Abstract
Background:
Chronic migraine is common but there is limited knowledge on associated comorbidities.
Objectives:
To examine mental and physical comorbidities in chronic migraine and the influence of socioeconomic status in a large, nationally representative dataset.
Design:
Analysis of cross-sectional primary healthcare data from 1,468,404 adults in Scotland. Chronic migraine, 31 other physical conditions, and seven mental health conditions we examined. Prevalence rates were standardized by age groups, sex, and socioeconomic deprivation, and adjusted odds ratio (aOR) and 95% confidence intervals (CI) calculated for those with chronic migraine compared with those without.
Results:
Chronic migraine patients had more conditions, with the biggest difference found for five or more conditions (chronic migraine 11.7% vs. controls 4.9%; aOR 3.00; 95% CI 2.78–3.22). Twenty-five of the 31 physical conditions were significantly more prevalent in the chronic migraine group. The biggest difference was for chronic pain (aOR 4.33; 95% CI 4.12–4.55). For mental health conditions, the biggest differences were for anxiety (aOR 2.95; 95% CI 2.76–31.5) and depression (aOR 2.94; 95% CI 2.81–3.08). Increasing deprivation was associated with more severe and complex comorbidity (five or more conditions), and with more combined mental and physical comorbidity in the chronic migraine group.
Conclusions:
In a large nationally representative sample in primary care, comorbidity was most common in those with chronic migraine compared with standardized controls, and this was exacerbated by living in areas of higher deprivation.
Keywords: chronic migraine, comorbidity, socioeconomic, primary care, mental health
Introduction
Migraine is a common neurological disorder that usually begins in adolescence or early adult life. The World Health Organization has classified headache as a major health disorder and it has been rated as the sixth highest cause of disability worldwide [1]. Higher prevalence of migraine has also been associated with those living in poorer households [2,3] who have also been found to be more likely to have comorbidity and higher numbers of comorbid conditions [4]. Individuals with migraine have been reported to be more likely to have comorbidity than the general population for a range of different types of conditions (e.g. stroke, chronic heart disease) [4,5].
However, most studies on comorbidity in migraine have focused on a limited number of individual comorbid conditions, such as migraine and comorbidity in stroke [6,7], heart disease [8,9], and certain mental health conditions [10–12]. There has been little work that has examined the level of physical and mental health comorbidity in those with chronic migraine compared with those without, across a wide range of conditions.
Therefore, the aim of this study was to quantify the extent of comorbidity experienced by adults with chronic migraine compared with the general population, and to measure the associations between socioeconomic deprivation and comorbidity in those with chronic migraine.
Methods
We obtained data from the Primary Care Clinical Informatics Unit at the University of Aberdeen for all 1,468,404 patients aged ≥16 years who were alive and permanently registered with one of 314 Scottish general practices on March 31, 2007 [13]. We divided individuals into two groups according to whether they had chronic migraine or not, based on prescribing data (see below). Data on the presence of chronic migraine, 31 other common chronic physical health conditions, and seven mental health conditions were extracted. The dataset is representative of the whole Scottish population in terms of age, sex, and socioeconomic deprivation, and has been detailed elsewhere [14]. We defined chronic migraine as being present in patients who had four or more anti-migraine prescriptions in the previous 12 months, as explained in our previous work on this dataset [14].
To control for differences between the two populations in age, sex, and deprivation levels, we adopted a similar approach to that undertaken in previous papers [15–17], and generated standardized prevalence rates by age groups (16–24; 25–34; 35–44; 45–54; 55–64; 65–74 and ≥75 years), sex, and deprivation quintile using the direct method. These age–sex–deprivation standardized rates were then used to calculate adjusted odds ratio (aORs) and 95% confidence intervals (95% CI) for adults with chronic migraine compared with those without (controls), for the prevalence of 31 other physical conditions, seven other mental health conditions as well as by the number of overall conditions and the number of physical and mental health conditions.
Socioeconomic deprivation was measured using the Carstairs deprivation score divided into quintiles from the most affluent (1) to the most deprived (5). The Carstairs score is based on postcode of residence and is widely used in healthcare research as a measure of socioeconomic status [13].
There is no gold standard way of measuring severity or complexity of comorbidity. However, those with mental and physical comorbidity, or with five or more conditions in total are likely to have more severe and complex problems requiring greater clinical support [18].
We used t-tests to analyse differences between groups and one-way analysis of variance for differences across age groups and deprivation quintiles. For all statistical analyses, a p-value less than 0.05 was considered statistically significant. All analyses were performed in Stata version 13. The NHS Grampian Research Ethics Service approved the anonymous use of these data for research purposes.
Results
Demographics
There were 9,370 patients with chronic migraine based on our definition (Table 1). The chronic migraine group was more likely to be women compared with unadjusted controls (85.0% vs. 50.6% for controls; p<0.001). Individuals with chronic migraine were on average older (mean age 50.5 years vs. 47.0 years for unadjusted controls; p<0.001). Little difference was found by distributions in deprivation quintiles (Table 1).
Table 1.
Age, sex, and deprivation status for individuals with migraine versus controls.
| Variable | Migraine, n (%) | No migraine, n (%) | p* |
|---|---|---|---|
| Total | 9,370 (0.6) | 1,459,034 (99.4) | |
| Female sex | 7,961 (85.0) | 738,197 (50.6) | <0.001 |
| Mean age (SD), years | 50.5 (13.2) | 47.0 (18.7) | <0.001 |
| Age group, years | |||
| 16–24 | 363 (3.9) | 195,356 (13.4) | <0.001 |
| 25–34 | 718 (7.7) | 228,678 (15.7) | <0.001 |
| 35–44 | 1,710 (18.3) | 277,283 (19.0) | 0.06 |
| 45–54 | 2,874 (30.7) | 250,920 (17.2) | <0.001 |
| 55–64 | 2,482 (26.5) | 216,851 (14.9) | <0.001 |
| 65–74 | 923 (9.9) | 154,357 (10.6) | 0.02 |
| ≥75 | 300 (3.2) | 135,589 (9.3) | <0.001 |
| Deprivation quintile | |||
| 1 (least deprived) | 1,706 (18.2) | 278,508 (19.1) | 0.03 |
| 2 | 1,928 (20.6) | 311,710 (21.4) | 0.06 |
| 3 | 2,172 (23.2) | 330,113 (22.6) | 0.2 |
| 4 | 1,839 (19.6) | 277,924 (19.0) | 0.15 |
| 5 (most deprived) | 1,725 (18.4) | 260,779 (17.9) | 0.17 |
*Based on t-tests. SD, standard deviation.
Comorbidities
Overall, 24.8% of individuals with chronic migraine had no other condition compared with 52.6% of adjusted controls (aOR 0.33; 95% CI 0.32–0.35) (Table 2). Significant differences between the chronic migraine and adjusted control groups showed that those with chronic migraine were more likely to have three, four, five, or more conditions. Differences widened with increasing number of conditions, with the biggest difference found for five or more conditions (chronic migraine 11.7% vs. controls 4.9%; aOR 3.00; 95% CI 2.78–3.22) (Table 2).
Table 2.
Prevalence and adjusted odds ratios for number and type of comorbidities (standardized by age, sex, and deprivation score).
| Variable | Migraine n (%) |
No migraine n (%) |
Adjusted odds ratio (95% CI) |
|---|---|---|---|
| Total number of individuals | 9,370 (0.6) | 1,459,034 (99.4) | |
| Total number of morbidities* | |||
| 0 | 2,325 (24.8) | 767,609 (52.6) | 0.33 (0.32–0.35) |
| 1 | 2,092 (22.3) | 304,281 (20.9) | 0.90 (0.86–0.95) |
| 2 | 1,717 (18.3) | 161,388 (11.1) | 1.47 (1.39–1.55) |
| 3 | 1,276 (13.6) | 96,049 (6.6) | 1.97 (1.85–2.10) |
| 4 | 868 (9.3) | 57,821 (4.0) | 2.35 (2.18–2.54) |
| ≥5 or more | 1,092 (11.7) | 71,886 (4.9) | 3.00 (2.78–3.22) |
| Total number of physical conditions* | |||
| 0 | 2,903 (31.0) | 838,170 (57.5) | 0.36 (0.34–0.37) |
| 1 | 2,494 (26.6) | 295,757 (20.3) | 1.14 (1.09–1.19) |
| 2 | 1,750 (18.7) | 147,780 (10.1) | 1.68 (1.59–1.77) |
| 3 | 1,076 (11.5) | 82,723 (5.7) | 2.04 (1.90–2.18) |
| 4 | 596 (6.4) | 46,540 (3.2) | 2.19 (2.00–2.35) |
| ≥5 | 551 (5.9) | 48,064 (3.3) | 2.45 (2.22–2.70) |
| Total number of mental health conditions | |||
| 0 | 5,953 (63.5) | 406,379 (85.3) | 0.37 (0.36–0.39) |
| 1 | 2,417 (25.8) | 163,390 (11.2) | 2.18 (1.92–2.27) |
| ≥2 | 1,000 (10.7) | 50,777 (3.5) | 2.67 (2.49–2.85) |
All differences significant at p<0.001. *Excluding migraine. CI, confidence interval.
Restricting the analysis to only physical health comorbidities showed a similar trend to that of total number of conditions, although the differences were slightly smaller. Those with chronic migraine were far less likely to have no physical conditions (migraine 31.0% vs. controls 57.5%; aOR 0.36; 95% CI 0.34–0.37), with the biggest difference found for five or more physical conditions (chronic migraine 5.9% vs. controls 3.3%; aOR 2.45; 95% CI 2.20–2.70).
Table 2 also shows high levels of co-occurring mental health in the chronic migraine group, with 36.5% having at least one mental health condition. People with chronic migraine were less likely to have no recorded mental health condition compared with controls (chronic migraine 63.5% vs. controls 85.0%; aOR 0.37; 95% CI 0.36–0.39) and more than twice as likely to have one (chronic migraine 25.8% vs. controls 11.2%; aOR 2.18; 95% CI 1.92–2.27) and two or more (chronic migraine 10.7% vs. controls 3.5%; aOR 2.67; 95% CI 2.49–2.85).
Physical health conditions
For the chronic migraine group, 25 out of 31 physical conditions were significantly more prevalent relative to controls after standardization for age, sex, and deprivation, with six conditions showing no significant difference and no conditions significantly less prevalent (Table 3). The biggest differences after standardization for age, sex, and deprivation were for painful condition (aOR 4.33; 95% CI 4.12–4.55) and constipation (aOR 2.98; 95% CI 2.72–3.26). Five other conditions (dyspepsia, prostate disease, irritable bowel syndrome, epilepsy, chronic sinusitis) were more than twice as likely to be found in those with chronic migraine following standardization for age, sex, and deprivation. High raw prevalence rates were found for the chronic migraine group for painful condition (32.1%), hypertension (19.2%), and dyspepsia (13.9%).
Table 3.
Prevalence and adjusted odds ratios for individual physical conditions (standardized by age, sex, and deprivation score). Conditions are ordered by size of odds ratio (largest to smallest).
| Condition | Migraine n (%) |
No migraine n (%) |
Adjusted odds ratio (95% CI) |
|---|---|---|---|
| Total number of individuals | 9,370 (0.6) | 1,459,034 (99.4) | |
| Painful condition | 3,005 (32.1) | 123,229 (8.5) | 4.33 (4.12–4.55) |
| Constipation | 587 (6.3) | 35,913 (2.5) | 2.98 (2.72–3.26) |
| Dyspepsia | 1,300 (13.9) | 77,993 (5.4) | 2.42 (2.27–2.57) |
| Prostate disease | 58 (0.6) | 15,176 (1.0) | 2.24 (1.72–2.91) |
| Irritable bowel syndrome | 1,047 (11.2) | 51,286 (3.5) | 2.22 (2.08–2.37) |
| Epilepsy | 186 (2.0) | 12,379 (0.9) | 2.19 (1.89–2.54) |
| Chronic sinusitis | 167 (1.8) | 9,027 (0.6) | 2.11 (1.81–2.47) |
| Active asthma | 1,163 (12.4) | 86,587 (5.9) | 1.95 (1.84–2.08) |
| Multiple sclerosis | 81 (0.9) | 3,768 (0.3) | 1.94 (1.55–2.42) |
| Stroke or transient ischaemic attack | 281 (3.0) | 36,279 (2.5) | 1.66 (1.47–1.88) |
| Psoriasis or eczema | 103 (1.1) | 10,451 (0.7) | 1.57 (1.29–1.91) |
| Bronchiectasis | 31 (0.3) | 2,793 (0.2) | 1.53 (1.07–21.9); p=0.01 |
| Inflammatory arthritis and related conditions, including gout | 581 (6.2) | 57,600 (4.0) | 1.52 (1.39–1.65) |
| Inflammatory bowel disease | 111 (1.2) | 9,686 (0.7) | 1.52 (1.25–1.83) |
| Thyrotoxicosis/thyroid disorders, including hypothyroidism | 1,006 (10.7) | 71,077 (4.9) | 1.44 (1.35–1.54) |
| Diverticular disease | 286 (3.1) | 33,530 (2.3) | 1.43 (1.26–1.61) |
| Any new cancer in the last 5 years | 404 (4.3) | 43,422 (3.0) | 1.36 (1.23–1.50) |
| Hearing loss | 398 (4.3) | 55,333 (3.7) | 1.34 (1.21–1.49) |
| Peripheral vascular disease | 170 (1.8) | 23,122 (1.6) | 1.29 (1.10–1.50) |
| Diabetes | 569 (6.1) | 74,524 (5.1) | 1.23 (1.13–1.35) |
| Viral hepatitis | 8 (0.1) | 1,167 (0.1) | 1.23 (0.61–2.48); p=0.55 |
| Chronic kidney disease | 205 (2.2) | 33,362 (2.3) | 1.23 (1.07–1.43) |
| Visual impairment | 50 (2.9) | 8,485 (0.6) | 1.22 (0.92–1.62) |
| Coronary heart disease | 64 (0.7) | 81,019 (5.6) | 1.21 (1.10–1.34) |
| Hypertension | 1,798 (19.2) | 232,538 (15.9) | 1.18 (1.11–1.25) |
| Atrial fibrillation | 91 (1.0) | 23,887 (1.6) | 1.02 (0.82–1.26); p=0.83 |
| Glaucoma | 84 (0.9) | 15,844 (1.1) | 1.01 (0.81–1.26); p=0.88 |
| Parkinson’s disease | 10 (0.1) | 2,732 (0.2) | 1.00 (0.53–1.87); p=0.99 |
| Heart failure | 178 (6.7) | 18,840 (1.3) | 0.80 (0.62–1.02); p=0.08 |
| Cirrhosis/chronic liver disease/alcoholic liver disease | 11 (0.1) | 2,603 (0.2) | 0.64 (0.35–1.45); p=0.14 |
All differences significant at p<0.001, except where stated.
Mental health conditions
Table 4 shows that those with chronic migraine had significantly higher prevalence for three of the seven mental health conditions, with no significant difference found for the remaining four. After standardization for age, sex, and deprivation, both anxiety and depression were almost three times as likely to be found in the chronic migraine group; anxiety (chronic migraine 12.1% vs. controls 3.8%; aOR 2.95; 95% CI 2.76–3.15) and depression (migraine 31.3% vs. controls 9.7%; aOR 2.94; 95% CI 2.81–3.08).
Table 4.
Prevalence and adjusted odds ratios for individual mental health conditions (standardized by age, sex, and deprivation score). Conditions are ordered by size of odds ratio (largest to smallest).
| Condition | Migraine, n (%) |
No migraine, n (%) |
Adjusted odds ratio (95% CI) |
|---|---|---|---|
| Total number of individuals | 9,370 (0.6) | 1,459,034 (99.4) | |
| Anxiety and other neurotic conditions, stress-related and somatoform disorders | 1,131 (12.1) | 54,763 (3.8) | 2.95 (2.76–3.15) |
| Depression | 2,932 (31.3) | 141,245 (9.7) | 2.94 (2.81–3.08) |
| Anorexia or bulimia | 71 (0.8) | 5,295 (0.4) | 1.48 (1.17–1.87) |
| Learning disability | 43 (0.5) | 5,040 (0.4) | 1.28 (0.94–1.73); p=0.11 |
| Schizophrenia (and related non-organic psychosis) or bipolar disorder | 102 (1.1) | 12,396 (0.9) | 1.09 (0.89–1.33); p=0.38 |
| Alcohol misuse | 220 (2.4) | 42,340 (2.9) | 0.92 (0.81–1.05); p=0.24 |
| Dementia | 39 (0.4) | 11,664 (0.8) | 0.83 (0.60–1.15); p=0.27 |
All differences significant at p<0.001 except where stated.
Effect of deprivation
Figure 1 shows the effect of deprivation on the number of individuals with five or more conditions (upper panel) and those with at least one physical and one mental health condition using age–sex standardized rates (lower panel). For both measures, the trend is similar with steeper rises in prevalence, with increasing deprivation found for chronic migraine compared with controls (p<0.001). For example, for those with five or more conditions, the difference between the least and most deprived quintiles in those with chronic migraine was least deprived 377 (22.1%) vs. most deprived 649 (37.6%); p<0.00, and in the controls, it was least deprived 20,553 (7.2%) vs. most deprived 32,597 (12.5%); (p<0.001). For those with at least one physical and at least one mental health condition and with chronic migraine, it was least deprived 106 (6.2%) vs. most deprived 236 (13.7%); (p<0.001), and in the controls, it was least deprived 10,583 (3.8%) vs. most deprived 15,386 (5.9%); (p<0.001).
Figure 1.
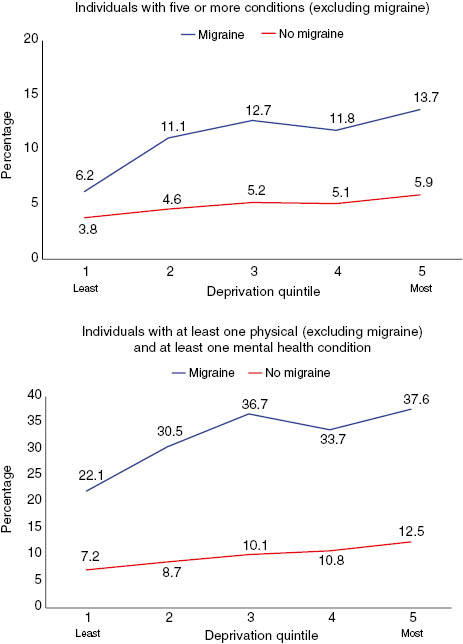
Age–sex-standardized prevalence rates by deprivation quintile.
Discussion
We analysed comorbidity using primary healthcare data on patients with chronic migraine compared with those without chronic migraine (adjusted for age, sex, and deprivation), in a large nationally representative dataset that included a wide range of physical and mental comorbid conditions. The chronic migraine group were significantly more likely to have more comorbidity, with the biggest difference found for five or more conditions. In total, 25 of the 31 physical conditions were significantly more prevalent in the chronic migraine group. The biggest difference was for chronic pain, which was over four times more common in the chronic migraine group compared with (adjusted) controls. Three of the seven mental health conditions were also significantly more prevalent in the chronic migraine group. Increasing deprivation was associated with more comorbidity that was likely to be more severe and complex (five or more conditions, and combined mental and physical conditions) in the chronic migraine group [18].
The current study had strengths and weaknesses. Strengths included the large and nationally representative nature of the dataset, the fact it was primary care based, and the wide range of physical and mental conditions included. Analysis of the data by deprivation level was also a strength, as this is an often-neglected area of enquiry. Weaknesses include the fact that the data were cross-sectional, and there were no outcome data available. The classification of chronic migraine was also made on the basis of prescribing data, which may not give a true prevalence. Prescribing data in Scotland are highly accurate as all prescriptions are computer-based, but it is possible that there were some patients with chronic migraine who were not receiving anti-migraine medication.
In relation to the published literature on the topic, comparison of the current study with others is difficult, as many of the previous studies have been smaller, have not been primary care based, and have not included such a wide range of mental and physical comorbid conditions. They also have seldom differentiated between acute and chronic migraine. In a population-based study, Jette et al. [2] found that migraine prevalence was higher in people with lower socioeconomic status, but that comorbidities were not related to socioeconomic status; whereas in the current study, we found the opposite of this. In a review of comorbidities in migraine, Wang et al. [5] found that migraine was related to stroke, and possibly to coronary heart disease, which was found in the current study. They also reported increases in several mental health conditions, such as depression and anxiety, which were reflected in the current study. Psychiatric problems in migraine are common and worsen outcomes of treatment [19]. We found a higher prevalence of anorexia, which has not previously been reported. In relation to the other physical conditions, our increased prevalence of epilepsy, pain, irritable bowel syndrome, diabetes, and asthma in chronic migraine in the present study is in line with previous findings [5]. Higher rates of constipation, cancer, thyroid disease, dyspepsia, diverticular disease, inflammatory bowel disease, inflammatory arthritis, and psoriasis/eczema were also detected in those with migraine compared with standardized controls.
In terms of implications for research and practice, there is much research needed to be done to explore the mechanisms behind the higher level of comorbidity in patients with chronic migraine. Health service providers need to deliver a holistic approach to the care of such patients, with an appropriate balance of specialist and generalist input. The social patterning of complex comorbidities in chronic migraine, with much higher levels in more deprived areas, is important in view of the continuing problem of the inverse care law [18]. This inverse care law states that because of the mismatch of unmet need and supply in deprived areas, doctors are more stressed and less patient-centred, and consultations are shorter compared with more affluent settings, and patients with complex needs are less enabled and have poorer outcomes [20,21]. Studies have not been specifically conducted on chronic migraine sufferers in deprived areas, but it seems very likely that the inverse care law will operate in the same way in this group as it does generally. Experimentally reversing the inverse care law by providing longer consultation, a patient-centred approach, continuity of care, and support for practitioners has recently been shown to improve quality of life in patients with severe multimorbidity in deprived areas, in a cost-effective way [22].
Conclusions
In conclusion, in a large nationally representative sample in primary care, comorbidity was more common in people living with chronic migraine compared with standardized controls, and this was exacerbated by living in areas of higher deprivation. Such patients need integrated patient-centred care that provides a holistic generalist approach.
Acknowledgments
This work was originally funded by the Chief Scientist Office of Scottish Government Health Directorate (Applied Research Programme Grant ARPG/07/1), with additional support funding from the R.S. MacDonald Trust (with S.W.M. as the grant holder) who supported G.M. to undertake the analysis. Study design, data analysis, interpretation, and publication were the responsibility of the research team who had sole access to the data. The data contained herein were provided by the Primary Care Clinical Informatics Unit (PCCIU) at the University of Aberdeen. The views in this publication are not necessarily the views of the University of Aberdeen, its agents, or employees. Particular thanks go to Katie Wilde and Fiona Chaloner, University of Aberdeen, who carried out the initial data extraction and management.
Conflicts of interest
S.W.M. is a Co-Editor-in-Chief of the Journal of Comorbidity.
Funding
Chief Scientist Office of Scottish Government Health Directorate (Applied Research Programme Grant ARPG/07/1), and the R.S. MacDonald Trust, Scotland.
References
- 1.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Global burden of disease study 2013 collaborators. Lancet. 2013;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jette N, Patten S, Williams J, Becker W, Wiebe S. Comorbidity of migraine and psychiatric disorders–a national population-based study. Headache. 2007;48:501–16. doi: 10.1111/j.1526-4610.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Roy J, Lipton RB. Migraine prevalence, socioeconomic status, and social causation. Neurology. 2013;81:948–55. doi: 10.1212/WNL.0b013e3182a43b32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean G, Gunn J, Wyke S, Guthrie B, Watt GC, Blane DN, et al. The influence of socioeconomic deprivation on multimorbidity at different ages: a cross-sectional study. Br J Gen Pract. 2014;64(624):e440–7. doi: 10.3399/bjgp14X680545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S-J, Chen P-K, Fuh J-L. Comorbidities of migraine. Front Neurol. 2010;1:16. doi: 10.3389/fneur.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidetti D, Rota E, Morelli N, Immovilli P. Migraine and stroke: “Vascular” comorbidity. Front Neurol. 2014;5:193. doi: 10.3389/fneur.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stang PE, Carson AP, Rose KM, Mo J, Ephross SA, Shahar E, et al. Headache, cerebrovascular symptoms, and stroke: the Atherosclerosis Risk in communities study. Neurology. 2005;64:1573–7. doi: 10.1212/01.WNL.0000158326.31368.04. [DOI] [PubMed] [Google Scholar]
- 8.Bigal ME, Kurth T, Santanello N, Buse D, Golden W, Robbins M, et al. Migraine and cardiovascular disease: a population-based study. Neurology. 2010;74(8):628–35. doi: 10.1212/WNL.0b013e3181d0cc8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurth T, Schürks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. Br Med J. 2008;337:a636. doi: 10.1136/bmj.a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh K, Cho S-J, Chung YK, Kim J-M, Chu MK. Combination of anxiety and depression is associated with an increased headache frequency in migraineurs: a population-based study. BMC Neurol. 2014;14:238. doi: 10.1186/s12883-014-0238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yavuz BG, Aydinlar EI, Dikmen PY, Incesu C. Association between somatic amplification, anxiety, depression, stress and migraine. Headache. 2013;14(1):53. doi: 10.1186/1129-2377-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders EFH, Nazir R, Kamali M, Ryan KA, Evans S, Langenecker S, et al. Gender differences, clinical correlates and longitudinal outcome of bipolar disorder with co-morbid migraine. J Clin Psych. 2014;75(5):512–9. doi: 10.4088/JCP.13m08623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elder R, Kirkpatrick M, Ramsay W, MacLeod M, Guthrie B, Sutton M, et al. Measuring quality in primary medical services using data from SPICE Edinburgh: NHS National Services Scotland. 2007 Available from: http://bit.ly/2tBjYbH [Last accessed Jun 30, 2017] [Google Scholar]
- 14.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 15.Smith DJ, Martin DJ, McLean G, Langan Martin J, Guthrie B, Mercer SW. Multimorbidity in bipolar disorder and under treatment of cardiovascular disease: cross sectional study. BMC Med. 2013;11:263. doi: 10.1186/1741-7015-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Court H, McLean G, Guthrie B, Mercer S, Smith D. Visual impairment is associated with physical and mental comorbidities in older adults: a cross-sectional study. BMC Med. 2014;12:181. doi: 10.1186/s12916-014-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper SA, McLean G, Guthrie B, McConnachie A, Mercer S, Sullivan F, et al. Multiple physical and mental health comorbidity in adults with intellectual disabilities: population-based cross-sectional analysis. BMC Fam Pract. 2015;16:110. doi: 10.1186/s12875-015-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean G, Guthrie B, Mercer SW, Watt GC. General practice funding underpins the persistence of the inverse care law: cross-sectional study in Scotland. Br J Gen Pract. 2015;65(641):799–805. doi: 10.3399/bjgp15X687829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seng EK, Seng CD. Understanding migraine and psychiatric comorbidity. Curr Opin Neurol. 2016;29(3):309–13. doi: 10.1097/WCO.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 20.Mercer SW, Watt GMC. The inverse care law: clinical primary care encounters in deprived and affluent areas of Scotland. Ann Fam Med. 2007;5:503–10. doi: 10.1370/afm.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercer SW, Higgins M, Bikker AM, Fitzpatrick B, Lloyd SM, Little P, et al. General Practitioners’ empathy and health outcome: prospective observational study of consultations in areas of high and low deprivation. Ann Fam Med. 2016;14:117–24. doi: 10.1370/afm.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercer SW, Fitzpatrick B, Guthrie B, Fenwick E, Grieve E, Lawson K, et al. The Care Plus study – a whole system intervention to improve quality of life of primary care patients with multimorbidity in areas of high socioeconomic deprivation: cluster randomised controlled trial. BMC Med. 2016;14:88. doi: 10.1186/s12916-016-0634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


