Abstract
Extremely premature infants are at great risk for poor neurodevelopmental outcomes, in part because neurologic structures designed to mature in the womb must now do so in the extrauterine environment. Reliable biomarkers of neurodevelopment are especially critical in this population, as behavioral measures can be unreliable due to immaturity of the premature infant nervous system. Oxytocin (OT) has the potential to be a marker of neurobiological processes that offer infant neuroprotection. However, no studies have measured OT in the plasma and urine of premature infants. The purposes of this study were to describe plasma and urine OT levels of premature infants through 34 weeks corrected gestational age (CGA), determine whether plasma and urine OT are correlated, and explore associations between infant demographics and OT trajectories. Plasma and urine from 37 premature infants, born at gestational ages 25–28 6/7 weeks, were longitudinally collected at 14 days of life, then weekly until 34 weeks CGA. Plasma OT decreased with age, at a rate of 15% per week, and exhibited strong stability within infants. Urine OT was not correlated with plasma OT and did not show a significant trend over time; thus, urine may not be a reliable, noninvasive measurement in this population. Apgar score was the only infant demographic characteristic associated with plasma OT. Given the novelty of this work, replication is needed to confirm these findings, and future research should explore potential mechanisms (e.g., stress, normal maturation, and social experiences) that contribute to declining plasma OT levels in premature infants.
Keywords: premature infant, oxytocin, neurobiology, neurodevelopment, NICU
Prematurity is the largest contributor to morbidity and mortality in perinatal health care, with neurologic deficits being one of the most significant morbidities. Extremely premature infants, those born at 28 weeks gestation or less, are the most vulnerable population for experiencing brain injury and/or altered brain development that result in a range of neurodevelopmental deficits. For example, up to 70% of these infants display white matter abnormalities on magnetic resonance imaging, and 75% display atypical neurodevelopment at 5 years of age (Jarjour, 2015). Moreover, improvements in the rate of neurologic morbidities have not kept pace with decreases in mortality. These morbidities place considerable burden on families, the health-care system, and society.
Because of the severe immaturity of the nervous systems of extremely premature infants, the effectiveness of behavioral evaluation of neurodevelopmental status is limited during the first months of life. Extremely premature infants have difficulty coordinating motor responses to the stimuli of behavioral evaluation, and behavioral exams can be very stressful for the infants. Thus, there is a critical need for noninvasive neurobiological markers in this infant population to allow for the accurate evaluation of neurologic risk, developmental progression, and response to nursing interventions.
We hypothesize that the hormone oxytocin (OT) has the potential to be a neurobiological marker for early identification of neurodevelopmental risk in these vulnerable infants. OT is neuroprotective at birth through its effect of decreasing neuronal excitability to perinatal events such as anoxia and seizures (Leonzino et al., 2016; Tyzio et al., 2006; Vargas-Martínez, Uvnäs-Moberg, Petersson, Olausson, & Jiménez-Estrada, 2014). OT contributes to a range of neurologic functions, including modulation of infant social behaviors (Weisman, Zagoory-Sharon, & Feldman, 2012), affiliation (Feldman, 2012), and bonding (Nagasawa, Okabe, Mogi, & Kikusui, 2012). OT also has significant vascular effects, including reducing blood pressure (Gutkowska, Jankowski, & Antunes-Rodrigues, 2014), inflammation (Yuan et al., 2016), and metabolic rates (Chaves, Tilelli, Brito, & Brito, 2013). In animal models, these protective vascular effects promote healing after brain insults such as infarction (Karelina et al., 2011) or hypoxia (Ceanga, Spataru, & Zagrean, 2010), insults that are common in the extremely preterm population. Researchers have proposed that the OT-based physiologic regulation of autonomic and neurovascular parameters is the foundation of infant self-regulation (Feldman, 2006).
In animal models, the OT system undergoes significant maturation during early fetal life and infancy (Vargas-Martínez et al., 2014). OT is present in human fetal brain tissues as early as 14 weeks gestation, and its levels significantly increase throughout the course of gestation in areas of the brain such as the pituitary and the hypothalamus (Burford & Robinson, 1982). Investigators have not measured OT longitudinally in fetal umbilical plasma, but cross-sectional measurements have been successful in fetuses near the time of labor (Kuwabara, Takeda, Mizuno, & Sakamoto, 1987). In healthy, term human infants, OT is measurable in plasma (Kuwabara et al., 1987), urine (White-Traut, Powlesland, Gelhar, Chatterton, & Morris, 1998), saliva (Feldman, Gordon, & Zagoory-Sharon, 2010), and cerebral spinal fluid (CSF; Clark et al., 2013). While the neuroprotective properties of OT suggest promising applications for vulnerable infant populations, we found only one study in which researchers reported measuring OT in the saliva of premature infants (Kommers et al., 2017). Unfortunately, the saliva samples that researchers obtained in the Kommers’s study had insufficient volumes, resulting in the decision to pool saliva samples from multiple infants for analysis. Our team also had difficulty obtaining sufficient saliva for OT analysis, and thus, we did not include the saliva collection method in the current study design. There is still considerable controversy about the accuracy of measuring salivary OT in human populations.
Measurement questions need to be addressed before future studies measure OT in younger infant populations. First, longitudinal studies of infant OT levels have not been conducted, and thus, it is not known whether maturational changes exist in peripheral OT. Controlling for maturational changes in research designs enrolling premature infants is especially critical because brain growth is at its highest rate during neonatal intensive care unit (NICU) hospitalization. Second, individual stability of OT during early infancy is unknown, although peripheral levels of OT (i.e., plasma, urine, and saliva) are stable in children and adults (Feldman, Gordon, Influs, Gutbir, & Ebstein, 2013). Additional research on the stability of OT during infancy is needed because rapid developmental changes in the infant’s nervous and endocrine systems may affect stability of peripheral OT levels.
Finally, it is not known whether using specimens that require less invasive collection methods, such as urine, can be used as a substitute for plasma when measuring OT in infant populations. Using urine as a means of measuring OT levels in premature infants is especially appealing because of low circulating blood volumes in this population (∼90 ml/kg) and the increased risk of anemia due to additional blood draws. Urinary clearance studies administering intravenous OT show that OT is reliably transferred from plasma and excreted in the urine of adult human males (Amico, Ulbrecht, & Robinson, 1987), primates (Seltzer & Ziegler, 2007), dogs (Mitsui et al., 2011), and rodents (Polito, Goldstein, Sanchez, Cool, & Morris, 2006). Results of human correlational studies are mixed, with some indicating positive correlations between plasma and urinary OT (Amico et al., 1987; Francis, Kirkpatrick, de Wit, & Jacob, 2016) and others showing no relationships (Feldman, Gordon, & Zagoory-Sharon, 2011; Hoffman, Brownley, Hamer, & Bulik, 2012). Additionally, research has shown that urinary OT levels increase after exposure to conditions known to stimulate OT release: Urinary OT level increased from baseline in human children after social contact with their mothers (Wismer Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005), in human adults after social interaction with their dogs (Nagasawa, Kikusui, Onaka, & Ohta, 2009), and in primates after group interaction (Seltzer & Ziegler, 2007), though urinary OT levels did not increase in foster mothers cuddling their infants for 30 min (Bick, Dozier, Bernard, Grasso, & Simons, 2013) or in term-born infants receiving massage (White-Traut et al., 1998). Because of the difficulty of serially timing urine collection without invasive catheterization, urinary OT may be more appropriate for correlation studies in premature infants or designs that measure changes in basal levels over time. Additional studies examining the correlation between OT levels in plasma and urine need to be conducted in the premature infant population because immaturity of their kidneys may affect processing of neuropeptides such as OT.
Because measurement of plasma and urinary OT in premature infants has not been reported in the literature, the feasibility and reliability of OT measurement in this population are not known. The purpose of the present study was to measure plasma OT in the extremely preterm population and describe its longitudinal developmental trajectory and stability within the complex NICU environment. We explored associations between infant demographics and plasma OT levels to determine whether plasma OT differed by infant characteristics. The second purpose of this study was to compare OT levels in plasma and urine to assess whether urine can be used as a noninvasive measure of OT in premature infants. This study represents a critical first step for future studies that might use OT as a biomarker in vulnerable infant populations by addressing measurement issues related to maturational changes, stability over time, and specimen substitutes for plasma.
Method
Sample
We recruited a convenience sample of premature infants from three Midwestern Level III NICUs. Inclusion criteria included English-speaking mothers who gave birth to premature infants at ages ranging from 25 to 28 6/7 weeks (i.e., 28 weeks and 6 days or less than 29 weeks) gestation. Exclusion criteria were chosen due to their influence on infant brain development and/or neurobiological processes: history of maternal drug abuse, presence of major congenital or chromosomal abnormality, Grade 3 or 4 intraventricular hemorrhage, hypoxic ischemic encephalopathy, metabolic disorders involving the adrenal system, and necrotizing enterocolitis requiring surgical intervention.
We conducted a power analysis using a standard formula (Laird, Donnelly, & Ware, 1992) in which we inferred conservative estimates from cross-sectional studies with term infants (Feldman et al., 2010; Weisman et al., 2012). Using these studies, we assumed OT measurements would have a standard deviation of 31.6 pg/ml, a linear change over time of 9 pg/ml per week, and a correlation of 0.2 between repeated measurements from the same infant. With these levels and, on average, five measures per infant, we determined that a sample of 50 infants would provide at least 80% power to detect a positive rate of change in OT level.
Procedure
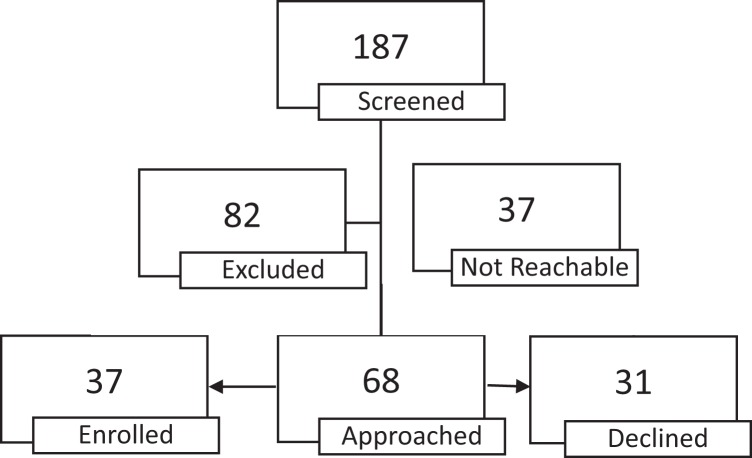
The principal investigator approached mothers directly during their infants’ first 2 weeks of life to invite them to participate. Figure 1 presents the results of the recruitment process. Over a period of 2 years, 187 infants were screened, with 44% of them meeting study exclusion criteria. This exclusion rate highlights the extreme vulnerability of our patient population. In addition, we did not approach the parents of 16% of infants who were eligible for the study due to inconsistency in parent schedules and visitation. Among those parents whom we approached to participate in our study, our recruitment rate was 57%, a percentage that is quite high for this vulnerable population. Parents declined participation generally due to overwhelming stress or lack of interest in the study. All participating mothers signed a written consent form approved by the institutional review boards of the academic institution and participating clinical sites.
Figure 1.
Infant recruitment and enrollment for study.
Sample collection started on approximately Day of Life 14, then continued weekly until the infant reached 34 weeks corrected gestational age (CGA). We stopped measurements at 34 weeks CGA to ensure that all infants would still be hospitalized and available for sample collection. Sample collections occurred between the hours of 2300 and 0200 to control for potential diurnal variation of OT. Although a nocturnal increase in plasma OT exists in human pregnant women (Fuchs, Behrens, & Liu, 1992), it is not known whether a diurnal variation in OT exists for infants. We collected urine (about 1–3 ml) using a cotton ball placed in the diaper during the diaper change just prior to data collection. Blood collection was concurrent with blood draws required for clinical treatment and occurred by heel stick unless the infant had an arterial line. We performed no additional blood draws for the purposes of this research. We collected approximately 0.3 ml of blood into a chilled ethylenediaminetetraacetic acid (1mg/ml) microtainer tube (Becton, Dickson, & Company, Franklin Lakes, NJ) containing aprotinin (10 µl/ml of blood). We collected all samples before feedings, as OT levels are influenced by the act of suckling and digestion. Continuous feeds were not a standard of care for the recruitment sites, thus no participating infants were receiving continuous feeds. After collection, we immediately placed samples on ice, processed them, and transferred them to a locked −80°C freezer.
Measures
Plasma and urinary OT
We measured plasma and urinary OT using an extraction-free, commercially available kit (KIT S-1355.0001; Peninsula Laboratories International, Inc., San Carlos, CA). Samples were not extracted due to safety and ethical concerns of taking larger amounts of body fluids from medically fragile infants. Samples were immediately placed on ice, transported to the laboratory, and then centrifuged at 4°C and 2,000 g for 10 min within 1 hr of collection. Supernatants were collected and stored at −80°C until analysis. We assayed samples in batches to minimize the time they spent in storage. To avoid interassay variation, we assayed samples from the same infant simultaneously, and we performed all measurements in duplicate. Intraassay coefficient of variation for the study was 12%. We measured creatinine concentrations using a commercially available kit (QuantiChrom Creatinine Assay Kit: DICT-500; Bioassay Systems, Hayward, CA) and normalized urinary OT levels to total creatinine. We did not normalize blood for protein content, as we had measured a portion of samples for protein and found that levels changed minimally and would not affect analysis. We analyzed data using GraphPad Prism software (version 6).
Demographics
We extracted demographic characteristics for infants and their mothers from the electronic medical record or, for those that were not documented, we obtained information through a questionnaire (e.g., maternal education, income).
Data Analysis
We chose an overall α level of .05 for analysis. We transformed OT levels using the natural logarithm to mitigate skew in the distribution of the data. To describe the overall mean trajectory of OT levels as well as infant-level trajectories over time, we used linear mixed models (LMMs). LMMs account for the possible correlation of repeated measurements from infants while allowing for missing data as well as variability in the number and spacing of observations from different subjects. We assumed CGA (Age) to be a continuous time variable centered at 27 weeks CGA to coincide with the earliest time we would collect data in our study.
The model form is as follows:
In the model, i indexes the infant and t the CGA. The two β terms (β0, β1) are fixed effects providing the mean intercept and slope coefficients. The two b terms (b 0i, b 1i) are random effects, which adjust the mean parameters for infant-specific variation in OT trajectory. Infant-specific trajectories provide estimates of the between-infant variation in overall OT level (random intercepts) and its rate of change over time (random slopes). We estimated fluctuation of OT levels about an infant’s trajectory by the variation in ε, which estimates scatter of infant i’s data about his or her linear fit.
For our exploratory analysis investigating associations between plasma OT levels and infant demographics, we used a series of LMMs similar to the model above to study the effect of each infant demographic and its respective interaction with age. In each model, CGA (Age) was again centered at 27 weeks. We individually tested the following covariates and their interactions for significance: birth weight (centered at 500 g), gestational age at birth (centered at 25 weeks), gender (female = 0), and Apgar score. The model form is as follows:
In the model, Xji is the value of a predictor j for infant i (e.g., birth weight) and βkj is the model parameter for the kth term (k = 0 or 1) for the jth predictor.
We used a bivariate random intercepts model to estimate the correlations between plasma and urinary OT levels on average and over time. The model form is as follows:
Y denotes the sample source as plasma and W as urine. The model permits an unstructured covariance structure for the ε terms, from which the correlation estimates between plasma and urine within an infant over time were derived. The covariance of the random effects permitted estimates of the average correlation between plasma and urine in an infant.
Results
After recruiting 37 premature infants over a period of 2 years, we conducted an interim statistical analysis to determine whether additional recruitment would be necessary to adequately address the study aims. In comparison to our original power analysis parameters, we found a larger standard deviation of 50 pg/ml in plasma OT levels, a smaller number of observations per infant (three time points), and a larger correlation between repeated measurements of 0.3. We also found a much larger effect in the change of OT levels per week (see below). Results of this interim analysis showed that we had achieved enough statistical power to detect clinically significant results if they existed in our sample after enrolling 37 infants and their mothers, so we closed recruitment. We present demographic characteristics and descriptive statistics for our sample in Table 1 and summary statistics for the values of plasma and urinary OT in Table 2. Figure 2 depicts the distribution of plasma and urinary OT observations across CGA, with an overlapping Lowess curve indicating trend across CGA. When considering all the possible time points after infant enrollment (n = 187), intermittent missingness was 47% and 26% for plasma and urinary OT levels, respectively. Numerous factors contributed to missingness for plasma samples, including labs not being ordered for the week (heel sticks were not conducted in infants exclusively for the study), low hemoglobin and hematocrit (blood was not taken in these instances due to patient safety), bleeding from the heel stick stopped prematurely (additional heel sticks were not allowed), or labs were ordered at nonstandard times due to changes in patient health status or the plan of care. Missingness factors for urine samples included low urine output, movement of the cotton balls out of the diaper, contamination with stool or barrier cream, and protocol deviation due to the bedside nurse not placing the cotton ball in the diaper.
Table 1.
Maternal and Infant (N = 37) Demographic Characteristics.
| Characteristic | Descriptive Statistics |
|---|---|
| Maternal | |
| Age (years), mean (SD)/min–max | 31.2 (8.1)/18–48 |
| Education (years), mean (SD)/min–max | 14.8 (2.8)/11–22 |
| Family income ($, thousands), mean (SD)/min–max | 55.1 (41.7)/0–100 |
| Births/para (no.), mean (SD)/min–max | 1.9 (1.6)/1–8 |
| Race, % | |
| Caucasian | 81 |
| African American | 16 |
| Other | 3 |
| Marital status, n (%) | |
| Single, never married | 8 (21.62) |
| Married | 19 (51.35) |
| Partnered, not living together | 3 (8.11) |
| Partnered, living together | 7 (18.92) |
| Infant | |
| Gestational age at birth (weeks), mean (SD)/min–max | 27.2 (1.14)/25.6–28.9 |
| Birth weight (g), mean (SD)/min–max | 1,015 (242)/380–1,470 |
| Apgar score at 5 min | 6.8 (2.2)/2–9 |
| Gender (female), % | 54 |
| Race, % | |
| Caucasian | 71 |
| African American | 26 |
| Other | 3 |
| Multiples, % | 10 |
Table 2.
Descriptive Statistics for Oxytocin (OT) Levels in Extremely Premature Infants.
| OT Measure | Number of Observations | Mean (SD) | Min | Max |
|---|---|---|---|---|
| Log plasma (pg/ml) | 3.93 (0.67) | 1.95 | 5.97 | |
| Plasma (pg/ml) | 98 | 63.67 (50.49) | 7.02 | 392.22 |
| CGA 27 weeks | 1 | 24.53 | 24.53 | 24.53 |
| CGA 28 weeks | 2 | 57.97 (32.39) | 35.07 | 80.87 |
| CGA 29 weeks | 8 | 80.52 (54.79) | 7.02 | 156.91 |
| CGA 30 weeks | 15 | 93.46 (95.18) | 19.19 | 392.22 |
| CGA 31 weeks | 15 | 69.04 (40.69) | 29.05 | 194.47 |
| CGA 32 weeks | 23 | 62.08 (35.36) | 20.45 | 178.36 |
| CGA 33 weeks | 19 | 45.75 (24.65) | 7.32 | 93.33 |
| CGA 34 weeks | 15 | 48.02 (25.76) | 18.88 | 100.86 |
| Log urine (pg/ml/ng creatinine) | 8.65 (1.16) | 5.33 | 10.84 | |
| Urine (pg/ml/ng creatinine) | 129 | 9,588.75 (9,695.21) | 206.25 | 50,838.70 |
| CGA 27 weeks | 1 | 206.25 | 206.25 | 206.25 |
| CGA 28 weeks | 5 | 12,417.01 (12,889.36) | 275.90 | 32,225.83 |
| CGA 29 weeks | 9 | 10,086.27 (10,335.98) | 774.31 | 34,751.45 |
| CGA 30 weeks | 19 | 9,840.20 (9,741.49) | 263.57 | 39,198.45 |
| CGA 31 weeks | 18 | 6,794.70 (9,498.97) | 560.16 | 42,171.63 |
| CGA 32 weeks | 31 | 8,459.72 (6,387.97) | 214.64 | 25,765.53 |
| CGA 33 weeks | 22 | 9,881.67 (9,221.26) | 618.31 | 43,974.80 |
| CGA 34 weeks | 24 | 12,290.17 (12,806.24) | 509.28 | 50,838.70 |
Note. N = 37. CGA = corrected gestational age.
Figure 2.

Plasma and urinary oxytocin trajectories in extremely premature infants. As seen in the figure, plasma oxytocin (OT) levels significantly decreased with increasing weeks of corrected gestational age (CGA), whereas urinary OT levels did not show a trend with age. Mean urinary OT levels were higher at 33–34 weeks CGA than at previous ages, but this difference was not statistically significant.
Table 3 presents the LMM describing the trajectory of plasma OT levels. These levels significantly decreased with the increase in chronological age at a rate of about 15% per week (b = −.15, p = .006), and values demonstrated strong stability within infants, as calculated by an intraclass correlation coefficient (ICC) of .75 (95% CI [0.44, 0.92]). Due to the lower incidence of birth at 25–26 weeks gestation, we captured less data from 27 to 29 weeks gestation than from subsequent weeks, as depicted in Table 2 and Figure 2. During these early weeks with fewer data points, plasma OT appears to increase. However, LMM is not only robust to missing data but also accounts for significant variability in observations per week by weighting weeks with less data toward the sample mean.
Table 3.
Linear Mixed Model of Log-Transformed Oxytocin (OT) Trajectories.
| Parameter | Coefficient | p > z | 95% CI |
|---|---|---|---|
| Log plasma OT (pg/ml) | |||
| Corrected gestational age | −0.15 | <.01 | [−0.25, −0.04] |
| Intercept | 4.72 | <.01 | [4.15, 5.29] |
| Random-effects parameters | Estimate | ||
| σb 0i (random intercepts) | 0.18 | [0.09, 0.35] | |
| σb 1i (random slopes) | 0.98 | [0.53, 1.84] | |
| (correlation of random effects) | −0.99 | [−1.00, 0.22] | |
| σε it (residual) | 0.56 | [0.46, 0.68] | |
| Log urine OT (pg/ml/ng creatinine) | Coefficient | p > z | |
| Corrected gestational age | 0.04 | .58 | [−0.10, 0.18] |
| Intercept | 8.44 | <.01 | [7.67, 9.21] |
| Random-effects parameters | Estimate | ||
| σb 0i (random intercepts) | 1.24 | [0.56, 2.76] | |
| σb 1i (random slopes) | 0.23 | [0.11, 0.49] | |
| (correlation of random effects) | −0.91 | [−0.99, −0.49] | |
| σε it (residual) | 0.96 | [0.82, 1.14] | |
Note. CI = confidence interval.
Infant Apgar scores at 5 min of life, and the interaction with age, were significantly associated with plasma OT levels (Table 4). Specifically, higher Apgar scores were associated with higher OT levels at 27 weeks CGA (b = .28, p = .02), and infants with higher Apgar scores had greater declines in plasma OT levels with age (b = −.06, p = .01). Infant birth weight (b = .002, p = .13, 95% CI [−0.001, 0.004]), gestational age at birth (b = −.023, p = .25, 95% CI [−0.22, 0.84]), gender (b = .49, p = .39, 95% CI [−0.63, 1.62]), and the interactions of age with birth weight (b = −.001, p = .13, 95% CI [−0.001, 0.001]), with gestational age at birth (b = −.06, p = .20, 95% CI [−0.19, 0.03]), and with gender (b = −.10, p = .33, 95% CI [−0.31, 0.10]) were not related to plasma OT levels.
Table 4.
Associations Between Infant Apgar Scores and Plasma Oxytocin (OT) Levels.
| Parameter | Coefficient | p > z | 95% CI |
|---|---|---|---|
| Log plasma OT (pg/ml) | |||
| Corrected gestational age (weeks) | 0.20 | .15 | [−0.07, 0.47] |
| Apgar score at 5 min | 0.28 | .02 | [0.05, 0.52] |
| Apgar × CGA | −0.06 | .01 | [−0.10, −0.01] |
| Intercept | 3.01 | <0.01 | [1.56, 4.47] |
| Random-effects parameters | Estimate | ||
| σb 0i (random intercepts) | 0.87 | [0.07, 0.32] | |
| σb 1i (random slopes) | 0.15 | [0.44, 1.71] | |
| ρ^ (correlation of random effects) | −0.99 | [−1.00, 0.75] | |
| σεit (residual) | 0.56 | [0.46, 0.68] | |
Note. Corrected gestational age, the time-indicating variable for the study, is centered at 27 weeks. CGA = corrected gestational age.
Neither the correlation between the average levels of plasma and urinary OT (r = .31, p = .14) nor the correlation of plasma and urinary OT levels within an infant over time (r = .19, p = .54) was statistically significant. There was no evidence that urinary OT levels changed with age (b = .04, p = .58; Table 3). Urinary OT levels exhibited wide variability across infants, but values were stable within infants, with an ICC of .62 (95% CI [0.23, 0.90]). Table 5 presents the variances and covariances of the random effects and residuals of the bivariate random intercept model, from which we derived the estimates of the correlations.
Table 5.
The Covariance Matrix of the Bivariate Random Intercept Model.
| Parameter | Estimate | SE | t | p Value |
|---|---|---|---|---|
| Covariance matrix | ||||
| Variance () | 0.06 | .05 | 1.1 | .14 |
| Covariance () | 0.04 | .07 | 0.62 | .54 |
| Variance () | 0.33 | .16 | 2.02 | .02 |
| Variance () | 0.41 | .07 | 5.84 | <.0001 |
| Covariance () | 0.13 | .09 | 1.47 | .14 |
| Variance () | 1.20 | .17 | 7.13 | <.0001 |
Note. = random intercepts, where plasma is denoted by Y, and urine is denoted by W; = within-infant residuals, where plasma is denoted by Y, and urine is denoted by W; = random effects of plasma and urine with which the correlation between average levels of plasma and urinary oxytocin is derived (r = .31, p = .14); = residuals with which the correlation of within-infants’ estimates of plasma and urinary oxytocin levels over time is derived (r = .19, p = .54); SE = standard error.
Discussion
The purpose of the present study was to measure plasma OT in extremely preterm infants and describe its developmental trajectory and stability over time. We also explored associations between infant demographic characteristics and plasma OT levels to see whether OT levels differed by characteristics of the infant. Finally, we compared OT levels in plasma and urine to explore the possibility of using urine as a valid specimen for measuring OT noninvasively in premature infants. This study represents a critical step in neurobiological research in premature infants by confirming that OT levels are measurable in plasma and urine of premature infants and by providing preliminary evidence that plasma OT can be used as a reliable biomarker in this population.
Regarding our first aim, results demonstrate that plasma OT significantly decreased with age with a large effect size. To our knowledge, this is the first assessment of the trajectory of plasma OT levels in living infants. Our findings suggest that plasma OT exhibits significant maturational changes in extremely preterm infants. This finding has important implications for future OT studies, namely, that researchers should incorporate strict age controls into their research designs because our results suggest that even a small difference of 1 week in age could equate to as much as a 15% difference in plasma OT. Our findings are consistent with those of prior cross-sectional sampling studies in term infants. Researchers studying 76 full-term infants found that plasma OT levels were lower at 4 days of life than at 0.5 hr of life, though the result was not statistically significant (Leake, Weitzman, & Fisher, 1981). Others found significantly lower plasma OT levels in term infants at 7 versus 2 days of life (Kuwabara et al., 1987). Future research is needed to find physiologic explanations for the maturational decrease in plasma OT levels. It may be that, over time, the immature infant OT system gains greater control over peripheral OT release from the pituitary, allowing buildup of OT in the brain and decline of circulating OT in the periphery. Alternatively, perhaps maternal OT, increased during labor and birth, crosses the placenta and contributes to elevated infant plasma OT levels early in the infant’s OT trajectory. This explanation may also explain why urinary OT levels did not change with time. These questions could be answered with animal models comparing OT levels in the brain, plasma, and urine at different ages.
Interestingly, several cross-sectional studies conducted with expired fetuses have demonstrated significant increases in central OT levels between 11 and 42 weeks gestation, particularly in the hypothalamus and pituitary (Burford & Robinson, 1982). An important research question remains as to whether OT levels in plasma are reflective of central OT. Microdialysis studies in rats show simultaneous release of OT in the hypothalamus and blood during nursing (Neumann, Ludwig, Engelmann, Pittman, & Landgraf, 1993). Both plasma and CSF OT levels increase after intranasal and intravenous exogenous OT administration in primates (Dal Monte, Noble, Turchi, Cummins, & Averbeck, 2014; Freeman et al., 2016) and humans (Striepens et al., 2013), so there is evidence that the central and peripheral OT systems communicate with and respond to each other. In animal models, the central and peripheral OT systems can communicate with each other and influence behavior through afferent inputs, including the vagal nerve (Brown, Bains, Ludwig, & Stern, 2013). Human correlational studies of endogenous OT levels in plasma and CSF under basal conditions have been contradictory, with one study showing significant correlations in children (Carson et al., 2015), but others showing no significant relationships in adults (Kagerbauer et al., 2013; Martin et al., 2014; Veening, de Jong, & Barendregt, 2010). Regardless of whether peripheral OT is representative of central OT release, the ability of the peripheral OT system to communicate with and influence the central system suggests that plasma OT may be a useful biomarker in human infants if correlated with infant outcomes.
In our sample, infant plasma OT levels indicated high individual stability over time, as evidenced by our computed ICC (r = .75). Previous research has also confirmed strong stability of OT levels in plasma, including in pregnant women across each trimester (Levine, Zagoory-Sharon, Feldman, & Weller, 2007) and across a 5-month period in mothers (r = .91) and fathers (r = .88) when their infants were 1–6 months of age (measures were taken at the beginning and end of the period; Feldman et al., 2013). Our results in the present study provide the first documented support for the reliable measurement of plasma OT during infancy, especially in premature infants during a time in which they are undergoing rapid maturational changes in the central nervous system. Our exploratory analysis revealed that plasma OT levels declined more readily in infants with higher Apgar scores. Higher plasma OT levels at 27 weeks CGA and subsequent declining OT levels in infants with higher Apgar scores may serve as support for the hypothesis that OT is neuroprotective at birth and declines after severe birthing stress is removed (Kenkel, Yee, & Carter, 2014; Khazipov, Tyzio, & Ben-Ari, 2008; Tyzio et al., 2006). In rodent models, OT changes the action of γ-aminobutyric acid to an inhibitory neurotransmitter (Leonzino et al., 2016), which reduces neuronal activity and metabolism and provides fetal neuroprotection during episodes of perinatal hypoxia and glucose deprivation (Tyzio et al., 2006). It is possible that for infants with a poor outcome at delivery, the OT system is dysregulated and cannot provide the same neuroprotection as it does in infants with normal Apgar scores and greater physiologic stability at birth. However, due to the exploratory nature of the present analysis, more research is needed to replicate our findings.
The data did not support our hypothesis that plasma and urinary OT levels would be correlated over time. Urinary OT levels did not follow the significant declining trend over time that we observed in plasma. Moreover, the individual stability of urinary OT levels (r = .62) was lower than that of plasma levels (r = .75). Thus, urine may not be an acceptable candidate for measurement of OT in extremely premature infants. However, replication studies with more refined collection protocols are necessary before drawing any definite conclusions. While there have been studies showing a lack of correlation between endogenous plasma and urine OT levels in pregnant women (Feldman et al., 2011) and anorexic individuals (Hoffman et al., 2012), animal (Polito et al., 2006; Romero, Nagasawa, Mogi, Hasegawa, & Kikusui, 2014) and human (Francis et al., 2016) mechanistic studies show that both plasma and urinary OT levels increase reliably after exogenous OT administration. Limitations in our research procedures may have contributed to increased variability in urinary OT levels. For example, small remnants of stool or diaper cream could have contaminated our specimens, although our team was careful to discard visibly contaminated specimens. A urine bag collection would have reduced potential interaction or contamination, but we chose not to use this method because of potential damage to the infant’s immature skin and because stress to the infant would have been increased. Furthermore, urinary OT levels represent an average measure during the time required to fill the bladder and void, as opposed to the instantaneous measure of plasma OT levels. Researchers have claimed that the acidity of the urine acts as a preserving agent and prevents OT from degrading prior to assay (Reyes et al., 2014). In order to minimize stressful procedures, we did not catheterize infants for a more instantaneous measure. Researchers need to determine how unstable urinary OT samples are due to degradation and to variability in sampling procedure.
One study limitation was the amount of missing data. However, our statistical analysis strategy using LMM is robust to intermittent missing data, and missing data are common phenomenon in longitudinal research designs with premature infants. An additional limitation was the smaller number of samples we collected at earlier weeks of CGA, during which mean OT values increased in contrast to our overall study finding of decreases in plasma OT levels. The paucity of data in infants <30 weeks CGA precludes separate interpretation by age-group. In future studies, a stratified sampling procedure would assist in balancing numbers across gestational ages, enabling analyses related to specific gestational ages. In spite of these limitations, we were able to detect a significant effect of age in plasma OT levels. A significant strength of our sample was the demonstration of good generalizability for our geographic region, with excellent variability in maternal demographics, including maternal age, education, income, and race. Infants for our sample also demonstrated good variability in birth weight, gender, race, and Apgar score.
Our results in the present study provide promise for the use of plasma OT as a biomarker in future studies, as differences between infants in their relative OT levels may predict neurodevelopmental outcomes. Future studies should also consider the measurement of plasma OT for the evaluation of nursing-based interventions that are hypothesized to harness the mother–infant dyad’s natural OT systems (e.g., breastfeeding and skin-to-skin contact). Implementation of these nursing interventions might support OT processes, mitigate OT dysregulation, and ultimately promote infant neurodevelopment.
Conclusion
The addition of OT to existing measures of neurobiological processes underlying development represents progress toward a more comprehensive, scientific investigation of early human development. Our study is the first to monitor plasma and urinary OT levels over time in any population of infants. Our study is also the first to demonstrate strong stability of OT in premature infants. More research is needed to determine norms for peripheral levels of OT across different gestational ages. Our findings provide a foundation for future research that uses OT as a biomarker of hormonal processes that provide neuroprotection and promote neuromaturation in premature infants.
Acknowledgment
The authors would like to thank Dr. Mary Cismowski for generously providing her time to assay the samples required for this research. The authors would also like to thank Dr. Courtney DeVries for her mentorship and critical appraisal of this research project.
Authors’ Note: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Authors’ Contribution: Ashley Weber contributed to conception, design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Tondi M. Harrison contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Loraine Sinnott contributed to design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Abigail Shoben contributed to design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Deborah Steward contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health; National Institute of Nursing Research; National Research Service Award (F31NR-014985) and a T32 postdoctoral fellowship (1T32NR015433-01); the Association of Women’s Health, Obstetric, and Neonatal Nurses and the Every Woman, Every Baby Campaign; the Epsilon chapter of Sigma Theta Tau; Ohio Nurses Foundation; Sigma Theta Tau International in partnership with the Midwestern Nursing Research Society; National Association of Neonatal Nurses Small Grants program; Nurses Educational Funds; the Ohio Perinatal Research Network; and The Ohio State University Graduate School’s Alumni Grants for Graduate Research and Scholarship. The project was also supported by the National Center for Advancing Translational Sciences (award no. UL1TR001070).
References
- Amico J. A., Ulbrecht J. S., Robinson A. G. (1987). Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. Journal of Clinical Endocrinology and Metabolism, 64, 340–345. doi:10.1210/jcem-64-2-340 [DOI] [PubMed] [Google Scholar]
- Bick J., Dozier M., Bernard K., Grasso D., Simons R. (2013). Foster mother-infant bonding: Associations between foster mothers’ oxytocin production, electrophysiological brain activity, feelings of commitment, and caregiving quality. Child Development, 84, 826–840. doi:10.1111/cdev.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. H., Bains J. S., Ludwig M., Stern J. E. (2013). Physiological regulation of magnocellular neurosecretory cell activity: Integration of intrinsic, local and afferent mechanisms. Journal of Neuroendocrinology, 25, 678–710. doi:10.1111/jne.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford G. D., Robinson I. C. (1982). Oxytocin, vasopressin and neurophysins in the hypothalamo-neurohypophysial system of the human fetus. Journal of Endocrinology, 95, 403–408. [DOI] [PubMed] [Google Scholar]
- Carson D. S., Berquist S. W., Trujillo T. H., Garner J. P., Hannah S. L., Hyde S. A.…Parker K. J. (2015). Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Molecular Psychiatry, 20, 1085–1090. doi:10.1038/mp.2014.132 [DOI] [PubMed] [Google Scholar]
- Ceanga M., Spataru A., Zagrean A.-M. (2010). Oxytocin is neuroprotective against oxygen-glucose deprivation and reoxygenation in immature hippocampal cultures. Neuroscience Letters, 477, 15–18. doi:10.1016/j.neulet.2010.04.024 [DOI] [PubMed] [Google Scholar]
- Chaves V. E., Tilelli C. Q., Brito N. A., Brito M. N. (2013). Role of oxytocin in energy metabolism. Peptides, 45, 9–14. doi:10.1016/j.peptides.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Clark C. L., St. John N., Pasca A. M., Hyde S. A., Hornbeak K., Abramova M.…Penn A. A. (2013). Neonatal CSF oxytocin levels are associated with parent report of infant soothability and sociability. Psychoneuroendocrinology, 38, 1208–1212. doi:10.1016/j.psyneuen.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O., Noble P. L., Turchi J., Cummins A., Averbeck B. B. (2014). CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PloS One, 9, e103677 doi:10.1371/journal.pone.0103677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. (2006). From biological rhythms to social rhythms: Physiological precursors of mother-infant synchrony. Developmental Psychology, 42, 175–188. doi:10.1037/0012-1649.42.1.175 [DOI] [PubMed] [Google Scholar]
- Feldman R. (2012). Oxytocin and social affiliation in humans. Hormones and Behavior, 61, 380–391. doi:10.1016/j.yhbeh.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Influs M., Gutbir T., Ebstein R. P. (2013). Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology, 38, 1154–1162. doi:10.1038/npp.2013.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Zagoory-Sharon O. (2010). The cross-generation transmission of oxytocin in humans. Hormones and Behavior, 58, 669–676. doi:10.1016/j.yhbeh.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Zagoory-Sharon O. (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding. Developmental Science, 14, 752–761. doi:10.1111/j.1467-7687.2010.01021.x [DOI] [PubMed] [Google Scholar]
- Francis S. M., Kirkpatrick M. G., de Wit H., Jacob S. (2016). Urinary and plasma oxytocin changes in response to MDMA or intranasal oxytocin administration. Psychoneuroendocrinology, 74, 92–100. doi:10.1016/j.psyneuen.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Freeman S. M., Samineni S., Allen P. C., Stockinger D., Bales K. L., Hwa G. G. C., Roberts J. A. (2016). Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology, 66, 185–194. doi:10.1016/j.psyneuen.2016.01.014 [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Behrens O., Liu H. C. (1992). Correlation of nocturnal increase in plasma oxytocin with a decrease in plasma estradiol/progesterone ratio in late pregnancy. American Journal of Obstetrics and Gynecology, 167, 1559–1563. [DOI] [PubMed] [Google Scholar]
- Gutkowska J., Jankowski M., Antunes-Rodrigues J. (2014). The role of oxytocin in cardiovascular regulation. Brazilian Journal of Medical and Biological Research [Revista Brasileira De Pesquisas Médicas E Biológicas/Sociedade Brasileira De Biofísica…(et Al.)], 47, 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. R., Brownley K. A., Hamer R. M., Bulik C. M. (2012). Plasma, salivary, and urinary oxytocin in anorexia nervosa: A pilot study. Eating Behaviors, 13, 256–259. doi:10.1016/j.eatbeh.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour I. T. (2015). Neurodevelopmental outcome after extreme prematurity: A review of the literature. Pediatric Neurology, 52, 143–152. doi:10.1016/j.pediatrneurol.2014.10.027 [DOI] [PubMed] [Google Scholar]
- Kagerbauer S. M., Martin J., Schuster T., Blobner M., Kochs E. F., Landgraf R. (2013). Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. Journal of Neuroendocrinology, 25, 668–673. doi:10.1111/jne.12038 [DOI] [PubMed] [Google Scholar]
- Karelina K., Stuller K. A., Jarrett B., Zhang N., Wells J., Norman G. J., DeVries A. C. (2011). Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke, 42, 3606–3611. doi:10.1161/STROKEAHA.111.628008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel W. M., Yee J. R., Carter C. S. (2014). Is oxytocin a maternal-foetal signalling molecule at birth? Implications for development. Journal of Neuroendocrinology, 26, 739–749. doi:10.1111/jne.12186 [DOI] [PubMed] [Google Scholar]
- Khazipov R., Tyzio R., Ben-Ari Y. (2008). Effects of oxytocin on GABA signalling in the foetal brain during delivery. Progress in Brain Research, 170, 243–257. doi:10.1016/S0079-6123(08)00421-4 [DOI] [PubMed] [Google Scholar]
- Kommers D. R., Broeren M., Andriessen P., Oei S. G., Feijs L., Bambang Oetomo S. (2017). Pilot study demonstrates that salivary oxytocin can be measured unobtrusively in preterm infants. Acta Paediatrica, 106, 34–42. doi:10.1111/apa.13606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara Y., Takeda S., Mizuno M., Sakamoto S. (1987). Oxytocin levels in maternal and fetal plasma, amniotic fluid, and neonatal plasma and urine. Archives of Gynecology and Obstetrics, 241, 13–23. [DOI] [PubMed] [Google Scholar]
- Laird N. M., Donnelly C., Ware J. H. (1992). Longitudinal studies with continuous responses. Statistical Methods in Medical Research, 1, 225–247. [DOI] [PubMed] [Google Scholar]
- Leake R. D., Weitzman R. E., Fisher D. A. (1981). Oxytocin concentrations during the neonatal period. Biology of the Neonate, 39, 127–131. [DOI] [PubMed] [Google Scholar]
- Leonzino M., Busnelli M., Antonucci F., Verderio C., Mazzanti M., Chini B. (2016). The timing of the excitatory-to-inhibitory GABA switch is regulated by the oxytocin receptor via KCC2. Cell Reports, 15, 96–103. doi:10.1016/j.celrep.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A., Zagoory-Sharon O., Feldman R., Weller A. (2007). Oxytocin during pregnancy and early postpartum: Individual patterns and maternal-fetal attachment. Peptides, 28, 1162–1169. doi:10.1016/j.peptides.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Martin J., Kagerbauer S. M., Schuster T., Blobner M., Kochs E. F., Landgraf R. (2014). Vasopressin and oxytocin in CSF and plasma of patients with aneurysmal subarachnoid haemorrhage. Neuropeptides, 48, 91–96. doi:10.1016/j.npep.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Mitsui S., Yamamoto M., Nagasawa M., Mogi K., Kikusui T., Ohtani N., Ohta M. (2011). Urinary oxytocin as a noninvasive biomarker of positive emotion in dogs. Hormones and Behavior, 60, 239–243. doi:10.1016/j.yhbeh.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Nagasawa M., Kikusui T., Onaka T., Ohta M. (2009). Dog’s gaze at its owner increases owner’s urinary oxytocin during social interaction. Hormones and Behavior, 55, 434–441. doi:10.1016/j.yhbeh.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Nagasawa M., Okabe S., Mogi K., Kikusui T. (2012). Oxytocin and mutual communication in mother-infant bonding. Frontiers in Human Neuroscience, 6, 31 doi:10.3389/fnhum.2012.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I., Ludwig M., Engelmann M., Pittman Q. J., Landgraf R. (1993). Simultaneous microdialysis in blood and brain: Oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology, 58, 637–645. [DOI] [PubMed] [Google Scholar]
- Polito A. B., Goldstein D. L., Sanchez L., Cool D. R., Morris M. (2006). Urinary oxytocin as a non-invasive biomarker for neurohypophyseal hormone secretion. Peptides, 27, 2877–2884. doi:10.1016/j.peptides.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Reyes T. L., Galinsky A. M., Hoffmann J. N., You H. M., Ziegler T. E., McClintock M. K. (2014). Social peptides: Measuring urinary oxytocin and vasopressin in a home field study of older adults at risk for dehydration. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69, S229–S237. doi:10.1093/geronb/gbu104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero T., Nagasawa M., Mogi K., Hasegawa T., Kikusui T. (2014). Oxytocin promotes social bonding in dogs. Proceedings of the National Academy of Sciences of the United States of America, 111, 9085–9090. doi:10.1073/pnas.1322868111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer L. J., Ziegler T. E. (2007). Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): A radiolabeled clearance study and endogenous excretion under varying social conditions. Hormones and Behavior, 51, 436–442. doi:10.1016/j.yhbeh.2006.12.012 [DOI] [PubMed] [Google Scholar]
- Striepens N., Kendrick K. M., Hanking V., Landgraf R., Wüllner U., Maier W., Hurlemann R. (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports, 3, 3440 doi:10.1038/srep03440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R., Cossart R., Khalilov I., Minlebaev M., Hübner C. A., Represa A.…Khazipov R. (2006). Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science, 314, 1788–1792. doi:10.1126/science.1133212 [DOI] [PubMed] [Google Scholar]
- Vargas-Martínez F., Uvnäs-Moberg K., Petersson M., Olausson H. A., Jiménez-Estrada I. (2014). Neuropeptides as neuroprotective agents: Oxytocin a forefront developmental player in the mammalian brain. Progress in Neurobiology, 123C, 37–78. doi:10.1016/j.pneurobio.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Veening J. G., de Jong T., Barendregt H. P. (2010). Oxytocin-messages via the cerebrospinal fluid: Behavioral effects; a review. Physiology & Behavior, 101, 193–210. doi:10.1016/j.physbeh.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Weisman O., Zagoory-Sharon O., Feldman R. (2012). Oxytocin administration to parent enhances infant physiological and behavioral readiness for social engagement. Biological Psychiatry, 72, 982–989. doi:10.1016/j.biopsych.2012.06.011 [DOI] [PubMed] [Google Scholar]
- White-Traut R., Powlesland J., Gelhar D., Chatterton R., Morris M. (1998). Methodologic issues in the measurement of oxytocin in human neonates. Journal of Nursing Measurement, 6, 155–174. [PubMed] [Google Scholar]
- Wismer Fries A. B., Ziegler T. E., Kurian J. R., Jacoris S., Pollak S. D. (2005). Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences of the United States of America, 102, 17237–17240. doi:10.1073/pnas.0504767102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Liu S., Bai X., Gao Y., Liu G., Wang X.…Wang Z. (2016). Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. Journal of Neuroinflammation, 13, 77 doi:10.1186/s12974-016-0541-7 [DOI] [PMC free article] [PubMed] [Google Scholar]