Summary
Acetaminophen (APAP) is the most commonly used drug for the treatment of pain and fever around the world. At the same time, APAP is capable of causing dose-related hepatocellular necrosis, responsible for nearly 500 deaths annually in the U.S. alone, as well as 100,000 calls to US Poison Control Centers, 50,000 emergency room visits and 10,000 hospitalizations per year. As an over-the-counter and prescription product (with opioids), APAP toxicity dwarfs all other prescription drugs as a cause for acute liver failure in the United States and Europe, but is not regulated in any significant way. This review will highlight the ongoing controversy as to the proper role for this ubiquitous pain reliever: its history, pathogenesis, clinical challenges in recognition and management, and current regulatory status and propose a new solution to a 50-year-old problem.
Introduction
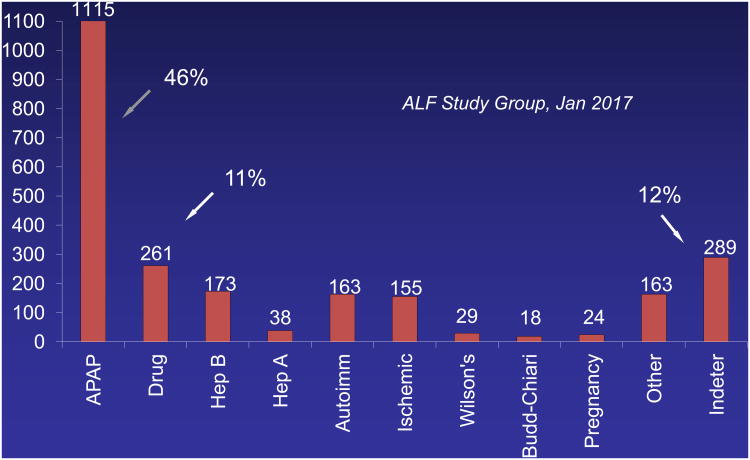
Acetaminophen (N-acetyl-p-aminophenol, APAP, paracetamol, Tylenol®) is a ubiquitous and highly utilized over-the-counter medication for relief of pain and fever that is also a dose-related toxin.1 APAP toxicity accounts for 46% of all acute liver failure (ALF) in the United States2 and between 40 and 70% of all cases in Great Britain and Europe.3 Acetaminophen toxicity dwarfs by several-fold the number of deaths related to acute liver failure (ALF) resulting from all prescription drugs combined (Figure 1), and has been the subject of two United States Food and Drug Administration (FDA) Advisory Committee meetings in the past 15 years.
Figure 1.
Bar graph showing breakdown by percent for each of the major ALF etiologies over 18 years. Over this time period, there has been little change in the percentages for each etiology, save a decline in hepatitis A and B.
Acetaminophen is very safe when used in limited doses but the margin of safety is relatively narrow, leading to dose-dependent liver injury in all mammalian species. The opioid combination medications containing hydrocodone/acetaminophen (Vicodin®, Norco®, others) represent the most frequently prescribed generic in the U.S. with 139 million prescriptions written in 2012.4 Overall, acetaminophen represents a multi-billion-dollar product and Tylenol®, a well-protected brand. Coupled with its reputation as being extremely safe, the public and regulatory authorities are faced with an unusual situation: over-the-counter, yet deadly. Meanwhile, acetaminophen remains a vital tool for basic scientists seeking to better understand hepatic metabolism and mechanisms of liver injury.5, 6 Thus, for researchers and clinicians alike, APAP currently provides indefinite job security. How did a ubiquitous pain reliever achieve this unusual status? What can be done to better understand the risk and avoid the consequences of APAP overdosing? And is there a long-term solution here?
History
As early as 1960, paracetamol, as it is referred to in Europe and the United Kingdom (UK), had become a popular analgesic for the treatment of headache and mild pain, possessing few of the side effects associated with aspirin (acetylsalicylic acid, ASA). By 1966, reports began to appear concerning its association with liver injury resulting in fatal outcomes. By the 1970's, paracetamol was the most frequently used suicidal agent7 in the UK; the Liver Unit at Kings College Hospital London, in 1972 set up the first 2-bed Liver Failure Intensive Care Unit, typically filled with young women on life support following attempts at self-harm. A single-time-point APAP overdose of 12-15 grams (24-30 ‘extra strength’ (500 mg) tablets), is associated with an approximately 50% mortality.8 By 1973, Mitchell and Jollow at the US National Institutes of Health (NIH) had delineated the APAP metabolic pathway,9 and suggested that N-acetylcysteine (NAC) was a suitable antidote. Oral NAC (Mucomyst®) came into common usage within a few years and intravenous NAC shortly thereafter, although considerably later in the US, in 2004.10
Acetaminophen crosses the Atlantic
Acetaminophen was virtually non-existent in the United States until the early 1980's when, after the association of aspirin with Reyes syndrome in children was recognized,11 acetaminophen was seen as a suitable substitute and became marketed actively as Tylenol® as well as other brands. This was followed by development of convenience combinations such as acetaminophen/diphenhydramine (Tylenol PM®, Nyquil®, others), as well as opioid/APAP combinations. Acetaminophen's popularity rose dramatically, despite the fact that virtually all Reyes cases were confined to children, not adults.12 In the late 1970's and early 1980's, numerous reports surfaced regarding severe liver injury associated not with suicide attempts but as so-called ‘therapeutic misadventures.’13-15 These represented inadvertent overdoses in the setting of acute or chronic pain, often accompanied by alcohol use and without suicidal intent. Over the next decade, U.S. hepatologists became increasingly aware of this entity. Zimmerman and Maddrey published a comprehensive article in 1995 describing 67 cases of inadvertent toxicity, most of whom had ingested therapeutic or supra-therapeutic doses without evident suicidal intent, but accompanied by alcohol use/abuse and poor outcomes.16
A review of acute liver failure (ALF) in 1993 included mention that APAP was fast becoming the most frequent cause of ALF in the US.17 While not substantiated with specifics, a subsequent article provided results of a comprehensive review of APAP-related toxicity at a large urban hospital, substantiating the prior claim: 71 hospital admissions for acetaminophen toxicity (not necessarily ALF) were identified over a 40-month period.18 This article established criteria to distinguish the intentional (suicide) from the unintentional (therapeutic misadventure) phenotype (Table 1).
Table 1. Parkland Hospital Study of APAP overdoses.19.
Over a 40-month period, in an urban county hospital, 71 cases were identified without confounding features that qualified as acetaminophen toxicity. The features of the intentional and unintentional cases were clearly different. While there were fewer unintentional cases identified, they had poorer outcomes, likely the result of late presentations.
| Suicidal: n=50 | Unintentional: n=21 |
|---|---|
|
|
| Schiødt FV et al., NEJM 1997:337:1112-17 | |
| Only 9 of 71 had ALF, 8 of 9 were unintentional | |
The unique features of the two groups were evident: suicides typically were young people with relationship problems, taking between 12 and 50 gms at one time, but once they admitted to having overdosed, were brought to the Emergency Department quickly (within 4-6 hours), and, for the most part, received N-acetylcysteine (NAC) promptly, precluding serious injury. Use of the NAC antidote within 12-18 hours precludes most severe liver injury, whereas later presentations demonstrate massive liver injury roughly proportional to the dose taken. By contrast, unintentional overdoses typically involved ingestion of 6-10 gm/day over several days for post-op pain, pancreatitis, low back pain, frequently involving opioid combinations, with denial of suicidal intent. Patients not aware of having done something risky presented late, after symptoms (nausea, vomiting, abdominal pain and eventually drowsiness) had developed and had worse outcomes.
Unique pattern of toxicity
APAP toxicity has a characteristic ‘hyperacute’ evolutionary pattern, reproducible in virtually all subjects regardless of intentionality. After an acetaminophen overdose at a single time point, there is no immediate sedative effect, and few symptoms initially, until abdominal pain and nausea develop between 12 and 24 hours. In the following 24 hours, symptoms may appear to improve but aminotransferases (aspartate aminotransferase-AST and alanine aminotransferase-ALT) and an international normalized ratio (INR) rise abruptly to very high levels, frequently above 10,000 U/L, normal (< 40 U/L), with INR ≥ 4.0, respectively. By 72-96 hours, biochemical elevation will have peaked along with hyperammonemia, somnolence, stupor and coma, accompanied by lactic acidosis, cerebral edema, brain stem herniation and vascular collapse.7,8 Concomitant acute (tubular) kidney injury (AKI, 70%) and varying degrees of skeletal muscle cytolysis also occur.19 If the multi-organ failure syndrome does not evolve by this juncture, then recovery ensues equally quickly, with rapid resolution of AST/ALT and INR. Virtually no permanent injury has been identified after severe overdoses or long-term chronic use. The kidney injury resolves in a week or two, although occasionally dialysis is required for up to a month a more.20 By comparison, idiosyncratic drug-induced liver injury (DILI), and most other forms of acute liver injury leading to ALF except ischemic hepatic injury, have a subacute course, evolving over 1-4 weeks and features lower aminotransferase and higher bilirubin levels, with poorer overall outcomes, fewer spontaneous recoveries but more time to await a liver graft and undergo transplantation (Table 2).
Table 2. Comparison of Different ALF Etiology Groups.
ALFSG data summary between January 1998 and December 2016. The APAP group is younger than the DILI, more likely to have advanced coma grade but shorter duration of jaundice to coma, much higher aminotransferases and lower bilirubin levels than the DILI case or most other groups. There are significant differences in outcomes as well.
| N = 2,436 | |||||
|---|---|---|---|---|---|
| APAP N=1115 | Drug n=261 | Indeterminate n=282 | HepA/HepB n=38/173 | All Others N=560 | |
| Age (median) | 37 | 46 | 40 | 50/43 | 45 |
| Sex (% F) | 76 | 69 | 61 | 45/46 | 70 |
| Jaundice to coma (Days) | 1 | 12 | 10 | 4/8 | 7 |
| Coma ≥3 (%) | 53 | 36 | 47 | 53/50 | 40 |
| ALT (median IU) | 3798.5 | 648 | 870 | 2316.5/1415 | 774 |
| Bili (median) | 4.3 | 19.2 | 20.1 | 12.3/18.8 | 12.7 |
| Tx (%) | 8.6 | 38 | 42 | 34/39 | 29 |
| Spontaneous Survival (%) | 64.4 | 25 | 23 | 50/19 | 31 |
| Overall Survival (%) | 71.5 | 59 | 61 | 74/53 | 55 |
Centri-lobular hepatocellular necrosis, the hallmark lesion of acetaminophen injury is indistinguishable by routine light microscopy from ischemic necrosis, since both affect zone 3 of the hepatic lobule, where oxygen tension is lowest.20 The metabolic pathway outlined indicates that the parent compound is readily esterified to glucuronides and/or sulfates unless the capacity for esterification is saturated, in which case the secondary pathway via cytochrome P450 enzymes comes into play, principally Cyp 2E1, leading to formation of a highly reactive and toxic intermediate metabolite, N-acetyl-p-benzoquinone imine (NAPQI-Figure 2).9 NAPQI can be readily de-toxified via glutathione to mercapturic acid that is water soluble, harmless and readily excreted in urine. However, once glutathione is depleted, NAPQI binds directly to cell proteins via cysteine residues, disrupts cellular integrity yielding hepatocyte necrosis. This injury likely takes place very rapidly once glutathione depletion is accomplished, leading to the extraordinary levels of aminotransferases but also a very rapid decline upon cessation of liver injury. However, given the relatively long half-life of both aspartate and alanine aminotransferase (AST and ALT, respectively), enzyme levels fully resolve only after 3-9 days, depending on the severity of the injury. The injury is so uniform in nature that a mathematical model has been created to predict outcome, using only the AST, ALT and INR values at one time point.21
Figure 2.
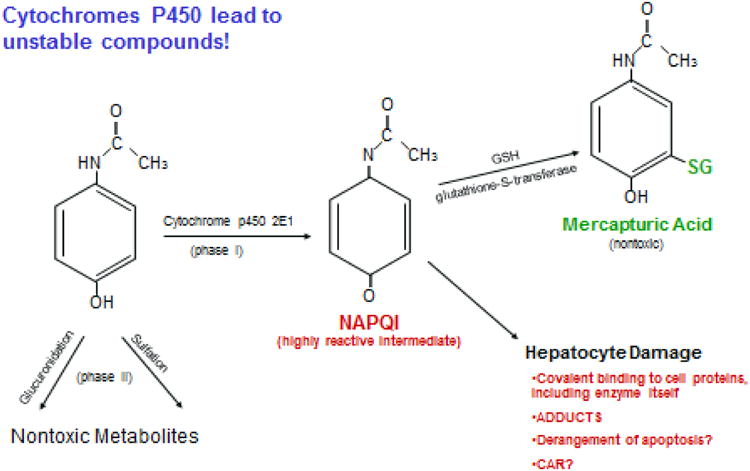
Biochemical pathways of acetaminophen metabolism. Only small amounts of NAPQI are formed unless the capacity for glucuronidation and sulfation is exceeded. Even then, glutathione supplies sulfhydryl groups that detoxify NAPQI to mercapturic acid, which is excreted in the urine. When glutathione is exhausted, then NAPQI binds to cell proteins disrupting cell function, the full details of which remain poorly understood.
Between 1990 and 1998, the percentage of cases of ALF related to APAP in the US rose from ca. 20%22,23 to its current 46%,24 where it has remained with no evident decline for nearly two decades. There has been no decline in other etiologies, save perhaps a small drop in hepatitis A and B.24 (Very recently, the hepatitis B percentage has begun to increase once again, likely secondary to the opioid drug use epidemic.)
Beginning of regulatory action
Although first used ca. 1966, the dangers of paracetamol had been recognized in case reports as early as 1970, at least in the UK.25 An unsigned Lancet editorial in 1975 stated: “Surely the time has come to replace paracetamol with an effective analogue which cannot cause liver damage.”26 How ironic to read this 42 years later! Professor Keith Hawton, a suicidologist at Oxford University, has chronicled the situation over the past 40 years.27,28 Initially, little was known about paracetamol's toxicity as a suicide agent-- patients who had overdosed were not necessarily aware that it had risks.29 Two decades later, this had evolved so that paracetamol was understood, at least in the UK, as responsible for a rising number of deaths with extensive media publicity describing the problem.30 Efforts to curb package size were encouraged by the Hawton group, based on surveys of overdose patients conducted in the 1990's that suggested that impulsive behavior was responsible for most suicides: utilization of whatever was readily available in the home. These initiatives culminated in Parliament passing legislation in 1998 limiting the package size to 16 in convenience stores and 32 in pharmacies, and a requirement for blister packing to further inhibit the likelihood of impulsive behavior. Whether these measures have been effective has been debated over the following two decades.31-34 Most evidence suggests that the number of deaths, and number of registered self-harm incidents has declined considerably while the number of liver transplants has declined more modestly, and there has been no evident change in Scotland for unclear reasons. If the anticipated results were somewhat limited, this is likely due to enforcement limitations—those intending to garner large numbers of tablets can readily do so, since the chemist (pharmacy) or store cashier serves, in effect, as the only gatekeeper. The original intent, of course, was to limit quantities found around the home that might be then used impulsively. Perhaps the modest diminution in incident cases reflects a decline in ‘impulsive’ cases with no diminution in those where more planning is involved.
Intentional vs. unintentional overdoses
While most paracetamol overdoses were assumed to be attempts at self-harm as studied by the University of Oxford Centre for Suicide Research, when the problem of acetaminophen overdoses emigrated to the US in the 1980's, it became apparent that most of the severe injuries were not related to intentional self-harm, perhaps casting the whole overdose conundrum in a different light: if one dies after inadvertently overdosing, does this carry a different significance vis-a-vis self-inflicted cases? In short, should be public care more or differently about unintentional overdoses? And do the different clinical phenotypes differ in other ways such as outcomes? The US FDA certainly considered the unintentional cases a more compelling argument toward regulatory oversight than intentional overdoses (see below).
Despite the seemingly contrasting clinical scenarios associated with unintentional and suicidal APAP ingestions, patients who develop ALF due to either phenotype resemble each other in many ways. In an early descriptive study of 662 patients enrolled by the US Acute Liver Failure Study Group, 275 (41.5%) were found to have APAP overdoses, all meeting ALF criteria: coagulopathy (prolonged INR ≥ 1.5) and encephalopathy.35 In nearly all, the intentionality could be discerned; more were found to have unintentional than intentional overdoses. Seven percent gave a history of taking less than 4 gm, suggesting that there might in fact be increased sensitivity to APAP's toxic effects in certain patients, perhaps enhanced by alcohol or starvation, both known to deplete glutathione. By contrast, in Europe the unintentional overdose remained relatively unrecognized or was thought to constitute very few instances of ALF, until 201036 when a report focused on outcomes of intentional vs. unintentional overdoses, suggesting that unintentional overdoses constituted a significant number (16.6%), still lower than the US reports. The term ‘staggered overdose,’ was used, indicating that no longer were single time point ingestions the rule. Although seemingly unintentional, since toxicity occurred only after repeat sub-toxic ingestions over several days, the intentionality of staggered overdoses has remained somewhat ambiguous.37 Some have questioned whether a staggered overdose might simply be another form of suicidal behavior.37 Clinically, both forms are associated with nearly equally high aminotransferases and similar frequency of anti-depressant and other substance use (Table 3).35 Unintentional overdose patients with chronic pain may be given anti-depressants as part of pain management since several have been granted chronic pain indications, Duloxetine® for example. More frequently, 2/3 of unintentional patients reported taking high doses of hydrocodone/APAP products because of habituation/addiction to the opioid; others (one third of unintentional patients, roughly) ingest more than one APAP-containing ‘convenience’ medication such as Nyquil® along with Tylenol PM®, plain Tylenol® perhaps as well as a hydrocodone/APAP combination (Table 3), unaware that they are overdosing by not reading labels carefully.35 As has been stated, the damage is uniquely sudden and severe, resolving in an equally rapid fashion once APAP has been metabolized. APAP has a relatively short half-life of about 2-3 hours (although it is somewhat prolonged with significant liver injury).38
Table 3. Comparison of the APAP Phenotypes.
ALFSG data summary between January 1998 and December 2008.36 Most APAP overdoses are women, and many features between the two phenotypes are similar although the percentage with opioid use or that used multiple preparations is higher in the unintentional group.
| N=606 (56=unk) | Intentional (n=251) | Unintentional (n=296) | p-value |
|---|---|---|---|
| Female (%) | 77 | 71 | NS |
| Age | 35 | 39 | < 0.001 |
| ACM dose(g) | 38/38 | 47/7.5 | NS |
| Coma (% ≥3) | 39 | 55 | < 0.026 |
| ALT (IU/L) | 6053 | 4207 | < 0.0001 |
| Alcohol use/abuse (%) | 50/18 | 50/17 | NS |
| Antidepress't (%) | 39 | 34 | NS |
| History of depression (%) | 45 | 24 | < 0.001 |
| Opioid cpd (%) | 18 | 63 | < 0.001 |
| Multiple preps (%) | 5 | 38 | < 0.001 |
| Spont surv (%) | 70 | 65 | NS |
Further characterizing the unintentional patient
Originally called the therapeutic misadventure or the ‘alcohol/Tylenol® syndrome’, unintentional overdose patients have long been recognized as having substance use issues.13-16 This has been underlined in recent studies showing high rates of polysubstance abuse, including cocaine and benzodiazepines, in addition to alcohol and opioids.39 Again, there are similarities between intentional and unintentional groups as shown in a recent questionnaire study: the incidence of depressive disorder at any time, use of SSRI and SNRI medication, as well as use of alcohol were similar between the two groups, although opioid use was more prevalent in the unintentional group (Table 3).35 Long term outcomes for patients surviving all forms of APAP overdoses are poorer than for other forms of ALF; they tend to have lower socioeconomic status, less education and are less likely to be married, with no differences apparent between the two phenotypes.40
Outcomes in APAP ALF
While spontaneous resolution of injury occurs with or without NAC in nearly 2/3 of cases, many die or require transplantation (Table 2). There is little difference in transplant selection or outcomes between intentional and unintentional phenotype, and all progress to death, transplant or recovery in a similarly short time interval. In the ALFSG experience, virtually all APAP patients reached an end point by 4 days following admission to study, while DILI patients continued to die or receive liver grafts over the ensuing 7-10 days (Figure 3).41 Overall, 36% of ALF patients were listed for transplantation, only 22% of those with APAP ALF vs. 56% of the non-APAP ALF group. While this might be thought to be related to better outcomes with APAP, the listed APAP patients were actually sicker in terms of clinical and biochemical features. Only 36% of those listed received a graft vs. 74% of the non-APAP group. The very sick APAP patients often die because a liver cannot be found in time--favorably impacting the death rate for APAP cases will remain extremely challenging. It will involve more rapid evaluation for transplantation and quicker organ availability as well.42
Figure 3.
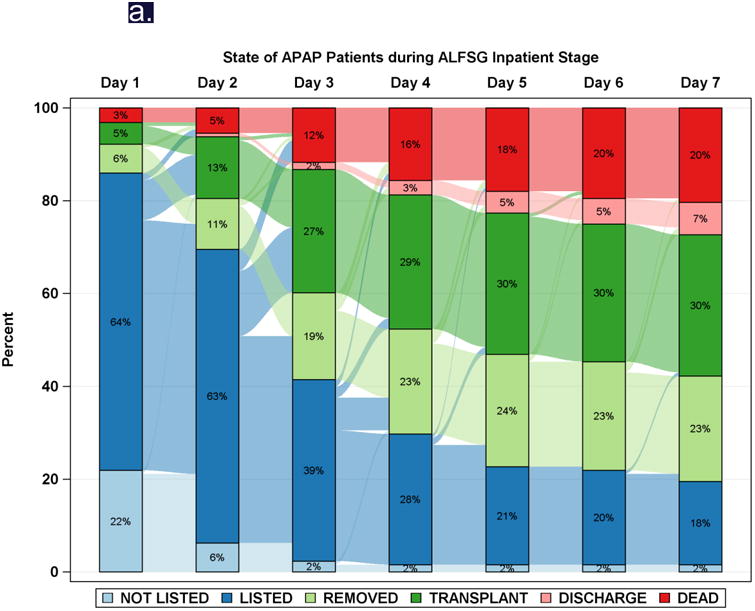
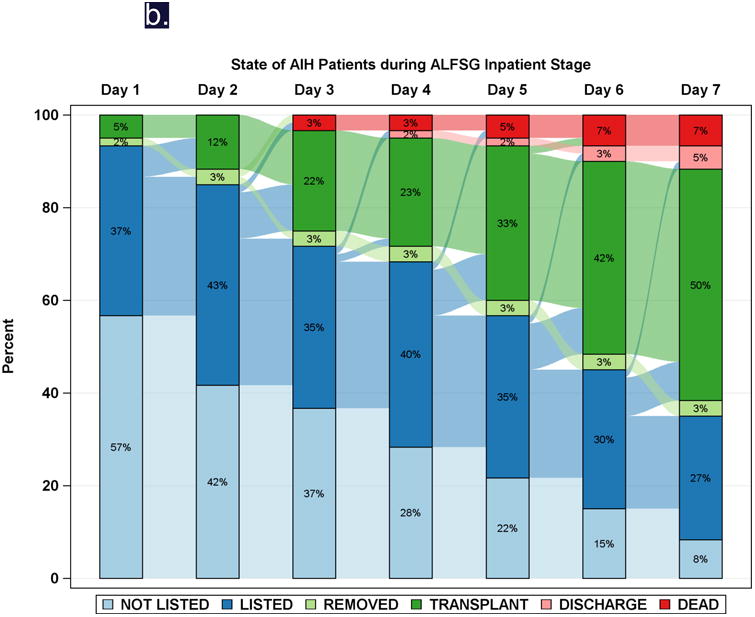
Diagrammatic representation of events by day (Sankey plot), after registry enrollment/listing for transplantation, according to etiology groups: a: APAP, and b: Drug-induced liver injury (DILI). Most of the deaths and transplants in the APAP group (3a) took place within the first 48-72 hours, while both deaths and transplants evolved more slowly in the non-APAP categories such as DILI (3b).
FDA responses
In 2002 and 2009, FDA held advisory committee meetings with the goal of tackling the issue of APAP hepatotoxicity. It should be noted that FDA has little authority to act regarding over-the-counter medications in comparison with its authority over prescription drugs. However, data regarding APAP toxicity had been presented at an FDA-sponsored educational meeting in 2001.43 The 2002 FDA meeting principally dealt with package labeling:44 were the cautions about use with alcohol and other products plain enough and was the full name appropriately placed on the front of the package? By 2009, FDA had formed an internal group to review the ongoing problem.45 This second advisory committee addressed more cogent issues (Table 4).39 Was 4 gm/day too large a dose? Should the hydrocodone-APAP combinations be unbundled? Were convenience medications (TylenolPM®, Dayquil®, other cough syrups) a significant problem? While the committee did in fact vote to lower the daily dose recommendation, a specific amount was not given; the committee also voted to unbundle the opioid-APAP combinations, but did not believe that convenience medications needed additional regulation.45
Table 4. Summary of the questions and votes posed at the FDA Advisory Committee meeting in 2009 concerning APAP toxicity.
Results of voting are shown after each question.
| Raduce current dosage strengths for OTC: maximum total daily dose, maximum adult single dose, maximum strength. |
| 1. Maximum dose per day: less than 4 g, exact amount unspecified. A11, B10, C16.(Yes) |
| 2. Maximum single dose: 650 mg (2 × 325 mg). A 12, B 12, C13. (Yes) |
| If the above is approved should 500-mg tablet, 1,000-mg tablet, and/or 4g/day dosing be prescription only? |
| 3. Maximum dose of 500 mg × 2 should be prescription only. A 8, B 18, C 11. (Yes) |
| Establish pack size limits for OTC acetaminophen products? |
| 4. Pack size limits? A 2, B 15, C 20. (No) |
| Eliminate nonprescription combination products (e.g., Nyqull, Dayqull)? |
| 5. Eliminate these products? A 2, B 11, C 24.(No) |
| Limit formulations of liquids to only one concentration (this has to do with pediatric dosing)? |
| 6. Do you recommend that only one nonprescription concentration of liquid be available? A 19, B 17, C 1. (Yes) |
| Eliminate prescription combination products (oplold–acetaminophen compounds)? |
| 7. Do you recommend eliminating the prescription combination products? A 10, B10, C 17. (Yes) |
| If not eliminated should prescription combinations be sold In “unit of use” packaging or with additional warning labels? |
| 8. Do you recommend “unit of use” packaging? A 5, B 22, C 10. (Yes) |
| 9. Do you recommend box warning? A 25, B 11, C 1. (Yes) |
| 10. What of the above is your highest priority? [These last two questions required subjective answers from the panelists.] |
| 11. Discuss other options you would suggest. |
| A, B and C refer to preferences: A, strongly In favor; B, In favor; C. against. Vote tallies follow each letter, and the final tally is Indicated as yes or no: A+ B vs. C. |
OTC, over the counter.
Following the 2009 Advisory Committee meeting, FDA issued a mandate (in January 2011)46 that any prescription form of APAP (basically oxycodone- or hydrocodone-APAP) combinations sold after January 2014 should only contain 325 mg per tablet as opposed to the prior combinations that were 500, 650 or even 750 mg per tablet. This rule is now in place in the US. Current package labeling mentions severe liver injury as a possible outcome if one takes more than 4000 mg in 24 hours or with other APAP-containing compounds or with alcohol. While there has been a certain amount of public outcry about the problem, further efforts to more fully apply the committee's recommendations have not occurred.
Criticism for FDA has been moderate.47 In 2013, the problems surrounding acetaminophen toxicity were featured by Propublica,48 a U.S. public interest journalist website, and subsequently in an hour-long radio show, This American Life. In addition, a large class action lawsuit with over 100 plaintiffs was recently settled by McNeil in June 2016, until recently the over-the-counter arm of Johnson & Johnson, and responsible for Tylenol® and its many related products.49 Thus far, any increased visibility afforded to the problem has appeared to have little impact. There is no apparent slowing of cases, although very recent data, other than ALFSG annual snapshots has been somewhat sparse.50,52 The blame has variously been laid to the slowness of FDA as well as, for the unintentional cases, to the opioid combination products. All this has been dwarfed by the larger problem of the opioid epidemic.52 Further regulation at this point seems unlikely since it is very difficult to accomplish for over-the-counter products, particularly for successful brands. Thus, 42 years after the call for its replacement in Lancet, acetaminophen remains the most commonly taken analgesic and there is no relief in sight.
Regulation will never solve this problem
Regulation of acetaminophen to bring about a diminution of the number of intentional or unintentional cases appears impossible, given a highly successful product in a very cautious regulatory environment. Beyond further regulation which seems highly unlikely, what might be done to diminish the overall cost in money and lives? Efforts to combine APAP with an antidote that would preclude toxicity began more than 30 years ago but have never gained traction, for unclear reasons. Compounds such as cysteamine, 53 or methionine were considered as well as cimetidine,54 which would compete for Cyp 2E1 with APAP blocking NAPQI accumulation, while allowing continued detoxification via glucuronidation and sulfation to occur at a slower pace. The challenge with this approach would be to find a compound that has no intrinsic effects except protecting against APAP, and it would have to be truly very safe. It is likely that the cost of developing such a product is outweighed by the pressure to continue what is currently the standard, APAP as we currently know it.
What about a totally new pain pill?
The most promising strategy would be to find a new analgesic that had the same properties of APAP but without the toxicity. If we can design biologics that interact with specific cell-surface receptors, why can't we apply some basic pharmacologic chemistry to analgesic development? APAP has a central CNS effect that is presumed related to the benzene ring structure, and although classed as an NSAID, does not share ulcerogenic or cardiac toxicity with other NSAID compounds. Other benzene ring structures should be explored. Basically, there has not been a new class of analgesics since the COX-2 inhibitors arrived 15 years ago, and these were not really new, but simply an improvement on existing drugs.55 The rewards to the company identifying such a new analgesic would be tremendous!!
Challenges to implementation of a new safer product also would be enormous, but challenging the risk of acetaminophen's popularity could be worth it. Pushback from existing analgesic providers would be formidable, particularly when marketing something unknown as being safer than APAP, as APAP maintains a reputation for safety though not be well-deserved. It would be up to FDA and a grateful (educated) public to embrace a truly safe and effective analgesic that is not saddled with the baggage of opioids (habituation, constipation, somnolence) or NSAIDs (gastrointestinal bleeding) or APAP (deaths from acute liver failure). One parallel that comes to mind is the barbiturate class of sleeping pills that were highly popular in the 1960's and ‘70's but caused innumerable overdose deaths. Once benzodiazepines came along, barbiturates disappeared with remarkable rapidity. The question becomes: Who will come forth and make this happen for acetaminophen--and fill this very large need?
Key Points.
Acetaminophen is uniquely situated within over the counter drugs: a dose-related toxin, that is readily available and can be lethal.
For nearly 50 years, acetaminophen hepatotoxicity has been recognized, its metabolism understood and an excellent antidote is available.
Acetaminophen toxicity follows a uniquely uniform pattern, such that the damage is either fatal or requires a liver transplant or recovery ensues within 4-5 days.
Thus, efforts to manage toxicity once it develops requires very rapid assessment.
Prognostic indexes, including an ‘app’, are available.
The number of deaths in North America and Europe shows no sign of decreasing, despite some efforts to limit package size in the United Kingdom.
Regulatory efforts by the U.S. Food and Drug Administration (and worldwide) have been ineffective thus far and are likely to remain so.
What is needed is a new paradigm: development of a totally safe congener of acetaminophen that would provide effective analgesia with no risk of toxicity.
Acknowledgments
Many of the studies referred to in this paper were the cumulative result of the data and bio-sample collection of Acute Liver Failure Study Group registry. The author gratefully acknowledges the contributions and support of the ALFSG investigators, coordinators and the patients and their families who agreed to participate in this important study. The support of the NIDDK administrative and repository staff has been essential to the success of this study. Support has been provided by NIDDK U-01 DK58369 with additional support from the Southwestern Medical Foundation.
References
- 1.Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetamol)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- 2.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SBH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 4.http://www.pharmacytimes.com/publications/issue/2013/July2013/Top-200-Drugs-of-2012, accessed May 10, 2016
- 5.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, et al. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol. 2009;37:193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- 7.Proudfoot AT, Wright N. Acute paracetamol poisoning. Br Med J. 1970;3:557–8. doi: 10.1136/bmj.3.5722.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rumack BH, Peterson RC, Koch GG, Amara IA. Acetaminophen overdose 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141:380–385. doi: 10.1001/archinte.141.3.380. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H. Acetaminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Therap. 1974;16:676–84. doi: 10.1002/cpt1974164676. [DOI] [PubMed] [Google Scholar]
- 10.https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-539_Acetadote.cfm, accessed on June 26, 2017
- 11.Federal Register 1982: Labeling for Salicylate Compounds. [accessed on June 15, 2017]; http://cdn.loc.gov/service/ll/fedreg/fr047/fr047249/fr047249.pdf#page=214.
- 12.http://cdn.loc.gov/service/ll/fedreg/fr047/fr047249/fr047249.pdf#page=214 FDA monograph suggesting a warning to be put on aspirin in regard to use in children under 16 for influenza symptoms—1982
- 13.McClain CJ, Kromhout JP, Peterson FJ, Holtzman JL. Potentiation of acetaminophen hepatotoxicity by alcohol. JAMA. 1980;244:251–3. [PubMed] [Google Scholar]
- 14.Seeff LB, Cuccherini BA, Zimmerman HJ, Adler E, Benjamin SB. Acetaminophen toxicity in alcoholics. Ann Intern Med. 1986;104:399–404. doi: 10.7326/0003-4819-104-3-399. [DOI] [PubMed] [Google Scholar]
- 15.Wootton FT, Lee WM. Acetaminophen hepatotoxicity in the alcoholic. Southern Med J. 1990;83:1047–1049. doi: 10.1097/00007611-199009000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22:767–73. [PubMed] [Google Scholar]
- 17.Lee WM. Medical Progress: Acute liver failure. N Engl J Med. 1993;329:1862–72. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 18.Schiødt FV, Rochling FJ, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112–17. doi: 10.1056/NEJM199710163371602. [DOI] [PubMed] [Google Scholar]
- 19.Tujios SR, Hynan LS, Vazquez MA, Larson AM, Seremba E, Sanders CM, et al. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol. 2015;13:352–9. doi: 10.1016/j.cgh.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James LP. Drug-Induced Liver Disease. Third. Amsterdam: 2013. Acetaminophen: Pathology and clinical presentation of hepatotoxicity, in Kaplowitz N, Deleve LD; pp. 331–341. [Google Scholar]
- 21.Remien CH, Adler FR, Waddoups L, Box TD, Sussman NL. Mathematical modeling of liver injury and dysfunction after acetaminophen overdose: Early discrimination between survival and death. Hepatology. 2012;56:727–734. doi: 10.1002/hep.25656. [DOI] [PubMed] [Google Scholar]
- 22.Shakil AO, Kramer D, Mazariegos GV, Fung JJ, Rakela J. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163–9. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 23.Schiødt FV, Atillasoy E, Shakil O, Lee WM, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transplant Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 24.Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, et al. Outcomes in adults with acute liver failure (ALF) from 1998-2013: An observational cohort study. Ann Intern Med. 2016;164:724–32. doi: 10.7326/M15-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proudfoot AT, Wright N. Acute paracetamol poisoning. BMJ. 1970;3:557–58. doi: 10.1136/bmj.3.5722.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lancet Editorial (unsigned) 1975 Dec 13;:1189. [Google Scholar]
- 27.Hawton K, Fagg J. Deliberate self-poisoning and self-injury in adolescents A study of characteristics and trends in Oxford, 1976-89. Br J Psychiatry. 1992;161:816–23. doi: 10.1192/bjp.161.6.816. [DOI] [PubMed] [Google Scholar]
- 28.Hawton K, Ware C, Mistry H, Hewitt J, Kingsbury S, Roberts D, et al. Paracetamol self-poisoning characteristics, prevention and harm reduction. Brit J Psych. 1996;168:43–48. doi: 10.1192/bjp.168.1.43. [DOI] [PubMed] [Google Scholar]
- 29.Gazzard BG, Davis M, Spooner J, Williams R. Why do people use paracetamol for suicide? BMJ. 1976;i:212–15. doi: 10.1136/bmj.1.6003.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawton K, Bergen H, Casey D, Simkin S, Palmer B, Cooper J, et al. Self-harm in England: a tale of three cities. Soc Psychiatr Epidemiol. 2007;42:513–21. doi: 10.1007/s00127-007-0199-7. [DOI] [PubMed] [Google Scholar]
- 31.Hawton K, Bergen H, Simkin S, Dodd S, Pocock P, Bernal W, et al. Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyses. BMJ. 2013;346:403–10. doi: 10.1136/bmj.f403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bateman DN. Limiting paracetamol pack size: has it worked in the UK? Clin Toxicol (Phila) 2009;47:536–41. doi: 10.1080/15563650903093192. [DOI] [PubMed] [Google Scholar]
- 33.Morgan O, Hawkins L, Edwards N, Dargan P. Paracetamol (acetaminophen) pack size restrictions and poisoning severity: time trends in enquiries to a UK poisons centre. J Clin Pharm Ther. 2007;32:449–55. doi: 10.1111/j.1365-2710.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 34.Hawton K, Simkin S, Deeks J, Cooper J, Johnston A, Waters K, et al. UK legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ. 2004;329:1076–82. doi: 10.1136/bmj.38253.572581.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson AM, Fontana RJ, Davern TJ, Polson J, Lalani EK, Hynan LS, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2005;42:1367–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 36.Craig DGN, Bates CM, Davidson JS, Martin KG, Hayes PC, Simpson KJ. Overdose pattern and outcome in paracetamol-induced acute severe hepatotoxicity. Brit J Clin Pharm. 2010;71:273–82. doi: 10.1111/j.1365-2125.2010.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craig DGN, Bates CM, Davidson JS, Martin KG, Hayes PC, Simpson KJ. Staggered overdose pattern and delay to hospital presentation are associated with adverse outcomes following paracetamol-induced hepatotoxicity. Br J Clin Pharmacol. 2012;73:285–94. doi: 10.1111/j.1365-2125.2011.04067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiødt FV, Ott P, Christensen E, Bondesen S. The value of plasma acetaminophen half-life in antidote-treated acetaminophen overdose. Clin Pharmacol Ther. 2002;71:221–5. doi: 10.1067/mcp.2002.121857. [DOI] [PubMed] [Google Scholar]
- 39.Lee WM. Point-counterpoint: The case for limiting acetaminophen-related deaths: Smaller doses and unbundling the opioid-acetaminophen compounds. Clin Pharm Therap. 2010;88:289–92. doi: 10.1038/clpt.2010.164. [DOI] [PubMed] [Google Scholar]
- 40.Fontana RJ, Ellerbe C, Durkalski VE, Rangnekar A, Reddy KR, Stravitz T, et al. Two-year outcomes in initial survivors with acute liver failure: Results from a prospective, multicenter study. Liver Int. 2015;35:370–80. doi: 10.1111/liv.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy KR, Ellerbe C, Schilsky M, Stravitz RT, Fontana RJ, Durkalski V, et al. Determinants of outcome among patients with acute liver failure listed for liver transplantation in the US. Liver Transpl. 2015;22:505–515. doi: 10.1002/lt.24347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson KJ, Bates CM, Henderson NC, Wigmore SJ, Garden OJ, Lee A, et al. The utilization of liver transplantation in the management of acute liver failure: Comparison between acetaminophen and non-acetaminophen etiologies. Liver Transplant. 2009;15:600–09. doi: 10.1002/lt.21681. [DOI] [PubMed] [Google Scholar]
- 43.https://wayback.archive-it.org/7993/20161022165242/http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/ucm091365.htm, accessed June 14, 2017
- 44.www.fda.gov/ohrms/dockets/ac/02/slides/3882s1_01_ganley.pdf, accessed June 14, 2017
- 45.https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM174699.pdf, dated January 13, 2011, accessed June 14, 2017
- 46.https://www.fda.gov/Drugs/DrugSafety/ucm239821.htm, accessed June 19, 2017
- 47.Graham GG, Day RO, Graudins A, Mohamudally A. FDA proposals to limit the hepatotoxicity of paracetamol (acetaminophen): are they reasonable? Inflammopharmacol. 2010;18:47–55. doi: 10.1007/s10787-010-0036-6. [DOI] [PubMed] [Google Scholar]
- 48.https://www.propublica.org/article/tylenol-mcneil-fda-use-only-as-directed, accessed June 19, 2017
- 49.https://www.propublica.org/article/johnson-johnson-emerges-victorious-in-lawsuit-on-tylenols-risks, accessed June 19, 2017
- 50.Bronstein AC, Spyker DA, Cantilena L, Rumack BH, Dart RC. 2011 Annual Report of the American Association of Poison Control Centers' National Poison Data System. Clin Toxicol. 2012;50:911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- 51.Bond GR, Ho M, Woodward RW. Trends in hepatic injury associated with unintentional overdose of paracetamol (acetaminophen) with or without opioid. Drug Saf. 2012;35:149–57. doi: 10.2165/11595890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR. 2016;65:1445–52. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 53.Miller MG, Jollow DJ. Acetaminophen hepatotoxicity: studies on the mechanism of cysteamine protection. Toxicol Appl Pharmacol. 1986;83:115–25. doi: 10.1016/0041-008x(86)90329-7. [DOI] [PubMed] [Google Scholar]
- 54.Jackson JE. Cimetidine protects against acetaminophen hepatoxicity. Vet Hum Toxicol. 1981;23(Suppl 1):7–9. [PubMed] [Google Scholar]
- 55.Brune K. Next generation of everyday analgesics. Am J Ther. 2002;9:215–23. doi: 10.1097/00045391-200205000-00007. [DOI] [PubMed] [Google Scholar]