Abstract
Postnatal thymic epithelial cell (TEC) homeostatic defect- or natural aging-induced thymic atrophy results in a decline in central T-cell tolerance establishment, which is constituted by thymocyte negative selection and cluster of differentiation (CD) 4+ thymic regulatory T (tTreg) cell generation. Emerging evidence shows this decline mainly results from defects in negative selection, but there is insufficient evidence regarding whether tTreg cell generation is also impaired. We mechanistically studied tTreg cell generation in the atrophied thymus by utilizing both postnatal TEC-defective (resulting from FoxN1-floxed conditional knockout [cKO]) and naturally aged mouse models. We found that the capacity of tTreg cell generation was not impaired compared to CD4+ thymic conventional T cells, suggesting thymic atrophy positively influences tTreg cell generation. This is potentially attributed to decreased T cell receptor (TCR) signaling strength due to inefficiency in promiscuous expression of self-antigens or presenting a neo-self-antigen by medullary TECs, displaying decreased negative selection-related marker genes (Nur77 and CD5high) in CD4 single positive (SP) thymocytes. Our results provide evidence that the atrophied thymus attempts to balance the defective negative selection by enhancing tTreg cell generation to maintain central T-cell tolerance in the elderly. Once the balance is broken, age-related diseases could take place.
Author summary
Chronic inflammation in the elderly is partially attributed to atrophy of the thymus—an organ that regulates the immune system—and in particular the ability of organisms to recognize their own cells—a phenomenon known as central tolerance. Immune central tolerance is established by two processes: first, immune cells that react strongly to self are eliminated in a process called negative selection, and second, thymic regulatory T (tTreg) cells are generated to suppress self-reactive immune reactions. The former has already been reported to be defective in the aged thymus, but whether the generation of new tTreg cells is also impaired has remained unclear. Here, we analyzed the effect of aging on tTreg cell generation and found that the atrophied thymus is still able to make new tTreg cells; indeed, we show that tTreg cell generation capacity is enhanced when compared with other naïve T cells from the same thymus. We conclude that the balance of defective negative selection with enhanced tTreg cell generation may be necessary to avoid autoimmune diseases during aging.
Introduction
Central T-cell tolerance, which refers to the mechanism by which newly developing T cells are rendered nonreactive to self [1], plays an important role in controlling autoimmunity [2]. Central T-cell tolerance is established in the thymus through two main processes: thymocyte negative selection to deplete self-reactive T clones [3, 4] and tTreg cell generation to execute suppressive function in the periphery [5, 6]. Both negative selection and tTreg cell generation are critically dependent on medullary thymic epithelial cell (mTEC)-presentation (promiscuous expression) of self-antigens/peptides binding to the MHC groove (self-peptide-Major Histocompatibility Complex [self-pMHC]) [4, 7]. Once the self-pMHC interacts with T cell receptors (TCRs) on the self-reactive T clones, a TCR signal is produced. Based on affinity/avidity of self-pMHC to self-TCR, this interaction may produce a strong signal, causing negative selection to lead to deletion of this self-T clone. However, if a weak signal is produced, it will allow this T clone to survive and differentiate into thymic conventional T (tTcon) cells [8]. Moreover, if the TCR receives a signal at an intermediate strength, or a strong but transient signal (hit-and-run), this self-T clone will undergo tTreg cell differentiation [9, 10]. Therefore, central T-cell tolerance establishment in the thymus is determined by the interaction between the self-pMHC on the thymic epithelial cells (TECs) and TCR on the developing thymocytes.
During aging, the thymus undergoes a progressively age-related atrophy, or involution, which leads to the impairment of central T-cell tolerance establishment, resulting in increased self-reactivity in the periphery, which is one of the etiologies that induce the manifestation of chronic inflammation in the elderly (termed inflammaging) [11]. This atrophy is attributed both to the deterioration of TEC homeostasis, particularly mTEC, regulated by the FOXN1 (Forkhead box protein N1) gene [12], and in part, the decline of the autoimmune regulator Aire gene in mTECs, which causes a prominent dysfunction in negative selection [13, 14]. The generation of tTreg cells are critical for the maintenance of self-antigen tolerance and immune homeostasis [6]. Although evidence shows that aging is associated with enhancement of the peripheral regulatory T cell (pTreg) population as well as its function [15, 16], it is largely unclear whether the process of tTreg cell development in the age-related atrophied thymus is enhanced or impaired. Although newly generated tTreg cells were reported to be substantially declined with age in a recent report [17], the experimental evidence on this point is still insufficient. Therefore, the question is whether tTreg cell generation in the aged thymus exhibits any decline or enhancement along with declined negative selection; whether thymic atrophy differentially impacts these two processes of central T-cell tolerance establishment; and what the underlying mechanism is.
In this study, we explored how tTreg cell generation in the atrophied thymus is affected and by what mechanism in mouse models. We demonstrated that although thymic atrophy perturbs negative selection, resulting in an inability to sufficiently deplete self-reactive T clones, it was compensated for by relative enhancement of tTreg cell generation, as shown by an increased ratio of percentage of tTreg cells to percentage of tTcon cells and unreduced absolute tTreg cell numbers despite a dramatically decreased whole thymic cellularity. This relative enhancement was attributed to the new generation/differentiation of tTreg cells, rather than any changes in apoptotic or anti-apoptotic features, which was demonstrated by enhanced phosphoralated-Zap70 (p-Zap70) and unchanged apoptotic (Annexin V+) cells in certain cell subsets. We determined that the mechanism is potentially due to a declined TCR signaling strength, shifting from high strength, which is required for negative selection, to intermediate strength, which favors tTreg cell generation, because negative selection-related marker gene signals (Nur77 and cluster of differentiation [CD] 5high) were decreased in certain cell subsets of the atrophied thymus compared to those in the young thymus. Additionally, this declined TCR signaling strength is due to an inefficient presentation of self-pMHC on mTECs, which was demonstrated in a neo/mock self-antigen rat insulin promoter (RIP)-driven membrane-bound ovalbumin (mOVA) transgenic (Tg) mouse model. Together, our results provide evidence that the atrophied thymus attempts to balance the defective negative selection by relatively enhancing tTreg cell generation to maintain central T-cell tolerance in the elderly.
Results
Thymic atrophy relatively enhanced tTreg generation associated with a reduced mature Treg thymic reentrance
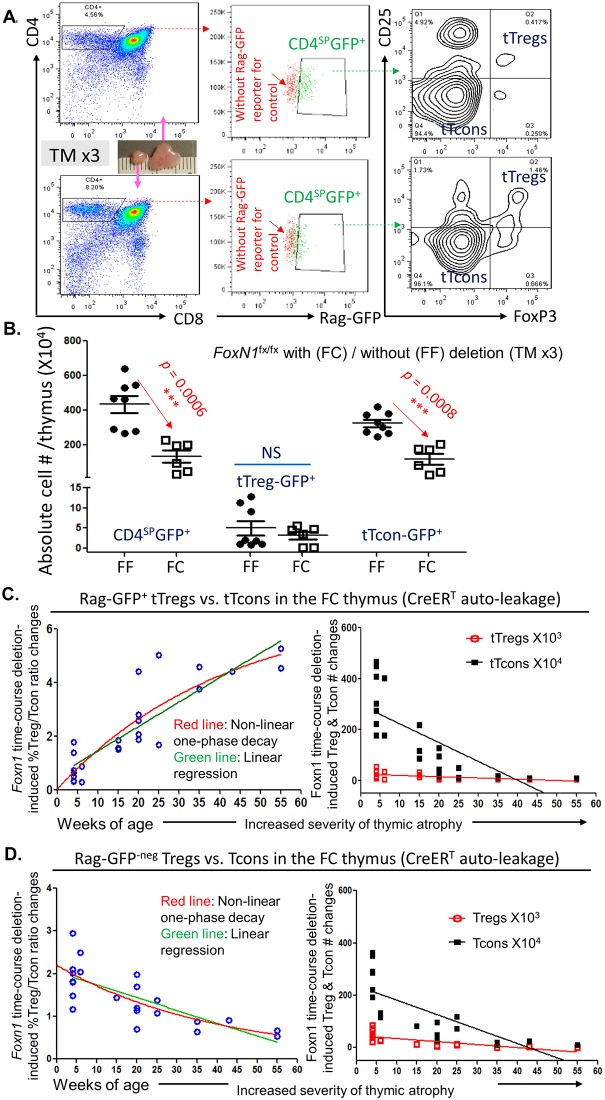
To determine whether the capacity of newly generated tTreg cells is impaired in aged thymus [17] and/or in the TEC defect-induced atrophied thymus, we utilized our previously generated mouse model with accelerated thymic atrophy (due to postnatal TEC homeostatic defect) [12], in which the loxp-flanked FoxN1 gene (FoxN1fx/fx) [18] can be deleted by ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein (CreERT) mediation through either a tamoxifen (TM) induction or a CreERT autoleakage with age. We named this model the FC (FoxN1fx/fx/CreERT) mouse and FF (FoxN1fx/fx without CreERT for controls) mouse. One-month-old FC thymi without any TM treatment exhibit the same thymocyte profiles as those in wild-type (WT) young mice. However, when young FC mice are treated with TM for 3 consecutive days (TM x3), or FC mice are housed for more than 6 months without any TM treatment but with an age-related CreERT autoleakage, their thymi exhibit thymic atrophy analogous to aged (approximately 18-month-old) WT thymi (details in S1 Fig). In addition, a rag-gfp reporter gene was utilized in this model to mark newly generated thymocytes [14], which we termed either FC-Rag-GFP or FF-Rag-GFP mice. We first observed absolute cell numbers of newly generated (Rag-GFP+) CD4 single positive (SP) (CD4SP) thymocytes, including tTreg cells and tTcon cells in CD4SP population, in the acute atrophied thymus (TM x3) (Fig 1A and 1B). We found that although thymic mass was dramatically reduced (Fig 1A image) with reduced numbers of newly generated CD4SP and tTcon cells, the newly generated tTreg cells were not decreased (Fig 1B), implying tTreg cells are unaffected by the acute thymic atrophy. We then analyzed integration of tTreg cells and tTcon cells in newly generated CD4SP population by measuring the age-dependent dynamic ratios of percentage of tTreg cells to percentage of tTcon cells in the accelerated atrophied thymus (FoxN1fx/fx deletion through a CreERT autoleakage over time [12]). The ratio was increased rather than decreased (Fig 1C, left panel). Under the background of a dramatically decreased total thymic cell number in the atrophied thymus, the absolute cell numbers of newly generated tTcon cells were dynamically decreased, while tTreg cell numbers were not (Fig 1C, right panel). Additionally, we gated the Rag-GFP reporter negative (Rag-GFP-neg) CD4SP population (gate shown in Fig 2A), which are not composed of newly generated T cells and were proposed to be primarily peripheral cells that had recirculated back to the thymus (i.e., thymic-reentered mature T cells) [17]. These thymic-reentered pTreg cells were also proposed to suppress development of newly generated tTreg cells [17]. The ratio of percentage of regulatory T cells (Treg) (mainly pTreg cells [17]) to percentage of conventional T (Tcon) cells of Rag-GFP-neg in the thymus was decreased with age (Fig 1D, left panel), while the absolute cell numbers of both Rag-GFP-neg populations (Fig 1D, right panel) mirrored the pattern observed with the Rag-GFP+ population (Fig 1C, right panel). Together, these results suggest that the severity of thymic atrophy positively influences new tTreg cell generation and negatively affects pTreg cells reentering the thymus.
Fig 1. Relatively enhanced activity of new (Rag-GFP+) tTreg cell generation along with reduced thymic-reentered (Rag-GFP-neg) Treg cells were related to the severity of thymic atrophy.
(A) A representative flow cytometric gate strategy from young mice of FF (normal thymus, top panels) or FC (atrophied thymus, bottom panels) with a Rag-GFP reporter shows gates of CD4SP (left panels), CD4SP-GFP+ (middle panels), and tTcon cells and tTreg cells in the CD4SP-GFP+ population from the same thymus (right panels). (B) Summarized absolute cell numbers per thymus from panel-A of newly generated (Rag-GFP+) CD4SP, tTreg cells, and tTcon cells from FF-Rag-GFP and FC-Rag-GFP mice treated with TM x3 (for induction of acute FoxN1fx/fx gene deletion), respectively. A Student t test was used to determine statistical significance between groups. (C) FoxN1fx/fx time-course deletion (due to the CreERT autoleakage over time) induced dynamic changes in ratio (left panel) and absolute cell numbers per thymus (right panel) of newly generated (Rag-GFP+) tTreg cells and tTcon cells in the same thymus. (D) Potential peripheral thymic-reentered (Rag-GFP-neg) Treg cells and Tcon cells in the same thymus. Two curves in left panels of C and D show linear regression (green lines) and nonlinear one-phase decay (red lines), respectively. Lines in right panels of C and D are linear regression, while data presented by nonlinear one-phase decay are shown in S2A Fig. GraphPad-5 statistic software was used for analysis. Each symbol represents an individual animal sample. Underlying data used in the generation of this figure can be found in S1 Data. CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FC, FoxN1fx/fx/CreERT; FF, FoxN1fx/fx without CreERT for controls; Foxn1, Forkhead box protein N1; FoxN1fx/fx, loxp-flanked FoxN1 gene; GFP, green fluorescent protein; Tcon, conventional T cell; TM, tamoxifen; tTcon, thymic conventional T cell; tTreg, thymic regulatory T cell.
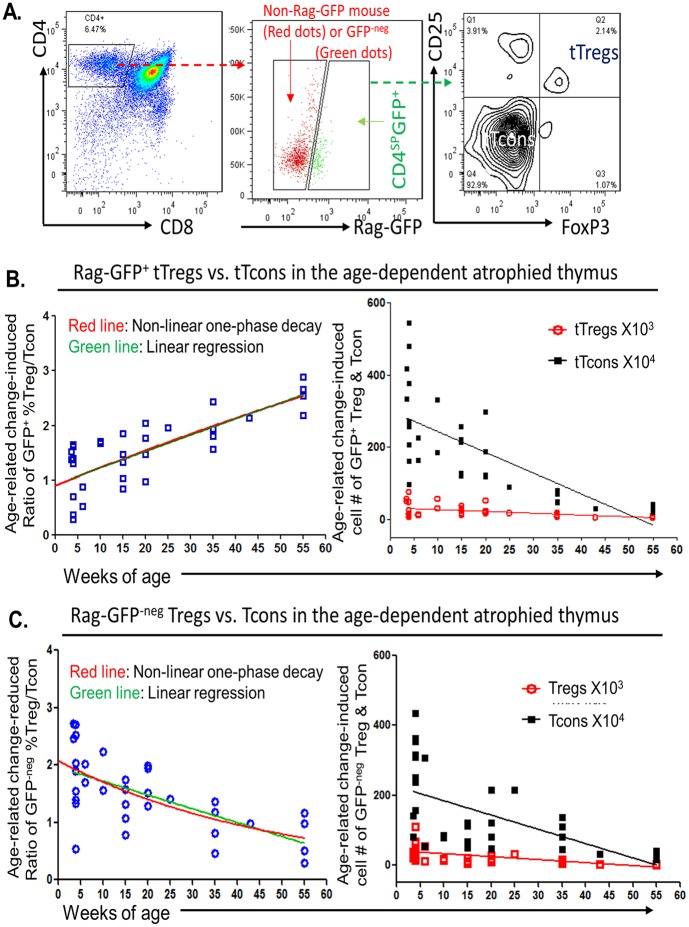
Fig 2. Activity of new (Rag-GFP+) tTreg cell generation was relatively enhanced, while thymic-reentering (Rag-GFP-neg) Treg cells were declined in an age-dependent manner in the naturally aged thymus.
(A) A representative flow cytometric gate strategy from Rag-GFP reporter mice shows gates of CD4SP (left panel), CD4SP-Rag-GFP+ and CD4SP-Rag-GFP-neg (middle panel, the red dots are cells from non-Rag-GFP reporter mouse for setting up a cutoff), and tTcon cells and tTreg cells in the CD4SP-Rag-GFP+ population (right panel). (B) In newly generated (Rag-GFP+) CD4SP population, age-dependent dynamic changes in ratio of tTreg cells versus tTcon cells (left panel) and absolute cell numbers of tTreg cells and tTcon cells per thymus (right panel). (C) Age-dependent dynamic changes in ratio (left panel) and absolute cell numbers per thymus (right panel) of thymic-reentering (Rag-GFP-neg) Treg cells and Tcon cells. Two curves in left panels of B and C show linear regression (green lines) and nonlinear one-phase decay (red lines), respectively. Lines in right panels of B and C are linear regression, while data presented by nonlinear one-phase decay are shown in S2B Fig. GraphPad-5 statistic software was used for analysis. Each symbol represents an individual animal sample. Underlying data used in the generation of this figure can be found in S1 Data. CD, cluster of differentiation; GFP, green fluorescent protein; Tcon, conventional T cell; TM, tamoxifen; Treg, regulatory T cell; tTcon, thymic conventional T cell; tTreg, thymic regulatory T cell.
In the naturally aged (WT, ≥ 18 months old) mouse, pTreg cells are increased [15] due to their accumulation, which is attributed to decreased Bim (a pro-apoptotic gene) expression [19, 20]. However, the FoxN1fx/fx-deleted mice have a relative young periphery since only the thymus undergoes an accelerated aging. Therefore, the young FC mouse does not have increased/accumulated pTreg cells. In order to account for the peripheral interference of new tTreg cell generation in the naturally age-related atrophied thymus, we investigated the same parameters in WT (containing Rag-GFP reporter) mouse thymi (Fig 2). As we expected, in the population of new Rag-GFP+CD4SP cells (Fig 2A, left and middle panels), the ratio of percentage of tTreg cells to percentage of tTcon cells was shown also to be increased in an age-dependent manner (Fig 2B, left panel). The absolute cell numbers of either tTreg cells or tTcon cells (Fig 2B, linear regression in right panel; for nonlinear one-phase decay, see S2 Fig) showed the same tendency as FC mice in Fig 1C right panel, as did the Rag-GFP-negCD4SP populations, i.e., potential thymic-reentered cells (Fig 2C). These results are consistent with the findings in FC mice shown in Fig 1. Therefore, we concluded that thymic atrophy does not impair the capacity of new tTreg cell generation and is associated with a reduction of pTreg cells reentering in an age-dependent manner.
Relatively enhanced tTreg cell generation in the atrophied thymus was driven by the thymic microenvironment, rather than tTreg cell intrinsic alterations
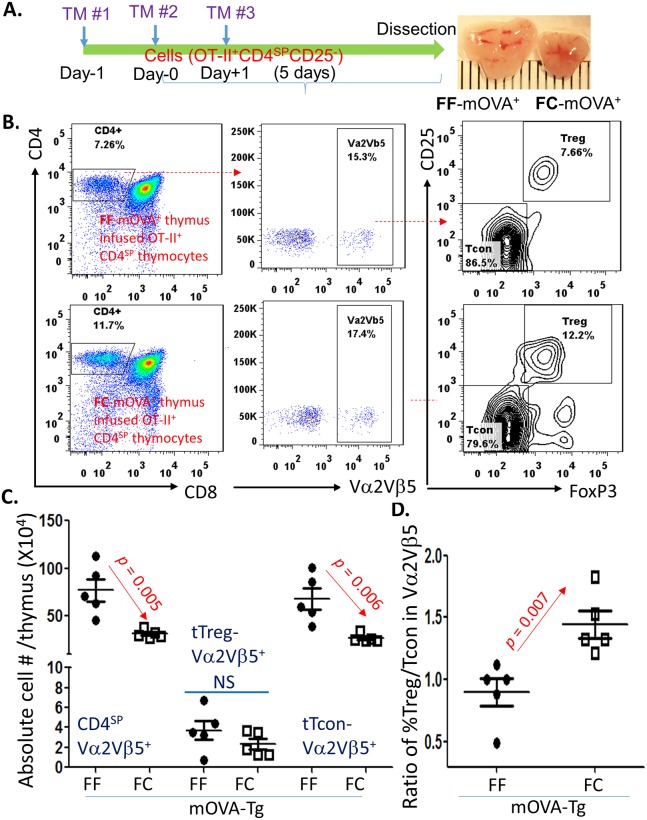
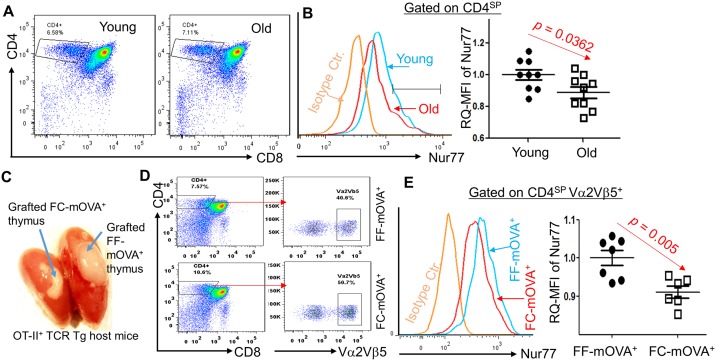
We believe that the atrophied thymic microenvironment promotes the activity of new tTreg cell generation. To exclude any intrinsic alterations from tTreg cells in the atrophied thymus, such as tTreg cells acquiring any refractory behavior for anti-apoptosis or loss of suppressive function, we designed an adaptive transfer experiment based on a published protocol [21]. We intrathymically (i.t.) injected sorted pre-tTreg cells (CD4SPCD25-neg thymocytes) from a pool of young OT-II+ TCR Tg (MHC class-II restricted ovalbumin-specific TCR transgenic) mice [22] (with Rag−/− background, details in S3 Fig) into either normal (FF control) or acute atrophied (FC, FoxN1fx/fxCreERT with a TM x3 induction) thymi containing RIP-mOVA Tg TECs [23, 24], termed FC- or FF-mOVA mice (Fig 3A). In this setting, with an interaction between mOVA-MHC and OT-II+ TCR, these grafted OT-II+ (bearing Vα2 and Vβ5 TCR subunits which recognize mOVA antigen) CD4SP clones from young mice, which do not have any aging-related imprints or characteristics, undergo either negative selection-driven apoptosis or tTreg cell development-related differentiation, dependent on TCR signaling strength produced by the interaction [25, 26].
Fig 3. Acute atrophied thymic microenvironment enhanced intrathymic generation of OT-II+ TCR-Tg tTreg cells in the FC-mOVA-Tg mouse thymus.
(A) A schematic workflow shows that young FF-mOVA-Tg (normal thymus) or FC-mOVA-Tg (atrophied thymus) mice were i.t. infused with sorted CD4SPCD25-neg thymocytes (pre-tTreg cells) from OT-II+/Rag −/− mice. These mice were injected with TM x3 i.p. at Day−1, Day0, and Day+1 to induce FoxN1fx/fx deletion under a CreERT mediation. Five days after the i.t. infusion of CD4SPCD25-neg pre-tTreg cells, the thymi of recipients were isolated and analyzed for infused OT-II+ TCR-Tg thymocyte-derived tTreg cells and tTcon cells with flow cytometry. (B) A representative flow cytometric gate strategy shows gates of CD4SP (left panels), CD4SP-Vα2+Vβ5+ (mainly from infused OT-II TCR-Tg thymocytes) (middle panels), and tTcon cells versus tTreg cells (right panels) in FF-mOVA-Tg (top panels) and FC-mOVA-Tg (bottom panels) mice. (C) Summarized absolute cell numbers of CD4SP-Vα2+Vβ5+, tTreg cells, and tTcon cells in the FF-mOVA-Tg and FC-mOVA-Tg mice. (D) The ratios of Vα2+Vβ5+ percentage of tTreg cells versus percentage of tTcon cells in FF-mOVA-Tg and FC-mOVA-Tg thymi, respectively. A Student t test was used to determine statistical significance between groups, and all level bars in panels C and D are expressed as mean ± SEM. Each symbol represents an individual animal sample. Underlying data used in the generation of this figure can be found in S1 Data. CD, cluster of differentiation; CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FC, FoxN1fx/fx/CreERT; FF, FoxN1fx/fx without CreERT for controls; Foxn1, Forkhead box protein N1; FoxN1fx/fx or FoxN1-floxed, loxp-flanked FoxN1 gene; i.p., intraperitoneally; i.t., intrathymically; mOVA, membrane-bound ovalbumin; NS, not significant; TCR, T cell receptor; Tg, transgenic; TM, tamoxifen; tTcon, thymic conventional T cell; tTreg, thymic regulatory T cell.
We first verified that the atrophied thymic microenvironment promoted the grafted pre-tTreg cells (CD4SPCD25-neg thymocytes) to differentiate into tTreg cells in an increased proportion compared to tTcon cells (Fig 3B bottom panels and Fig 3D) and that there were unchanged absolute cell numbers (Fig 3C) compared to the counterpart in the FF-mOVA normal thymic microenvironment. The results therefore confirm that changes in the thymic microenvironment, rather than changes intrinsic in T clones, enhance the differentiation toward tTreg cells.
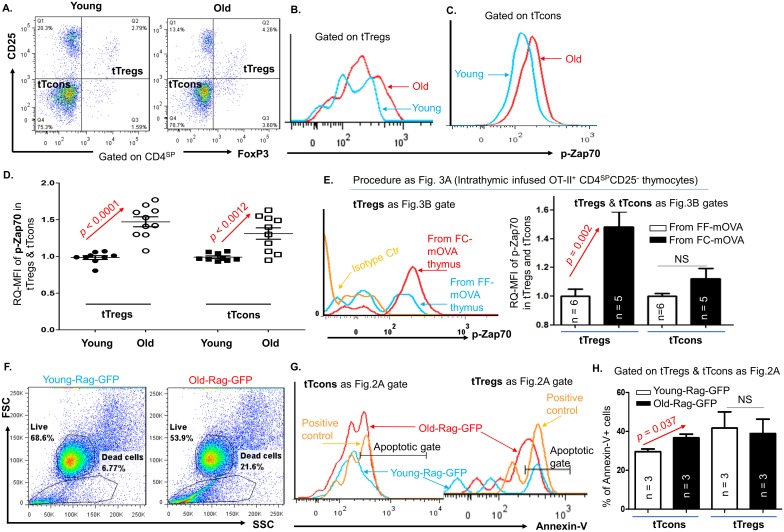
We then observed an increased intensity of p-Zap70 in the subsets of both newly generated tTreg cells and newly generated tTcon cells of the naturally aged thymi (Fig 4A–4D) and in tTreg cells but not in tTcon cells from the FC-mOVA atrophied thymi infused with OT-II+ TCR-Tg pre-tTreg cells (Fig 4E). The increase of p-Zap70 in T clones indicates that the TCRs are activated and these thymocytes are activated to undergo either apoptosis or differentiation. Therefore, we investigated the apoptosis in tTreg cells and tTcon cells from the young and aged thymi of Rag-GFP reporter mice. This approach is used to either exclude a possibility that the increased tTreg cell proportion is due to aquiring a refractory behavior and subsequently resisting apoptosis or to confirm that the decreased tTcon cell proportion is due to increased apoptosis after TCR activation (increased p-Zap70). The results showed that in the atrophied thymus, apoptosis in newly generated tTreg cells was not reduced or increased compared to those in the normal thymus, whereas apoptosis in newly generated tTcon cells was increased (Fig 4F–4H). The results imply that increased TCR activation in tTreg cells leads to tTreg cell generation, while in tTcon cells it results in tTcon reduction in the aged thymus. Finally, we tested tTreg cell suppressive function to further comfirm that tTreg cells in the atrophied thymus do not possess intrinsic defects in function. This was done by comparing sorted, newly generated tTreg cells (Rag-GFP+CD4SPCD25+) from young WT, middle-aged WT, and middle-aged FC mice (S4A Fig). Due to CreERT-mediated autoleakage-related deletion of FoxN1fx/fx, mice in this group have a similar thymic profile to 18-month-old WT, which was shown in S1 Fig. We found that both normal and atrophied thymus-generated tTreg cells possessed similar suppressive function to T effectors (S4B Fig).
Fig 4. p-Zap70 was increased in both tTreg cells and tTcon cells, but apoptosis was only increased in tTcon cells in the aged and atrophied thymus.
(A) Representative gate strategy shows the percentage of tTreg cells versus tTcon cells from the thymi of WT young and naturally aged mice. (B) A representative histogram shows expression of p-Zap70 in the tTreg cells of WT young (blue curve) and naturally aged (red curve) mice. (C) A representative histogram shows expression of p-Zap70 in the tTcon cells of WT young (blue curve) and naturally aged (red curve) mice. (D) A summary of RQ-MFI of p-Zap70 in the tTreg cells and tTcon cells of WT young and naturally aged mice. (E) The workflow is the same as Fig 3A. Left panel: A representative result shows expression of p-Zap70 in the tTreg cells derived from i.t. infused sorted CD4SPCD25-neg pre-tTreg cell thymocytes of OT-II+/Rag−/− mice in FF-mOVA (blue line) and FC-mOVA (red line) thymi. Right panel: A summary of RQ-MFI of p-Zap70 in the tTreg cells and tTcon cells from left penal mice. (F) Representative dot plots from young and old-Rag-GFP reporter mice show increased dead/necrotic cells in the old mice. (G) Histogram shows Annexin-V+ apoptotic cells in tTcon cells and tTreg cells (gates as in Fig 2A right panel). (H) A summarized result of percentages of Annexin-V+ apoptotic tTcon cells and tTreg cells in young and old-Rag-GFP mice, respectively. In panels D, E, and H, Student t test was used to determine statistical significance between groups; data are expressed as mean ± SEM; and each symbol represents an individual animal sample or n = animal numbers. Underlying data used in the generation of this figure (panels D, E, and H) can be found in S1 Data. CD, cluster of differentiation; FF, FoxN1fx/fx without CreERT for controls; i.t., intrathymically; mOVA-Tg, membrane-bound ovalbumin transgenic mouse model; OT-II+ TCR Tg, MHC class-II restricted ovalbumin-specific TCR transgenic; mOVA, membrane-bound ovalbumin; p-Zap70, phosphorylated-zeta-chain-associated protein kinase 70; RQ-MFI, relative quantitative Mean Fluorescence Intensity; tTcon, thymic conventional T cell; tTreg, thymic regulatory T cell; WT, wild-type.
Taken together, the unchanged Treg generation in the atrophied thymus is relatively enhanced in activity, which is driven by the atrophied thymic microenvironment but unlikely to be due to age-related intrinsic changes in apoptosis or in loss of suppressive function of tTreg cells.
Negative selection-related signals were decreased in CD4SP thymocytes in both naturally aged and acute atrophied thymi
To determine the underlying mechanism leading to the relatively enhanced tTreg cell generation in the atrophied thymic microenvironment, we focused on TCR signaling strength, since it determines self-T clone fates. First, we looked at Nur77 as a representative negative selection marker gene, since insufficient Nur77 signaling leads to reduced clonal deletion [27] and enhanced tTreg cell generation [28]. We found that negative selection signaling molecule Nur77, as measured by the relative quantitative Mean Fluorescence Intensity (RQ-MFI) was reduced in either WT CD4SP thymocytes of the naturally aged thymus (Fig 5A and 5B) or OT-II+ TCR-Tg CD4SP thymocytes from the acute atrophied FC-mOVA thymus grafted under the kidney capsule of OT-II+ TCR-Tg recipients (Fig 5C shows an illustration of this setting; results are shown in Fig 5D and 5E).
Fig 5. Thymic atrophy impaired negative selection signal Nur77 in CD4SP thymocytes.
Panels A and B: results from directly stained young and naturally aged thymocytes. (A) Flow cytometric gate strategy shows CD4SP gate. (B) Left panel shows Nur77 in CD4SP population; right panel is summarized RQ-MFI results of Nur77 levels in CD4SP thymocytes of WT young and naturally aged mice. Panels C–E: results from grafted FF- and FC-mOVA thymi, which were grafted under the kidney capsule of OT-II+ TCR-Tg mice with Rag−/− background (OT-II+/Rag−/−). (C) Image of grafted mOVA-Tg thymi under the kidney capsule of OT-II+/Rag−/− mice, treated with TM x3. (D) A representative flow cytometric gate strategy shows Vα2+Vβ5+ thymocytes in CD4SP population (these thymocytes were derived from OT-II+/Rag−/− host mouse bone marrow progenitors) in the grafted FF- and FC-mOVA-Tg thymi. (E) Left panel: a representative histogram of Nur77 in the gates of CD4SP and Vα2+Vβ5+ (primarily derived from OT-II+/Rag−/− progenitors) thymocytes in the grafted FF- and FC-mOVA-Tg thymi; right panel: summarized results of RQ-MFI of Nur77 levels from the left panel. In panels B and E, a Student t test was used to determine statistical significance between groups, and all data are expressed as mean ± SEM. Each symbol represents an individual animal sample. Underlying data used in the generation of this figure (panels B and E) can be found in S1 Data. CD, cluster of differentiation; FC, FoxN1fx/fx/CreERT; FF, FoxN1fx/fx without CreERT for controls; Foxn1, Forkhead box protein N1; FoxN1fx/fx, loxp-flanked FoxN1 gene; mOVA-Tg, membrane-bound ovalbumin transgenic mouse; Nur77, orphan nuclear receptor; OT-II, MHC class-II restricted ovalbumin-specific TCR transgenic mouse; RQ-MFI, relative quantitative Mean Fluorescence Intensity; WT, wild-type.
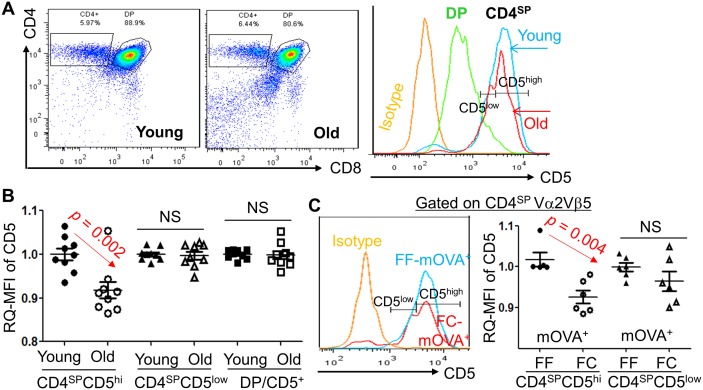
Although the role of CD5 in the thymus is for fine-tuning of TCR signaling, CD5 surface expression quantitatively correlates with TCR signal intensity [29], i.e., CD5high expression in T cells is produced by a strong interaction between a high affinity/avidity self-pMHC to self-TCR [29–32], which triggers negative selection in the thymus [29]. CD5 expression is conversely related to tTreg cell selection, since insufficient CD5 in CD5-deficient mice causes an increase in tTreg cells [33]. We measured the CD4SPCD5high thymocyte population either in the naturally aged thymus (Fig 6A and 6B) or in the acute atrophied FC-mOVA+ thymus under the kidney capsule of OT-II+ TCR-Tg mice, similar to the Fig 5C setting (Fig 6C). The MFI of CD5 in the CD4SPCD5high population in both settings was decreased, but there was no difference in CD4+CD8+ double positive (DP) and CD4SPCD5low populations (Fig 6B and 6C right panel).
Fig 6. CD4SPCD5hi thymocytes were decreased in either the naturally aged (≥ 18 months old) or the acute atrophied (FoxN1 cKO) thymi.
(A) A representative result from WT young and aged mice shows flow cytometric gate strategy for CD4SP, DP, and CD5low and CD5high. (B) Summarized RQ-MFI of CD5 in various populations from panel A. (C) Results from grafted FF- and FC-mOVA-Tg thymi, which were under the kidney capsule of OT-II+/Rag−/− host mice (Same setting as Fig 5C). Left panel: representative histograms of CD5low and CD5high in CD4SP thymocytes (developed from seeded OT-II+/Rag−/− bone marrow progenitors) in the grafted thymi of FF-mOVA-Tg (normal thymus, without CreERT) and FC-mOVA-Tg (atrophied thymus after TM x3) mice. Right panel: summarized results of RQ-MFI of CD5 from the left panel. A Student t test was used to determine statistical significance between groups, and all data are expressed as mean ± SEM. Each symbol represents an individual animal sample. Underlying data used in the generation of this figure (panels B and C) can be found in S1 Data. CD, cluster of differentiation; cKO, conditional knockout; CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; DP, double positive; FC, FoxN1fx/fx/CreERT; FF, FoxN1fx/fx without CreERT for controls; Foxn1, Forkhead box protein N1; FoxN1fx/fx, loxp-flanked FoxN1 gene; mOVA-Tg, membrane-bound ovalbumin transgenic mouse; OT-II, MHC class-II restricted ovalbumin-specific TCR transgenic mouse; RQ-MFI, relative quantitative Mean Fluorescence Intensity; WT, wild-type.
These results implied insufficient negative selection signals were received by self-reactive TCRs in the atrophied thymus, which leads to a functional handicap of both negative selection and deletion of self-reactive T clones from the developing thymocyte pool, as we reported in our previous publications [13, 14].
Capacity of presenting self-antigen on mTECs in the atrophied thymus declined
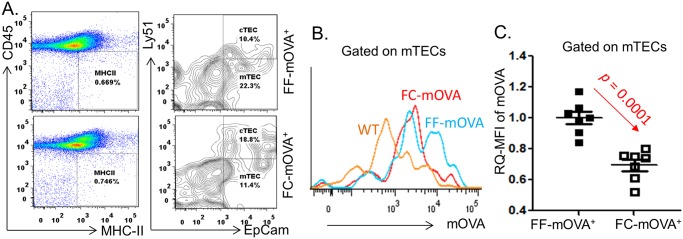
TCR signaling strength is produced through an interaction between the self-pMHC presented on mTECs and the self-recognizing TCR expressed on self-T clones. Self-peptide presentation in mTECs is also termed “promiscuous expression” of peripheral tissue-specific antigens (TSAs), which distinguishes the TEC-autonomous gene expression. In addition, expression of most TSAs on mTECs is Aire-dependent. Given that aging primarily occurs in TECs [34] and the Aire gene is declined in mTECs on a per-cell basis in the atrophied thymus [14], this suggests that mTECs in the age-related atrophied thymus are unable to normally promiscuously express self-pMHC. Therefore, we hypothesized that the reduced TCR signaling strength is due to ineffective self-pMHC expression on the surface of these mTECs, leading to a reduction of interactive signaling to the self-T clones. This is unlikely to be a defect in TCR avidity since transfer of young thymocytes into the atrophied thymus showed the same relatively enhanced tendency of new tTreg cell generation (Fig 3). Therefore, we detected expression of a neo/mock-self-antigen (mOVA) on mTECs to mimic TSA expression in FF-mOVA and FC-mOVA mouse models. In this model, mOVA expression on mTECs is controlled by Aire gene [24], and the thymus of FC-mOVA experiences accelerated aging (resulting from a FoxN1fx/fx deletion under a CreERT autoleakage over time [12], see S1 Fig). Our results showed that mOVA expression in the FC-mOVA+ thymus was significantly reduced (Fig 7), which verified our hypothesis that mTECs in the atrophied thymus are unable to present self-antigen efficiently. This notion is also supported by our previous finding that Aire-dependent self-antigens (such as salivary protein-1, Insulin-1 and -2, intestinal fatty acid binding protein) were decreased in mTECs of the FoxN1-conditional knockout (cKO) atrophied thymus [13].
Fig 7. Capacity of mTEC self-antigen presentation was declined in the atrophied thymus demonstrated by a neo/mock self-antigen mOVA model.
(A) A representative flow cytometric gate strategy shows CD45-negMHC-II+ TECs (left panel) and Ly51+EpCam+ cTECs and Ly51-negEpCam+ mTECs (right panel) from young 1.5-month-old FF thymus and 8-month-old FC (FoxN1fx/fx with a CreERT autoleakage) thymus. (B) A representative flow cytometric histogram shows expression of mOVA in mTECs of WT young (non-mOVA-Tg, orange), young 1.5-month-old FF-mOVA-Tg (blue), and 8-month-old FC-mOVA-Tg (red) mice. (C) A summary of mOVA expression in mTECs of young 1.5-month-old FF-mOVA-Tg thymus and 8-month-old FC-mOVA-Tg thymus. A Student t test was used to determine statistical significance between two groups, and in Panel C, the level bars are expressed as mean ± SEM. Each symbol represents an individual animal sample. Underlying data used in the generation of this figure can be found in S1 Data. CD, cluster of differentiation; cTEC, cortical thymic epithelial cells; FC, FoxN1fx/fx/CreERT; FF, FoxN1fx/fx without CreERT for controls; mTEC, medullary thymic epithelial cell; TEC, thymic epithelial cell; Tg, transgenic; mOVA-Tg, membrane-bound ovalbumin transgenic mouse.
The tTreg cell-required cytokines and NF-κB factor unlikely affected tTreg cell generation in the atrophied thymic microenvironment
To further identify the underlying mechanism by which thymic atrophy relatively enhances tTreg cell generation, we also considered that tTreg cell generation-required cytokines, such as interleukin-2 (IL-2) [35] and Transforming Growth Factor-Beta (TGFβ) [36, 37], as well as transcription factors, such as the Nuclear Factor kappa Beta (NF-κB) family [38, 39], which are primarily provided by the thymic microenvironment, could be altered in the atrophied thymus. We detected that in the naturally aged and acute atrophied FC thymi (S5 Fig), expression levels of IL-2 and TGFβ, as well as one of the NF-kB family of transcription factors, p105/p50, were unchanged compared to young and FF counterparts with normal thymi. Only expression of p105/p50 was slightly increased in the naturally aged thymus (S5 Fig, top panel), which was due to inflammation in the atrophied thymus. Although this increase could help tTreg cell maturation by inducing expression of forkhead box P3 (FoxP3) [38, 39], this is most likely not the primary mechanism for the enhancement of tTreg cell generation we observed in the atrophied thymus.
Discussion
Using a combination of naturally aged and multiple genetically engineered mouse models and investigating signaling molecules in either the thymocyte or mTEC thymic cellular compartments, we have demonstrated that the aged, atrophied thymic microenvironment does not impair, but may relatively promote, tTreg cell generation. This evidence has revealed how central tolerance is established in the age-related atrophied thymic microenvironment, which we determined to be a heterogeneous effect of defective negative selection accompanied by relatively enhanced tTreg cell generation. This is probably a potential attempt by the atrophied thymus to desperately balance the defective negative selection by enhancing tTreg cell generation to maintain central T-cell tolerance. As we know, under normal conditions, two arms—namely, negative selection and tTreg cell generation—work in tandem for central T-cell tolerance establishment. However, in the age-related atrophied thymus, as one arm of tolerance induction (negative selection) fails, we assert that the second arm (tTreg cell generation) attempts to compensate, resulting in relative tTreg cell enhancement.
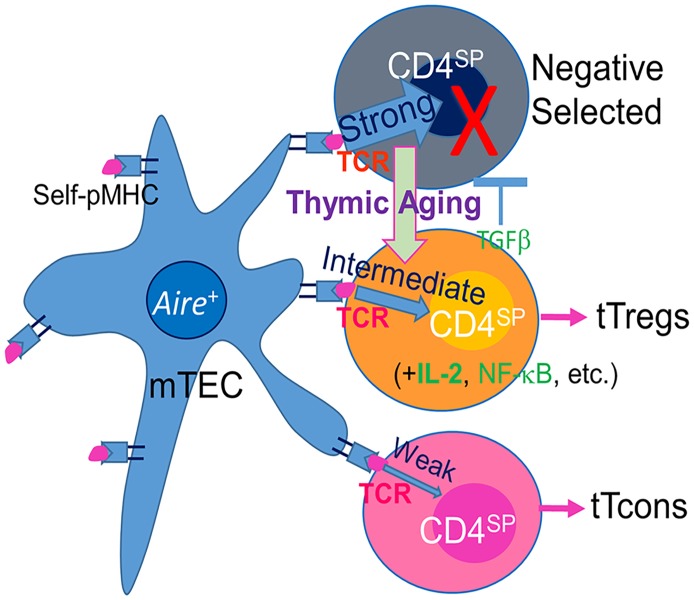
This mechanism can be explained by changes in TCR signaling strength in the thymus, which acts as a gate-keeper to determine thymocyte fate (diagrammed in Fig 8). Although this is the first time that this observation has been noted in the TEC-defective and age-related atrophied thymus, the phenomenon has been demonstrated by several loss-of-function young mouse models. The TCR signaling is produced by interaction between the two parties: mTECs and thymocytes. The first established mouse model for studying this signaling pathway contains an alteration in the thymocyte side. As we know, when the TCR responds to antigen recognition, the immunoreceptor tyrosine-based activation motifs (ITAMs) are activated and the signaling kinase Zap70 is subsequently phosphorylated. A mouse model with a knock-in allele of TCRξ chain gene with tyrosine-to-phenylalanine mutations in 6 out of 10 ITAMs leads to a 60% decrease in TCR signaling potential [40]. This mouse model exhibited a defect in negative selection but an increase in tTreg cell generation [40]. The second mouse model for the TCR signaling pathway contains a change in the TEC compartment. Tg expression of a microRNA targeting Major Histocompatibility Complex (MHC) class-II transactivator (CIITA) results in declined MHC-II on mTECs [26], which leads to an insufficiency for mTECs to present self-antigens for normal interaction with TCR. This also resulted in an enhancement of tTreg cell generation at the expense of negative selection [26]. These two models from both parties of the signaling interaction support one conclusion, namely, that shifting a strong signal to an intermediate signal due to any weakening of the interaction between self-pMHC and self-reactive TCR allows the self-reactive thymocytes, which should be deleted, to differentiate to tTreg cells [25]. In the age-related atrophied thymus, the capacity of self-antigen presentation (promiscuous expression of TSAs) on mTECs is reduced due to a defect in TEC homeostasis [12, 34] associated with a decline of Aire gene expression [13, 14]. Although there has been controversy about the role of Aire gene in positive selection of Treg cells [41], it has been accepted that Aire regulates Aire-dependent self-peptide expression on mTECs. Moreover, total [42] or certain specific [43] tTreg cells were found not to be decreased in Aire deficient models. Therefore, the interaction of self-pMHC and self-reactive TCR produces weak signals in the age-related atrophied thymus, which results in a similar outcome as illustrated by the second model: ineffective mTECs [26].
Fig 8. Schematic diagram of potential mechanism.
Interaction between self-pMHC on mTEC and TCR on thymocytes produces three types of signaling strength to determine self-T clone fates. A strong signal leads to negative selection and results in the self-T clone depletion; an intermediate signal leads to thymocyte differentiation to a tTreg cell population; and a weak signal results in thymocyte survival to differentiate into tTcon cells. These signals can be detected by negative selection marker genes, such as Nur77 and CD5 expression on CD4SP thymocytes. Thymic aging impairs mTECs, including a decline of Aire expression. It results in ineffective presentation of self-pMHC, thereby weakening the interactions between self-pMHC and TCR. Therefore, some self-T clones which should be depleted shift to tTreg cell generation due to strong signaling strength shifting toward intermediate signaling strength. CD, cluster of differentiation; mTEC, medullary thymic epithelial cell; self-pMHC, self-peptide-Major Histocompatibility Complex; TCR, T cell receptor; tTcon, thymic conventional T cell; tTreg, thymic regulatory T cell.
Our results presented here are inconsistent with a published report in which the phenotype of “substantially decreased new tTreg cells and augmented recirculating pTreg cells among thymic Treg cells with age” was observed [17]. These phenotypes were based on different observations by comparing Rag-GFP+ Treg cells versus Rag-GFP-neg Treg cells within a thymic Treg subset and Rag-GFP+ Tcon cells versus Rag-GFP-neg Tcon cells within a thymic Tcon subset, rather than comparing Treg cells to Tcon cells within the Rag-GFP+ CD4SP or Rag-GFP-neg CD4SP subsets. The results presented a proportion change between newly generated (Rag-GFP+) and reentered (Rag-GFP-neg) portions only in the same subset, rather than reflecting the changes in an integrated total CD4SP population. Therefore, it does not exhibit integrated changes in newly generated and reentered Treg cells compared to their counterpart Tcon cells and within the entire CD4SP cell population. Regarding inhibition of the de novo tTreg cell generation by reentered pTreg cells in the thymus, these cells may be importantly involved in a potential negative feedback loop of regulation [17]. Since pTreg cells are increased in aged mice and humans due to declined Bim gene-resulted accumulation [15], it is a concern that aged pTreg cells may reenter the aged thymus more than their young counterparts to augment inhibition of new tTreg cell generation. However, we did not see this, since there was no particularly different tendency of re-entry of young pTreg cells (FC model possesses a young periphery with no accumulated Treg cells and no decreased Bim gene [14]) or old pTreg cells (naturally aged model) in the atrophied thymus (Figs 1D and 2C). Therefore, the pTreg reentering mechanism should be the same in either young or aged individuals.
Development of tTreg cells in the thymus is primarily dependent on thymic microenvironments. We found that the atrophied thymic microenvironment favors tTreg cell development because they demonstrated more activity compared to their counterparts in the normal thymus (Figs 3 and 4A–4E) and compared to tTcon cells in the same atrophied thymus (Fig 4E). This is not due to apoptotic resistance by a refractory feature (Fig 4F–4H). Except for tTreg cells, apoptosis in tTcon cells (Fig 4F–4H) and in other populations of FC mice [18] is increased. Increased apoptotic microenvironment was reported to drive tTreg cell generation through the apoptosis-TGFβ-FoxP3 axis [21]. Although we did not find any increase of TGFβ (S5 Fig), we believe that increased apoptosis in the thymus induces an increased pro-inflammatory condition that will be favorable for tTreg cell development [38, 39].
It is well known that in aged humans and mice, an increased pTreg pool has been observed not only in proportion and absolute Treg numbers [15, 44] but also in function [16], which negatively affects anti-infection [45] and antitumor [46] immunity. Given the fact that tTreg cell generation is relatively enhanced in the aged atrophied thymus, we have a reason to deduce that output of tTreg cells may impact the pTreg pool in aged individuals. Although evidence is still insufficient, it was reported that in the pTreg pool, the proportion of natural Treg cells (i.e., tTreg cells, based on recommended Treg nomenclature [47]) versus inducible Treg cells is increased with age [48]. This not only implies that older individuals are less capable of generating inducible Treg cells [48] due to an intrinsic defect in these extrathymically induced Treg cells [49] but also indirectly suggests that aged thymus-derived tTreg cells are increased in the peripheral pTreg pool. Our peripheral data also provide evidence that recent thymic emigrant (Rag-GFP+) Treg cells in the spleens of aged mice were increased, while Rag-GFP+ CD4SP Tcon cells in the spleens of aged mice were decreased (S6 Fig). Another profound question is why this increased tTreg cell involvement in the aged pTreg pool cannot suppress self-reactivity-induced inflammaging [14], although it suppresses normal anti-infection and antitumor immunity [45, 46]. This can probably be accounted for because reduced capacity of presenting self-pMHC diverts the lineage of some tTreg cell-biased clones into self-reactive tTcon cells [50]. These tTcon cells are released to the periphery to elevate self-reactivity, which is our next in-depth work. There is also another possibility of an imbalance between increased total Treg cells and decreased specific Treg cells, i.e., a biased tTreg cell specificity, which is seen in unpublished studies of certain autoimmune diseases and will be substantially addressed in our future work as well. However, as we have reported here, the atrophied thymus indeed attempts to balance the defective negative selection by enhancing tTreg cell generation to try to maintain central T-cell immune tolerance in the elderly. This mechanism should contribute to an optimal balance in most aged people since most of them do not have an onset of age-related autoimmune diseases, despite most elderly people having increased chronic inflammation. However, once this balance is broken, age-related inflammatory diseases could take place.
Materials and methods
Ethics statement
All experiments were conducted on C57BL/6 genetic background mice, and the performance was in compliance with a protocol (IACUC-2016-0037) approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Texas Health Science Center, in accordance with guidelines on animal welfare of the National Institutes of Health. All efforts were taken to minimize mouse usage to maximize necessary results; provide the best veterinary care; and minimize discomfort, distress, and surgery with anesthetic procedures and euthanasia.
Mice, crossbreeding, and animal care
Various genetically manipulated mouse colonies (all on C57BL/6 genetic background) and their crossbreeding schemes have been described in our previous publication [14]. Briefly, FoxN1fx/fx [18] mice were crossbred with CMV-promoter-driven CreERT (ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein) Tg mice, then with rag-gfp reporter mice. The FoxN1fx/fx cKO was through either a TM-induced acute deletion or CreERT autoleakage-mediated time-course deletion [12]. Both deletions induce accelerated thymic atrophy. We term this model the FC mouse and FF mouse. The thymocyte profiles in a 1-month-old FC thymus without TM treatment is exactly the same as those in 1-month-old WT mice, whereas the thymocyte profiles in a young FC mouse treated with TM (1 mg/10 g body weight/day) for 3 consecutive days (TM x3) and FC mouse is housed for 6 months without any TM treatment with a CreERT age-related autoleakage are the same as in the naturally aged WT (≥18-month-old) thymus (details in S1 Fig). RIP-mOVA Tg mice (#005431) and OT-II+ TCR Tg mice (#004194) were purchased from Jackson Lab. OT-II+ TCR Tg mice were cross-bred with Rag−/− mice (Jackson Lab #002216). These mice were termed OT-II+/Rag −/− mice (see S3 Fig). Aged WT mice were ordered from the National Institute on Aging, aged rodent colonies. Mouse ages are indicated in each figure legend or defined young (1–2 months old) and naturally aged (in most situations, they were ≥ 18 months old).
Intrathymic transfer of pre-tTreg cells
CD8SP and CD8+CD4+ DP OT-II+ TCR-Tg thymocytes were depleted through an anti-CD8 cytotoxic antibody (clone HO-2.2) and Low-Toxic-M complement. Then, the live thymocytes were sorted by flow cytometry for pre-tTreg cells (CD4SP and CD25-neg thymocytes). The sorted pre-tTreg cells were i.t. injected into the thymus (details in our previous publication [51]) of young FF- or FC-mOVA-Tg mice (1 × 106 cells per recipient mouse), respectively. Five days after the pre-tTreg cell injection, the thymocytes with Vα2+Vβ5+ TCR chain types (mostly OT-II+ TCR-Tg thymocytes) in recipient FF- or FC-mOVA thymi were collected for analysis of newly generated tTreg cells (CD4SPCD25+FoxP3+) versus tTcon cells (CD4SPCD25-negFoxP3-neg). Meanwhile, recipient mice were intraperitoneally (i.p.) injected with TM (1 mg/10 g body weight/day) for 3 consecutive days (TM x3): one day before, one day along with i.t. injection, and one day after to induce thymic atrophy in FC-mOVA mice (detailed procedure is outlined in Fig 3A).
Kidney capsule transplantation of thymic lobes
The surgical operation of the kidney capsule transplantation was performed as previously described [14]. Briefly, intact thymic lobes from FF- or FC-mOVA-Tg newborn mice were directly transplanted into young host OT-II+ TCR-Tg mice under the kidney capsule (a diagram is shown in Fig 5C). One week after the transplantation, the host mice were i.p. injected with TM x3 to induce deletion of the FoxN1fx/fx gene in the grafted thymic lobes. Two weeks after the last TM injection, the grafted thymi were isolated for flow cytometric analysis (FACS) of Nur77 and CD5 in CD4SP cells carrying Vα2+Vβ5+ TCR chain type for detecting cells bearing OT-II+ TCR-Tg T cells.
Flow cytometric assay of thymocytes and TECs
For thymocyte staining, single-thymocyte suspensions were prepared from the thymi of mice using a 70-μm cell strainer. Samples were then stained with specific fluorochrome-conjugated antibodies of cell surface CD markers, indicated in each figure legend, and then fixed and permeabilized with fixation/permeabilization buffer (eBioscience Treg staining kit, #88-8115-40), per company’s instruction, followed by intracellular staining for PE-anti-FoxP3 (eBioscience Cat# 12-5773-82) or FITC-anti-FoxP3 (eBioscience Cat# 11-5773-82), and/or PE-anti-p-Zap70 (Cell Signaling, Cat# 14791, clone 65E4, recognizing phosphorylation at Tyr319/Syk [Tyr352]), PE-anti-Nur77 (eBioscience, Cat# 12-5965-82, clone 12.14), and AlexaFluor488-anti-GFP (BioLegend, Cat# FM264G). For TEC staining, the thymus was cut into pieces, then was digested with Collagenase-V/DNase-I, as per previously published methods [51], then stained with surface molecules: fluorochrome-conjugated anti-CD45 (clone 30-F11), -MHC-II (clone M5/114.15.2), -Ly51 (clone 6C3), and -EpCAM (clone G8.8), which were purchased from BioLegend, and FITC-anti-Ovalbumin from AbCam (Cat# ab85584). Flow cytometry was performed using an LSRII flow cytometer (BD Biosciences) with 6 colors, and data were analyzed using Flow-Jo software.
Annexin-V-based apoptosis assays of tTreg cells
Thymocytes were harvested from young (2 months old) and aged (16 months old) Rag-GFP reporter mice, and stained with surface CD markers as described above. Then, the cells were washed with Annexin-V binding buffer and incubated in APC-Annexin-V (BioLegend, Cat# 640920) at a 1:20 dilution with Annexin-V binding buffer (10 mM Hepes adjusted to pH 7.4, 140 mM NaCl and 2.5 mM CaCl2) for 15 min at room temperature in the dark. The cells were then washed with PBS and fixed and permeabilized with fixation/permeabilization buffer, followed by eBioScience intracellular staining protocol for FoxP3 and GFP. Positive control cells were aliquoted from the thymocytes and made by incubating at 55°C for 20 min to induce cell death before staining.
Suppressive function assay of tTreg cells
Antigen presenting cells (APCs) were prepared from young WT mouse bone marrow-derived dendritic cells (with rGM-CSF and rIL-4 induction to get 85% CD11c+ cells) and were treated with irradiation of 2,000 Rads. T effector (Teff) cells were prepared from young WT spleen through direct flow cytometric sorting of CD4+CD25-neg spleen T cells; details were outlined in our and others’ previous publications [14, 16]. The tested newly generated tTreg cells (CD4SPCD25+Rag-GFP+) were sorted from 3 groups of thymocytes: young (approximately 6–8 weeks old) Rag-GFP WT; middle-aged (approximately 12 months old) Rag-GFP WT; and middle-aged (≥ 8 months old) FC-Rag-GFP mice (similar to ≥18-month-old WT with the atrophied thymus, See S1 Fig). The sorting purity check showed 95%–99% (S4A Fig). A mixed culture with APCs (5 × 103/well), Teff cells (5 × 104/well), and tTreg cells (2.5 × 104/well) supplied with CD3ε (1 μg/ml) and CD28 (2 μg/ml) antibodies were set in 96-well U-bottom plates for 72 hours. Proliferation of cells was determined using CellTiter 96 Aqueous One Solution Reagent (Promega, Cat#TB245) following the company’s protocol: adding 20 μl of solution per well for additional 2-hour culture, and absorbance was measured at 490 nm using an ELISA 96-well plate reader (BioTek ELx800).
Real-time RT-PCR for cytokine expression assay
Total RNAs were isolated with TRIzol reagent and reverse transcribed (RT) to cDNA with the SuperScriptIII cDNA kit (Invitrogen/ThermoFisher Scientific). Real-time RT-PCR was performed with TaqMan reagents and primers (TGFβ, NFκBs, IL-2, and housekeeping GAPDH and 18sRNA; the primers and probes were purchased from Invitrogen) [18]. The relative expression levels of mRNAs from the thymi were internally normalized to both GAPDH and 18sRNA levels, then compared to a ΔΔCT value from pooled young samples, which was always arbitrarily set as 1.0 in each real-time PCR reaction.
Statistics
For evaluation of group differences, the unpaired two-tailed Student t test was used, assuming equal variance. Differences were considered statistically significant at values of * p < 0.05; ** p < 0.01; and *** p < 0.001. Curves in Figs 1 and 2 and S2 Fig are nonlinear one-phase decay assay and linear regression assay, as indicated in the figure legends. All statistics were analyzed with Prism software (GraphPad).
Supporting information
(A) Flow cytometric profile of CD4 versus CD8 from the 5 groups of various mice. (B) Total thymocyte number in four thymocyte subsets of 5 mouse groups. (C) Proportions of thymocytes in four thymocyte subsets of 5 mouse groups. Mouse numbers in each group are 5–15 animals. The thymus of FoxN1fx/fx-CreERT (FC)-Young (1 month old) was not treated with tamoxifen (TM), showed the same profile as WT-young; while treated with TM 3 times (TM x3), showed the same profile as WT-aged (18 Months old) thymus. FC-6-month-old thymus without treatment with TM (with CreERT-mediated auto-leaky deletion of FoxN1fx/fx), also showed the same profile as WT-aged (18 Months old) thymus. NS = not significant between groups; SDs = significant differences (either p < 0.05 or p < 0.01) between any of these groups (ANOVA analysis). The results suggest that WT-young and FC-young (without TM treatment) have the same profile, while WT-aged (18 Months old), FC-young (TM x3), and FC-6 Months old (without TM treatment) possess the same profile. Underlying data used in the generation of this figure can be found in S1 Data. CD, cluster of differentiation; CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FC, FoxN1fx/fx/CreERT; FF, FoxN1fx/fx without CreERT for controls; Foxn1, Forkhead box protein N1; FoxN1fx/fx, loxp-flanked FoxN1 gene; mOVA-Tg, membrane-bound ovalbumin transgenic mouse; TM, tamoxifen; WT, wild-type.
(TIF)
(A and B) The curves are nonlinear one-phase decay; the results demonstrated that absolute cell numbers of tTreg cells were not reduced with age, while the numbers of tTcon cells were dramatically reduced with age. (C) Summarized results of absolute cell numbers of tTcon and tTreg at the ages of 4 weeks and 55 weeks from the FoxN1fx/fx-CreERT (autoleakage-induced deletion with time) and naturally aged thymus, respectively, from which the tTreg cells were not different between the two age groups, while tTcon cells were significantly reduced in the aged group compared to young groups. SD = Standard Deviation; NS = Not Significant. Underlying data used in the generation of panels A and B can be found in S1 Data. CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FoxN1fx/fx, loxp-flanked FoxN1 gene; Tcon, conventional T cell; tTcon, thymic conventional T cell; tTreg, thymic regulatory T cell.
(TIF)
(A) Comparison of Vα2 and Vβ5 double TCR positive CD4SP thymocytes in WT, OT-II TCR-Tg only, Rag−/− only, and OT-II TCR-Tg with Rag−/− background mice; (B) Comparison of Vα2 and Vβ5 double TCR positive CD3+CD4+ splenic cells in four genotypic mice. The results indicated that we successfully generated OT-II TCR-Tg with Rag−/− background mice, in which Vα2Vβ5 TCR+ CD4SP population is dramatically increased, while CD8SP and B cells are dramatically decreased. CD, cluster of differentiation; OT-II+ TCR Tg, MHC class-II restricted ovalbumin-specific TCR transgenic; RAG, Recombination activating gene; TCR, T cell receptor; Tg, transgenic; WT, wild-type.
(TIF)
Sorted newly generated (Rag-GFP+) tTreg (CD4+CD25+) cells from young WT, middle-aged WT, and middle-aged FC (with CreERT-mediated auto-leaky deletion of FoxN1fx/fx, thymocyte profile is similar to aged WT—See S1 Fig) mice were cultured with APCs (bone marrow-derived DCs), supplied with anti-CD3ε (1μg/ml) and anti-CD28 (2μg/ml), with or without Teff cells (CD4+CD25-) sorted from young WT splenic cells. Cells were cultured for 3 days, then CellTiter 96 AQ reagent (Promega) was added for 2–4 hours of culture, then reaction was analyzed by Absorber Reader at 490 nm. (A) Results of tTreg cell sorting purity test; (B) Summarized results of tTreg cell suppressive capacity from the tTreg cell sorting of three times with at least four animals in each group. The results suggest that there is no difference of tTreg cell suppressive capacity among young, middle-aged, and middle-aged FC (similar to naturally old, i.e., ≥ 18-month-old) mice. Underlying data used in the generation of this figure (panel B) can be found in S1 Data. CD, cluster of differentiation; CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FC, FoxN1fx/fx/CreERT; FoxN1, Forkhead box protein N1; FoxN1fx/fx or FoxN1-floxed, loxp-flanked FoxN1 gene; GFP, green fluorescent protein; Teff, T effector cell; tTreg, thymic regulatory T cell; WT, wild-type.
(TIF)
(Top) The mRNAs from the thymi of WT young and naturally aged mice; (Bottom) The mRNAs from thymi of FF (FoxN1fx/fx, without uCreERT) and FC (FoxN1fx/fx with CreERT, conditional knockout) mice (both groups treated with TM x3). A TaqMan-based real-time RT-PCR was conducted with TaqMan primers and probes to TGFβ, NF-κB (p105/p50), and IL-2, along with house-keeping genes, GAPDH and 18sRNA, for normalization. A Student t-test was used to determine statistical significance between groups. Data are expressed as mean ± SEM. Each symbol represents an animal. Underlying data used in the generation of this figure can be found in S1 Data. CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FC, FoxN1fx/fx/CreERT; FoxN1, Forkhead box protein N1; FoxN1fx/fx, loxp-flanked FoxN1 gene; IL-2, interleukin-2; NF-κB, Nuclear Factor kappa Beta; RT, reverse transcribed; PCR, polymerase chain reaction; tTreg, thymic regulatory T cell.
(TIF)
(A) Flow cytometric gate strategy shows gates of splenic Treg cells and Tcon cells from isotype control sample (mixture of young and old spleen cells stained with isotype control antibody for FoxP3); Young and old Rag-GFP reporter mice. (B) A summary of percentages of splenic Tcon cells and Treg cells in young and old mice, showing decreased RTE Tcon cells and increased RTE Treg cells in the old spleens. Underlying data used in the generation of this figure can be found in S1 Data. FoxP3, forkhead box P3; GFP, green fluorescent protein; RTE, recent thymic emigrant; Tcon, conventional T cell; Treg, regulatory T cell; tTcon, thymic conventional T cell; tTreg, thymic regulatory T cell.
(TIF)
(XLSX)
Acknowledgments
We thank Dr. Leszek Ignatowicz (Georgia State University) for initiation of this project and insightful suggestions, Dr. Rance Berg (UNTHSC) for critiques of experiments, Ms. Lenore Price (UNTHSC) for editing of the manuscript, and Dr. Xiangle Sun (UNTHSC) for technical assistance with the flow cytometric cell sorter, as well as Ms. Youlim Kim (summer student from Duke University) and Ms. Olga Sizova (UNTHSC) for assistance with some early experiments.
Abbreviations
- APCs
antigen presenting cells
- CD
cluster of differentiation
- CIITA
class-II transactivator
- cKO
conditional knockout
- CreERT
ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein
- DP
double positive
- FACS
flow cytometric analysis
- FC
FoxN1fx/fx/CreERT
- FF
FoxN1fx/fx without CreERT for controls
- FoxN1
Forkhead box protein N1
- FoxP3
forkhead box P3
- GFP
green fluorescent protein
- i.t.
intrathymically
- IL-2
interleukin-2
- ITAMs
immunoreceptor tyrosine-based activation motifs
- MFI
mean fluorescent intensity
- MHC
Major Histocompatibility Complex
- mOVA
membrane-bound ovalbumin
- mTEC
medullary thymic epithelial cell
- NF-κB
Nuclear Factor kappa Beta
- pTreg
peripheral regulatory T cell
- p-Zap70
phosphoralated-Zap70
- Rag
recombination-activating gene
- Rag-GFP-neg
Rag-GFP reporter negative
- RIP
rat insulin promoter
- RQ-MFI
relative quantitative Mean Fluorescence Intensity
- RT
reverse transcribed
- self-pMHC
self-peptide-Major Histocompatibility Complex
- SP
single positive
- Tcon
conventional T cell
- TCR
T cell receptor
- TEC
thymic epithelial cell
- Teff
T effector cells
- Tg
transgenic
- TGFβ
Transforming Growth Factor-Beta
- TM
tamoxifen
- Treg
regulatory T cell
- TSA
tissue-specific antigen
- tTcon
thymic conventional T cell
- tTreg
thymic regulatory T cell
- WT
wild-type
- Zap70
Zeta-chain-associated protein kinase 70
Data Availability
Data used in the generation of figures are available from the Supporting Information file S1 Data. The unique FoxN1-floxed mouse allele, generated by us, was deposited in Jackson Labs (stock #012941) for public availability.
Funding Statement
NIH/NIAID https://projectreporter.nih.gov/reporter_searchresults.cfm (grant number 1R01AI121147-01). Received by D-M.S.. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5(10):772–82. doi: 10.1038/nri1707 . [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Bour-Jordan H, Cheng M, Anderson M. T cells in the control of organ-specific autoimmunity. J Clin Invest. 2015;125(6):2250–60. doi: 10.1172/JCI78089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer E. Negative selection—clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3(5):383–91. Epub 2003/05/27. doi: 10.1038/nri1085 . [DOI] [PubMed] [Google Scholar]
- 4.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol. 2014;14(6):377–91. doi: 10.1038/nri3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162(9):5317–26. . [PubMed] [Google Scholar]
- 6.Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497(7448):258–62. doi: 10.1038/nature12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramson J, Anderson G. Thymic Epithelial Cells. Annu Rev Immunol. 2017. doi: 10.1146/annurev-immunol-051116-052320 . [DOI] [PubMed] [Google Scholar]
- 8.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107 . [DOI] [PubMed] [Google Scholar]
- 9.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. Epub 2012/01/10. doi: 10.1146/annurev.immunol.25.022106.141623 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol. 2016;16(4):220–33. doi: 10.1038/nri.2016.26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giunta S. Is inflammaging an auto[innate]immunity subclinical syndrome? Immun Ageing. 2006;3:12 doi: 10.1186/1742-4933-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun L, Guo J, Brown R, Amagai T, Zhao Y, Su DM. Declining expression of a single epithelial cell-autonomous gene accelerates age-related thymic involution. Aging Cell. 2010;9(3):347–57. Epub 2010/02/17. doi: 10.1111/j.1474-9726.2010.00559.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia J, Wang H, Guo J, Zhang Z, Coder B, Su DM. Age-Related Disruption of Steady-State Thymic Medulla Provokes Autoimmune Phenotype via Perturbing Negative Selection. Aging Dis. 2012;3(3):248–59. Epub 2012/06/23. . [PMC free article] [PubMed] [Google Scholar]
- 14.Coder BD, Wang H, Ruan L, Su DM. Thymic Involution Perturbs Negative Selection Leading to Autoreactive T Cells That Induce Chronic Inflammation. J Immunol. 2015;194(12):5825–37. doi: 10.4049/jimmunol.1500082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raynor J, Lages CS, Shehata H, Hildeman DA, Chougnet CA. Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol. 2012;24(4):482–7. Epub 2012/05/09. doi: 10.1016/j.coi.2012.04.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg SK, Delaney C, Toubai T, Ghosh A, Reddy P, Banerjee R, et al. Aging is Associated with Increased Regulatory T-cell Function. Aging Cell. 2014;13(3):441–8. doi: 10.1111/acel.12191 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiault N, Darrigues J, Adoue V, Gros M, Binet B, Perals C, et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol. 2015;16(6):628–34. doi: 10.1038/ni.3150 . [DOI] [PubMed] [Google Scholar]
- 18.Cheng L, Guo J, Sun L, Fu J, Barnes PF, Metzger D, et al. Postnatal tissue-specific disruption of transcription factor FoxN1 triggers acute thymic atrophy. J Biol Chem. 2010;285(8):5836–47. Epub 2009/12/04. doi: 10.1074/jbc.M109.072124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P, et al. A major role for Bim in regulatory T cell homeostasis. J Immunol. 2011;186(1):156–63. Epub 2010/11/26. doi: 10.4049/jimmunol.1001505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J Immunol. 2010;185(8):4535–44. Epub 2010/09/17. doi: 10.4049/jimmunol.1001668 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konkel JE, Jin W, Abbatiello B, Grainger JR, Chen W. Thymocyte apoptosis drives the intrathymic generation of regulatory T cells. Proc Natl Acad Sci U S A. 2014;111(4):E465–73. doi: 10.1073/pnas.1320319111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x . [DOI] [PubMed] [Google Scholar]
- 23.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184(3):923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, et al. Aire regulates transfer of antigen from mTEC to dendritic cells for induction of thymic tolerance. Blood. 2011. Epub 2011/04/21. doi: 10.1182/blood-2010-06-286393 . [DOI] [PubMed] [Google Scholar]
- 25.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2011;89(1):45–53. Epub 2010/11/03. doi: 10.1038/icb.2010.123 . [DOI] [PubMed] [Google Scholar]
- 26.Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11(6):512–9. doi: 10.1038/ni.1874 . [DOI] [PubMed] [Google Scholar]
- 27.Zhou T, Cheng J, Yang P, Wang Z, Liu C, Su X, et al. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J Exp Med. 1996;183(4):1879–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fassett MS, Jiang W, D'Alise AM, Mathis D, Benoist C. Nuclear receptor Nr4a1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proc Natl Acad Sci U S A. 2012;109(10):3891–6. doi: 10.1073/pnas.1200090109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, et al. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166(9):5464–72. . [DOI] [PubMed] [Google Scholar]
- 30.Palin AC, Love PE. CD5 helps aspiring regulatory T cells ward off unwelcome cytokine advances. Immunity. 2015;42(3):395–6. doi: 10.1016/j.immuni.2015.02.018 . [DOI] [PubMed] [Google Scholar]
- 31.Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. Sensory adaptation in naive peripheral CD4 T cells. J Exp Med. 2001;194(9):1253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172(1):40–4. . [DOI] [PubMed] [Google Scholar]
- 33.Ordonez-Rueda D, Lozano F, Sarukhan A, Raman C, Garcia-Zepeda EA, Soldevila G. Increased numbers of thymic and peripheral CD4+ CD25+Foxp3+ cells in the absence of CD5 signaling. Eur J Immunol. 2009;39(8):2233–47. doi: 10.1002/eji.200839053 . [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Brown R, Chen S, Zhuge Q, Su DM. Aging induced decline in T-lymphopoiesis is primarily dependent on status of progenitor niches in the bone marrow and thymus. Aging (Albany NY). 2012;4(9):606–19. doi: 10.18632/aging.100487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–11. Epub 2008/01/18. doi: 10.1016/j.immuni.2007.11.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32(5):642–53. doi: 10.1016/j.immuni.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Konkel JE. Development of thymic Foxp3(+) regulatory T cells: TGF-beta matters. Eur J Immunol. 2015;45(4):958–65. doi: 10.1002/eji.201444999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31(6):921–31. doi: 10.1016/j.immuni.2009.09.022 . [DOI] [PubMed] [Google Scholar]
- 39.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31(6):932–40. doi: 10.1016/j.immuni.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang S, Song KD, Lesourne R, Lee J, Pinkhasov J, Li L, et al. Reduced TCR signaling potential impairs negative selection but does not result in autoimmune disease. J Exp Med. 2012;209(10):1781–95. doi: 10.1084/jem.20120058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson MS, Su MA. AIRE expands: new roles in immune tolerance and beyond. Nat Rev Immunol. 2016;16(4):247–58. doi: 10.1038/nri.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23(2):227–39. doi: 10.1016/j.immuni.2005.07.005 . [DOI] [PubMed] [Google Scholar]
- 43.Zhu ML, Nagavalli A, Su MA. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res. 2013;73(7):2104–16. doi: 10.1158/0008-5472.CAN-12-3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140(3):540–6. Epub 2005/06/04. doi: 10.1111/j.1365-2249.2005.02798.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181(3):1835–48. Epub 2008/07/22. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177(12):8348–55. Epub 2006/12/05. . [DOI] [PubMed] [Google Scholar]
- 47.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14(4):307–8. doi: 10.1038/ni.2554 . [DOI] [PubMed] [Google Scholar]
- 48.Jagger A, Shimojima Y, Goronzy JJ, Weyand CM. Regulatory T cells and the immune aging process: a mini-review. Gerontology. 2014;60(2):130–7. doi: 10.1159/000355303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carpentier M, Chappert P, Kuhn C, Lalfer M, Flament H, Burlen-Defranoux O, et al. Extrathymic induction of Foxp3(+) regulatory T cells declines with age in a T-cell intrinsic manner. Eur J Immunol. 2013;43(10):2598–604. doi: 10.1002/eji.201343532 . [DOI] [PubMed] [Google Scholar]
- 50.Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, Savage PA. Aire Enforces Immune Tolerance by Directing Autoreactive T Cells into the Regulatory T Cell Lineage. Immunity. 2016;44(5):1102–13. doi: 10.1016/j.immuni.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnley P, Rahman M, Wang H, Zhang Z, Sun X, Zhuge Q, et al. Role of the p63-FoxN1 regulatory axis in thymic epithelial cell homeostasis during aging. Cell Death Dis. 2013;4:e932 doi: 10.1038/cddis.2013.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Flow cytometric profile of CD4 versus CD8 from the 5 groups of various mice. (B) Total thymocyte number in four thymocyte subsets of 5 mouse groups. (C) Proportions of thymocytes in four thymocyte subsets of 5 mouse groups. Mouse numbers in each group are 5–15 animals. The thymus of FoxN1fx/fx-CreERT (FC)-Young (1 month old) was not treated with tamoxifen (TM), showed the same profile as WT-young; while treated with TM 3 times (TM x3), showed the same profile as WT-aged (18 Months old) thymus. FC-6-month-old thymus without treatment with TM (with CreERT-mediated auto-leaky deletion of FoxN1fx/fx), also showed the same profile as WT-aged (18 Months old) thymus. NS = not significant between groups; SDs = significant differences (either p < 0.05 or p < 0.01) between any of these groups (ANOVA analysis). The results suggest that WT-young and FC-young (without TM treatment) have the same profile, while WT-aged (18 Months old), FC-young (TM x3), and FC-6 Months old (without TM treatment) possess the same profile. Underlying data used in the generation of this figure can be found in S1 Data. CD, cluster of differentiation; CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FC, FoxN1fx/fx/CreERT; FF, FoxN1fx/fx without CreERT for controls; Foxn1, Forkhead box protein N1; FoxN1fx/fx, loxp-flanked FoxN1 gene; mOVA-Tg, membrane-bound ovalbumin transgenic mouse; TM, tamoxifen; WT, wild-type.
(TIF)
(A and B) The curves are nonlinear one-phase decay; the results demonstrated that absolute cell numbers of tTreg cells were not reduced with age, while the numbers of tTcon cells were dramatically reduced with age. (C) Summarized results of absolute cell numbers of tTcon and tTreg at the ages of 4 weeks and 55 weeks from the FoxN1fx/fx-CreERT (autoleakage-induced deletion with time) and naturally aged thymus, respectively, from which the tTreg cells were not different between the two age groups, while tTcon cells were significantly reduced in the aged group compared to young groups. SD = Standard Deviation; NS = Not Significant. Underlying data used in the generation of panels A and B can be found in S1 Data. CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FoxN1fx/fx, loxp-flanked FoxN1 gene; Tcon, conventional T cell; tTcon, thymic conventional T cell; tTreg, thymic regulatory T cell.
(TIF)
(A) Comparison of Vα2 and Vβ5 double TCR positive CD4SP thymocytes in WT, OT-II TCR-Tg only, Rag−/− only, and OT-II TCR-Tg with Rag−/− background mice; (B) Comparison of Vα2 and Vβ5 double TCR positive CD3+CD4+ splenic cells in four genotypic mice. The results indicated that we successfully generated OT-II TCR-Tg with Rag−/− background mice, in which Vα2Vβ5 TCR+ CD4SP population is dramatically increased, while CD8SP and B cells are dramatically decreased. CD, cluster of differentiation; OT-II+ TCR Tg, MHC class-II restricted ovalbumin-specific TCR transgenic; RAG, Recombination activating gene; TCR, T cell receptor; Tg, transgenic; WT, wild-type.
(TIF)
Sorted newly generated (Rag-GFP+) tTreg (CD4+CD25+) cells from young WT, middle-aged WT, and middle-aged FC (with CreERT-mediated auto-leaky deletion of FoxN1fx/fx, thymocyte profile is similar to aged WT—See S1 Fig) mice were cultured with APCs (bone marrow-derived DCs), supplied with anti-CD3ε (1μg/ml) and anti-CD28 (2μg/ml), with or without Teff cells (CD4+CD25-) sorted from young WT splenic cells. Cells were cultured for 3 days, then CellTiter 96 AQ reagent (Promega) was added for 2–4 hours of culture, then reaction was analyzed by Absorber Reader at 490 nm. (A) Results of tTreg cell sorting purity test; (B) Summarized results of tTreg cell suppressive capacity from the tTreg cell sorting of three times with at least four animals in each group. The results suggest that there is no difference of tTreg cell suppressive capacity among young, middle-aged, and middle-aged FC (similar to naturally old, i.e., ≥ 18-month-old) mice. Underlying data used in the generation of this figure (panel B) can be found in S1 Data. CD, cluster of differentiation; CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FC, FoxN1fx/fx/CreERT; FoxN1, Forkhead box protein N1; FoxN1fx/fx or FoxN1-floxed, loxp-flanked FoxN1 gene; GFP, green fluorescent protein; Teff, T effector cell; tTreg, thymic regulatory T cell; WT, wild-type.
(TIF)
(Top) The mRNAs from the thymi of WT young and naturally aged mice; (Bottom) The mRNAs from thymi of FF (FoxN1fx/fx, without uCreERT) and FC (FoxN1fx/fx with CreERT, conditional knockout) mice (both groups treated with TM x3). A TaqMan-based real-time RT-PCR was conducted with TaqMan primers and probes to TGFβ, NF-κB (p105/p50), and IL-2, along with house-keeping genes, GAPDH and 18sRNA, for normalization. A Student t-test was used to determine statistical significance between groups. Data are expressed as mean ± SEM. Each symbol represents an animal. Underlying data used in the generation of this figure can be found in S1 Data. CreERT, ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein; FC, FoxN1fx/fx/CreERT; FoxN1, Forkhead box protein N1; FoxN1fx/fx, loxp-flanked FoxN1 gene; IL-2, interleukin-2; NF-κB, Nuclear Factor kappa Beta; RT, reverse transcribed; PCR, polymerase chain reaction; tTreg, thymic regulatory T cell.
(TIF)
(A) Flow cytometric gate strategy shows gates of splenic Treg cells and Tcon cells from isotype control sample (mixture of young and old spleen cells stained with isotype control antibody for FoxP3); Young and old Rag-GFP reporter mice. (B) A summary of percentages of splenic Tcon cells and Treg cells in young and old mice, showing decreased RTE Tcon cells and increased RTE Treg cells in the old spleens. Underlying data used in the generation of this figure can be found in S1 Data. FoxP3, forkhead box P3; GFP, green fluorescent protein; RTE, recent thymic emigrant; Tcon, conventional T cell; Treg, regulatory T cell; tTcon, thymic conventional T cell; tTreg, thymic regulatory T cell.
(TIF)
(XLSX)
Data Availability Statement
Data used in the generation of figures are available from the Supporting Information file S1 Data. The unique FoxN1-floxed mouse allele, generated by us, was deposited in Jackson Labs (stock #012941) for public availability.