Abstract
Purpose
For women with stage IV breast cancer (BC), the association between survival time (ST) and use of aggressive end-of-life (EOL) care is unknown.
Methods
We used the SEER-Medicare database to identify women with stage IV BC diagnosed 2002–2011 who died by 12/31/2012. Aggressive EOL care was defined as receipt in the last month of life: >1 ED visit, >1 hospitalization, ICU admission, life-extending procedures, hospice admission within 3 days of death, IV chemotherapy within 14 days of death, and/or ≥10 unique physician encounters in the last 6 months of life. Receipt of aggressive EOL care and hospice in the last month of life were determined using claims, and multivariable analysis was used to identify factors associated with receipt. Costs of care were also evaluated.
Results
We identified 4521 eligible patients. Of these, 2748 (60.8%) received aggressive EOL care. Factors associated with aggressive EOL care were race (OR 1.45, 95% CI 1.19–1.81 for blacks compared to whites) and more frequent oncology office visits (OR 1.56, 95% CI 1.28–1.90). Patients who lived >12 months after diagnosis were less likely to receive aggressive EOL care (OR 0.44, 95% CI 0.38–0.52), and more likely to utilize hospice (OR 1.43, 95% CI 1.21–1.69) compared to patients who lived ≤6 months. Patients with a shorter ST had significantly higher costs of care per-month-alive compared to patients with longer ST.
Conclusion
Patients with a shorter ST were more likely to receive aggressive EOL care and had higher costs of care compared to patients who lived longer.
Keywords: End-of-life care, Health services overuse, Healthcare quality, Hospice, Breast cancer
Introduction
Compared to other types of cancer, patients with metastatic breast cancer (MBC) may utilize more aggressive end-of-life (EOL) care. A population-based study of 215,484 elderly patients who died of cancer between 1993 and 2000 showed that patients with breast cancer compared to patients with lung cancer were 63% more likely to receive chemotherapy within the last 14 days of death, 21% more likely not to utilize hospice care, and 21% more likely to be admitted to hospice within 3 days of death [1]. Similarly, a single-institution evaluation of patients with MBC reported that one-third of women did not utilize hospice services, and of the patients who were admitted to hospice, only 22% had a documented advanced directive discussion in their medical record [2].
The National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) recommend against aggressive care at the EOL [3–5]. Aggressive EOL care includes in the last month of life: more than one emergency department (ED) visit, more than one hospital admission, admission to the intensive care unit (ICU), receipt of life-extending procedures such as a feeding tube, ventilation, or cardiopulmonary resuscitation (CPR), admission to hospice within 3 days of death, receipt of intravenous (IV) chemotherapy within 14 days of death, and/or ≥10 unique physician encounters in the last 6 months of life [6–8, 12]. Despite these recommendations, 30–65% of patients with advanced cancer receive aggressive EOL care [6–10]. Use of hospice has been shown to reduce the number of ED visits, hospitalizations, and total costs of care, and is associated with improved quality of EOL care [3, 11–13]. NCCN guidelines call for consideration of hospice care in the management of patients with advanced cancer who experience disease progression or unacceptable toxicity from treatment; despite these recommendations, hospice care is only utilized in about 60% of patients with advanced cancer, and when utilized, 10.9% enroll within three days of death [3, 14, 15]. A population-based study of 6956 women with ovarian cancer found that, despite a significant increase of hospice use from 1997 to 2007, aggressive EOL care also increased significantly, suggesting that hospice may be introduced too late in the course of disease [8].
Survival time may also impact the receipt of aggressive EOL care. A population-based analysis showed that at least 65% of patients with poor prognosis cancers, including lung and pancreatic cancers, utilized aggressive EOL care. It is unknown how survival time (ST) impacts receipt of aggressive EOL care among patients with stage IV breast cancer (BC). The objectives of this study were to determine the association between length of time alive with stage IV BC and aggressive EOL care, and its associated costs.
Methods
Data source
We used data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database [16]. The SEER registries encompass approximately 28% of the US population and the database contains information on patient sociodemographic characteristics, tumor details, and survival outcomes for cancers occurring within those populations [17]. Linkage with the Medicare database allows the longitudinal evaluation of cancer care and characterizes inpatient, outpatient, and physician-billed services, including diagnoses, and healthcare costs [18].
Cohort selection
We identified all women ≥66 years of age with histologically confirmed stage IV breast cancer diagnosed between January 1, 2002 and December 31, 2011, who died by December 31, 2012. We excluded patients who were enrolled in a Medicare health maintenance organization or who were not continuously enrolled by Medicare Parts A and B for a period of 12 months before diagnosis through death or the end of the study period. We also excluded patients who were enrolled in Medicare due to end-stage renal disease, as well as patients with other primary cancers. We also excluded patients whose reporting source of death was autopsy or death certificate, whose reason for entitlement was not age, whose date of death differed by >3 months between SEER and Medicare, and those with missing claims from 45 days before diagnosis through 180 days after diagnosis. Patients who died within 30 days of diagnosis were also excluded.
End-of-life care
Aggressive EOL care was defined as in the last month of life having any one of the following: more than one ED visit, more than one hospital admission, greater than 14 days hospitalized, admission to the ICU, admission to hospice within 3 days of death, receipt of IV chemotherapy within 14 days of death, or the use of life-extending procedures (feeding tube placement, ventilation, and/or cardiopulmonary resuscitation) [6–8, 10]. We also included ten or more encounters with unique physicians in the last 6 months of life in our definition of aggressive EOL care [10]. We identified ICU admissions using International Classification of Diseases, Ninth Revision (ICD-9) codes (96.7x) and diagnosis-related group codes (475 or 483) for mechanical ventilation and the ICU indicator variable in the Medicare inpatient file [19, 20]. Hospice admissions were identified through billing claims in the Medicare hospice files and were categorized as home and/or facility hospice (hospital and/or skilled nursing facility [SNF]). In addition, we examined length of stay (LOS) among patients admitted to the hospital, ICU, and hospice in the last month of life [8].
Among patients who utilized hospice, the source of referral (inpatient, SNF, or outpatient) was identified using billing claims; patients who enrolled in hospice within 2 days of hospital discharge were characterized as inpatient referrals, patients enrolled within 2 days of SNF discharge were characterized as SNF referrals, and other enrollments were characterized as outpatient referrals [8, 21].
Survival time (ST)
Patients were categorized by the time from diagnosis of breast cancer until death. This was classified as: ≤6 months, 6–12 months, and >12 months.
Costs of care
Costs of care were calculated from Medicare reimbursement claims from physician, hospital, outpatient, durable medical equipment, and hospice filings between the date of diagnosis and the date of death [22, 23]. Costs were categorized as costs per-month-alive after diagnosis, which were calculated as totals costs divided by the number of months alive after diagnosis; and costs in the last month of life.
Location of death
Location of death (LOD) was determined using discharge status codes on claims from the death date, including care received in the hospital, SNF, facility and home hospice. Patients without a hospice discharge prior to their death date were considered to have died in hospice. If there was a hospital or SNF claim on their death date their LOD was categorized as facility hospice; if there was no facility claim on their death date their LOC was categorized as home hospice. If patients were never enrolled in hospice or discharged prior to their death date, with hospital or SNF claims on their death date their LOD was considered hospital or SNF, respectively; if no claims on their death date their LOD was considered home without hospice [24].
Covariates
Demographic covariates included age at diagnosis (66–69, 70–74, 75–79, and ≥80 years), year of diagnosis, marital status (married, single, unknown), race (white, black, other), hospital location (urban, rural), region (East, Midwest, West), Charlson comorbidity score (0, 1, ≥2), and socioeconomic status. Tumors were categorized as hormone receptor (estrogen receptor [ER] and/or progesterone receptor [PR]) positive, negative, or unknown. Other covariates included medical oncology office visits, defined as the number of visits per-month alive by tertile (low, medium, high). To define medical oncology office visits, physician files were used to determine provider specialty and date of office visit. Visits were identified through HCPCS codes [23].
Statistical analysis
Univariate analyses comparing patient and tumor characteristics with patient prognosis were performed with t tests for continuous variables and χ2 tests for categorical variables. We developed logistic regression models to determine the associations between clinical, demographic, and prognostic factors and receipt of aggressive EOL care and hospice care in the last month of life. For analyses of hospice care in the last month of life, hospice enrollment within 3 days of death was excluded from the analysis. Trends over time in median EOL expenditures and expenditure per-month-alive were evaluated by ST. Logistic regression was used to assess the association of year of death with aggressive EOL care utilization and location of death, which was adjusted for patient characteristics described to predict the yearly proportion of patients with each outcome. Then a linear trend test over time using orthogonal polynomial coefficients was conducted to determine if there was significant change in the adjusted proportions between 2002 and 2011 [8, 25]. All analyses were conducted with SAS version 9.4 software (SAS Institute, Cary, NC) and STATA 14 software (College Station, TX). All statistical tests were two-sided, with α <0.05.
Results
We identified 4521 women with an initial diagnosis of MBC between 2002 and 2011 who were eligible for analysis. The cohort was predominantly white (83.0%), without comorbidities (48.7%), and had hormone receptor (HR)-positive MBC (56.3%, Table 1). The median survival of the total cohort was 9.8 months, while 2040 (45.1%) patients lived at least 12 months from their diagnosis. The majority of patients 2748 (60.8%) received aggressive EOL care as defined (Supplemental Table 1). The most common LOD was home hospice (37.7%) followed by the hospital (22.0%).
Table 1.
Patient and hospital characteristics of women with metastatic breast cancer diagnosed 2002–2011
| Total | Length of time with MBC (time from diagnosis until death) | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| ≤6 months | 6–12 months | > 12 months | |||||||
|
|
|
|
|||||||
| N | % | N | % | N | % | N | % | ||
| 4521 | 100 | 1752 | 38.8 | 729 | 16.1 | 2040 | 45.1 | ||
| Median survivala | 9.8 | 2.5 | 8.6 | 27.2 | |||||
| Age | < 0.0001 | ||||||||
| 66–69 | 736 | 16.3 | 207 | 11.8 | 109 | 14.9 | 420 | 20.6 | |
| 70–74 | 953 | 21.1 | 309 | 17.6 | 169 | 23.2 | 475 | 23.3 | |
| 75–79 | 994 | 22.0 | 377 | 21.5 | 161 | 22.1 | 456 | 22.3 | |
| 80–84 | 935 | 20.7 | 387 | 22.1 | 151 | 20.7 | 397 | 19.5 | |
| ≥85 | 903 | 19.9 | 472 | 27.0 | 139 | 19.1 | 292 | 14.3 | |
| Diagnosis year | < 0.0001 | ||||||||
| 2002 | 519 | 11.5 | 196 | 11.2 | 77 | 10.6 | 246 | 12.2 | |
| 2003 | 488 | 10.8 | 180 | 10.3 | 62 | 8.5 | 246 | 12.1 | |
| 2004 | 527 | 11.7 | 174 | 9.9 | 86 | 11.8 | 267 | 13.1 | |
| 2005 | 530 | 11.7 | 170 | 9.7 | 97 | 13.3 | 263 | 12.9 | |
| 2006 | 493 | 10.9 | 200 | 11.4 | 66 | 9.0 | 227 | 11.1 | |
| 2007 | 488 | 10.8 | 177 | 10.1 | 68 | 9.3 | 243 | 11.9 | |
| 2008 | 448 | 9.9 | 184 | 10.5 | 70 | 9.6 | 194 | 9.5 | |
| 2009 | 412 | 9.1 | 156 | 8.9 | 77 | 10.6 | 179 | 8.8 | |
| 2010 | 355 | 7.8 | 163 | 9.3 | 64 | 8.8 | 128 | 6.2 | |
| 2011 | 261 | 5.8 | 152 | 8.7 | 62 | 8.5 | 47 | 2.3 | |
| Race | 0.02 | ||||||||
| White | 3753 | 83.0 | 1426 | 81.4 | 602 | 82.6 | 1725 | 84.5 | |
| Black | 592 | 13.1 | 263 | 15.0 | 97 | 13.3 | 232 | 11.4 | |
| Other/unknown | 176 | 3.9 | 63 | 3.6 | 30 | 4.1 | 83 | 4.1 | |
| Marital status | < 0.0001 | ||||||||
| Married | 3040 | 67.2 | 1258 | 71.8 | 500 | 68.6 | 1282 | 62.8 | |
| Single | 1277 | 28.3 | 417 | 23.8 | 184 | 25.2 | 676 | 33.2 | |
| Unknown | 204 | 4.5 | 77 | 4.4 | 45 | 6.2 | 82 | 4.0 | |
| Hospital location | 0.42 | ||||||||
| Urban | 4072 | 90.1 | 1565 | 89.3 | 660 | 90.5 | 1847 | 90.5 | |
| Rural | 449 | 9.9 | 187 | 10.7 | 69 | 9.5 | 193 | 9.5 | |
| Region | 0.40 | ||||||||
| East | 1217 | 26.9 | 471 | 26.9 | 180 | 24.7 | 566 | 27.7 | |
| Midwest | 1868 | 41.3 | 741 | 42.3 | 305 | 41.8 | 822 | 40.3 | |
| West | 1436 | 31.8 | 540 | 30.8 | 244 | 33.5 | 652 | 32.0 | |
| Charlson comorbidity | < 0.0001 | ||||||||
| 0 | 2198 | 48.7 | 730 | 41.8 | 339 | 46.5 | 1129 | 55.4 | |
| 1 | 1149 | 25.4 | 450 | 25.7 | 187 | 25.7 | 512 | 25.1 | |
| ≥2 | 1169 | 25.9 | 568 | 32.5 | 203 | 27.8 | 398 | 19.5 | |
| Socioeconomic status | 0.45 | ||||||||
| 1 (low) | 1376 | 30.5 | 556 | 31.7 | 228 | 31.3 | 592 | 29.0 | |
| 2 | 1018 | 22.5 | 404 | 23.1 | 144 | 19.8 | 470 | 23.1 | |
| 3 | 650 | 14.4 | 242 | 13.8 | 111 | 15.2 | 297 | 14.6 | |
| 4 | 952 | 21.1 | 355 | 20.3 | 155 | 21.3 | 442 | 21.7 | |
| 5 (high) | 522 | 11.5 | 195 | 11.1 | 90 | 12.4 | 237 | 11.6 | |
| ER/PR status | < 0.0001 | ||||||||
| Positive | 2545 | 56.3 | 764 | 43.6 | 366 | 50.2 | 1415 | 69.4 | |
| Negative | 822 | 18.2 | 390 | 22.3 | 182 | 25.0 | 250 | 12.2 | |
| Unknown | 1154 | 25.5 | 598 | 34.1 | 181 | 24.8 | 375 | 18.4 | |
| Location of death | < 0.0001 | ||||||||
| Home hospice | 1703 | 37.7 | 562 | 32.1 | 297 | 40.7 | 844 | 41.4 | |
| Facility hospice | 902 | 19.9 | 346 | 19.7 | 146 | 20.0 | 410 | 20.1 | |
| Hospital | 995 | 22.0 | 454 | 25.9 | 137 | 18.8 | 404 | 19.8 | |
| SNF | 330 | 7.3 | 175 | 10.0 | 47 | 6.5 | 108 | 5.3 | |
| Home without hospice | 591 | 13.1 | 215 | 12.3 | 102 | 14.0 | 274 | 13.4 | |
All bolded values are p < 0.05
Median survival in months
In a multivariable model (Table 2), race (OR 1.46, 95% CI 1.19–1.81 for blacks compared to whites), and higher oncology office visit volume (OR 1.56 95% CI 1.28–1.90 compared to low volume) were associated with aggressive EOL care. Patients who never saw an oncologist (OR 0.67, 95% CI 0.55–0.81) and patients with a longer time alive since diagnosis (OR 0.47, 95% CI 0.39–0.58 among patients who lived 6–12 months, and OR 0.44 95% CI 0.38–0.52 among patients who lived >12 months) were less likely to receive aggressive EOL care than those who lived <6 months.
Table 2.
Multivariable analysis of factors associated with aggressive end-of-life care and hospice utilization in the last month of life (N = 4521)
| Factor | Aggressive EOL carea | Hospice use last month of lifeb | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | ||||||
| 66–69 | 0.94 | 0.76–1.17 | 0.58 | 0.85 | 0.69–1.05 | 0.14 |
| 70–74 | Reference | Reference | ||||
| 75–79 | 0.86 | 0.71–1.05 | 0.13 | 1.10 | 0.90–1.34 | 0.36 |
| 80–84 | 0.66 | 0.54–0.80 | < 0.0001 | 1.01 | 0.83–1.25 | 0.89 |
| ≥85 | 0.49 | 0.40–0.60 | < 0.0001 | 0.93 | 0.75–1.16 | 0.52 |
| Diagnosis year | 1.02 | 0.99–1.04 | 0.19 | 1.10 | 1.07–1.12 | < 0.0001 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 1.46 | 1.19–1.81 | 0.0004 | 0.82 | 0.66–1.01 | 0.06 |
| Other/unknown | 1.17 | 0.83–1.63 | 0.37 | 0.68 | 0.48–0.96 | 0.03 |
| Marital Status | ||||||
| Married | 1.04 | 0.90–1.21 | 0.59 | 0.94 | 0.81–1.09 | 0.41 |
| Single | Reference | Reference | ||||
| Unknown | 1.29 | 0.94–1.77 | 0.12 | 0.99 | 0.72–1.36 | 0.97 |
| Hospital location | ||||||
| Urban | Reference | Reference | ||||
| Rural | 0.75 | 0.60–0.93 | 0.01 | 0.74 | 0.58–0.93 | 0.01 |
| Region | ||||||
| East | Reference | Reference | ||||
| Midwest | 0.64 | 0.54–0.76 | < 0.0001 | 1.65 | 1.38–1.98 | < 0.0001 |
| West | 0.60 | 0.50–0.71 | < 0.0001 | 1.02 | 0.86–1.22 | 0.82 |
| Charlson comorbidity | ||||||
| 0 | Reference | Reference | ||||
| 1 | 1.20 | 1.03–1.39 | 0.02 | 0.95 | 0.81–1.12 | 0.53 |
| ≥ 2 | 2.00 | 1.70–2.35 | < 0.0001 | 0.86 | 0.73–1.01 | 0.07 |
| Socioeconomic status | ||||||
| 1 (low) | Reference | Reference | ||||
| 2 | 0.81 | 0.68–0.97 | 0.02 | 1.10 | 0.91–1.33 | 0.32 |
| 3 | 0.91 | 0.74–1.13 | 0.39 | 1.18 | 0.95–1.47 | 0.13 |
| 4 | 0.99 | 0.81–1.20 | 0.89 | 1.43 | 1.17–1.76 | 0.0006 |
| 5 (high) | 0.95 | 0.75–1.21 | 0.68 | 1.56 | 1.21–2.00 | 0.0005 |
| ER/PR status | ||||||
| Positive | Reference | Reference | ||||
| Negative | 0.99 | 0.83–1.18 | 0.90 | 1.06 | 0.90–1.29 | 0.42 |
| Unknown | 0.65 | 0.56–0.76 | < 0.0001 | 1.05 | 0.88–1.22 | 0.71 |
| Survival time | ||||||
| ≤6 months | Reference | Reference | ||||
| 6–12 months | 0.47 | 0.39–0.58 | < 0.0001 | 1.27 | 1.02–1.53 | 0.03 |
| >12 months | 0.44 | 0.38–0.52 | < 0.001 | 1.45 | 1.21–1.69 | < 0.0001 |
| Oncology office visit volume | ||||||
| Never saw | 0.67 | 0.55–0.81 | < 0.0001 | 1.00 | 0.82–1.21 | 0.97 |
| Low | Reference | Reference | ||||
| Medium | 1.02 | 0.85–1.23 | 0.82 | 1.13 | 0.93–1.38 | 0.22 |
| High | 1.56 | 1.28–1.90 | < 0.0001 | 1.03 | 0.84–1.26 | 0.78 |
| Number of aggressive EOL resources utilized | ||||||
| 0 | – | – | – | 1.94 | 1.64–2.28 | < 0.0001 |
| 1 | – | – | – | Reference | ||
| ≥2 | – | – | – | 0.30 | 0.25–0.35 | < 0.0001 |
All bolded values are p < 0.05
Aggressive end-of-life care included: in the last month of life:>1 emergency department visit,>1 hospital admission,>14 days hospitalized, admission to the intensive care unit (ICU), admission to hospice within 3 days of death, receipt of intravenous (IV) chemotherapy within 14 days of death, life-extending procedures such as a feeding tube placement, ventilation, and/or cardiopulmonary resuscitation, or ten or more encounters with unique physicians in the last 6 months of life
In the multivariable model of hospice, aggressive end-of-life care included all measures listed above except admission to hospice within 3 days of death
Among the total cohort, 62.8% of patients enrolled in hospice care at some point during their treatment course. The majority of patients enrolled in hospice had outpatient referrals (55.4%, Table 3), and received home hospice without facility hospice (50.9%). Patients who died within 6 months of diagnosis compared to patients who lived 6–12 months and >12 months were more likely to receive inpatient hospice referrals (44.9% vs. 31.5%, vs. 28.9%, respectively, p < 0.0001); and were more likely to utilize facility hospice (50.9% vs. 37.0% vs. 31.7%, respectively, p <0.0001).
Table 3.
Hospice referral source and location of services among elderly patients with metastatic breast cancer who enrolled in hospice (N = 2838, 62.8% of total cohort)
| Total | Prognosis (time from diagnosis until death) | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ≤6 months | 6–12 months | > 12 months | |||||||
|
|
|
|
|
||||||
| N | % | N | % | N | % | N | % | ||
| 4521 | 100 | 1752 | 38.8 | 729 | 16.1 | 2040 | 45.1 | ||
| Median survival (months) | 9.8 | 2.5 | 8.6 | 27.2 | |||||
| Hospice enrollment | 2821 | 100 | 975 | 34.6 | 476 | 16.9 | 1370 | 48.5 | < 0.0001 |
| Source of referral | < 0.0001 | ||||||||
| Inpatient | 984 | 34.9 | 438 | 44.9 | 150 | 31.5 | 396 | 28.9 | |
| SNF | 273 | 9.7 | 143 | 14.7 | 29 | 6.1 | 101 | 7.4 | |
| Outpatient | 1564 | 55.4 | 394 | 40.4 | 297 | 62.4 | 873 | 63.7 | |
| Hospice location | < 0.0001 | ||||||||
| Home | 1436 | 50.9 | 418 | 42.9 | 253 | 53.1 | 765 | 55.8 | |
| Facility | 1106 | 39.2 | 496 | 50.9 | 176 | 37.0 | 434 | 31.7 | |
| Both | 279 | 9.9 | 61 | 6.2 | 47 | 9.9 | 171 | 12.5 | |
All bolded values are p < 0.05
Receipt of hospice in the last month of life was evaluated in a multivariable model (Table 2), the following variables were associated with hospice utilization in the last month of life: diagnosis year (OR 1.10 95% CI 1.07–1.12), higher socioeconomic (SES) status (OR 1.43 95% CI 1.17–1.76 SES 4 vs. SES 1, and OR 1.56 95% CI 1.21–2.00 SES 5 vs. SES 1), patients with longer ST (OR 1.25, 95% CI 1.02–1.053 among patients who lived 6–12 months, and OR 1.43 95% CI 1.21–1.69 among patients who lived >12 months versus those who lived <6 months), and patients who did not utilize aggressive EOL care (OR 1.94 95% CI 1.64–2.28). Patients who received at least two measures of aggressive EOL care (OR 0.30 95% CI 0.25–0.35) were less likely to utilize hospice.
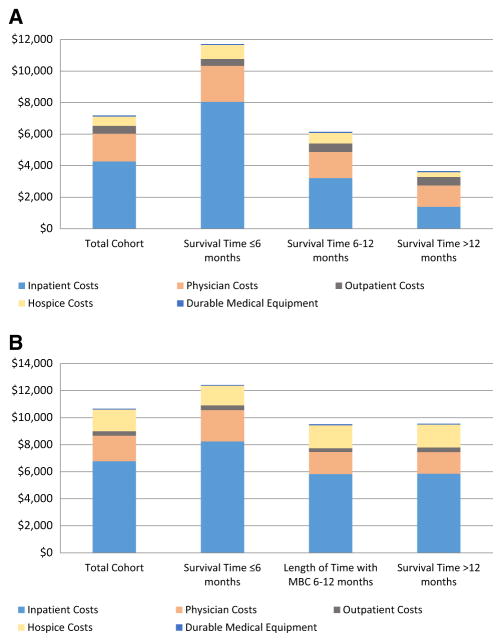
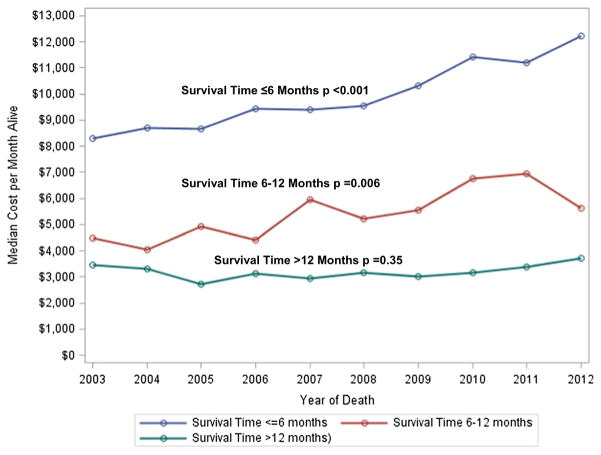
Total costs per-month-alive and in the last month of life were significantly higher among patients who lived ≤6 months compared to patients who lived 6–12 months and >12 months (Fig. 1). The majority (65.5%) of total costs per-month-alive were related to inpatient care, and inpatient care costs were significantly higher among patients who lived ≤6 months compared to patients who lived 6–12 months and >12 months ($8923 vs. $3639 vs. $1512, respectively, p < 0.001). The results were similar for costs in the last month of life. Overall median costs per-month-alive significantly increased during this time frame among patients who lived ≤6 months from $7649 to $11,184 (p < 0.0001, Fig. 2), and among patients who lived 6–12 months from $4182 to $6937 (p = 0.006), costs did not significantly change from 2002 to 2011 among patients who lived >12 months from diagnosis.
Fig. 1.
a Mean direct costs of care per-month alive for patients with MBC (N = 4521). Costs of care in each category were significantly different between groups by length of time with metastatic breast cancer. b Mean direct costs of care in the last month of life for patients with MBC (N = 4521). Costs of care in each category were significantly different between groups by length of time with metastatic breast cancer except for outpatient costs of care
Fig. 2.
Unadjusted trends in median expenditures per-month-alive by survival time with stage IV breast cancer (N = 4521)
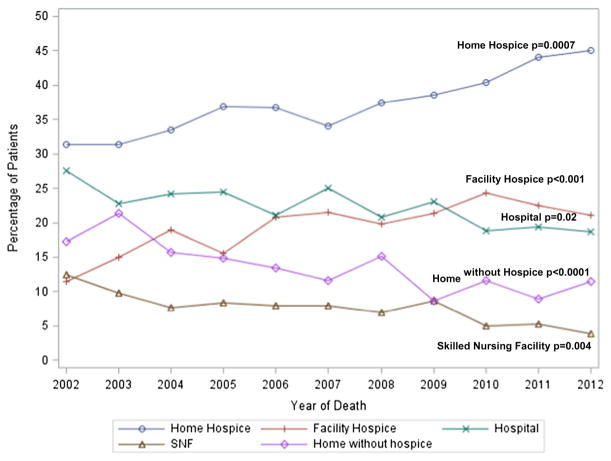
Despite increasing costs over time, the proportion of patients who received aggressive EOL care did not vary much from 2002 to 2011 6.0 versus 5.95% (p = 0.62,), and only significantly increased with regard to ICU admission 11.2 to 18.3% (p < 0.0001) and life sustaining measures 6.7 to 9.6% (p = 0.007). In contrast, from 2002 to 2011 the proportion of patients who died in home hospice increased from 34.7 to 43.0% (p = 0.0007, Fig. 3) and the proportion of patients decreased who died in the hospital 25.2 to 19.3% (p = 0.02).
Fig. 3.
Adjusted yearly proportion of location of death from 2002 to 2012 (N = 4521). Logistic regression models were adjusted for age, diagnosis year, race, marital status, hospital location, region, comorbidities, socioeconomic status, hormone receptor status, oncology office volume, survival time. Linear trend test over time of the predicted value using orthogonal polynomial coefficients
Discussion
In our population-based study of women with stage IV breast cancer who expired, we found that approximately 60% of patients diagnosed with BC received aggressive EOL care, and that patients with a shorter survival time were more likely to receive aggressive EOL care and less likely to receive hospice. Patients who live ≤6 months from their diagnosis have higher EOL costs compared to those who live beyond 6 months, and the bulk of these costs are attributed to inpatient care. Despite an increase in hospice use over time, costs per-month alive have increased among all patients with MBC, and particularly among lose who live ≤6 months.
Hospice care at the end-of-life is considered an essential component of high quality care [7, 24, 26]. In an analysis of cancer patients using Medicare data from 2003 to 2010, approximately one quarter of deaths occurred in the hospital, and over 60% of patients were hospitalized in the last month of life [27]. In 2010, 61.3% of patients enrolled in hospice, and 10.9% enrolled in the last three days of life; the average hospice LOS was only 9 days [27]. Patients with longer admissions to hospice (>3 days) reported higher quality of life, and their caregivers were significantly less likely to experience major depressive disorder after their loved one’s death [28–30]. We found a significant increase in the number of patients with MBC who died in hospice from 2002 to 2011, and a significant decrease in hospital and skilled nursing facility deaths. Despite this trend, the mean hospice LOS was only 10.9 days, suggesting that the majority of patients are still enrolling within two weeks of death.
Patients who lived a short time with MBC (<6 months) were more than twice as likely to utilize aggressive EOL care and one-third less likely to utilize hospice care, compared to patients who lived longer than 6 months from diagnosis. Compared to patients who lived longer, patients who died within 6 months of diagnosis were more likely to die in the hospital or in a skilled nursing facility and less likely to die at home. These results are similar to a population-based analysis of EOL care among elderly patients with poor prognosis cancers which found frequent hospitalizations and ICU admissions in the last month of life, and an average hospice LOS of 8.4 days [10].
It is possible that patients who die quickly after diagnosis don’t establish a relationship with a multidisciplinary care team to discuss advanced care planning and, therefore, they utilize more aggressive EOL care. Interestingly, we found that patients who saw their oncologists more frequently were more likely to utilize aggressive EOL care. In our previous work, we found that patients who had more frequent oncology visits were up to three times more likely to be extreme-users of disease monitoring tests, and that patients who were extreme-users of disease monitoring tests were more likely to utilize aggressive EOL care [23]. These findings suggest that it is possible that patients and/or physicians who are overly-aggressive with breast cancer treatment may drive healthcare utilization apart from disease severity.
Previous research has shown significant racial/ethnic disparities related to adequacy of pain and symptom control, and perceived unmet needs for supportive services [31–34]. In addition, other studies have found that among patients with MBC at the EOL, elderly black women were less likely to utilize hospice [34]. In addition to underuse of services, disparities also exist with overuse of treatment in the EOL setting. Compared to elderly white women, elderly black women with MBC are more likely to die in the hospital, and be admitted to the ICU, hospital, or ED within the last month of life [34]. These results are also similar to our own results which found that elderly black women with MBC were more likely to utilize aggressive EOL care compared to elderly white women with MBC.
Our work has several important limitations. The SEER-Medicare database includes only patients who are 65 years or older with Medicare insurance and may not be generalizable to all patient populations. We only included patients who died in our total cohort which may result in selection bias, meaning that patients who survived for a long period of time were excluded from our analysis; however, we expect this effect to be small. We included patients who died of all causes, but the majority, 3375 (74.7%) of patients included died from breast cancer. Additionally, a sensitivity analysis was performed on all analyses among only those patients whose cause of death was attributed to breast cancer, and all results were similar. Our cost estimates did not include costs associated with oral therapies, and, therefore, may be an underestimate of total cancer costs, but are similar to EOL care costs reported in the literature [35, 36]. Survival time was determined retrospectively by the interval from diagnosis until death; there is currently no objective prospective method to determine which patients will live longer and this may present a challenge to apply our results in clinical practice.
In summary, we found approximately 60% of elderly women with MBC received aggressive EOL care. Patients who died quickly after diagnosis were more likely to receive aggressive EOL care and have higher EOL care costs compared to patients who lived longer. Patients with rapidly progressive MBC should be targeted for interventions to curb aggressive EOL care and introduce advanced care planning earlier in the treatment course.
Supplementary Material
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. Dr. Accordino is a recipient of Dr. Charles A. Coltman, Jr. Fellowship from the Hope Foundation. Dr. Hershman (NCI R01 CA186084) and Dr. Wright (NCI R01CA169121) are recipients of Grants from the National Cancer Institute. Dr. Hershman is a recipient of a Grant from The American Society of Clinical Oncology/Breast Cancer Research Foundation.
Footnotes
Compliance with ethical standards
Conflicts of interest The authors have no conflicts of interest to declare.
Electronic supplementary material The online version of this article (doi:10.1007/s10549-017-4420-4) contains supplementary material, which is available to authorized users.
References
- 1.Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Connor TL, Ngamphaiboon N, Groman A, Luczkiewicz DL, Kuszczak SM, Grant PC, Kerr CW. Hospice utilization and end-of-life care in metastatic breast cancer patients at a comprehensive cancer center. J Palliat Med. 2015;18(1):50–55. doi: 10.1089/jpm.2014.0238. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology: Palliative Care. Version 1. 2016 doi: 10.6004/jnccn.2009.0031. https://www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. [DOI] [PubMed]
- 4.Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni TA, Basch EM, Ferrell BR, Loscalzo M, Meier DE, Paice JA, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Clinical Oncology End of Life Task Force. Cancer care during the last phase of life. J Clin Oncol. 1998;16(5):1986–1996. doi: 10.1200/JCO.1998.16.5.1986. [DOI] [PubMed] [Google Scholar]
- 6.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 7.Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21(6):1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 8.Wright AA, Hatfield LA, Earle CC, Keating NL. End-of-life care for older patients with ovarian cancer is intensive despite high rates of hospice use. J Clin Oncol. 2014;32(31):3534–3539. doi: 10.1200/JCO.2014.55.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Check DK, Rosenstein DL, Dusetzina SB. Early supportive medication use and end-of-life care among Medicare beneficiaries with advanced breast cancer. Support Care Cancer. 2016;24(8):3463–3472. doi: 10.1007/s00520-016-3174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morden NE, Chang CH, Jacobson JO, Berke EM, Bynum JP, Murray KM, Goodman DC. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff. 2012;31(4):786–796. doi: 10.1377/hlthaff.2011.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitkopf CR, Stephens EK, Jatoi A. Hospice in end-of-life patients with cancer: does it lead to changes in nonhospice health care utilization after stopping cancer treatment? Am J Hospice Palliat Care. 2014;31(4):392–395. doi: 10.1177/1049909113488927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley AS, Deb P, Du Q, Aldridge Carlson MD, Morrison RS. Hospice enrollment saves money for Medicare and improves care quality across a number of different lengths-of-stay. Health Aff. 2013;32(3):552–561. doi: 10.1377/hlthaff.2012.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallston KA, Burger C, Smith RA, Baugher RJ. Comparing the quality of death for hospice and non-hospice cancer patients. Med Care. 1988;26(2):177–182. doi: 10.1097/00005650-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy EP, Burns RB, Ngo-Metzger Q, Davis RB, Phillips RS. Hospice use among Medicare managed care and fee-for-service patients dying with cancer. JAMA. 2003;289(17):2238–2245. doi: 10.1001/jama.289.17.2238. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Lindenauer PK, Fitzgerald JL, Benjamin EM. Forecasting the impact of a clinical practice guideline for peri-operative beta-blockers to reduce cardiovascular morbidity and mortality. Arch Intern Med. 2002;162(1):63–69. doi: 10.1001/archinte.162.1.63. [DOI] [PubMed] [Google Scholar]
- 16.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–748. [PubMed] [Google Scholar]
- 17.NCI. Overivew of the SEER Program. http://seer.cancer.gov/about/overview.html.
- 18.SEER-Medicare Linked Database. http://healthcaredelivery.cancer.gov/seermedicare/
- 19.Cooke CR, Feemster LC, Wiener RS, O’Neil ME, Slatore CG. Aggressiveness of intensive care use among patients with lung cancer in the Surveillance, Epidemiology, and End Results-Medicare registry. Chest. 2014;146(4):916–923. doi: 10.1378/chest.14-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 21.Carlson MD, Herrin J, Du Q, Epstein AJ, Cherlin E, Morrison RS, Bradley EH. Hospice characteristics and the disenrollment of patients with cancer. Health Serv Res. 2009;44(6):2004–2021. doi: 10.1111/j.1475-6773.2009.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsey SD, Henry NL, Gralow JR, Mirick DK, Barlow W, Etzioni R, Mummy D, Thariani R, Veenstra DL. Tumor marker usage and medical care costs among older early-stage breast cancer survivors. J Clin Oncol. 2015;33(2):149–155. doi: 10.1200/JCO.2014.55.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Accordino MK, Wright JD, Vasan S, Neugut AI, Hillyer GC, Hu JC, Hershman DL. Use and costs of disease monitoring in women with metastatic breast cancer. J Clin Oncol. 2016;34(24):2820–2826. doi: 10.1200/JCO.2016.66.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack JW, Chen K, Boscoe FP, Gesten FC, Roohan PJ, Weeks JC, Schymura MJ, Schrag D. Underuse of hospice care by Medicaid-insured patients with stage IV lung cancer in New York and California. J Clin Oncol. 2013;31(20):2569–2579. doi: 10.1200/JCO.2012.45.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962–970. doi: 10.1093/ije/dyu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Palliative Care. Version 1. 2016 https://www.nccn.org/professionals/physician_gls/pdf/palliative.pdf.
- 27.Goodman DC, Morden NE, Change C, Fisher ES, Wennberg JE. Trends in cancer care near the end of life. A Dartmouth Atlas of Health Care Brief. http://www.dartmouthatlas.org/downloads/reports/Cancer_brief_090413.pdf. [PubMed]
- 28.Bradley EH, Prigerson H, Carlson MD, Cherlin E, Johnson-Hurzeler R, Kasl SV. Depression among surviving care-givers: does length of hospice enrollment matter? Am J Psychiatry. 2004;161(12):2257–2262. doi: 10.1176/appi.ajp.161.12.2257. [DOI] [PubMed] [Google Scholar]
- 29.Kris AE, Cherlin EJ, Prigerson H, Carlson MD, Johnson-Hurzeler R, Kasl SV, Bradley EH. Length of hospice enrollment and subsequent depression in family caregivers: 13-month follow-up study. Am J Geriatr Psychiatry. 2006;14(3):264–269. doi: 10.1097/01.JGP.0000194642.86116.ce. [DOI] [PubMed] [Google Scholar]
- 30.Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, Mitchell SL, Jackson VA, Block SD, Maciejewski PK, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisch MJ, Lee JW, Weiss M, Wagner LI, Chang VT, Cella D, Manola JB, Minasian LM, McCaskill-Stevens W, Mendoza TR, et al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol. 2012;30(16):1980–1988. doi: 10.1200/JCO.2011.39.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeill JA, Reynolds J, Ney ML. Unequal quality of cancer pain management: disparity in perceived control and proposed solutions. Oncol Nurs Forum. 2007;34(6):1121–1128. doi: 10.1188/07.ONF.1121-1128. [DOI] [PubMed] [Google Scholar]
- 33.Stephenson N, Dalton JA, Carlson J, Youngblood R, Bailey D. Racial and ethnic disparities in cancer pain management. J Natl Black Nurses’ Assoc. 2009;20(1):11–18. [PubMed] [Google Scholar]
- 34.Check DK, Samuel CA, Rosenstein DL, Dusetzina SB. Investigation of racial disparities in early supportive medication use and end-of-life care among medicare beneficiaries with stage IV breast cancer. J Clin Oncol. 2016;34(19):2265–2270. doi: 10.1200/JCO.2015.64.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang B, Wright AA, Huskamp HA, Nilsson ME, Maciejewski ML, Earle CC, Block SD, Maciejewski PK, Prigerson HG. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.