Abstract
Cardiovascular disease (CVD) complications are important causes of morbidity and mortality after orthotopic liver transplantation (OLT). There is currently no preoperative risk assessment tool that allows physicians to estimate the risk for CVD events following OLT. We sought to develop a point-based prediction model (risk score) for CVD complications after OLT, the CAR-OLT risk score, among a cohort of 1024 consecutive patients aged 18-75 years who underwent first OLT in a tertiary-care teaching hospital (2002-2011). The main outcome measures were major 1-year CVD complications, defined as death from a CVD cause or hospitalization for a major CVD event (myocardial infarction, revascularization, heart failure, atrial fibrillation, cardiac arrest, pulmonary embolism, and/or stroke). The bootstrap method yielded bias-corrected 95% confidence intervals for the regression coefficients of the final model. Among 1024 first OLT recipients, major CVD complications occurred in 329 (32.1%). Variables selected for inclusion in the model (using model optimization strategies) included pre-operative recipient age, sex, race, employment status, education status, history of hepatocellular carcinoma, diabetes, heart failure, atrial fibrillation, pulmonary or systemic hypertension, and respiratory failure. The discriminative performance of the CAR-OLT point-based score (C statistic=0.78, bias-corrected C statistic=0.77) was superior to other published risk models for postoperative CVD morbidity and mortality, and it had appropriate calibration (Hosmer-Lemeshow p=0.33).
Conclusion
The point-based CAR-OLT risk score can identify patients at risk for CVD complications after OLT surgery (available at: www.carolt.us). This score may be useful for identification of candidates for further risk stratification or other management strategies to improve CVD outcomes after OLT.
Keywords: heart disease, mortality, metabolic syndrome, survival, risk
Cardiovascular disease (CVD) complications are a leading cause of morbidity and mortality among adult recipients of an orthotopic liver transplantation (OLT)(1-3). Reported risk factors for adverse CVD outcomes after OLT have included traditional CVD risk factors such as high blood pressure, cigarette smoking, and diabetes(4). Factors such as fatty liver(5, 6), portal vein thrombosis(1), atrial fibrillation(3), tricuspid regurgitation on echocardiogram(7), prolonged QTc interval on electrocardiogram (ECG)(8), and biomarkers, including serum troponin-I(9, 10) and ferritin(11), have also been associated individually with higher CVD risk after OLT. Data from these studies have enabled limited prediction of CVD complications after OLT during a follow-up interval of months to years. However, these prediction algorithms have not been adapted to a user-friendly risk assessment tool that will allow physicians to estimate CVD risk in OLT candidates.
In the present study, we rigorously developed a simple CVD risk prediction model for use in first OLT recipients based on widely available clinical data. We considered numerous donor, recipient and operative factors that we hypothesized could affect CVD morbidity and mortality after OLT. We evaluated the utility and accuracy of this model for CVD risk prediction after OLT using data from a comprehensive institutional database of consecutive OLT recipients linked to data from the Organ Procurement and Transplantation Network (OPTN) over a 10-year period, with outcomes ascertainment up to 1 year after transplantation. This approach emphasizes the established, powerful, and independent, important factors for major CVD complications within a year of liver transplantation.
Patients and Methods
Protocol, Design, and Inclusion Criteria
All adult (age ≥ 18 years) patients who underwent first OLT at Northwestern Medicine in Chicago, IL from February 1, 2002 to December 31, 2011, with follow up data through December 31, 2012, were included in the study cohort (n=1024). Recipient waitlist and donor-related clinical data were drawn from the OPTN standard transplant analysis and research (STAR) files (created on March 15, 2013), which contain data regarding every organ donation and transplant event occurring in the U.S. since October 1, 1987. These data were then linked to the same recipient's preoperative, operative and postoperative clinical data from the Northwestern Medicine Enterprise Data Warehouse (NMEDW). The NMEDW currently stores 35 terabytes of data on ∼6.4 million unique patients, including 30 million outpatient encounters, 4 million inpatient hospitalizations, and 272 million clinical and laboratory diagnostic results since approximately 1998. Each night it loads 4.2 million new data elements from 129 separate computing systems and sources, including electronic health records, pathology data from the hospital and research laboratories, biomarker data from research databases, and research transactional data from our electronic institutional review board and other administrative and financial institutional systems. The linkage between OPTN data and NMEDW data was completed using date of transplant, date of birth, and recipient's sex as linking variables. Duplicate matches were verified by residential zip codes based on previously published methodology(3, 12). All direct identifiers were removed before the final data set was available for analysis.
For the present study, inclusion was restricted to adult patients who received a first liver or liver–kidney transplant for chronic liver disease or primary liver cancer at our institution. Patients deemed to be at unacceptably high cardiovascular risk by the care team based on the following criteria were clinically excluded from transplant and thus not included in our study sample, since they did not undergo OLT. At our institution, we consider it prohibitive to accept the patient as a transplant candidate with the following conditions: (1) decreased left ventricular systolic function (ejection fraction < 45%), (2) decreased right ventricular function and/or significant right ventricular dilation, (3) uncontrolled pulmonary hypertension defined as pulmonary arterial systolic pressure (PASP) ≥ 35 mmHg at rest despite maximal medical management, (4) significant uncorrectable structural valvular abnormalities (aortic stenosis, mitral stenosis, aortic regurgitation, tricuspid regurgitation), (5) uncorrectable coronary artery disease with induced ischemia on stress testing, (6) significant carotid disease in particular if symptomatic, and (7) diffuse atherosclerotic disease involving multiple organs.
Clinical Covariate Definitions
Prevalent comorbid conditions at the time of transplant were assessed by ICD-9 codes present prior to the initial transplant admission. Obesity was defined as body mass index (BMI) ≥ 30 kg/m2 or an ICD-9 code prior to the transplant admission for obesity (ICD-9=278.00, 278.01, 278.02 or 278.03). Transplantation for nonalcoholic steatohepatitis (NASH) was defined as a primary or secondary listing diagnosis of NASH or cryptogenic cirrhosis with at least one risk factor for the metabolic syndrome (pretransplant obesity, diabetes, hypertension and/or dyslipidemia). Smoking status was assessed using natural language processing, which is a technique that converts free text information relevant to smoking status that is commonly found in clinical documentation into a structured form that can be readily processed and analyzed, as described previously(13).
Potential risk factors or risk markers for major CVD complications after OLT were examined based on a comprehensive set of a priori clinical hypotheses. These risk variables included known traditional (e.g., diabetes, smoking status) and previously published novel CVD risk markers (e.g., serum troponin-I, ferritin) as well as transplant-specific critical illness indicators known to contribute to competing mortality risk (e.g., Model for End-Stage Liver Disease (MELD) score). The full range of recipient, donor, operative and perioperative potential risk markers for CVD complications that were evaluated in this study is listed in Supplemental Table 2.
Routine pretransplant cardiac testing included ECG and echocardiography in all patients. Echocardiography was used to assess right (RV) and left (LV) ventricular systolic and diastolic function, any wall motion abnormalities, intracardiac or intrapulmonary shunting, presence and grading of valvular abnormalities and for estimation of chamber size. It is routine practice at our institution for all patients to be seen by a cardiologist prior to listing. Individuals with a history of coronary artery disease (CAD) or those with ≥ 2 traditional risk factors for CVD (e.g., age (male > 45 years; female > 55 years), hypercholesterolemia, hypertension, tobacco use, and family history of early CAD (first-degree relative male < 55 years; female < 65 years)) undergo a pharmacologic stress examination prior to listing. Reports from completed stress examinations were available in 538 (52.5%) patients. Rate-pressure double product (heart rate × systolic blood pressure) at peak dobutamine stress echocardiography (DSE) test was evaluated in all patients who underwent DSE. Any stress-induced ischemia on the stress test warranted a left heart catheterization. If the DSE was nondiagnostic due to inability to achieve > 85% maximal predicted heart rate, further workup to rule out significant CAD was left to the discretion of the treating cardiologist. In recent years, reflecting advances in cardiac imaging, evaluation for coronary artery disease at our institution has also included performance of coronary multidetector computer tomographic angiography (CTA)(14). Left heart catheterization was performed in all patients with 1) any stress-induced ischemia, 2) significant coronary artery disease on CTA or 3) was left up to the discretion of the treating cardiologist with or without prior functional testing or imaging present. Right heart catheterization was performed to assess for portopulmonary hypertension in all patients with an estimated resting right ventricular systolic pressure (RVSP) on echocardiogram ≥ 35 mmHg.
Study Outcomes
The primary outcome was a major CVD complication occurring within 1 year of OLT, defined as death from a CVD cause or hospitalization for a major CVD event as defined by a discharge international classification of diseases ninth revision (ICD-9) or common procedural terminology (CPT) code within the first three diagnosis positions that indicated myocardial infarction (MI), revascularization (e.g., percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)), heart failure (HF), atrial fibrillation (AF), cardiac arrest, pulmonary embolism (PE), transient ischemic attack (TIA) and/or stroke during the initial transplant admission, or subsequent hospitalization, at a Northwestern Medicine member hospital (Supplemental Table 1). The discharge diagnosis was used in an attempt to capture in-hospital and rehospitalization events. In those patients in whom troponin was measured postoperatively, MI was also defined by a peak serum troponin I > 0.05 ng/mL. Recipient cause of death was determined by a physician's review (L.B.V.) of primary and contributory causes of death (including all free text inputs) listed in the OPTN database, which is matched to the social security death master file index. Any potential case with a CVD death or event was then adjudicated by trained study personnel using the Northwestern Medicine medical record and then reviewed by an independent panel of three physicians (one cardiologist, one hepatologist, one surgeon). Each patient is represented only once in the cohort. Documentation of a major CVD complication was based on the first documented event in the medical record following OLT. Secondary outcomes included other CVD complications such as documented hypertension, pulmonary hypertension, internal cardiac defibrillator (ICD) placement, permanent pacemaker (PPM) placement, amputation or peripheral arterial revascularization. Of 1024 recipients, 88% (n=904) were alive at 1-year post OLT with complete follow up data available for analysis.
Statistical Analysis
Clinical characteristics of primary OLT recipients from the integrated dataset were described using frequency counts and percentages for categorical variables and means ± standard deviations for continuous variables. Analysis of variance or a chi-square test was used to examine the relationship between clinical characteristics and 1-year CVD complications for continuous or categorical variables as appropriate. Twenty-nine preoperative candidate variables that were statistically significant (p<0.05) in univariate models, and that were available in the entire population with a CVD complication (n=329), were entered into a multivariable logistic regression model using forward stepwise selection. Age, race/ethnicity, and gender were forced into the final model. Twelve covariates were selected for the final model based on significance and additive contribution to the model based on Akaike Information Criteria (AIC). Further covariates did not improve model fit. Missing data that were categorized as “unknown” were excluded from analysis. Adjusted relative risks (RR) were converted from the adjusted odds ratios (OR) using the Zhang & Yu method(15). For internal validation, the bootstrap method was used to account for the generalizability error, and bias-corrected 95% confidence intervals were calculated based on 10,000 resamples with replacement.
The final model, the CArdiovascular Risk in Orthotopic Liver Transplantation (CAR-OLT) score was developed by assigning points for each risk factor based on the weighted value of the beta coefficients corresponding to the relative risks for each covariate in the model (range 0-25 points)(16). We calculated a point total for all participants in the data set using the point system. A score sheet for prediction of 1-year CVD complications is provided and absolute risk estimates are shaded, ranging from very low risk to high risk. Such distinctions are arbitrary but provide a foundation to determine the potential need for clinical intervention. The discrimination of the point-based model was estimated using the area under the receiver operating curve (AUROC). The calibration, a measure of the goodness of model fit, was assessed by comparing the observed and predicted number of events in deciles of predicted risk, as calculated by the Hosmer and Lemeshow statistic. A p-value of 0.05 was used for all significance tests. All analysis was performed using SAS 9.3 (SAS institute, Cary, NC).
Results
The baseline characteristics of 1024 orthotopic liver transplant recipients who were included in the final analysis are shown in Table 1. Mean age of our study sample was 56.3 ± 9.7 years, 65.9% (n=675) were male, and 78.6% (n=805) were non-Hispanic White. The majority of the patients were transplanted for hepatitis C (33.5%, n=343), followed by alcohol (23.5%, n=241) and NASH (16.1%, n=165). The average MELD score at transplant for this sample was 20.9 ± 11.6. Preexisting CVD conditions were common: 59.3% of patients (n=607) had hypertension, 40.6% (n=416) met criteria for obesity, 32.5% (n=333) had diabetes, and 34.1% (n=349) had chronic kidney disease at the time of OLT. The overall characteristics and risk profile of our cohort were similar to a national sample from the OPTN who were transplanted during the same time period (2002-2011, Supplemental Table 3). Compared to the OPTN cohort, the NMEDW cohort was slightly older (56.3 vs. 53.4 years) and was more likely to be college educated (64.1% vs. 47.4%, p<0.0001 for both). A larger proportion of patients within the NMEDW received a simultaneous liver-kidney transplant (16.9% vs. 5.7%) or a living donor transplant (15.2% vs. 4.2%), and were less likely to have hepatocellular carcinoma (HCC) as a primary indication for OLT compared to the OPTN cohort (28.0% vs. 40.1%, p<0.0001 for all). There was no statistically significant difference in functional status, mean MELD score, prevalence of diabetes, or prevalence of ischemic heart disease.
Table 1. Baseline Prevalence and Univariate Analysis of Potential Risk Factors for Cardiovascular Complications within 1 year of Liver Transplantation, Northwestern Medicine Enterprise Data Warehouse, 2002-2012.
| Characteristic | All patients (N=1024) | No CVD complication (N=695) | + CVD complication (N=329) | P value* | ||||
|---|---|---|---|---|---|---|---|---|
| Age, years, mean ± SD | 56.3 ± 9.7 | <0.0001 | ||||||
| <45, n (%) | 114 (11.1) | 90 (13.0) | 24 (7.3) | |||||
| 45-49 | 109 (10.6) | 86 (12.4) | 22 (6.7) | |||||
| 50-54 | 198 (19.3) | 157 (22.6) | 41 (12.5) | |||||
| 55-59 | 222 (21.7) | 152 (21.9) | 70 (21.3) | |||||
| 60-64 | 186 (18.2) | 111 (16.0) | 75 (22.8) | |||||
| 65+ | 196 (19.1) | 99 (14.2) | 97 (29.5) | |||||
|
| ||||||||
| Sex, % | 0.16 | |||||||
| Men | 675 (65.9) | 468 (67.3) | 207 (62.9) | |||||
| Women | 349 (34.1) | 227 (32.7) | 122 (37.1) | |||||
|
| ||||||||
| Recipient race & ethnicity, % | 0.02 | |||||||
| Non-Hispanic White | 805 (78.6) | 538 (77.4) | 267 (81.2) | |||||
| Hispanic | 112 (10.9) | 88 (12.7) | 24 (7.3) | |||||
| Non-Hispanic Black | 71 (6.9) | 43 (6.2) | 28 (8.5) | |||||
| Asian | 32 (3.1) | 23 (3.3) | 9 (2.7) | |||||
| Other | 4 (0.4) | 3 (0.4) | 1 (0.3) | |||||
|
| ||||||||
| Socioeconomic Status, % | ||||||||
| Less than high school education | 24 (2.3) | 17 (2.5) | 6 (1.8) | 0.03 | ||||
| Working for income at transplant | 173 (16.9) | 146 (21.0) | 27 (8.2) | <0.0001 | ||||
|
| ||||||||
| Primary Payer, % | 0.0002 | |||||||
| Medicare | 501 (48.9) | 314 (45.2) | 189 (56.8) | |||||
| Private | 419 (40.9) | 306 (44.0) | 113 (34.4) | |||||
| Medicaid | 77 (7.5) | 57 (8.2) | 20 (6.1) | |||||
| Other | 27 (2.6) | 18 (2.6) | 9.0 (2.7) | |||||
|
| ||||||||
| Hospitalization status at transplant, % | <0.0001 | |||||||
| Not hospitalized | 712 (69.5) | 528 (76.0) | 184 (55.9) | |||||
| Hospitalized not in ICU | 237 (23.1) | 135 (19.4) | 101 (30.7) | |||||
| In ICU | 75 (7.4) | 32 (4.6) | 44 (13.4) | |||||
|
| ||||||||
| Recipient functional status at transplant, % | <0.0001 | |||||||
| Independent | 470 (45.9) | 374 (53.8) | 117 (35.6) | |||||
| Partially dependent | 231 (22.6) | 179 (25.8) | 62 (18.9) | |||||
| Totally dependent | 278 (27.1) | 142 (20.4) | 150 (45.5) | |||||
|
| ||||||||
| Calculated MELD at LT, mean ± SD | 20.9 ± 11.6 | 19.5 ± 11.5 | 24.0 ± 11.4 | <0.0001 | ||||
| <15 | 382 (37.3) | 306 (44.0) | 76 (23.2) | |||||
| 15-30 | 515 (50.3) | 379 (54.5) | 136 (41.2) | |||||
| 35-40 | 68 (6.6) | 39 (5.6) | 28 (8.5) | |||||
| 40+ | 66 (6.4) | 34 (4.9) | 31 (9.5) | |||||
|
| ||||||||
| Cause of ESLD, % | <0.0001 | |||||||
| Hepatitis C | 343 (33.5) | 249 (35.8) | 94 (28.6) | |||||
| Alcohol | 241 (23.5) | 161 (23.2) | 80 (24.3) | |||||
| NASH | 165 (16.1) | 89 (12.5) | 78 (23.7) | |||||
| Other | 275 (26.9) | 198 (28.5) | 77 (23.4) | |||||
|
| ||||||||
| Hepatocellular Carcinoma, % | 411 (40.1) | 314 (45.2) | 97 (9.5) | <0.0001 | ||||
|
| ||||||||
| Complications of ESLD at LT, % | ||||||||
| Hepatic Encephalopathy | 981 (95.8) | 658 (94.7) | 323 (98.2) | 0.009 | ||||
| Ascites at transplant | 782 (76.4) | 519 (74.7) | 263 (79.9) | 0.06 | ||||
| Spontaneous bacterial peritonitis | 200 (19.5) | 119 (17.1) | 81 (24.6) | 0.005 | ||||
| Variceal Bleed | 255 (24.9) | 169 (24.3) | 86 (26.1) | 0.53 | ||||
| Hepatorenal syndrome | 226 (22.1) | 126 (18.1) | 100 (30.4) | <0.0001 | ||||
| Hyponatremia | 176 (17.2) | 114 (16.4) | 62 (18.8) | 0.33 | ||||
| TIPS | 163 (15.9) | 100 (14.4) | 63 (19.2) | 0.055 | ||||
| Portal Vein Thrombosis | 97 (9.5) | 68 (9.8) | 29 (8.8) | 0.62 | ||||
| Hepatopulmonary syndrome | 4 (0.4) | 0 (0) | 4 (1.2) | 0.01 | ||||
|
| ||||||||
| CVD comorbidities present at LT, % | ||||||||
| Hypertension | 607 (59.3) | 374 (53.8) | 233 (70.8) | <0.0001 | ||||
| Hyperlipidemia | 167 (16.3) | 103 (14.8) | 64 (19.5) | 0.06 | ||||
| Heart failure | 153 (14.9) | 69 (9.9) | 84 (25.5) | <0.0001 | ||||
| Ischemic heart Disease | 67 (6.5) | 31 (4.5) | 36 (10.9) | <0.0001 | ||||
| Myocardial infarction | 28 (2.7) | 11 (1.6) | 17 (5.2) | 0.001 | ||||
| Prior revascularization | 45 (4.4) | 22 (3.2) | 23 (7.0) | 0.005 | ||||
| Prior CABG | 10 (0.98) | 3 (0.43) | 7 (2.1) | 0.02 | ||||
| Cerebrovascular disease (stroke/TIA) | 63 (6.2) | 34 (4.9) | 29 (8.8) | 0.01 | ||||
| Atrial Fibrillation | 62 (6.1) | 9 (1.3) | 53 (16.1) | <0.0001 | ||||
| Any other dysrhythmia | 32 (3.1) | 15 (2.2) | 17 (5.2) | 0.01 | ||||
| Peripheral Vascular Disease | 11 (1.1) | 8 (1.2) | 3 (0.91) | 0.73 | ||||
|
| ||||||||
| Other comorbidities present at LT, % | ||||||||
| Chronic kidney disease | 349 (34.1) | 208 (29.9) | 141 (42.9) | <0.0001 | ||||
| Renal Failure requiring RRT | 177 (17.3) | 88 (12.7) | 89 (27.1) | <0.0001 | ||||
| Diabetes | 333 (32.5) | 202 (29.1) | 131 (39.8) | 0.0006 | ||||
| COPD | 132 (12.9) | 78 (11.2) | 54 (16.4) | 0.02 | ||||
| Pulmonary hypertension | 109 (10.6) | 51 (7.3) | 57 (17.3) | <0.0001 | ||||
| Respiratory failure on ventilator | 98 (9.6) | 38 (5.5) | 61 (18.4) | <0.0001 | ||||
| Venous thromboembolism, any | 85 (8.3) | 45 (6.5) | 40 (12.2) | 0.002 | ||||
| Deep venous thrombosis | 28 (2.7) | 12 (1.7) | 16 (4.9) | 0.004 | ||||
| Pulmonary Embolism | 10 (0.98) | 4 (0.58) | 6 (1.8) | 0.06 | ||||
| Peptic ulcer disease | 38 (3.7) | 23 (3.3) | 15 (4.6) | 0.32 | ||||
| Obstructive sleep apnea | 36 (3.5) | 27 (3.9) | 9 (2.7) | 0.35 | ||||
| Connective tissue disease | 28 (2.7) | 20 (2.9) | 8 (2.4) | 0.68 | ||||
| Dementia | 2 (0.2) | 0 (0) | 2 (0.61) | 0.10 | ||||
|
| ||||||||
| Recipient BMI (kg/m2) at transplant, mean | 29.6 ± 6.9 | 0.29 | ||||||
| Underweight (BMI ≤ 18 kg/m2) | 22 (2.1) | 13 (1.9) | 8 (2.4) | |||||
| Normal weight (BMI 18.1-24.9 kg/m2) | 255 (24.9) | 182 (26.2) | 73 (22.2) | |||||
| Overweight (BMI 25-29.9 kg/m2) | 332 (32.4) | 230 (33.1) | 102 (31.0) | |||||
| Obese (BMI ≥ 30 kg/m2) | 416 (40.6) | 270 (38.9) | 146 (44.4) | |||||
|
| ||||||||
| Smoking status | 0.06 | |||||||
| Never | 676 (66.0) | 445 (64.0) | 231 (70.1) | |||||
| Former or current | 348 (34.0) | 250 (36.0) | 98 (29.9) | |||||
|
| ||||||||
| Simultaneous liver-kidney, % | 173 (16.9) | 92 (13.2) | 81 (24.6) | <0.0001 | ||||
|
| ||||||||
| Living donor, % | 156 (15.2) | 123 (17.7) | 33 (10.0) | 0.001 | ||||
Abbreviations: NMEDW, Northwestern Medicine Enterprise Data Warehouse; No, number; ESLD, end-stage liver disease; LT, liver transplant; MELD, model for end-stage liver disease; NASH, nonalcoholic steatohepatitis; SD, standard deviation
Results are presented as mean ± standard deviation or %
Analysis of variance for continuous variables and chi-square or Fischer exact for categorical variables
Results from preoperative CVD testing and medication usage for the study cohort are shown in Table 2. The majority of patients had ECG (64.1%, n=656), transthoracic echocardiogram (77.4%, n=793) and stress imaging (52.5%, n=538) available for review from the NMEDW (other patients may have had outside testing performed). Of 656 patients with ECG data, 47.4% (n=311) had a prolonged QTc interval (defined as >450ms in males and >470ms in females); 15.4% (n=101) were taking a quinolone medication, which is associated with prolonged QTc. Of 793 patients with an available echocardiogram, the mean ejection fraction was 62.4%, 26.0% (n=206) had evidence of diastolic dysfunction, and 86.3% (n=684) had tricuspid regurgitation of any severity present prior to OLT. DSE was the most common type of stress imaging performed and 44.2% (n=238) of the 538 patients with stress imaging available had an abnormal test with a mean maximal predicted heart rate of 83.1%. Left heart catheterization was performed prior to OLT in 43.8% (n=449) of the study cohort. Of the 449 patients with left heart catheterization performed, only 6.5% (n=29) underwent coronary revascularization prior to OLT at a mean (SD) of 110.7 (135.0) days prior to transplant. The remaining 275 patients had nonobstructive CAD that did not require intervention, of which 7 had a prior history of CABG and 5 had a prior history of PCI. Mean time between prior CABG/PCI and transplant was 3.3 (1.2) years. Right heart catheterization was performed in 25.8% (n=264) of the study cohort and portopulmonary hypertension diagnosed in 1.3% (n=6) of those patients who underwent right heart catheterization. A minority of patients underwent CT angiography (3.9%, n=30) as screening for asymptomatic CAD prior to OLT. Notably, fewer than 2% of patients were receiving treatment with a statin, aspirin, antiplatelet agent, or antihypertensive agent prior to OLT.
Table 2. Preoperative Cardiac Testing and Medication Usage in 1024 First Liver Transplant Recipients, Northwestern Medicine Enterprise Data Warehouse, 2002-2012.
| Cardiac testing | Total N=1024* |
|---|---|
| ECG performed, No(%)* | 656 (64.1) |
| QTc, ms, mean ± SD | 457.2 ± 33.6 |
| Abnormal QTC (>450m, >470f) | 311 (30.3) |
| Ventricular rate, bpm, mean | 75.6 ± 14.9 |
| >=100bpm | 44 (4.3) |
| QRS duration, ms, mean | 92.2 ± 15.1 |
|
| |
| Pretransplant Echo performed, No(%) | 793 (77.4) |
| Ejection fraction, %, mean ± SD | 62.4 ± 5.7 |
| Diastolic dysfunction, any | 206 (20.1) |
| Grade I | 167 (16.3) |
| Grade II-III | 39 (3.8) |
| Tricuspid regurgitation, any | 684 (66.8) |
| Trace/Mild | 595 (58.1) |
| Mild-mod | 20 (2.0) |
| Moderate | 53 (5.2) |
| Mod-Severe | 16 (1.6) |
| Septal flattening present | 32 (3.1) |
|
| |
| Stress test performed, No(%) | 538 (52.5) |
| Dobutamine stress echo | 382 (37.3) |
| Myocardial perfusion imaging | 155 (15.1) |
| Stress test result | |
| Normal | 300 (29.2) |
| Abnormal | 238 (23.2) |
| Maximal predicted heart rate, mean ± SD | 83.1 ± 16.3 |
|
| |
| Left heart catheterization performed, No(%)** | 449 (43.8) |
| Normal | 145 (14.2) |
| Left main not intervened | 45 (4.4) |
| Any other CAD not intervened | 230 (22.5) |
| Intervened on CAD | 29 (2.8) |
|
| |
| Right heart catheterization performed, No(%) | 264 (25.8) |
| Pulmonary capillary wedge pressure, mmHg, mean ± SD | 15.8 ± 7.5 |
| Mean pulmonary artery pressure, mmHg, mean ± SD | 24.2 ± 9.3 |
| Portopulmonary hypertension present***, No(%) | 6 (0.6) |
|
| |
| CT angiography performed, No(%) | 40 (3.9) |
| Abnormal | 21 (2.1) |
|
| |
| Medication usage within 48 hours LT, No (%) | |
| Quinolone | 158 (15.4) |
| Vasopressor | 141 (13.8) |
| Insulin | 112 (10.9) |
| Antiarrhythmic | 80 (7.8) |
| Beta blocker | 76 (7.4) |
| Diuretic | 66 (6.5) |
| Steroid | 15 (1.5) |
| Statin | 14 (1.4) |
| Aspirin | 13 (1.3) |
| Antiplatelet | 13 (1.3) |
| Antihypertensive | 12 (1.2) |
| Calcium channel blocker | 7 (0.68) |
| Pulmonary vasodilator | 5 (0.49) |
| Other anticoagulant | 5 (0.49) |
Abbreviations: bpm, beats per minute; No, number; SD, standard deviation; LT, liver transplantation; CAD, coronary artery disease; CT, computed tomography
% are expressed using the common denominator of n=1024 for the entire sample.
Of the 449 recipients with a left heart catheterization performed, only 238 had a prior abnormal stress test (see above). Of the remaining 211 patients, 176 had failed to achieve a MPHR > 85% on stress imaging, 21 had an abnormal CT angiography and 14 were missing documentation of a noninvasive stress imaging prior to left heart catheterization.
Defined as mean pulmonary artery pressure >25 mmHg and pulmonary capillary wedge pressure <15 mmHg
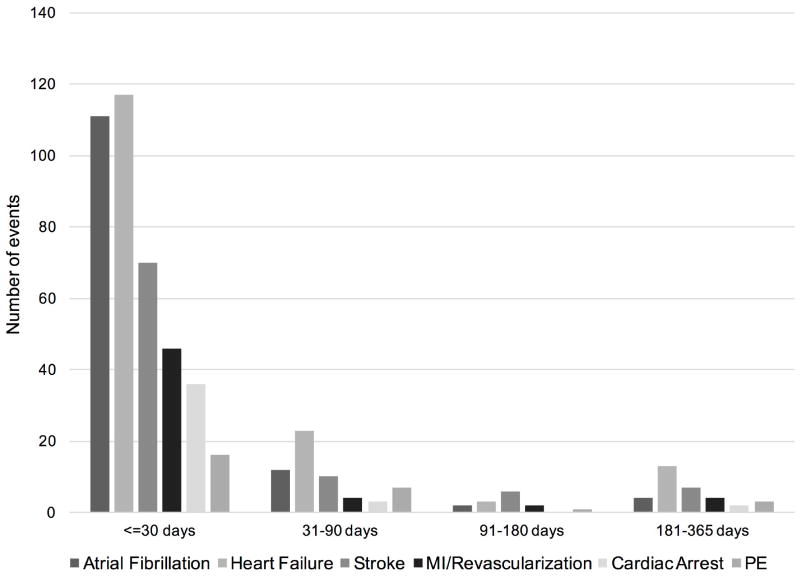
Four hundred ninety-eight CVD complications occurred among 329 (32.1%) patients within 1 year of OLT, the majority of which (75.1%) occurred in the early perioperative period (Figure 1). The most common CVD complication was HF (n=154, 31%), followed by AF (n=130, 26%) and stroke/TIA (n=90, 18%). MI or need for revascularization accounted for only 12% (n=60) of all CVD complications after OLT (Figure 1). There were 120 deaths within 1 year of OLT, of which 17 (14.2%) were attributable to CVD. The all-cause and CVD-related 1-year mortality rates were 11.7% and 1.7%, respectively. Univariate analyses of baseline variables identified 26 potential risk markers (Table 1) for 1-year CVD complications after OLT.
Figure 1.
Distribution of 1-year CVD complications among adult first liver transplant recipients (NMEDW, 2002-2011). There were 498 complications among 329 individuals within 1 year of liver transplantation. Abbreviations: PE, pulmonary embolism; MI, myocardial infarction
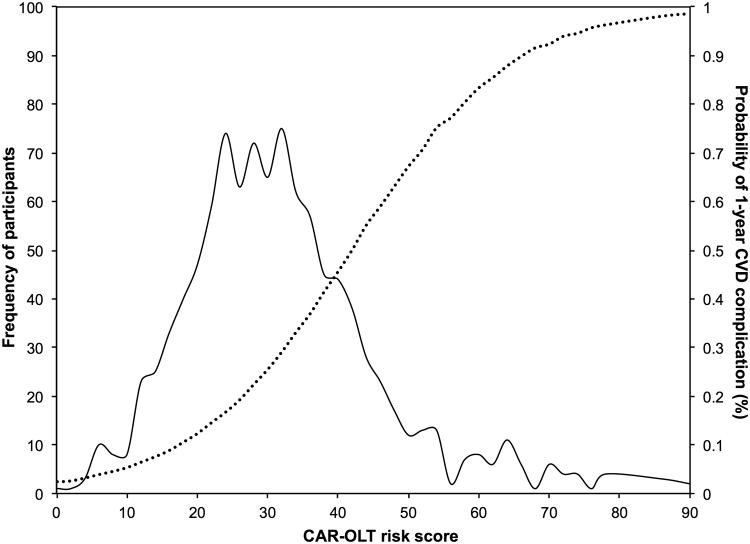
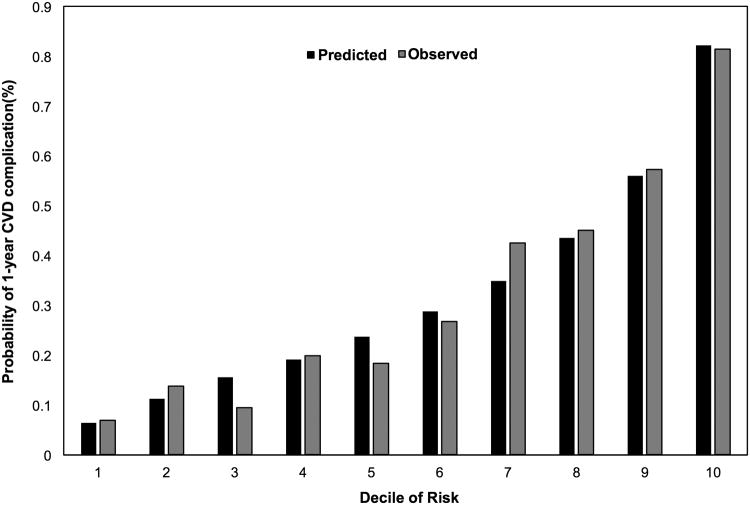
The regression coefficients, ORs, converted RR, and calculated integer scores assigned for all risk factors in the final multivariate model are summarized in Table 3. Variables selected for inclusion included preoperative recipient age, sex, race, employment status, education status, history of HCC, diabetes, HF, AF, pulmonary or systemic hypertension, and respiratory failure. The bootstrap method yielded bias-corrected 95% confidence intervals for the regression coefficients. All of the aforementioned variables except variables we forced in (age, sex and race/ethnicity) were statistically significant in the final model supporting the inclusion of the aforementioned variables in the final regression model. The predicted probability of a 1-year CVD complication after OLT by total points (score) is presented in Table 4. The majority of patients (n=691) had a CAR-OLT score < 40 points and a predicted probability of a 1-year CVD complication < 40% (Figure 2). However, a significant proportion of patients (30.1%) were at very high risk (predicted risk > 45%) for a 1-year CVD complication after OLT (Figure 2). The model demonstrated good discrimination (C statistic, 0.78; bias-corrected C statistic, 0.77). The numbers of predicted CVD complications generally matched the number of observed CVD complications in 1-year risk deciles indicating excellent calibration (Hosmer-Lemeshow, P=0.33; Figure 3). A web-based calculator and link to download the mobile application for the CAR-OLT score is available here: www.carolt.us.
Table 3. Results of the Multivariate Logistic Regression Analysis model of 1-year CVD complications and the Bootstrap Method.
| Pretransplant Risk Factor* | Regression Coefficient (95% Bias-Corrected CI) | OR (95% CI) | RR** (95% CI) | Points Assigned |
|---|---|---|---|---|
| Age Group, yr | ||||
| < 45 | - | 1.00 (referent) | 1.00 (referent) | 0 |
| 45-49 | -0.50 (-1.24-0.29) | 0.61 (0.29-1.33) | 0.69 (0.37-1.20) | -6 |
| 50-54 | -0.34 (-0.98-0.33) | 0.71 (0.37-1.39) | 0.78 (0.47-1.24) | -4 |
| 55-59 | 0.21 (-0.40-0.85) | 1.24 (0.67-2.34) | 1.15 (0.75-1.63) | 2 |
| 60-64 | 0.46 (-0.19-1.10) | 1.58 (0.82-3.00) | 1.33 (0.87-1.82) | 5 |
| 65+ | 0.72 (0.10-1.37) | 2.06 (1.11-3.93) | 1.53 (1.07-2.02) | 8 |
| Sex | ||||
| Men | - | 1.00 (referent) | 1.00 (referent) | 0 |
| Women | 0.08 (-0.23-0.42) | 1.09 (0.79-1.52) | 1.06 (0.84-1.31) | 1 |
| Race | ||||
| White | 0.64 (0.16-1.11) | 1.90 (1.17-3.06) | 1.48 (1.11-1.84) | 7 |
| Black | 0.93 (0.20-1.63) | 2.53 (1.22-5.11) | 1.70 (1.28-2.11) | 10 |
| Others | - | 1.00 (referent) | 1.00 (referent) | 0 |
| Working status | ||||
| Working for income | - | 1.00 (referent) | 1.00 (referent) | 0 |
| Not working for income | 0.93 (0.39-1.49) | 2.54 (1.48-4.45) | 1.70 (1.28-2.11) | 10 |
| Education | ||||
| <= High school /unknown | 0.45 (0.10-0.78) | 1.56 (1.10-2.19) | 1.32 (1.07-1.58) | 5 |
| College+ | - | 1.00 (referent) | 1.00 (referent) | 0 |
| Atrial Fibrillation | ||||
| Yes | 2.19 (1.31-3.09) | 8.96 (3.70-22.0) | 2.52 (1.98-2.84)) | 25 |
| No | - | 1.00 (referent) | 1.00 (referent) | 0 |
| Respiratory failure on ventilator at transplant | ||||
| Yes | 1.17 (0.64-1.69) | 3.22 (1.90-5.40) | 1.88 (1.47-2.23) | 13 |
| No | - | 1.00 (referent) | 1.00 (referent) | 0 |
| Pulmonary hypertension | ||||
| Yes | 0.83 (0.31-1.27) | 2.30 (1.35-3.59) | 1.62 (1.22-1.96) | 9 |
| No | - | 1.00 (referent) | 1.00 (referent) | 0 |
| HCC | ||||
| Yes | - | 1.00 (referent) | 1.00 (referent) | 0 |
| No | 0.57 (0.23-0.90) | 1.77 (1.27-2.47) | 1.42 (1.17-1.67) | 6 |
| Hypertension | ||||
| Yes | 0.37 (0.03-0.72) | 1.45 (1.03-2.06) | 1.27 (1.02-1.53) | 4 |
| No | - | 1.00 (referent) | 1.00 (referent) | 0 |
| Diabetes | ||||
| Yes | 0.36 (0.01-0.69) | 1.43 (1.01-1.98) | 1.26 (1.01-1.51) | 4 |
| No | - | 1.00 (referent) | 1.00 (referent) | 0 |
| Heart Failure | ||||
| Yes | 0.59 (0.13-1.01) | 1.80 (1.14-2.75) | 1.43 (1.09-1.76) | 7 |
| No | - | 1.00 (referent) | 1.00 (referent) | 0 |
All comorbidities (e.g., atrial fibrillation, hypertension, HCC, diabetes, heart failure, pulmonary hypertension) are coded as history of the condition prior to transplant
Adjusted RR are converted from the adjusted OR using Zhang & Yu method 1998(15)
C statistics = 0.784; Hosmer and Lemeshow Goodness-of-Fit Test (p = 0.33)
Table 4. Predicted 1-year Post Liver Transplant Cardiovascular Complication Risk by Risk Score, Northwestern Medicine Enterprise Data Warehouse, 2002-2012.
| Score | 1-year Absolute Predicted Risk (%)* |
|---|---|
| <9 | <5 |
| 10-12 | 6 |
| 13-15 | 8 |
| 16-17 | 10 |
| 18-19 | 12 |
| 20-22 | 14 |
| 23-24 | 17 |
| 25-26 | 20 |
| 27-28 | 22 |
| 29-30 | 26 |
| 31-32 | 30 |
| 33-34 | 34 |
| 35-36 | 38 |
| 37-38 | 40 |
| 39-40 | 43 |
| > 40 | 45+ |
| Color | Risk |
| Green | Very low |
| White | Low |
| Yellow | Moderate |
| Orange | High |
| Red | Very high |
Risk levels are estimated based on actual event rates in the sample population. Risk levels are shaded, ranging from very low absolute risk to very high. Such distinctions are arbitrary but provide a foundation to determine potential need for clinical intervention.
Figure 2.
Distribution of CAR-OLT scores among liver transplant recipients and their relation to probability of a 1-year post-transplant cardiovascular event.
Figure 3.
Calibration of the prediction model by decile of risk. Observed (solid bar) and expected (gray bar) of the probability of a cardiovascular event within 1 year of liver transplant showed good agreement. P-value from the Hosmer and Lemeshow Goodness-of-Fit test was 0.33.
Discussion
The goals of predicting CVD risk after OLT are twofold: 1) To estimate the risk of intraoperative and early postoperative CVD-related death and complications; and 2) To estimate longer-term risk of significant CVD morbidity that may mitigate against use of a scarce donor organ or indicate careful postoperative management and monitoring to avoid a CVD event. In the current study, we have rigorously evaluated over 200 recipient, operative, and donor variables for their association with risk of death or hospitalization from a CVD complication within 1 year of OLT. We observed that an optimized clinical model including preoperative recipient age, sex, race, education, employment status, history of AF, pulmonary hypertension, systemic hypertension, diabetes, HF, presence of HCC and ventilator dependence at the time of OLT was predictive of 1-year CVD complications after OLT with good discrimination (c-statistic=0.78) and excellent calibration. To further assist clinicians in accurately predicting CVD risk after OLT, we have developed a point-based risk score (the CAR-OLT score) and risk calculator for clinical use.
To date, there are four published studies that provide information on risk markers for CVD complications specific to the OLT population with the c-statistic ranging from 0.66 to 0.73(1, 3, 17, 18). Josefsson et al. derived a point-based model comprised of renal impairment, prolonged QTc and age>52 in 202 Swedish OLT recipients (mean age 52 years, 69% male, race NR) with a c-statistic of 0.73 for 1-year CVD mortality, arrhythmia or acute coronary syndrome(17). The model by Umphrey et al. specifically evaluated the role of DSE among 157 OLT recipients (mean age 54.5 years, 71% male, 94% white) for the prediction of cardiac death, MI, HF, asystole or symptomatic ventricular tachycardia within 4 months of OLT though no c-statistic was reported(18). The main limitations of these models are the small sample sizes, lack of calibration statistic reporting and limited generalizability. Two other published risk factor models were developed by our group among a larger national sample in order to identify risk markers for CVD complications specific to OLT recipients(1, 3). However, these national models are not easily applied in routine clinical practice due to 1) lack of information on pretransplant cardiac testing, 2) inability to adjudicate CVD outcomes rigorously and 3) inability to assess both CVD mortality and morbidity within a single nationally representative data source(1, 3). The current study includes over 1000 diverse OLT recipients from a large urban academic health system, includes comprehensive cardiac testing data, adjudicates CVD outcomes, predicts both CVD-related morbidity and mortality and the risk model performs much more robustly than those previously published with good discrimination (c-statistic= 0.78) and excellent calibration (link: www.carolt.us).
Established perioperative CVD risk algorithms have been applied to the liver transplant population, however, none was specifically developed in patients with end-stage liver disease(19). For example, the commonly used Revised Cardiac Risk Index (RCRI) was developed in patients undergoing non-cardiac surgery that excluded those with end-stage liver disease(20). In addition, the RCRI was designed primarily to detect underlying ischemic heart disease. Importantly, we have demonstrated that ischemic heart disease is responsible for a relatively small proportion (12%) of 1-year CVD complications after OLT compared to non-coronary events, including HF (31%), AF (23%), and stroke (18%). The low incidence of ischemic heart disease events is at least in part due to the fact that significant nonintervenable coronary artery disease is considered an absolute contraindication to OLT. On the other hand, we hypothesize that a high prevalence of pretransplant underlying cirrhotic cardiomyopathy, which is characterized by a compromised ventricular response to stress, may place OLT recipients at risk for postoperative arrhythmias and heart failure, which accounted for over 50% of all events in our cohort(21). In addition, employment at the time of OLT, a surrogate for functional status, remained a multivariable predictor of CVD complications. Poor functional status may be a marker of accumulating physiologic decline that makes OLT recipients less able to handle physiologic stressors, including surgery.
The 1-year CVD complication rate in the current study was 32.1%. The incidence rates for CVD complications post OLT in the published literature vary widely according to outcome definition and follow-up duration. For example, the incidence rate of CVD complications within 30 days of OLT ranges from 1.1% to 23.2%(1, 8, 17, 22-24); within 30 days to 6 months of OLT from 1.1% to 50%(1, 3, 8, 17, 18, 22-27); and > 6 months post OLT from 0% to 31.4%(2, 4, 8-10, 17, 19, 28-34). The wide discrepancy in rates is at least partially attributable to the heterogeneity in outcome definitions across these studies. We defined a CVD complication as death from a CVD cause or hospitalization due to a major CVD event, including MI, revascularization (e.g., PCI or CABG), HF, AF, cardiac arrest, PE or TIA/stroke. Thus, the model derived in this study is applicable to the broad range of major CVD complications observed after OLT and focuses on those complications most likely to affect 1-year patient and graft survival, which is an important quality metric in OLT(35).
Not surprisingly, several traditional CVD risk factors, including older age, diabetes, and prior CVD (systemic and pulmonary hypertension, HF, and AF), were predictive of CVD complications in our cohort. However, some traditional candidate variables did not enter the prediction equation, including a family history of premature CVD, smoking status, prior ischemic heart disease and BMI. Our findings are consistent with multiple other published studies that have failed to find an association between BMI, smoking status or family history of CVD and poor post-OLT CVD outcomes(1, 3, 8, 17, 24, 29). This is likely due to the small residual impact of these metabolic variables on CVD prediction after the other aforementioned variables have been incorporated into the regression model(36).
We identified HCC status and employment status as novel markers for improved post-OLT CVD morbidity and mortality. Both of these variables are presumed markers for a “healthier” OLT candidate with a high functional status, and a relatively low calculated MELD score compared to other indications for OLT. In contrast, patients with respiratory failure (e.g., on a ventilator) were nearly twice as likely as those without respiratory failure at OLT to have a CVD complication. This finding is particularly relevant in the context of the increasing critical illness burden of the modern transplant population, which is reflected in the increasing median MELD score for which a given patient receives a liver transplant across the country(37). These high MELD patients likely have a high prevalence of subclinical cardiac disease, and a blunted cardiac response to the hemodynamic stress of liver transplantation. Therefore, candidates who are older and sicker with poor underlying functional status may have a limited underlying cardiac reserve, leading to poor post-transplant CVD outcomes.
Strengths of the present study include its large sample size, rigorous assessment of a multitude of recipient, operative and donor-related factors including harmonized data from multiple sources to increase the robustness of the model covariate pool, generalizability given the overall similarity to the OPTN general population sample and similar median event rates to other published studies, and the use of state-of-the-art methods to evaluate the performance of the prediction model for CVD outcomes after OLT.
There are limitations that warrant mention. First, CVD complication assessment was limited to events that occurred at a Northwestern Medicine member hospital. Therefore, not all CVD events may have been captured if a recipient presented to a non-member organization. However, OLT recipients are very likely to be readmitted or transferred to their transplant hospital within the first year. Our ascertainment of CVD complications were limited to those listed in the top 3 discharge diagnosis codes in order to maximize code specificity for acute CVD complications(38). Thus, our estimates may underestimate the true incidence of CVD complications particularly if events occurred after surgery during the index transplant hospitalization where other transplant-related complications may be coded higher in the taxonomy. Second, certain CVD risk factors, such as recipient family history of CVD, are historically unreliable based on claims data and may not be accurately captured here(39). In addition, laboratory values that are not part of routine evaluation for OLT at our center (e.g., serum troponin I, BNP, ferritin, CRP) were unavailable in the vast majority (>75%) of participants, thus limiting assessment of their utility for prediction of post-transplant CVD complications. Because these are not routinely measured, their inclusion in the analysis would also lead to concerns about propensity for testing or reverse causation. Finally, the risk model was derived from a single academic medical center. Associations observed may be due to specific practice patterns and patient admixture of Northwestern Medicine. Thus, this score requires prospective validation in other centers. To assist centers with validation we have provided online and mobile CAR-OLT score applications.
Conclusion
We have developed and calibrated a point-based prediction model for 1-year CVD complications using a large cohort of diverse OLT recipients at an urban academic transplant center. The CAR-OLT score risk calculator is available for download at: www.carolt.us. Whether use of the CAR-OLT score for risk stratification and the decision to proceed with more intensive cardiovascular testing or targeted risk factor reduction would be helpful to improve CVD outcomes after OLT requires external validation in a future multicenter clinical trial.
Supplementary Material
Acknowledgments
The authors thank Jaylen Thomas, Tanvi Subramanian, Tricia Pendergrest, Darren Boyd and Sarah Uttal for their assistance with data collection and case adjudication. The authors thank Bing-Bing Weitner and Fred Rademaker for their assistance with initial data cleaning and analysis. Portions of the data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Grants and Financial Support: This work was supported by the National Institutes of Health (1 F32 HL116151-01), the American Liver Foundation (New York, NY), and an Alpha Omega Alpha Postgraduate Award. Dr. VanWagner is currently supported by the National Institutes of Health's National Center for Advancing Translational Sciences, Grant Number KL2TR001424. The Northwestern Medicine Enterprise Data Warehouse (NMEDW) is funded, in part, by the National Center for Advancing Translational Sciences (NCATS) of the NIH research grant UL1TR001422 to the Northwestern University Clinical and Translational Sciences (NUCATS) Institute.
List of Abbreviations (in order of appearance)
- CVD
Cardiovascular Disease
- OLT
Orthotopic Liver Transplantation
- CAR-OLT
Cardiovascular Risk in Orthotopic Liver Transplantation
- ECG
Electrocardiogram
- OPTN
Organ Procurement and Transplantation Network
- STAR
Standard Transplant Analysis and Research
- NMEDW
Northwestern Medicine Enterprise Data Warehouse
- PASP
pulmonary arterial systolic pressure
- ICD-9
International Classification of Diseases Ninth Revision
- CPT
Common Procedural Terminology
- MI
Myocardial Infarction
- PCI
Percutaneous Coronary Intervention
- CABG
Coronary Artery Bypass Grafting
- HF
Heart Failure
- AF
Atrial Fibrillation
- PE
Pulmonary Embolism
- TIA
Transient Ischemic Attack
- ICD
Internal Cardiac Defibrillator
- PPM
Permanent Pacemaker
- BMI
Body Mass Index
- NASH
Nonalcoholic Steatohepatitis
- MELD
Model for End-Stage Liver Disease
- RV
Right Ventricle
- LV
Left Ventricle
- CAD
Coronary Artery Disease
- DSE
Dobutamine Stress Echocardiography
- RVSP
Right Ventricular Systolic Pressure
- AIC
Akaike Information Criteria
- RR
Relative Risk
- OR
Odds Ratio
- HCC
Hepatocellular Carcinoma
- RCRI
Revised Cardiac Risk Index
Footnotes
Author Contributions: Lisa B. VanWagner MD MSc: study design, analysis, interpretation of results, drafting of manuscript
Hongyan Ning MS: statistical analysis, interpretation of results, manuscript editing
Maureen Whitsett, MD: data collection, manuscript editing
Josh Levitsky, MD: study design, interpretation of results, manuscript editing
Sarah Uttal, BS: data collection, manuscript editing
John T. Wilkins, MD MSc: case adjudication, interpretation of results, manuscript editing
Michael M. Abecassis, MD MBA: manuscript editing
Anton I. Skaro, MD PhD: study design, interpretation of results, manuscript editing
Donald Lloyd-Jones MD ScM: study design, analysis, interpretation of results, manuscript editing
Disclosures: The authors have no conflicts of interest pertinent to this study. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.VanWagner LB, Lapin B, Levitsky J, Wilkins JT, Abecassis MM, Skaro AI, Lloyd-Jones DM. High early cardiovascular mortality after liver transplantation. Liver Transpl. 2014;20:1306–1316. doi: 10.1002/lt.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fussner LA, Heimbach JK, Fan C, Dierkhising R, Coss E, Leise MD, Watt KD. Cardiovascular disease after liver transplantation: When, What, and Who Is at Risk. Liver Transpl. 2015;21:889–896. doi: 10.1002/lt.24137. [DOI] [PubMed] [Google Scholar]
- 3.VanWagner LB, Serper M, Kang R, Levitsky J, Hohmann S, Abecassis M, Skaro A, et al. Factors Associated With Major Adverse Cardiovascular Events After Liver Transplantation Among a National Sample. Am J Transplant. 2016;16:2684–2694. doi: 10.1111/ajt.13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albeldawi M, Aggarwal A, Madhwal S, Cywinski J, Lopez R, Eghtesad B, Zein NN. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012;18:370–375. doi: 10.1002/lt.22468. [DOI] [PubMed] [Google Scholar]
- 5.Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56:1741–1750. doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- 6.VanWagner LB, Lapin B, Skaro AI, Lloyd-Jones DM, Rinella ME. Impact of renal impairment on cardiovascular disease mortality after liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Int. 2015;35:2575–2583. doi: 10.1111/liv.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kia L, Shah SJ, Wang E, Sharma D, Selvaraj S, Medina C, Cahan J, et al. Role of pretransplant echocardiographic evaluation in predicting outcomes following liver transplantation. Am J Transplant. 2013;13:2395–2401. doi: 10.1111/ajt.12385. [DOI] [PubMed] [Google Scholar]
- 8.Josefsson A, Fu M, Bjornsson E, Kalaitzakis E. Prevalence of pre-transplant electrocardiographic abnormalities and post-transplant cardiac events in patients with liver cirrhosis. BMC Gastroenterol. 2014;14:65. doi: 10.1186/1471-230X-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt KD, Coss E, Pedersen RA, Dierkhising R, Heimbach JK, Charlton MR. Pretransplant serum troponin levels are highly predictive of patient and graft survival following liver transplantation. Liver Transpl. 2010;16:990–998. doi: 10.1002/lt.22102. [DOI] [PubMed] [Google Scholar]
- 10.Coss E, Watt KD, Pedersen R, Dierkhising R, Heimbach JK, Charlton MR. Predictors of cardiovascular events after liver transplantation: a role for pretransplant serum troponin levels. Liver Transpl. 2011;17:23–31. doi: 10.1002/lt.22140. [DOI] [PubMed] [Google Scholar]
- 11.Walker NM, Stuart KA, Ryan RJ, Desai S, Saab S, Nicol JA, Fletcher LM, et al. Serum ferritin concentration predicts mortality in patients awaiting liver transplantation. Hepatology. 2010;51:1683–1691. doi: 10.1002/hep.23537. [DOI] [PubMed] [Google Scholar]
- 12.Salvalaggio PR, Dzebisashvili N, MacLeod KE, Lentine KL, Gheorghian A, Schnitzler MA, Hohmann S, et al. The interaction among donor characteristics, severity of liver disease, and the cost of liver transplantation. Liver Transplantation. 2011;17:233–242. doi: 10.1002/lt.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalifa A, Meystre S. Adapting existing natural language processing resources for cardiovascular risk factors identification in clinical notes. J Biomed Inform. 2015;58(1):S128–132. doi: 10.1016/j.jbi.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeling AN, Flaherty JD, Davarpanah AH, Ambrosy A, Farrelly CT, Harinstein ME, Flamm SL, et al. Coronary multidetector computed tomographic angiography to evaluate coronary artery disease in liver transplant candidates: methods, feasibility and initial experience. Journal of Cardiovascular Medicine. 2011;12:460–468. doi: 10.2459/JCM.0b013e3283483916. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Yu KF. What's the relative risk?: A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Josefsson A, Fu M, Bjornsson E, Castedal M, Kalaitzakis E. Pre-transplant renal impairment predicts posttransplant cardiac events in patients with liver cirrhosis. Transplantation. 2014;98:107–114. doi: 10.1097/01.TP.0000442781.31885.a2. [DOI] [PubMed] [Google Scholar]
- 18.Umphrey LG, Hurst RT, Eleid MF, Lee KS, Reuss CS, Hentz JG, Vargas HE, et al. Preoperative dobutamine stress echocardiographic findings and subsequent short-term adverse cardiac events after orthotopic liver transplantation. Liver Transpl. 2008;14:886–892. doi: 10.1002/lt.21495. [DOI] [PubMed] [Google Scholar]
- 19.Guckelberger O, Mutzke F, Glanemann M, Neumann UP, Jonas S, Neuhaus R, Neuhaus P, et al. Validation of cardiovascular risk scores in a liver transplant population. Liver Transpl. 2006;12:394–401. doi: 10.1002/lt.20722. [DOI] [PubMed] [Google Scholar]
- 20.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 21.Wiese S, Hove JD, Bendtsen F, Moller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2013 doi: 10.1038/nrgastro.2013.210. [DOI] [PubMed] [Google Scholar]
- 22.Dare AJ, Plank LD, Phillips AR, Gane EJ, Harrison B, Orr D, Jiang Y, et al. Additive effect of pretransplant obesity, diabetes, and cardiovascular risk factors on outcomes after liver transplantation. Liver Transpl. 2014;20:281–290. doi: 10.1002/lt.23818. [DOI] [PubMed] [Google Scholar]
- 23.Kong YG, Kang JW, Kim YK, Seo H, Lim TH, Hwang S, Hwang GS, et al. Preoperative coronary calcium score is predictive of early postoperative cardiovascular complications in liver transplant recipients. Br J Anaesth. 2015;114:437–443. doi: 10.1093/bja/aeu384. [DOI] [PubMed] [Google Scholar]
- 24.Safadi A, Homsi M, Maskoun W, Lane KA, Singh I, Sawada SG, Mahenthiran J. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation. 2009;120:1189–1194. doi: 10.1161/CIRCULATIONAHA.108.847178. [DOI] [PubMed] [Google Scholar]
- 25.Fouad TR, Abdel-Razek WM, Burak KW, Bain VG, Lee SS. Prediction of cardiac complications after liver transplantation. Transplantation. 2009;87:763–770. doi: 10.1097/TP.0b013e318198d734. [DOI] [PubMed] [Google Scholar]
- 26.Konerman MA, Price JC, Campbell CY, Eluri S, Gurakar A, Hamilton J, Li Z. Pre-Liver Transplant Transthoracic Echocardiogram Findings and 6-Month Post-Transplant Outcomes: A Case-Control Analysis. Ann Transplant. 2016;21:416–427. doi: 10.12659/aot.897425. [DOI] [PubMed] [Google Scholar]
- 27.Raevens S, De Pauw M, Geerts A, Berrevoet F, Rogiers X, Troisi RI, Van Vlierberghe H, et al. Prevalence and outcome of diastolic dysfunction in liver transplantation recipients. Acta Cardiol. 2014;69:273–280. doi: 10.1080/ac.69.3.3027830. [DOI] [PubMed] [Google Scholar]
- 28.Weick A, Chacra W, Kuchipudi A, Elbatta M, Divine G, Burmeister C, Moonka D. Incidence of cardiovascular and cerebrovascular events associated with sirolimus use after liver transplantation. Transplant Proc. 2015;47:460–464. doi: 10.1016/j.transproceed.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Nicolau-Raducu R, Gitman M, Ganier D, Loss GE, Cohen AJ, Patel H, Girgrah N, et al. Adverse cardiac events after orthotopic liver transplantation: a cross-sectional study in 389 consecutive patients. Liver Transpl. 2015;21:13–21. doi: 10.1002/lt.23997. [DOI] [PubMed] [Google Scholar]
- 30.Di Maira T, Rubin A, Puchades L, Aguilera V, Vinaixa C, Garcia M, De Maria N, et al. Framingham score, renal dysfunction, and cardiovascular risk in liver transplant patients. Liver Transpl. 2015;21:812–822. doi: 10.1002/lt.24128. [DOI] [PubMed] [Google Scholar]
- 31.Bushyhead D, Kirkpatrick JN, Goldberg D. Pretransplant echocardiographic parameters as markers of posttransplant outcomes in liver transplant recipients. Liver Transpl. 2016;22:316–323. doi: 10.1002/lt.24375. [DOI] [PubMed] [Google Scholar]
- 32.Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17:15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- 33.Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, et al. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109–1114. doi: 10.1002/lt.21126. [DOI] [PubMed] [Google Scholar]
- 34.Borg MA, van der Wouden EJ, Sluiter WJ, Slooff MJ, Haagsma EB, van den Berg AP. Vascular events after liver transplantation: a long-term follow-up study. Transpl Int. 2008;21:74–80. doi: 10.1111/j.1432-2277.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 35.Abecassis MM, Burke R, Cosimi AB, Matas AJ, Merion RM, Millman D, Roberts JP, et al. Transplant center regulations--a mixed blessing? An ASTS Council viewpoint. Am J Transplant. 2008;8:2496–2502. doi: 10.1111/j.1600-6143.2008.02434.x. [DOI] [PubMed] [Google Scholar]
- 36.Graversen P, Abildstrom SZ, Jespersen L, Borglykke A, Prescott E. Cardiovascular risk prediction: Can Systematic Coronary Risk Evaluation (SCORE) be improved by adding simple risk markers? Results from the Copenhagen City Heart Study. Eur J Prev Cardiol. 2016;23:1546–1556. doi: 10.1177/2047487316638201. [DOI] [PubMed] [Google Scholar]
- 37.Wedd JP, Harper AM, Biggins SW. MELD score, allocation, and distribution in the United States. Clinical Liver Disease. 2013;2:148–151. doi: 10.1002/cld.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring Diagnoses: ICD Code Accuracy. Health Services Research. 2005;40:1620–1639. doi: 10.1111/j.1475-6773.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.