Abstract
In sickle cell disease (SCD), abnormal microvascular function combined with chronic anaemia predisposes patients to perfusion-demand mismatch. We hypothesized that skeletal muscle and myocardial perfusion, normalized to the degree of anaemia, is reduced at basal-state compared to controls, and that this defect is ameliorated by hydroxycarbamide (HC; also termed hydroxyurea) therapy.
Twenty-one SCD patients, of whom 15 were treated with HC, and 27 controls underwent contrast-enhanced ultrasound (CEU) perfusion imaging of the forearm as well as the myocardium.
HC treatment was associated with lower white cell and reticulocyte counts, and higher fetal haemoglobin and total haemoglobin levels. When corrected for the degree of anaemia in SCD patients, skeletal flow in HC-treated patients was significantly higher than in untreated SCD patients (217.7±125.4 vs 85.9±40.2, p=0.018). Similarly, when normalized for both anaemia and increased myocardial work, resting myocardial perfusion was also significantly higher in HC-treated patients compared with untreated SCD patients (0.53±0.47 vs 0.13±0.07, p=0.028). Haemoglobin F (HbF) levels correlated with skeletal muscle microvascular flow (r=0.55, p=0.01).
In conclusion, patients with SCD not on HC therapy have resting flow deficits in both skeletal muscle and myocardial flow. HC therapy normalizes flow and there is a direct correlation with HbF levels.
Keywords: sickle cell disease, hydroxycarbamide, perfusion imaging, contrast ultrasound, microvasculature
INTRODUCTION
Sickle cell disease (SCD) is a genetic condition caused by a point mutation in the beta-globin gene (HBB), producing haemoglobin S (HbS). HbS polymerizes upon deoxygenation resulting in changes in the rheology of sickled erythrocytes. The pathophysiology of tissue ischaemia in SCD is multifactorial (Rees et al, 2010;Gladwin, 2016;Piel et al, 2017;Ware et al, 2017) involving (i) microvascular loss (from repetitive vaso-occlusive events or increased endothelial adhesion of leucocytes and erythrocytes), (ii) abnormal rheology in the distal microcirculation and (iii) functional abnormalities of the microcirculation which are partly attributable to haemolysis and loss of nitric oxide (NO) bioavailability. Cumulatively, these functional or structural abnormalities could lead to either overt hyoperfusion of key tissues, such as the myocardium or skeletal muscle, or to a lack of ability to augment perfusion as a compensatory response that normally occurs with non-SCD forms of anaemia (Kubes et al, 1988;Piel et al, 2017;Ware et al, 2017). Recent studies have suggested that hydroxycarbamide (HC), also called hydroxyurea, a cornerstone in the treatment of SCD, can have beneficial effects by increasing the level of non-polymerizing HbF and by increasing NO availability (Poillon et al, 1993;Charache et al, 1995;McGann & Ware, 2015). However, little is known about the effects of HC on tissue perfusion in SCD.
In this study we hypothesized that microvascular flow impairment may be present in skeletal muscle or myocardium in patients with SCD even under non-crisis conditions and that HC therapy ameliorates perfusion deficits. To test this hypothesis, quantitative microvascular perfusion imaging with contrast enhanced ultrasound (CEU) was performed due to its ability to quantify perfusion at the capillary level in both the myocardium and skeletal muscle. Given that the left ventricle in SCD is likely to have higher basal oxygen demand due to adaptive increases in cardiac output that occur with anaemia and increased left ventricular (LV) end-systolic wall stress (Lamers et al, 2006), we corrected for these haemodynamic factors. In addition, we characterized the balance between myocardial work and myocardial perfusion, and assessed the ability to augment myocardial perfusion using vasodilator stress in controls and SCD patients.
METHODS
Study Population
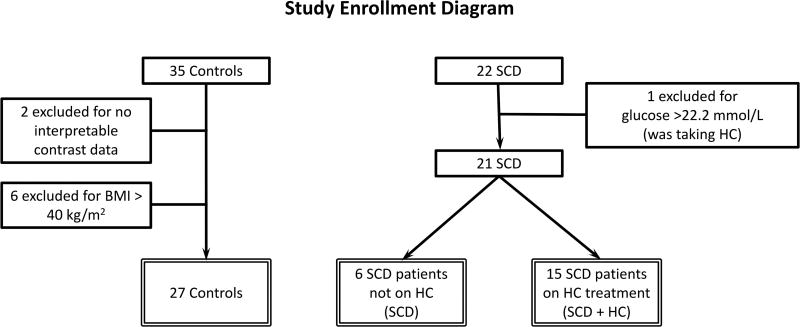
The study was approved by the National Heart, Lung and Blood Iinstitute (NHLBI) Institutional Review Board (ClinicalTrials.gov Identifier: NCT01602809), and subjects gave written informed consent. Twenty-two adult African-American patients with SCD (21 HbSS, one with sickle β°-thalassemia) who were free of acute crisis for the previous four weeks were prospectively recruited and completed study procedures. Sixteen of these patients were on HC therapy at the discretion of their referring physicians. Thirty-five healthy African-American control subjects with no history of SCD or sickle cell trait were also studied (Figure 1).
Figure 1. Study Enrolment Diagram.
A total of 57 subjects signed consent and completed study procedures. Two had no interpretable contrast data for technical reasons. Six controls were excluded for a BMI > 40 kg/m2. One SCD patient was excluded for a glucose level of > 22.2 mmol/l. There were 48 evaluable patients in the study (27 controls, 21 SCD patients of whom 15 were on HC). BMI: body mass index; HC: hydroxycarbamide; SCD: sickle cell disease.
Exclusion criteria included a history of obstructive coronary or peripheral artery disease, heart failure, uncontrolled hypertension, uncontrolled diabetes, pregnancy and allergy or intolerance to ultrasound contrast agent or adenosine-related vasodilators. Subjects were excluded for the presence of wall motion abnormality or moderate or greater valve stenosis or regurgitation during the study echocardiogram. Beyond the prospective inclusion and exclusion criteria, four control subjects with a body mass index (BMI) ≥40 kg/m2 were excluded because there was a substantial difference in BMI between the two groups. One SCD patient with a fasting glucose level >22.2 mmol/l was also excluded. As obesity and diabetes can decrease microvascular blood volume, exclusion of these subjects from both groups represented a conservative approach to data analysis. The final study population consisted of 27 controls, 6 SCD patients not taking HC and 15 SCD patients on HC (Figure 1). Analysis of all data without exclusions did not change study findings and conclusions.
Study Design
Subjects were studied after an overnight fast and blood was drawn that morning for electrolytes and blood counts. Transthoracic echocardiography was performed for evaluation of cardiac structure and function. Brachial artery ultrasound was performed to assess macrovascular blood flow. Forearm skeletal muscle and myocardial perfusion imaging with contrast-enhanced ultrasound was performed at rest and during vasodilator stress produced by regadenoson (0.4 mg intravenously over 10 s).
Echocardiographic Measurements
Transthoracic 2-dimensional echocardiography (IE33, Philips Medical System, Andover, MA) was performed by a single sonographer using a phased-array probe. Quantitative measurements of LV dimension and thickness were performed according to American Society of Echocardiography guidelines (Lang et al, 2015). Stroke volume (SV) was calculated as the product of LV outflow tract cross-sectional area and velocity time integral, and cardiac output was calculated as the product of stroke volume and heart rate. LV end-systolic wall stress was calculated as: (0.334*SBP*LVESD) /[PWTs (1+PWTs/LVESD)] where SBP=systolic blood pressure, LVESD=LV end-systolic diameter and PWTs=posterior wall thickness in systole (Reichek et al, 1982). LV stroke work was calculated as the product of SBP and SV and LV total work was calculated as the product of LV stroke work and heart rate.
Ultrasound Imaging
Ultrasound of the brachial artery was performed above the antecubital fossa with a linear array probe (Acuson Sequoia C512, Mountainview, CA). Brachial artery diameter was measured and pulse-wave spectral Doppler was used to obtain peak flow velocities in the artery. Brachial artery flow was calculated as the product of the cross-sectional area and the average velocity time integral.
Contrast-Enhanced Ultrasound
CEU can parametrically assess microvascular blood volume (MBV) and microvascular flux rate and is particularly useful for parsing flow abnormalities that may be caused by abnormal haemorheology, increased arterial tone, or functional or structural loss of terminal microvascular units. For CEU perfusion imaging, a transaxial view of the proximal forearm deep flexor group was used for skeletal muscle and an apical 4-chamber view was used for myocardium. One vial of lipid-shelled octafluoropropane microbubbles (Definity, Lantheus Medical Imaging, North Billerica, MA) diluted to a total volume of 30 ml was infused intravenously at a rate of 1.5–2.0 ml/min. A multipulse contrast-specific imaging protocol (IE33, Philips Ultrasound, Andover, MA) was performed at a transmission frequency of 1.8 MHz with a phased-array transducer at a mechanical index (MI) of 0.1–0.2 for myocardium and 0.8–0.9 for skeletal muscle. Skeletal muscle images were acquired at end-diastole during incremental prolongation of the pulsing interval from one to 15 cardiac cycles. For the myocardium, microbubbles within the sector were destroyed with a 5 frame sequence (MI 1.0–1.1) and end-systolic frames were subsequently recorded for 10–15 consecutive cardiac cycles.
Image analysis was performed off-line as previously described (Wei et al, 1998). Videointensity was measured from a large region of interest placed over the forearm flexor muscles. For skeletal muscle imaging, averaged frames from a pulsing interval (PI) of one cardiac cycle were digitally subtracted from averaged frames at longer PIs to eliminate signal from the majority of non-capillary vessels(Dawson et al, 2002). For myocardial flow, videointensity was measured from a region of interest placed over the mid-septum as previously described (Le et al, 2002;Wei et al, 1998). Time versus videointensity (VI) data were fit to the function y=A(1-e−βt), where y is the VI at time or pulsing interval t, A is the plateau of the VI curve and reflects relative microvascular blood volume, and β is the mean microvascular blood flux rate. Muscle perfusion was calculated as the product of myocardial blood volume (A) and microvascular flow velocity (β). Perfusion analysis was not possible in two control subjects due to poor images.
Statistics
Statistical analyses were performed with GraphPad Prism (version 6.00 for Windows, GraphPad Software, La Jolla, California). Data are expressed as mean ± standard deviation unless otherwise specified. Differences were considered significant at p<0.05. One way analysis of variance using the non-parametric Kruskal-Wallis test and Dunn’s post-test for multiple comparisons were used to compare differences between control subjects, SCD patients not on HC, and SCD patients taking HC. Differences in HbF and HbS levels between the SCD groups were compared with the non-parametric Mann-Whitney test. The association between HbF and measures of perfusion was assessed using the Spearman rank correlation.
RESULTS
Demographic and Laboratory Results
Demographic variables were similar between controls and SCD patients with the exception of lower BMI in the HC-treated SCD cohort (Table I). Haemodynamics at baseline and with regadenoson showed no significant differences between groups. A chronic haemolytic state in SCD subjects was evident by anaemia and increased total bilirubin, lactate dehydrogenase (LDH) and reticulocyte count. The control subjects were on no medications other than oral contraceptives (2 subjects). In the SCD group, 4 patients (19%) were on an ace-inhibitor or an angiotensin receptor blocker, 2 (10%) were on a calcium channel blocker, one was on a beta-blocker and one was on the endothelin receptor antagonist bosentan. The majority of SCD patients (17/21 or 81%) had a history of fewer than 3 vaso-occlusive crises yearly and a similar number had a history of <10 lifetime transfusions (Supplementary Table I).
Table I.
Clinical Characteristics and Laboratory Data
| Controls n=27 |
SCD - HC n=6 |
SCD + HC n=15 |
|
|---|---|---|---|
| Age (median, years [IQR]) | 27 (23–44) | 32 (24–35) | 35 (27–46) |
| Gender (%female) | 41 | 50 | 53 |
| BMI (kg/m2) | 27 ± 5 | 23 ± 7 | 23 ± 3* |
| Baseline heart rate (/min) | 62 ± 12 | 71 ± 10 | 66 ± 9 |
| Baseline systolic BP (mmHg) | 117 ± 11 | 113 ± 14 | 114 ± 12 |
| Baseline diastolic BP (mmHg) | 63 ± 10 | 59 ± 6 | 63 ± 9 |
| Creatinine (µmol/l) | 88.4 ± 17.7 | 53.0 ± 8.8* | 61.9 ± 35.4*** |
| Glucose (mmol/l) | 5.3 ± 0.8 | 5.1 ± 1.1 | 5.3 ± 0.9 |
| Total bilirubin (µmol/l) | 8.6 ± 5.1 | 53.0 ± 12.0*** | 44.5 ± 30.8*** |
| Alkaline phosphatase (u/l) | 65 ± 16 | 122 ± 39** | 82 ± 21 |
| LDH (u/l) | 181 ± 33 | 513 ± 156*** | 427 ± 135*** |
| WBC (109/l) | 4.9 ± 1.3 | 10.0 ± 2.1*** | 6.7 ± 2.1 * |
| Hemoglobin (g/l) | 132 ± 13 | 79 ± 11*** | 94 ± 14*** |
| MCV (fl) | 84.9 ± 6.1 | 82.5 ± 8.6 | 103.6 ± 11.5***^^ |
| Reticulocyte Count (%) | 1.4 ± 0.4 | 15.7 ± 5.6*** | 9.3 ± 3.4*** |
p<0.05,
p<0.01,
p<0.001 for controls vs SCD
p<0.05,
p<0.01 for SCD vs SCD+HC
BMI: body mass index; BP: blood pressure; HC: hydroxycarbamide; IQR: interquartile range; LDH: lactate dehydrogenase; MCV: mean corpuscular volume; SCD: sickle cell disease; WBC: white blood cell.
In the SCD patients taking HC, the average daily dose was 18±8 mg/kg/day (range 6 to 31 mg/kg/day). All patients had been taking HC for longer than one year and the average duration of treatment was 4.5 years. The daily timing of HC administration was not recorded or altered for this study. Compared with patients not on HC, HC-treated patients had higher mean corpuscular volume (MCV), and a trend towards lower white blood cell (WBC) count, lower reticulocyte count and higher haemoglobin (Table I). HC-treated patients had higher levels of HbF (15.0 ± 8.3 % vs 5.9 ± 4.4 %, p=0.014) and lower levels of HbS (78.7 ± 8.5 % vs 87.9 ± 3.0 %, p=0.018) compared with untreated patients.
Echocardiography and Brachial Artery Flow
On echocardiography, SCD patients were characterized by larger LV dimensions and greater LV mass index compared to controls consistent with pathological eccentric hypertrophy (Table II). Routine parameters of LV systolic function, including ejection fraction and global longitudinal strain, showed no group-wise differences. Stroke work, total myocardial work and cardiac output, however, were significantly increased in SCD patients, consistent with expected cardiac adaptation to a high cardiac output state. SCD patients treated with HC showed no significant differences in LV structure or function compared to untreated patients other than a mild decrease in E/A ratio (1.9±0.7 vs 2.8±0.5, p=0.022).
Table II.
Ultrasound Data
| Controls n=27 |
SCD n=6 |
SCD + HC n=15 |
|
|---|---|---|---|
| LVED dimension (mm) | 46 ± 4 | 52 ± 2* | 53 ± 4*** |
| LVES dimension (mm) | 31 ± 4 | 35 ± 3 | 35 ± 4* |
| LVED volume index (ml/m2) | 53 ± 16 | 70 ± 17 | 68 ± 13* |
| LVES volume index (ml/m2) | 21 ± 7 | 30 ± 10 | 27 ± 9 |
| LVES wall stress (103 dynes/cm2) | 63 ± 14 | 58 ± 14 | 60 ± 13 |
| LVMI (g/m2) | 67 ± 13 | 84 ± 16 | 94 ± 22*** |
| E/A ratio | 2.1 ± 0.7 | 2.8 ± 0.5 | 1.9 ± 0.7^ |
| Septal e' (cm/s) | 10.9 ± 2.9 | 11.5 ± 2.3 | 10.3 ± 1.7 |
| Septal E/e' | 6.4 ± 1.7 | 9.6 ± 1.3** | 9.2 ± 3.0*** |
| RVSP (mmHg) | 23 ± 4 | 27 ± 6 | 28 ± 7 |
| Ejection fraction (%) | 62 ± 5 | 61 ± 3 | 61 ± 6 |
| Stroke volume (ml) | 62 ± 13 | 76 ± 13 | 78 ± 17** |
| Stroke work (×10−3), (ml · mmHg) | 7.2 ± 1.7 | 8.7 ± 1.7 | 9.1 ± 2.6* |
| Total cardiac work (ml · mmHg/min) | 413 ± 123 | 609 ± 80** | 568 ± 129** |
| Cardiac output (l/min) | 3.5 ± 0.9 | 5.4 ± 0.6*** | 5.0 ± 0.8*** |
| Cardiac index (l/min/m2) | 1.9 ± 0.5 | 3.1 ± 0.6*** | 2.8 ± 0.3*** |
| Global longitudinal strain(%) | −19 ± 3 | −19 ± 3 | −20 ± 2 |
| Brachial diameter (cm) | 3.4 ± 0.5 | 3.2 ± 0.6 | 3.2 ± 0.4 |
p<0.05,
p<0.01,
p<0.001 for controls vs SCD
p<0.05,
p<0.01 for SCD vs SCD+HC
e': tissue Doppler mitral annular velocity; E/A: ratio of early (E) to late (A) left ventricular filling; HC: hydroxycarbamide; LVED: left ventricular end-diastolic; LVES: left ventricular end-systolic; LVMI: left ventricular mass index; RVSP: right ventricular systolic pressure; SCD: sickle cell disease.
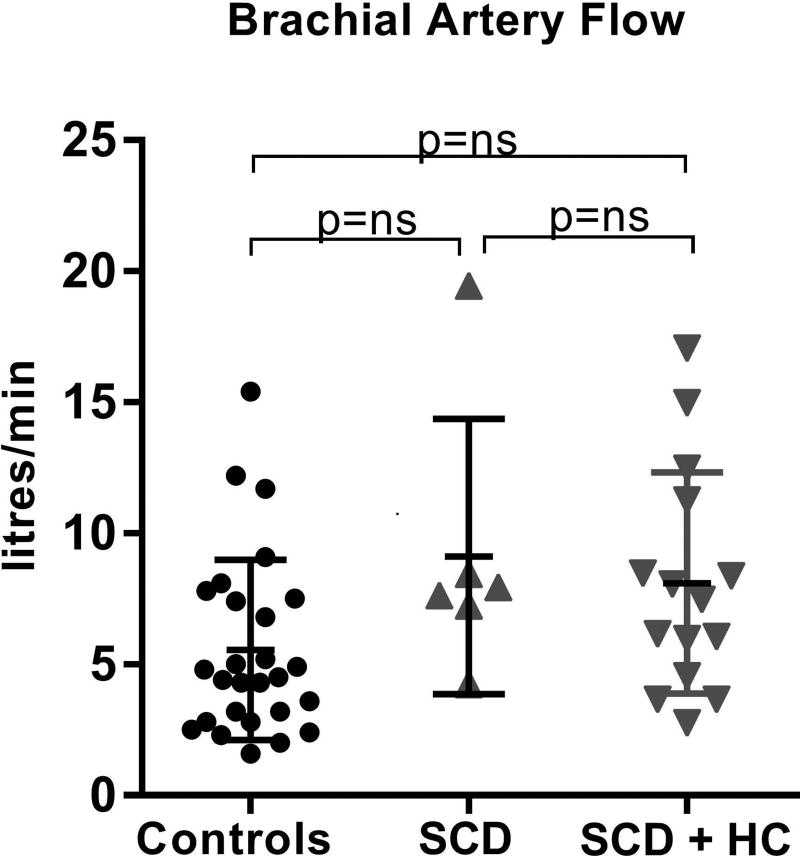
Brachial artery diameter and flow was not significantly different between controls and SCD, nor were there any differences within the SCD group based on HC therapy (Table II, Figure 2).
Figure 2. Brachial artery flow.
Calculated brachial artery flow in controls, SCD patients not on HC and SCD patients on HC therapy demonstrates no significant differences between groups. HC: hydroxycarbamide; ns: not significant; SCD: sickle cell disease.
Skeletal Muscle Perfusion
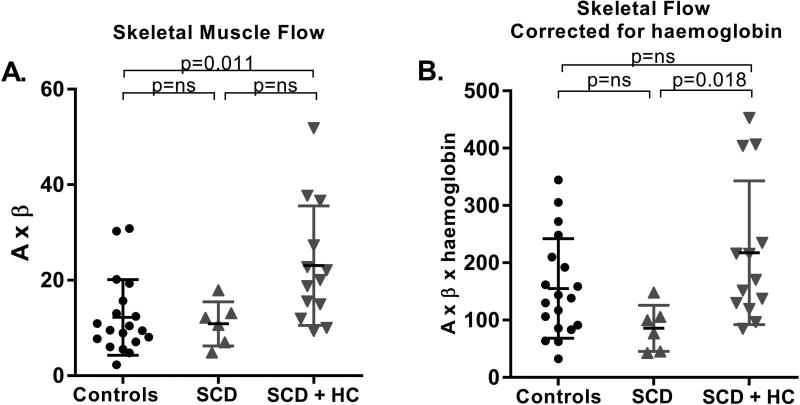
CEU of skeletal muscle demonstrated that the HC-treated SCD patients had significantly higher muscle perfusion than controls (Figure 3A). Parametric analysis of CEU data indicated a trend toward improvement in both functional microvascular blood volume and microvascular flux rate in SCD subjects treated with HC. This finding suggests both an improvement in the regulation of functional patency of capillary units, which has been shown to be largely under the regulatory control of NO (Vincent et al, 2003), and improvement in rheology (Rim et al, 2001), possibly due to the increased HbF level and decreased HbS polymerization.
Figure 3. Skeletal Muscle Microvascular Flow.
A. Resting contrast-enhanced ultrasound-derived skeletal muscle flow data is shown in controls, SCD patients not on HC and SCD patients on HC therapy. B. Skeletal muscle flow normalized to haemoglobin levels is shown in all three groups. HC: hydroxycarbamide; ns: not significant; SCD: sickle cell disease.
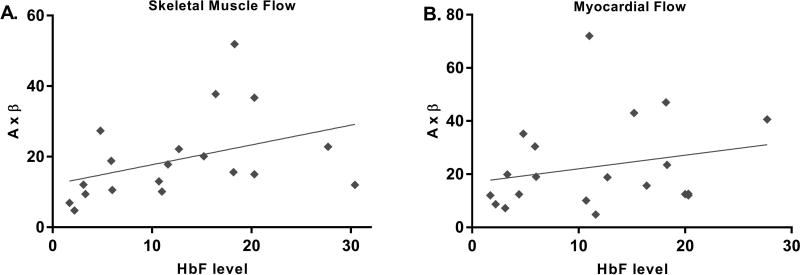
Muscle and myocardial perfusion are under tight metabolic control so that oxygen delivery meets demand under various working conditions. Accordingly, all of our measurements of tissue blood flow were normalized to the degree of anaemia in order to assess true efficiency of perfusion (perfusion efficiency = A×β×haemoglobin level). Skeletal muscle perfusion efficiency remained high in sickle cell patients treated with HC and was significantly higher than in the untreated SCD patients (Figure 3B). There was a good correlation between HbF levels and measurements of skeletal muscle perfusion (r=0.55, p=0.01, Figure 4A) and perfusion efficiency (r=0.60, p=0.007). With vasodilator stress, skeletal muscle perfusion measurements increased to a similar extent in controls and SCD patients (Supplementary Figure 1A).
Figure 4. HbF levels and Skeletal Muscle and Myocardial Flow.
A. There was a direct correlation between HbF levels and skeletal muscle flow (r=0.55, p=0.01). B. No significant correlation was seen between HbF levels and myocardial flow (r=0.31, p=0.191).
Myocardial Blood Flow
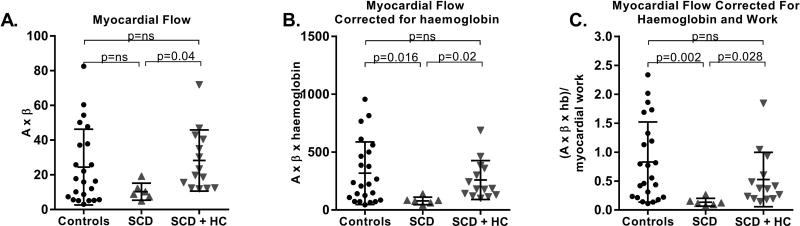
Resting myocardial perfusion in HC-treated SCD patients was significantly higher than in untreated patients (Figure 5A). With normalization for haemoglobin levels, resting flow in untreated SCD patients was lower than in healthy controls, but HC therapy appeared to restore myocardial perfusion to the same level as controls (Figure 5B).
Figure 5. Myocardial Blood Flow.
A. Myocardial perfusion data is shown in controls, SCD patients not on HC and SCD patients on HC therapy. B. Myocardial perfusion is normalized to haemoglobin levels. C. Myocardial perfusion is normalized to both haemoglobin and myocardial work. HC: hydroxycarbamide; ns: not significant; SCD: sickle cell disease.
Given that resting myocardial blood flow is closely coupled to myocardial oxygen consumption, which in turn is determined by heart rate, load and contractility, CEU measurements of myocardial perfusion were also normalized for myocardial work ((A×β×haemoglobin)/(stroke volume×systolic blood pressure×heart rate)). Normalized myocardial microvascular flow was significantly lower in untreated SCD patients compared with controls (Figure 5C). Microvascular flow in the myocardium was significantly higher in HC-treated patients, which again was attributable to increases in both functional microvascular blood volume and flux rate. However, there was no significant correlation between HbF levels and myocardial perfusion measurements (r=0.31, p=0.191, Figure 4B). Myocardial perfusion during vasodilator stress improved to a similar extent in both controls and SCD patients (Supplementary Figure 1B).
DISCUSSION
Cardiovascular complications are common in SCD patients with no evidence of disease in conduit vessels (Manci et al, 2003;Fitzhugh et al, 2010). Microvascular occlusion or dysfunction has been implicated as an important contributor to these complications (Desai et al, 2014) but direct measurements of this in SCD patients has been difficult due to inadequate techniques to measure microvascular flow. We used ultrasound to assess flow in the brachial artery; however, brachial artery blood flow is not an adequate surrogate for muscle perfusion because many other non-muscle tissue types are supplied by the brachial artery and because non-nutritive intramuscular vascular pathways that exist (Vincent et al, 2006). Hence, CEU perfusion imaging was performed to quantify microvascular flow in skeletal muscle and the myocardium. In our study of SCD patients, we found that perfusion measurements in SCD patients not taking HC were comparable to those of healthy controls. By normalizing blood flow to the degree of anaemia and disease-related increases in myocardial work, we demonstrated resting myocardial perfusion deficits in SCD patients not taking HC. In addition, we have shown a clear increase in both skeletal muscle and myocardial microvascular flow with HC therapy and a significant correlation between HbF levels and skeletal muscle microvascular flow.
Skeletal muscle blood flow is regulated by a complex interplay of factors that match oxygen and nutrient delivery with metabolic demand. Haemoglobin level is a critical component of this process. Leg blood flow studies in young healthy men following acute lowering of haemoglobin concentration have demonstrated increased systemic and muscle blood flow (Koskolou et al, 1997;Gonzalez-Alonso et al, 2006) ). Studies using strain gauge plethysmography (Eberhardt et al, 2003)and near infra-red spectroscopy (Waltz et al, 2012) have consistently shown increased resting overall forearm blood flow in SCD patients compared with controls. Animal studies of non-SCD anaemia show that resting skeletal muscle microvascular flow is significantly higher in the anaemic state compared with controls (Kubes et al, 1988). For patients with the level of anaemia found in our study, the lack of increased microvascular flow in SCD patients not treated with HC may represent an important and abnormal finding showing an inability to compensate for the anaemia at the microvascular level.
Beneficial effects of HC treatment in SCD have been attributed to ATP- and NO-mediated vasodilation and improvements in erythrocyte rheology, membrane deformability and cell hydration, possibly related to increases in HbF (Cokic et al, 2006;Cokic et al, 2008;Gladwin et al, 2002). Given that intracellular levels of HbF may not rise for one to two weeks after initiation of therapy(Rodgers et al, 1990), it is possible that other mechanisms also play an important role. HC-treatment also decreases soluble vascular adhesion molecule 1 levels, resulting in reduced adhesion of endothelial cells to sickled erythrocytes (Saleh et al, 1999), and inhibits neutrophil recruitment and activation through the NO/cyclic guanosine monophosphate (GMP) pathway (Canalli et al, 2008;Almeida et al, 2012). Improvements in erythrocyte hydration status and cell deformability have also been seen with HC treatment (Halsey & Roberts, 2003). Previous in vivo investigations of blood flow in relation to hydroxycarbamide therapy in SCD have involved assessment of transcranial flow (Helton et al, 2014;Zimmerman et al, 2007) or forearm blood flow (Gladwin et al, 2003). Assessment of microvascular flow in SCD has only been possible in the skin (Charlot et al, 2016) and the retina (Minvielle et al, 2016) to a limited degree. To our knowledge, this is the first study to directly evaluate differences in muscle and myocardial perfusion at the microvascular level associated with HC treatment in SCD patients.
Our CEU measurements of microvascular perfusion in skeletal muscle are novel with regards to the assessment of muscle blood flow and flux rate in a quantitative fashion. Investigations into structural alterations have been more common. Muscle biopsies in patients with SCD indicate capillary rarefaction in the skeletal muscle(Ravelojaona et al, 2015). Ravelojaona et al (2015) showed that despite the presence of capillary rarefaction, there is an increase in the number of non-capillary microvessels >5 µm in diameter. Importantly, the morphological appearance or density of microvessels does not necessary reflect functional status in terms of the number of microvascular units that are actively perfused as there may be dissociation between the degree of abnormalities in skeletal muscle microvascular structural and functional density(Clerk et al, 2007). Given that only a minority of skeletal muscle capillaries are open at rest in normal subjects, capillary recruitment to increase microvascular blood volume is a normal skeletal muscle response to increased oxygen requirements and low oxygen carrying capacity. Our parametric analysis of blood flow suggests that improvements in microvascular blood flow in HC-treated patients are a manifestation of improvements in both microvascular blood volume and blood flow velocity.
In the myocardium, we found that microvascular flow in HC-treated SCD patients is similar to that of controls at rest and following regadenoson infusion. In normal hearts, resting myocardial blood flow is closely coupled to myocardial oxygen consumption (MVO2), which is determined by multiple parameters including heart rate, blood pressure, contractility and wall stress. As expected, sickle cell patients had higher levels of stroke work and total myocardial work compared with controls. When we normalized myocardial blood flow for anaemia and increased myocardial work, we again found no significant flow abnormalities overall in HC-treated SCD patients. In contrast, we saw significant perfusion deficits in SCD patients not treated with HC. Our findings are in agreement with an earlier CEU study in SCD patients which found normal indices of microvascular flux rate and volume at baseline (Almeida et al, 2008), however, that study did not delineate differences in flow associated with HC-treatment.
Limitations
Our study’s sample size is small and we excluded two initial patients with poor quality data for perfusion assessment. The controls were significantly overweight compared with SCD patients and although this may have affected their microvascular flow, fasting glucose levels were similar in both groups. Although we adjusted perfusion measurements for the severity of anaemia and normalized for differences in cardiac work, it was not possible to normalize for efficiency of oxygen delivery.
The timing and dosing of HC administration and other medications that may affect perfusion haemodynamics were not controlled in our non-randomized observational study. The SCD subjects in the current study reported few yearly vaso-occlusive events and lifetime transfusion numbers and may represent a relatively stable group. We had a small number of patients not taking HC, therefore, these findings are hypothesis-generating and should be investigated further with larger patient numbers.
When we compared laboratory parameters in Table 1 between HC untreated and treated groups, only MCV reached statistical significance using the Kruskal-Wallis non-parametric ANOVA test. This is probably due to the small sample size because haemoglobin improved with HC-treatment as expected, and there were trends towards normalization of numerous other laboratory parameters (total bilirubin, alkaline phosphatase, LDH, WBC count, reticulocyte count).
Conclusions
Quantitative assessment of skeletal muscle and myocardial perfusion with CEU imaging shows that resting perfusion in SCD patients treated with HC is significantly better than in untreated patients and is similar to that of controls. Flow improvements in HC-treated patients were related to increases in both blood volume and flux rate and skeletal flow directly correlated with HbF levels. More definitive evidence of HC-related microvascular flow improvements will be needed through larger studies that evaluate temporal changes in perfusion occurring after HC administration.
Supplementary Material
Acknowledgments
The authors would like to thank Jim Nichols, RN, Anna Conrey, NP, and Kevin Smith, RN for assistance with recruitment and clinical evaluations.
Sources of Funding
This research was supported by the Intramural Research Program of the National Heart Lung and Blood Institute, NIH, DHHS.
Disclosures
Dr. Arai has a research agreement with Siemens for MRI imaging. Dr Kato has received research funding from AesRx, LLC and has been a consultant to Mast Therapeutics, Biogen Idec, Baxter Healthcare, Bayer HealthCare and CSL Behring (none pertinent to this manuscript).
Footnotes
CLINICAL TRIAL REGISTRATION ClinicalTrials.gov Identifier: NCT01602809; https://clinicaltrials.gov/ct2/show/NCT01602809?term=sACHDEV&rank=9
Each author contributed significantly to this manuscript including the conception and design of the study (JRL), analysis and interpretation of data (VS, SS, HH, MDW, CB, MAW, ANS, JRL), drafting and revision of the manuscript (VS, CM, AA, GK, ANS, JRL), and final approval of the manuscript (VS, JRL).
References
- Almeida AG, Araujo F, Rego F, David C, Lopes MG, Ducla-Soares J. Abnormal myocardial flow reserve in sickle cell disease: a myocardial contrast echocardiography study. Echocardiography. 2008;25:591–599. doi: 10.1111/j.1540-8175.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- Almeida CB, Scheiermann C, Jang JE, Prophete C, Costa FF, Conran N, Frenette PS. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood. 2012;120:2879–2888. doi: 10.1182/blood-2012-02-409524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalli AA, Franco-Penteado CF, Saad ST, Conran N, Costa FF. Increased adhesive properties of neutrophils in sickle cell disease may be reversed by pharmacological nitric oxide donation. Haematologica. 2008;93:605–609. doi: 10.3324/haematol.12119. [DOI] [PubMed] [Google Scholar]
- Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N.Engl.J.Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- Charlot K, Romana M, Moeckesch B, Jumet S, Waltz X, Divialle-Doumdo L, Hardy-Dessources MD, Petras M, Tressieres B, Tarer V, Hue O, Etienne-Julan M, Antoine-Jonville S, Connes P. Which side of the balance determines the frequency of vaso-occlusive crises in children with sickle cell anemia: Blood viscosity or microvascular dysfunction? Blood Cells Mol.Dis. 2016;56:41–45. doi: 10.1016/j.bcmd.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am.J.Physiol Endocrinol.Metab. 2007;293:E1804–E1809. doi: 10.1152/ajpendo.00498.2007. [DOI] [PubMed] [Google Scholar]
- Cokic VP, Beleslin-Cokic BB, Tomic M, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea induces the eNOS-cGMP pathway in endothelial cells. Blood. 2006;108:184–191. doi: 10.1182/blood-2005-11-4454. [DOI] [PubMed] [Google Scholar]
- Cokic VP, Andric SA, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood. 2008;111:1117–1123. doi: 10.1182/blood-2007-05-088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am.J.Physiol Endocrinol.Metab. 2002;282:E714–E720. doi: 10.1152/ajpendo.00373.2001. [DOI] [PubMed] [Google Scholar]
- Desai AA, Patel AR, Ahmad H, Groth JV, Thiruvoipati T, Turner K, Yodwut C, Czobor P, Artz N, Machado RF, Garcia JG, Lang RM. Mechanistic insights and characterization of sickle cell disease-associated cardiomyopathy. Circ.Cardiovasc.Imaging. 2014;7:430–437. doi: 10.1161/CIRCIMAGING.113.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt RT, McMahon L, Duffy SJ, Steinberg MH, Perrine SP, Loscalzo J, Coffman JD, Vita JA. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. Am.J.Hematol. 2003;74:104–111. doi: 10.1002/ajh.10387. [DOI] [PubMed] [Google Scholar]
- Fitzhugh CD, Lauder N, Jonassaint JC, Telen MJ, Zhao X, Wright EC, Gilliam FR, De Castro LM. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am.J.Hematol. 2010;85:36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT. Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 2016;387:2565–2574. doi: 10.1016/S0140-6736(16)00647-4. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Shelhamer JH, Ognibene FP, Pease-Fye ME, Nichols JS, Link B, Patel DB, Jankowski MA, Pannell LK, Schechter AN, Rodgers GP. Nitric oxide donor properties of hydroxyurea in patients with sickle cell disease. Br.J.Haematol. 2002;116:436–444. doi: 10.1046/j.1365-2141.2002.03274.x. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Schechter AN, Ognibene FP, Coles WA, Reiter CD, Schenke WH, Csako G, Waclawiw MA, Panza JA, Cannon RO., III Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J.Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey C, Roberts IA. The role of hydroxyurea in sickle cell disease. Br.J.Haematol. 2003;120:177–186. doi: 10.1046/j.1365-2141.2003.03849.x. [DOI] [PubMed] [Google Scholar]
- Helton KJ, Adams RJ, Kesler KL, Lockhart A, Aygun B, Driscoll C, Heeney MM, Jackson SM, Krishnamurti L, Miller ST, Sarnaik SA, Schultz WH, Ware RE. Magnetic resonance imaging/angiography and transcranial Doppler velocities in sickle cell anemia: results from the SWiTCH trial. Blood. 2014;124:891–898. doi: 10.1182/blood-2013-12-545186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskolou MD, Roach RC, Calbet JA, Radegran G, Saltin B. Cardiovascular responses to dynamic exercise with acute anemia in humans. Am.J.Physiol. 1997;273:H1787–H1793. doi: 10.1152/ajpheart.1997.273.4.H1787. [DOI] [PubMed] [Google Scholar]
- Kubes P, Cain SM, Chapler CK. Hindlimb skeletal muscle blood flow during sympathetic nerve block before and during acute anemia. Can.J.Physiol Pharmacol. 1988;66:1148–1153. doi: 10.1139/y88-189. [DOI] [PubMed] [Google Scholar]
- Lamers L, Ensing G, Pignatelli R, Goldberg C, Bezold L, Ayres N, Gajarski R. Evaluation of left ventricular systolic function in pediatric sickle cell anemia patients using the end-systolic wall stress-velocity of circumferential fiber shortening relationship. J.Am.Coll.Cardiol. 2006;47:2283–2288. doi: 10.1016/j.jacc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J.Am.Soc.Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Le DE, Bin JP, Coggins MP, Wei K, Lindner JR, Kaul S. Relation between myocardial oxygen consumption and myocardial blood volume: a study using myocardial contrast echocardiography. J.Am.Soc.Echocardiogr. 2002;15:857–863. doi: 10.1067/mje.2002.121275. [DOI] [PubMed] [Google Scholar]
- Manci EA, Culberson DE, Yang YM, Gardner TM, Powell R, Haynes J, Jr, Shah AK, Mankad VN. Causes of death in sickle cell disease: an autopsy study. Br.J.Haematol. 2003;123:359–365. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert.Opin.Drug Saf. 2015;14:1749–1758. doi: 10.1517/14740338.2015.1088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minvielle W, Caillaux V, Cohen SY, Chasset F, Zambrowski O, Miere A, Souied EH. Macular Microangiopathy in Sickle Cell Disease Using Optical Coherence Tomography Angiography. Am.J.Ophthalmol. 2016;164:137–144. doi: 10.1016/j.ajo.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. N.Engl.J.Med. 2017;376:1561–1573. doi: 10.1056/NEJMra1510865. [DOI] [PubMed] [Google Scholar]
- Poillon WN, Kim BC, Rodgers GP, Noguchi CT, Schechter AN. Sparing effect of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S at physiologic ligand saturations. Proc.Natl.Acad.Sci.U.S.A. 1993;90:5039–5043. doi: 10.1073/pnas.90.11.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelojaona M, Feasson L, Oyono-Enguelle S, Vincent L, Djoubairou B, Ewa'Sama EC, Messonnier LA. Evidence for a profound remodeling of skeletal muscle and its microvasculature in sickle cell anemia. Am.J.Pathol. 2015;185:1448–1456. doi: 10.1016/j.ajpath.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Reichek N, Wilson J, St John SM, Plappert TA, Goldberg S, Hirshfeld JW. Noninvasive determination of left ventricular end-systolic stress: validation of the method and initial application. Circulation. 1982;65:99–108. doi: 10.1161/01.cir.65.1.99. [DOI] [PubMed] [Google Scholar]
- Rim SJ, Leong-Poi H, Lindner JR, Wei K, Fisher NG, Kaul S. Decrease in coronary blood flow reserve during hyperlipidemia is secondary to an increase in blood viscosity. Circulation. 2001;104:2704–2709. doi: 10.1161/hc4701.099580. [DOI] [PubMed] [Google Scholar]
- Rodgers GP, Dover GJ, Noguchi CT, Schechter AN, Nienhuis AW. Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N.Engl.J.Med. 1990;322:1037–1045. doi: 10.1056/NEJM199004123221504. [DOI] [PubMed] [Google Scholar]
- Saleh AW, Hillen HF, Duits AJ. Levels of endothelial, neutrophil and platelet-specific factors in sickle cell anemia patients during hydroxyurea therapy. Acta Haematol. 1999;102:31–37. doi: 10.1159/000040964. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am.J.Physiol Endocrinol.Metab. 2003;285:E123–E129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am.J.Physiol Endocrinol.Metab. 2006;290:E1191–E1197. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- Waltz X, Pichon A, Lemonne N, Mougenel D, Lalanne-Mistrih ML, Lamarre Y, Tarer V, Tressieres B, Etienne-Julan M, Hardy-Dessources MD, Hue O, Connes P. Normal muscle oxygen consumption and fatigability in sickle cell patients despite reduced microvascular oxygenation and hemorheological abnormalities. PLoS.One. 2012;7:e52471. doi: 10.1371/journal.pone.0052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware RE, de MM, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390:311–323. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- Zimmerman SA, Schultz WH, Burgett S, Mortier NA, Ware RE. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007;110:1043–1047. doi: 10.1182/blood-2006-11-057893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.