Abstract
Lateral flow assays (LFAs) are highly attractive for point of care diagnostics for infectious disease, food safety, and many other medical uses. The unique optical, electronic and chemical properties that arise from the nanostructured and material characteristics of nanoparticles provide an opportunity to increase LFA sensitivity and impart novel capabilities. However, interfacing to nanomaterials in complex biological environments is challenging and can result in undesirable side effects such as non-specific adsorption, protein denaturation, and steric hindrance. These issues are even more acute in LFAs, where there are many different types of inorganic-biological interfaces, often of complex nature. Therefore, the unique properties of nanomaterials for LFAs must be exploited in a way that addresses these interface challenges.
Keywords: Nano-bio interfaces, protein coronas, rapid diagnostics, paper-based assays
The need for lateral flow diagnostics
Epidemic outbreaks are a major global health threat, and the frequency of outbreaks has been steadily increasing.[1] Diagnosis is a critical step in confining and treating infected patients. There are several approaches for detecting viruses in patient samples, ranging from detecting the virus itself to the antibodies developed in the immune response [2]. Methods for diagnosis range in achievable specificity and sensitivity, where each approach has advantages and disadvantages. Lateral flow assays (LFAs, see Glossary) have been promising as point of care (POC) devices because they are easy to use, robust, inexpensive, and implementable in rugged environments. [3] LFAs consist of paper strips to which a biological sample is added, and the fluid wicks through, resulting in two colored lines for a positive test, or one for a negative test. Readout relies on formation of a sandwich immunoassay when a biomarker is present, which is visible to the naked eye because of the accumulation of gold nanoparticle (NP)-antibody conjugates at the test line, though other nanomaterials outside of gold NPs have also been utilized such as dye-infused beads.[4]
Because of their simplicity and rugged format, LFAs have been used for many diverse applications not just including infectious disease, but also water and food safety, [5] routine clinical tests, and food allergy testing [6]. Thus, they have made a significant impact on public health. [7] There are many commercially available LFAs, some of which are summarized in Table 1. Available tests are primarily for testing serology by detecting IgG, IgM, or IgA antibodies produced by the immune system in response to pathogens (i.e., serological assays), and use a predominantly gold NP colorimetric readout. However, rapid tests can also detect metabolites (as in the case of drug testing) or the protein biomarkers themselves (i.e., direct assays).
Table 1.
Table of rapid tests for different types of biomarkers, their manufacturers, and the detection target.
| pathogen | sources | Detection |
|---|---|---|
| Blood Borne Diseases/Infectious Diseases | ||
| Cytomegalovirus | Biocan, Biogate Labs | IgG/IgM |
| Hepatitis A | Standard Diagnostics, Biogate Labs | IgG/IgM |
| Hepatitis B | Standard Diagnostics, Biocan, Cortez Diagnostics, Biogate Labs, Maternova, NTBIO | antigen, IgG/IgM |
| Hepatitis C | Standard Diagnostics, Orasure, Biocan, Cortez Diagnostics, Biogate Labs, Maternova, NTBIO | IgG/IgM |
| Herpes Simplex Virus | Biocan, Alere, Biogate Labs | IgG/IgM |
| HIV | Standard Diagnostics, InBios, Orasure, Biocan, Alere, biolytical, Biogate Labs, Maternova, NTBIO, Biopanda | antigen, IgG/IgM |
| Toxoplasma Gondi | Biocan, Biopanda | IgG/IgM |
| Infectious Disease | ||
| Anti-EV71 | Standard Diagnostics | IgM |
| Anti-Onchocerciasis | Standard Diagnostics | IgG |
| Chagas (Trypanosoma cruzi) | Standard Diagnostics, InBios, Biocan, Maternova | IgG/IgM/IgA |
| Chikungunya | Standard Diagnostics, Biocan, Maternova | IgG/IgM |
| Dengue | Standard Diagnostics, InBios, Biocan, Cortez Diagnostics, Access Bio, Biogate Labs, Maternova, Mediven, NTBIO, Biopanda, Focus Diagnostics | antigen, IgG/IgM, IgA |
| Ebola | Orasure, Biocan, Corgenix | antigen, IgG/IgM |
| Hanta | Standard Diagnostics | IgG/IgM/IgA |
| Japanese Encephalitis | Standard Diagnostics | IgM |
| Leishmaniasis | Standard Diagnostics, InBios, Biocan, Cortez Diagnostics | IgG/IgM |
| Leptospira | Standard Diagnostics, Biocan | IgG/IgM |
| Lymphatic Filariasis | Standard Diagnostics, Biocan | IgG/IgM |
| Malaria | Standard Diagnostics, Biocan, Alere, Cortez Diagnostics, Access Bio, Biogate Labs, Maternova, NTBIO, Biopanda | antigen, IgG/IgM/IgA |
| Melioidosis | InBios | antigen |
| Mononucleosis | Alere, Cortez Diagnostics | IgG/IgM |
| MRSA | Alere | antigen |
| Rubella | Standard Diagnostics, Biocan, Biogate Labs | IgG/IgM |
| S. aureus | Alere | antigen |
| Scrub Tyhpus | InBios, Cortez Diagnostics, Access Bio | IgG/IgM |
| Tetanus | Standard Diagnostics | IgG/IgM |
| Typhoid | Standard Diagnostics, Biocan, Biogate Labs, Mediven, Biopanda, Cortez Diagnostics | antigen, IgG/IgM |
| Zika | Biocan, Mediven | IgG/IgM |
| Sexually Transmitted Diseases | ||
| Chlamydia | Standard Diagnostics, Cortez Diagnostics, Maternova | antigen |
| Neisseria Gonorrhoeae | Cortez Diagnostics, Maternova | antigen |
| Syphilis | Standard Diagnostics, Biocan, Alere, biolytical, Cortez Diagnostics, Biogate Labs, Maternova, Biopanda | IgG/IgM |
| Gastrointestinal | ||
| Adeno | Standard Diagnostics, Biocan, Ameridx, Mediven | antigen |
| Cholera | Standard Diagnostics, Maternova | antigen |
| H. pylori | Standard Diagnostics, Biocan, Ameridx, Cortez Diagnostics, Biogate Labs, NTBIO, Biopanda | antigen, IgG/IgM |
| Rotavirus | Standard Diagnostics, Mediven | antigen |
| Fecal Antigens | ||
| Calprotectin | Biocan | antigen |
| Clostridium difficile | Biocan | antigen |
| E. coli O157 | Biocan | antigen |
| Giardia | Biocan, Alere, Ameridx | antigen |
| Lactoferrin | Biocan | antigen |
| Norovirus | Standard Diagnostics, Biocan | antigen |
| Procalcitonin | Biocan, Maternova, NTBIO | |
| Transferrin | Biocan | |
| Respiratory | ||
| Influenza | Standard Diagnostics, Orasure, Biocan, Alere, Cortez Diagnostics, Mediven, Biopanda | antigen |
| Legionella | Standard Diagnostics, Alere, Cortez Diagnostics | antigen |
| Pneumonia | Alere, Biogate Labs | antigen, IgG/IgM |
| Respiratory Syncytial Virus (RSV) | Standard Diagnostics, Alere | antigen |
| Strep A | Standard Diagnostics, Alere, Cortez Diagnostics | antigen |
| Tuberculosis (TB) | Standard Diagnostics, Cortez Diagnostics, Biogate Labs | antigen |
| Cancer Markers | ||
| Alpha-Fetprotein (AFP) | Standard Diagnostics, Biocan, Cortez Diagnostics, NTBIO | antigen |
| Carcinoembryonic Antigen (CEA) | Biocan, NTBIO | antigen |
| Fecal Occult Blood | Standard Diagnostics, Biocan, Cortez Diagnostics, Mediven, NTBIO | antigen |
| Prostate Specific Antigen (PSA) | Standard Diagnostics, Biocan, Cortez Diagnostics, Biogate Labs, NTBIO | antigen |
| Cardiac Markers | ||
| C-reactive protein | NTBIO | |
| Creatine Kinase Isoenzymes | Biocan, Cortez Diagnostics, Biogate Labs, NTBIO, Biopanda | antigen |
| Myoglobin | Standard Diagnostics, Biocan, Cortez Diagnostics, Biogate Labs, NTBIO, Biopanda | antigen |
| N-terminal B-type natriuretic peptide precursor (NT-proBNP) | Biopanda | antigen |
| Troponin I | Standard Diagnostics, Biocan, Cortez Diagnostics, Biogate Labs, NTBIO, Biopanda | antigen |
| Fertility Hormones | ||
| human Follicular Stimulating Hormone | Cortez Diagnostics | direct |
| hCG | Standard Diagnostics, Biocan, Cortez Diagnostics, Mediven | direct |
| Luteinizing Hormone | Standard Diagnostics, Biocan | direct |
Antigen indicates direct tests, IgG/IgM/IgA indicate serological tests.
Lateral flow diagnostics are enhanced by nanotechnology
LFAs rely on different modes of detection (Fig. 1), where readout can be optical-colorimetric, fluorescent, surface enhanced Raman spectroscopy (SERS), electrochemical, or biochemical. [8, 9] The species on the label antibody is often a gold nanoparticle due to its strong optical absorption. Other labels include dye-loaded polymeric beads, or fluorescent beads or quantum dots, magnetic nanoparticles, and carbon-based nanoparticles. [10] The combination of nanotechnology with point of care diagnostics has resulted in significant advances in device technology. [11–13] Nanomaterials possess unique material and size dependent properties that can enhance LFA detection by optical as well as fluorescent, spectroscopic, and electrochemical readouts. These advances can help create lower-cost, higher sensitivity, and field-forward devices.
Figure 1.
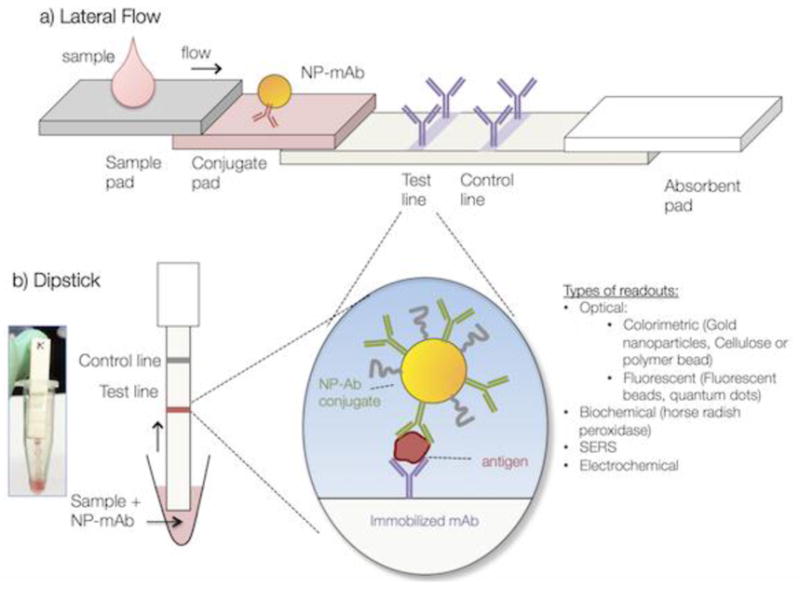
Basic structure of a) an LFA and b) a dipstick immunoassay.
However, the intersection of nanotechnology with biology also creates numerous challenges. Furthermore, the simplicity of LFA operation makes them appear deceivingly straightforward to construct. A device that uses a simplified format of the LFAs is the dipstick assay, where the conjugate pad and sample pad are substituted with a solution into which the nitrocellulose with the wick is immersed. This eliminates the need to dry down the NP-biomolecule conjugate. Unfortunately, LFAs and the related dipstick immunoassays suffer numerous interface issues such as non-specific adsorption of proteins and gold NPs to the test substrate or test materials. These surface effects can have negative ramifications such as ambiguous readouts and reduction in sensitivity. Despite their importance, interface issues are often overlooked and underappreciated. While any inorganic-biological interface can have undesirable side effects, these cannot be ignored for nanomaterials because of their high surface to volume ratios. Solving interface issues can be tedious and/or viewed as having limited innovative aspects, so often they are not published in the literature.
These interface issues have long plagued the nanomedicine and nanobiotechnology communities, which have faced complications that result from putting nano- and micro-scale inorganic materials in contact with biology. These complications include surface fouling, corrosion, and protein denaturation. For LFAs and dipstick immunoassays, these complications are amplified because there are two biological-inorganic interfaces, one for the NP and another for the nitrocellulose substrate. However, these surface effects can result in either false positives or false negatives, which can have life or death consequences.
Interface issues have been problematic in other nanotechnology fields such as cancer nanomedicine. Non-specific protein adsorption and protein corona formation (see Box 1) have been shown to reduce the specificity of ligand-cell receptor interactions, deteriorate the carrier efficacy, or cause undesirable biodistribution. While interface issues had been one of the biggest barriers for cancer nanomedicine, advances in recent years have promoted clinical applications of nanomaterials. Thus, these advances can help improve the development of LFAs. Despite the progress in nano-bio interface issues in cancer nanomedicine, they have not been extensively discussed for dipstick and lateral flow immunoassays. Therefore, this Review seeks to highlight the importance of nano-bio interface issues in point of care immunoassays, and what can be learned from other areas of nanomedicine where interface issues have been successfully overcome.
Box 1. Protein coronas.
Protein coronas are clouds of weakly bound proteins that form around nanoparticles (NPs) when they are introduced into biological fluids. The protein corona, and not the NPs themselves or the NP surface chemistry, is known to be the most important factor in biological processes such as cell uptake and cell association. Understanding and manipulating protein coronas has been challenging, though concerted efforts in the field have resulted in major progress. Protein corona properties such as size, composition, etc. are difficult to measure because coronas are held together by non-covalent bonds. Predicting what corona will form has remained elusive because coronas are complex and heterogeneous. However, advances in proteomics of coronas has allowed insight as to what proteins tend to be in coronas and how they influence some biological process such as cell association[55]. Additionally, corona composition and size evolves with time, and depends on the history of the sample. There have been many attempts to make NP surfaces that are completely non-fouling, which have met sporadic or limited success. However, there has been some progress with zwitterionic molecules. The composition of the protein corona that forms around a NP differs for every biological fluid (serum vs. blood vs. urine vs. saliva), though coronas have been characterized predominantly for human serum, cell media, and blood.
Interactions at the nano-bio interface in lateral flow immunoassays
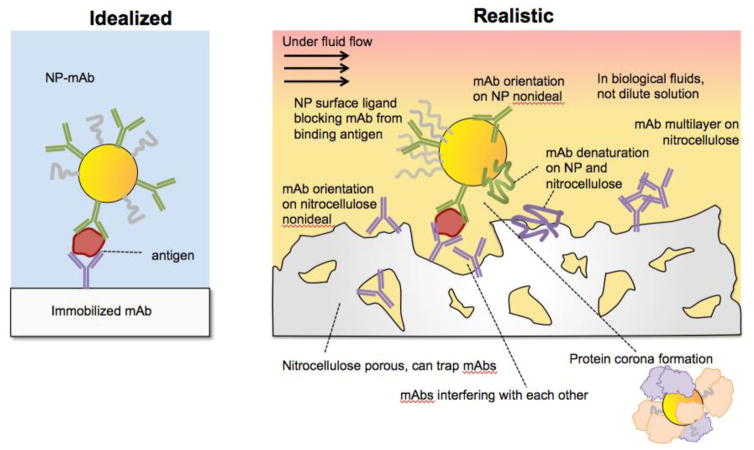
Several different interface issues in LFAs can affect the central phenomenon of antibody-antigen binding in the LFA. Often sandwich immunoassay formation is idealized as a single antigen binding cleanly to an antibody on a flat surface and another on a NP (Fig. 2a). However, undesirable interface issues can occur (Fig. 2b), including non-specific adsorption and steric hindrance on both the NP and the nitrocellulose. These can be categorized into different types of interface interactions.
Figure 2.
Idealized vs. realistic sandwich immunoassays that use nanoparticle antibody conjugates for sensing.
Interface issues for the NP-antibody conjugates
Like with any NP-biomolecule conjugate, the ability of the antibody (Ab) to bind to its target antigen can be diminished because of its attachment to a NP.[14] Most often this is manifested by a change in binding affinity, K. Studies of immobilized DNA have shown that surface immobilization can either increase or decrease the DNA’s affinity for its complement, changing K over 29 orders of magnitude [15, 16]. The Ab can denature due to perturbing effects of the NP and it surface coating ligand, preventing biomarker binding. The Ab can be potentially oriented incorrectly on the NP surface, where its binding epitopes for the biomarker are obscured, lowering or even preventing target binding. Finally, the surface ligands on the NP surface can sterically obscure the binding epitopes of the Ab.
Furthermore, the interaction of the NP-Ab with its environment can also impact Ab-antigen binding. Samples added to LFAs contain complex mixtures of proteins and small molecules in the presence of which the binding event must take place. Often biological samples for POC assays are not cleaned up, and even if they are, proteins and salts can still be present at high concentrations. When NPs are introduced into biological fluids, a protein corona forms around them, where the proteins non-covalently adsorb to the NP surface, forming a weakly bound “cloud” (Box 1). [17] [18] Often protein coronas are studied for cancer delivery agents, [19] but they are also present in LFA devices and undoubtedly influence antigen-antibody interactions.
The properties of the corona that forms around a NP is influenced by NP size, material, and shape, and strongly influenced by NP surface chemistry.[20] Unfortunately the molecules used to passive NP surfaces are typically a trade secret for commercially available NPs, which makes it difficult for end users to optimize the system for Ab conjugation.
Protein corona formation is rapid: proteins adsorb to the NP surface within seconds.[21] In typical LFAs the fluid front takes minutes to reach the test line, and often the test is allowed additional time to “develop,” so NP-antibody antigen conjugates most certainly have a protein corona. To further complicate matters, the biological fluids for LFAs are diverse, and can be blood, serum, urine, saliva, or others, all of which will have very different compositions, and thus will all form different protein coronas.
Interface issues for the immobilized antibodies
The interface issues for the NP-Ab conjugate are mirrored for the immobilized Ab. Because paper has several unique properties as a substrate, paper analytical devices and bioactive paper have had a surge of interest. There is a major advantage to immobilizing reactions on paper as opposed to having them in solution as it eliminates the need to transport fluids and a cold chain. Due to their chip-based format, paper-based devices are easily miniaturized, can be manufactured at scale, and are often considered to be the most widely deployed microfluidic devices. [22] There has been extensive work studying biomolecule conjugation to cellulose for paper supported assays [23]. Antibody immobilization onto nitrocellulose is most often achieved simply by spotting it down, where the Ab adheres by hydrophobic interactions. Alternative strategies include chemical conjugation to the nitrocellulose or to streptavidin have also been used successfully. [24] Generally, immobilization onto paper can result in different target affinities. Again, antibody behavior on paper can differ significantly from solution or ELISA. Paper is a much more complex substrate compared to flat glass, polystyrene, and metal surfaces that are typically used for ELISA, surface plasmon resonance (SPR) sensing, and other assays. Its porosity provides the driving force for the capillary flow, but this means that net surface area is high, amplifying interface effects. Like with the NP-Ab conjugate, the nitrocellulose can also have a “protein corona” or surface adsorption of the proteins from the biological fluid. Additionally, biomolecules can be trapped inside the pores and decrease target binding efficiency. Typically these adsorption issues are mitigated by membrane blocking via adsorption of other proteins such as albumin, casein, and other proteins. However, blocking paper can come at the cost of reducing assay signal and thus sensitivity. [25]
Antibody-biomarker interactions
The antibodies on the NP and test line must be able to bind to the target. For a given disease, the antibodies must be able to recognize targets even when there are differences due to strain and serotype. Furthermore, antigens from different geographic areas can present different epitopes or glycosylation, making it difficult to have one specific antibody for each disease, but an antibody cocktail can be utilized to ensure binding to all variations. To complicate matters, viruses evolve during an outbreak, which can result in the antibodies in the test strip to become less effective at detecting the antigen, or cross reactivity with closely related viruses.
For sandwich formation, the antibodies on the NP and nitrocellulose must be able to bind to the antigen of interest simultaneously. Thus, they must work in pairs, so the antibody on the NP cannot bind to the same epitope as the nitrocellulose antibody or interfere with each other. Therefore, even though a target antigen can have multiple antibodies that recognize it, not all of them will work in pairs.
In reality, antibodies do not have mutually exclusive targets, and can bind to multiple species, especially if biomarkers are closely related. Cross reactivity can be minimized by increasing stringency in screening, but because the nature of epidemics, the target antigen can evolve, resulting in unanticipated cross-reactivity. For diagnostics to be useful, they must be multiplexed, [26] which can become complicated by cross-reactivity within a device.
Finally, patient samples are more complicated than pure solutions. Testing in the lab is often performed on purified protein in buffer or spiked into human serum, which is not nearly as complex as samples obtained from patients in the field. Sample preparation can also greatly influence assay functionality and accuracy, and while minimization of processing steps is more convenient, “raw” samples can be much more variable. These factors can contribute to assay variability.
Overall device
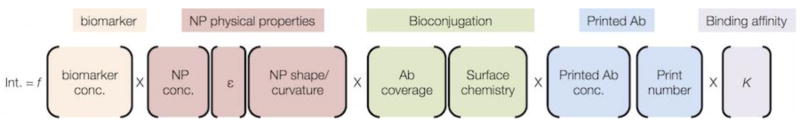
The overall device design requirements are that it needs to be simple to use, where the result can be readable by eye and easily interpreted by the user. This places constraints on the signal intensity at the test line, as it must be detectable by eye. The colorimetric intensity in a LFA is a function of multiple parameters (Fig. 3). Some of these parameters are straightforward and predictable, such as antigen concentration, and others such as NP size have been measured. [27] In addition, the number of antibodies on the NP surface (i.e., antibody coverage) is also a critical parameter. However, many of the parameters are not intuitively obvious, such as Ab coverage and NP surface chemistry. Nevertheless, this equation serves as a guide for considering all of the NP-Ab and other factors that are at play in generating a test line signal.
Figure 3.
The test line intensity of a LFA that uses a visual readout is a function (f) of multiple parameters, which are categorized by color.
The LFA also presents challenges in that target recognition must occur under fluid flow, so the binding event does not occur at equilibrium. [28] Finally, the device operation is subjected to environmental conditions where temperature, humidity, and lighting conditions differ greatly from the laboratory.
Design considerations for LFAs
Because LFAs and sandwich immunoassays are now a mature technology, there are numerous industry-written guides and workshops that discuss best practices for designing and manufacturing LFAs with high sensitivity and specificity, can be scaled up, and are appropriate for consumer use. [29] However, the interface challenges mentioned above still remain. Going forward, what is the best strategy for making LFAs that rely on nanotechnology? These issues need to be considered for the next outbreak that emerges. Even though LFA technology is not new, we still must consider issues that may present design constraints for each new or re-emerging disease (Fig. 4). Factors include the behavior of the disease itself, such as how infectious it is and the nature of the symptoms and outcomes. Also, characteristics of the endemic areas need to be considered, such as whether it is urban or rural, available health care, and if similar diseases are currently co-circulating. Then, the technical aspects of detection need to be considered, such as the identity of the biomarker that will be detected, what concentration it exists at, and how distinct it is from biomarkers of similarly related diseases. In addition, the availability of bioreagents to detect the biomarker of interest is critical. Generating and selecting antibodies, and their mass production requires 16–24 months, [30] [31] which is much longer than the critical initial period when an outbreak is emerging. Additionally, the nature of the patient sample can influence test design, as sample collection issues are different for urine collection vs. blood. Finally, readout modality should be chosen to optimize sensitivity, or whether a reader is required, or if readout by eye is important. Making a point of care device is inherently a multidisciplinary effort, as it involves technical expertise in immunology, molecular biology, infectious disease, materials science, fluid mechanics, and manufacturing. In addition, critical aspects of device usage go beyond the technical, as human interactions, socioeconomic issues, and public health aspects can strongly influence device design. For success, challenges in each of the technical fields must be fully appreciated by the others.
Figure 4.
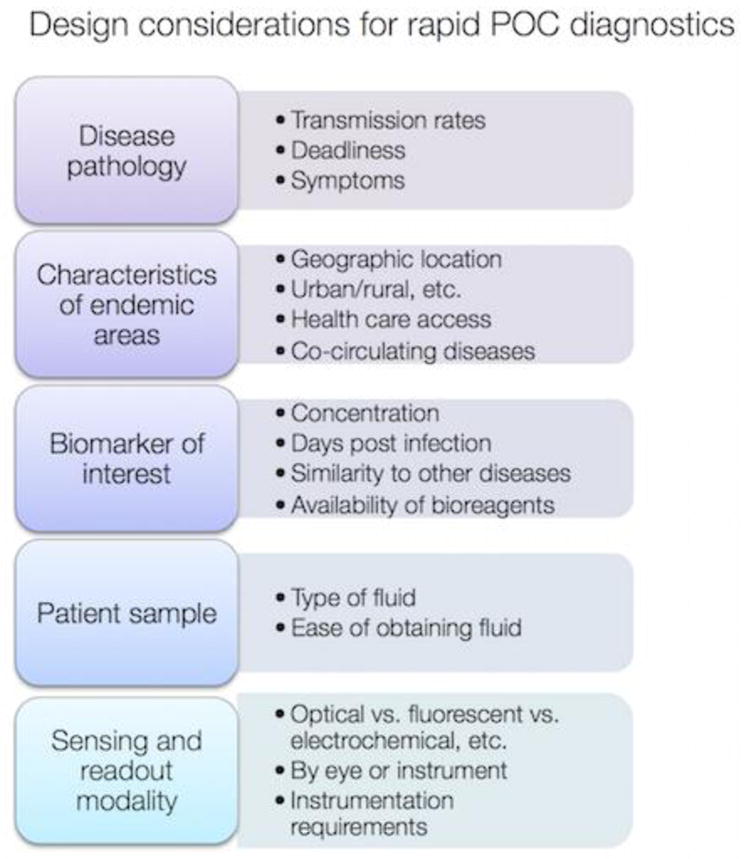
Design considerations for point of care diagnostics for infectious disease.
The World Health Organization (WHO) has issued criteria under the acronym ASSURED (see Box 2) which are guidelines that point of care diagnostics must meet in order to be useful in the developing world. Even though the needs for resource-poor settings share common characteristics, such being low-cost and rugged, constraints will differ for every outbreak. For example, in the 2014 Ebola outbreak, the high contagion level and human-to-human transmission emphasized the need for a differential multiplexed diagnostic that could diagnose Ebola and also confirm that symptoms were not due to another co-circulating disease, such as malaria. In addition, sample handling can also place constraints on the design of how biological fluids are introduced to minimize contamination of the health care worker administering or assay readout.
Box 2. World Health Organization ASSURED criteria.
Disease diagnostics are the most useful for populations in the developing world, where disease burden is the highest. Traditional lab tests are not feasible because they require infrastructure typically not available in these resource-poor settings. Consequently, the World Health Organization has issued guiding criteria for diagnostics, with the acronym ASSURED:
Affordable
Sensitive
Specific
User-friendly
Rapid & Robust
Equipment-Free
Delivered
Devices that meet ASSURED criteria are amenable for developing countries and rugged environments where many infectious diseases are endemic.
Towards improved nanotechnology-enabled LFAs
To overcome interface issues requires an understanding of them. Fortunately, the field has made significant progress overcoming and adapting to nano-bio interface issues. For example, our understanding of surface modification strategies of nanoparticles is now highly sophisticated, which has facilitated the development of nanoparticle drug carriers or imaging agents for cancer therapy. [32] Clearly, a multidisciplinary approach is critical for overcoming these challenges. This means that material scientists must appreciate the complexity of the biological samples and the behavior of antibody-antigen binding. Likewise, immunologists and virologists must recognize the unique challenges of NP-biomolecule conjugates and NPs in biological environments.
Currently, information on NP functionalization for LFAs has been growing. Commercial NP sources such as Nanocomposix [33] are now publishing guides that discuss tips for using NPs in LFAs in an effort to bridge the gap. Handbooks and protocol chapters have been helpful in disseminating step by step recipes and best practices for making LFAs.[34] However, one major impediment is that surface treatment of the NPs and the nitrocellulose/conjugate and surface pads in commercial assays are predominantly proprietary information. This greatly hinders troubleshooting, as NP surface chemistries and stabilization molecules are critical to the success of a paper-based immunoassay.
Thinking beyond best practices, it is also important to look towards new and emerging approaches. There have been some innovative ways to utilizing the unique properties of nanoparticles for LFAs, listed below:
Further ways to exploit nanotechnology
Even though NPs have been used in LFAs for decades, there are still many unique opportunities for exploiting their properties to improve LFA performance and also enhance their capabilities. For example, photothermal heating of the NPs [35] can be used to increase the signal of the test line, where a laser is used to excite the gold NPs at their SPR and increase image contrast by thermal detection. Signal enhancement has been achieved by enzymes such as horseradish peroxidase (HRP) [36], silver staining, [37] or even the use of multiple gold NPs binding species [38], all of which increase assay sensitivity. Induced NP aggregation has also been used to increase the signal or effect a color change. [39, 40]
NPs can be used for other modes of readouts besides visual imaging. Surface enhanced Raman spectroscopy (SERS) has been attractive for analyte detection because it can enhance the signal of molecules in its vicinity by several orders of magnitude.[41] For biological sensing and imaging, a format where the gold NP is decorated with a small molecule reporter, resulting in a “nanotag.” While this has been used for SERS imaging, it has recently been explored for LFAs.[42, 43] Superparamagnetic iron oxide nanoparticles (SPIONs) have also been investigated for a magnetic LFA readout [44].
Additionally, nanotechnology can enable new capabilities to assays. For example, test line colors have been traditionally only a single color, red, due to the size and shape gold NPs used in the assay. That NPs change their color with their shape and size has been well-studied, but only recently has it been used to enable spectral multiplexing by using multicolor test lines, as has been demonstrated for antibiotic detection using fluorescence of quantum dots [45], for Dengue/Yellow Fever/Ebola using visual readout of silver NPs,[46] and also dye-labeled cellulose particles for detection of Dengue/Chikungunya IgG/IgM. [47]
Exploiting protein coronas
As mentioned previously, protein corona formation is typically viewed as a hindrance (Box 1). Proteins in solution adsorb to the NP, obscuring ligands on the NP surface to bind to its target. For LFAs, the corona can potentially block the Ab from binding to the target antigen in the sandwich immunoassay. However, protein coronas do not produce only negative side effects, and it is now known that they can be exploited for their unique properties. Recently, the impact of the protein corona around NP-Ab in paper-based immunoassays was examined. [48] Anecdotally it is known that running strips in buffer solution often does not work because false test lines occur; this effect can be prevented by running strips in human serum. The NP-Ab in the serum forms a protein corona, and the corona is partially responsible for preventing non-specific adsorption to the test line, reducing false positives. These corona proteins actually mediate the interaction between the NP-Ab antigen complex or the NP-Ab and the immobilized Ab in a favorable way.
Fluid flow manipulation
Approaches to increase sensitivity by manipulating the fluid flow have also been explored, some of which can be used to improve NP-Ab interaction with the antigen and test line. For example, isotachaphoresis,[49] an electrokinetic preconcentration/separation technique, can improve transport of target analytes to the LFA capture line, resulting in a dramatic increase in sensitivity. There have been also efforts to modify fluid flow by use of wax pillars or shaping of paperfluidic channels [50, 51]. However, this aspect of LFAs has great potential for innovation, and can be used in addition to other methods for improving performance and sensitivity via NP-Ab optimization. [28]
Quantitative descriptions of binding events
Finally, a molecular level understanding of the binding event and the surface effects and perturbations is critical for improving antigen binding, which is at the heart of the immunoassay. Even though Ab-antigen binding does not occur at equilibrium, binding affinity constants (K) are useful for comparing the behavior of different tests when optimizing the properties of the NP, Ab, and test strip. For example, Langmuir isotherms modified to include surface terms can be used to separate the impact of the surface modifications on antibody-antigen binding in planar and NP surfaces, and can also be applied to LFAs.[48, 52, 53]
Concluding remarks and perspectives for the future
In summary, LFA and paper-based assays have been proven to be useful as diagnostics, with tremendous potential to improve response to disease outbreaks. In addition, there have been many innovations in using them for many other applications in unique ways. Despite the fact that LFAs have been around for many years, they can continue to take advantage the unique capabilities of nanotechnology, as both fields are still evolving.[54] Consequently, we have to be cognizant of bio-interface issues as they continue to emerge. Many outstanding issues remain (see Outstanding Questions), but as with other biological fields that intersect with nanotechnology, understanding and overcoming interface issues has facilitated the success of innovative applications.
Outstanding Questions.
How can we properly exploit nanotechnology to enable robust and sensitive POC devices?
How can we engineer optimal devices for the next epidemic outbreak in a timely manner?
LFAs rely on nanoparticle-biomolecule conjugates. With the increasing number of technical advances in nanomaterial manufacturing, can scale up also be reached for nanoparticle-biomolecule conjugates?
Can the rapid sample-to-answer time of LFAs be ultimately leveraged for epidemic control, such as making real-time maps of epidemic outbreaks based on actual patient data?
Will competing technologies such as nucleic amplification techniques render LFAs obsolete? With the increasing innovation in LFAs, what characteristics of LFAs vs. nucleic acid devices complement or compete with each other?
Trends.
Lateral flow assays (LFAs) for infectious disease, food safety, and many other applications have been enhanced by nanotechnology.
However, interface effects in LFAs are much more complicated, which is problematic because POCs have to be robust, simple, easy to use.
To fully utilize the unique properties of nanotechnology, these interface issues must be understood, controlled, and also leveraged.
Acknowledgments
Supported by the NIH NIAID (AI100190). HdP was supported by the Rafael del Pino fellowship, Broshy Fellowship, a Tata Center for Technology and design Fellowship and a MIT-SUTD IDC grant.
Glossary
- Dipstick assay
a paper-based assay for detecting that is a simplified version of the lateral flow assay (LFA), which consists of a nitrocellulose strip which has immobilized antibodies that is put in contact with a solution containing the biological sample mixed in with the nanoparticle-antibody conjugates
- Lateral flow assay (LFA)
a paper-based device for detecting the presence of a biomarker, antigen, or other analyte. A fluid is added to a sample pad and then wicks through a conjugate pad that has dried into it a nanoparticle-antibody conjugate or similar species that can specifically bind to the biomarker. The complex wicks through a nitrocellulose strip, which has immobilized antibodies or other binding species that capture the complex, providing the readout
- Nanoparticle (NP)
a particle with dimensions in the range of 1–100s of nanometers
- Protein corona
the “cloud” of proteins and other small molecules that forms around nanomaterials when they are introduced to biological fluids and physiological environments
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith KF, et al. Global rise in human infectious disease outbreaks. Journal of The Royal Society Interface. 2014;11(101) doi: 10.1098/rsif.2014.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeling RW, et al. Evaluation of diagnostic tests: dengue. Nature Reviews Microbiology. 2010 doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 3.Martinez AW, et al. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Analytical Chemistry. 2010;82(1):3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 4.O’Farrell B. Evolution in Lateral Flow–Based Immunoassay Systems. In: Wong R, Tse H, editors. Lateral Flow Immunoassay. Humana Press; 2009. pp. 1–33. [Google Scholar]
- 5.Sicard C, et al. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Research. 2015;70:360–369. doi: 10.1016/j.watres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Zheng C, et al. Rapid detection of fish major allergen parvalbumin using superparamagnetic nanoparticle-based lateral flow immunoassay. Food Control. 2012;26(2):446–452. [Google Scholar]
- 7.Vashist SK, et al. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends in Biotechnology. 2015;33(11):692–705. doi: 10.1016/j.tibtech.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Nie Z, et al. Electrochemical sensing in paper-based microfluidic devices. Lab on a Chip. 2010;10(4):477–483. doi: 10.1039/b917150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, et al. Rapid and Sensitive Detection of Protein Biomarker Using a Portable Fluorescence Biosensor Based on Quantum Dots and a Lateral Flow Test Strip. Analytical Chemistry. 2010;82(16):7008–7014. doi: 10.1021/ac101405a. [DOI] [PubMed] [Google Scholar]
- 10.Chun P. Colloidal Gold and Other Labels for Lateral Flow Immunoassays. In: Wong R, Tse H, editors. Lateral Flow Immunoassay. Humana Press; 2009. pp. 1–19. [Google Scholar]
- 11.Quesada-González D, Merkoçi A. Mobile phone-based biosensing: An emerging “diagnostic and communication” technology. Biosensors and Bioelectronics. 2017;92:549–562. doi: 10.1016/j.bios.2016.10.062. [DOI] [PubMed] [Google Scholar]
- 12.Syedmoradi L, et al. Point of care testing: The impact of nanotechnology. Biosensors and Bioelectronics. 2017;87:373–387. doi: 10.1016/j.bios.2016.08.084. [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro M, et al. Gold Nanoparticles for Diagnostics: Advances towards Points of Care. Diagnostics. 2016;6(4) doi: 10.3390/diagnostics6040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aubin-Tam ME, Hamad-Schifferli K. Structure and Function of Nanoparticle-Protein Conjugates. Biomedical Materials. 2008;3:034001. doi: 10.1088/1748-6041/3/3/034001. [DOI] [PubMed] [Google Scholar]
- 15.Federici S, et al. On the thermodynamics of biomolecule surface transformations. Journal of Colloid and Interface Science. 2012;375(1):1–11. doi: 10.1016/j.jcis.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Levicky R, Horgan A. Physicochemical perspectives on DNA microarray and biosensor technologies. Trends in Biotechnology. 2005;23(3):143–149. doi: 10.1016/j.tibtech.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Cedervall T, et al. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proceedings of the National Academy of Science. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pino Pd, et al. Protein corona formation around nanoparticles - from the past to the future. Materials Horizons. 2014;1(3):301–313. [Google Scholar]
- 19.Mahon E, et al. Designing the nanoparticle–biomolecule interface for “targeting and therapeutic delivery”. Journal of Controlled Release. 2012;161(2):164–174. doi: 10.1016/j.jconrel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Walkey CD, et al. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. Journal of the American Chemical Society. 2012;134(4):2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 21.Casals E, et al. Time Evolution of the Nanoparticle Protein Corona. ACS Nano. 2010;4(7):3623–3632. doi: 10.1021/nn901372t. [DOI] [PubMed] [Google Scholar]
- 22.Shafiee H, et al. Paper and Flexible Substrates as Materials for Biosensing Platforms to Detect Multiple Biotargets. Scientific Reports. 2015;5:8719. doi: 10.1038/srep08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelton R. Bioactive paper provides a low-cost platform for diagnostics. Trends in Analytical Chemistry. 2009;28(8):925–942. doi: 10.1016/j.trac.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, et al. Visual detection of microRNA with lateral flow nucleic acid biosensor. Biosensors and Bioelectronics. 2014;54:578–584. doi: 10.1016/j.bios.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones K. FUSION 5: A New Platform For Lateral Flow Immunoassay Tests. In: Wong R, Tse H, editors. Lateral Flow Immunoassay. Humana Press; 2009. pp. 1–15. [Google Scholar]
- 26.Li J, Macdonald J. Multiplexed lateral flow biosensors: Technological advances for radically improving point-of-care diagnoses. Biosensors and Bioelectronics. 2016;83:177–192. doi: 10.1016/j.bios.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Lou S, et al. A gold nanoparticle-based immunochromatographic assay: The influence of nanoparticulate size. Analyst. 2012;137(5):1174–1181. doi: 10.1039/c2an15844b. [DOI] [PubMed] [Google Scholar]
- 28.Fu E, et al. Two-Dimensional Paper Network Format That Enables Simple Multistep Assays for Use in Low-Resource Settings in the Context of Malaria Antigen Detection. Analytical Chemistry. 2012;84(10):4574–4579. doi: 10.1021/ac300689s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin CD, et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat Med. 2011;17(8):1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 30.Gronvall GK, et al. Next-Generation Monoclonal Antibodies: Challenges and Opportunities. Center for Biosecurity of UPMC; 2013. [Google Scholar]
- 31.Chartrain M, Chu L. Development and Production of Commercial Therapeutic Monoclonal Antibodies in Mammalian Cell Expression Systems: An Overview of the Current Upstream Technologies. Current Pharmaceutical Biotechnology. 2008;9(6):447–467. doi: 10.2174/138920108786786367. [DOI] [PubMed] [Google Scholar]
- 32.von Roemeling C, et al. Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends in Biotechnology. 2017;35(2):159–171. doi: 10.1016/j.tibtech.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Lateral Flow Assay Development Guide, Lateral Flow Handbook, Nanocomposix, 2016.
- 34.Wong RC, Tse HY, editors. Lateral Flow Immunoassay. Humana Press; 2009. [Google Scholar]
- 35.Qin Z, et al. Significantly Improved Analytical Sensitivity of Lateral Flow Immunoassays by Using Thermal Contrast. Angewandte Chemie International Edition. 2012;51(18):4358–4361. doi: 10.1002/anie.201200997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parolo C, et al. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosensors and Bioelectronics. 2013;40(1):412–416. doi: 10.1016/j.bios.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 37.Cho IH, et al. Immunogold–silver staining-on-a-chip biosensor based on cross-flow chromatography. Journal of Chromatography B. 2010;878(2):271–277. doi: 10.1016/j.jchromb.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Shen G, et al. Signal enhancement in a lateral flow immunoassay based on dual gold nanoparticle conjugates. Clinical Biochemistry. 2013;46(16–17):1734–1738. doi: 10.1016/j.clinbiochem.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Hu J, et al. Oligonucleotide-linked gold nanoparticle aggregates for enhanced sensitivity in lateral flow assays. Lab on a Chip. 2013;13(22):4352–4357. doi: 10.1039/c3lc50672j. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, et al. Lateral Flow Immunochromatographic Assay for Sensitive Pesticide Detection by Using Fe3O4 Nanoparticle Aggregates as Color Reagents. Analytical Chemistry. 2011;83(17):6778–6784. doi: 10.1021/ac201462d. [DOI] [PubMed] [Google Scholar]
- 41.Stiles PL, et al. Surface-Enhanced Raman Spectroscopy. Annual Review of Analytical Chemistry. 2008;1(1):601–626. doi: 10.1146/annurev.anchem.1.031207.112814. [DOI] [PubMed] [Google Scholar]
- 42.Fu X, et al. A SERS-based lateral flow assay biosensor for highly sensitive detection of HIV-1 DNA. Biosensors and Bioelectronics. 2016;78:530–537. doi: 10.1016/j.bios.2015.11.099. [DOI] [PubMed] [Google Scholar]
- 43.Hwang J, et al. Application of a SERS-based lateral flow immunoassay strip for the rapid and sensitive detection of staphylococcal enterotoxin B. Nanoscale. 2016;8(22):11418–11425. doi: 10.1039/c5nr07243c. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, et al. Study of superparamagnetic nanoparticles as labels in the quantitative lateral flow immunoassay. Materials Science and Engineering: C. 2009;29(3):714–718. [Google Scholar]
- 45.Taranova NA, et al. ‘Traffic light’ immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosensors and Bioelectronics. 2015;63:255–261. doi: 10.1016/j.bios.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 46.Yen CW, et al. Multicolored Silver Nanoparticles for Multiplexed Disease Diagnostics: Distinguishing Dengue, Yellow Fever, and Ebola Viruses. Lab on a Chip. 2015;15:1638–1641. doi: 10.1039/c5lc00055f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, et al. Two-Color Lateral Flow Assay for Multiplex Detection of Causative Agents Behind Acute Febrile Illnesses. Analytical Chemistry. 2016;88:8359–8363. doi: 10.1021/acs.analchem.6b01828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Puig H, et al. Effect of the protein corona on antibody-antigen binding in nanoparticle sandwich immunoassays. Bioconjugate Chemistry. 2017;28(1):230–238. doi: 10.1021/acs.bioconjchem.6b00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babak Y, Moghadam KTC, Posner Jonathan D. Two Orders of Magnitude Improvement in Detection Limit of Lateral Flow Assays Using Isotachophoresis. Analytical Chemistry. 2015;87:1009–1017. doi: 10.1021/ac504552r. [DOI] [PubMed] [Google Scholar]
- 50.Rivas L, et al. Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics. Lab on a Chip. 2014;14(22):4406–4414. doi: 10.1039/c4lc00972j. [DOI] [PubMed] [Google Scholar]
- 51.Parolo C, et al. Simple paper architecture modifications lead to enhanced sensitivity in nanoparticle based lateral flow immunoassays. Lab Chip. 2013;13(3):386–90. doi: 10.1039/c2lc41144j. [DOI] [PubMed] [Google Scholar]
- 52.de Puig H, et al. Quantifying the nanomachinery of the nanoparticle-biomolecule interface. Small. 2011;7(17):2477–2484. doi: 10.1002/smll.201100530. [DOI] [PubMed] [Google Scholar]
- 53.Tam JO, et al. A Comparison of Nanoparticle-Antibody Conjugation Strategies in Sandwich Immunoassays. Journal of Immunoassay and Immunochemistry. 2017 doi: 10.1080/15321819.2016.1269338. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen S. Market Trends in Lateral Flow Immunoassays. In: Wong R, Tse H, editors. Lateral Flow Immunoassay. Humana Press; 2009. pp. 1–15. [Google Scholar]
- 55.Walkey CD, et al. Protein Corona Fingerprinting Predicts the Cellular Interaction of Gold and Silver Nanoparticles. ACS Nano. 2014;8(3):2439–2455. doi: 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]