Abstract
Study Objectives:
Postoperative respiratory complications (PRCs) are common among children with obstructive sleep apnea (OSA) after adenotonsillectomy. We analyzed postoperative admission guidelines to determine which optimally balanced patient safety and cost.
Methods:
Retrospective study of children aged 12 years or younger undergoing adenotonsillectomy for OSA after polysomnography at a tertiary academic care center over 2 years. Demographics, medical history, and hospital course were collected. Advanced Excel modeling was used to assess the number of children with PRCs identified with guideline admission criteria and to validate the significance of these findings in our patient population with logistic regression.
Results:
Six hundred thirty children were included; 116 had documented PRCs. Children with PRCs were younger (P = .024) and more frequently male (P = .012). There were no significant differences in race (P = .411) or obesity (P = .265). More children with PRCs had an apnea-hypopnea index (AHI) > 24 events/h (P < .001). Following guidelines from the American Academy of Pediatrics, American Academy of Otolaryngology - Head and Neck Surgery, and Nationwide Children's Hospital, 82%, 87%, and 99% of children with PRCs would be identified, costing $535,962, $647,165, and $1,053,694 for admission, respectively. Using a non-validated, forced model to refine predictors described in published guidelines, our model would have identified 95% of children with one or more PRCs, with a moderate cost.
Conclusions:
Current admission guidelines attempt to identify children with OSA at high risk for PRCs after adenotonsillectomy; however, none consider the economic cost to the health care system. We present a comparison of the number of patients identified with PRCs after adenotonsillectomy and the cost of expected admissions using currently published guidelines.
Commentary:
A commentary on this article appears in this issue on page 1371.
Citation:
Smith DF, Spiceland CP, Ishman SL, Engorn BM, Donohue C, Park PS, Benke JR, Frazee T, Brown RH, Dalesio NM. Admission criteria for children with obstructive sleep apnea after adenotonsillectomy: considerations for cost. J Clin Sleep Med. 2017;13(12):1463–1472.
Keywords: adenotonsillectomy, obstructive sleep apnea, pediatric OSA, postoperative respiratory complications, safety, sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by partial or complete nocturnal upper airway obstruction and occurs in 1% to 4% of children in the United States,1,2 with peak incidence in preschool-aged children.3 Multiple factors, including race, affect the prevalence of this disease in children.1 Untreated OSA in children has been associated with poor school performance,4,5 metabolic abnormalities,6,7 cardiovascular disease,8–11 and pulmonary complications.12 Adenotonsillectomy (T&A) is the currently recommended first-line therapy for children with OSA.13 In a recently published guideline, polysomnography (PSG) is presented as the best diagnostic test for OSA14; however, in a recent survey of pediatric otolaryngologists, fewer than 10% of respondents routinely obtained PSG for children with sleep-disordered breathing.15 Postoperative complications of T&A include pain, nausea, vomiting, dehydration, hemorrhage, and respiratory compromise.14 In a recent meta-analysis, children with OSA had a fivefold increase in postoperative respiratory complications (PRCs) after T&A compared with those without OSA.16
BRIEF SUMMARY
Current Knowledge/Study Rationale: Postoperative respiratory complications (PRCs) are common among children with obstructive sleep apnea (OSA) after airway surgery. Several published guidelines utilize patient demographics, comorbid diseases, and sleep apnea severity to determine which patients are at highest risk for PRCs. Of the current admission guidelines, none are universally accepted.
Study Impact: We applied three published guidelines as a means to screen a population of children who underwent adenotonsillectomy for OSA to identify those children with PRCs and to determine if the models could be refined to balance patient safety with cost. Although safety should always be considered first, we recognize that cost is a growing concern for patients, physicians, and hospital administrators.
A number of investigators have examined the frequency of PRCs and identified predictors of postsurgical respiratory morbidity in children with OSA who undergo T&A. These predictors include high apnea-hypopnea index (AHI), younger age, and obesity.17 Validated risk factor scoring systems have even been developed based on the number and depth of desaturation events seen on overnight pulse oximetry studies.18 Although risk factors for these respiratory complications have been proposed, there are no universally accepted standards to help identify patients with OSA at highest risk for these complications. Additionally, none of the current recommendations have been evaluated for both safety and cost.
The most recent revised recommendations for the diagnosis and management of childhood OSA from the American Academy of Pediatrics (AAP) also include criteria for admission after T&A based on factors that put children at higher risk for PRCs.14 Criteria for admission of pediatric patients after T&A were expanded to decrease the number of unanticipated admissions based on PRCs.14 Similarly, admission criteria for children with OSA undergoing T&A are also offered by the American Academy of Otolaryngology - Head and Neck Surgery (AAOHNS).19 More recently, modified pediatric adenotonsillectomy guidelines were instituted at the Nationwide Children's Hospital to determine areas for practice improvement and limit unanticipated admissions.20 Although these guidelines use a similar list of predictors, the criteria are variable. No large-scale comparison exists in a pediatric population to match the ability of all three guidelines to predict PRCs, determine the number of admissions that each would require, or the cost of such admissions.
It is imperative that anesthesiologists, otolaryngologists, and pediatric pulmonologists determine a standard of care that can be used to identify those children at risk for PRCs and establish general admission criteria that are both cost-effective and safe for the patient. We hypothesized that certain risk factors would be predictive of PRCs and that current models could be refined in order to balance cost and patient safety. To test these hypotheses, we applied admission criteria to a population of children with OSA who previously underwent T&A in order to compare predictive ability and diagnostic accuracy of previously published guidelines.
METHODS
Study Participants
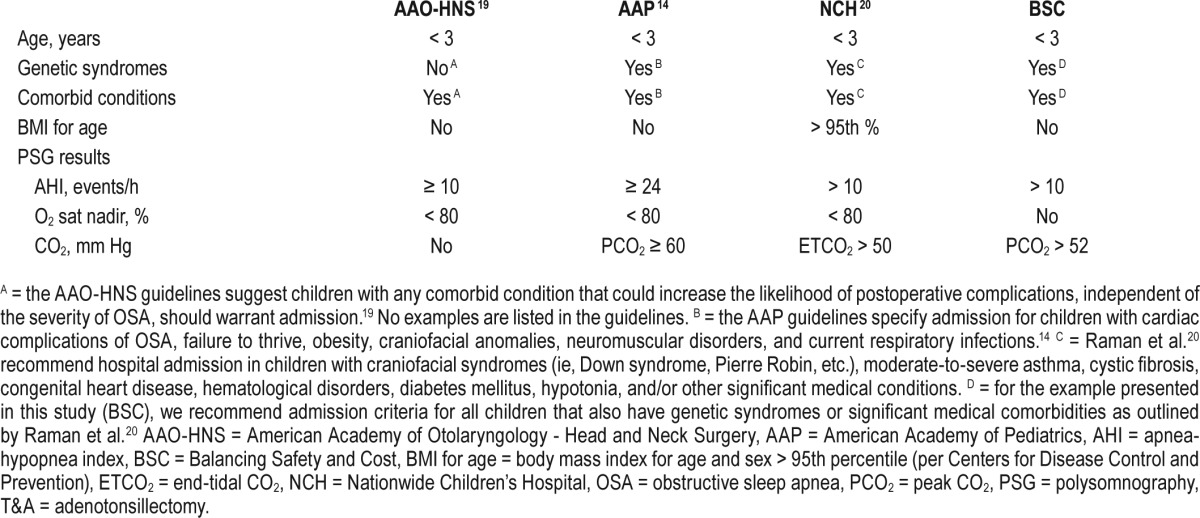
This study was approved by the Johns Hopkins institutional review board (NA_00065297). A retrospective database query used ICD-9 codes for obstructive sleep apnea, tonsillectomy, and adenotonsillectomy, to identify children undergoing T&A for OSA between January 1, 2011 and December 31, 2012 at the Johns Hopkins Hospital. Children were included only once in the dataset after review of the children undergoing procedures in the electronic medical record. All study participants received a complete tonsillectomy or adenotonsillectomy. Children age 12 years or younger in whom OSA was diagnosed using preoperative nocturnal PSG prior to T&A were included in the study. Children were excluded if they were older than 12 years, did not have a preoperative PSG, or had an adenoidectomy without a tonsillectomy. Per criteria established by the AAO-HNS,19 planned admissions were generally made for children if they were younger than 3 years, had an AHI ≥ 10 events/h, had an oxygen saturation nadir (O2 sat nadir) during the PSG < 80%, and/ or demonstrated postoperative sleep-disordered breathing after surgery (Table 1). Although the AAO-HNS guidelines mention considering postoperative admission for children with comorbid conditions,19 no examples of these comorbid conditions are given and interpretation is left to the treating physicians.
Table 1.
Current recommendations for hospital admission in children with OSA undergoing T&A.
PSG was obtained for children with sleep-disordered breathing per guidelines previously published through the AAO-HNS.19 These guidelines were uniformly followed by the pediatric otolaryngologists at this academic institution. PSG was evaluated and scored by pediatric sleep physicians at the Johns Hopkins Hospital using the criteria of the American Academy of Sleep Medicine. OSA was determined by the following parameters: mild OSA was defined by an obstructive AHI (oAHI) of 1 to < 5 events/h; moderate OSA was defined as an oAHI ≥ 5 and < 10 events/h; severe OSA was defined as an oAHI ≥ 10 events/h.21–23
Electronic medical records were reviewed for demographic data, including age, sex, race, weight, and body mass index (BMI) on the day of the procedure, as well as medical comorbidities, PSG parameters, hospital course (including intraoperative and postoperative complications), and readmissions within the first 2 weeks after discharge. If a readmission occurred in the first 2 weeks after surgery, the readmission was recorded even if it was a secondary admission unrelated to a postoperative complication. As a retrospective analysis, individuals reviewing the electronic medical records for data were not blinded from patient outcomes. Medical comorbidities that were tracked included those described in the other guidelines including neuromuscular disorders, Down syndrome, Pierre Robin sequence, other craniofacial disorders, and genetic syndromes not otherwise specified. For children age 2 years or older, the BMI for age (and sex) was calculated using the formulas derived from the United States Centers for Disease Control and Prevention24; obesity was defined as a BMI for age ≥ 95th percentile.
Comparison of Models
Children were divided into two groups: (1) patients without recorded PRCs and (2) patients with recorded PRCs. PRCs were defined as: persistent oxygen saturations of hemoglobin lower than 90% or respiratory distress that required intervention. Intermittent oxygen desaturations were included only if they required an intervention. However, their effect on the patient's course in the hospital was not determined. Interventions for PRCs included any one or more of the following: oxygen supplementation, respiratory support requiring medical intervention or noninvasive positive pressure ventilation, and/or the need for reintubation for severe respiratory complications.
Published guidelines describing admission criteria for children with OSA after T&A were reviewed. Guidelines included those from the aforementioned AAO-HNS,19 the AAP,14 and the Nationwide Children's Hospital (NCH).20 The AAP recommends admission for children after T&A if they meet the following criteria: age younger than 3 years, AHI ≥ 24 events/h on PSG, O2 sat nadir of < 80%, peak CO2 (PCO2) ≥ 60 mm Hg (Table 1).14 The guidelines also recommend admitting patients if they have cardiac complications of OSA, failure to thrive, obesity, craniofacial anomalies, neuromuscular disorders, or current respiratory infections.14 However, no distinction is made concerning those comorbidities on this list that cause the highest risk of PRCs, and other comorbidities, such as asthma, are not mentioned.14 Guidelines from the NCH recommend admission for children younger than 3 years, AHI > 10 events/h, O2 sat nadir < 80%, end-tidal CO2 (ETCO2) > 50 mm Hg, BMI for age > 95th percentile, and/or those children with craniofacial syndromes, moderate to severe asthma, cystic fibrosis, congenital heart disease, hematological disorders, diabetes mellitus, hypotonia, and any other major medical conditions (Table 1).20
Using advanced Excel modeling (Microsoft, Redmond, Washington, United States), we applied guidelines from the AAP,14 AAO-HNS,19 and NCH20 to our dataset to determine their ability to identify children who had known PRCs. In addition, we performed a cost analysis of the expected admission for each model. A univariate analysis was then performed on physical examination characteristics, demographics, and PSG findings commonly used in other admission guidelines to determine if these criteria could be refined to improve identification of children with PRCs while minimizing associated admission costs. As a forced model, outcome measures for each variable were examined as a group to determine the best outcomes. By evaluating each scenario, the recommendations with the highest number of identified PRCs and the lowest number of admissions were identified. This model, titled “Balancing Safety and Cost,” or BSC, proposes admission if children are younger than 3 years old, have an AHI > 10 events/h, have an O2 sat nadir < 80%, ETCO2 > 52 mm Hg, and/or have the same comorbid conditions outlined in the NCH guidelines.20
Cost Analysis
The daily charge for a floor admission at our institution averaged $1,823. These costs include only the facility charge, and do not include physician charges, medications, or supplies. The overall cost of admission per guideline was calculated by determining the number of patients who would be admitted using each guideline criteria. The total cost was then estimated by multiplying the average daily charge for a floor admission for the first night by the number of patients admitted.
Statistical Analysis
Descriptive statistics were used to characterize demographic data. Categorical measures were summarized using frequencies and percentages. Univariate analysis using logistic regression was used to assess the association of demographic and PSG measures with respiratory complications. Values of P < .05 were considered significant.
Multinomial logistic regression analyses were performed for each of the four models to determine how well the observed outcomes are replicated by each model. A Hosmer and Lemeshow (H&L) goodness-of-fit classification statistic was used to assess the fit of the models against actual outcomes, with actual outcomes representing those children who had PRCs. The P value of the chi-square in the H&L goodness-of-fit table should be large to indicate a good fit (not to demonstrate significance). Therefore, when comparing models, a larger P value indicates a better fit.25 The Akaike Information Criteria (AIC) is used as an additional indicator of relative goodness-of-fit. As with the H&L goodness-of-fit, the larger AIC when comparing models also indicates a better fit.26 The Nagelkerke R2 represents the percentage of variance of the dependent variables explained by the independent variables. This measure only accounts for the factors that are identified by the models that could cause PRCs. However, it does not incorporate the costs of admission for each guideline. The following formulas were used to analyze the ability of each model to identify PRCs.
AAO-HNS Model (Table 2)
Table 2.
Logistic regression of the AAO-HNS model.
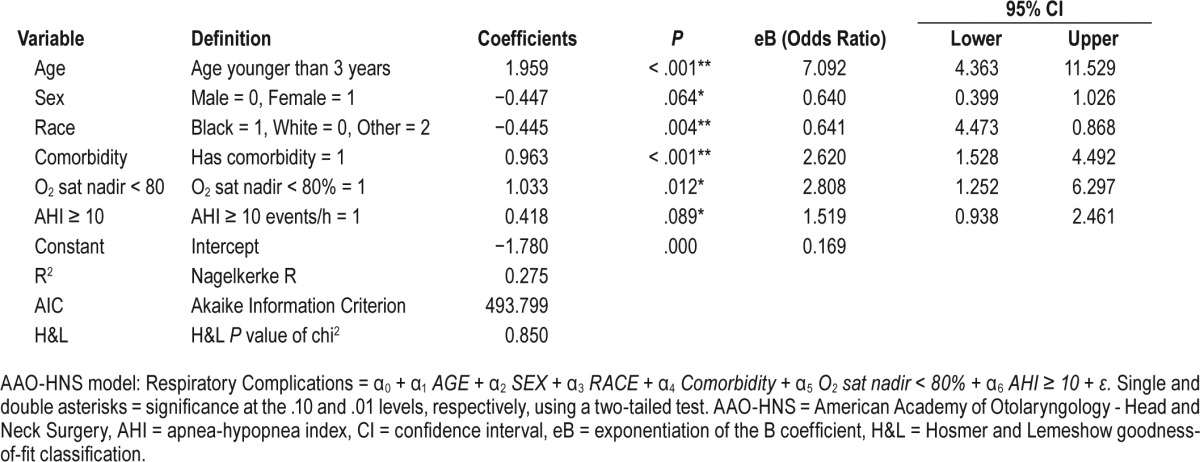
Respiratory Complications = α0 + α1 AGE + α2 SEX + α3 RACE + α4 Comorbidity + α5 O2 sat nadir < 80% + α6 AHI ≥ 10 + ε
AAP Model (Table 3)
Table 3.
Logistic regression of the AAP model.
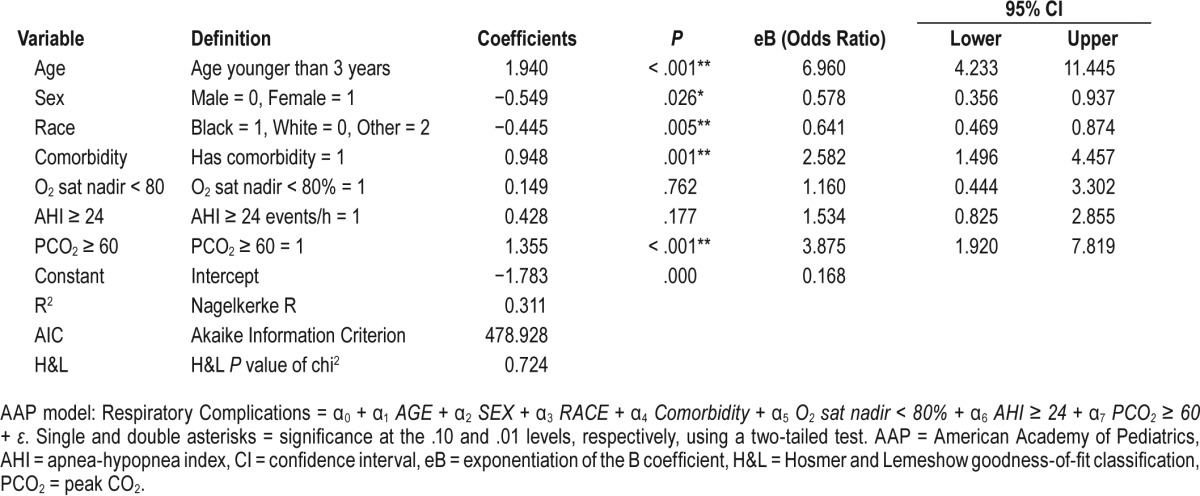
Respiratory Complications = α0 + α1 AGE + a2 SEX + a3 RACE + α4 Comorbidity + α5 O2 sat nadir < 80% + α6 AHI ≥ 24 + α7 PCO2 ≥ 60 + ε
NCH Model (Table 4)
Table 4.
Logistic regression of the NCH model.
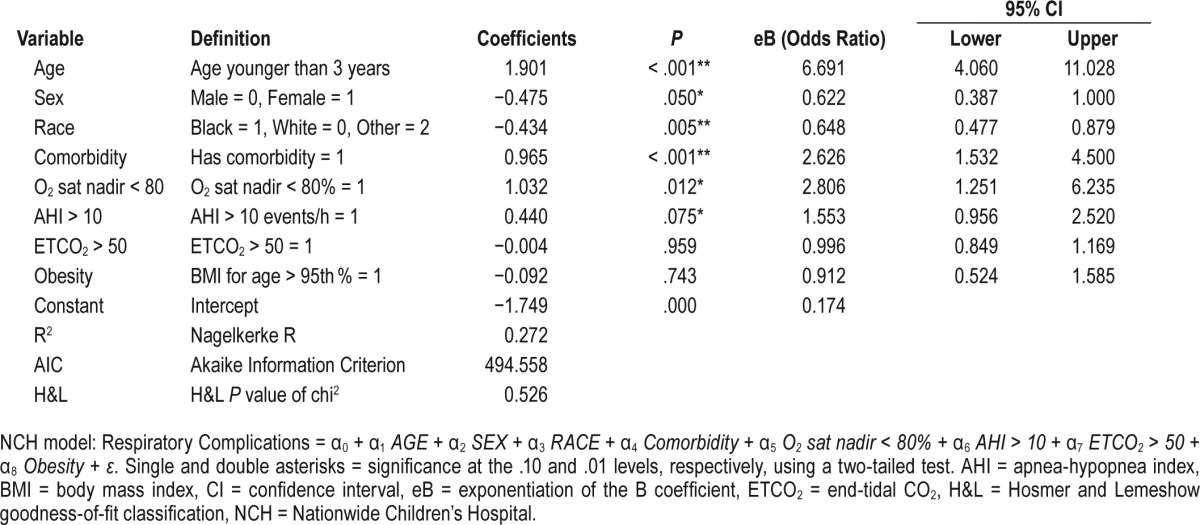
Respiratory Complications = α0 + α1 AGE + a2 SEX + a3 RACE + α4 Comorbidity + α5 O2 sat nadir < 80% + α6 AHI > 10 + α7 ETCO2 > 50 + α8 Obesity + ε
BSC Model (Table 5)
Table 5.
Logistic regression of the BSC model.
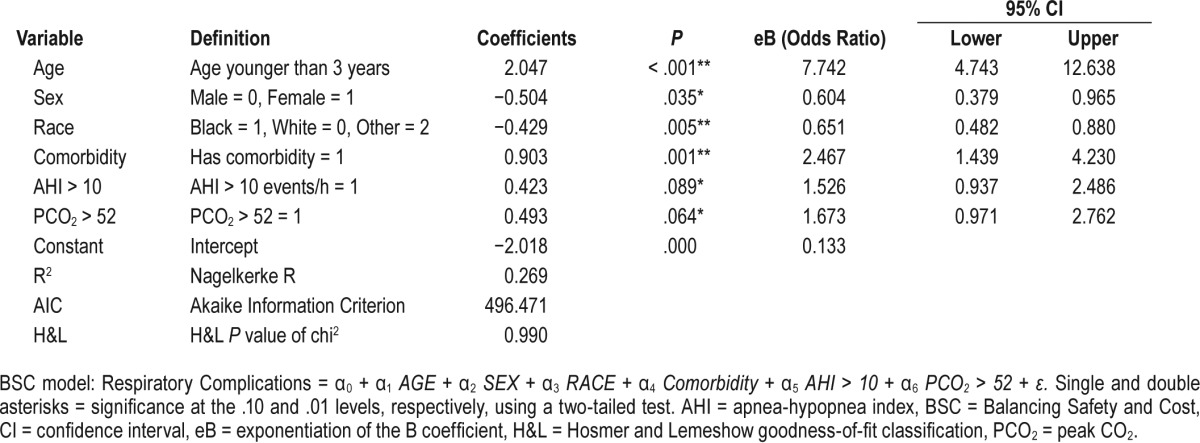
Respiratory Complications = α0 + α1 AGE + a2 SEX + a3 RACE + α4 Comorbidity + α5 AHI > 10 + α6 PCO2 > 52 + ε
where AGE = 1 if < 3 or 0 otherwise, SEX = 1 if the patient is female, or 0 otherwise, RACE = 1 if the patient is black, 0 if the patient is white, or 2 otherwise, Comorbidity = 1 if the patient has a craniofacial syndrome or pertinent comorbid condition, or 0 otherwise, O2 sat nadir < 80% = 1 if oxygen saturation nadir < 80% on PSG, or 0 otherwise, AHI > 10 = AHI > 10 events/h, AHI ≥ 24 = AHI ≥ 24 events/h, ETCO2 > 50 = ETCO2 > 50 mm Hg, PCO2 > 52 = PCO2 > 52 mm Hg, PCO2 ≥ 60 = PCO2 > 60 mm Hg, and Obesity = 1 if BMI for age > 95th percentile, or 0 otherwise.
Additionally, sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV) were also completed for comparison. These statistical analyses were performed using the following equations.
Sensitivity
Children with PRCs / (Children with PRCs + Children with PRCs not admitted)
Specificity
Children without PRCs / (Children without PRCs + Children without PRCs that were admitted)
PPV
Children with PRCs / (Children with PRCs + Children without PRCs that were admitted)
NPV
Children without PRCs / (Children without PRCs + Children with PRCs not admitted)
RESULTS
A total of 630 children (333 males, 52.9%) were included in our study (Table 6). Of these, 116 (74 males, 63.8%) had a PRC whereas 514 (259 males, 50.4%) did not. The average age of children with PRCs was 3.7 ± 2.3 years compared to 5.9 ± 2.9 years, and a significantly greater number of children with PRCs were younger than the age of 3 years (P = .024). No significant difference was seen in race (P = .411) or obesity (P = .265) between those with and without PRCs. However, more children were younger than 3 years of age (P = .024) or had a genetic syndrome (P < .001) among the PRC group. The most common genetic syndromes in our patient population were neuromuscular disorders (24/37), Down syndrome, Pierre Robin sequence, and other craniofacial disorders. Among the children in our study, 312 had a planned admission preoperatively prior to the scheduled T&A (Table 6). Further, 8 children in our study group required reintubation in the postoperative period, and 7 of these children (87.5%) had a comorbid genetic syndrome and 6 (75%) had a neuromuscular disorder (Table 7).
Table 6.
Demographic information, medical history, and PSG data for children with OSA undergoing T&A.
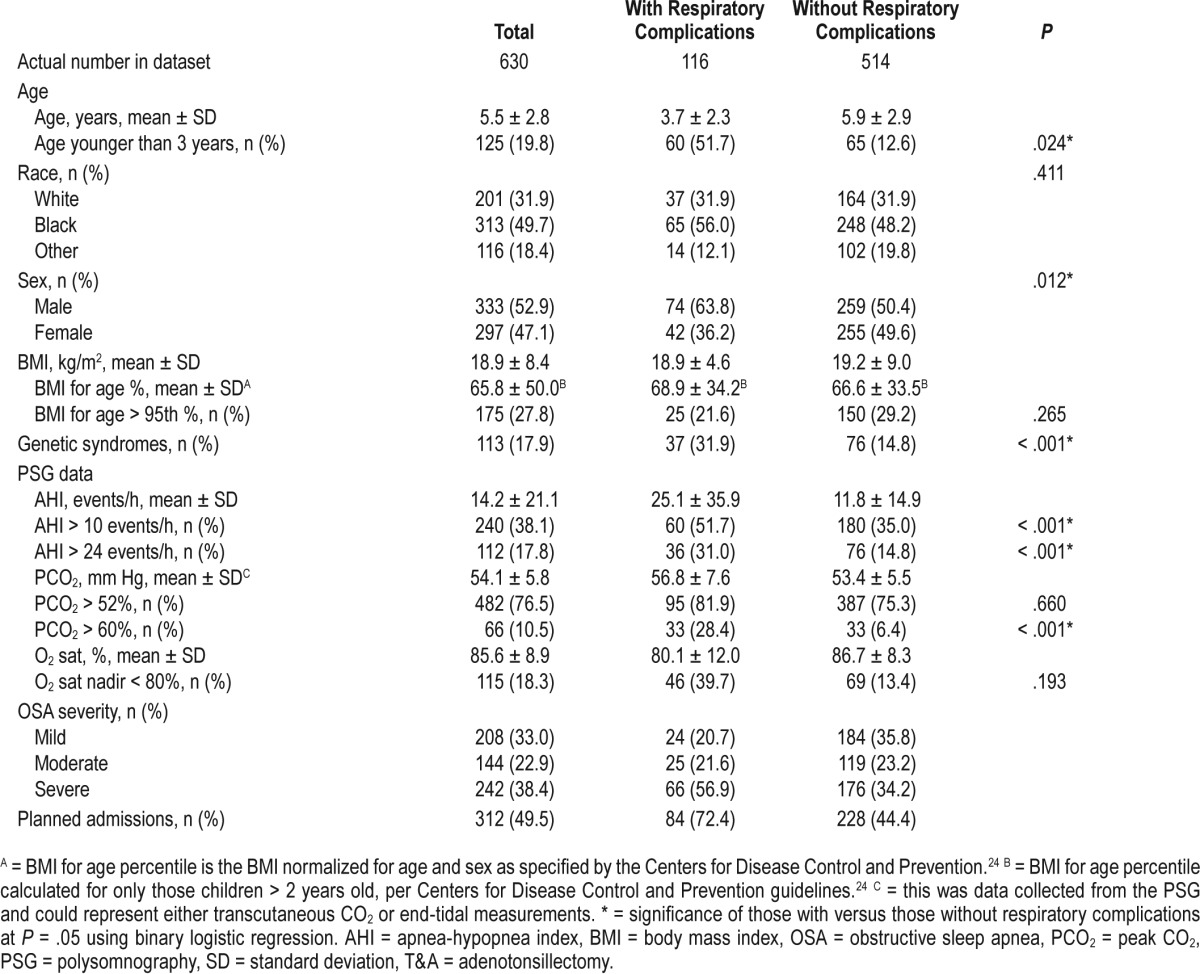
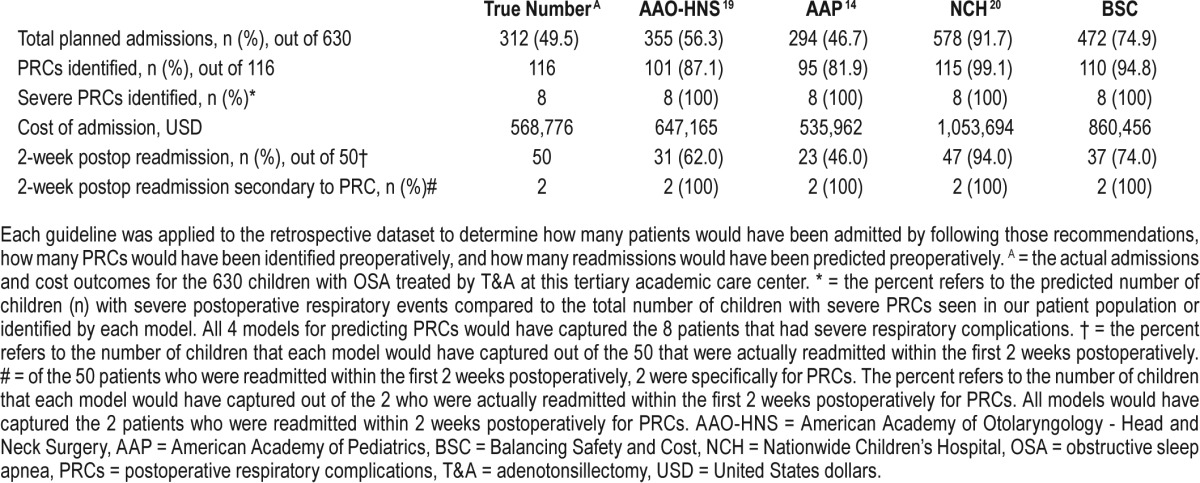
Table 7.
Planned admission, cost comparisons, predicted postoperative respiratory complications, and predicted readmissions for each of the guidelines.
The average AHI for children with PRCs (25.1 ± 35.9 events/h) was significantly higher than in those without PRCs (11.8 ± 14.9 events/h) (P < .001). Similarly, more children with PRCs also had a PCO2 > 60 mm Hg (P < .001) compared to those without PRCs. Seventy-two percent of those with PRCs (84 children) had planned admissions prior to the T&A (Table 1).
The admission criteria for each guideline is listed in Table 1. Applying the AAP criteria to our cohort, 46.7% of the children who underwent T&A would be admitted postoperatively (Table 7). Further, 95 children with PRCs (81.9%) would have been identified, the lowest identification rate of the three published guidelines; the Nagelkerke R2 for the AAP guidelines was 0.311. The H&L and AIC goodness-of-fit scores for the AAP model were 0.724 and 478.9, respectively (Table 3). The AAO-HNS guideline criteria would have required admission for 355 children (56.3%), and 101 children with PRCs (87.1%) would have been identified (Table 7); the Nagelkerke R2 for the AAO-HNS guidelines was 0.275, whereas the H&L and AIC goodness-of-fit scores were 0.850 and 493.8, respectively (Table 2). Approximately 92% of the cohort would require admission based on the NCH guidelines (Table 7), and 115 of the children with PRCs (99.1%) would be identified (Table 7); the Nagelkerke R2 for the NCS guidelines was 0.272, whereas the H&L and AIC goodness-of-fit scores were 0.526 and 494.6, respectively (Table 4). The NCH guidelines had the highest sensitivity (0.991) and NPV (0.998) (Table 8). The AAP guidelines had the highest specificity (0.743) and PPV (0.395). If admission criteria were followed for the AAP, AAO-HNS, and NCH guidelines, cost for admission would be $535,962, $647,165, and $1,053,962, respectively (Table 7).
Table 8.
Diagnostic accuracy of the various admission tools to predict postoperative respiratory complications.
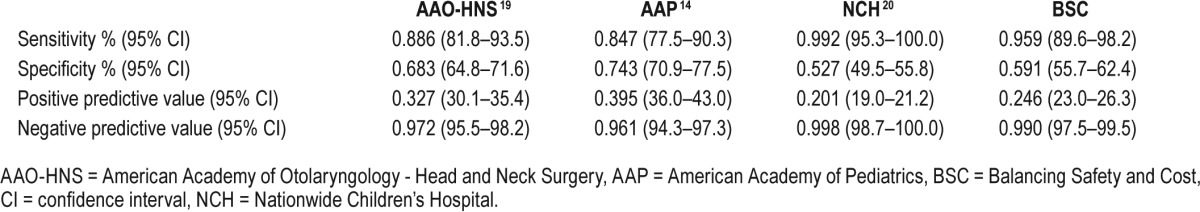
After each guideline was evaluated, the BSC model was created by balancing individual parameters, which contribute to PRCs and the cost of admission, as a forced model. The BSC model would recommend admission for 472 children (74.9%), and 110 children (94.8%) with PRCs would be identified (Table 7). The Nagelkerke R2 for our model was 0.269, whereas the H&L and AIC goodness-of-fit scores were 0.990 and 496.5, respectively. The application of this model to our cohort would result in an admission cost of $860,456, far less than what was recommended by Raman et al. in the NCH model.20 The BSC model presented here had a high sensitivity and NPV compared with most of the other models (Table 8).
Among our study population, 50 children were readmitted within the first 2 weeks after discharge (Table 7). Only 2 of these patients were readmitted for PRCs. The other 48 patients were readmitted for pain, dehydration, postoperative bleeding, or other complications such as upper respiratory infections. Of note, each of the four models would have predicted PRCs in 8 patients who had severe PRCs requiring reintubation (Table 7).
DISCUSSION
Our study is the first to compare the number of children with PRCs that would be identified and the cost of admission for those children using the three published guidelines for admission after T&A for OSA. Following guidelines from the AAP,14 AAO-HNS,19 and NCH,20 82%, 87%, and 99% of children from a large, retrospective cohort with PRCs would be identified and cost $535,962, $647,165, and $1,053,694 for admission, respectively. After examining the admission criteria, we found that refining the guidelines would result in an admission rate of 75%, costing $860,456, and the identification of 95% of those children from our cohort with known PRCs.
PRCs are common among children with OSA after airway surgery. Given the large number of children in the United States with OSA that undergo T&A each year, it is imperative that we can accurately predict which children are at highest risk for PRCs and appropriately monitor them postoperatively. Several published guidelines define standards for admission after T&A for those children who should be closely monitored; however, none of these parameters are universally accepted. We hypothesized that significant variability would exist in the number of children with PRCs that would be identified using each of the guidelines to screen our patient population. As expected, if guidelines from the NCH20 were followed, 99% of those children in our cohort with known PRCs would have been detected preoperatively, compared with 82% following criteria published by the AAP.14 However, the NCH guidelines would also require admission for almost 92% of those children undergoing T&A. If cost were not a concern, we would theoretically admit every patient after T&A for monitoring. Given the current health care climate, we must deliver quality health care through cost-effective means.
Previous work has examined the ability of some of the published guidelines to predict perioperative respiratory complications. A large prospective, observational cohort study demonstrated that a model using age, O2 sat nadir, and PCO2 predicted respiratory complications better than those guidelines outlined by the AAP and AAO-HNS.27 However, the authors also concluded that their model had limitations in predicting the need for postoperative admission. The nonvalidated model presented in our study also identified age and PCO2 as good predictors of these PRCs. Variation in the other predictors could also be due to the fact that our model included an analysis of a different patient population. Although preliminary, our study also included a cost comparison between the models. It is important to note that our method of capturing events included a retrospective analysis of any recorded PRC. Given that this method included respiratory events that do not necessarily translate to a change in clinical course, we may be overestimating the number of clinically relevant respiratory events. As such, this study is exploratory in nature to better understand risk factors for PRCs and to determine how this may affect cost.
Due to recent expansion in coverage through state and federal insurance programs, there is increased pressure on health care systems to establish best-practice models for cost-effectiveness and patient safety. Physicians are now tasked with limiting unnecessary tests, preventing readmission for patients recently discharged from the hospital, and decreasing costs.28 As an example of the importance of analyzing cost in the health care system, the Panel on Cost-Effectiveness in Health and Medicine has even developed recommendations to improve the quality of cost-effectiveness studies using consequences of interventions.29 Some states have even moved to all-payer approaches where the model of cost savings is of utmost importance.30 Therefore, it is imperative that anesthesiologists, otolaryngologists, and pediatric pulmonologists determine a standard of care that can be used to identify those children at risk for PRCs and establish general admission criteria that are both cost-effective and safe. We hypothesized that, along with the identification of children with PRCs, the cost for admission dictated by each guideline would be variable. In parallel with the number of admissions, a policy that reflects guidelines outlined by the NCH would cost almost $1.1 million, compared with $535,000 by the AAP. After modifying the admission criteria, admissions based on our BSC model would cost $860,000 but capture almost all of the patients who had known PRCs (95%).
Our results demonstrated that age, AHI, PCO2, and comorbid conditions including cystic fibrosis, craniofacial anomalies, asthma, and cardiac and other major medical conditions, can be accurately used to identify children with PRCs while limiting the number of unnecessary hospital admissions. Further, non-whites were more likely to experience PRCs in our patient cohort (P = .005, Table 5). Of note, our model does not use obesity or oxygen saturation in our admission criteria. This does not suggest that these parameters are poor predictors of PRCs after T&A for children with OSA. In fact, obesity is well established as a predictor of PRCs. In a retrospective study of 100 severely obese children undergoing tonsillectomy, Gleich et al. demonstrated that severe obesity was independently associated with an increased risk of perioperative airway complications.31 The predictors outlined in our model captured most of the children who had known PRCs because we were able to analyze this retrospective cohort multiple times using different formulas to select the best combination. Using the criteria that we presented, almost all of the obese children were already included based on their other high-risk criteria. Because our criteria capture them regardless, we did not have to specifically include obesity as an individual admission criteria.
In our study, 8 children had critical postoperative respiratory events requiring reintubation. In 7 of these patients (87.5%), a genetic syndrome was diagnosed. Only 37.5% of the patients had a BMI percentile for age > 95%. Given that only one child requiring reintubation did not have a genetic condition, it is possible that the PRC had nothing to do with the parameters listed in the admission criteria but was entirely related to other occurrences during admission (administered drug combinations, other undiagnosed medical comorbidities, etc). However, if the occurrence of this PRC was not at least indirectly related to risks outlined in the different admission criteria, this PRC would not necessarily have been identified using the guidelines (and all four guidelines identified all 8 patients who required reintubation).
Interestingly, all children requiring reintubation did so within 2 to 3 hours after the procedures were completed. This would suggest that clinical judgments could be based on the immediate postoperative course in the recovery room. However, many other PRCs requiring at least oxygen supplementation did occur over the first full 24 hours postoperatively. It is difficult to predict if the intervention provided at the time of PRCs prevented other worse outcomes or more serious respiratory difficulties. Future prospective studies should examine the time course of respiratory events in these children undergoing T&A.
There are several limitations of our study worth discussing. First, this study was used to compare the number of hospital admissions that would have been performed based on the already published admission guidelines available for clinicians. The forced model presented in this paper was not validated for use clinically because it only serves to more clearly delineate those most significant risk factors outlined in all of the admission guidelines. This illustration was offered as an example for considering both safety and cost. Using the data obtained retrospectively to support the model is a potential source for error, limiting validity of the information extracted from the database. However, the use of a patient population known to have PRCs allowed us to create models based on actual outcomes. Because this study was performed by gathering information from electronic medical records retrospectively, there is the potential for loss of data as some of the necessary scoring criteria information may be misinterpreted or not measured. As a post hoc analysis, we did not perform internal or external validation of our data. It is a single-center study at a large, tertiary academic hospital that may limit generalizability to other settings. In order to include the largest number of participants possible, no exclusion criteria were established for the type of anesthesia administered perioperatively. For this reason, drug choices, dosing, and opiate administration varied between patients. Given the large variability in anesthesia practices, there are likely significant differences in the combinations of anesthetics and opiates administered in the operating rooms. Further, it is also difficult to appropriately track the specific opiates and dosing regiments used in the recovery rooms. Without the ability to standardize these practices, it is important to understand that differences in the administration of these drugs could dramatically affect the risks for PRCs in children with OSA. Additionally, anesthesia risk (Cormack and Lehane score32) was not routinely recorded, and this measure could not be used in our analysis to predict PRCs postoperatively. Although a cost analysis was performed, this was based only on the charge-per-patient for a 1-night admission to the floor. The total costs do not include the costs of PSG and do not compare 23-hour observation versus formal admission. Because the models do not make recommendations for elevation of care, no admissions to the pediatric intensive care unit were included in these cost analyses. Also, no cost analysis was performed for those patients with multiple nights of admission. The respiratory complications were obtained from nursing staff reports, and thus it is possible that respiratory complications were treated but not recorded. Our collection method also likely includes respiratory events that did not result in a change in clinical course, overestimating the number of events that are clinically relevant. Although we included a large cohort of children for our study, we did not have enough variability to stratify the importance of individual comorbidities. All current guidelines only mention a few medical comorbidities that should warrant admission; however, other comorbid conditions not yet considered in admission guidelines could increase the risk of PRCs after T&A. Additionally, comorbidities were not substratified to determine the exact risk associated with each factor. However, future studies should be designed to determine the specific level of risk for each of the comorbidities. The risk associated with the presence of certain combinations of these disease comorbidities would be especially useful for practicing clinicians who treat these patients perioperatively. Larger prospective studies are needed to more thoroughly examine these conditions.
CONCLUSIONS
PRCs after T&A are common, and identifying at-risk children is crucial so that disposition planning can be appropriately conducted. Examining currently published guidelines using known admissions either did not identify enough children having PRCs or admitted too many patients unnecessarily, leading to increased cost. At that same time, more stringent regulations and decreases in reimbursement have forced physicians to limit excessive costs to the health care system, thus making it imperative that we identify those children at risk for PRCs to optimize patient safety while limiting total costs. Additional prospective studies are necessary to validate more current guidelines that identify patients at risk for PRCs while also taking into account the burden on the health care system.
DISCLOSURE STATEMENT
Work for this study was performed at Johns Hopkins Hospital, Baltimore, MD. The authors report no conflicts of interest.
ABBREVIATIONS
- AAO-HNS
American Academy of Otolaryngology – Head and Neck Surgery
- AAP
American Academy of Pediatrics
- AHI
apnea hypopnea index
- AIC
Akaike Information Criteria
- BMI
body mass index
- BSC
Balancing Safety and Cost
- eB
exponentiation of the B coefficient
- ETCO2
end tidal carbon dioxide
- H&L
Hosmer and Lemeshow
- NCH
Nationwide Children's Hospital
- O2 sat nadir
oxygen saturation nadir
- oAHI
obstructive apnea hypopnea index
- OSA
obstructive sleep apnea
- PCO2
peak carbon dioxide
- PRCs
postoperative respiratory complications
- PSG
polysomnography
- T&A
adenotonsillectomy
REFERENCES
- 1.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behavior in 4-5 year-olds. Arch Dis Child. 1993;68:360–366. doi: 10.1136/adc.68.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Resp Crit Care Med. 1999;159(5 Pt 1):1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 3.Farber JM. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;110(6):1255–1257. doi: 10.1542/peds.110.6.1255-a. [DOI] [PubMed] [Google Scholar]
- 4.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3 Pt 1):616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 5.Weissbluth M, Davis AT, Poncher J, Reiff J. Signs of airway obstruction during sleep and behavioral, developmental, and academic problems. J Dev Behav Pediatr. 1983;4(2):119–121. doi: 10.1097/00004703-198306000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among non-obese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142–1149. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Eva RC, Baur LA, Donaghue KC, Waters KA. Metabolic correlates with obstructive sleep apnea in obese subjects. J Pediatr. 2002;140(6):654–659. doi: 10.1067/mpd.2002.123765. [DOI] [PubMed] [Google Scholar]
- 8.Amin RS, Carroll JL, Jeffries JL, et al. Twenty-four ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169(8):950–956. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 9.Enright PL, Goodwin JL, Sherrill DL, Quan JR, Quan SF Tucson Children's Assessment of Sleep Apnea Study. Blood pressure elevation associated with sleep-related breathing disorder in a community sample of white and Hispanic children: the Tucson Children's Assessment of Sleep Apnea Study. Arch Pediatr Adoles Med. 2003;157(9):901–904. doi: 10.1001/archpedi.157.9.901. [DOI] [PubMed] [Google Scholar]
- 10.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1098–1103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 11.Quan SF, Gersh BJ National Center on Sleep Disorders Research, National Heart, Lung, and Blood Institute. Cardiovascular consequences of sleep-disordered breathing: past, present and future: report of a workshop from the National Center on Sleep Disorders Research and the National Heart, Lung, and Blood Institute. Circulation. 2004;109(8):951–957. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 12.Singer LP, Saenger P. Complications of pediatric obstructive sleep apnea. Otolaryngol Clin North Am. 1990;23(4):665–676. [PubMed] [Google Scholar]
- 13.Baugh RF, Archer SM, Mitchell RB, et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 Suppl):S1–S30. doi: 10.1177/0194599810389949. [DOI] [PubMed] [Google Scholar]
- 14.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell RB, Pereira KD, Friedman NR. Sleep-disordered breathing in children: survey of current practice. Laryngoscope. 2006;116(6):956–958. doi: 10.1097/01.MLG.0000216413.22408.FD. [DOI] [PubMed] [Google Scholar]
- 16.De Luca Canto G, Pacheco-Pereira C, Aydinoz S, et al. Adenotonsillectomy complications: a meta-analysis. Pediatrics. 2015;136(4):702–718. doi: 10.1542/peds.2015-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye J, Liu H, Zhang G, Huang Z, Huang P, Li Y. Postoperative respiratory complications of adenotonsillectomy for obstructive sleep apnea syndrome in older children: prevalence, risk factors, and impact on clinical outcome. J Otolaryngol Head Neck Surg. 2009;38(1):49–58. [PubMed] [Google Scholar]
- 18.Nixon GM, Kermack AS, Davis GM, Manoukian JJ, Brown KA, Brouillette RT. Planning adenotonsillectomy in children with obstructive sleep apnea: the role of overnight oximetry. Pediatrics. 2004;113(1 Pt 1):e19–e25. doi: 10.1542/peds.113.1.e19. [DOI] [PubMed] [Google Scholar]
- 19.Roland PS, Rosenfeld RM, Brooks LJ, et al. Clinical practice guideline: polysonmography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 Suppl):S1–S15. doi: 10.1177/0194599811409837. [DOI] [PubMed] [Google Scholar]
- 20.Raman VT, Jatana KR, Elmaraghy CA, Tobias JD. Guidelines to decrease unanticipated hospital admission following adenotonsillectomy in the pediatric population. Int J Pediatr Otorhinolaryngol. 2014;78(1):19–22. doi: 10.1016/j.ijporl.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Schwengel DA, Sterni LM, Tunkel DE, Heitmiller ES. Perioperative management of children with obstructive sleep apnea. Anesth Analg. 2009;109(1):60–75. doi: 10.1213/ane.0b013e3181a19e21. [DOI] [PubMed] [Google Scholar]
- 22.McColley SA, April MM, Carroll JL, Naclerio RM, Loughlin GM. Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118(9):940–943. doi: 10.1001/archotol.1992.01880090056017. [DOI] [PubMed] [Google Scholar]
- 23.Wilson K, Lakheeram I, Morielli A, Brouillette R, Brown K. Can assessment for obstructive sleep apnea help predict postadenotonsillectomy respiratory complications. Anesthesiology. 2002;96(2):313–322. doi: 10.1097/00000542-200202000-00015. [DOI] [PubMed] [Google Scholar]
- 24.About Child & Teen BMI. Centers for Disease Control and Prevention website. [Accessed November 17, 2015]. http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html. Published May 15, 2015. [Google Scholar]
- 25.Harrell FE., Jr . Regression Modeling Strategies. 2nd ed. New York, NY: Springer International Publishing; 2015. Binary logistic regression; pp. 219–274. [Google Scholar]
- 26.Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociolog Methods Res. 2004;33:261–304. [Google Scholar]
- 27.Thongyam A, Marcus CL, Lockman JL, et al. Predictors of perioperative complications in higher risk children after adenotonsillectomy for obstructive sleep apnea: a prospective study. Otolaryngol Head Neck Surg. 2014;151(6):1046–1054. doi: 10.1177/0194599814552059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lower Costs, Better Care: Reforming Our Health Care Delivery System. [Accessed November 21, 2015]. Centers for Medicare and Medicaide Services website. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-Sheets/2013-Fact-Sheets-Items/2013-02-28.html. Published February 28, 2013. [Google Scholar]
- 29.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 30.Rajkumar R, Patel A, Murphy K, et al. Maryland's all-payer approach to delivery-system reform. N Engl J Med. 2014;370(6):493–495. doi: 10.1056/NEJMp1314868. [DOI] [PubMed] [Google Scholar]
- 31.Gleich SJ, Olson MD, Sprung J, et al. Perioperative outcomes of severely obese children undergoing tonsillectomy. Paediatr Anaesth. 2012;22(12):1171–1178. doi: 10.1111/j.1460-9592.2012.03905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39(11):1105–1111. [PubMed] [Google Scholar]


