Abstract
MicroRNA-22 (miR-22) is a highly conserved microRNA that can regulate cell proliferation, oncogenesis, and cell maturation, especially during stress. In hematopoietic stem cells (HSCs) miR-22 has been reported to be involved in the regulation of key self-renewal factors including Tet2. Recent work demonstrates that miR-22 also participates in regulation of the interferon response, and expression profiling studies suggest that it is variably expressed at different stages in erythroid differentiation. We thus hypothesized that miR-22 regulates maturation of erythroid progenitors during stress hematopoiesis through its interaction with interferon. We compared the blood and bone marrow of wild type (WT) and miR-22-deficient mice at baseline and upon infectious challenge with systemic lymphochoriomeningitis (LCMV) virus. MiR-22-deficient mice maintained platelet counts better than WT mice during infection, but they showed significantly reduced red blood cells (RBC) and hemoglobin. Analysis of bone marrow progenitors demonstrated better overall survival and improved HSC homeostasis in infected miR-22-null mice compared to WT, attributable to a blunted interferon response to LCMV challenge in the miR-22-null mice. We found that miR-22 was exclusively expressed in stage II erythroid precursors and was downregulated upon infection in WT mice. Our results indicate that miR-22 promotes the interferon response to viral infection and that it functions at baseline as a brake to slow erythroid differentiation and maintain adequate erythroid potential. Impaired regulation of erythrogenesis in the absence of miR-22 can lead to anemia during infection.
Keywords: microRNA, mir-22, erythropoiesis, interferon alpha, stress hematopoiesis
Introduction
Inflammatory signaling and hematopoiesis are closely intertwined. Hematopoietic cells are rapidly consumed during infectious stress by clearance of immune complexes, apoptosis, or direct infection1, and their replacement is critical for survival. Hematopoietic progenitors in the bone marrow respond to this increased need for blood production by multiple routes. Decreased density of cells in the bone marrow can trigger proliferation by bone marrow progenitors2. Alternatively, exocrine signaling either directly to progenitor cells or to supportive cells of the bone marrow niche can promote cell cycle activity. For instance, G-CSF is induced during infection and promotes hematopoietic stem cell (HSC) expansion3. Finally, inflammatory cytokines such as interferons are known to activate immune cells and can have direct stimulatory effects on hematopoietic progenitors. For example, interferon alpha (IFNα) and interferon gamma (IFNγ) have been shown to induce HSC division in murine and human systems4–6. Inflammatory signaling pathways are thought to be critical in certain hematologic malignancies7. Still, many of the mechanisms by which inflammatory signals control division and differentiation of hematopoietic progenitors remain unknown.
Recent work indicates that microRNA-22 (miR-22) could be a pivotal regulator at the nexus of hematopoiesis and inflammation8. MiR-22 is an evolutionarily conserved microRNA that has been shown to play a role in diverse cellular functions including cell proliferation, oncogenesis, tumor suppression, and cell maturation, particularly in response to stress 9–13. In blood, miR-22 acts as a tumor suppressor in T cell lymphoma by inhibiting expression of oncogenic targets such as PTEN and CDK2. Meanwhile, JAK3, STAT3, and STAT5 repress miR-22 expression; thus, miR-22 provides an important link to explain how activating mutations in the JAK-STAT pathway promote cancer14. Additionally, increased expression of miR-22 correlates with poor survival in myelodysplastic syndrome (MDS) and leukemia15, and the tumor suppressor and epigenetic modifier Tet2 is a key target of miR-2216, 17. Patients with MDS express high miR-22 levels and HSCs over-expressing miR-22 show increased replating and repopulation capacity, indicative of more aggressive disease17. In contrast, patients with acute myelogenous leukemia (AML) have been reported to have decreased levels of miR-22, likely reflecting expression variability across cell types and stages of differentiation18, 19.
Using transgenic expression of miR-22 in quiescent fibroblasts, Polioudakis et al. showed that miR-22 suppresses IRF5 and HMGB1, two factors important to activating an interferon-mediated pro-inflammatory response through NK-κB and IRF320. MiR-22 overexpression enhances development of conventional dendritic cells (cDC) through suppression of the interferon response gene Irf8, and miR-22 is required for DC activation of TH17 responses through direct inhibition of the histone deacetylase HDAC421, 22. Importantly, miR-22 has also been implicated in erythroid maturation, as expression of miR-22 was found to correlate with increasingly mature states of erythroid maturation in ex vivo culture of human CD34+ and K562 cells23. This finding has been corroborated in murine progenitors in vivo24. Given prior work showing that interferons can promote HSC proliferation and myeloid differentiation and may also affect erythroid differentiation4, 6, we hypothesized that miR-22 regulates erythroid maturation during stress hematopoiesis.
Since we predicted that miR-22 function is particularly important under stress conditions and that these effects may be evident during in vivo infection, we compared blood and bone marrow hematopoietic progenitor populations of wild type (WT) and miR-22 knock out (KO) mice in the setting of acute viral infection with lymphochoriomeningitis virus (LCMV). LCMV is a model pathogen that has been widely used to study interferon-mediated immune responses to viral infections25, 26. We found that following infection erythropoiesis was impaired in miR-22 KO animals while megakaryopoiesis was enhanced, suggesting that miR-22 functions to control the balance of erythroid and megakaryocyte differentiation from their common precursor. This study thus provides in vivo evidence that miR-22 plays a critical role in regulating erythroid differentiation during infectious stress.
Materials and Methods
Mice
Mir22-KO mice were generated as previously described in 129 background and backcrossed to C57Bl/6 albino mice for at least 6 generations13. Wild-type C57Bl/6 were used as controls. All mouse strains were maintained at an AALAC-accredited, specific-pathogen-free animal facility at Baylor College of Medicine. Genotypes were confirmed by PCR of genomic DNA13. All experiments used gender-matched mice that were between 8 and 12 weeks of age.
Microbial Infections
Lymphochoriomeningitis virus subtype Armstrong was obtained from Michael Jordan (U. Cincinnati). LCMV propagated in BHK21 cells was titered using a standard plaque assay as well as realtime quantitative PCR analysis27, 28. LCMV titers from bone marrow nucleated hematopoietic cells were determined by realtime quantitative PCR analysis using a standard curve. Mice were infected by intraperitoneal (IP) injection of 1×105 plaque-forming units (PFU) and blood, serum, and bone marrow were harvested after 6 days unless otherwise noted. Survival studies were done by intravenously (IV) injecting miR-22 KO and WT mice with 4×105 PFU LCMV. Mice were monitored for 15 days and sacrificed if they were determined to be moribund.
Peripheral blood analysis
Complete blood counts were done at baseline and at various times post LCMV infection using an Advia 120 Hematology System (Siemens).
Cytokine analysis
Interferon alpha ELISA was conducted 2.5 days post LCMV infection (1×105 PFU IP). Serum was collected from infected and mock-infected miR-22 KO and WT animals and ELISA was completed with the BD Platinum IFNa kit.
Flow cytometry
Hematopoietic progenitors, erythroid precursors, and mature leukocytes were assessed from the bone marrow of infected and uninfected miR-22 KO animals and WT controls 6 days post infection. Flow cytometric markers used to analyze various populations were as follows: HSCs (Lin− cKit+ CD150+ CD48− CD34−), myeloid (GR1+), B cells (B220+), and T cells (CD4+ or CD8+), common myeloid progenitors (CMPs) (Lin− cKit+ IL7ra− CD34+ CD16/32−), granulocyte-monocyte progenitors (GMPs) (Lin− cKit+ IL7ra− CD34+ CD16/32+), megakaryocyte-erythroid progenitors (MEPs) (Lin− cKit+ IL7ra− CD34− CD16/32−), megakaryocyte progenitors (Lin− CD34+ CD41+), and stages in erythroid development (Lin− CD71+/− Ter119+/−); plasmacytoid DCs (CD11c+ SiglecH+ B220+ CD11b−); conventional DCs (CD11c+ CD11b+ B220−); T cells (CD3+); B cells (CD19+); mature neutrophils (Gr1hi CD11b+); immature neutrophils (Gr1lo CD11b+).
Antibodies used included: CD45.2 (104), CD48 (HM48-1), CD71 (R17217), Ter119, CD117 (2B8), Sca1 (D7), CD4 (GK1.5), CD8 (53-6.7), Mac1/CD11b (M1/70), F4/80 (BM8), CD150 (MSHAD150), CD11c (N418), SiglecH (eBio440c), B220 (RA3-6B2), CD11b (M1/70), CD19 (eBio1D3), CD3 (145-2C11) obtained from eBiosciences. CD45.1 (A20) was obtained from BioLegend and Gr1 (RB6-8C5) was obtained from BD.
Methylcellulose assays
104 whole bone marrow leukocytes were suspended in 1 mL methylcellulose (Methocult 3434, Stem Cell Technologies) and incubated at 37°C. Colonies were counted and typed by morphology after 8 days of incubation.
Quantitative PCR
Tet2 transcript levels were assessed in WBM cells using quantitative realtime PCR using Taqman assay mm00524395 (ThermoFisher) normalized to 18S rRNA.
Statistical analysis
All data were analyzed using using Student’s t-test, 2-way ANOVA with Tukey’s multiple comparisons test or Kaplan-Meier analysis in GraphPad Prism 6.
Results
MiR-22 KO mice maintain WBC counts but lose RBCs during infection
Initially we examined miR-22 null mice for potential baseline changes in immune cells populations in peripheral blood. Among the total leukocytes, there were only subtle differences between the genotypes, with an expanded monocyte population and slightly more neutrophils in miR-22 KO animals at baseline (Figure 1A–E).
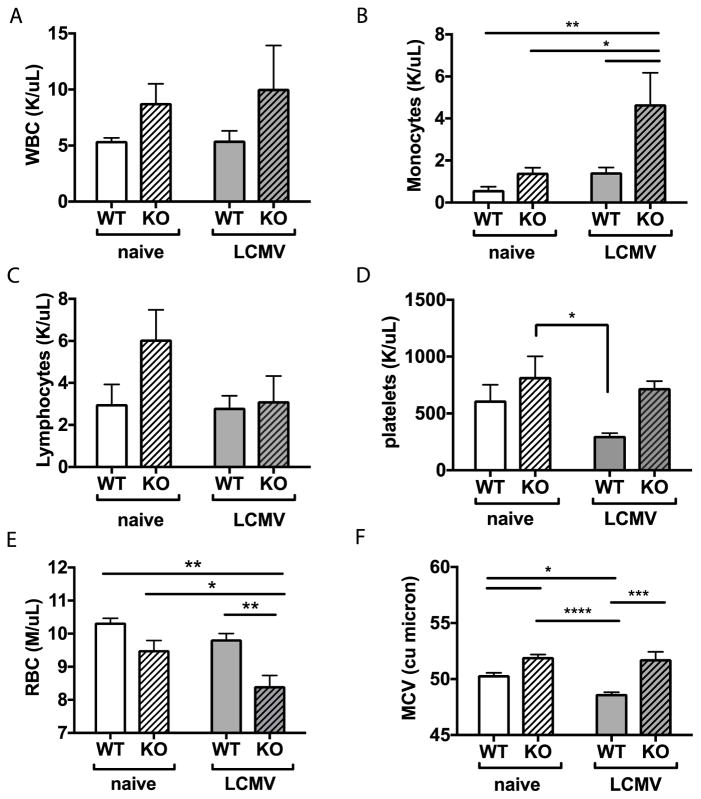
Figure 1. Peripheral blood counts reveal decreased erythrocytes in miR-22 KO mice during infection.
Peripheral blood counts from WT or miR-KO mice at baseline or 6 days post LCMV infection were measured using an automated hematology system. (A) There were no significant changes in total white blood count. (B) Monocytes increased upon infection; whereas (C) lymphocytes trended downwards. (D) Platelets decreased in WT but not miR-22 KO animals during infection. (E) Red blood cells (RBC) were diminished at baseline and declined further in miR-22 KO mice upon infection. (F) Mean corpuscular volume of RBCs (MCV) declined in WT mice but not in miR-22 KO mice. n=3–5 per group. Data are representative of 2–3 independent experiments. Mean+SEM. * p<0.05; ** p<0.01, **** p<0.0001 by 2-way ANOVA. Differences that do not meet statistical significance are not labelled.
MicroRNAs are frequently devoid of phenotypes in absence of stress, therefore we next compared peripheral blood immune cell numbers in miR-22 KO versus WT mice 6 days after a non-lethal short-term LCMV infection which is near the peak of response in blood29. Notably, platelets were depleted in WT animals during LCMV infection but not in miR-22 KO animals (Figure 1D). Conversely, the red blood cell (RBC) count was significantly depleted in miR-22 KO animals during infection (Figure 1E), with a drop in hemoglobin that was more pronounced in miR-22 KO animals compared to WT (Supplementary Figure 2). The mean corpuscular volume (MCV) was elevated in mir-22 KO animals, consistent with an enrichment in immature erythrocytes (Figure 1F). On the other hand, lymphocyte counts including T and B cells were not different between WT and miR-22 KO mice suggesting that the changes were more specific rather than general (Supplementary Figure 1B–C). The cDC population increased normally in miR-22 KO mice upon LCMV infection, indicating that miR-22 is not required for cDC expansion in this setting (Supplementary Figure 1D). In summary, peripheral blood analysis revealed that miR-22 KO animals maintain WBC and platelet numbers during infection but become significantly anemic during infection.
MiR-22 KO mice survive a lethal dose of LCMV
Given the severe anemia evident in miR-22 KO animals upon infection, we wondered if these animals would be more susceptible to a lethal challenge with LCMV. We therefore exposed miR-22 KO animals and age- and gender-matched WT controls to a lethal dose of LCMV by IV injection. All animals became robustly infected, and miR-22 KO animals had a viral load comparable to WT animals at 2.5 and 6 days post-infection (Figure 2A), with a trend towards higher titers suggesting impaired viral clearance by these animals. Surprisingly, miR-22 KO animals survived a lethal LCMV challenge at a significantly greater frequency than WT mice (Figure 2B).
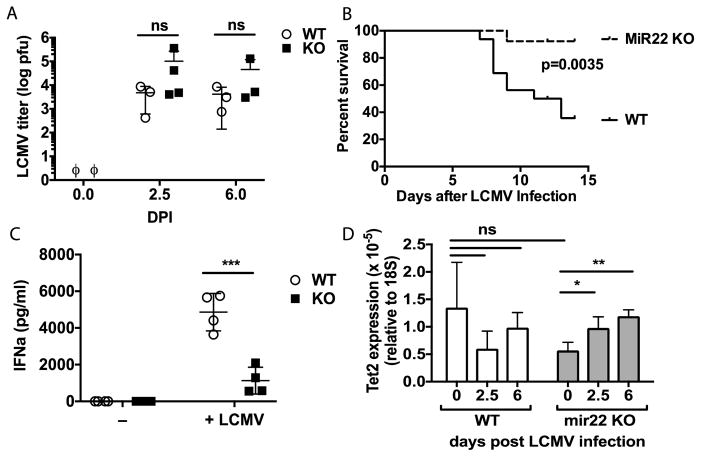
Figure 2. MiR-22 KO mice show immune tolerance and improved survival to LCMV challenge.
(A) LCMV titer in nucleated bone marrow cells of miR-22 KO and WT mice 6 days post-infection with 1 × 105 pfu LCMV IP, as determined by quantitative PCR and a standard curve. n=3 per group. ϕ indicates below the limits of detection. Data are representative of two independent experiments. (B) miR-22 KO mice and WT controls were infected with 5 × 105 pfu LCMV Armstrong by IV injection and monitored for 15 days. N=13–16; data compiled from 3 independent experiments and analyzed by Kaplan-Meier. (C) IFNa levels in serum at days 2.5 and 6 post-infection with 1 × 105 pfu LCMV IP. n=2–5 for each experimental group. Data are representative of two independent experiments. (D) Tet2 expression in whole blood relative to 18S RNA expression as determined by qPCR. Mean and SEM from 3–4 samples are shown. * p<0.05, ** p<0.01, *** p<0.001 by 2-way ANOVA.
LCMV is a noncytolytic virus that has been shown to cause lethality in mouse models not by direct killing of host tissues but rather by inducing a severe immunological reaction that is primarily mediated by type I interferon30. Normally LCMV elicits a pronounced IFNa response in mice that peaks 2–3 days after infection29. IFNa can then induce a cascade of cytokines including TNFa and IL1 that promote vasodilation and circulatory collapse.30 We thus measured the serum IFNa concentration in miR-22 KO and WT animals on day 3 following infection. Notably, WT animals mounted a strong IFNa response, while the IFNa levels in miR-22 KO were significantly lower (Figure 2C)29. Additionally, miR-22 KO animals demonstrated no baseline elevation in IFNa and no induction of IFNa at day 6 post-infection (Figure 2C, data not shown). Thus, we postulate that miR-22 KO mice survived LCMV lethal challenge because of a deficiency in IFNa production.
Given that miR-22 has been reported to mediate its effects through regulation of Tet2, we quantified the expression of Tet2 in the whole blood of WT versus miR-22 KO mice during infection. Whereas Tet2 expression decreased slightly during infection in WT mice, Tet2 expression increased significantly among miR-22 KO mice upon LCMV infection. These findings suggest that miR-22, either directly or indirectly, has a role in suppressing Tet2 expression during infection (Figure 2D).
MiR-22 KO maintain hematopoietic stem cell (HSC) numbers during infection
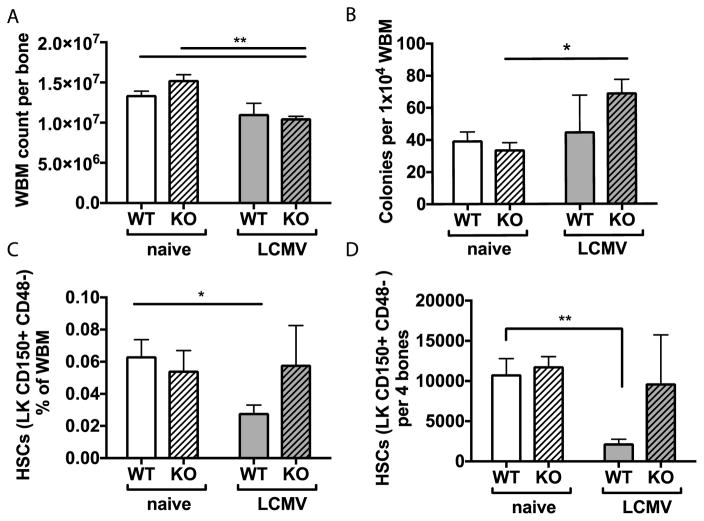
Since IFNa is known to impair HSC self-renewal4, 31, and since miR-22 KO mice produced less IFNa in response to LCMV infection, we predicted that HSCs may be protected from the harmful effects of LCMV infection in miR-22 KO mice. In order to study the role of miR-22 in depletion of hematopoietic progenitor populations in the bone marrow, we analyzed the whole bone marrow (WBM) counts and abundance of colony forming units in the bone marrow. Compared to WT, miR-22 KO mice showed a more significant drop in WBM count upon infection (Figure 3A). Nonetheless, there was no statistical difference in the WBM counts of infected WT versus miR-22 KO mice (Figure 3A). When WBM cells were cultured in methylcellulose, the number of colonies increased in miR-22 KO animals during infection (Figure 3B). Further characterization of the colonies indicated that all colony types were increased in the miR-22 KO mice, with burst forming unit-erythroid (BFU-E) colonies showing the most significant increase (Supplementary Figure 3). These findings suggest that hematopoietic stem and myeloid progenitor cells (HSPCs) are enriched in the marrow of miR-22 KO mice during infection due in part to loss of differentiated cells.
Figure 3. Stem cells are preserved in miR-22 KO mice during infection.
(A) Whole bone marrow (WBM) cell count decreased in miR-22 KO mice at 6 days post-infection. (B) Total colonies in methylcellulose cultures of 104 whole bone marrow leukocytes increased during infection in KO mice. (C) The percentage of total bone marrow cellularity and (D) absolute number of Lin-cKit+CD150+CD48− long-term HSCs decreased in WT animals at day 6 post-infection but remained unchanged in miR-22 KO animals. For all panels, n=3–5 per experimental group. Data are representative of at least 2 independent experiments. * p<0.05; ** p<0.01 by 2-way ANOVA or Student’s t-test. Differences that do not meet statistical significance are not labelled.
We next investigated the frequency of HSPCs by fluorescence activated cell sorting (FACS). We avoided the use of the stem cell marker Sca1, since this marker can be non-specifically activated during inflammatory responses32. The percentage and absolute number of HSCs were diminished in WT animals during infection but not in miR-22 KO animals (Figures 3B–C). Altogether, these results indicate that HSPCs are better preserved in miR-22 KO mice during infection compared to WT, possibly due to dampened IFNa inflammatory responses in these mice.
miR-22 restrains erythroid differentiation and preserves erythroid reserve during steady state hematopoiesis
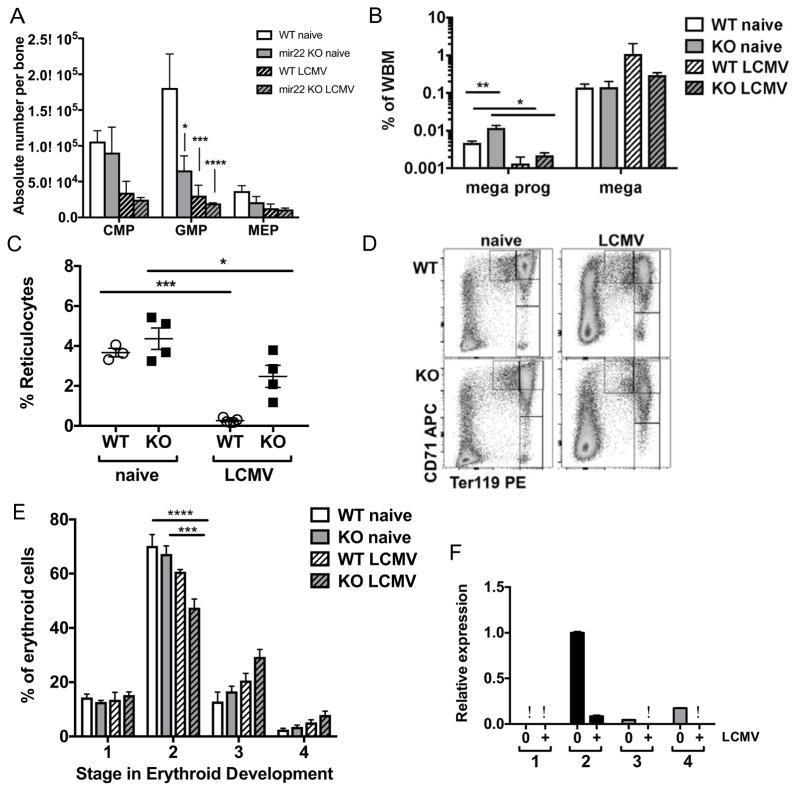
Given our observations that miR-22 mice, unlike WT, have low RBCs but normal platelet numbers in the setting of infection, we investigated myeloid progenitors in the bone marrow of these mice in greater detail. Primitive myeloid progenitors, including common myeloid progenitors (CMP) and granulocyte-monocyte progenitors (GMP), also analyzed with the exclusion of the Sca1 marker, were diminished during LCMV infection regardless of genotype (Figure 4A). The megakaryocyte-erythroid progenitors (MEPs) also trended down for both genotypes with infection, but these differences did not reach statistical significance (Figure 4A).
Figure 4. Erythopoiesis is dysregulated in miR-22 KO animals during infection.
(A) Myeloid progenitors were diminished in both WT and miR-22 KO mice following infection. (B) Megakaryocyte progenitors (mega prog) as a percentage of whole bone marrow were increased at baseline in miR-22 KO mice and diminished during infection. Megakaryocytes (mega) were maintained during infection in both genotypes. (C) Reticulocytes (as a percentage of total leukocytes) were decreased in miR-22 KO mice both at baseline and during infection. (D) Representative flow plot showing stages I–IV in erythroid differentiation in WT (top) versus miR-22 KO (bottom) mice at baseline (left) or in presence of LCMV infection (right). (E) Breakdown of stages in erythroid differentiation as a percentage of Lin (GR-1, Mac1, CD4, CD8, B220) negative leukocytes. (F) Expression of miR-22 relative to stage II baseline erythroid progenitors as determined by qPCR and normalized to internal control. n=3–5 per group. Data are representative of at least 2 independent experiments. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001 by 2-way ANOVA. Differences that do not meet statistical significance are not labelled.ϕ indicates below the limits of detection.
Since platelet numbers were maintained in miR-22 KO animals, we quantified megakaryocytes and megakaryocyte progenitors in the bone marrow by FACS. Megakaryocyte progenitors were diminished in both WT and miR-22 KO, while their progeny megakaryocytes were maintained during infection in both genotypes (Figure 4B, Supplementary Figure 4). Notably, the baseline percentage of Lin- CD34+ CD41+ megakaryocyte progenitors was elevated in miR-22 KO mice (Figure 4B), suggesting that megakaryocyte differentiation may be enhanced in miR-22 KO mice.
Next we characterized erythroid differentiation in the miR-22 KO mice. Reticulocytes were present at a lower frequency in miR-22 KO animals at baseline, and fell during LCMV infection in both WT and KO animals (Figure 4C). This baseline deficit in reticulocytes contrasted with the increase in megakaryocyte progenitors in miR-22 KO mice, suggesting that miR-22 may play a role in the differentiation of erythroid and megakaryocyte progenitors from their common progenitor, the MEP.
Erythrocytes undergo a standard process of differentiation progressing from CD71+Ter119− Stage I progenitors to CD71− Ter119+ Stage IV progenitors. We found that stage II erythroid progenitors were depleted in WT mice in the setting of LCMV infection while stage III and stage IV erythroid precursors were increased, and these differences were exaggerated in miR-22 KO mice (Figure 4D–E). Thus, the distribution of erythroid precursors in healthy miR-22 KO animals mimicked that of infected WT mice. Strikingly, the expression pattern of miR-22 mirrored this distribution, with the highest level of expression per cell detected at erythroid precursor stage II. The expression of miR-22 in WT stage II erythroid precursors was diminished upon infection (Figure 4F). Collectively, these data suggest that in normal conditions miR-22 restrains erythroid differentiation at stage II, and that this restraint is lifted during infection to promote adequate RBC production.
Discussion
In this study, we used miR-22 KO animals to determine the role of miR-22 in interferon responses and hematopoiesis during acute LCMV infection. MiR-22 KO animals showed a blunted interferon response despite a high viral titer following infection, indicating that miR-22 is critical in the generation of inflammatory responses to LCMV. Consistent with this blunted inflammatory response, hematopoietic stem and progenitor cells were better preserved in miR-22 KO mice during infection compared to WT. Furthermore, miR-22 animals showed increased megakaryocyte progenitors and decreased RBC counts following infection. Platelets and erythrocytes are thought to arise from a common progenitor, the megakaryocyte-erythrocyte progenitor (MEP), and our findings suggest that miR-22 may affect the balance between platelet and RBC production at the MEP stage. Further evaluation of miR-22 KO erythroid precursors revealed a stress-like distribution with fewer cells in stage II and more cells at stages III–IV, indicating that miR-22 normally acts as a brake to restrain erythroid development at stage II. We postulate that in the absence of this restraint, miR-22 KO animals do not maintain an adequate reserve of immature populations and are thus especially susceptible to anemia during infectious stress.
A number of microRNAs have been reported to participate in the regulation of stress erythropoiesis33. Ours is the first specific investigation of the role of miR-22 in this process using viral infection in KO animals. These in vivo studies allowed us to evaluate key functional roles of miR-22 during stress, thereby expanding on prior in vitro studies that overexpressed or knocked down miR-22 in specific cell lines. Our studies yielded two findings that answer unresolved questions in the field.
First, miR-22 animals did not mount a normal interferon response to LCMV infection. Prior studies using murine fibroblasts suggested that miR-22 is normally involved in repression of Irf5 and NFkB, two factors that amplify interferon responses.8 Thus, we originally expected that interferon levels in miR-22 KO animals would be excessively high. Quite to the contrary, interferon levels were significantly suppressed following LCMV infection in miR-22 KO animals, even though these animals contained high titers of virus. Dendritic cells are key antigen presenting cells important to activate interferon expression by T and NK cells, and MiR-22 has been implicated in normal dendritic cell development, possibly through repression of the interferon response factor 8 (IRF8).21 Furthermore, miR-22 has also been shown to be essential for bone marrow derived-DC and lung CD11c+ APC activation to endotoxin and other stimulants in a mechanism involving inappropriate induction of HDAC4. Indeed, impaired DC activation has been shown to interfere with cross stimulation of the TH17 response in a mouse model of emphysema22. In our model, both cDCs and pDCs were present in miR-22 KO animals during infection, albeit pDCs at somewhat lower levels. Further studies will be needed to evaluate their functional capacity.
While interferon is a key immune signal required for viral clearance after acute infection, it also causes deleterious effects on the host, most notably triggering lymphocyte apoptosis, inflammation, and an immunosuppressed state34. Indeed, a CD8-T cell-mediated pathologic cascade triggered by DC presentation can be lethal to mice during LCMV infection35. We postulate that the absence of miR-22 may cause defective APC cross activation of T cells in response to infection, which may have contributed to the improved survival of miR-22 KO mice compared to WT. Thus, loss of miR-22 does not significantly affect basal interferon expression in vivo and instead leads to reduced interferon responses during acute viral infection. We therefore speculate that antagomirs to inhibit miR-22 activity may be useful to reduce inflammatory responses, such as during toxic cytokine storm in response to viral infections or during use of cytotoxic T cell therapies for cancer.
The second key finding in our study is that miR-22 KO mice had a significant decrease in RBC levels during infection, whereas platelets were maintained. These reciprocal effects on platelets and RBCs suggest a role for miR-22 in the branchpoint differentiation of MEPs. Whether of not miR-22 expression is specifically diminished in primitive megakaryocyte progenitors with stem cell-like properties remains to be seen36. Furthermore, our data show a decrease in the percentage of stage II erythroid progenitors among miR-22 KO mice, suggesting that miR-22 normally acts as a brake to keep the majority of erythroid precursors at stage II. miR-22 expression decreased during infection in WT mice, effectively releasing the brake to allow erythroid maturation beyond stage II and thereby meet the demands of infection. These findings validate a prior report by Song et al. in which over-expression of miR-22 led to an accumulation of stage II erythroid progenitors17. Thus, similar to many developmental systems that contain auto-regulatory features to control the pace of differentiation 37, miR-22 appears to serve a regulatory role to control the pace of erythropoiesis – by slowing the stage II–III erythroid transition. Our data are consistent with the potential for rapid exhaustion of early erythrocyte progenitors in stressed mice lacking such inhibitory regulation.
IFNa has been shown to significantly alter HSC function, driving these cells out of quiescence and potentially leading to replicative stress and DNA damage over the long term. Since miR-22 KO mice have a blunted IFNa response following LCMV infection, we predicted that their HSCs would be better preserved relative to WT mice. Indeed, miR-22 KO mice demonstrated steady HSC numbers and preserved progenitors in colony forming assays. These results highlight the powerful effects that IFNa can exert on HSPCs and suggest that there may be a therapeutic role for targeting miR-22 in people suffering from bone marrow suppression due to chronic inflammatory conditions such as rheumatoid arthritis.
MiR-22 has been shown to regulate epigenetic programming through the methylcytosine deoxygenase Tet217. Our data support a role for miR-22 in suppressing Tet2 expression. These data indicate that epigenetic programming could be a key component of hematopoietic homeostasis during infectious stress. Tet2 is one of the most commonly mutated genes in normal karyotype AML and MDS38. Inflammatory signaling is a major component of the pathogenesis of MDS, and Tet2 mutations may promote pathogenesis by causing dysregulated inflammatory signaling39. Our data showing that Tet2 expression is not different when comparing WT and miR-22 KO mice at baseline suggest that miR-22 is not the only regulator of Tet2 expression. However, increased Tet2 expression in the miR-22 KO mice during infection suggests that miR-22 can suppress Tet2 expression under inflammatory conditions and thus could have an important therapeutic role in myeloid malignancies.
In summary, strategies to modify miR-22 expression during infection may be useful to curb excessive inflammatory and deleterious hematologic changes during severe viral infections and inflammatory conditions and may have applications in cancer therapy.
Supplementary Material
Highlights.
MiR-22 knock-out mice have a blunted interferon response to viral infection.
MiR-22 exerts reciprocal effects on megakaryocyte and erythrocyte maintenance during infection.
MiR-22 is expressed in Stage II erythroid progenitors to regulate the pace of differentiation.
Acknowledgments
The authors would like to thank V. Sheehan and T. Horton for sharing research equipment, and K. Matatall and K. Josefsdottir for critical reading of the manuscript. This project depended on the support of the BCM Cytometry and Cell Sorting Core with funding from the NIH (NCRR grant S10RR024574, NIAID AI036211 and NCI P30CA125123). CTL is a Hudson Scholar in the Baylor College of Medicine Medical Scientist Training Program. SSW and HSL were supported by NIH NIAID R01AI109294 and seed funding from the MD Anderson Center for Inflammation and Cancer. KYK and CSK were supported by a Texas Children’s Hospital Pediatric Pilot Award, NIH K08HL098898, R01HL136333, R01HL134880 (KYK), the Aplastic Anemia and MDS International Foundation Liviya Anderson Award (KYK), and a March of Dimes Basil O’Connor Starter Scholar Award (KYK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang M, Li CK, Li K, Hon KL, Ng MH, Chan PK, Fok TF. Hematological findings in SARS patients and possible mechanisms (review) Int J Mol Med. 2004;14:311–5. [PubMed] [Google Scholar]
- 2.Cain DW, Snowden P, Sempowski G, Kelsoe G. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS ONE. 2011;6:e19957. doi: 10.1371/journal.pone.0019957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuettpelz LG, Borgerding JN, Christopher MJ, Gopalan PK, Romine MP, Herman AC, Woloszynek JR, Greenbaum AM, Link DC. G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling. Leukemia. 2014 doi: 10.1038/leu.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Essers MAG, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 5.King KY, Matatall KA, Shen CC, Goodell MA, Swierczek SI, Prchal JT. Comparative long-term effects of interferon α and hydroxyurea on human hematopoietic progenitor cells. Experimental Hematology. 2015;43:912–918. doi: 10.1016/j.exphem.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breccia M, Alimena G. NF-kappaB as a potential therapeutic target in myelodysplastic syndromes and acute myeloid leukemia. Expert opinion on therapeutic targets. 2010;14:1157–76. doi: 10.1517/14728222.2010.522570. [DOI] [PubMed] [Google Scholar]
- 8.Polioudakis D, Bhinge AA, Killion PJ, Lee BK, Abell NS, Iyer VR. A Myc-microRNA network promotes exit from quiescence by suppressing the interferon response and cell-cycle arrest genes. Nucleic Acids Res. 2013;41:2239–54. doi: 10.1093/nar/gks1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan S, Li F, Meng Q, Zhao Y, Chen L, Zhang H, Xue L, Zhang X, Lengner C, Yu Z. Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by miR-22. PLoS Genet. 2015;11:e1005253. doi: 10.1371/journal.pgen.1005253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li BX, Yu Q, Shi ZL, Li P, Fu S. Circulating microRNAs in esophageal squamous cell carcinoma: association with locoregional staging and survival. Int J Clin Exp Med. 2015;8:7241–50. [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q, Jiang W, Zhuang C, Geng Z, Hou C, Huang D, Hu L, Wang X. microRNA-22 downregulation of galectin-9 influences lymphocyte apoptosis and tumor cell proliferation in liver cancer. Oncol Rep. 2015;34:1771–8. doi: 10.3892/or.2015.4167. [DOI] [PubMed] [Google Scholar]
- 12.Fiumara F, Rajasethupathy P, Antonov I, Kosmidis S, Sossin WS, Kandel ER. MicroRNA-22 Gates Long-Term Heterosynaptic Plasticity in Aplysia through Presynaptic Regulation of CPEB and Downstream Targets. Cell Rep. 2015;11:1866–75. doi: 10.1016/j.celrep.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Gurha P, Abreu-Goodger C, Wang T, Ramirez MO, Drumond AL, van Dongen S, Chen Y, Bartonicek N, Enright AJ, Lee B, Kelm RJ, Reddy AK, Taffet GE, Bradley A, Wehrens XH, Entman ML, Rodriguez A. Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation. 2012;125:2751–61. doi: 10.1161/CIRCULATIONAHA.111.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibbesen NA, Kopp KL, Litvinov IV, Jønson L, Willerslev-Olsen A, Fredholm S, Petersen DL, Nastasi C, Krejsgaard T, Lindahl LM, Gniadecki R, Mongan NP, Sasseville D, Wasik MA, Iversen L, Bonefeld CM, Geisler C, Woetmann A, Odum N. Jak3, STAT3, and STAT5 inhibit expression of miR-22, a novel tumor suppressor microRNA, in cutaneous T-Cell lymphoma. Oncotarget. 2015;6:20555–69. doi: 10.18632/oncotarget.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solary E, Bernard OA, Tefferi A, Fuks F, Vainchenker W. The Ten-Eleven Translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseases. Leukemia. 2014;28:485–96. doi: 10.1038/leu.2013.337. [DOI] [PubMed] [Google Scholar]
- 16.Coutinho DF, Monte-Mor BC, Vianna DT, Rouxinol ST, Batalha AB, Bueno AP, Boulhosa AM, Fernandez TS, Pombo-de-Oliveira MS, Gutiyama LM, Abdelhay E, Zalcberg IR. TET2 expression level and 5-hydroxymethylcytosine are decreased in refractory cytopenia of childhood. Leuk Res. 2015 doi: 10.1016/j.leukres.2015.07.005. [DOI] [PubMed]
- 17.Song SJ, Ito K, Ala U, Kats L, Webster K, Sun SM, Jongen-Lavrencic M, Manova-Todorova K, Teruya-Feldstein J, Avigan DE, Delwel R, Pandolfi PP. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell stem cell. 2013;13:87–101. doi: 10.1016/j.stem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen C, Chen MT, Zhang XH, Yin XL, Ning HM, Su R, Lin HS, Song L, Wang F, Ma YN, Zhao HL, Yu J, Zhang JW. The PU.1-Modulated MicroRNA-22 Is a Regulator of Monocyte/Macrophage Differentiation and Acute Myeloid Leukemia. PLoS Genet. 2016;12:e1006259. doi: 10.1371/journal.pgen.1006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X, Hu C, Arnovitz S, Bugno J, Yu M, Zuo Z, Chen P, Huang H, Ulrich B, Gurbuxani S, Weng H, Strong J, Wang Y, Li Y, Salat J, Li S, Elkahloun AG, Yang Y, Neilly MB, Larson RA, Le Beau MM, Herold T, Bohlander SK, Liu PP, Zhang J, Li Z, He C, Jin J, Hong S, Chen J. miR-22 has a potent anti-tumour role with therapeutic potential in acute myeloid leukaemia. Nat Commun. 2016;7:11452. doi: 10.1038/ncomms11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polioudakis D, Bhinge AA, Killion PJ, Lee BK, Abell NS, Iyer VR. A Myc-microRNA network promotes exit from quiescence by suppressing the interferon response and cell-cycle arrest genes. Nucleic acids research. 2013;41:2239–54. doi: 10.1093/nar/gks1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li HS, Greeley N, Sugimoto N, Liu Y, Watowich SS. miR-22 controls Irf8 mRNA abundance and murine dendritic cell development. PloS one. 2012;7:e52341. doi: 10.1371/journal.pone.0052341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu W, You R, Yuan X, Yang T, Samuel E, Marcano D, Sikkema W, Tour J, Rodriguez A, Kheradmand F, Corry D. The microRNA miR-22 inhibits the histone deacetylase HDAC4 to promote T(H)17 cell-dependent emphysema. Nat Immunol. 2015;16:1185–94. doi: 10.1038/ni.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choong ML, Yang HH, McNiece I. MicroRNA expression profiling during human cord blood-derived CD34 cell erythropoiesis. Exp Hematol. 2007;35:551–64. doi: 10.1016/j.exphem.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Opalinska JB, Bersenev A, Zhang Z, Schmaier AA, Choi J, Yao Y, D’Souza J, Tong W, Weiss MJ. MicroRNA expression in maturing murine megakaryocytes. Blood. 2010;116:e128–38. doi: 10.1182/blood-2010-06-292920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buechler M, Gessay G, Srivastava S, Campbell D, Hamerman J. Hematopoietic and nonhematopoietic cells promote Type I interferon- and TLR7-dependent monocytosis during low-dose LCMV infection. European Journal of Immunology. 2015;45:3064–3072. doi: 10.1002/eji.201445331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teijaro J, Ng C, Lee A, Sullivan B, Sheehan K, Welch M, Schreiber R, Carlos De La Torre J, Oldstone M. Persistent LCMV Infection Is Controlled by Blockade of Type I Interferon Signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildeman D, Janssen E. IFN-gamma and self-absorbed CD4+ T cells: a regulatory double negative. Nat Immunol. 2008;9:1210–2. doi: 10.1038/ni1108-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mccausland M, Crotty S. Quantitative PCR technique for detecting lymphocytic choriomeningitis virus in vivo. Journal of Virological Methods. 2008;147:167–76. doi: 10.1016/j.jviromet.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matatall KA, Shen CC, Challen GA, King KY. Type II interferon promotes differentiation of myeloid-biased hematopoietic stem cells. Stem Cells. 2014;32:3023–30. doi: 10.1002/stem.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penaloza-MacMaster P, Barber DL, Wherry EJ, Provine NM, Teigler JE, Parenteau L, Blackmore S, Borducchi EN, Larocca RA, Yates KB, Shen H, Haining WN, Sommerstein R, Pinschewer DD, Ahmed R, Barouch DH. Vaccine-elicited CD4 T cells induce immunopathology after chronic LCMV infection. Science. 2015;347:278–82. doi: 10.1126/science.aaa2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, Moehrle B, Brocks D, Bayindir I, Kaschutnig P, Muedder K, Klein C, Jauch A, Schroeder T, Geiger H, Dick TP, Holland-Letz T, Schmezer P, Lane SW, Rieger MA, Essers MAG, Williams DA, Trumpp A, Milsom M. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520:549–52. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- 32.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32:57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azzouzi I, Schmugge M, Speer O. MicroRNAs as components of regulatory networks controlling erythropoiesis. Eur J Haematol. 2012;89:1–9. doi: 10.1111/j.1600-0609.2012.01774.x. [DOI] [PubMed] [Google Scholar]
- 34.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–40. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou R, Zhang M, Huang L, Flavell RA, Koni PA, Moskophidis D. Regulation of immune response and inflammatory reactions against viral infection by VCAM-1. Journal of Virology. 2008;82:2952–65. doi: 10.1128/JVI.02191-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas S, Hansson J, Klimmeck D, Loeffler D, Velten L, Uckelmann H, Wurzer S, Prendergast ÁM, Schnell A, Hexel K, Santarella-Mellwig R, Blaszkiewicz S, Kuck A, Geiger H, Milsom M, Steinmetz LM, Schroeder T, Trumpp A, Krijgsveld J, Essers MAG. Inflammation-Induced Emergency Megakaryopoiesis Driven by Hematopoietic Stem Cell-like Megakaryocyte Progenitors. Cell stem cell. 2015;17:422–34. doi: 10.1016/j.stem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Rué P, Martinez Arias A. Cell dynamics and gene expression control in tissue homeostasis and development. Molecular Systems Biology. 2015;11:792. doi: 10.15252/msb.20145549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie M, Lu C, Wang J, Mclellan M, Johnson KJ, Wendl M, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, Ozenberger BA, Welch J, Link DC, Walter MJ, Mardis ER, DiPersio JF, Chen F, Wilson RK, Ley TJ, Ding L. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nature Medicine. 2014;20:1472–8. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.