Abstract
Study Objectives:
To examine whether change in caregiving status and intensity among community-dwelling older women was associated with sleep characteristics at follow-up, and whether perceived stress modified these associations.
Methods:
The sample included 800 women aged 65 years or older who completed baseline and second follow-up interviews in the Caregiver-Study of Osteoporotic Fractures (Caregiver-SOF). Respondents were categorized into four groups based on change in caregiving status and intensity between the two time points: continuous noncaregivers, ceased caregivers, low-intensity caregivers (continuous caregivers with low/decreased intensity), and high-intensity caregivers (continuous caregivers with high/increased intensity or new caregivers). Perceived Stress Scale scores at the second follow-up were dichotomized into high versus low stress. Sleep outcomes at SOF Visit 8 (which overlapped with Caregiver-SOF second follow-up) included the Pittsburgh Sleep Quality Index total score; and actigraphy-measured total sleep time, sleep efficiency, wake after sleep onset, and sleep latency.
Results:
Multivariate-adjusted sleep characteristics did not differ significantly across caregiving groups. Among high-intensity caregivers, however, those with high stress levels had significantly longer wake after sleep onset (mean 82.3 minutes, 95% confidence interval = 70.9–93.7) than those with low stress levels (mean 65.4 minutes, 95% confidence interval = 55.2–75.7). No other sleep outcomes were modified by stress levels. Further, higher stress was significantly associated with worse Pittsburgh Sleep Quality Index scores, regardless of the caregiving group.
Conclusions:
Overall, sleep characteristics did not differ among noncaregivers, ceased caregivers, or those with high-/low-intensity caregiving among older women. However, subgroups of caregivers may be vulnerable to developing sleep problems, particularly those with high stress levels.
Citation:
Song Y, Harrison SL, Martin JL, Alessi CA, Ancoli-Israel S, Stone KL, Fredman L. Changes in caregiving status and intensity and sleep characteristics among high and low stressed older women. J Clin Sleep Med. 2017;13(12):1403–1410.
Keywords: caregiving, older women, perceived stress, sleep
INTRODUCTION
Caregivers often experience more sleep problems than noncare-givers. These problems include poorer sleep quality,1,2 shorter total sleep time,3 lower sleep efficiency,1,3,4 and greater wake after sleep onset (WASO).1,3 Sleep problems among caregivers may result from their involvement in caregiving tasks, as well as greater stress and depression associated with caregiving. It is generally assumed that psychological distress mediates the relationship between caregiving and sleep disturbance, yet it may also modify the effect of caregiving status or intensity on sleep. Several studies have found that depressive symptoms5–7 and positive affect8 modified the association between caregiving and sleep problems, but to our knowledge no study has investigated whether perceived stress also modifies this association. The current study examined whether the effect of changes in the caregiving role (eg, caregiving status and intensity) on sleep problems differed depending on the level of perceived stress among older women.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Current knowledge of sleep problems and psychological distress among caregivers are based on cross-sectional studies or subjective sleep measures. We examined associations between change in caregiving role, and subjective and objective sleep characteristics among older community-dwelling women, and explored the potential modifying role of stress on these relationships.
Study Impact: Sleep characteristics were not associated with ceasing caregiving or the intensity of the caregiving role. However, among high-intensity caregivers, those with high stress levels spent significantly longer time in wake after sleep onset than the caregivers with low stress levels. Further studies are needed to examine whether relationship between caregiving-related stress and caregivers' sleep would change over time, particularly those with high level of perceived stress.
Perceived stress may modify the association between care-giving and sleep problems for several reasons. Previous studies by our group (Caregiver-Study of Osteoporotic Fractures, SOF) and others have found that perceived stress influenced mortality risk9 and health-related quality of life10 in population-based samples of caregivers and noncaregivers. Caregiving may increase perceived stress and depressive symptoms. Caregivers with depressive symptoms have poorer sleep than those without depressive symptoms.5–7 In a longitudinal study, caregivers with high levels of depressive symptoms slept longer than caregivers with low levels of depressive symptoms, whereas no significant difference in sleep was found over time in terms of caregiving status.6 In another study, care-givers had poorer sleep quality than noncaregivers; however, this relationship was no longer significant when negative affect (ie, depression, hopelessness, perceived stress, and anxiety) and perceived social support were accounted.7 Feeling more stressed may exacerbate the effect of the caregiving challenges (eg, disruptive behaviors, agitation, or apathy in care recipients with dementia)11,12 or feeling burdened by caregiving responsibilities6,13,14 on sleep. Understanding how stress may modify the relationship between caregiving intensity and sleep problems would be important for identifying high-risk caregivers and areas of intervention to improve sleep in caregivers.
Most studies of caregiving and sleep have been cross-sectional, thereby precluding analysis of whether sleep problems existed prior to caregiving.1,4,12,15–17 Assessment of sleep characteristics have primarily focused on subjective measures using a variety of patient questionnaires.4,5,12,16 Although self-reported sleep quality is important, it does not necessarily correspond with objective sleep metrics, and studies show that subjective18,19 and objective20,21 sleep measures are associated with different health outcomes. Studies of caregivers3,6 found discrepancies between self-report and objective sleep, yet both types of sleep measures have contributed to our knowledge of factors associated with sleep problems in caregivers and noncaregivers. Studies that include both subjective and objective sleep measures may inform intervention strategies that aim to improve caregivers' perceived sleep quality as well as objective sleep characteristics, such as WASO.
Moreover, transitions in the caregiving role, due to beginning or ceasing caregiving, or changes in caregiving intensity, are associated with changes in perceived stress22 and physical function.23 Yet, assessing caregiver status at a single time point prevents determining whether moving into and out of caregiving roles24,25 or change in caregiving intensity affects sleep.23 The current longitudinal study examined associations between change in caregiving status and intensity, and subjective and objective sleep among older community-dwelling women. It also explored the potential moderating role of stress on these relationships.
We hypothesized that caregivers whose caregiving tasks increased or remained high, or who became new caregivers over 1 year (ie, high-intensity caregiver group) would experience the worst sleep outcomes, and those who remained low or decreased in caregiving intensity (ie, low-intensity caregiver group) or who ceased caregiving between annual interviews (ie, ceased caregivers) would have moderately worse sleep than women who were noncaregivers at both time points (ie, continuous noncaregivers). We also hypothesized that high-intensity caregivers would have worse sleep if they experienced high levels of stress than those with low levels of stress.
METHODS
Participants
Study participants came from the Caregiver-SOF, an ancillary study to the SOF study,26 which is an ongoing, prospective multicenter study of women aged 65 years or older that aims to evaluate risk factors for osteoporosis, falls, and fractures.26 Participants were recruited between 1986 and 1988 in 4 areas of the United States: Baltimore County, Maryland; Minneapolis, Minnesota; Monongahela Valley, Pennsylvania; and Portland, Oregon. A total of 9,704 white women were enrolled and followed with comprehensive clinical visits approximately every 2 years. Women were excluded if they were unable to walk without the assistance of another person or had a history of bilateral hip replacement.26 African-American women were originally excluded because of the low incidence of hip fractures in this group. At SOF Visit 6 in 1997, 662 African American women aged 65 years and older who met the same inclusion criteria were added to the study. The Caregiver-SOF included participants from both SOF cohorts.
Caregiver-SOF is a prospective cohort study that aims to compare changes in physical health among informal older caregivers (eg, family, friends) and noncaregivers.27 Participants were identified in two phases. In each phase, a Caregiver Screening Questionnaire was administered to SOF participants to determine if they helped a relative or friend, without pay, with any of seven basic activities of daily living (ADLs; ie, walking across a room, grooming, transferring from bed to chair, eating, dressing, bathing, and using a toilet)28 or seven instrumental activities of daily living (IADLs; ie, using a telephone, getting to places out of walking distance, shopping, preparing meals, managing medications, managing finances, and doing heavy housework)29 because that person was physically, emotionally, or cognitively unable to do these tasks independently.30 Caregivers were defined as participants who helped one or more persons with one or more ADLs and/or IADLs; noncaregivers were participants who did not provide help with ADLs and/or IADLs to anyone. One or two noncare-givers were matched to each caregiver on SOF site, age, race, and ZIP code. The baseline Caregiver-SOF sample included 1,069 participants (375 caregivers and 694 noncaregivers).
Data Collection
Baseline data were collected at Caregiver-SOF baseline (1999– 2001) and at the second annual follow-up interviews (2002– 2004). Sleep data were collected at SOF Visit 8 (2002–2004), which overlapped with the second follow-up of the Caregiver-SOF. The institutional review boards at each SOF site and the Boston University Medical Center approved this study. Written informed consent was obtained from all participants.
Variables
Study groups
We combined caregiving status with caregiving intensity from the Caregiver-SOF baseline and second follow-up interviews to create a four-category variable. At each time point respondents were classified as caregivers or noncaregivers as described previously. Caregiving intensity was determined by the median number of ADLs and/or IADLs caregivers performed for care recipients.23 High-intensity was defined as helping with ≥ 2 ADLs, or ≥ 6 IADLs; low-intensity was defined as helping with < 2 ADLs and < 6 IADL tasks.23 Participants were categorized as “continuous noncaregivers” if they were noncaregivers at both interviews, as “ceased caregivers” if they were caregivers at baseline but noncaregivers at the second follow-up, and as “continuous caregivers” if they were caregivers at both interviews.
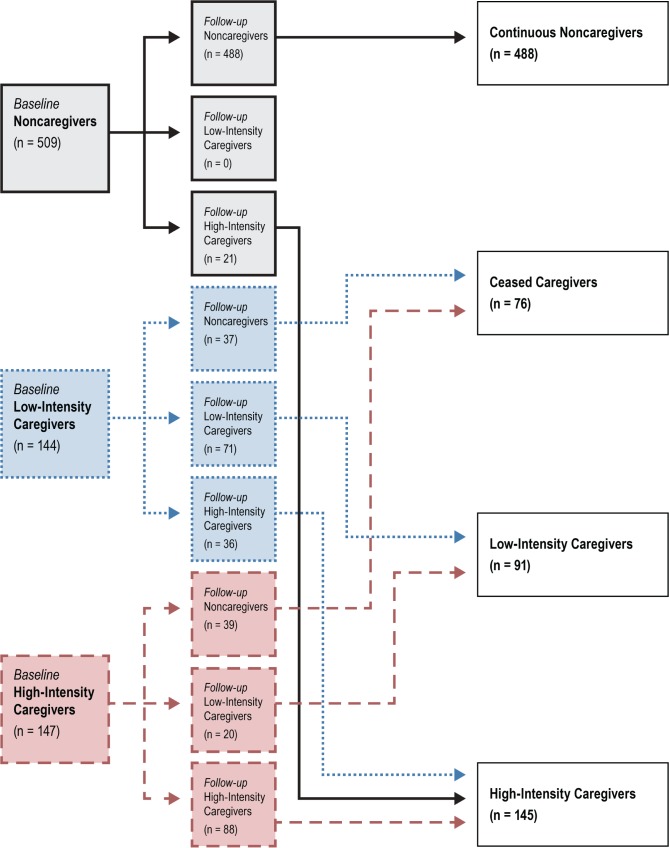
We further created subgroups of the continuous caregivers to reflect transitions in caregiver status: high- to low-intensity; low- to high-intensity, and noncaregiver to caregiver. Because of the small number of participants in these transition groups, and finding that their sleep characteristics were similar to those of caregivers who were low- or high-intensity continuous caregivers, respectively, we combined these groups with the continuous caregiver groups as follows. “High-intensity care-givers” included continuous high-intensity caregivers, those who transitioned from low- to high-intensity, and noncare-givers who became high-intensity caregivers. “Low-intensity caregivers” included those who were low-intensity caregivers at the second follow-up interview, regardless of their caregiving intensity at baseline; no noncaregivers transitioned into this group. Figure 1 shows transitions of caregiving status and intensity from baseline to the follow-up.
Figure 1. Flow diagram of study groups (n = 800).
Those who were identified as noncaregivers at baseline and follow-up were categorized as continuous noncaregivers. Those who were caregivers at baseline, but noncaregivers at follow-up were categorized as ceased caregivers. Those who were caregivers at baseline and follow-up (either low- or high-intensity; represented as blue and red in the figure) were categorized as low-intensity caregivers and high-intensity caregivers.
Sleep measures
Subjective sleep was measured by the Pittsburgh Sleep Quality Index (PSQI),31 which assesses self-reported sleep quality over the past month. Eighteen items are used to generate 7 component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction, with 0–3 points for each domain. Component scores and are summed for a PSQI total score (range 0–21). Total scores for the PSQI were used for these analyses. Objective sleep was measured by wrist actigraphy. Participants wore the Sleepwatch-O (Ambulatory Monitoring, Inc., Ardsley, New York, United States) on their nondominant wrist for a minimum of 3 consecutive 24-hour periods. Action W-2 software (Ambulatory Monitoring, Inc., Ardsley, New York, United States)32 was used to analyze the raw data with a validated sleep scoring algorithm.33 Sleep diaries were used to identify the nighttime period. Four actigraphy-measured sleep outcomes were calculated: total sleep time (between bedtime and rise time), sleep efficiency (ie, time asleep over time between bedtime and rise time), WASO (wake time between first sleep onset to rise time), and sleep latency (time from bedtime to first onset of sleep).
Perceived stress
The 14-item Perceived Stress Scale34,35 was used to assess the frequency of stressful experiences in the past month. Each item was rated on a 5-point, Likert-type scale from 0 = never to 4 = very often (total score range 0–56; higher scores indicate greater stress). For this analysis, respondents were categorized as high or low stress based on the cutpoint for the top quartile of the distribution in noncaregivers in the Caregiver-SOF sample (total score ≥ 20).9
Covariates
Race and education level were measured at the Caregiver-SOF baseline interview. All other covariates were measured at the Caregiver-SOF second follow-up interview. Comorbid conditions were based on participants' self-report that she had ever received a diagnosis of: arthritis, diabetes, heart disease, high blood pressure, lung disease, or stroke. Respondents were categorized into those who endorsed 0–1 versus 2 or more co-morbid conditions. Self-reported use of medication for anxiety or depression (yes/no) and for sleep (yes/no) was assessed. Participants were asked if they needed help with ADLs and/ or IADLs and a dichotomous variable to indicate if a woman reported > 1 ADL and/or IADL limitations was created. Depression was measured by the 20-item Center for Epidemio-logic Studies Depression scale (CESD),36 which assesses the frequency of depressive symptomatology during the previous week using 4-point Likert items ranging from 0 (rarely or none of the time) to 3 (most or all of the time) with total scores of 0–60 (high scores indicate greater depressive symptoms).
Statistical Analyses
Participant characteristics were compared across caregiving intensity groups using analyses of variance and chi-square tests. Differences in caregiving characteristics between the two continuous caregiver groups (high- versus low-intensity) were assessed using t tests and chi-square tests. To examine the relationship between caregiving intensity and sleep outcomes, we performed age- and multivariate-adjusted linear regression models. Covariates for the multivariate models were chosen if they were associated with caregiver status at a value of P < .10. The Tukey-Kramer pairwise comparison test was used to detect significant differences between caregiver intensity categories for all sleep outcomes.
The interaction between level of perceived stress and care-giving intensity group on sleep outcomes was determined by including an interaction term. We obtained age- and multivariate-adjusted least square means for the combination of high/ low stress with each caregiving intensity group for each sleep outcome. SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, United States) was used for all analyses.
RESULTS
Participant Characteristics
Of the 1,069 Caregiver-SOF participants, 269 were excluded from analysis (58 deceased, 43 withdrawn from SOF or Care-giver-SOF, 168 lacked data on key variables), leaving a total of 800 participants in the analytic sample. These women were mainly white (88%) with a mean ± standard deviation (SD) age of 81.1 ± 3.5 years. Half the sample had at least two medical comorbidities. Compared with the 269 excluded participants, those included were more likely to be white, were slightly younger, had higher body mass index, fewer ADL and/ or IADL limitations, were taking more sleep medications, and had more depressive symptoms, high blood pressure, and arthritis. They did not differ on other variables.
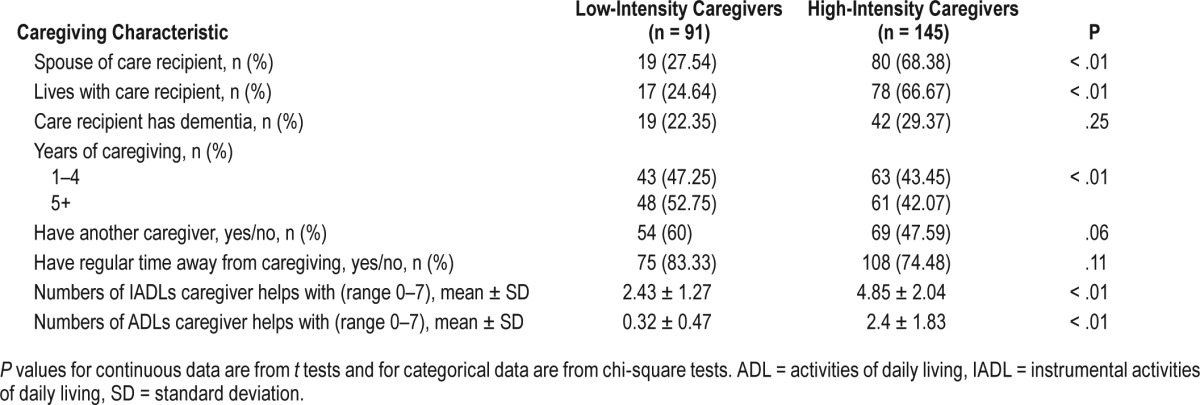
Most participants were continuous noncaregivers (61%); 76 participants had ceased caregiving between baseline and the second follow-up interview; 145 were high-intensity caregivers and 91 were low-intensity caregivers. Continuous noncaregivers and ceased caregivers were slightly older and had more medical comorbidities than the other groups. High-intensity care-givers were more likely to be white and had greater perceived stress than others (Table 1). These caregivers were more likely to be caring for a spouse and living with the care recipient than low-intensity caregivers (Table 2). Twenty-eight caregivers in the high-stress, high-intensity caregiver group (42.4%) were caring for a person with dementia, compared to 27% in the low-stress, high-intensity caregiver group and 36% in high-stress, low-intensity caregiver group.
Table 1.
Baseline characteristics by caregiving intensity groups (800 Caregiver-Study of Osteoporotic Fractures participants).
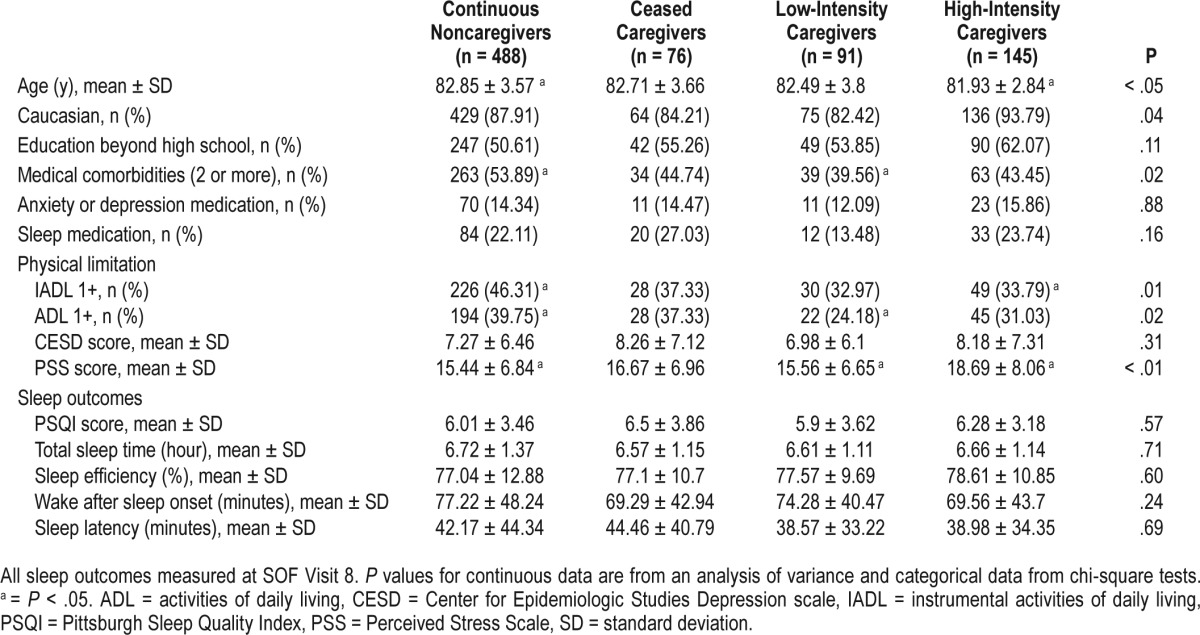
Table 2.
Differences in caregiving characteristics between high-intensity and low-intensity caregivers.
Caregiving Intensity Group and Sleep
The mean number of days between the second follow-up interview and SOF Visit 8 was 354 (SD 258). Poor sleep was common across the groups. The mean total PSQI score was 6.1 (SD 3.5), indicating poor quality of sleep. Mean ± SD objective sleep measures were: total sleep time 6.7 ± 1.3 hours, sleep efficiency 77.4 ± 12.0%, WASO 74.8 ± 46.2 minutes, and sleep latency 41.4 ± 41.2 minutes. No significant differences among caregiving intensity groups were found for subjective or objective sleep measures in unadjusted analysis (Table 1) or in age or multivariate adjusted analyses that adjusted for age, race, comorbidities, ADLs/IADLs, and perceived stress (results not shown).
Caregiving Intensity Group and Sleep, Stratified by Perceived Stress
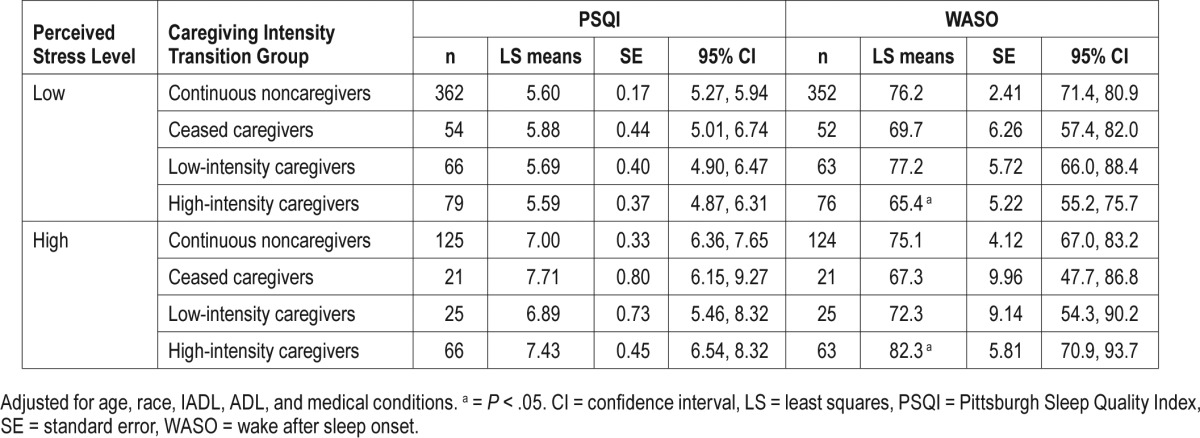
The interaction term between perceived stress and caregiving intensity group was statistically significant for objectively measured WASO (P = .03), but not for other sleep outcomes. In multivariate-adjusted models (Table 3), among high-intensity caregivers, those with high perceived stress had a significantly higher WASO than those with low perceived stress: mean WASO was 82.3 minutes (95% confidence interval = 70.9–93.7) versus 65.4 minutes (95% confidence interval = 55.2–75.7). Among participants with high stress, caregiving intensity was not associated with either subjective or objective sleep measures. Perceived stress did not significantly modify other objectively measured total sleep time, sleep efficiency or sleep latency, or PSQI score among caregiving intensity groups. Participants with high stress levels reported significantly higher PSQI score (indicating poorer sleep quality) than those with low stress levels, regardless of caregiving intensity status (P < .01).
Table 3.
Multivariate adjusted associations between the combined effects of perceived stress levels and caregiving intensity with sleep at Study of Osteoporotic Fractures Visit 8.
DISCUSSION
In this sample of older women, high-intensity caregivers (including continuous high-intensity caregivers, those whose caregiving intensity increased, and new caregivers) did not have worse sleep than continuous noncaregivers or other groups. This finding is consistent with a recent longitudinal study finding that although caregivers reported worse sleep quality than noncaregivers at baseline, caregiver status was not associated with subjective or objective sleep over 3 years.6 We also found that among high-intensity caregivers, those experiencing high stress had longer WASO than those with low stress. Perceived stress did not modify associations between intensity of caregiving with other sleep outcomes. Thus, our results partially supported our hypotheses.
There are several possible reasons why we observed no association between intensity of caregiving and sleep characteristics. One reason is that our sleep data were not collected at the same time as the Caregiver-SOF second follow-up interview, even though they were collected close in time. Other events may have occurred between the Caregiver-SOF second follow-up visit and the sleep assessment that affected sleep outcomes. Similarly, the Caregiver-SOF second follow-up visit was conducted 2 years after the baseline interview. Transitions in care-giver status could have occurred at any point during this period and high-intensity continuous caregivers could have adjusted to caregiving demands. These factors may have obscured differences that might have been observed closer to the transition in caregiving intensity. The similar mean PSQI scores across all caregiving intensity groups, except for those who ceased caregiving, support this possibility. Another reason may be that all the groups had poor sleep duration and sleep quality at the SOF Visit 8; these findings were similar to those of other cohort studies of women aged 70 years or older.37 Poor sleep in our participants may be due to their having one or more risk factors for poor sleep, such as older age and comorbid conditions. In fact, more than half of SOF participants were 70 years or older,26 and half had 2 or more medical comorbidities at the Caregiver-SOF baseline interview. In addition, our measure of caregiving intensity was based on median number of ADL/ IADL tasks performed, which may have obscured associations between very high-intensity caregiving and sleep problems. The heterogeneity of our caregivers is both a strength and a weakness. We may have seen differences between caregivers and noncaregivers if we had restricted our sample to dementia caregivers, who are generally more stressed than caregivers to persons with other conditions.38,39 Finally, it is possible that caregiving intensity are not associated with sleep problems. Multiple assessment time points for caregiving intensity with a longer follow-up would further our understanding of the association between changes in caregiving status and intensity and sleep patterns.
Perceived stress significantly modified the effect of high-intensity caregiving on objectively measured WASO. One possible mechanism is that caregivers experience high levels of stress when they lack sufficient resources (eg, family network, community-based program availability) to adapt to various caregiving situations,40,41 thus contributing to negative health outcomes such as sleep problems. Greater WASO also may have resulted from characteristics of the care recipient, because high-intensity continuous caregivers were more likely to co-reside with their care recipient. These care recipients also required assistance with more basic ADLs, which might have included assistance with transferring in and out of bed. For care recipients who needed assistance during the night, this might also have led to longer awakenings for the caregivers (and therefore increased WASO). Prolonged time awake during the night may then have led to increased stress at the follow-up time point. More importantly, this is supported by the fact that more numbers of high-intensity caregiver groups with high stress were caregivers for patients with dementia than other groups. Other stress-related factors (eg, biomarkers of inflammation) might play a role in sleep and stress in complex caregiving situations. Because this finding was statistically significant only in the high-intensity caregiver group, it must be interpreted with caution given that it was nonsignifi-cant in other groups. The high-intensity caregivers may be at elevated risk for stress in the context of prolonged time awake during the night. This concept is supported by previous studies in which high stress, rather than caregiving per se, was associated with higher risk of mortality9 and poor health-related quality of life.10
Although we found a difference in objective WASO, the absence of an effect of perceived stress on self-reported sleep quality (PSQI) suggests that time awake at night by itself may be a more relevant metric. It is also possible that objective WASO varies more over time, whereas a measure of self-reported global sleep quality (PSQI) fluctuates less in response to changes in external factors, such as caregiving intensity. Actigraphically measured WASO is more strongly correlated with polysomnography measured WASO than subjectively measured WASO, suggesting it is a more reliable measure than, for example, sleep onset latency.42
This study had several limitations. Our sample was limited to women aged 65 or older in the United States and most of Caregiver-SOF participants were older non-Hispanic white women. Thus, the results may not be generalizable to caregivers who are younger women, male, non-white, or older women in other cultural groups. Considering that most informal care-givers in the United States are older women, however, our findings are likely relevant to most United States caregivers. In these analyses, we measured perception of global stress because it applied to both caregivers and noncaregivers; however, caregiving-specific measures would also be informative.9,10 This study also did not conduct polysomnographic screening; thus, we were unable to identify and exclude participants with sleep apnea from our analyses. Given the higher prevalence of sleep apnea in older women,43 we cannot rule out potential effect of this on our study findings.
This study also had notable strengths. To our knowledge, this was the first study to examine the relationship between caregiving intensity over time with sleep outcomes. This study was conducted in a large, multisite community-based longitudinal study, and caregivers and noncaregivers came from the same population, which reduces potential biases that may result from recruiting caregivers and noncaregivers from separate sources.9,41 Our study used rigorous, task-based methods to categorize caregiving status over time. Additionally, this study measured sleep using both self-report and objective actigraphy measures.
In conclusion, we found no association between transitions in caregiving status and intensity over time and sleep characteristics, nor effect modification by perceived stress, with the exception of WASO in high-intensity caregivers among older women. Further studies are needed to evaluate the relationship between caregiving-related stress and sleep over time in the subgroups of caregivers (eg, those with high levels of stress, those caring for dementia patients). Studies are also needed to determine whether stress reduction interventions could improve sleep in older female caregivers.
DISCLOSURE STATEMENT
This study was supported by the National Institutes of Health, National Institute on Aging (AG18037, AG05407, T32-AG-00262), and National Institute of Arthritis and Musculoskeletal and Skin Disease (AR35582, AG05394, AR35584, AR35583). Other funding support for authors include the National Institute on Aging of the National Institutes of Health (K23AG055668, PI: Song). Preparation of this publication was also supported by the VA Advanced Geriatrics Fellowship Program (Song), Office of Academic Affiliations, Department of Veterans Affairs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs, National Institutes of Health, or the United States Government.
ABBREVIATIONS
- ADL
activities of daily living
- CESD
Center for Epidemiologic Studies Depression scale
- IADL
instrumental activities of daily living
- PSQI
Pittsburgh Sleep Quality Index
- SD
standard deviation
- SOF
Study of Osteoporotic Fractures
- WASO
wake after sleep onset
REFERENCES
- 1.von Kanel R, Ancoli-Israel S, Dimsdale JE, et al. Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology. 2010;56(1):41–50. doi: 10.1159/000264654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro CM, Lee KA, Bliwise DL, Urizar GG, Woodward SH, King AC. Sleep patterns and sleep-related factors between caregiving and non-caregiving women. Behav Sleep Med. 2009;7(3):164–179. doi: 10.1080/15402000902976713. [DOI] [PubMed] [Google Scholar]
- 3.Rowe MA, McCrae CS, Campbell JM, Benito AP, Cheng J. Sleep pattern differences between older adult dementia caregivers and older adult noncaregivers using objective and subjective measures. J Clin Sleep Med. 2008;4(4):362–369. [PMC free article] [PubMed] [Google Scholar]
- 4.McKibbin CL, Ancoli-Israel S, Dimsdale J, et al. Sleep in spousal caregivers of people with Alzheimer's disease. Sleep. 2005;28(10):1245–1250. doi: 10.1093/sleep/28.10.1245. [DOI] [PubMed] [Google Scholar]
- 5.Kochar J, Fredman L, Stone KL, Cauley JA. Sleep problems in elderly women caregivers depend on the level of depressive symptoms: results of the Caregiver--Study of Osteoporotic Fractures. J Am Geriatr Soc. 2007;55(12):2003–2009. doi: 10.1111/j.1532-5415.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 6.von Kanel R, Mausbach BT, Ancoli-Israel S, et al. Sleep in spousal Alzheimer caregivers: a longitudinal study with a focus on the effects of major patient transitions on sleep. Sleep. 2012;35(2):247–255. doi: 10.5665/sleep.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brummett BH, Babyak MA, Siegler IC, et al. Associations among perceptions of social support, negative affect, and quality of sleep in caregivers and noncaregivers. Health Psychol. 2006;25(2):220–225. doi: 10.1037/0278-6133.25.2.220. [DOI] [PubMed] [Google Scholar]
- 8.Fredman L, Gordon SA, Heeren T, Stuver SO. Positive affect is associated with fewer sleep problems in older caregivers but not noncaregivers. Gerontologist. 2014;54:559–569. doi: 10.1093/geront/gnt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredman L, Cauley JA, Hochberg M, Ensrud KE, Doros G. Mortality associated with caregiving, general stress, and caregiving-related stress in elderly women: results of caregiver-study of osteoporotic fractures. J Am Geriatr Soc. 2010;58(5):937–943. doi: 10.1111/j.1532-5415.2010.02808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litzelman K, Skinner HG, Gangnon RE, Nieto FJ, Malecki K, Witt WP. Role of global stress in the health-related quality of life of caregivers: evidence from the Survey of the Health of Wisconsin. Qual Life Res. 2014;23(5):1569–1578. doi: 10.1007/s11136-013-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson C, Carter PA. Pilot study of a brief behavioral sleep intervention for caregivers of individuals with dementia. Res Gerontol Nurs. 2010;3(1):19–29. doi: 10.3928/19404921-20090731-02. [DOI] [PubMed] [Google Scholar]
- 12.Creese J, Bedard M, Brazil K, Chambers L. Sleep disturbances in spousal caregivers of individuals with Alzheimer's disease. Int Psychogeriatr. 2008;20(1):149–161. doi: 10.1017/S1041610207005339. [DOI] [PubMed] [Google Scholar]
- 13.Happe S, Berger K FAQT Study Investigators. The association between caregiver burden and sleep disturbances in partners of patients with Parkinson's disease. Age Ageing. 2002;31(5):349–354. doi: 10.1093/ageing/31.5.349. [DOI] [PubMed] [Google Scholar]
- 14.McCurry SM, Gibbons LE, Logsdon RG, Teri L. Longitudinal changes in sleep and psychosocial function in Alzheimer's patients and family caregivers. Sleep. 2004;27:A121. abstract suppl. [Google Scholar]
- 15.Fonareva I, Amen AM, Zajdel DP, Ellingson RM, Oken BS. Assessing sleep architecture in dementia caregivers at home using an ambulatory polysomnographic system. J Geriatr Psychiatry Neurol. 2011;24(1):50–59. doi: 10.1177/0891988710397548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cupidi C, Realmuto S, Lo Coco G, et al. Sleep quality in caregivers of patients with Alzheimer's disease and Parkinson's disease and its relationship to quality of life. Int Psychogeriatr. 2012;24(11):1827–1835. doi: 10.1017/S1041610212001032. [DOI] [PubMed] [Google Scholar]
- 17.Mausbach BT, Ancoli-Israel S, von Kanel R, et al. Sleep disturbance, norepinephrine, and D-dimer are all related in elderly caregivers of people with Alzheimer disease. Sleep. 2006;29(10):1347–1352. doi: 10.1093/sleep/29.10.1347. [DOI] [PubMed] [Google Scholar]
- 18.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012;13(10):1261–1270. doi: 10.1016/j.sleep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 20.Dam TT, Ewing S, Ancoli-Israel S, Ensrud K, Redline S, Stone K. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56(9):1665–1673. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paudel ML, Taylor BC, Ancoli-Israel S, et al. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiol Int. 2010;27(2):363–377. doi: 10.3109/07420520903419157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons JG, Cauley JA, Fredman L. The effect of transitions in caregiving status and intensity on perceived stress among 992 female caregivers and noncaregivers. J Gerontol A Biol Sci Med Sci. 2015;70(8):1018–1023. doi: 10.1093/gerona/glv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fredman L, Doros G, Ensrud KE, Hochberg MC, Cauley JA. Caregiving intensity and change in physical functioning over a 2-year period: results of the caregiver-study of osteoporotic fractures. Am J Epidemiol. 2009;170(2):203–210. doi: 10.1093/aje/kwp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirst M. Carer distress: a prospective, population-based study. Social science & medicine (1982) 2005;61(3):697–708. doi: 10.1016/j.socscimed.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Burton LC, Zdaniuk B, Schulz R, Jackson S, Hirsch C. Transitions in spousal caregiving. Gerontologist. 2003;43(2):230–241. doi: 10.1093/geront/43.2.230. [DOI] [PubMed] [Google Scholar]
- 26.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263(5):665–668. [PubMed] [Google Scholar]
- 27.Fredman L, Tennstedt S, Smyth KA, et al. Pragmatic and internal validity issues in sampling in caregiver studies: a comparison of population-based, registry-based, and ancillary studies. J Aging Health. 2004;16(2):175–203. doi: 10.1177/0898264303262639. [DOI] [PubMed] [Google Scholar]
- 28.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 29.Fillenbaum G. Multidimensional Functional Assessment of Older Adults: The Duke Older Americans Resources and Services Procedures. Hillsdale, New Jersey: Lawrence Erlbaum; 1988. [Google Scholar]
- 30.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;28(23):2, 2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Ambulatory Monitoring, Inc. Action-W User's Guide. Ardsley, NY: Ambulatory Monitoring, Inc; Version 2.0. [Google Scholar]
- 33.Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105(2):185–191. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 35.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 36.Radloff L. The CES-D Scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 37.Unruh ML, Redline S, An MW, et al. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc. 2008;56(7):1218–1227. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 38.Ory MG, Hoffman RR, 3rd, Yee JL, Tennstedt S, Schulz R. Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39(2):177–185. doi: 10.1093/geront/39.2.177. [DOI] [PubMed] [Google Scholar]
- 39.Bertrand RM, Fredman L, Saczynski J. Are all caregivers created equal? Stress in caregivers to adults with and without dementia. J Aging Health. 2006;18(4):534–551. doi: 10.1177/0898264306289620. [DOI] [PubMed] [Google Scholar]
- 40.Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30(5):583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 41.Roth DL, Fredman L, Haley WE. Informal caregiving and its impact on health: a reappraisal from population-based studies. Gerontologist. 2015;55(2):309–319. doi: 10.1093/geront/gnu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239. [PubMed] [Google Scholar]
- 43.Franklin KA, Sahlin C, Stenlund H, Lindberg E. Sleep apnoea is a common occurrence in females. Eur Respir J. 2013;41(3):610–615. doi: 10.1183/09031936.00212711. [DOI] [PubMed] [Google Scholar]