Abstract
Background
Autism spectrum disorder (ASD) is associated with deficits in adaptively orienting attention to behaviorally-relevant information. Neural oscillatory activity plays a key role in brain function and provides a high-resolution temporal marker of attention dynamics. Alpha band (8–12 Hz) activity is associated with both selecting task-relevant stimuli and filtering task-irrelevant information.
Methods
The present study used electroencephalography (EEG) to examine alpha-band oscillatory activity associated with attentional capture in nineteen children with ASD and twenty-one age- and IQ-matched typically developing (TD) children. Participants completed a rapid serial visual presentation paradigm designed to investigate responses to behaviorally-relevant targets and contingent attention capture by task-irrelevant distractors, which either did or did not share a behaviorally-relevant feature. Participants also completed six minutes of eyes-open resting EEG.
Results
In contrast to their TD peers, children with ASD did not evidence posterior alpha desynchronization to behaviorally-relevant targets. Additionally, reduced target-related desynchronization and poorer target detection were associated with increased ASD symptomatology. TD children also showed behavioral and electrophysiological evidence of contingent attention capture, whereas children with ASD showed no behavioral facilitation or alpha desynchronization to distractors that shared a task-relevant feature. Lastly, children with ASD had significantly decreased resting alpha power, and for all participants increased resting alpha levels were associated with greater task-related alpha desynchronization.
Conclusions
These results suggest that in ASD under-responsivity and impairments in orienting to salient events within their environment are reflected by atypical EEG oscillatory neurodynamics, which may signify atypical arousal levels and/or an excitatory/inhibitory imbalance.
Keywords: autism, attention, EEG, alpha, event-related desynchronization, resting state
Introduction
Given the infinite number of objects and events to which one can attend and the finite nature of the human brain’s processing capacity, a key function of attention is to select items for further processing. To function adaptively, we must select and respond to behaviorally-relevant information within our environment, while filtering irrelevant details. This selection may occur reflexively to salient items within the environment (i.e. bottom-up modulation), may be based on the goals of the individual (i.e. top-down control), or, more commonly, reflect the integration of these two processes. Contingent attentional capture - when a stimulus-driven shift of attention (e.g., looking at a person in a bright yellow sweater) is contingent upon on a pre-existing top-down attentional setting (e.g., searching a crowd for your friend in the yellow hat) - is one such example of a combination of top-down and bottom-up processes (1). Thus, this form of attentional orienting, which is a focus of the present study, may provide insight into both top-down and bottom-up systems.
Autism spectrum disorder (ASD) is associated with early and pervasive deficits in selective attention (2), including impaired bottom-up (3) and top-down (4) modulation of attention. Children with ASD have deficits filtering irrelevant information (5–7), which may be due, in part, to increased perceptual capacity (8–10). Prior research has also demonstrated that individuals with ASD are atypically over-focused (11, 12), possibly linked to a narrower attentional spotlight (13, 14) and deficits in increasing attentional breadth (15, 16). These more fundamental deficits in non-social attentional processes may result, for example, in reduced orienting of attention to behaviorally-relevant stimuli in children with ASD (e.g., 17, 18), and have important implications for the development of the disorder (2).
Neural oscillatory dynamics play a key role in brain function and are associated with a variety of perceptual and cognitive processes (19). In particular, event-related synchronization (ERS) and desynchronization (ERD) of the alpha band (8–12 Hz) have been associated with perception and awareness (20), visual spatial orienting (21), filtering irrelevant information (22), and working memory (23). Neural responses linked to enhancing or selecting external information are associated with greater alpha-band desynchronization, whereas responses related to suppressing or filtering external stimuli are associated with increased alpha synchrony (24). The neural generators underlying modulation in alpha power are currently unknown; however, animal (25) and human pharmacological challenge studies (26–28) suggest that inhibitory GABAergic interneurons drive synchrony in EEG oscillations. An excitatory-inhibitory neural imbalance that involves reduced GABAergic inhibition has been hypothesized to be a primary feature in the neurobiology of ASD (29, 30). Reduction in GABA levels in ASD has been supported by both histological (31, 32) and in vivo measurements (33, 34). Therefore, investigating synchronized neural oscillations within the alpha band may be of particular interest in ASD.
Although findings from studies investigating alpha power using resting EEG have been inconsistent, a recent review suggests that ASD is more commonly associated with decreased resting alpha power (35). For example, infants at high-risk for ASD (36), as well as children (37, 38) and adults (39) with ASD have shown reduced power in the alpha-band frequencies. Studies examining task-related changes in alpha power in ASD have reported reduced alpha-band ERS, indicative of impaired suppression of task-irrelevant sensory information (5), reduced ERD during the preparation and execution of a motor control task (40), and increased frontal ERS, which may be related to the use of alternative strategies in ASD (41). Together, these findings suggest that ASD is associated with spontaneous and task-related differences in alpha power.
Our goal in the present study was to further investigate the pathophysiological mechanisms associated with atypical attention in ASD using behavioral, eye-tracking, and electrophysiological measures. Given the links between alpha oscillatory activity, GABAergic inhibitory function, and attentional processes outlined above, and their relationship to ASD, the present study examined spontaneous and task-related changes in alpha power associated with attentional capture. We have previously showed that children with ASD exhibit: 1) reduced target-related activation in the right temporal parietal junction, a key node in the ventral attentional network, and 2) attentional capture by irrelevant information that did and did not share a task-relevant feature (42). Therefore, in the present study we hypothesized that compared to their typically developing (TD) peers, children with ASD would show reduced alpha-band ERD to behaviorally-relevant targets and no difference in ERD to distractors regardless of whether they shared a task-relevant feature. Additionally, that resting alpha power would be reduced in ASD and that lower resting state alpha-band power would be associated with reduced task-related alpha desynchronization and insensitivity to behaviorally-relevant information.
Methods and Materials
Participants
Twenty-one children with ASD and 21 age- and nonverbal IQ-matched TD children participated. After exclusion of two children with ASD for poor task performance (accuracy < 2SD below the mean), the final sample included 19 ASD and 21 TD children (Table 1). Clinical diagnoses were confirmed using the Autism Diagnostic Interview-Revised (ADI-R; 43), the Autism Diagnostic Observation Schedule (ADOS; 44), and expert clinical judgment according to DSM-5 criteria. Children with ASD-related medical conditions (e.g., Fragile-X syndrome, tuberous sclerosis) were excluded. Participants in the TD group had no reported personal or family history of ASD and were confirmed via parent report to be free of ASD-related symptoms or any other neurological or psychiatric conditions. Normal color vision was confirmed for all participants using the Ishihara Tests for Colour Deficiency (45). Informed assent and consent were obtained from all participants and caregivers in accordance with the University of California, San Diego and San Diego State University Institutional Review Boards.
Table 1.
Participant Characteristics
| ASD | TD | t-value | p | ||
|---|---|---|---|---|---|
| n (M:F) | 19 (17:2) | 21 (17:4) | - | - | |
| Handedness | 18 right; 1 left | 17 right; 4 left | - | - | |
| Age (years) | 14.4 (1.6); | 14.3 (1.4); | 0.23 | 0.82 | |
| 12.4–17.8 | 12.0–16.8 | ||||
| Verbal IQ | 109 (19); | 107 (10); | 0.38 | 0.71 | |
| 72–147 | 87–126 | ||||
| Nonverbal IQ | 109 (15); | 108 (10); | 0.16 | 0.87 | |
| 81–140 | 90–129 | ||||
| SRS Total score (t-score) | 78 (10); | 42 (5); | 14.77 | < .001 | |
| 57–94 | 35–52 | ||||
| ADOS | Communication | 3 (1); | - | - | - |
| 0–5 | |||||
| Social Interaction | 8 (3); | - | - | - | |
| 3–14 | |||||
| Repetitive Behavior | 2 (2); | - | - | - | |
| 0–6 | |||||
IQ determined using the Wechsler Abbreviated Scale of Intelligence (WASI; 70). Mean (SD); range.
Experimental Paradigms
RSVP
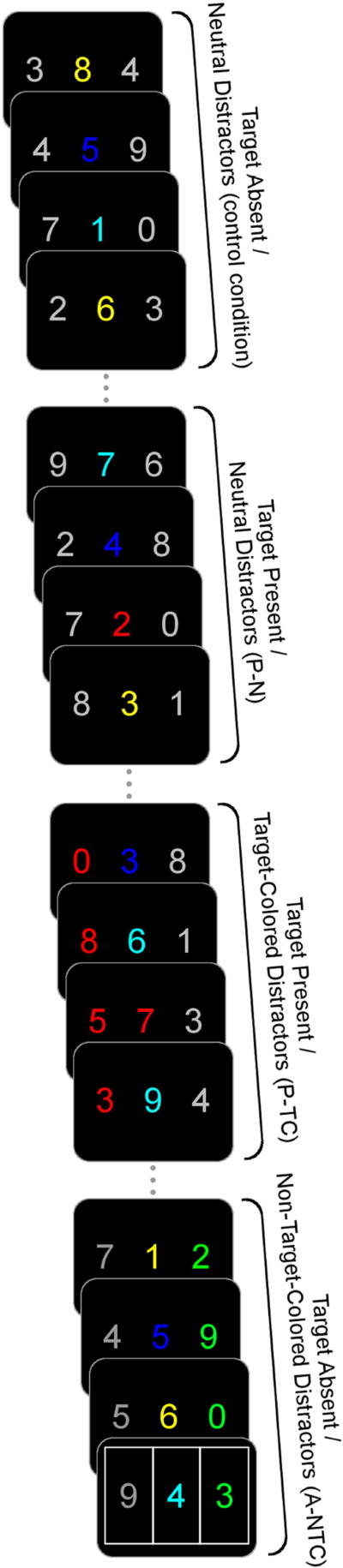
The paradigm was modified from Serences and colleagues (46) and was similar to Keehn et al. (42) (Figure 1). To simplify the task for younger participants, it included numbers instead of letters, presented in three simultaneously varying streams. The task was to identify red numbers appearing in the central stream of colored numbers. Participants were instructed to look only at the central stream and respond with their dominant hand, using a two-choice button-box, pressing the left button if the target (i.e., red number) was a low number (0–4) or the right button if the target was a high number (5–9). Digits presented in the peripheral streams were gray in most trials (Supplementary Table 1); colored distractors appeared infrequently in either left or right peripheral streams and were either the same color as the target (TC; red) or a non-target color that never appeared in the center stream (NTC; green). On target present trials, a red number occurred in the center stream on the third frame with or without the appearance of target- or non-target-colored peripheral distractors (which were presented on either the left or the right for all four frames). For target absent trials, no red number appeared in the center stream with the appearance of either TC or NTC peripheral distractors (which appeared for all four frames). Lastly, null trials consisted of target absent trials with gray distractors (Supplementary Materials).
Figure 1.
Example of experimental trials. Each trial lasted 480ms and consisted of four frames of simultaneously varying numbers (120ms per frame; no ISI between trials). Dots (“…”) indicate presentation of control trials that separated target present and absent conditions. The target present non-target colored condition (P-NTC) is not displayed, but is identical to target present target-colored condition (P-TC) with the exception that peripheral distractors were green. Likewise, the target absent target-colored (A-TC) is not displayed, but identical to the target absent non-target-colored condition (A-NTC), except that peripheral distractors were red. Eye-tracking regions of interest presented on the final frame (not visible to participants). Note: target and non-target colored distractors appeared equally in either left or right peripheral stream.
Participants were instructed to respond as quickly as possible without making errors. Eye-tracking data were acquired to assess attention to the central stream and saccades to peripheral distractors. To rule out group differences in numerical processing, participants completed a baseline number task after the experimental paradigm (Supplementary Materials).
Resting state
In the same session and after the RSVP task, participants also completed six minutes of eyes-open resting EEG (two, 3-minute blocks). During each block, a black central fixation crosshair was presented on a grey background. Participants were instructed to relax, remain as still as possible, and look at the crosshair.
Electroencephalography (EEG)
Continuous EEG was recorded using a Biosemi ActiveTwo system with 68 Ag/AgCl active electrodes. Sixty-four electrodes were mounted in an elastic cap according to locations in the modified International 10–20 system. The remaining electrodes were placed below the right eye, on the outer canthus of the left eye (to monitor blinks and saccades), and over the left and right mastoids (reference). EEG data were recorded at a sampling rate of 256Hz and DC offsets were kept below 25 mV at all channels. Data were processed using EEGLAB (47), re-referenced to the average of the left and right mastoid electrodes, and high-pass filtered at 0.5Hz.
RSVP
Sections of the continuous EEG data containing non-stereotyped artifacts (e.g., movement artifacts) were removed. EEG data were decomposed using extended infomax Independent Component Analysis (ICA). Components reflecting highly stereotyped oculomotor artifacts (e.g., blinks, saccades) were removed. Three-second epochs (−1000 to 2000msec around trial onset) were extracted from the artifact-cleaned continuous EEG data for correct trials for the three conditions of interest (P-N, A-TC, A-NTC). Finally, epochs were rejected if they exceeded a threshold of ±150 µV or a sample-to-sample threshold of 100 µV.
Time-frequency analyses were performed implementing Event-Related Spectral Perturbations (ERSP) on each channel, for the three-second epochs. The ERSP measures mean event-related power spectrum changes (in decibels; dB); we used the sinusoidal wavelet transform with a minimum of three cycles (increasing with frequency) and a one-second pre-stimulus baseline. The STUDY function in EEGLAB was used to analyze ERSP. Based on peak ERD for both groups combined and prior alpha ERD research (48), event-related changes in alpha power (8–12 Hz) 400 to 700ms after the onset of the target/distractors were extracted from posterior left (P5, CP5, C5, TP7), central (Pz, POz, PO3, PO4), and right (P6, CP6, C6, TP8) regions of interest (ROI).
Resting state
Each 3-minute block was segmented into 1-second epochs. Alpha power (8–12 Hz), expressed as decibels (dB), was extracted from midline frontal (Fz, AFz, AF3, AF4), central (Cz, C3, C4), and parietal (Pz, POz, PO3, PO4) ROIs.
Results
Behavior
Mean accuracy rates and median response times (RT) for correct target present trials were entered into a 2 (group: ASD, TD) × 3 (distractor type: neutral, TC, NTC) mixed-model repeated measures ANOVA. There were no significant differences between groups in overall accuracy (ASD = 77%; TD = 83%) and there were no differences in accuracy as a function of distractor type, nor was there an interaction between group and distractor type (all p > .05). Similarly, there were no significant differences between groups in RT (ASD = 741ms; TD = 727ms) and no interaction between group and distractor type (all p >.05). However, there was a significant main effect of distractor type, F(2,76) =33.83, p <.001, ηp2 = .47, reflecting faster RT for P-TC compared to P-N and P-NTC conditions and faster RT for the P-NTC compared to P-N (i.e., P-TC<P-NTC<P-N; all p <.01). Similar to Keehn et al., (42) separate exploratory paired t-tests for ASD and TD groups showed that RT for P-TC (M = 705ms) were faster than for P-NTC (M = 726ms) in the TD group, t(20) = 4.56 p <.001, but not the ASD group (TC = 728ms; NTC = 737ms), t(18) = −0.96, p = .35.
EEG
Analysis of ERSP examined target-related processing in the absence of TC and NTC peripheral distractors (i.e., P-N condition) and distractor-related processing in the absence of target- and response-related processes (i.e., A-TC and A-NTC conditions). Of particular interest in the target absent trials was the TC versus NTC comparison, for which greater TC compared to NTC desynchronization may be associated with contingent attentional capture. Proportion of usable epochs did not differ between ASD and TD groups for RSVP or resting EEG data (Supplementary Table 2).
Target-related processing
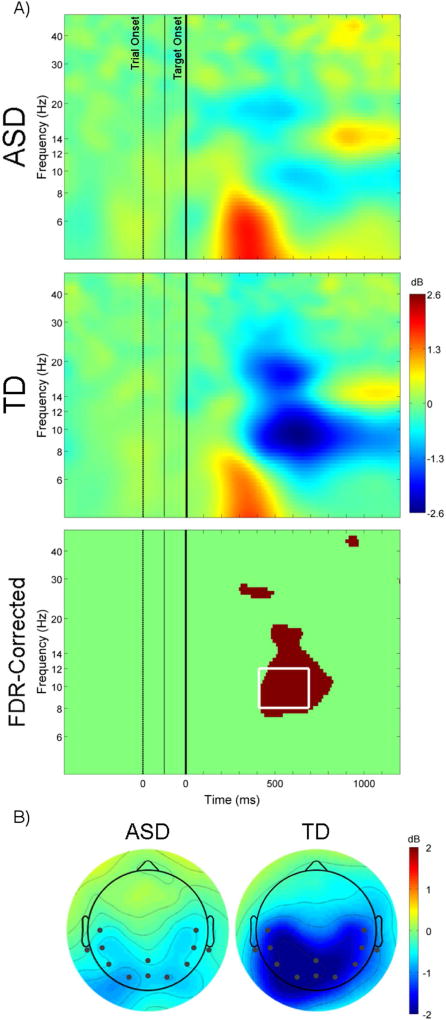
As illustrated in Figure 2A, ERSP showed that TD children exhibited significantly greater posterior event-related alpha desynchronization compared to children with ASD. A 2 (group: ASD, TD) × 3 (ROI: left, center, right) mixed-model repeated measures ANOVA showed that alpha desynchronization from 400 to 700ms post-target onset was significantly greater in the TD compared to the ASD group, F(1,38) = 12.09, p = .001, ηp2 = .24 (Figure 2B). There was also a significant main effect of ROI and interaction between group and ROI (p <.05). Follow-up independent-samples t-tests showed that alpha ERD was greater for the TD group at each ROI (all p <.05); paired-samples t-tests revealed no difference in ERD across the three ROIs for the ASD group, but significantly greater ERD for central ROI compared to left and right ROIs for the TD group (Supplementary Figure 1).
Figure 2.
(A) Event-related spectral perturbations for target present neutral (P-N) condition at electrode location Pz for the ASD group (top; x-axis first zero corresponds to onset of the trial, second zero corresponds to onset of the target [3rd frame]), TD group (middle), and difference between two groups (bottom; FDR-corrected p < .1, white box corresponds to time and frequency range use for further analyses). (B) Scalp maps of average alpha power (8 – 12 Hz) from 400 to 700ms after the onset of the target. Channels included in regions of interest displayed as black dots.
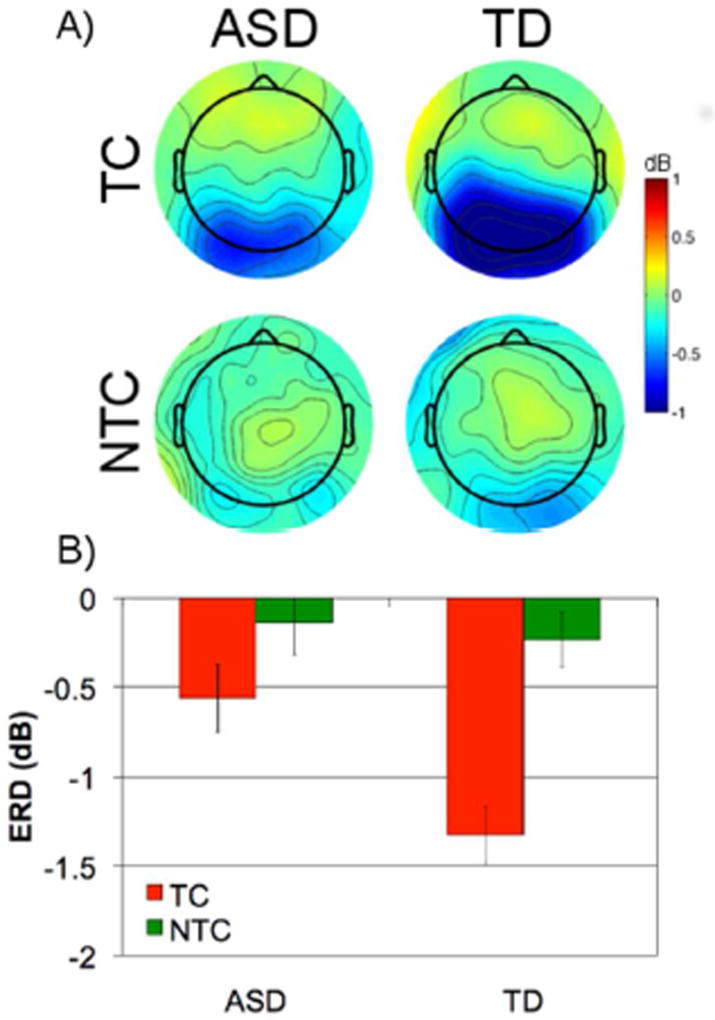
Contingent attentional capture in target absent trials
As shown in Figure 3A, comparison of target absent TC and NTC distractors revealed that TD children showed greater alpha desynchronization to TC compared to NTC distractors, whereas children with ASD did not. For ERD at the central ROI, a 2 (group: ASD, TD) × 2 (condition: TC, NTC) mixed-model repeated measures ANOVA showed that, similar to the P-N condition, the TD group exhibited significantly greater alpha desynchronization compared to the ASD group, F(1,38) = 4.84, p = .034, ηp2 = .11. There was a significant main effect of condition, F(1,38) = 28.85, p < .001, ηp2 = .43, with greater alpha desynchronization to TC compared to NTC distractors indicative of increased attentional capture to TC compared to NTC distractors. Additionally, there was a significant interaction between group and condition, F(1,38) = 5.62, p = .023, ηp2 = .13. Follow-up independent samples t-tests showed that, compared to individuals with ASD, TD individuals had greater alpha desynchronization for TC, t(38) = 2.93, p = .006, but not NTC distractors, t(38) = 0.44, p = .67. Paired t-tests showed greater alpha desynchronization to TC compared to NTC distractors for the TD, t(20) = −6.79, p < .001, but not the ASD group, t(18) = −1.78, p = .09.
Figure 3.
(A) Scalp maps of average alpha (8 −12 Hz) from 400 to 700ms after the onset of the distractors for ASD and TD groups for target-colored (TC) and non-target-colored (NTC) distractors. (B) Average alpha (8 – 12 Hz) from 400 to 700ms at posterior ROI (error bars represent ± 1 SEM).
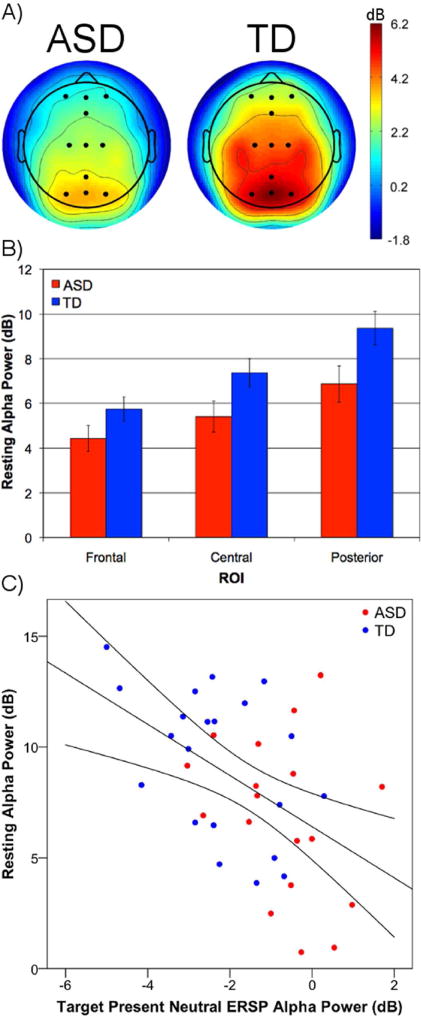
Resting state EEG
Resting state EEG data were not collected for one ASD participant. TD participants exhibited increased alpha power compared to children with ASD (Figure 4A). Absolute power values were entered into a 2 (group: ASD, TD) × 3 (ROI: frontal, central, posterior) mixed-model repeated measures ANOVA. There was a significant main effect of ROI, F(2,74) = 99.26, p < .001, ηp2 = .73, as alpha power was greatest at posterior, lower at central, and lowest at frontal ROIs. Children with ASD showed significantly lower alpha power compared to TD children, F(1,37) = 4.34, p = .04, ηp2 = .11. There was also a significant interaction between group and ROI, F(2,74) = 3.71, p = .03. Follow-up t-tests revealed significant group differences at posterior, t(37) = −2.26, p = .03, and central, t(37) = −2.08, p = .04, but not frontal ROIs, t(37) = −1.66, p = .11 (Figure 4B).
Figure 4.
(A) Scalp maps of resting-state alpha (8–12 Hz) power for the ASD and TD groups. Channels included in regions of interest displayed as black dots. (B) Group differences in alpha power at frontal, central, posterior ROIs (error bars represent ± 1 SEM). (C) Scatterplot of resting state alpha power and alpha-band ERD to the target present neutral (P–N) condition (from posterior ROI) for both ASD (red) and TD (blue) groups.
Correlational analyses were conducted to determine whether differences in resting alpha power levels were associated with alpha-band ERD to targets, measured at the posterior ROI. For both groups combined, greater resting alpha power was associated with increased alpha desynchronization to target present neutral trials, r(37) = −.486, p = .002 (Figure 4C), and target absent target-colored, r(37) = −.482, p = .002, but not non-target-colored distractors, r(37) = −.123, p = .455.
Relationship with ASD Symptomatology
Summary scores from the ADOS diagnostic algorithm were used as symptom measures, with higher ADOS scores reflecting greater severity. Accuracy and RT for P-N condition were converted into inverse efficiency scores (IES; RT/percent correct). To examine whether ASD symptomatology was associated with behavioral and neural indices of target processing Spearman correlations between ADOS and IES and alpha ERD within left, center, and right ROIs for P-N condition were conducted. Increased repetitive behaviors were associated with IES, r(17) = .60, p = .007, and alpha ERD at left, r(17) = .53, p = .02, and right ROIs, r(17) = .47, p = .04 (Supplementary Figure 5; Supplementary Table 3). No significant correlations existed for measures of contingent attentional capture or resting state EEG.
Discussion
The present study examined resting and event-related changes in alpha power associated with attentional capture. TD children showed: 1) robust alpha-band desynchronization to the onset of targets (reflecting attention capture by behaviorally-relevant information); 2) behavioral and electrophysiological evidence of contingent attentional capture (i.e., response to distractors that shared the target color but not to distractors of a different color). In contrast, children with ASD did not show electrophysiological evidence of attentional capture by behaviorally-relevant targets or distractors regardless of whether they shared a task-relevant feature. Our data also suggest that the resting brain state is related to the efficiency of attentional capture. We found that higher resting alpha power was associated with greater attention-related EEG response to behaviorally-relevant information (i.e., greater alpha desynchronization to targets and target-colored distractors). Compared to their TD peers, ASD children showed reduced resting alpha power and reduced EEG response reflecting attention capture by behaviorally-relevant information (i.e., lower alpha desynchronization to relevant stimuli). Finally, inefficient behavioral responsivity and reduced target-related alpha ERD were associated with increased ASD symptomatology. Together, these findings may provide some insight into the neural underpinnings of attentional impairments in ASD.
Target-Related Processing
Behavioral and EEG findings from this study are consistent with those from our previous fMRI investigation using a similar RSVP paradigm (42). In that study, as in the current study, we found no group differences in accuracy or RT to target present neutral condition, but significant group differences in target-related neural activity. TD children evidenced significant target-related alpha-band desynchronization, which has been associated with phasic alertness and target-related processing (49) and allocation of attentional resources (50). Children with ASD did not show similar neural indices of target-related processing, suggesting that they may not be allocating attentional resources or processing targets in the same way as their TD peers.
Further, the presence of equivalent performance despite atypical neurophysiological response in ASD is not unique to the present paradigm, and has been highlighted elsewhere (51). These divergent findings suggest that children with ASD may be achieving similar performance by atypical, potentially less efficient means. For example, previous fMRI and ERP odd-ball studies have reported equivalent behavioral performance, and yet robust differences in brain activation between ASD and TD groups (51–53). Alpha desynchronization is associated with amplitude and latency of the averaged ERP P3 component, an electrophysiological marker of attention processes (54), and, thus, our findings are also in accord with numerous ERP studies, which have shown reduced average amplitude of the P3b component in ASD (55).
Consistent with our previous findings (42) in which impairments in target detection and atypical target-related activation in right temporal parietal junction (rTPJ) were associated with increased sociocommunicative impairments, we found that reduced target-related desynchronization and response efficiency were related to increased ASD symptomatology. Activation of rTPJ and alpha ERD to targets may reflect re-orienting attention to behaviorally-relevant information (56) and/or contextual updating (57). Together with previous reports using resting alpha power (58) and fMRI (59), the present results suggest that social impairments and repetitive behaviors in ASD may develop, in part, due to deficits in non-social attentional processes and/or common disruption in brain formation that affects contiguous areas of cortex.
Lastly, baseline behavioral performance and eye-tracking results (Supplementary Materials) suggest that these electrophysiological differences are not related to numerical processing or attention to the central stream. Rather, both previous fMRI results and current electrophysiological findings indicate that onset of behaviorally-relevant information does not elicit robust activation of the ventral attentional network (42) or alpha ERD in children with ASD, which suggests atypical attention capture and allocation of attentional resources.
Contingent Attentional Capture
TD children showed behavioral and electrophysiological evidence of contingent attentional capture. In TD children, target-colored distractors were more likely to capture attention and facilitate response than were non-target colored distractors. In contrast, children with ASD did not show behavioral facilitation or electrophysiological evidence of contingent attentional capture. These findings complement our previous results using fMRI to examine contingent attentional capture (42) in which children with ASD exhibited increased activation to both TC and NTC distractors. That is, while both studies demonstrated an absence of the neural indices of contingent attentional capture in ASD (i.e., no difference between TC and NTC conditions), our present findings indicate a lack of activation to both TC and NTC distractors whereas our previous study showed similar occipital activation to both. Evidence of less susceptibility to distraction, as indexed by reduced alpha ERD to both TC and NTC irrelevant distractors in ASD, is inconsistent with prior research demonstrating increased perceptual capacity (8–10). Therefore, in the present paradigm and using a different index of neural activation, children with ASD are less susceptible to distraction from an attentional focus, but in the absence of distraction are generally under-reactive to behaviorally-relevant information.
Resting Alpha Power and the Role of Alpha and Attention in ASD
In agreement with previous reports (36–39), we found significantly reduced resting alpha power in our sample of high-functioning children with ASD. Furthermore, across both groups, lower resting alpha power was associated with reduced alpha ERD evoked by targets and by TC, but not NTC distractors. These findings are consistent with prior work demonstrating that larger tonic alpha power is related greater task-related alpha ERD (60, 61) and increased attention-related ERP amplitudes (62) in neurotypical adults. Together, these findings suggest that resting alpha levels are related to phasic changes in electrophysiological responses.
How might differences in resting alpha power and task-related alpha desynchronization be related to impaired attention in ASD? Belmonte (51) previously hypothesized that hyper-arousal associated with atypical sensory processing may result in impaired early selection of relevant stimuli. Previous research has shown that resting and pre-stimulus alpha levels are associated with peripheral measures of autonomic activity in children (63) and adults (64, 65). Furthermore, animal (25) and human pharmacological studies (26–28) have linked decreased GABAergic activity with reduced resting alpha power. In ASD, atypically increased arousal (66) and reduced GABA (33) levels have been associated with sensory dysfunction. Furthermore, research on TD adults has shown that visual perception is enhanced if the cortex is activated (i.e., lower baseline alpha) before the task is performed, whereas improved performance of more complex cognitive processes is associated with pre-task cortical deactivation (i.e., higher baseline alpha). Thus, while it remains to be determined, atypical arousal levels and/or an aberrant excitatory/inhibitory balance, as indexed by decreased resting alpha power, may result in atypical sensory processing and under-responsivity to behaviorally-relevant stimuli in those with ASD.
An alternative explanation for the present findings may relate to entrainment of neural oscillations by the rhythmic nature of our task, in which numbers within each stream were updated every 120ms (~8Hz). Intrinsic oscillatory patterns in the brain activation may entrain to external stimuli, resulting in optimal processing of rhythmic, predictable events (67). In TD adults, resting alpha levels are associated with task-related entrainment of alpha oscillations and detection of entrained targets (20). Thus, TD children, who exhibited greater resting alpha levels, may have been more likely to become entrained to presentation of the stimuli, which may have facilitated perception of and ERD to task-relevant information. Decreased resting alpha power in children with ASD may have resulted in a lack of entrainment, and could potentially lead to reduced detection and task-related alpha modulation to targets (68). Neural entrainment may be important for sensory processing and selective attention to other inputs such as speech (69), and may have broader implications for understanding the neural underpinnings and development of ASD.
Conclusion
ASD is associated with deficits in orienting attention to behaviorally-relevant information be it a subtle shift in eye gaze, a new person entering a room, or a caregiver’s name call. Although these examples are social in nature, they likely require, in part, more fundamental non-social attention mechanisms in order to recognize and execute adaptive, socially appropriate responses (and develop those responses in the first place). Our findings shed light on neurophysiological mechanisms related to these basic, non-social attentional impairments and may have implications for understanding the development of ASD. Children with ASD show atypically reduced neural response to behaviorally-relevant information, which was significantly associated with clinical features of the disorder. Additionally, children with ASD had significantly decreased resting alpha power, and for all participants increased resting alpha levels were associated with greater task-related alpha desynchronization. These results indicate that under-responsivity and impairments in orienting to salient events within the environment may be due to deficits in oscillatory neurodynamics, which may be in turn related to both atypical arousal levels and/or an excitatory/inhibitory imbalance in ASD.
Supplementary Material
Acknowledgments
Supported by R01-MH081023 (RAM) and RO1-NS42639 (JT). Special thanks to the children and families who generously participated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18(4):1030–44. Epub 1992/11/01. [PubMed] [Google Scholar]
- 2.Keehn B, Muller RA, Townsend J. Atypical attentional networks and the emergence of autism. Neurosci Biobehav Rev. 2013;37(2):164–83. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keehn B, Joseph RM. Impaired prioritization of novel onset stimuli in autism spectrum disorder. J Child Psychol Psychiatry. 2008;49(12):1296–303. doi: 10.1111/j.1469-7610.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- 4.Greenaway R, Plaisted K. Top-down attentional modulation in autistic spectrum disorders is stimulus-specific. Psychological Science. 2005;16(12):987–94. doi: 10.1111/j.1467-9280.2005.01648.x. [DOI] [PubMed] [Google Scholar]
- 5.Murphy JW, Foxe JJ, Peters JB, Molholm S. Susceptibility to distraction in autism spectrum disorder: probing the integrity of oscillatory alpha-band suppression mechanisms. Autism Res. 2014;7(4):442–58. doi: 10.1002/aur.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burack JA. Selective attention deficits in persons with autism: Preliminary evidence of an inefficient attentional lens. Journal of Abnormal Psychology. 1994;103(3):535–43. doi: 10.1037//0021-843x.103.3.535. [DOI] [PubMed] [Google Scholar]
- 7.Ronconi L, Gori S, Giora E, Ruffino M, Molteni M, Facoetti A. Deeper attentional masking by lateral objects in children with autism. Brain Cogn. 2013;82(2):213–8. doi: 10.1016/j.bandc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Remington A, Swettenham J, Campbell R, Coleman M. Selective attention and perceptual load in autism spectrum disorder. Psychological Science. 2009;20(11):1388–93. doi: 10.1111/j.1467-9280.2009.02454.x. [DOI] [PubMed] [Google Scholar]
- 9.Remington A, Swettenham JG, Lavie N. Lightening the load: perceptual load impairs visual detection in typical adults but not in autism. J Abnorm Psychol. 2012;121(2):544–51. doi: 10.1037/a0027670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohta H, Yamada T, Watanabe H, Kanai C, Tanaka E, Ohno T, Takayama Y, Iwanami A, Kato N, Hashimoto R. An fMRI study of reduced perceptual load-dependent modulation of task-irrelevant activity in adults with autism spectrum conditions. NeuroImage. 2012;61(4):1176–87. doi: 10.1016/j.neuroimage.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 11.Liss M, Saulnier C, Fein D, Kinsbourne M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10(2):155–72. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- 12.Lovaas OI, Koegel RL, Schreibman L. Stimulus overselectivity in autism: A review of research. Psychological Bulletin. 1979;86(6):1236–54. [PubMed] [Google Scholar]
- 13.Robertson CE, Kravitz DJ, Freyberg J, Baron-Cohen S, Baker CI. Tunnel vision: sharper gradient of spatial attention in autism. J Neurosci. 2013;33(16):6776–81. doi: 10.1523/JNEUROSCI.5120-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend J, Courchesne E. Parietal damage and narrow "spotlight" spatial attention. J Cogn Neurosci. 1994;6(3):220–32. doi: 10.1162/jocn.1994.6.3.220. [DOI] [PubMed] [Google Scholar]
- 15.Mann TA, Walker P. Autism and a deficit in broadening the spread of visual attention. Journal of child psychology and psychiatry, and allied disciplines. 2003;44(2):274–84. doi: 10.1111/1469-7610.00120. [DOI] [PubMed] [Google Scholar]
- 16.Ronconi L, Gori S, Ruffino M, Molteni M, Facoetti A. Zoom-out attentional impairment in children with autism spectrum disorder. Cortex. 2013;49(4):1025–33. doi: 10.1016/j.cortex.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–85. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- 18.Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–83. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- 19.Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7(12):553–9. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Mathewson KE, Prudhomme C, Fabiani M, Beck DM, Lleras A, Gratton G. Making waves in the stream of consciousness: entraining oscillations in EEG alpha and fluctuations in visual awareness with rhythmic visual stimulation. J Cogn Neurosci. 2012;24(12):2321–33. doi: 10.1162/jocn_a_00288. [DOI] [PubMed] [Google Scholar]
- 21.Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26(37):9494–502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95(6):3844–51. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- 23.Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12(8):877–82. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- 24.Klimesch W. alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16(12):606–17. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorincz ML, Kekesi KA, Juhasz G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63(5):683–96. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreckenberger M, Lange-Asschenfeldt C, Lochmann M, Mann K, Siessmeier T, Buchholz HG, Bartenstein P, Grunder G. The thalamus as the generator and modulator of EEG alpha rhythm: a combined PET/EEG study with lorazepam challenge in humans. Neuroimage. 2004;22(2):637–44. doi: 10.1016/j.neuroimage.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 27.Ahveninen J, Lin FH, Kivisaari R, Autti T, Hamalainen M, Stufflebeam S, Belliveau JW, Kahkonen S. MRI-constrained spectral imaging of benzodiazepine modulation of spontaneous neuromagnetic activity in human cortex. Neuroimage. 2007;35(2):577–82. doi: 10.1016/j.neuroimage.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 28.Lozano-Soldevilla D, ter Huurne N, Cools R, Jensen O. GABAergic modulation of visual gamma and alpha oscillations and its consequences for working memory performance. Curr Biol. 2014;24(24):2878–87. doi: 10.1016/j.cub.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain and Behavior. 2003;2(5):255–67. doi: 10.1034/j.1601-183x.2003.00037.x. Epub 2003/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson SB, Valakh V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron. 2015;87(4):684–98. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashemi E, Ariza J, Rogers H, Noctor SC, Martinez-Cerdeno V. The Number of Parvalbumin-Expressing Interneurons Is Decreased in the Medial Prefrontal Cortex in Autism. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009;39(2):223–30. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puts NA, Wodka EL, Harris AD, Crocetti D, Tommerdahl M, Mostofsky SH, Edden RA. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res. 2016 doi: 10.1002/aur.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TP. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Barstein J, Ethridge LE, Mosconi MW, Takarae Y, Sweeney JA. Resting state EEG abnormalities in autism spectrum disorders. J Neurodev Disord. 2013;5(1):24. doi: 10.1186/1866-1955-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLoS One. 2012;7(6):e39127. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawson G, Klinger LG, Panagiotides H, Lewy A, Castelloe P. Subgroups of autistic children based on social behavior display distinct patterns of brain activity. J Abnorm Child Psychol. 1995;23(5):569–83. doi: 10.1007/BF01447662. [DOI] [PubMed] [Google Scholar]
- 38.Chan AS, Sze SL, Cheung MC. Quantitative electroencephalographic profiles for children with autistic spectrum disorder. NeuroPsychology. 2007;21(1):74–81. doi: 10.1037/0894-4105.21.1.74. [DOI] [PubMed] [Google Scholar]
- 39.Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry. 2007;62(3):270–3. doi: 10.1016/j.biopsych.2006.11.012. Epub 2007/03/06. doi: S0006-3223(06)01471-5[pii] 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ewen JB, Lakshmanan BM, Pillai AS, McAuliffe D, Nettles C, Hallett M, Crone NE, Mostofsky SH. Decreased Modulation of EEG Oscillations in High-Functioning Autism during a Motor Control Task. Front Hum Neurosci. 2016;10:198. doi: 10.3389/fnhum.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milne E, Scope A, Pascalis O, Buckley D, Makeig S. Independent component analysis reveals atypical electroencephalographic activity during visual perception in individuals with autism. Biol Psychiatry. 2009;65(1):22–30. doi: 10.1016/j.biopsych.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Keehn B, Nair A, Lincoln AJ, Townsend J, Muller RA. Under-reactive but easily distracted: An fMRI investigation of attentional capture in autism spectrum disorder. Dev Cogn Neurosci. 2016;17:46–56. doi: 10.1016/j.dcn.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview - Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 44.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Obervation Schedule - WPS (ADOS-WPS) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- 45.Ishihara S. Ishihara’s tests for colour deficiency: 38 plates edition. Tokyo, Japan: Kanehara and Co. Ltd; 1999. [Google Scholar]
- 46.Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychological Science. 2005;16(2):114–22. doi: 10.1111/j.0956-7976.2005.00791.x. Epub 2005/02/03. doi: PSCI791 [pii]10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 47.Delorme A, Mullen T, Kothe C, Akalin Acar Z, Bigdely-Shamlo N, Vankov A, Makeig S. EEGLAB, SIFT, NFT, BCILAB, and ERICA: new tools for advanced EEG processing. Comput Intell Neurosci. 2011;2011:130714. doi: 10.1155/2011/130714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci Lett. 1998;244(2):73–6. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 50.Spencer KM, Polich J. Poststimulus EEG spectral analysis and P300: attention, task, and probability. Psychophysiology. 1999;36(2):220–32. [PubMed] [Google Scholar]
- 51.Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Cognitive Brain Research. 2003;17(3):651–64. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- 52.Clery H, Andersson F, Bonnet-Brilhault F, Philippe A, Wicker B, Gomot M. fMRI investigation of visual change detection in adults with autism. NeuroImage Clinical. 2013;2:303–12. doi: 10.1016/j.nicl.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courchesne E, Lincoln AJ, Yeung-Courchesne R, Elmasian R, Grillon C. Pathophysiologic findings in nonretarded autism and receptive developmental language disorder. Journal of Autism and Developmental Disorders. 1989;19(1):1–17. doi: 10.1007/BF02212714. Epub 1989/03/01. [DOI] [PubMed] [Google Scholar]
- 54.Yordanova J, Kolev V, Polich J. P300 and alpha event-related desynchronization (ERD) Psychophysiology. 2001;38(1):143–52. [PubMed] [Google Scholar]
- 55.Jeste SS, Nelson CA., 3rd Event related potentials in the understanding of autism spectrum disorders: an analytical review. J Autism Dev Disord. 2009;39(3):495–510. doi: 10.1007/s10803-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. Epub 2008/05/10. doi: S0896-6273(08)00369-3 [pii] 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng JJ, Vossel S. Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci Biobehav Rev. 2013;37(10 Pt 2):2608–20. doi: 10.1016/j.neubiorev.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathewson KJ, Jetha MK, Drmic IE, Bryson SE, Goldberg JO, Schmidt LA. Regional EEG alpha power, coherence, and behavioral symptomatology in autism spectrum disorder. Clin Neurophysiol. 2012;123(9):1798–809. doi: 10.1016/j.clinph.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 59.Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry. 2008;63(10):974–80. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doppelmayr MM, Klimesch W, Pachinger T, Ripper B. The functional significance of absolute power with respect to event-related desynchronization. Brain Topogr. 1998;11(2):133–40. doi: 10.1023/a:1022206622348. [DOI] [PubMed] [Google Scholar]
- 61.Klimesch W, Doppelmayr M, Hanslmayr S. Upper alpha ERD and absolute power: their meaning for memory performance. Prog Brain Res. 2006;159:151–65. doi: 10.1016/S0079-6123(06)59010-7. [DOI] [PubMed] [Google Scholar]
- 62.Dockree PM, Kelly SP, Foxe JJ, Reilly RB, Robertson IH. Optimal sustained attention is linked to the spectral content of background EEG activity: greater ongoing tonic alpha (approximately 10 Hz) power supports successful phasic goal activation. The European journal of neuroScience. 2007;25(3):900–7. doi: 10.1111/j.1460-9568.2007.05324.x. [DOI] [PubMed] [Google Scholar]
- 63.Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Rushby JA, Ploskova E. EEG differences in children as a function of resting-state arousal level. Clin Neurophysiol. 2004;115(2):402–8. doi: 10.1016/s1388-2457(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 64.Lim CL, Barry RJ, Gordon E, Sawant A, Rennie C, Yiannikas C. The relationship between quantified EEG and skin conductance level. Int J Psychophysiol. 1996;21(2–3):151–62. doi: 10.1016/0167-8760(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 65.Lim CL, Gordon E, Rennie C, Wright JJ, Bahramali H, Li WM, Clouston P, Morris JG. Dynamics of SCR, EEG, and ERP activity in an oddball paradigm with short interstimulus intervals. Psychophysiology. 1999;36(5):543–51. [PubMed] [Google Scholar]
- 66.Palkovitz RJ, Wiesenfeld AR. Differential autonomic responses of autistic and normal children. Journal of Autism and Developmental Disorders. 1980;10(3):347–60. doi: 10.1007/BF02408294. [DOI] [PubMed] [Google Scholar]
- 67.Calderone DJ, Lakatos P, Butler PD, Castellanos FX. Entrainment of neural oscillations as a modifiable substrate of attention. Trends Cogn Sci. 2014;18(6):300–9. doi: 10.1016/j.tics.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belmonte MK. Abnormal attention in autism shown by steady-state visual evoked potentials. Autism. 2000;4(3):269–85. [Google Scholar]
- 69.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32(1):9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wechsler D. Wechsler's Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.