Abstract
The research of mitochondrial dysfunction is of great importance and implicated in a range of neurodegenerative diseases. Traditionally, to investigate mitochondrial dynamics and functions, mitochondria are usually stimulated indirectly by treating cells with exogenous chemicals like oxidative agents. Such treatment lacks precision and controllability, and will simultaneously activate unknown complex cell processes. In this study, we report that two-photon 100-μs line scan by a femtosecond laser can induce restorable fragmentation or swelling of any targeted mitochondria instead of ablation or disruption. It can be defined by a customized two-photon line scan and inserted into any microscopy sequence as a single frame. The mitochondrial response is dependent on the peak power of laser pulses, cellular oxidative environment, and membrane permeability transition pores of mitochondria. The translocation of cytochrome C and Bax can be regulated by the photostimulation. Moreover, significant upregulation of Bcl-2 can be observed if the whole cell is stimulated. Those results suggest the mitochondrial and molecular response to photostimulation is quite complex. This femtosecond-laser stimulation method can thus provide a very noninvasive, precise, and controllable method to stimulate single target mitochondria for related biological research.
OCIS codes: (170.1530) Cell analysis, (170.7160) Ultrafast technology, (180.4315) Nonlinear microscopy
1. Introduction
Mitochondria are essential organelles for cell life, known as key roles in metabolism, respiration, and regulation of cell signaling pathways [1–3]. A lot of molecular activities in mitochondria are involved in those processes, accompanied with complex signaling transduction to maintain cell functions and life [4]. In particular, mitochondrial dysfunction in neuron may induce serious diseases such as Parkinson’s disease [5–7]. Great progresses on understanding the mechanism of mitochondria in those diseases at cellular and molecular level have been made in the last decades by classic biochemical methods. Generally cells were treated with various drugs/chemicals or the genes and proteins were manipulated by advanced gene techniques to access the information of mitochondrial processes and functions [8–11]. For example, caged calcium compound treatment is able to induce reactive oxygen species (ROS) production, mitochondrial permeability transition pore (mPTP) opening, and eventual death in PINK1-dificient neurons through calcium overloading for cell model of Parkinson’s disease [12]. But research at the organelle level are more difficult to further probably because the biochemical methods cannot specifically stimulate single mitochondria without interfering with other subcellular elements or cell processes. For example, adding oxidative agent into cell buffer can stimulate mitochondria to generate superoxide flashes but cells will develop to the early stage of apoptosis [13]. Hence the chemical stimulation is lack of spatial and temporal precision and may simultaneously activate unknown complex cell processes. In this regard, it is challenging to stimulate mitochondria precisely in live target cells for mitochondrial research.
The tightly-focused femtosecond-laser beam has been shown with great capability working as a precise, clean, and noninvasive “lancet” for cell surgery at micron level [14–17]. The optical axotomy on single neuron provides new methods and insights for regeneration of neurons [18-19]. Furthermore, by optical breakdown effect, subcellular structures, including single mitochondria, cell skeletons, and some nuclear structures, can be ablated directly with little damage to other parts of the cell [20–24]. Nevertheless, such kind of single-organelle disruption/ablation can hardly provide biological information, such as molecular dynamics, physiological status, and some other responses of the disrupted organelle to extra stimulation, for related research. Recently, we showed that the long-duration (0.1-s level) exposure of mitochondria to tightly-focused femtosecond laser that was coupled directly into the objective could induce their fragmentation with complex physiological dynamics rather than disruption of them [25, 26]. In this study, we present that 100-μs stimulation by a line scan of femtosecond laser can induce mitochondrial deformation and series of molecular dynamics in live cells. The stimulation can be defined by a customized two-photon line scan and inserted into any microscopy sequence as a single frame without any extra beam shaping or pulse modulation. We propose that this short-duration femtosecond-laser stimulation that can be easily accomplished at any two-photon microscope system is of great potential for mitochondrial research at single-organelle level. The mechanism of those complex responses of mitochondria under weak laser stimulations also needs further investigations.
2. Methods and materials
2.1 Cell culture and materials
HeLa cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and incubated at 37 °C with 5% CO2. For photostimulation experiments, cells will be seeded on petri dishes with glass slides (0.17-mm thick) on the bottom. In an experiment, three dishes of cells were used. The first dish was a control. Cells were randomly selected from the other two dishes and mitochondria were stimulated. If the mitochondrial response from three dishes were generally consistent, the data in the latter two dishes was used. Otherwise all three dishes of cells would be abandoned.
The tetramethylrhodamine, methyl ester, perchlorate (TMRM) for indication of the mitochondrial membrane potential, and tert-Butyl hydroperoxide (TBHP) were purchased from Life Technology, N-acetyl-L-cysteine (NAC) from Beyotime, and Cyclosporin A (CsA) from TCI Shanghai, respectively. All materials were used following the protocols given by suppliers.
2.2 Transfections
Plasmids of enhanced green fluorescent protein (GFP), and enhanced mitochondrial GFP (mitoGFP) that can combined solely with mitochondria were transfected using 8 μL Cell Light Reagent BacMam 2.0 of Life Technology (C10508) per dish (0.5 mL) and incubated at 37 °C for 16 hours before experiments. The validity of enhanced mitochondrial GFP localization was tested and verified by co-staining cells with MitoTrackerRed and TMRM (Life Technology).
2.3 Immunofluorescence confocal microscopy
HeLa cells were at first cultured in petri dishes (ibidi) with grid (numeric coordinates) on the bottom glass slides (0.17-mm thick). Immediately after laser treatment, cells were quickly fixed for 10 min with a precooled mixture of methanol and acetone (v/v = 7:3) at room temperature. The specimen was then washed with PBS for 5 min and blocked with a 5% defatted milk for 30 min at 37 °C. Next, the sample was added with diluted anti-cytochrome c monoclonal antibody (Novex), mouse anti-Bax antibody (Novex), and anti-Bcl-2 monoclonal antibody (Abcam, ab692, with secondary antibody, anti-mouse DyLight 488 polyclonal antibody ab96879), respectively. After the overnight incubation in 4 °C environment, the sample was washed with PBS for 5 min and incubated with diluted secondary antibody 488 donkey anti-mouse IgG (H + L) (Molecular Probes) for one hour at room temperature. The stimulated cells would be located by the grids on the bottom and observed by confocal microscopy.
2.4 Confocal microscopy and femtosecond-laser stimulation
The whole system was set on a two-photon confocal microscope (Nikon, MP A1+) coupled with a Ti:Sapphire femtosecond laser (80 MHz, MaiTai DeepSee, Spectra-Physics). The wavelength of the Ti:Sapphire laser was tunable from 700 nm to 1000 nm. The laser was focused by a water-immersed 40 × objective with the numerical aperture of 1.0.
For confocal scanning, all green dyes or GFP were excited by a 488-nm laser and the red ones by a 561-nm laser. The green fluorescence was detected with a bandpass filter (500-540 nm) and the red with also another filter (600-640 nm). To minimize photobleaching, the power of 488-nm laser was set at around 0.1 mW, and the 561-nm laser at 0.2 mW at the output (laser powers at specimen can be estimated by multiplying the transmission efficiency of around 20% of the microscope system). Images were acquired at a speed of one frame (512 × 512 pixels)/1.1 s.
The femtosecond-laser stimulation was delivered by a self-defined line scan of the femtosecond laser at the targeted mitochondrion, as demonstrated in Fig. 1(a). The line was defined by a scanning across the mitochondrial tubule transversely with no overlapping with other mitochondria to ensure only a single mitochondrial tubule stimulated. In this study, only one mitochondrial tubule was selected from one single cell, i.e., the cells were stimulated for only one time. To take the real-time microscopy, the stimulation was set as a single frame of two-photon microscopy and inserted into the continuous confocal microscopy sequence. After setting the scanning line, the microscopy sequence would be started. In this case, immediately before and after the stimulation, confocal microscopy was running to record images continuously. During the microscopy and photostimulation, there was no observable mitochondrial movement.
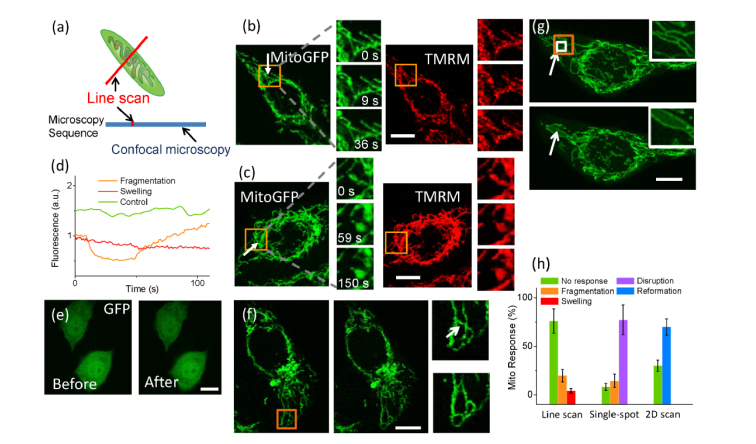
Fig. 1.
Fragmentation and swelling were found in photostimulated mitochondria. (a) The two-photon line scan scheme to a single mitochondrion. Red line: the scanning line across the mitochondrial tubule. Blue line: the microscopy sequence. The stimulation (red) can be inserted into the confocal microscopy sequence to provide a continuous observation. (b) Fragmentation of the mitochondrial tubule structure was found immediately after the phototimulation at the 9th s. At the 36th s, recovery of the fragmented mitochondrion was observed. (c) Swollen mitochondria were induced by chance and could not recover. (d) The MMP of the stimulated mitochondrion during fragmentation and recovery. (e) No significant photobleaching of GFP was found by continuous two-photon scanning on cells. (f) Femtosecond-laser stimulation in a 1.5-μm spot at 30 mW disrupted the mitochondria inside. (g) Two-photon scanning in a microregion (3 × 3 μm2) induced complex changes of several mitochondria nearby. Arrow: photostimulation events. (h) Mitochondrial responses to different photostimulations with different scanning types in (b,c), (f), and (g) respectively. White box: 2D scanning. Orange box: the area magnified in the right. Bar: 10 μm.
2.5 Statistics
After photostimulation, all the mitochondria were observed for 200 s, and if there was no morphological change, they would be counted as “no response”. All fragmented mitochondria showed recovery. Otherwise the un-recoverable mitochondria would be defined as “swelling”. The fragmentation duration of stimulated mitochondria was counted in this way: the period from the photostimulation to the moment when the mitochondria showed most significant fragmentation. Then the recovery duration was recorded from that moment to the first significant recovery. The data were presented as mean ± standard error of mean (S.E.M.) and analyzed with GraphPad Prism 7 software throught the one-tailed paired t test except where stated otherwise.
3. Results
Mitochondria in HeLa cells were at first genetically labelled by mitoGFP ensuring stable fluorescent observation of them. For experiments, cells can be co-labeled with other fluorophores with different fluorescent spectrums.
3.1 Femtosecond-laser stimulation induces mitochondrial fragmentation and swelling
HeLa cells were at first observed by confocal microscopy and single mitochondria were selected randomly. A two-photon line scanning by the femtosecond laser was manually set across the target. We defined 64 pixels in the line as shown in Fig. 1(a), where around 32 pixels were occupied by the mitochondrial tubule. The scan duration inside the mitochondrial tubule would take around 120 μs (4.2 μs/pixel). To monitor the mitochondrial dynamics at real time, the confocal scanning was running continuously while the femtosecond-laser stimulation was set as a single frame and inserted into the scanning sequence at a defined time slot as in Fig. 1(a). The confocal microscopy would stop and be switched to two-photon line scan at that time, and then back to confocal microscopy automatically. The femtosecond laser power was 16 mW at the specimen and the wavelength was 800 nm. After the femtosecond-laser stimulation, the targeted mitochondria were not disrupted. Instead, the major part (76.1% ± 12.5%, n = 50 mitochondria in 3 independent experiments) of stimulated mitochondria showed no morphological change (after photostimulation, all mitochondria were observed for 200 s). Some mitochondria showed fragmentation at the scanning site (19.8% ± 6.5%, n = 50 mitochondria in 3 independent experiments). But soon after tens of seconds, the fragmented mitochondria would recover as shown in Fig. 1(b). The rest of stimulated mitochondria turned swollen and could not be observed with any recovery (4.1% ± 2.4%, n = 50 mitochondria), as shown in Fig. 1(c). It should be noted that in the fragmented mitochondria, the mitochondrial membrane potential (MMP) also recovered along with the morphology (as shown in Fig. 1(d)). During this process, there was no observable mitochondrial movement. We tested if disappearance of mitoGFP or TMRM fluorescence in the fragmentation was resulted from photobleaching by the single-time two-photon line scan. To this purpose, cells transfected with GFP were scanned at 16 mW continuously for 10 frames at 4.2 μs/pixel. The GFP fluorescence was 93.3 ± 4.5% of the original (n = 65 cells in three trials). Hence no GFP photobleaching was found as shown in Fig. 1(e).
We decreased the pixels of the line to 10 and length to 1.5 μm which was approximately a single-spot stimulation with only 40 μs. In this case, no mitochondria showed any change after the stimulation (n = 30 mitochondria). If the laser power was increased to 30 mW, disruption of the targeted mitochondria could be observed as shown in Fig. 1(f) (77.4% ± 15.4%, n = 40 mitochondria in 3 trials). If expanding the line scan to a 2D area scan, the spatial resolution was decreased. We defined an area of 3 × 3 μm2 with 32 × 32 pixels for two-photon scanning at 16 mW. As shown in Fig. 1(g), some mitochondria inside or near the scanning area would be influenced with quite unpredictable and complex changes (70.0% ± 8.3%, n = 60 mitochondria in 3 trials). Several tubular structures of mitochondria in an area (around 6 × 6 μm2) that was much larger than the scanning area could be found with new formations after the 2D area stimulation. Fragmentations and swellings could be also found in some mitochondria inside the area. The different mitochondrial responses induced by those different photostimulations were summarized in Fig. 1(h). According to those results, the photostimulation by two-photon line scan had higher spatial resolution and was able to bring more information of mitochondrial changes.
3.2 Key factors that affect mitochondrial changes
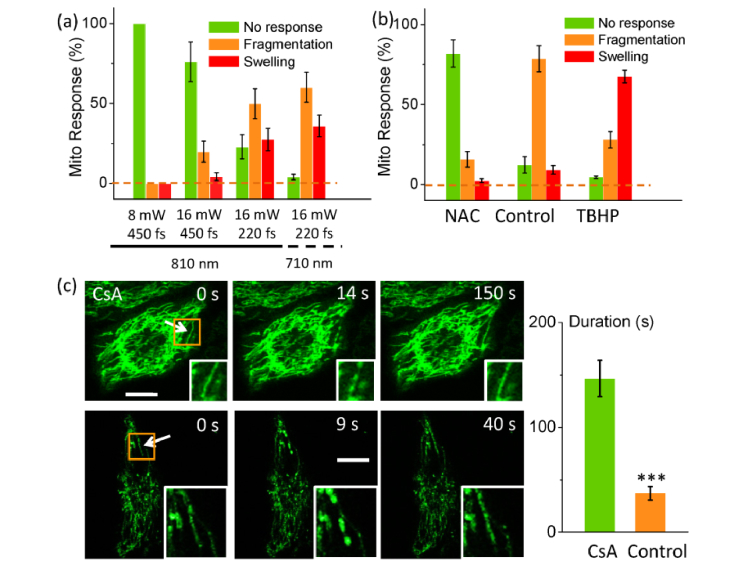
The parameters of the femtosecond laser influenced the mitochondrial response a lot. As shown in Fig. 2(a), at 8 mW (repetition rate: 80 MHz, 450 fs), the femtosecond-laser stimulation could not induce any change of mitochondria. Only when the laser power was increased to 16 mW, some stimulated mitochondria turned fragmented (19.8% ± 6.5%) or swollen (4.1% ± 2.4%). It should be noted that if the pulse width was compressed from 450 fs to 220 fs (measured at the specimen), the rate of fragmented (49.7% ± 9.5%, n = 50 mitochondria) and swollen (22.8% ± 7.6%, n = 50 mitochondria) mitochondria increased dramatically. This result then implied the peak power of femtosecond laser pulses, which generally determines the multi-photon excitation efficiency, contributed more to the mitochondrial fragmentation and swelling. Moreover, if the wavelength was tuned to 710 nm, the fragmentation rate could increase to 60.2% ± 9.5% and swelling to 35.9% ± 6.8% because the two-photon excitation at 710 nm could provide higher photon energy with more photodamage. All stimulated cells were tested by propidium iodide (PI) for the integrity of cell membrane six hours after photostimulation. No PI fluorescence was found in them. All cells showed good membrane integrity. In general, the shorter pulse width and higher photon energy could generate higher multiphoton excitation efficiency and photon energy.
Fig. 2.
Mitochondrial response to photostimulation was dependent on the peak power and wavelength of laser pulses (a), the cellular oxidative environment (b), and mPTP (c).The inhibition of mPTP by CsA greatly prolonged the recovery duration of fragmented mitochondria as in (c). Arrow: the position of femtosecond-laser stimulation. Bar: 10 μm. *** P<0.0005.
Since the thermal effect of femtosecond laser with shorter wavelength (at 700-1000 nm range) and pulse width is lower, according to the data above, it can be deduced that the multi-photon excitation played the major role in photostimulation rather than the thermal effect. According to previous studies, the femtosecond laser excitation can generate oxidative stress to the cell and mitochondria [27–29]. In this regard, we then tested if the oxidative environment influenced mitochondrial responses to the laser stimulation. NAC, a scavenger of cellular reactive oxygen species (ROS), was at first added into cell buffer. After 30-minute incubation, mitochondria were again selected and stimulated at 20 mW (810 nm, 220 fs). Compared with the control, much more mitochondria did not response to the photostimulation (Fig. 2(b), n = 50 mitochondria in 3 independent experiments in each group). In contrast, if the cells were treated with TBHP, which can enter cytosol to provide high oxidative stress to the whole cell, the rate of swollen mitochondria increased to 67.6%. Hence the mitochondrial response was also dependent on the oxidative level of the cell.
Considering ROS were mainly generated in mitochondria and then released into cytosol, the mitochondrial permeability transition pores (mPTP) were investigated if with contribution to fragmentation or swelling of the stimulated mitochondria (at 16 mW, 220 fs). CsA was then used to inhibit the mPTP opening. As shown in Fig. 2(c), with the presence of CsA, the rate of fragmented (46%) and swollen (35%) mitochondria did not show significant change. But the recovery duration of fragmented mitochondria was prolonged to 146.6 ± 17.3 s while it only needed 37.2 ± 6.4 s in the control (significantly different). Hence the opening of mPTP was involved in mitochondrial recovery.
3.3 Molecular changes of stimulated mitochondria
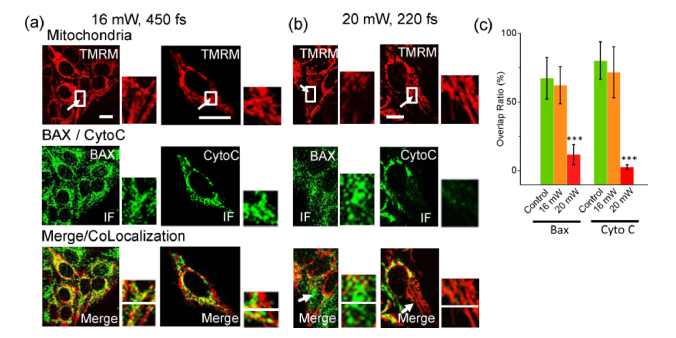
The photostimulated fragmentation and swelling of mitochondria might be accompanied with complex molecular dynamics. In this study, location of proapoptotic molecules Bax and cytochrome C (Cyto C) were carefully monitored before and after the photostimulation. In control experiments, mitochondria in the cells with no treatment were labeled and the immunofluorescence (IF) microscopy of Bax and Cyto C was then performed. The overlap ratio of the IF of Bax and Cyto C respectively with the mitochondrial fluorescence was then used to define the translocation of those two molecules. Theoretically there would be no molecular translocation of Bax or Cyto C and the overlap ratio should be 100%. We calculated the overlapping pixels from Bax/Cyto C and mitochondrial images in a line (64 pixels along the photostimulation as shown in Fig. 3(a)). In control tests, the overlap ratio of Bax and mitochondria (labeled by TMRM) was 67.4% ± 15.1%, and it of Cyto C and mitochondria was 80.2% ± 13.6% (n = 40 mitochondria in both measurements). In photostimulation experiments, the mitochondria were at first only labeled with TMRM and stimulated at 16 mW (450 fs). In each targeted cell, only one mitochondrial tubule was selected and stimulated for only one time. After stimulation, the MMP was soon recovered. The cells were fixed immediately. As shown in Fig. 3(a), the IF microscopy of Bax and Cyto C was then performed respectively. By colocalization of the TMRM fluorescence and IF of Bax and Cyto C, no significant translocation of those two molecules was found (The overlap ratio = 62.3% ± 13.5%, 71.7% ± 18.6% for Bax and Cyto C respectively; n = 20 mitochondria in each group). In contrast, if the mitochondria were stimulated at 20 mW with 220-fs pulse width, the MMP of mitochondria near the two-photon scanning line was depolarized. As in Fig. 3(b) the aggregation of Bax and release of Cyto C in the stimulated mitochondria could be observed (The overlap ratio = 11.8% ± 7.4%, 2.7% ± 1.7% for Bax and Cyto C respectively; n = 30 mitochondria in each group), suggesting the initiation of programmed cell death. Therefore, the translocation of Bax and Cyto C could be controlled by different photostimulation parameters (Fig. 3(c)).
Fig. 3.
Translocation of Bax and Cyto C by different photostimulations. (a) Bax did not translocate at 16-mW stimulation (n = 30). No release of Cyto C from the stimulated mitochondrion was found (n = 30). (b) Bax concentrated to the stimulated mitochondria (n = 30) and Cyto C release (n = 30) was found at 20-mW stimulation. IF: immunofluorescence microscopy, performed 20 minutes after photostimulation. Arrow: the position of femtosecond-laser stimulation. Bar: 10 μm. (c) The Co-Localization rate of Bax and Cyto C calculated by the number of overlapping pixels in the crossing line in the zoom-in views (n = 30). *** P<0.0005.
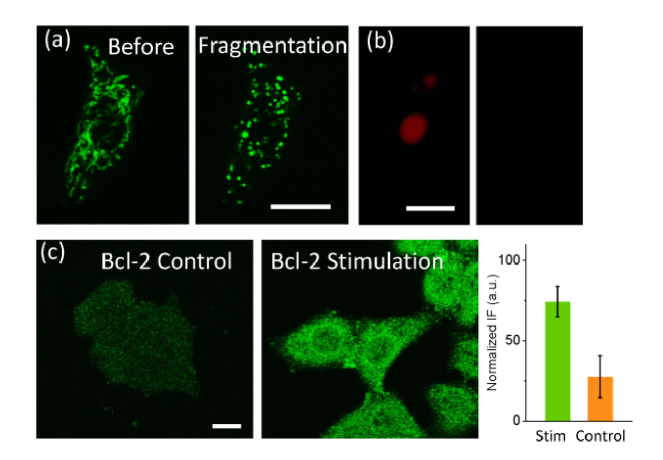
The initiation of apoptosis will simultaneously induce expression of anti-apoptosis molecules, the Bcl-2 family [30]. In this regard, we further used this parameter (20 mW, 220 fs) to scan the whole cell to study the dynamics of mitochondria and Bcl-2. As shown in Fig. 4(a), after such intense stimulation, all mitochondria inside the cell showed fragmentation. The fragmented mitochondria could not recover, further implying the initiation of cell death. PI was then added into cell buffer after 6 hours and red fluorescence could be found in 63.5% cells (n = 50 stimulated cells, Fig. 4(b)). We also measured the level of Bcl-2 6 hours after the photostimulation. As shown in Fig. 4(c), the greatly increased IF intensity in all stimulated cells indicates the upregulation of Bcl-2, which works to oppose apoptosis to keep cells alive. Therefore, the aggregation of Bax, release of Cyto C, and upregulation of Bcl-2 can all be controlled by the femtosecond-laser stimulation with different parameters.
Fig. 4.
Cellular response to the whole-cell photostimulation. (a) Un-recoverable fragmentation of mtiochondria was induced if the intense stimulation was performed to the whole cell. (b) The PI fluorescence in HeLa cells with 100 μM H2O2 treated for 1 hour (left) and control (right). (c) Upregulation of Bcl-2 was found in the stimulated cells. Bar: 20 μm.
4. Conclusion
In this study, we report a simple femtosecond-laser photostimulation method to any targeted single mitochondria based on a two-photon microscope. It can be easily accomplished by a self-defined line scan of two-photon microscopy in all two-photon microscopes. Different from optical ablation or disruption of organelles, this femtosecond-laser stimulation with only around 100-μs duration can provide a relatively gentle stimulation to mitochondria and hence induce quite complex physiological and molecular dynamics. The stimulation can share the light path of two-photon microscopy and does not need any additional beam shaping, shutter control, or coupling separately to the objective, as in previous studies. It can be found that the photostimulation is dependent on the peak power of laser pulses rather than thermal effect. The femtosecond pulses provide high multiphoton excitation efficiency and thus the stimulation effect. The oxidative stress and the mPTP are also involved in the morphological response as well as the recovery. Bax and Cyto C translocation can be activated by intense stimulation at single-mitochondrion level while the cells can keep good viability. Significant Bcl-2 upregulation can be found in the whole cell if it is stimulated by such intense two-photon scanning. Our results therefore suggest the cell response to laser stimulation can be quite complex, not only at structural level, but at molecular level. This type of photostimulation therefore provides a previse and noninvasive method to stimulate single mitochondria to study the physiological and molecular mechanism of mitochondrial dynamics.
Conflict of interest
The authors declare that there are no conflicts of interest related to this article.
Funding
This work is supported by National Natural Science Foundation of China (NSFC) (81661168014 and 81571719), and Shanghao Jiao Tong University YG2015QN08.
References and links
- 1.Newmeyer D. D., Ferguson-Miller S., “Mitochondria: releasing power for life and unleashing the machineries of death,” Cell 112(4), 481–490 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Ferri K. F., Kroemer G., “Organelle-specific initiation of cell death pathways,” Nat. Cell Biol. 3(11), E255–E263 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Sahin E., DePinho R. A., “Axis of ageing: telomeres, p53 and mitochondria,” Nat. Rev. Mol. Cell Biol. 13(6), 397–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butow R. A., Avadhani N. G., “Mitochondrial signaling: the retrograde response,” Mol. Cell 14(1), 1–15 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Beal M. F., “Mitochondria take center stage in aging and neurodegeneration,” Ann. Neurol. 58(4), 495–505 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Henchcliffe C., Beal M. F., “Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis,” Nat. Clin. Pract. Neurol. 4(11), 600–609 (2008). [DOI] [PubMed] [Google Scholar]
- 7.McFarland R., Taylor R. W., Turnbull D. M., “A neurological perspective on mitochondrial disease,” Lancet Neurol. 9(8), 829–840 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Lin M. T., Beal M. F., “Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases,” Nature 443(7113), 787–795 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Yan M. H., Wang X., Zhu X., “Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease,” Free Radic. Biol. Med. 62, 90–101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abou-Sleiman P. M., Muqit M. M., Wood N. W., “Expanding insights of mitochondrial dysfunction in Parkinson’s disease,” Nat. Rev. Neurosci. 7(3), 207–219 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Tufi R., Gandhi S., de Castro I. P., Lehmann S., Angelova P. R., Dinsdale D., Deas E., Plun-Favreau H., Nicotera P., Abramov A. Y., Willis A. E., Mallucci G. R., Loh S. H., Martins L. M., “Enhancing nucleotide metabolism protects against mitochondrial dysfunction and neurodegeneration in a PINK1 model of Parkinson’s disease,” Nat. Cell Biol. 16(2), 157–166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi S., Wood-Kaczmar A., Yao Z., Plun-Favreau H., Deas E., Klupsch K., Downward J., Latchman D. S., Tabrizi S. J., Wood N. W., Duchen M. R., Abramov A. Y., “PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death,” Mol. Cell 33(5), 627–638 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Q., Fang H., Shang W., Liu L., Xu Z., Ye T., Wang X., Zheng M., Chen Q., Cheng H., “Superoxide Flashes: Early Mitochondrial Signals For Oxidative Stress-Induced Apoptosis,” J. Biol. Chem. 286(31), 27573–27581 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tirlapur U. K., König K., “Targeted transfection by femtosecond laser,” Nature 418(6895), 290–291 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Chung S. H., Mazur E., “Surgical applications of femtosecond lasers,” J. Biophotonics 2(10), 557–572 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Maxwell I., Chung S., Mazur E., “Nanoprocessing of subcellular targets using femtosecond laser pulses,” Med. Laser Appl. 20, 193–200 (2005). [Google Scholar]
- 17.Nishimura N., Schaffer C. B., Friedman B., Tsai P. S., Lyden P. D., Kleinfeld D., “Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke,” Nat. Methods 3(2), 99–108 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Yanik M. F., Cinar H., Cinar H. N., Chisholm A. D., Jin Y., Ben-Yakar A., “Neurosurgery: functional regeneration after laser axotomy,” Nature 432(7019), 822 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Guo S. X., Bourgeois F., Chokshi T., Durr N. J., Hilliard M. A., Chronis N., Ben-Yakar A., “Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies,” Nat. Methods 5(6), 531–533 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe W., Arakawa N., Matsunaga S., Higashi T., Fukui K., Isobe K., Itoh K., “Femtosecond laser disruption of subcellular organelles in a living cell,” Opt. Express 12(18), 4203–4213 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Kumar S., Maxwell I. Z., Heisterkamp A., Polte T. R., Lele T. P., Salanga M., Mazur E., Ingber D. E., “Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics,” Biophys. J. 90(10), 3762–3773 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brugués J., Nuzzo V., Mazur E., Needleman D. J., “Nucleation and transport organize microtubules in metaphase spindles,” Cell 149(3), 554–564 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Heisterkamp A., Baumgart J., Maxwell I. Z., Ngezahayo A., Mazur E., Lubatschowski H., “Fs-laser scissors for photobleaching, ablation in fixed samples and living cells, and studies of cell mechanics,” Methods Cell Biol. 82, 293–307 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Shimada T., Watanabe W., Matsunaga S., Higashi T., Ishii H., Fukui K., Isobe K., Itoh K., “Intracellular disruption of mitochondria in a living HeLa cell with a 76-MHz femtosecond laser oscillator,” Opt. Express 13(24), 9869–9880 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Shi F., He H., Wang Y., Liu D., Hu M., Wang C., “Mitochondrial swelling and restorable fragmentation stimulated by femtosecond laser,” Biomed. Opt. Express 6(11), 4539–4545 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., He H., Wang S., Liu Y., Hu M., Cao Y., Kong S., Wei X., Wang C., “Photostimulation by femtosecond laser triggers restorable fragmentation in single mitochondrion,” J. Biophotonics 10(2), 286–293 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Tirlapur U. K., König K., Peuckert C., Krieg R., Halbhuber K. J., “Femtosecond Near-Infrared Laser Pulses Elicit Generation of Reactive Oxygen Species in Mammalian Cells Leading to Apoptosis-like Death,” Exp. Cell Res. 263(1), 88–97 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Baumgart J., Kuetemeyer K., Bintig W., Ngezahayo A., Ertmer W., Lubatschowski H., Heisterkamp A., “Repetition rate dependency of reactive oxygen species formation during femtosecond laser-based cell surgery,” J. Biomed. Opt. 14(5), 054040 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Yan W., He H., Wang Y., Wang Y., Hu M., Wang C., “Controllable generation of reactive oxygen species by femtosecond-laser irradiation,” Appl. Phys. Lett. 104, 083703 (2014). [Google Scholar]
- 30.Green D. R., Kroemer G., “The pathophysiology of mitochondrial cell death,” Science 305(5684), 626–629 (2004). [DOI] [PubMed] [Google Scholar]