRH neuron activity.
Action potential timing in GnRH neurons changes with age, sex, and PNA treatment
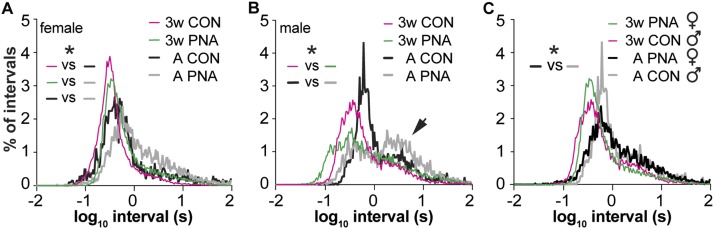
The firing rate data are mean values over recordings lasting up to 1 hour. This provides a good overview of activity levels, but differences in spike timing are also important to neuronal function, in particular for the release of neuropeptides in adults (32, 38) and synapse formation during development (39). We investigated action potential patterning of GnRH neurons from mice of each sex and treatment at 3 weeks of age and in adults as these ages had the largest PNA-induced difference in overall activity in females, which are of primary interest with regards to PCOS. First, we compared the probability distribution of ISIs among groups. In histograms of ISI probability, a sharp peak indicates a fairly regular firing pattern, multiple peaks indicate strict burst firing (long interval between bursts and shorter intervals within bursts) and flatter distributions indicate irregularly firing neurons that may exhibit a combination of bursts and individual spikes (40); ISI distributions for most groups of GnRH neurons studied fell into this latter category. In control females, ISI distributions shifted toward longer intervals in adults (Fig. 4A; KS, P < 0.0004, 3 weeks vs adult; note KS tests only permit two distributions to be compared). There was no difference between PNA and control females at 3 weeks of age (KS, P = 0.3927). In adulthood, however, PNA mice exhibited a longer interval shoulder that resulted in a shift in ISI distributions compared with controls (KS, P < 0.05). These data suggest that spike intervals shift with age in female mice and that PNA treatment leads to altered ISI distribution in adults.
Figure 4.
Spike timing in GnRH neurons changes with age in females and differs with PNA treatment in adults. (A) Log10 ISI distributions of GnRH recordings for each group of female mice. (B) Log10 ISI distribution for each group of male mice. Arrow indicates longer interval shoulder (see text). (C) Log10 ISI distributions comparing male control and female PNA mice, *P < 0.05, KS. Note change in color in panel (C) vs (A) and (B) for adult animals. CON, control.
In control males, ISI distributions did not quite differ with age (Fig. 4B, KS, P = 0.0522, 3 weeks vs adult), trending toward longer intervals in adults, but did differ with PNA treatment in both three-week-old (KS, P = 0.0004) and adult mice (KS, P = 0.0062). Further, a distinct shoulder appeared at longer intervals in both control and PNA adult males (arrow in Fig. 4B), suggesting emergence of a greater percentage of burst firing with age. These results indicate that despite a lack of effect of PNA on mean firing rate in males, this treatment does affect short-term organization of spikes. To further address the question of whether PNA induces masculinization of GnRH neuron firing in females, we compared ISI distributions between cells from PNA female and control male mice. These distributions did not differ at 3 weeks of age (KS, P = 0.27) but a difference emerged in adulthood (KS, P = 0.03; Fig. 4C). PNA treatment thus induces changes in spike organization in females that are distinct from masculinization.
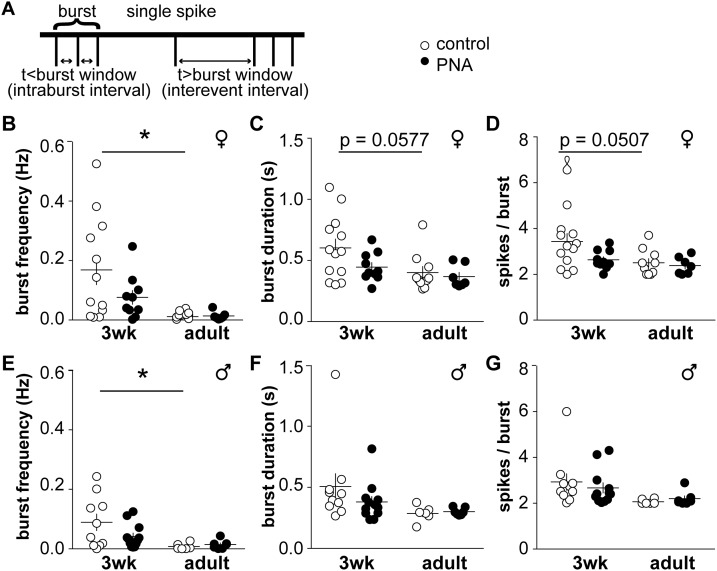
Burst patterning in GnRH neurons is affected by age but not PNA treatment
We also examined how sex, age and PNA treatment affect organization of action potentials into bursts (Fig. 5A; Tables 3 and 4). In control female mice, burst frequency was higher at 3 weeks of age than in adults (Fig. 5B, two-way-ANOVA/Tukey, P < 0.05). Burst duration and spikes/burst approached significance with age in control mice, with younger animals having longer bursts with more spikes (Fig. 5C and 5D: two-way ANOVA/Tukey, P = 0.0577 burst duration, P = 0.0507 spikes/burst). In females, intraburst interval did not differ among groups, but interevent interval differed with age in controls, and between adult control and PNA groups (Table 3). In males, burst frequency was increased in 3-week-old control animals compared with adults (Fig. 5E, two-way ANOVA/Tukey, P < 0.05); however, although there was an effect with age on burst duration and spikes/burst, post hoc analysis revealed no differences among groups (Fig. 5F and 5G; P > 0.16 for all comparisons). Intraburst interval and the interevent interval were not different among male groups, and no effect of PNA was detected. Burst frequency is thus higher at younger ages in both sexes; in females, the properties of these bursts are also shifted with age.
Figure 5.
Burst patterns of GnRH neurons differ with age but are not affected by PNA. (A) Schematic showing burst parameter determination. (B) Burst frequency, (C) duration, (D) and spikes/burst in cells from female mice. (E) Burst frequency, (F) duration, and (G) spikes/burst in cells from male mice. *P < 0.05, two-way-ANOVA/Tukey.
Table 3.
Mean ± SEM Intraburst and Interevent Intervals
| Parameter | 3-wk ♀ Control | 3-wk ♀ PNA | 3-wk ♂ Control | 3-wk ♂ PNA | Adult ♀ Control | Adult ♀ PNA | Adult ♂ Control | Adult ♂ PNA |
|---|---|---|---|---|---|---|---|---|
| Intraburst interval, s | 0.26 ± 0.01 | 0.27 ± 0.01 | 0.26 ± 0.01 | 0.24 ± 0.01 | 0.27 ± 0.02 | 0.27 ± 0.01 | 0.27 ± 0.02 | 0.26 ± 0.02 |
| Interevent interval, s | 2.86a ± 0.58 | 8.43 ± 3.35 | 71.2 ± 67.6 | 16.4 ± 5.11 | 62.1 ± 19.9 | 14.3b ± 3.90 | 163.4 ± 117.6 | 46.9 ± 29.0 |
P < 0.001 vs adult control females, two-way ANOVA/Tukey.
P < 0.05 vs adult control females, two-way ANOVA/Tukey.
Table 4.
Statistical Parameters for Burst Analysis
| Comparison | Parameter | Age | Treatment | Interaction |
|---|---|---|---|---|
| ♀ Control vs ♀ PNA | Frequency | F (1, 38) = 11.72a | F (1, 38) = 2.522 | F (1, 38) = 2.867; P = 0.09 |
| Duration | F (1, 36) = 5.612b | F (1, 36) = 2.591 | F (1, 36) = 1.093 | |
| Spikes/burst | F (1, 36) = 4.931b | F (1, 36) = 2.996; P = 0.09 | F (1, 36) = 1.608 | |
| Intraburst interval | F (1, 36) = 0.277 | F (1, 36) = 0.321 | F (1, 36) = 0.506 | |
| Interevent interval | F (1, 38) = 8.814a | F (1, 38) = 3.711; P = 0.06 | F (1, 38) = 5.926b | |
| ♂ Control vs ♂ PNA | Frequency | F (1, 34) = 8.368a | F (1, 34) = 0.7651 | F (1, 34) = 1.832 |
| Duration | F (1, 31) = 4.331b | F (1, 31) = 0.6023 | F (1, 31) = 0.9594 | |
| Spikes/burst | F (1, 31) = 5.874b | F (1, 31) = 0.05163 | F (1, 31) = 0.502 | |
| Intraburst interval | F (1, 31) = 0.9099 | F (1, 31) = 0.9165 | F (1, 31) = 0.2845 | |
| Interevent interval | F (1, 33) = 0.8984 | F (1, 33) = 1.751 | F (1, 33) = 0.2273 |
P < 0.01.
P < 0.05.
Discussion
PCOS is the most common infertility disorder in reproductive-aged women. Although the underlying causes are still emerging, studies suggest that in addition to genetic associations (41), high levels of circulating androgens and altered placental steroidogenesis in pregnant women with PCOS could expose the fetus to an altered endocrine milieu (9, 10). We and others have used prenatal exposure to androgens (PNA) to construct animal models that recapitulate many aspects of PCOS, enabling potential underlying mechanisms to be studied (11–16). Based on growing clinical evidence that altered neuroendocrine activity manifests in hyperandrogenemic girls during the pubertal transition (19–21, 42), we used the PNA mouse model to examine GnRH neuron activity during development and whether this is altered by PNA and/or is sexually differentiated. GnRH neurons are remarkably active in prepubertal mice, and their activity is affected by PNA treatment, sex, and age. These results suggest GnRH neurons are not merely “turned on” at the pubertal transition, but play a role throughout development; disruption of this early-life activity may contribute to PCOS.
Striking sex differences were observed in the development of GnRH neuron firing activity. Firing rate of GnRH neurons from control females increased steadily through the first 3 weeks of development. In contrast, male firing activity decreased from 1 to 2 weeks of age. This result is consistent with the previously observed decline in GnRH release from 1 to 2 weeks of age in brain slices from male mice (23). The pattern of GnRH release during prepubertal development in females has not been characterized, but the observation that release at the median eminence is largely dependent upon action potentials (43) suggests release may follow a pattern similar to firing in females, and thus be different than males. It is notable that despite these sex differences in GnRH neuron activity during development, females and males share the surprising finding that activity of GnRH neurons peaks well before any outward signs of puberty are evident.
Because PNA increases reproductive neuroendocrine output in adults in all species studied, we postulated GnRH neuron firing rate would be greater in PNA than control females during the prepubertal period. Contrary to this hypothesis, PNA exposure reduced GnRH neuron activity during prepubertal development, most notably at 3 weeks of age, although the decline appears to manifest earlier. This result is of interest with regard to studies examining pulsatile LH release in young obese girls. LH pulse frequency is reduced in these girls compared with controls in early Tanner stages (Tanner stages 1 to 2). This was reversed, however, in later stages (Tanner stages 4 to 5) with LH pulse frequency being higher than controls (44). Circulating androgens increase with body mass index quintile (45), thus obese girls have a higher tendency to be hyperandrogenemic, although the small numbers in McCartney et al. (44) precluded a statistical identification of hyperandrogenemia. No animal model can perfectly recapitulate a human disease, but the present data showing an initial suppression of GnRH neuron activity during development coupled with elevated GnRH neuron activity (17) and increased LH-pulse frequency in adult PNA females (17, 36) seem to parallel these clinical findings. Of note, no difference was detected in firing rate of GnRH neurons from adult PNA vs control mice in the current study; we attribute this to the loss of statistical power with the high number of comparisons made. The means are similar to the previous report and a two-way test of adult values achieves significance.
In contrast to females, PNA had no effect on firing rate in cells from male mice. This supports and extends previous work showing that few neuroendocrine deficits causing fertility problems have been observed in male offspring of women with PCOS (46). These offspring do exhibit metabolic deficits and higher anti-Müllerian hormone levels (46, 47), but these are downstream and/or tangentially related to the reproductive neuroendocrine axis. Whether PNA treatment in females merely masculinizes the GnRH neurosecretory system is an obvious question, given androgens are typically elevated in male fetuses during development to facilitate differentiation of the genitalia in a male pattern (48). In the present studies, the suppression of overall firing rate before puberty in PNA compared with control females suggests masculinization. Differences between PNA females and control males were observed, however, in action potential firing patterns. In controls from both sexes, burst frequency declined with age. ISI also shifted to longer intervals with age and became more irregular. Although burst properties were not affected by PNA, the interval distribution in adult PNA females was different than control males. This difference in action potential patterning argues against masculinization as a simple explanation for effects of PNA and suggests finer details of GnRH neuron firing may be altered by PNA exposure.
The observation that GnRH neuron firing rate peaks before puberty indicates that the main role of GnRH neuron activity before activation of the downstream reproductive system may be unrelated to reproduction per se. In adults, GnRH is released not only in the median eminence, but also from the perisomatic region and proximal processes (43, 49). The former regulates pituitary gonadotropin output, but the latter acts as a neuromodulator to alter central circuits, including regulating GABAergic transmission to GnRH neurons (50). High-frequency depolarization of GnRH neurons can also affect short-term plasticity in GABAergic transmission to these cells via glia and/or endocannabinoid intermediates (51). Neuronal activity plays many roles in the development of neuronal circuits, including formation, maturation, and refinement of synapses (52–54). Synapse formation is driven by a competitive process involving strong activity input from the postsynaptic cell (55, 56). The present observations of higher burst frequency in GnRH neurons from younger animals are consistent with this. Classic studies in the cat visual cortex demonstrated that blocking activity leads to reorganization of synaptic connections and functional output of retinal ganglion cells (57). Decreasing activity of cultured hippocampal neurons before synapse formation decreased functional inputs to that cell. In contrast, decreasing activity after formation of synaptic connections increases the number of synapses to the cell as a way to restore activity (58).
An alternative explanation for the PNA-induced decline in GnRH neuron activity during development is that changes in GnRH neuron activity reflect postsynaptic changes initiated within the GnRH neuron to compensate for altered synaptic input. In this regard, a preliminary report indicates prepubertal GABAergic transmission to GnRH neurons is increased at 3 weeks of age in PNA mice (59), corresponding to the time when PNA reduced GnRH firing rate in the current study. GABAergic transmission is typically excitatory in these cells (18, 60). Consistent with the observed increase in GABAergic transmission, appositions from GABAergic processes that originate in the arcuate nucleus of the hypothalamus are increased in PNA mice as early as postnatal day 25 (61). Whether changes GnRH neuron activity or the presynaptic network come first is unknown. Both pre- and postsynaptic components play a role in the final maturation and establishment of a synapse (62), supporting the idea that either altered activity and/or GABAergic inputs in PNA animals may affect synaptogenesis within the GnRH network. GABA can act as a paracrine factor to help establish synapses (63, 64). Activity of the postsynaptic cell can also modify postsynaptic scaffolding proteins that stabilize receptors (65). Additionally, both activity and GABA are thought to play a role in the retraction of synapses. Reducing GABA release in basket interneurons by knockdown of the vesicular loading transporters decreases branch pruning and increases the number of synaptic boutons (66), and postnatal activity in the visual cortex drives the engulfment of synapses via microglia (67). Together, these observations suggest the postulate that GnRH neuron activity before puberty is important for attracting, pruning, and refining synaptic connections in the postnatal GnRH network; the suppression of activity in PNA females is therefore likely to alter these processes.
Prenatal exposure to androgens may act centrally to program changes in the circuitry and/or activity of GnRH neurons. GnRH neurons, however, do not express detectable levels of the androgen receptor (68), suggesting androgens initiate the cascade of events that program PNA-induced changes in another cell type. Androgen receptor expression in the early embryonic brain has not been well characterized. In rats, mRNA has been detected at E20 in multiple brain regions (69), whereas immunohistochemical studies in the mouse hypothalamus detected androgen receptors at P5 but not E19 (70). Neither of those studies examined androgen receptor within GABAergic neurons; however, this receptor is expressed in GABAergic cell populations of the adult hypothalamus (71). Another possibility is that PNA alters other processes that then affect neural circuits; for example, postnatal androgen production. Hyperandrogenemic women with PCOS have mild elevations of androgen, lower than typical male levels (5). In primates, exposure to a similar mild elevation in testosterone during the prepubertal period increases LH pulse frequency around puberty (22). Of interest in this regard, androgens increase GnRH neuron firing in brain slices from adult mice of both sexes (27, 28) and also upregulate GABA transmission to adult GnRH neurons in an activity-dependent manner (72). Programming and activational effects of androgens in this system are not mutually exclusive and both may be contributing to the PNA phenotype during development and in adulthood. Of note, it is not known when changes in androgen levels occur in PNA mice during development, thus whether the observed developmental changes are solely attributable to prenatal androgen exposure cannot be determined from these studies.
The present demonstration that the final common pathway of the reproductive neuroendocrine axis is active prior to puberty, is altered by PNA, and is sexually differentiated provides insight into both typical and pathological prepubertal development. If GnRH activity during this early postnatal developmental stage does indeed sculpt the afferent network, sex differences in activity may be critical for attraction of inputs needed for sexually differentiated reproductive functions, such as female-specific generation of a GnRH surge in most mammals. The early suppression of GnRH neuron activity by PNA in females may produce changes that ultimately lead to upregulation of the reproductive neuroendocrine axis observed in many adult PNA models and women with PCOS. An interesting, albeit speculative, possibility raised by these observations is that changes in neural activity could be the initial insult in a cascade that, in some cases, independently leads to PCOS, perhaps particularly in individuals in which one of several gene associations that have now been made are not identifiable (41, 73–75). Together these findings suggest the postulate that GnRH neurons have a typical neuronal role, setting up the neural network needed for later reproductive function, before they assume the neuroendocrine role regulating fertility with which they are traditionally associated.
Acknowledgments
We thank Laura L. Burger and Elizabeth R. Wagenmaker for expert technical assistance and Christopher McCartney, University of Virginia, for comments on the manuscript.
Financial Support: This work was supported by National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant P50 HD28934 and a National Science Foundation Graduate Research Program Fellowship (to E.A.D.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance
- GnRH
- gonadotropin-releasing hormone
- ISI
- interspike interval
- KS
- Kolmogorov-Smirnov
- LH
- luteinizing hormone
- LSD
- least significant difference
- PCOS
- polycystic ovary syndrome
- PNA
- prenatally androgenized
- SEM
- standard error of the mean.
References
- 1.Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130(1):503–510. [DOI] [PubMed] [Google Scholar]
- 2.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. [DOI] [PubMed] [Google Scholar]
- 3.Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. [DOI] [PubMed] [Google Scholar]
- 4.Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128(1):509–517. [DOI] [PubMed] [Google Scholar]
- 5.McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20(4):317–326. [DOI] [PubMed] [Google Scholar]
- 6.Marshall JC, Eagleson CA, McCartney CR. Hypothalamic dysfunction. Mol Cell Endocrinol. 2001;183(1-2):29–32. [DOI] [PubMed] [Google Scholar]
- 7.Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF Jr. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66(1):165–172. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, Hall JE. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2248–2256. [DOI] [PubMed] [Google Scholar]
- 9.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17(10):2573–2579. [DOI] [PubMed] [Google Scholar]
- 10.Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):151–155. [DOI] [PubMed] [Google Scholar]
- 11.Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67(1):155–163. [DOI] [PubMed] [Google Scholar]
- 12.Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72(6):1475–1483. [DOI] [PubMed] [Google Scholar]
- 13.Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1-2):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207(2):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9(2):62–67. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101(18):7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011;152(2):618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16(12):2872–2891. [DOI] [PubMed] [Google Scholar]
- 19.Collins JS, Beller JP, Burt Solorzano C, Patrie JT, Chang RJ, Marshall JC, McCartney CR. Blunted day-night changes in luteinizing hormone pulse frequency in girls with obesity: the potential role of hyperandrogenemia. J Clin Endocrinol Metab. 2014;99(8):2887–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab. 2005;90(5):2810–2815. [DOI] [PubMed] [Google Scholar]
- 21.Yoo RY, Dewan A, Basu R, Newfield R, Gottschalk M, Chang RJ. Increased luteinizing hormone pulse frequency in obese oligomenorrheic girls with no evidence of hyperandrogenism. Fertil Steril. 2006;85(4):1049–1056. [DOI] [PubMed] [Google Scholar]
- 22.McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27(2):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34(45):15060–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selmanoff MK, Goldman BD, Ginsburg BE. Developmental changes in serum luteinizing hormone, follicle stimulating hormone and androgen levels in males of two inbred mouse strains. Endocrinology. 1977;100(1):122–127. [DOI] [PubMed] [Google Scholar]
- 25.Michael SD, Kaplan SB, Macmillan BT. Peripheral plasma concentrations of LH, FSH, prolactin and GH from birth to puberty in male and female mice. J Reprod Fertil. 1980;59(1):217–222. [DOI] [PubMed] [Google Scholar]
- 26.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–633. [DOI] [PubMed] [Google Scholar]
- 27.Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod. 2006;74(5):931–937. [DOI] [PubMed] [Google Scholar]
- 28.Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology. 2006;147(3):1474–1479. [DOI] [PubMed] [Google Scholar]
- 29.Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online. 2003;5:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcami P, Franconville R, Llano I, Marty A. Measuring the firing rate of high-resistance neurons with cell-attached recording. J Neurosci. 2012;32(9):3118–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidman RLAJ, Pierce ET. Atlas of the Mouse Brain and Spinal Cord. Cambridge, MA: Harvard University Press; 1971. [Google Scholar]
- 32.Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290(2):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu Z, Tomaiuolo M, Bertram R, Moenter SM. Two types of burst firing in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2012;24(7):1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunemaker CS, Straume M, DeFazio RA, Moenter SM. Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology. 2003;144(3):823–831. [DOI] [PubMed] [Google Scholar]
- 35.Gaskins GT, Moenter SM. Orexin a suppresses gonadotropin-releasing hormone (GnRH) neuron activity in the mouse. Endocrinology. 2012;153(8):3850–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci USA. 2015;112(2):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witham EA, Meadows JD, Shojaei S, Kauffman AS, Mellon PL. Prenatal exposure to low levels of androgen accelerates female puberty onset and reproductive senescence in mice. Endocrinology. 2012;153(9):4522–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76(1):98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratt KG, Dong W, Aizenman CD. Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci. 2008;11(4):467–475. [DOI] [PubMed] [Google Scholar]
- 40.Romanò N, Yip SH, Hodson DJ, Guillou A, Parnaudeau S, Kirk S, Tronche F, Bonnefont X, Le Tissier P, Bunn SJ, Grattan DR, Mollard P, Martin AO. Plasticity of hypothalamic dopamine neurons during lactation results in dissociation of electrical activity and release. J Neurosci. 2013;33(10):4424–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Zhao J, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Li Y, Ma J, Zhao Y. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55–59. [DOI] [PubMed] [Google Scholar]
- 42.Collins JS, Marshall JC, McCartney CR. Differential sleep-wake sensitivity of gonadotropin-releasing hormone secretion to progesterone inhibition in early pubertal girls. Neuroendocrinology. 2012;96(3):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glanowska KM, Moenter SM. Differential regulation of GnRH secretion in the preoptic area (POA) and the median eminence (ME) in male mice. Endocrinology. 2015;156(1):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91(2):492–497. [DOI] [PubMed] [Google Scholar]
- 46.Recabarren SE, Sir-Petermann T, Rios R, Maliqueo M, Echiburú B, Smith R, Rojas-García P, Recabarren M, Rey RA. Pituitary and testicular function in sons of women with polycystic ovary syndrome from infancy to adulthood. J Clin Endocrinol Metab. 2008;93(9):3318–3324. [DOI] [PubMed] [Google Scholar]
- 47.Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburú B, Codner E, Cassorla F, Rojas P, Sir-Petermann T. Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(5):1820–1826. [DOI] [PubMed] [Google Scholar]
- 48.Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol. 2010;31(3):341–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuenzalida LC, Keen KL, Terasawa E. Colocalization of FM1-43, Bassoon, and GnRH-1: GnRH-1 release from cell bodies and their neuroprocesses. Endocrinology. 2011;152(11):4310–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen P, Moenter SM. GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. J Neurosci. 2009;29(31):9809–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glanowska KM, Moenter SM. Endocannabinoids and prostaglandins both contribute to GnRH neuron-GABAergic afferent local feedback circuits. J Neurophysiol. 2011;106(6):3073–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4(Suppl):1207–1214. [DOI] [PubMed] [Google Scholar]
- 53.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. [DOI] [PubMed] [Google Scholar]
- 54.Andreae LC, Burrone J. The role of neuronal activity and transmitter release on synapse formation. Curr Opin Neurobiol. 2014;27:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Favero M, Massella O, Cangiano A, Buffelli M. On the mechanism of action of muscle fibre activity in synapse competition and elimination at the mammalian neuromuscular junction. Eur J Neurosci. 2009;29(12):2327–2334. [DOI] [PubMed] [Google Scholar]
- 56.Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434(7036):1022–1026. [DOI] [PubMed] [Google Scholar]
- 57.Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6(8):2117–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420(6914):414–418. [DOI] [PubMed] [Google Scholar]
- 59.Berg T, Moenter SM. Prenatal androgenization alters prepubertal development of gonadotropin-releasing hormone (GnRH) neuronal network function and connectivity. In Neuroscience Meeting Planner; November 12–16; San Diego, CA; Program No. 60.15 (Abstract). [Google Scholar]
- 60.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23(7):557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva MS, Prescott M, Campbell R. Development of GABAergic altered brain wiring and plasticity in a mouse model of polycystic ovary syndrome (PCOS). In: Neuroscience Meeting Planner; November 12–16; San Diego, CA; Program No. 60.20. (Abstract). [Google Scholar]
- 62.Lardi-Studler B, Fritschy JM. Matching of pre- and postsynaptic specializations during synaptogenesis. Neuroscientist. 2007;13(2):115–126. [DOI] [PubMed] [Google Scholar]
- 63.Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36(6):1051–1061. [DOI] [PubMed] [Google Scholar]
- 64.Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28(6):278–283. [DOI] [PubMed] [Google Scholar]
- 65.Okabe S, Kim HD, Miwa A, Kuriu T, Okado H. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nat Neurosci. 1999;2(9):804–811. [DOI] [PubMed] [Google Scholar]
- 66.Wu X, Fu Y, Knott G, Lu J, Di Cristo G, Huang ZJ. GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. J Neurosci. 2012;32(1):331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang X, Harlan RE. Absence of androgen receptors in LHRH immunoreactive neurons. Brain Res. 1993;624(1-2):309–311. [DOI] [PubMed] [Google Scholar]
- 69.McAbee MD, DonCarlos LL. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139(4):1738–1745. [DOI] [PubMed] [Google Scholar]
- 70.Brock O, De Mees C, Bakker J. Hypothalamic expression of oestrogen receptor α and androgen receptor is sex-, age- and region-dependent in mice. J Neuroendocrinol. 2015;27(4):264–276. [DOI] [PubMed] [Google Scholar]
- 71.Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sullivan SD, Moenter SM. GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod. 2005;72(1):33–41. [DOI] [PubMed] [Google Scholar]
- 73.Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Stener-Victorin E, Legro RS, Dunaif A; Reproductive Medicine Network . Corrigendum: Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2016;7:10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mutharasan P, Galdones E, Peñalver Bernabé B, Garcia OA, Jafari N, Shea LD, Woodruff TK, Legro RS, Dunaif A, Urbanek M. Evidence for chromosome 2p16.3 polycystic ovary syndrome susceptibility locus in affected women of European ancestry. J Clin Endocrinol Metab. 2013;98(1):E185–E190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louwers YV, Stolk L, Uitterlinden AG, Laven JS. Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98(12):E2006–E2012. [DOI] [PubMed] [Google Scholar]