Abstract
Study Objectives:
The relationship among obstructive sleep apnea (OSA), body mass index (BMI), and testosterone levels has long been suggested. Obese men have shown a negative correlation between testosterone level and sleep apnea severity. Yet, little is known about the association between testosterone levels and sleep apnea in men who are not obese. This study evaluated the association between the total testosterone (TT) level and OSA in patients who are not obese.
Methods:
A retrospective review of 523 records of patients in whom OSA was diagnosed from 2013–2016 was performed. The study included men with a BMI < 30 kg/m2 and with TT levels measured in a blood sample collected the morning after a sleep study.
Results:
In all, 153 nonobese men met inclusion criteria, of whom 47 (30.7%) had testosterone levels below the reference values; 44 of these individuals (93.6%) were overweight (P = .029). Reduced testosterone levels showed significant correlations with the oxygen desaturation index, the lowest oxygen saturation < 80% (O2 nadir < 80%), and rapid eye movement (REM) sleep duration, after adjusting for BMI. Among patients with normal weight, only 3 who had O2 nadir < 80% and were older than 50 years presented with a reduced TT level.
Conclusions:
In a large population of nonobese men with OSA, we demonstrated that hypoxemia (O2 nadir < 80%) and overweight are associated with reduced testosterone levels. This association was only observed among normal-weight individuals older than 50 years.
Citation:
Viana A Jr, Daflon AC, Couto A, Neves D, de Araujo-Melo MH, Capasso R. Nocturnal hypoxemia is associated with low testosterone levels in overweight males and older men with normal weight. J Clin Sleep Med. 2017;13(12):1395–1401.
Keywords: body mass index, hypoxia, obstructive sleep apnea, sex hormone, testosterone
INTRODUCTION
Progressive decreases in androgen production are observed in at least 20% of males aged 60 to 70 years.1 Total testosterone (TT) levels were found to be stable in men up to the ages of 50 to 55 years, while a reduction in TT of approximately 35% has been described in males between the ages of 25 and 75 years.2,3 In healthy patients aged 20 to 100 years, subnormal levels of TT and free testosterone were observed in only 1% of the population younger than 40 years, whereas this percentage increased to more than 10%, 20%, and 40% in those aged 40–60 years, 60–80 years, and older than 80 years, respectively.2 Hypogonadal testosterone levels have been found in approximately 12% of men older than 50 years and in 50% of men older than 80 years.4,5 The etiology of this testosterone decline is multifactorial and involves disturbed neuroendocrine gonadotropin regulation.1,6
BRIEF SUMMARY
Current Knowledge/Study Rationale: The relationship between obesity, sleep apnea, and testosterone has been widely addressed in multiple studies, describing a negative correlation between polysomnographic parameters (apnea-hypopnea index, oxygen desaturation index and O2 nadir) and testosterone levels, but studies assessing this relationship in a nonobese population with obstructive sleep apnea (OSA) are still needed. Our research evaluated associations between total testosterone (TT) levels in nonobese patients with OSA and overnight polysomnography measurements.
Study Impact: The study demonstrated that, among nonobese men with OSA, being overweight may aggravate the reduction of TT associated with severe hypoxia measured by O2 nadir < 80% and oxygen desaturation index, regardless of apnea-hypopnea index. This association was only observed among normal-weight individuals older than 50 years as well. All normal-weight men with O 2 nadir ≥ 80% presented with normal TT levels.
Obstructive sleep apnea (OSA) is a common disorder. In men 40–49 years old, the prevalence of apnea-hypopnea index [AHI] ≥ 5 events/h has been reported at 25%; furthermore, in this same age group, the prevalence of AHI ≥ 15 events/h has been reported at 11%.7 Peppard et al. reported that, among overweight men, the prevalence of AHI ≥ 5 events/h was approximately twofold higher in men 50–70 years old (37%) than in men 30–49 years old (18%). Peppard et al. analyzed data from two time points: the 1988–1994 and 2007–2010 United States National Health and Nutrition Examination Surveys. Using these data, Peppard et al. reported an increase in prevalence of AHI ≥ 5 events/h in men 30–49 years old from 20% to 26.6%, and in men 50–70 years old from 38.5% to 43.2%.8 It is well established that obesity is an important risk factor for OSA with metabolic repercussions,9 and it can also result in reduced testosterone levels. However, this association is less clear in overweight and normal-weight subjects.
It has been suggested that OSA affects the hypothalamic-pituitary-gonadal axis, and is associated with hormone secretions impairment in humans.10,11 Previous studies have described a complex relationship between OSA, testosterone levels, body mass index (BMI), and sexual dysfunction. Decreased morning testosterone levels have been shown in male patients with OSA.12–14 Karacan et al. demonstrated that OSA treatment via continuous positive airway pressure improved complaints of erectile dysfunction in one-third of patients with OSA.15 Obese men have also been shown to have lower TT and free testosterone levels.16,17 Semple et al. demonstrated that weight reduction reversed low testosterone levels observed in obese patients with OSA.18 Although some studies report that the low sex hormone levels in patients with OSA are mostly due to obesity,19,20 others indicate that sleep apnea negatively affects testosterone level21,22 independent of BMI.23–26
Overall, the research results on the relationship between OSA and the level of this sex hormone are inconsistent and thus require further investigation; therefore, the objective of this study was to evaluate the association between the TT levels in nonobese patients with OSA, as determined by different overnight polysomnography parameters.
METHODS
Participants
The medical records of 523 consecutive subjects in whom OSA was diagnosed between January 2013 and December 2016 at the Sleep Clinic, Department of Otorhinolaryngology, Naval Hospital Marcílio Dias, Rio de Janeiro, Brazil were reviewed. The inclusion criteria included a diagnosis of OSA, male sex, BMI < 30 kg/m2, and available plasma TT levels measured the morning following polysomnography. It is important to highlight that TT levels are routinely assessed in this subpopulation. The exclusion criteria were: presence of craniofacial abnormalities, active history of alcohol or substance abuse or dependency, severe or poorly controlled psychiatric illnesses such as depression or dementia, or severe/unstable comorbidities such as cancer, cardiovascular, inflammatory or autoimmune diseases, patients with well-known history of hypogonadism or erectile dysfunction. The study was approved by the Institutional Review and Research Ethics Committee of the Institution (N° 51104215.0.0000.5256).
Parameters
The following data were gathered: age at the time of polysomnography, BMI, serum TT level, and overnight polysomnography comprehensive assessment including AHI, apnea index (AI), hypopnea index (HI), rapid eye movement (REM) sleep duration, oxygen desaturation index (ODI) and lowest oxygen saturation (O2 nadir).
Patients were allocated into 2 age groups: 18–50 and > 50 years old. Height (cm) and weight (kg) were measured with the patient wearing light clothing without shoes. BMI was calculated as kg/m2. Patients were classified based on their BMI as underweight (< 18.5 kg/m2), normal-weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥ 30.0 kg/m2).27 The BMI < 30 limit was established so higher obesity grades would not bias the serum testosterone levels.
A peripheral venous blood sample was collected to measure TT quantitatively via the competitive immunoassay method (direct chemiluminescent technology) using ACCESS Testosterone Calibrators (Beckman Coulter, Minnesota, United States). The reference values for TT—normal: 20–49 years (270–827 ng/dL), ≥ 50 years (212–755 ng/dL)—in men are aligned with the protocols of the hormone dosage systems of the Central Laboratory of the executing institution.
OSA was classified based on the AHI as follows: mild (≥ 5 and < 15 events/h), moderate (≥ 15 and < 30 events/h), or severe (≥ 30 events/h). The sleep studies were scored at the hospital by certified technicians. Apnea was identified when the amplitude of the airflow was flat or nearly flat for longer than 10 seconds. Obstructive apnea was identified when the persistence of effort was noted by abdominal or thoracic inductance plethysmography. Central apnea was identified when there was no evident effort on both the abdominal and thoracic plethysmography bands. Hypopnea was identified when there was a 30% reduction in the airflow amplitude for at least 10 seconds that was associated with oxygen desaturation ≥ 3% or electroencephalographic arousal. The AHI, AI, HI, REM sleep, ODI, O2 nadir, and sleep time spent below 90% oxygen saturation were measured based on polysomnography recordings (EMSA Equipamentos Médicos, Rio de Janeiro, Brazil). REM sleep duration was considered normal when between 20% and 25% of the total sleep time.28
Statistics
All the continuous variables were examined for normality in their distributions. The two-tailed Student t test was applied for normally distributed variables and the Wilcoxon rank-sum test was used for skewed variables when the variables were compared with normal testosterone and reduced testosterone. The chi-square test or Fisher exact test was applied as appropriate for comparisons of categorical variables. Logistic regression models were used to examine the associations between testosterone level and sleep outcomes. Values of P ≤ .05 were considered statistically significant. All analyses were performed using SAS Software, version 9.4 (SAS Institute, Inc., Cary, North Carolina, United States).
RESULTS
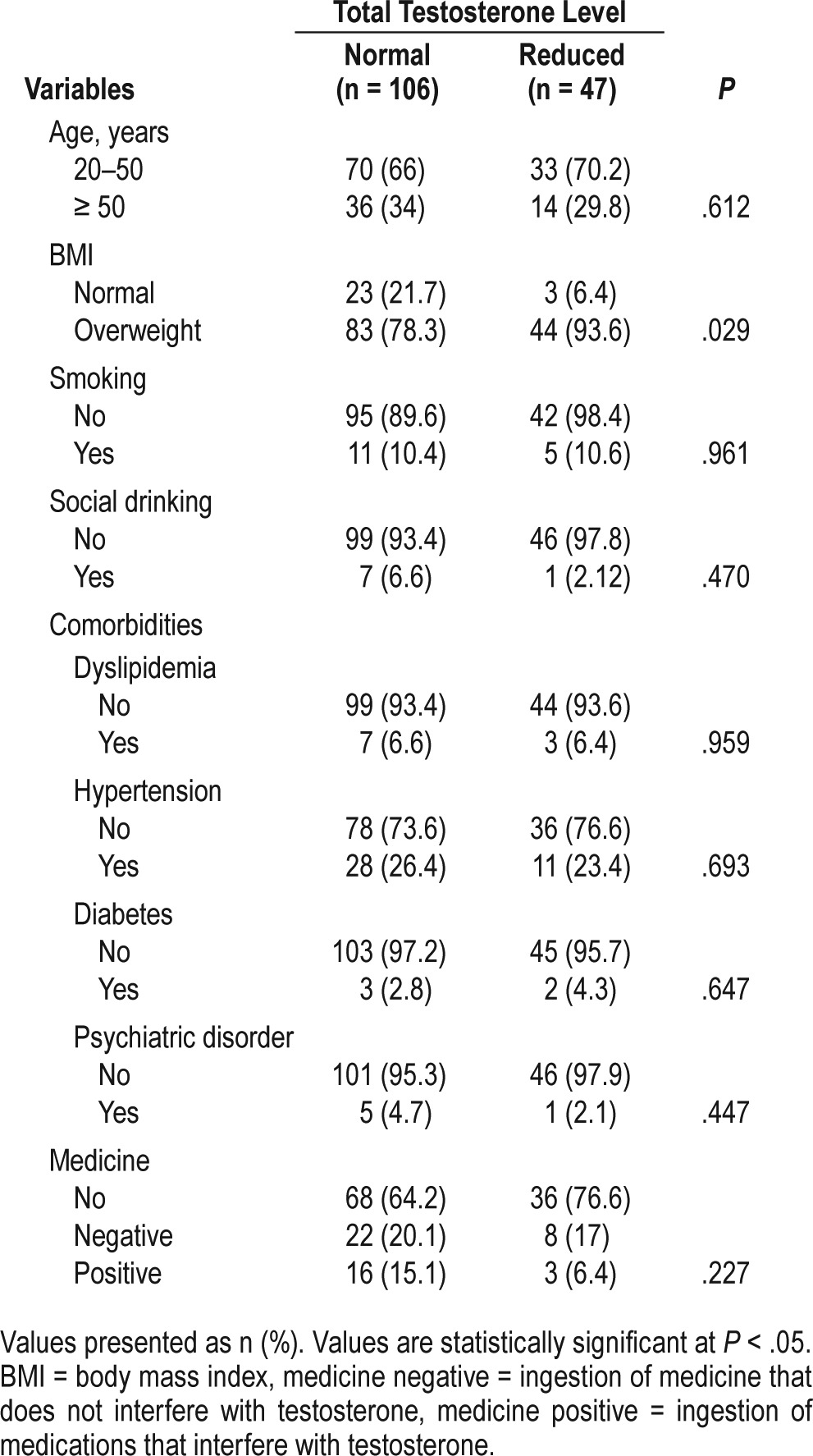
From 153 patients who met inclusion criteria, 47 (30.7%) had testosterone levels below the reference values. Among the 370 patients who were excluded, the most common reason was BMI > 30. The average age was 46.3 years (standard deviation [SD] = 12.71). A total of 127 out of 153 (83%) were determined to be overweight. Table 1 shows the baseline characteristics in this population. TT levels were reduced in 47 men, and 44 of the 47 (93.6%) were overweight [P = .029, odds ratio (OR) = 4.06 (1.1–13.2)]. No significant trends were found for age, current cigarette smoking, alcohol consumption, dyslipidemia, hyper-tension, diabetes, psychiatric disorders, or medicines.
Table 1.
Distribution of the epidemiological characteristics of the study sample.
Compared with individuals with normal TT, those with reduced TT had higher average BMI values, Epworth Sleepiness Scale scores, AHI, and increased nocturnal hypoxemia (O2 nadir and ODI). These individuals also had lower average REM sleep duration. The average AI was significantly higher than the average HI between the two groups of patients (Table 2). The AI was higher than the HI among patients with reduced testosterone levels (Figure 1).
Table 2.
Distribution of continuous variables in relation to total testosterone.
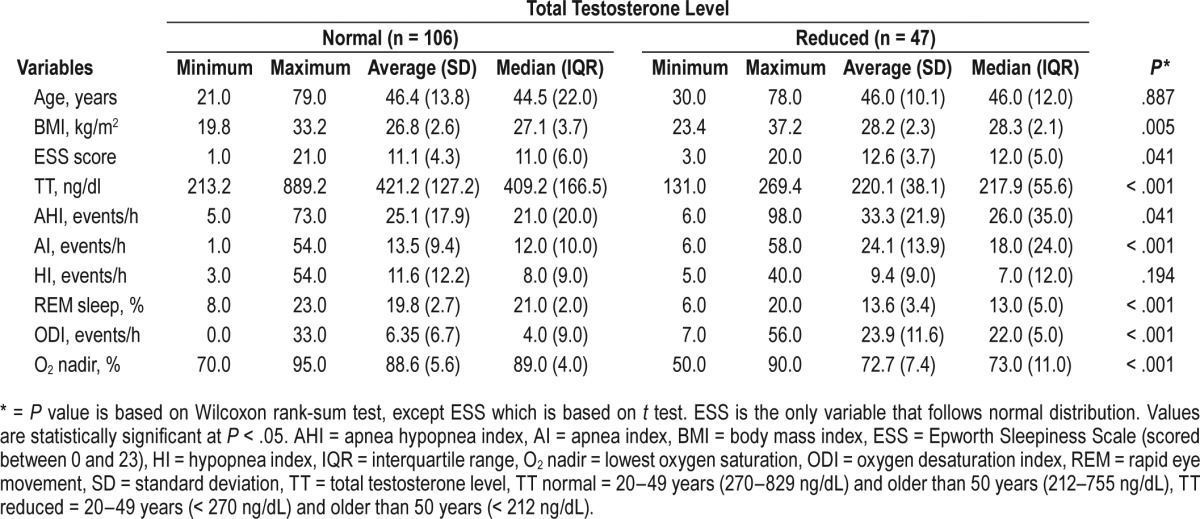
Figure 1. Relationship between testosterone level and apnea-hypopnea index, apnea index, and hypopnea index.
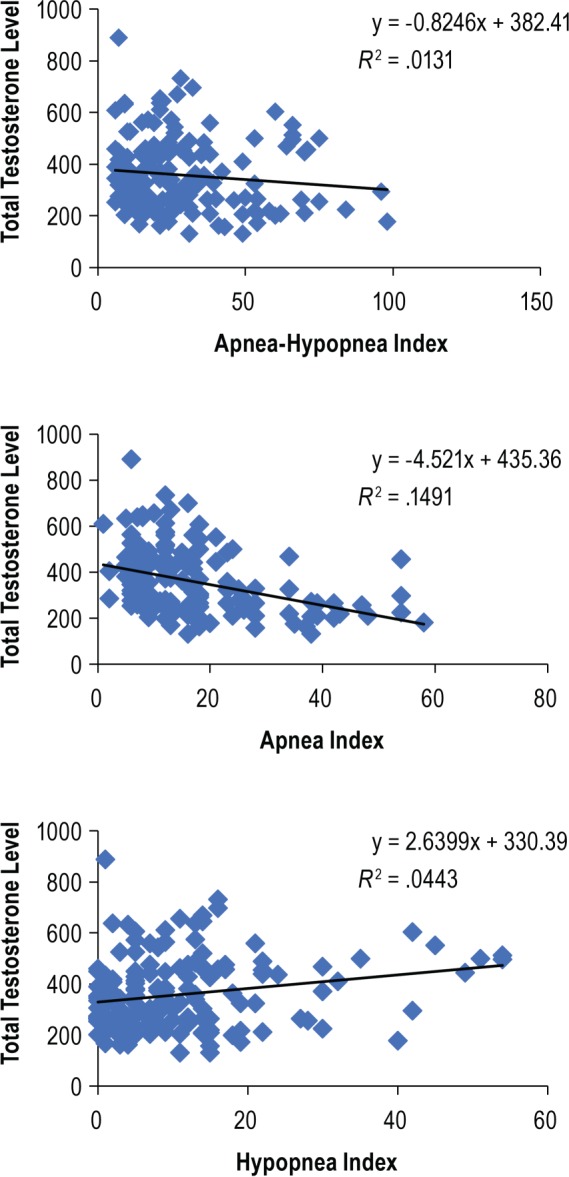
As shown in Table 3, the unadjusted analyses revealed a significant correlation between OSA severity (mild, moderate, and severe) and TT levels (P = .048, OR = 1.88). However, when the AHI ≥ 30 and AHI < 30 groups were considered, the significance of this correlation was more robust in subjects with severe OSA (P = .018, OR = 2.08). A strong correlation was observed among reduced TT, severe OSA, and reduced oxygen values (including O2 nadir and ODI) and subjects with O2 nadir < 80% (OR = 106.1). Subjects with O2 nadir < 80% had a higher OR than subjects with O2 nadir < 90% (OR = 49.24).
Table 3.
Associations between total testosterone level and sleep outcomes.
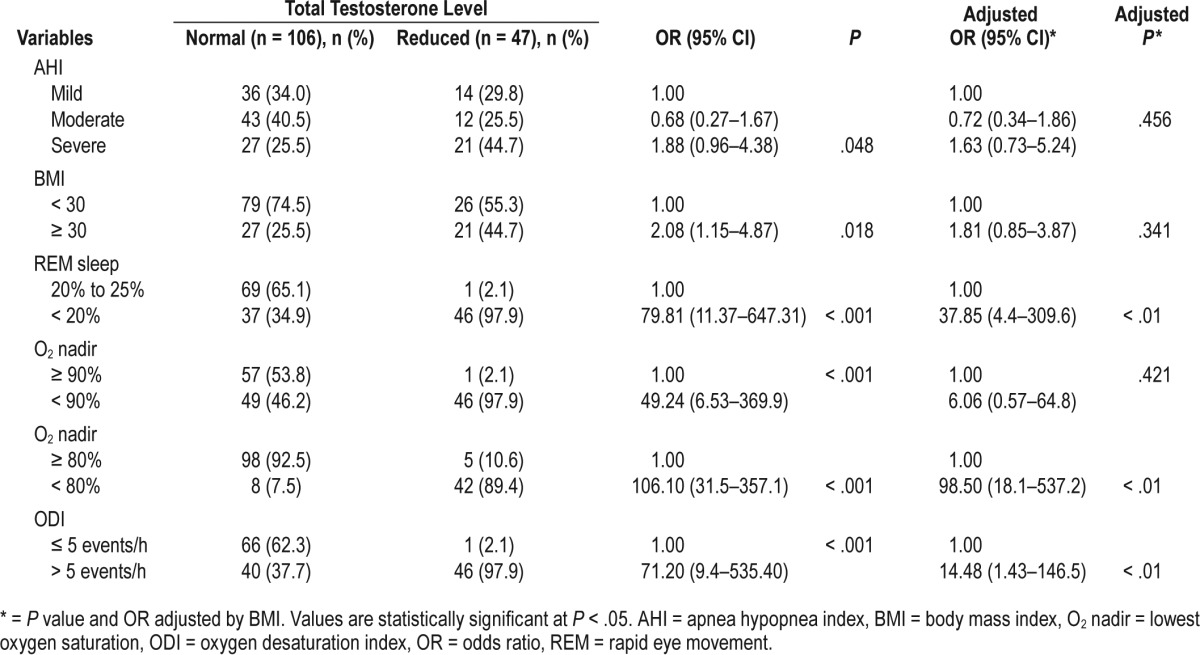
Models with different combinations of age, BMI, and AHI were tested. They were entered in the form that maximized predictive power. BMI was the best and only significant predictor. As shown in Table 3, after adjusting for BMI, the variables AHI and O2 nadir < 90% lost significance. The variables O2 nadir < 80%, ODI, and REM sleep duration remained statistically significant.
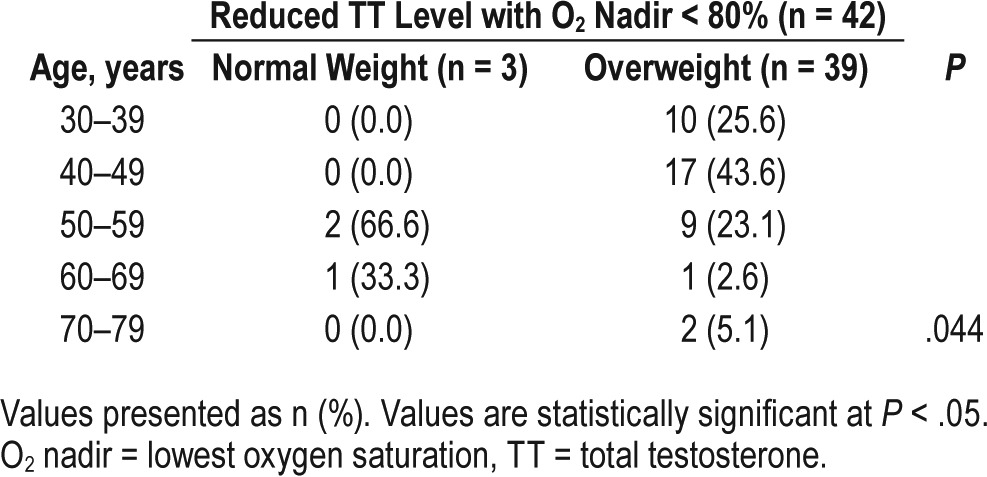
Table 4 shows the association between age groups and weight (normal and overweight) in individuals with reduced testosterone levels. It was possible to identify a significant difference between normal-weight and overweight individuals in relation to their respective ages. With overweight patients, the distribution into age groups was more homogeneous than among normal-weight individuals. We observed that among patients with normal weight (n = 26), 15 (57.7%) and 4 (15.4%) had O2 nadir < 90% and < 80%, respectively. The patients who had a reduced TT level were older than 50 years with O2 nadir < 80%.
Table 4.
Individuals with reduced testosterone levels and hypoxemia by age group.
DISCUSSION
In a population of nonobese men, we found a significant association between OSA severity and presence of hypoxemia, being overweight, and having a reduced TT level. Previous data demonstrated that decreased TT levels were associated with obesity and advanced age, and to a lesser extent with sleep fragmentation and hypoxia.25 A case report showed that just a reduction in body weight led to improvements in respiratory function and blood oxygenation; moreover, the patient's testosterone level returned to normal.18 A multicenter study with men aged 65 years or older (mean = 72.6 years, SD = 5.4) found that lower testosterone was independently associated with adverse sleep effects only in men with higher BMI, suggesting that the associations among testosterone, obesity, and sleep are likely complex.29
In the current study, we observed an association in non-obese younger men (mean = 46.3 years, SD = 12.71), between reduced TT and being overweight. Furthermore, out of the 153 individuals who met inclusion criteria, 83 (54.2%) were over-weight and exhibited OSA without a reduction in TT. However, only 8 of 153 (5.2%) had a O2 nadir < 80%. The severity of hypoxia, established by the ODI and O2 nadir, was found to be an important factor correlated with the reduction in testosterone. The severity of hypoxia might be another factor contributing to reduced testosterone levels, regardless of BMI.30
AHI lost significance when adjusted for BMI. Similar findings have been demonstrated elsewhere.31 However, a negative correlation was found between the severity of sleep apnea and TT level. Higher AHI values correlated with lower TT levels, suggesting that increased apnea severity is related to decreased testosterone secretion in those with OSA.14,25,30 The significant association of low TT levels with high AHI values suggest that gonadal dysfunction is a consequence of OSA rather than a primary condition independent of the hypothalamic-pituitary-gonadal axis.4,32,33 Several factors may account for lower testosterone secretion levels in middle-aged men with OSA, including hypoxemia,14,29 fragmented sleep, obesity,21,34 and advanced age.2
There has been growing recognition that sex hormones play important roles in almost all physiological processes, including breathing. With an emphasis on estrogen, progesterone, and testosterone, sex hormones may affect respiratory function in animals and humans.35 One study suggested that measuring testosterone levels, which is easier than performing nocturnal polysomnography, may be useful for monitoring patients with OSA.34 The incidence of OSA has been shown to be higher in men with reduced serum testosterone levels and in middle-aged men.4,36
This concept remains controversial. Reduced testosterone levels have also been found in patients with mild37 or no OSA.38 In our study, TT would not be an optimal alternative measure for identifying patients with OSA. Of the 106 with normal testosterone levels, 49 (46%) had O2 nadir < 90%, whereas those with reduced testosterone already had significant desaturation levels (ODI and O2 nadir < 80%).
Previous studies have shown that obesity is common in patients with OSA and is associated with increased sleep apnea severity.39 In our nonobese sample, being overweight was not associated with increased OSA severity, however; there was a significant correlation with reduced TT.
Studies have shown that sleep deprivation is associated with the suppression of gonadal steroids in normal young adults, affecting the circadian rhythm of testosterone secretion and resulting in decreased morning levels of this hormone.32,33,40 These studies also revealed a significant correlation between maximal testosterone levels and total oxygen desaturation time.32,33 In our study, this correlation was significant for both oxygen desaturation variables, ODI and O2 nadir. The relationship between the reduction in TT and elevated ODI persisted even after adjusting for BMI. Similar findings have been previously shown in obese but not overweight individuals.30,31,34,38,41 The mechanisms by which oxygen desaturation influences testosterone levels are poorly understood and require further investigation. The circadian rhythm of testosterone may be affected in patients exposed to repetitive episodes of upper airway obstruction with marked sleep fragmentation as well.32 Reduced oxygen availability during sleep may be associated with a central inhibition of the hypothalamic-pituitary-gonadal axis, probably due to increased central levels of endorphins.11
The association between TT and percentage of time spent in REM sleep remained significant after adjusting for BMI. It has been demonstrated in rats that REM sleep deprivation leads to decreased testosterone concentrations,42 thus confirming the biological significance of sleep homeostasis for normal endocrine regulation. Studies have shown that reduced amounts of REM sleep reflect an unconsolidated sleep pattern and are also risk factors for complaints of erectile dysfunction.13,43
The small number of patients with OSA and normal weight who had reduced levels of total testosterone (n = 3, 6%) is a limitation in the current study that prevents from generalization on the hypoxemia effect among normal-weight individuals. However, these patients were observed to have O2 nadir < 80% and were older than 50 years. The reduced testosterone level can be associated with accentuated hypoxemia in sleep-disordered breathing independent of adiposity, and dependent on age. Intermittent hypoxia caused by the recurring episodes of apnea and near-apnea in OSA is a major cause of systemic effects. Studies have noted that the risk of sleep-disordered breathing appears to increase progressively with age.44–46 The elevated levels of cytokines, such as interleukin-6 and tumor necrosis factor-alpha, which also increase with age, are a common feature of both OSA and metabolic syndrome.47,48 Further prospective studies are needed to clarify the association of reduced levels of testosterone, normal weight and age.
In summary, our study demonstrated that among nonobese men with OSA, being overweight may aggravate the reduction of TT associated with the severity of hypoxia measured by O2 nadir < 80% and ODI. The association between O2 nadir < 80% and reduced TT levels was also observed among normal-weight individuals older than 50 years. Normal-weight men of all ages at with OSA and O2 nadir ≥ 80% presented with normal TT levels. Individuals with reduced TT levels also had a positive correlation with reduced REM sleep duration.
DISCLOSURE STATEMENT
Work for this study was performed at Marcílio Dias Naval Hospital and Stanford University. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Yifei Ma, MS, for his valuable assistance in the statistical analysis.
ABBREVIATIONS
- AI
apnea index
- AHI
apnea-hypopnea index
- BMI
body mass index
- HI
hypopnea index
- O2 nadir
lowest oxygen saturation
- ODI
oxygen desaturation index
- OR
odds ratio
- OSA
obstructive sleep apnea
- REM
rapid eye movement
- SD
standard deviation
- TT
total testosterone
REFERENCES
- 1.Kaufman JC, Vermeulen A. Androgens in male senescence. In: Nieschlag E, Behre HM, editors. Testosterone. Action, Deficiency, Substitution. Berlin: Springer; 1998. pp. 437–472. [Google Scholar]
- 2.Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indices on sex hormone binding globulin and androgen levels in aging and obese males. J Clin Endocrinol Metab. 1996;81:1821–1827. doi: 10.1210/jcem.81.5.8626841. [DOI] [PubMed] [Google Scholar]
- 3.Vermeulen A. Andropause. Maturitas. 2000;34(1):5–15. doi: 10.1016/s0378-5122(99)00075-4. [DOI] [PubMed] [Google Scholar]
- 4.Luboshitzky R, Aviv A, Hefetz A, et al. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab. 2002;87(7):3394–3398. doi: 10.1210/jcem.87.7.8663. [DOI] [PubMed] [Google Scholar]
- 5.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 6.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of decreased androgen levels in obese men. Clin Endocrinol Metab. 1994;79(4):997–1000. doi: 10.1210/jcem.79.4.7962311. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokturk O, Ciftci TU, Mollarecep E, Ciftci B. Elevated C-reactive protein levels and increased cardiovascular risk in patients with obstructive sleep apnea syndrome. Int Heart J. 2005;46(5):801–809. doi: 10.1536/ihj.46.801. [DOI] [PubMed] [Google Scholar]
- 10.Pugh LG. Physiological and medical aspects of the Himalayan scientific and mountaineering expedition, 1960-61. Br Med J. 1962;2(5305):621–627. doi: 10.1136/bmj.2.5305.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semple PA, Beastall GH, Watson WS, Hume R. Serum testosterone depression associated with hypoxia in respiratory failure. Clin Sci (Lond) 1980;58(1):105–106. doi: 10.1042/cs0580105. [DOI] [PubMed] [Google Scholar]
- 12.Guilleminault C, Simmons FB, Motta J, et al. Obstructive sleep apnea syndrome and tracheostomy long-term follow-up experience. Arch Intern Med. 1981;141(8):985–988. [PubMed] [Google Scholar]
- 13.Santamaria JD, Prior JC, Fleetham JA. Reversible reproductive dysfunction in men with obstructive sleep apnoea. Clin Endocrinol. 1988;28(5):461–470. doi: 10.1111/j.1365-2265.1988.tb03680.x. [DOI] [PubMed] [Google Scholar]
- 14.Grunstein RR, Handelsman DJ, Lawrence SJ, et al. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab. 1989;68(2):352. doi: 10.1210/jcem-68-2-352. [DOI] [PubMed] [Google Scholar]
- 15.Karacan I, Karatas M. Erectile dysfunction in sleep apnea and response to CPAP. J Sex Marital Ther. 1995;21(4):239–247. doi: 10.1080/00926239508414643. [DOI] [PubMed] [Google Scholar]
- 16.Ostbye T, Kolotkin R, He H, et al. Sexual functioning among obese adults enrolling in a weight loss study. J Sex Marital Ther. 2011;37(3):224–235. doi: 10.1080/0092623X.2011.564530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008;90(4):897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Semple PA, Graham A, Malcolm Y, et al. Hypoxia, depression of testosterone, and impotence in pickwickian syndrome reversed by weight reduction. Br Med J (Clin Res Ed) 1984;289(6448):801–802. doi: 10.1136/bmj.289.6448.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esposito K, Giugliano D. Obesity, the metabolic syndrome, and sexual dysfunction. Int J Impot Res. 2005;17(5):391–398. doi: 10.1038/sj.ijir.3901333. [DOI] [PubMed] [Google Scholar]
- 20.Biswas M, Hampton D, Newcombe RG, Rees DA. Total and free testosterone concentrations are strongly influenced by age and central obesity in men with type 1 and type 2 diabetes but correlate weakly with symptoms of androgen deficiency and diabetes-related quality of life. Clin Endocrinol. 2012;76(5):665–673. doi: 10.1111/j.1365-2265.2011.04196.x. [DOI] [PubMed] [Google Scholar]
- 21.Kirbas G, Abakay A, Topcu F, et al. Obstructive sleep apnoea, cigarette smoking and serum testosterone levels in a male sleep clinic cohort. J Int Med Res. 2007;35(1):38–45. doi: 10.1177/147323000703500103. [DOI] [PubMed] [Google Scholar]
- 22.Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008;90(4):897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Stannek T, Hürny C, Schoch OD, Bucher T, Münzer T. Factors affecting self-reported sexuality in men with obstructive sleep apnea syndrome. J Sex Med. 2009;6(12):3415–3424. doi: 10.1111/j.1743-6109.2009.01486.x. [DOI] [PubMed] [Google Scholar]
- 24.Budweiser S, Enderlein S, Jörres RA, et al. Sleep apnea is an independent correlate of erectile and sexual dysfunction. J Sex Med. 2009;6(11):3147–3157. doi: 10.1111/j.1743-6109.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 25.Luboshitzky R, Lavie L, Shen-Orr Z, et al. Altered luteinizing hormone and testosterone secretion in middle-aged obese men with obstructive sleep apnea. Obes Res. 2005;13(4):780–785. doi: 10.1038/oby.2005.88. [DOI] [PubMed] [Google Scholar]
- 26.Hammoud AO, Walker JM, Gibson M, et al. Sleep apnea, reproductive hormones and quality of sexual life in severely obese mem. Obesity. 2011;19(6):1118–1123. doi: 10.1038/oby.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Physical status: the use and interpretation of anthropometry. 1995. [Accessed October 3, 2017]. World Health Organization website. http://www.who.int/childgrowth/publications/physical_status/en/.Published. [Google Scholar]
- 28.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2012. [Google Scholar]
- 29.Barrett-Connor E, Dam TT, Stone K, et al. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008;93(7):2602–2609. doi: 10.1210/jc.2007-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gambineri A, Pelusi C, Pasquali R. Testosterone levels in obese male patients with obstructive sleep apnea syndrome: relation to oxygen desaturation, body weight, fat distribution and the metabolic parameters. J Endocrinol Invest. 2003;26(6):493–498. doi: 10.1007/BF03345209. [DOI] [PubMed] [Google Scholar]
- 31.Dong JQ, Chen X, Xiao Y, Zhang R, Niu X, Kong WJ. Serum sex hormone levels in different severity of male adult obstructive sleep apnea-hypopnea syndrome in East Asians. J Huazhong Univ Sci Technolog Med Sci. 2015;35(4):553–557. doi: 10.1007/s11596-015-1469-3. [DOI] [PubMed] [Google Scholar]
- 32.Kouchiyama S, Honda Y, Kuriyama T. Influence of nocturnal oxygen desaturation on circadian rhythm of testosterone secretion. Respiration. 1990;57(6):359–363. doi: 10.1159/000195872. [DOI] [PubMed] [Google Scholar]
- 33.Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001;86(3):1134–1139. doi: 10.1210/jcem.86.3.7296. [DOI] [PubMed] [Google Scholar]
- 34.Canguven O, Salepci B, Albayrak S, et al. Is there a correlation between testosterone leves and the severity of the disease in male patients with obstructive sleep apnea. Archivio Italiano di Urologia e Andrologia. 2010;82:143–147. [PubMed] [Google Scholar]
- 35.Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Resp Physiol Neurobiol. 2008;164(1-2):213–221. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meston N, Davies RJ, Mullins R, Jenkinson C, Wass JA, Stradling JR. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J. Intern Med. 2003;254(5):447–454. doi: 10.1046/j.1365-2796.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- 37.Araujo AB, ODonnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89(12):5920–5926. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 38.Molina DF, Suman M, Carvalho TBO, Piatto VM, Taboga RS, Maniglia JV, Tognola WA. Evaluation of testosterone serum levels in patients with obstructive sleep apnea syndrome. Braz J Otorhinolaryngol. 2011;77(1):88–95. doi: 10.1590/S1808-86942011000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima N, Cavaliere H, Knobel M, Halpern A, Mediros-Neto R. Decreased androgen levels in massively obese men may be associated with impaired function of the gonadostat. Int J Obes Relat Metab Disord. 2000;24(11):1433–1437. doi: 10.1038/sj.ijo.0801406. [DOI] [PubMed] [Google Scholar]
- 40.Singer F, Zumoff B. Subnormal serum testosterone levels in male internal medicine residents. Steroids. 1992;57(2):86–89. doi: 10.1016/0039-128x(92)90035-8. [DOI] [PubMed] [Google Scholar]
- 41.Bercea RM, Patacchioli FR, Ghiciuc CM, Cojocaru E, Mihaescu T. Serum testosterone and depressive symptoms in severe OSA patients. Andrologia. 2013;45(5):345–350. doi: 10.1111/and.12022. [DOI] [PubMed] [Google Scholar]
- 42.Andersen ML, Bignotto M, Machado RB, Tufik S. Does paradoxical sleep deprivation and cocaine induce penile erection and ejaculation in old rats? Addic Biol. 2002;7(3):285–290. doi: 10.1080/13556210220139497. [DOI] [PubMed] [Google Scholar]
- 43.Andersen ML, Santos-Silva R, Bittencourt LRA, Tufik S. Prevalence of erectile dysfunction complaints associated with sleep disturbances in Sao Paulo, Brazil: A population-based survey. Sleep Med. 2010;11(10):1019–1024. doi: 10.1016/j.sleep.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philip P, Dealberto MJ, Dartigues JF, Guilleminault C, Bioulac B. Prevalence and correlates of nocturnal desaturations in a sample of elderly people. J Sleep Res. 1997;6(4):264–271. doi: 10.1111/j.1365-2869.1997.00264.x. [DOI] [PubMed] [Google Scholar]
- 46.Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3(5):467–472. [PMC free article] [PubMed] [Google Scholar]
- 47.Salvioli S, Capri M, Valensin S, et al. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12(24):3161–3171. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- 48.He Q, Yang Q, Zhou Q, et al. Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS One. 2014;9(1):e86326. doi: 10.1371/journal.pone.0086326. [DOI] [PMC free article] [PubMed] [Google Scholar]


