Abstract
Prior research suggests that the construct of emotional instability may be salient to bulimia nervosa (BN), but no study to date has used ecological momentary assessment (EMA) to examine its temporal association with binge eating and purging. In the current study, 133 women with DSM-IV BN used portable digital devices to provide multiple daily negative affect (NA) and positive affect (PA) ratings and record eating disorder behaviors over 2 weeks. Two state-of-the art indices quantified affective instability: probability of acute change (PAC), which represents the likelihood of extreme affective increases, and mean squared successive difference (MSSD), which represents average change over successive recordings. For extreme affective change, results revealed that on bulimic behavior days, extreme NA increases were less likely after bulimic behaviors than before them, and extreme increases in PA were more likely after bulimic behaviors than during the same time period on non-bulimic behavior days. However, average NA instability (i.e., MSSD) was (a) greater on bulimic behavior days than non-bulimic behavior days, (b) greater after bulimic behaviors than during the same time period on non-bulimic behavior days, and (c) greater after bulimic behaviors than before them. Results lend support to the notion that bulimic behaviors are negatively reinforcing (i.e., via post-behavior acute affective changes), but also indicate that these behaviors may exacerbate overall affective dysregulation. These findings may improve understanding of BN maintenance and inform the development of novel interventions or refinement of existing treatments.
Keywords: bulimia nervosa, affective instability, ecological momentary assessment, emotion, binge eating, purging
1. Introduction
Bulimia nervosa (BN) is characterized by recurrent episodes of binge eating, defined most saliently by a sense of “loss of control” over eating (Mond et al., 2010; Shomaker et al., 2010; Vannucci et al., 2013; Wolfe et al., 2009), and compensatory behaviors (e.g., self-induced vomiting, laxative misuse; American Psychiatric Association, 2013). The disorder is associated with significant medical complications, high rates of comorbid psychopathology, and substantial psychosocial impairment (American Psychiatric Association, 2013; Wonderlich and Mitchell, 1997). Recent studies suggest that behaviors characteristic of BN and the development and persistence of the disorder may result, in part, from impairments in the ability to regulate cognitive and behavioral processes (ie., self-regulatory control; e.g., Marsh et al., 2011; Marsh et al., 2013; Marsh et al., 2009; Wu et al., 2013).
One self-regulatory process, emotion regulation, may be a particularly relevant etiological and/or maintenance variable for BN. Self-reported affect-regulation impairments in BN are well-documented (Gilboa-Schechtman et al., 2006; Harrison et al., 2010; Svaldi et al., 2012), and may relate to BN symptoms (Gilboa-Schechtman et al., 2006; Harrison et al., 2010; Svaldi et al., 2012). Individuals with BN report poorer behavioral control when in distress (Harrison et al., 2010; Svaldi et al., 2012), and self-reported emotion regulation difficulties have been associated with eating disorder cognitions and compensatory behaviors in BN (Lavender et al., 2015; Lavender et al., 2014).
Some evidence suggests that individuals engage in binge eating and purging as a means of regulating their affect (Bohon et al., 2009; Combs et al., 2011; Haedt-Matt and Keel, 2011; Pearson et al., 2015). Expectations of reduced negative affect (NA) after eating predict later development of binge eating (Combs et al., 2011), and the association of enhanced positive affect (PA) with eating (e.g., the belief that “eating is fun and enjoyable”) predicts a longer time to remission from BN (Bohon et al., 2009). Several studies using ecological momentary assessment (EMA), a method involving collection of momentary data in a participant’s natural environment, have demonstrated that trajectories of increasing NA and decreasing PA precede binge eating and purging (Alpers and Tuschen-Caffier, 2001; Berg et al., 2013; Hilbert and Tuschen-Caffier, 2007; Smyth et al., 2007). How NA changes after binge eating and purging, as well as the role of PA in bulimic symptoms, are less clear; however, results derived from a recent multilevel, autoregressive cross-lagged analysis of EMA data indicate that binge eating predicts subsequent decreases in NA at numerous time points across the day (Lavender et al., 2016). These results are consistent with those of within-day analyses that examined trajectories (i.e., temporal patterns of change) of NA and PA intensity ratings preceding and following eating disorder behaviors (Berg et al., 2015; Engel et al., 2007; Engel et al., 2013; Smyth et al., 2007) and suggest that binge eating reduces negative affect. Taken together, despite some remaining debate about post-binge eating and purging affective change (e.g., Haedt-Matt and Keel, 2011), these findings lend support to negative reinforcement models of bulimic behavior.
Previous studies of affect in BN have highlighted the importance of NA and PA intensity, but fluctuations in affective state, or affective instability, may align more closely with the construct of “emotional dysregulation” theorized to drive BN symptoms (Ebner-Priemer and Trull, 2009; Trull et al., 2008). Results of recent EMA research indicate that individuals with BN demonstrate increased overall affective instability relative to healthy controls: NA states occur more frequently, PA is unstable, and large drops from positive to negative affective states are frequent (Santangelo et al., 2014).
1.1. Current study
The current study uses a novel analytic approach in a large EMA dataset of women with BN to examine, for the first time, associations between affective instability and bulimic behaviors. Previous analyses using this dataset that have examined affect before and after bulimic behaviors have focused on affective intensity (Berg et al., 2013; Engel et al., 2007; Lavender et al., 2016; Smyth et al., 2007). Two prior analyses of this dataset have examined affective instability in relation to bulimic behavior frequency, but over time spans that prevented examination of whether affective instability temporally relates to BN symptoms. Results of the first study suggest that total frequency of binge eating and purging over two weeks is inversely related to average stability of NA over the same two-week period of time (Anestis et al., 2010). In the second study, “daily emotional variability,” calculated as each individual’s standard deviation around their daily mean affect rating, was used to operationally define average daily NA and PA lability (Selby et al., 2012). NA was more variable on days with bulimic events (bulimic behavior days) compared with non-bulimic behavior days, and average daily PA variability on bulimic behavior and non-bulimic behavior days did not differ (Selby et al., 2012). However, these previous analyses did not address within-person, within-day changes in affective instability.
We adopted two advanced affective instability indices (Jahng et al., 2008; Santangelo et al., 2014) to replicate and extend prior findings. We examined, in both between-day and within-person, within-day models, the relationship of bulimic behaviors to short-term average positive and negative affective instability (Mean Squared Successive Difference [MSSD]) and the likelihood of an extreme increase in NA or PA (Probability of Acute Change [PAC]). Higher MSSD indices reflect greater overall mean variance in affect, whereas higher PAC indices reflect more frequent severe shifts in affect. Both of these metrics have been used to document affective instability in individuals across a range of psychiatric disorders (Santangelo et al., 2014; Snir et al., 2016; Trull et al., 2008). Although Santangelo and colleagues (2014) used MSSD and PAC metrics to document increased affective instability in individuals with BN compared to healthy controls, the current study is the first to examine these two metrics of affective instability before and after bulimic behavior within a BN sample. This approach allowed us to examine affective instability as a potential momentary precipitant of bulimic behavior, as well as the possible reduction in affective instability following the behaviors (i.e., affective stabilization) as a potential reinforcer.
We were guided by the following hypotheses about both NA and PA: 1) The probability of acute affective increases (PAC) and mean affective instability (MSSD) would be greater on days that included a bulimic behavior than on non-bulimic behavior days; 2) the probability of acute affective increases (PAC) and mean affective instability (MSSD) would be greater prior to a bulimic behavior than during the same time period on a non-bulimic behavior day; 3) the probability of acute affective increases (PAC) and mean affective instability (MSSD) would be lower following a bulimic behavior than during the same time period on a non-bulimic behavior day; and 4) on bulimic behavior days, the probability of acute affective increases (PAC) and mean affective instability (MSSD) would be greater before the episode occurred than after.
2. Method
2.1. Participants
As previously described (Smyth et al., 2007), the full sample included 133 women who met diagnostic criteria for BN as defined by the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994). These women, all over the age of 18, medically stable, and with no changes in mental health treatment in the past 6 weeks, were recruited from the community, local clinics, and a university campus. The majority of the sample (95.5%) was Caucasian. Participants had a mean age of 25.3 ± 7.6 years and a mean BMI of 23.9 ± 5.2 kg/m2.
2.2. Procedure
The Institutional Review Boards of the University of North Dakota and MeritCare Hospital (Fargo, ND) approved this study. Participants completed an initial phone screen and then attended an informational session, where they learned more about the study and provided written informed consent. Baseline assessments and EMA training were completed during two, in-person visits. Participants completed two practice EMA days (data not used in current analyses) before beginning the two-week EMA protocol.
2.2.1. EMA protocol
Participants completed affect (i.e., PA and NA) and behavior ratings on a hand-held computer each time they engaged in an eating disorder behavior and in response to six semi-random signals throughout the day. In response to these signals, participants also reported any recent eating disorder behaviors that had not been previously recorded. Participants also provided end-of-day ratings.
2.3. Measures
2.3.1. Diagnostic assessment
A doctoral level psychologist administered the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P; First et al., 1997) to confirm DSM-IV BN diagnosis. Trained assessors independently recoded 25 randomly selected cases, and diagnostic interrater reliability was excellent (κ = 1.00).
2.3.2. EMA binge eating and purging
Participants were instructed during EMA training to report binge eating episodes any time they consumed “an amount of food that you consider excessive or an amount of food that other people would consider excessive, with an associated loss of control or the feeling of being driven or compelled to keep eating.” Participants were trained to identify “objectively large amounts of food” through examples individually tailored to participants’ episodes (based on intake assessments). Participants also provided signal- and event-contingent reports of self-induced vomiting and laxative misuse.
2.3.3. EMA negative and positive affect
Items from the Positive and Negative Affect Scale-Expanded Form (Watson and Clark, 1994), a measure of state emotion, were selected based on high factor loadings and theoretical relevance to bulimic symptomatology. NA items included: afraid, lonely, irritable, ashamed, disgusted, nervous, dissatisfied with self, jittery, sad, distressed, and angry with self (α = 0.92). PA items included: happy, alert, proud, cheerful, enthusiastic, confident, concentrating, energetic, calm, strong, determined, attentive, and relaxed (α = 0.91). Prior studies have combined NA and PA ratings from other measures to examine affective instability along a continuum (e.g., Santangelo et al., 2014). However, because NA and PA dysregulation likely play unique roles in BN (Wonderlich et al., October, 2014) and psychopathology in general (Carl et al., 2013), we examined NA and PA instability separately.
2.3.3.1. Probability of acute change (PAC)
The PAC index captures the likelihood or occurrence of extreme increases in NA and PA. Successive differences between affect ratings are calculated, and those above the 90th percentile of the distribution of all differences over all persons are defined as “acute.” We calculated PAC indices per person for both NA and PA as the number of acute changes divided by total number of changes. For the present study, NA PAC and PA PAC were calculated for each person on each day in three different ways: (1) using NA and PA scores across the entire day, (2) using NA and PA scores occurring prior to the first bulimic behavior on a bulimic behavior day, or for time-matched comparative time intervals non-bulimic behavior days, and (3) using NA and PA scores occurring after the first bulimic behavior, or for time-matched comparative intervals on non-bulimic behavior days (from the average time at which the first bulimic behaviors occurred for that individual to the end of the day). Calculations of PAC in many prior studies capture extreme increases or decreases in affective valence ratings along a single continuum (e.g., Santangelo et al., 2014). Because we examined NA and PA separately, we examined only extreme increases in affect ratings, but used an otherwise identical formula to calculate the index (Snir et al., 2016). In the event of multiple bulimic behaviors occurring on the same day, only those NA and PA scores occurring prior to the second bulimic behavior were included in the above calculations.
2.3.3.2. Mean square successive difference (MSSD)
The MSSD statistic measures average affective instability and is calculated as the squared successive differences between consecutive affect ratings. As such, the index captures both increases and decreases in affect. Like PAC, MSSD scores were calculated across the entire day, as well as before and after the first bulimic behavior and, on non-bulimic behavior days, before and after the individual’s average time of the first bulimic behavior.
PAC and MSSD values were calculated for modeling as level 1 events in multilevel analyses, and values were corrected for time intervals between observations using adjusted successive differences (Jahng et al., 2008).
2.4. Statistical analyses
Generalized estimating equations (GEE) with gamma with log link models (due to significant skew) were used to examine between- and within-day hypotheses. We used an independent correlation structure and a robust covariance matrix. All tests were two-tailed (α = 0.01 as a correction for multiple comparisons). Estimated effect sizes (estimated Cohen’s d and Hedges’ g) were calculated using estimated marginal means and standard errors from GEEs. Only available data were analyzed.
2.4.1. Between-day analyses
PAC and MSSD in NA or PA on days during which either binge eating, purging, or combined binge-purge episodes occurred (bulimic behavior days) were compared with those on days during which none of these behaviors occurred (non-bulimic behavior days). Subject was included as a repeated effect. Covariates included day number of recording (i.e., time), days squared, and the proportion of days for a given participant that included a bulimic behavior. Whether the day included a bulimic behavior was the categorical independent variable of interest, and PAC and MSSD values were the dependent variables.
GEEs also examined whether PACs and MSSDs calculated for the interval before any bulimic behavior occurred differed from the same time period on non-bulimic behavior days; similarly, PACs and MSSDs in the interval following a bulimic behavior were compared with those from matched time periods on non-bulimic behavior days. These models included the same first and second level variables as between-day models examining PAC and MSSD ratings across the entire day.
2.4.2. Within-person, within-day analyses
GEEs examined whether, within each participant, bulimic behavior day PACs and MSSDs in the time period before a bulimic behavior occurred differed from PACs and MSSDs after the bulimic behavior. These analyses were restricted to bulimic behavior days only. Models included subject as a repeated effect, and day and days squared as covariates. Whether the metric corresponded to the pre- or post-bulimic behavior period was the independent variable of interest, and PAC and MSSD in pre- and post-bulimic behavior time periods were the dependent variables.
3. Results
3.1. EMA descriptives
Participants provided 13,055 separate ratings over the two-week EMA protocol with an average of 86% compliance to semi-random signals. On average, participants reported 7.8 ± 6.5 total binge eating episodes (Range = 0–34) and 11.1 ± 9.6 total purging episodes (Range = 0–47). Roughly half (53.9 ± 25.6 %) of the days included at least one binge-purge episode (mode = 67%). Average NA across the two weeks was 24.5 ± 8.2 (Range = 11.8–50.2). Average PA was 34.6 ± 8.6 (Range =13.8–62.1).
Average daily NA PAC was 0.098 ± 0.065 (Range = 0–0.260), and average daily PA PAC was 0.100 ± 0.066 (Range = 0–0.290). Average daily NA MSSD across the two weeks was 49.7 ± 36.1 (Range = 2.8–169.4), and average daily PA MSSD was 60.1 ± 42.7 (Range = 1.5– 231.0). Daily PAC and MSSD ratings were positively correlated (rs = 0.54–0.89).
On average, 3.5 ± 1.3 observations were used to calculate MSSD and PAC pre-bulimic behaviors, 4.3 ± 1.9 were used for post-bulimic behavior calculations, and 6.7 ± 1.6 were used to calculate MSSD and PAC across the entire day.
3.2. Between-day affective instability
Results of between-day analyses are presented in Table 1 and Figures 1 and 2.
Table 1.
Between-Day Negative and Positive Affective Instability
| Non-Bulimic Behavior Day |
Bulimic Behavior Day |
Wald Chi- Square |
p | Estimated Cohen’s d |
Estimated Hedges’ g |
99% CI | ||
|---|---|---|---|---|---|---|---|---|
| EMM (SE) | EMM (SE) | Lower | Upper | |||||
| Negative Affect | ||||||||
| PAC | ||||||||
| Full Day | 0.070 (0.006) | 0.117 (0.007) | 37.47 | < 0.001 | 0.63 | 0.62 | −0.73 | −0.30 |
| Pre-Bulimic Behavior | 0.090 (0.010) | 0.118 (0.011) | 4.14 | 0.042 | 0.23 | 0.23 | −0.63 | 0.07 |
| Post-Bulimic Behavior | 0.061 (0.006) | 0.079 (0.006) | 5.15 | 0.023 | 0.26 | 0.26 | −0.54 | 0.03 |
| MSSD | ||||||||
| Full Day | 38.5 (3.3) | 57.4 (4.0) | 20.37 | < 0.001 | 0.45 | 0.45 | −0.62 | −0.17 |
| Pre-Bulimic Behavior | 41.6 (4.8) | 40.7 (3.7) | 0.04 | 0.85 | 0.02 | 0.02 | −0.28 | 0.32 |
| Post-Bulimic Behavior | 40.5 (3.8) | 59.3 (4.9) | 11.94 | 0.001 | 0.44 | 0.44 | −0.67 | −0.1 |
| Positive Affect | ||||||||
| PAC | ||||||||
| Full Day | 0.102 (0.007) | 0.097 (0.006) | 0.57 | 0.452 | 0.07 | 0.07 | −0.13 | 0.24 |
| Pre-Bulimic Behavior | 0.120 (0.012) | 0.093 (0.010) | 3.58 | 0.059 | 0.21 | 0.21 | −0.09 | 0.61 |
| Post-Bulimic Behavior | 0.050 (0.006) | 0.081 (0.006) | 19.05 | < 0.001 | 0.45 | 0.45 | −0.78 | −0.2 |
| MSSD | ||||||||
| Full Day | 55.7 (4.4) | 62.7 (4.1) | 2.41 | 0.12 | 0.14 | 0.14 | −0.31 | 0.78 |
| Pre-Bulimic Behavior | 58.8 (5.0) | 57.0 (4.6) | 0.09 | 0.766 | 0.03 | 0.03 | −0.24 | 0.30 |
| Post-Bulimic Behavior | 50.6 (4.4) | 59.3 (5.1) | 2.7 | 0.101 | 0.16 | 0.16 | −0.41 | 0.09 |
Estimated Cohen’s d and estimated Hedges’ g were calculated using estimated marginal means and standard errors.
Abbreviations: PAC = probability of acute change; MSSD = mean squared successive difference; EMM = estimated marginal mean; SE = standard error; 99% CI = 99% confidence interval for effect size, as our a priori alpha was set at 0.01.
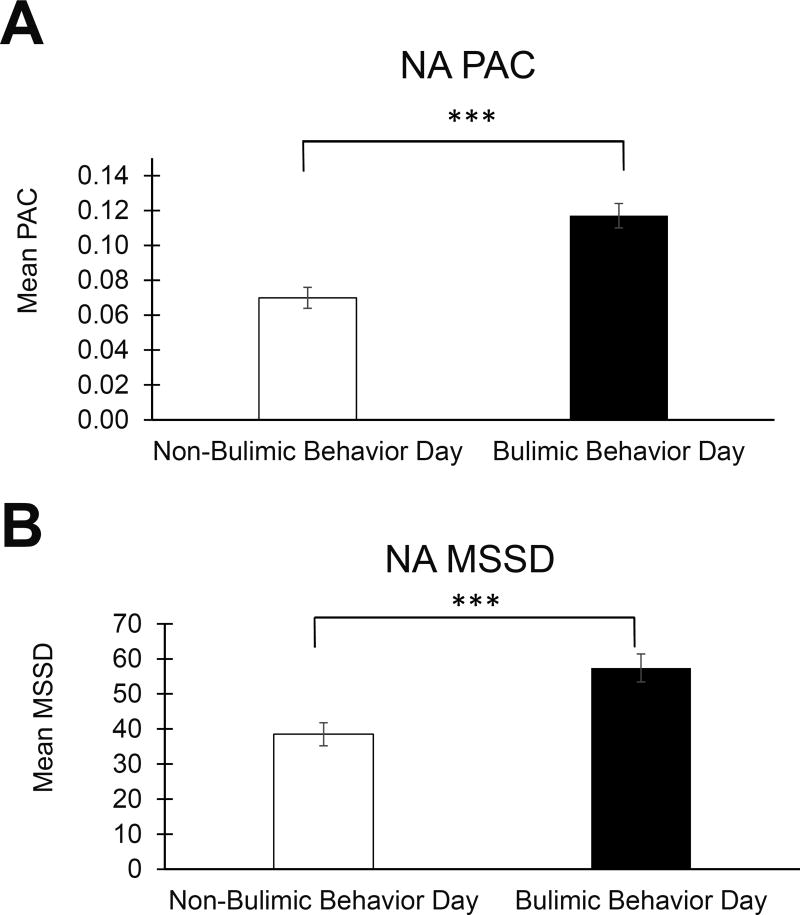
Figure 1. Negative Affect Instability on Days with and without Bulimic Behavior.
A) The likelihood of extreme increases in NA (PAC) was higher on bulimic behavior days compared with non-bulimic behavior days, and B) average NA instability (MSSD) was greater on bulimic behavior days than on non-bulimic behavior days. Error bars represent standard error of the mean. Abbreviations: NA = negative affect; PA = positive affect; PAC = probability of acute change; MSSD = mean squared successive difference. ***p < 0.001
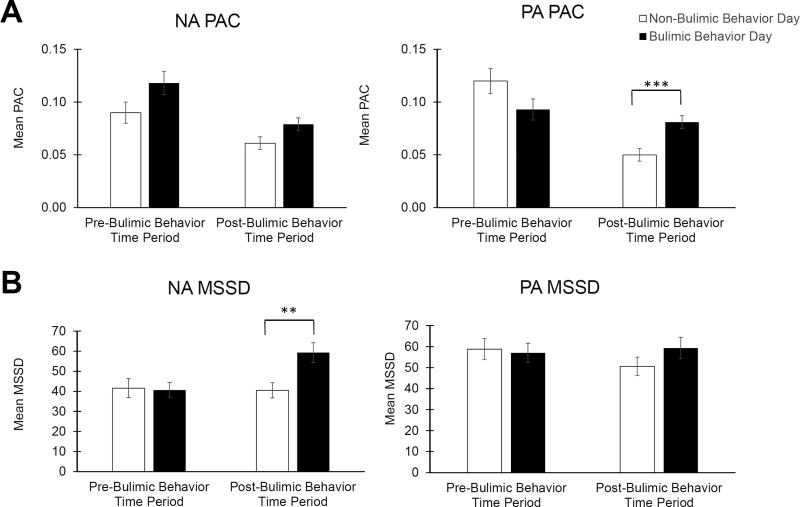
Figure 2. Negative and Positive Affective Instability Before and After Bulimic Behaviors Compared with Time-Matched Periods on Non-Bulimic Behavior Days.
A) The likelihood of an extreme increase in positive affect (PAC) was significantly higher after bulimic behaviors than during matched time periods on non-bulimic behavior days. B) Average negative affect instability (MSSD) was greater after bulimic behaviors than during matched time periods on non-bulimic behavior days, but average positive affect instability (MSSD) before and after bulimic behaviors did not statistically significantly differ from positive affect instability on non-bulimic behavior days. Error bars represent standard error of the mean. Abbreviations: NA = negative affect; PA = positive affect; PAC = probability of acute change; MSSD = mean squared successive difference. **p < 0.005, ***p < 0.001
3.2.1. NA PAC
NA PAC was significantly higher on bulimic behavior versus non-bulimic behavior days (p < 0.001, estimated d = 0.63; Figure 1A). NA PAC before bulimic behaviors was higher than during the same time period on non-bulimic behavior days (p = 0.042, estimated d = 0.23), and NA PAC after bulimic behaviors was higher compared with the same time period on non-bulimic behavior (p = 0.023, estimated d = 0.26), but these differences did not reach the threshold for statistical significance (α = 0.01; Figure 2A).
3.2.2. NA MSSD
NA MSSD on bulimic behavior days was significantly higher than on non-bulimic behavior days (p < 0.001, estimated d = 0.45; Figure 1B). However, there was no statistically significant difference in NA MSSD before bulimic behaviors on bulimic behavior days compared with the same time period on non-bulimic behavior days (p = 0.850, estimated d = 0.02). NA MSSD was greater after bulimic behaviors compared with the same time period on non-bulimic behavior days (p = 0.001, estimated d = 0.44; Figure 2B).
3.2.3. PA PAC
There were no statistically significant differences in PA PAC on bulimic behavior days compared with non-bulimic behavior days (p = 0.452, estimated d = 0.07; Table 1) or before bulimic behaviors compared with the same time period on non-bulimic behavior days (p = 0.059, estimated d = 0.21). However, PA PAC after bulimic behaviors was higher than PA PAC during same time period on non-bulimic behavior days (p < 0.001, estimated d = 0.45; Figure 2A).
3.2.4. PA MSSD
There was no statistically significant difference in PA MSSD on bulimic behavior days compared with non-bulimic behavior days (p = 0.120, estimated d = 0.14; Table 1), before bulimic behaviors compared with the same time period on non-bulimic behavior days (p = 0.766, estimated d = 0.03), or after bulimic behaviors compared with the same time period on non-bulimic behavior days (p = 0.101, estimated d = 0.16; Figure 2B).
3.3. Within-person pre- and post-bulimic behavior affective instability
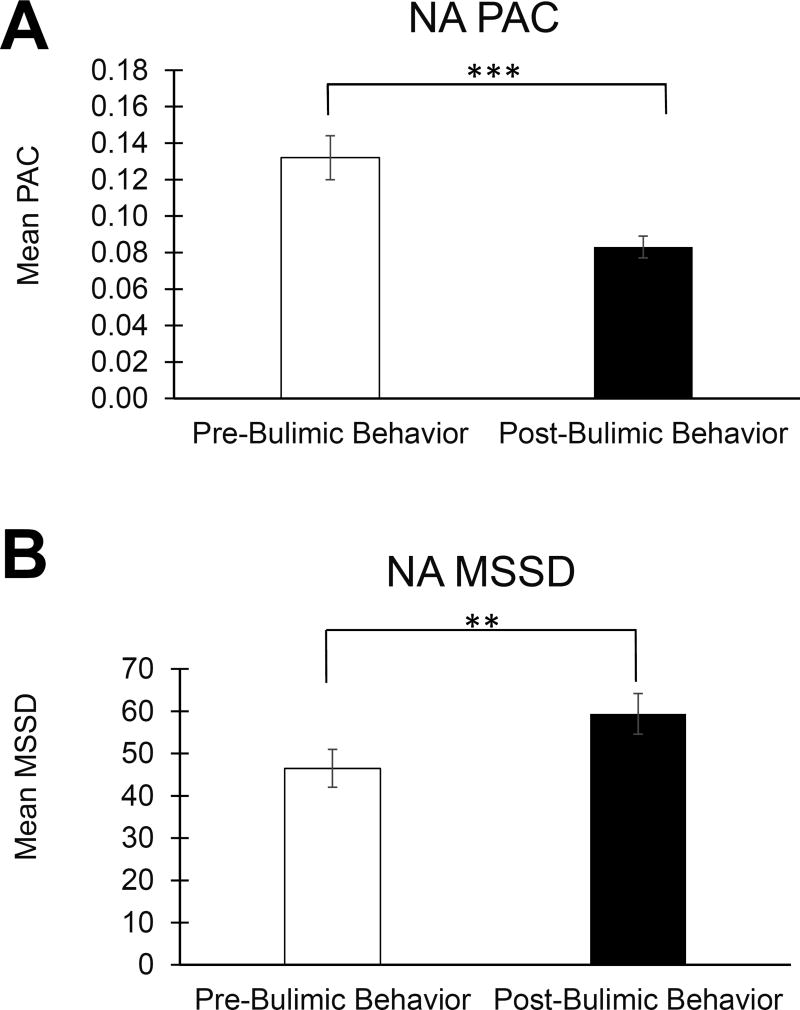
On bulimic behavior days, within-person NA PAC was higher before bulimic behaviors than after them (p < 0.001, estimated d = 0.45; see Table 2 and Figure 3A). In contrast to these NA PAC findings, NA MSSD was significantly greater after bulimic behaviors occurred than before (p = 0.007, estimated d = 0.26; Figure 3B). There was no statistically significant difference in PA PAC (p = 0.255) or MSSD (p = 0.646) before versus after bulimic behaviors on bulimic behavior days.
Table 2.
Within-Day, Within-Person Negative and Positive Affective Instability
| Pre-Bulimic Behavior |
Post-Bulimic Behavior |
Wald Chi- Square |
p | Estimated Cohen’s d |
Estimated Hedges’ g |
99% CI | ||
|---|---|---|---|---|---|---|---|---|
| EMM (SE) | EMM (SE) | Lower | Upper | |||||
| Negative Affect | ||||||||
| PAC | 0.132 (0.012) | 0.083 (0.006) | 25.23 | < 0.001 | 0.45 | 0.45 | 0.23 | 0.71 |
| MSSD | 46.5 (4.5) | 59.4 (4.8) | 7.25 | 0.007 | 0.26 | 0.26 | −0.48 | −0.01 |
| Positive Affect | ||||||||
| PAC | 0.100 (0.011) | 0.087 (0.008) | 1.29 | 0.255 | 0.12 | 0.12 | −0.18 | 0.45 |
| MSSD | 60.0 (4.7) | 62.7 (6.3) | 0.21 | 0.646 | 0.04 | 0.04 | −0.30 | 0.21 |
Estimated Cohen’s d and estimated Hedges’ g were calculated using estimated marginal means and standard errors.
Abbreviations: PAC = probability of acute change; MSSD = mean squared successive difference; EMM = estimated marginal mean; SE = standard error; 99% CI = 99% confidence interval for effect size, as our a priori alpha was set at 0.01.
Figure 3. Negative Affect Instability Before and After Bulimic Episodes.
A) The likelihood of an extreme increase in negative affect was reduced after bulimic behaviors. B) In contrast, average negative affect instability (MSSD) was greater after than before bulimic behaviors. Error bars represent standard error of the mean. Abbreviations: NA = negative affect; PA = positive affect; PAC = probability of acute change; MSSD = mean squared successive difference. **p < 0.005, ***p < 0.001
4. Discussion
The present study is the first, to our knowledge, to investigate NA and PA instability as they relate, naturalistically and in real time, to binge eating and purging behaviors among women with BN. Furthermore, this study is the first to examine two different metrics of affective instability in relation to bulimic behavior. There was a basic association, with a consistently small to moderate effect size, between our measure of acute increases in NA (i.e., NA PAC) and the presence of bulimic behavior. For example, NA PAC was higher on bulimic behavior days than on non-bulimic behavior days and was marginally higher in the time intervals before and after bulimic behaviors than in comparative time intervals on non-bulimic behavior days. Importantly, however, in comparisons of time intervals within the same day and within subjects (i.e., before and after bulimic behavior behavior), acute NA increases were less likely after than before bulimic behavior. In contrast, acute PA changes were generally less strongly associated with bulimic behavior. Although acute PA increases were more likely following bulimic behaviors than in time-matched intervals on non-bulimic behavior days, there was not a corresponding within-person, within-day acute PA increase after bulimic episodes compared with the time intervals before the episodes.
Analyses of average affective instability (i.e., MSSD) paralleled many of the PAC findings. Consistent with results of prior analyses of this dataset using each individual’s within-person standard deviation around their daily mean NA rating (Selby et al., 2012), we found that participants reported greater average NA instability, as measured by MSSD, on days during which bulimic behavior occurred. In addition, average NA during time intervals after bulimic behaviors was more unstable than during time-matched intervals on non-bulimic behavior days. However, in contrast to NA PAC within-day findings, and contrary to our hypotheses, average NA instability (MSSD) increased after bulimic behaviors. Consistent with within-day PA PAC results, within-day PA MSSD analyses failed to reveal significant shifts in average PA instability after bulimic behaviors.
Overall, results of PAC and MSSD analyses support a role for NA instability in reinforcement processes related to bulimic behavior. Our findings suggest that bulimic behavior may serve to reduce acute emotional shifts but not average emotional instability. Prior findings indicate that a trajectory of increasing NA intensity precipitates bulimic behaviors (Alpers and Tuschen-Caffier, 2001; Berg et al., 2013; Hilbert and Tuschen-Caffier, 2007; Smyth et al., 2007), and that post-behavior NA is lower than pre-behavior NA in absolute magnitude (Lavender et al., 2016). Our results indicate that the likelihood of extreme exacerbations of NA decreases after bulimic episodes, but that average NA instability increases after bulimic episodes. Therefore, although binge eating and purging may be negatively reinforced by a post-behavior downregulation of NA intensity and reduced likelihood of dramatic NA increases, the behavior ultimately may worsen average NA instability, potentially precipitating subsequent maladaptive behaviors.
In general, findings from EMA studies of PA in BN suggest a trajectory of decreasing PA preceding bulimic behavior behaviors, but our results do not indicate that PA instability precipitates these behaviors. Prior results from this dataset suggest that the magnitude of PA intensity increases after binge eating and purging, which may positively reinforce these behaviors (e.g., Smyth et al., 2007). However, our findings provide limited support for a role of PA instability in bulimic behavior reinforcement. Extreme increases in PA after bulimic episodes were more likely when compared with matched time intervals on non-bulimic behavior days, but this pattern was not upheld in within-day analyses comparing extreme or average shifts in PA before and after bulimic behavior. Consequently, further research is needed to clarify the role of PA and PA instability in precipitating or reinforcing BN behaviors.
4.1. Limitations
The current study is limited in a number of ways. First, our analyses account for the temporal order of affective instability and eating disorder behavior, but they do not address causality. Second, the nature of the sample may limit the generalizability of findings to other ethnicities or males. Third, although participants were trained to identify “objectively large amounts of food,” recorded “binge eating” may have included both objectively and subjectively large episodes. Finally, because affective instability metrics summarize multiple affect ratings within a timeframe, we lacked sufficient data to conduct analyses separately for binge-only, purge-only, and combined bulimic behavior events and days. Exploratory analyses suggested there were no differences in affective instability on days with only binge eating compared to days with only purging; however, EMA studies with longer data collection periods are needed to permit well-powered examination of PAC and MSSD surrounding isolated and combined bulimic behaviors. This is an important area for future research that will critically inform efforts to develop and refine treatments specifically tailored for each of these behaviors.
4.2. Clinical implications
There are several potential theoretical and treatment-related implications of our results. Consistent with affect regulation models of BN and binge eating (Hawkins and Clement, 1984; Heatherton and Baumeister, 1991; Hohlstein et al., 1998), our findings suggest that reduced likelihood of extreme increases in NA (i.e., PAC) could function to provide short-term reinforcement of bulimic behaviors; however, our findings also suggest that these behaviors are ineffective in terms of promoting ongoing emotional stability (i.e., MSSD). BN is associated with a preference for smaller-sooner rewards (Kekic et al., 2016), and recurrent binge eating and purging despite significant longer-term negative repercussions may reflect alterations in reward-based learning in BN (Berner and Marsh, 2014; Frank et al., 2011). Individuals with BN also may overvalue the immediate rewards of reduced NA intensity and reduced likelihood of an extreme NA increase after bulimic behaviors, while undervaluing the negative outcome of increased average NA instability. This may promote the development of an entrenched pattern of responding to emotionally dysregulated states by engaging in maladaptive behaviors that ultimately exacerbate emotional dysregulation.
With regard to the treatment of BN, the present findings suggest that NA and PA instability could represent useful targets for novel interventions or treatment adjuncts, particularly via an increased focus on maintaining a steady affective state and reducing the likelihood of extreme increases in NA (e.g., by encouraging use of therapeutic skills earlier in the emotion generation process or, in severe cases, adding a mood stabilizing medication; Trunko et al., 2014). If post-behavior affective instability drives the recurrence of binge eating and purging, a specific focus on implementing skills during the post-behavior time period could be particularly helpful.
4.3. Conclusion
Our results build upon the existing emotion-focused literature in BN. Previous findings indicate that BN is associated with increased affective instability relative to psychiatrically healthy individuals (Santangelo et al., 2014) but our results are the first to suggest that this instability may play a direct role in bulimic symptoms. Whereas prior analyses of this dataset have examined affective instability by using only between-day analyses (Selby et al., 2012) or examined the relationship between NA MSSD and binge eating and purging frequency over a full two-week period (Anestis et al., 2010), we examined affective instability in (a) between-day analyses, (b) between-day, within-person analyses, and (c) within-person, within-day analyses that compared the degree of affective instability before and after bulimic behavior behaviors. This analytic approach permitted comprehensive examination of how instability across affective valence may precipitate or reinforce bulimic behaviors. Most existing studies of affect and emotion dysregulation in eating disorders have focused on NA, but our findings add to those in anorexia nervosa (Selby et al., 2015) and suggest that both NA and PA instability may be related to eating disorder behaviors across diagnoses. Further research is needed to better understand the directionality of extreme changes in NA and PA in BN. In addition, future studies should investigate whether affective instability is similarly temporally associated with other maladaptive behaviors in BN (e.g., dietary restriction, excessive/driven exercise, substance use).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpers GW, Tuschen-Caffier B. Negative feelings and the desire to eat in bulimia nervosa. Eating Behaviors. 2001;2(4):339–352. doi: 10.1016/s1471-0153(01)00040-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fouth Edition (DSM-IV) American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition (DSM-5) American Psychiatric Association; Washington. D.C: 2013. [Google Scholar]
- Anestis MD, Selby EA, Crosby RD, Wonderlich SA, Engel SG, Joiner TE. A comparison of retrospective self-report versus ecological momentary assessment measures of affective lability in the examination of its relationship with bulimic symptomatology. Behaviour Research and Therapy. 2010;48(7):607–613. doi: 10.1016/j.brat.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Crosby RD, Cao L, Crow SJ, Engel SG, Wonderlich SA, Peterson CB. Negative affect prior to and following overeating-only, loss of control eating-only, and binge eating episodes in obese adults. International Journal of Eating Disorders. 2015 doi: 10.1002/eat.22401. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Crosby RD, Cao L, Peterson CB, Engel SG, Mitchell JE, Wonderlich SA. Facets of negative affect prior to and following binge-only, purge-only, and binge/purge events in women with bulimia nervosa. Journal of Abnormal Psychology. 2013;122(1):111–118. doi: 10.1037/a0029703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner LA, Marsh R. Frontostriatal circuits and the development of bulimia nervosa. Front Behav Neurosci. 2014;8:395. doi: 10.3389/fnbeh.2014.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohon C, Stice E, Burton E. Maintenance factors for persistence of bulimic pathology: A prospective natural history study. International Journal of Eating Disorders. 2009;42(2):173–178. doi: 10.1002/eat.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl JR, Soskin DP, Kerns C, Barlow DH. Positive emotion regulation in emotional disorders: A theoretical review. Clinical Psychology Review. 2013;33(3):343–360. doi: 10.1016/j.cpr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Combs JL, Smith GT, Simmons JR. Distinctions between two expectancies in the prediction of maladaptive eating behavior. Personality and Individual Differences. 2011;50(1):25–30. doi: 10.1016/j.paid.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Trull TJ. Ecological momentary assessment of mood disorders and mood dysregulation. Psychological assessment. 2009;21(4):463. doi: 10.1037/a0017075. [DOI] [PubMed] [Google Scholar]
- Engel SG, Boseck JJ, Crosby RD, Wonderlich SA, Mitchell JE, Smyth J, Miltenberger R, Steiger H. The relationship of momentary anger and impulsivity to bulimic behavior. Behaviour Research and Therapy. 2007;45(3):437–447. doi: 10.1016/j.brat.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Engel SG, Wonderlich SA, Crosby RD, Mitchell JE, Crow S, Peterson CB, Le Grange D, Simonich HK, Cao L, Lavender JM, Gordon KH. The role of affect in the maintenance of anorexia nervosa: Evidence from a naturalistic assessment of momentary behaviors and emotion. Journal of Abnormal Psychology. 2013;122(3):709–719. doi: 10.1037/a0034010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1997. Structured Clinical Interview for DSM-IV Axis I Disorders: Research version. [Google Scholar]
- Frank GKW, Reynolds JR, Shott ME, O’Reilly RC. Altered temporal difference learning in bulimia nervosa. Biological Psychiatry. 2011;70(8):728–735. doi: 10.1016/j.biopsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa-Schechtman E, Avnon L, Zubery E, Jeczmien P. Emotional processing in eating disorders: specific impairment or general distress related deficiency? Depression and Anxiety. 2006;23(6):331–339. doi: 10.1002/da.20163. [DOI] [PubMed] [Google Scholar]
- Haedt-Matt AA, Keel PK. Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychological Bulletin. 2011;137(4):660–681. doi: 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, Sullivan S, Tchanturia K, Treasure J. Emotional functioning in eating disorders: attentional bias, emotion recognition and emotion regulation. Psychological Medicine. 2010;40(11):1887–1897. doi: 10.1017/S0033291710000036. [DOI] [PubMed] [Google Scholar]
- Hawkins RC, Clement PF. Binge eating: Measurement problems and a conceptual model. In: Hawkins RC, Fremouw WJ, Clement PF, editors. The binge purge syndrome: Diagnosis, treatment, and research. Springer; New York: 1984. pp. 229–251. [Google Scholar]
- Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psychol Bull. 1991;110(1):86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Tuschen-Caffier B. Maintenance of binge eating through negative mood: A naturalistic comparison of binge eating disorder and bulimia nervosa. International Journal of Eating Disorders. 2007;40(6):521–530. doi: 10.1002/eat.20401. [DOI] [PubMed] [Google Scholar]
- Hohlstein LA, Smith GT, Atlas JG. An application of expectancy theory to eating disorders: Development and validation of measures of eating and dieting expectancies. Psychological Assessment. 1998;10(1):49. [Google Scholar]
- Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: Indices using successive difference and group comparison via multilevel modeling. Psychological Methods. 2008;13(4):354–375. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- Kekic M, Bartholdy S, Cheng J, McClelland J, Boysen E, Musiat P, O’Daly OG, Campbell IC, Schmidt U. Increased temporal discounting in bulimia nervosa. International Journal of Eating Disorders. 2016;49(12):1077–1081. doi: 10.1002/eat.22571. [DOI] [PubMed] [Google Scholar]
- Lavender JM, Utzinger LM, Cao L, Wonderlich SA, Engel SG, Mitchell JE, Crosby RD. Reciprocal associations between negative affect, binge eating, and purging in the natural environment in women with bulimia nervosa. Journal of abnormal psychology. 2016;125(3):381. doi: 10.1037/abn0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender JM, Wonderlich SA, Engel SG, Gordon KH, Kaye WH, Mitchell JE. Dimensions of emotion dysregulation in anorexia nervosa and bulimia nervosa: A conceptual review of the empirical literature. Clinical Psychology Review. 2015;40:111–122. doi: 10.1016/j.cpr.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender JM, Wonderlich SA, Peterson CB, Crosby RD, Engel SG, Mitchell JE, Crow SJ, Smith TL, Klein MH, Goldschmidt AB, Berg KC. Dimensions of emotion dysregulation in bulimia nervosa. European Eating Disorders Review. 2014;22(3):212–216. doi: 10.1002/erv.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Horga G, Wang Z, Wang P, Klahr KW, Berner LA, Walsh BT, Peterson BS. An fMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. American Journal of Psychiatry. 2011;168(11):1210–1220. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Stefan M, Bansal R, Hao X, Walsh BT, Peterson BS. Anatomical characteristics of the cerebral surface in bulimia nervosa. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Steinglass JE, Gerber AJ, O’Leary KG, Walsh BT, Peterson BS. Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Archives of General Psychiatry. 2009;66(1):1–13. doi: 10.1001/archgenpsychiatry.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond JM, Latner JD, Hay PH, Owen C, Rodgers B. Objective and subjective bulimic episodes in the classification of bulimic-type eating disorders: Another nail in the coffin of a problematic distinction. Behaviour Research and Therapy. 2010;48(7):661–669. doi: 10.1016/j.brat.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Pearson CM, Wonderlich SA, Smith GT. A risk and maintenance model for bulimia nervosa: From impulsive action to compulsive behavior. Psychological Review. 2015;122(3):516–535. doi: 10.1037/a0039268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo P, Reinhard I, Mussgay L, Steil R, Sawitzki G, Klein C, Trull TJ, Bohus M, Ebner-Priemer UW. Specificity of affective instability in patients with borderline personality disorder compared to posttraumatic stress disorder, bulimia nervosa, and healthy controls. Journal of Abnormal Psychology. 2014;123(1):258–272. doi: 10.1037/a0035619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby EA, Cornelius T, Fehling K, Kranzler A, Panza EA, Lavender J, Wonderlich S, Crosby R, Engel S, Mitchell J, Crow S, Peterson C, Le Grange D. A perfect storm: examining the synergistic effects of negative and positive emotional instability on promoting weight loss activities in anorexia nervosa. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby EA, Doyle P, Crosby RD, Wonderlich SA, Engel SG, Mitchell JD, Le Grange D. Momentary emotion surrounding bulimic behaviors in women with bulimia nervosa and borderline personality disorder. Journal of Psychiatric Research. 2012;46(11):1492–1500. doi: 10.1016/j.jpsychires.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, Elliott C, Wolkoff LE, Columbo KM, Ranzenhofer LM, Roza CA, Yanovski SZ, Yanovski JA. Salience of loss of control for pediatric binge episodes: Does size really matter? International Journal of Eating Disorders. 2010;43(8):707–716. doi: 10.1002/eat.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, Wonderlich SA, Heron KE, Sliwinski MJ, Crosby RD, Mitchell JE, Engel SG. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. Journal of Consulting and Clinical Psychology. 2007;75(4):629–638. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- Snir A, Bar-Kalifa E, Berenson KR, Downey G, Rafaeli E. Affective Instability as a Clinical Feature of Avoidant Personality Disorder. 2016 doi: 10.1037/per0000202. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Griepenstroh J, Tuschen-Caffier B, Ehring T. Emotion regulation deficits in eating disorders: A marker of eating pathology or general psychopathology? Psychiatry Research. 2012;197(1–2):103–111. doi: 10.1016/j.psychres.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Solhan MB, Tragesser SL, Jahng S, Wood PK, Piasecki TM, Watson D. Affective instability: measuring a core feature of borderline personality disorder with ecological momentary assessment. J Abnorm Psychol. 2008;117(3):647–661. doi: 10.1037/a0012532. [DOI] [PubMed] [Google Scholar]
- Trunko ME, Schwartz TA, Marzola E, Klein AS, Kaye WH. Lamotrigine use in patients with binge eating and purging, significant affect dysregulation, and poor impulse control. International Journal of Eating Disorders. 2014;47(3):329–334. doi: 10.1002/eat.22234. [DOI] [PubMed] [Google Scholar]
- Vannucci A, Theim KR, Kass AE, Trockel M, Genkin B, Rizk M, Weisman H, Bailey JO, Sinton MM, Aspen V, Wilfley DE, Taylor CB. What constitutes clinically significant binge eating? Association between binge features and clinical validators in college-age women. International Journal of Eating Disorders. 2013;46(3):226–232. doi: 10.1002/eat.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule--Expanded Form. The University of Iowa; 1994. [Google Scholar]
- Wolfe BE, Baker CW, Smith AT, Kelly-Weeder S. Validity and utility of the current definition of binge eating. International Journal of Eating Disorders. 2009;42(8):674–686. doi: 10.1002/eat.20728. [DOI] [PubMed] [Google Scholar]
- Wonderlich SA, Lavender JM, Crosby RD, Engel SG, Mitchell JE, Peterson C, Crow S, Le Grange D, Berner LA. The unique contributions of positive and negative affect to eating disorder behaviors; annual meeting of the Eating Disorders Research Society; San Diego. Oct, 2014. [Google Scholar]
- Wonderlich SA, Mitchell JE. Eating disorders and comorbidity: Empirical, conceptual, and clinical implications. Psychopharmacology Bulletin. 1997;33(3):381–390. [PubMed] [Google Scholar]
- Wu M, Hartmann M, Skunde M, Herzog W, Friederich H-C. Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PLoS ONE. 2013;8(12):e83412. doi: 10.1371/journal.pone.0083412. [DOI] [PMC free article] [PubMed] [Google Scholar]