Abstract
Chronic glomerular injury is associated with eventual development of tubulointerstitial fibrosis. Here we aimed to assess whether, and how, mild chronic tubulointerstitial injury affects glomeruli. For this, we generated mice expressing different toxin receptors, one on their proximal tubular epithelial cells (diphtheria toxin receptor (DTR)) and the other only on podocytes (human CD25 (IL-2R) driven by the nephrin promotor (Nep25)), allowing serial induction of tubule-specific and glomerular (podocyte)-specific injury, respectively. Six weeks after diphtheria toxin injection, mild interstitial fibrosis was found in Nep25+/DTR+, but not in Nep25+/DTR− mice. However, atubular glomeruli and neuronal nitric oxide synthase, a mediator of tubuloglomerular feedback, were higher in Nep25+/DTR+ than in DTR− mice and these atubular glomeruli had less podocyte density as assessed by WT-1 biomarker expression. Peritubular capillary density, hypoxia-inducible factor-1 and -2, and cyclooxygenase 2 expression were similar at week six in the two groups. At week seven, all mice were given the immunotoxin LMB2 which binds to CD25 to induce podocyte injury. Ten days later, proteinuria, podocyte injury and glomerulosclerosis were more severe in Nep25+/DTR+ than Nep25+/DTR− mice with more severe sclerosis in the tubule-connected glomeruli. This supports the concept that even mild preexisting tubulointerstitial injury sensitizes glomeruli to subsequent podocyte- specific injury. Thus, increased atubular glomeruli and abnormal tubuloglomerular feedback significantly contribute to the crosstalk between the tubulointerstitium and glomeruli.
Keywords: tubulointerstitial fibrosis, glomerular injury, atubular glomeruli, tubuloglomerular feedback
Introduction
Chronic glomerular injury is associated with eventual development of tubulointerstitial fibrosis, resulting in progressive loss of entire nephrons and chronic kidney disease. This glomerulo-tubular spread of injury has been observed in animal models and several potential mechanisms have been proposed.1 Although chronic progressive tubulointerstitial disease plays a critical role in the outcome of patients with primary glomerular lesions, the basic mechanisms that generate the tubulointerstitial damage remain unclear.2 Proposed mechanisms can be broadly classified as “tubular” and “glomerular” hypotheses.3 The tubular hypothesis states that excessive tubular protein load due to glomerular protein leakage induces inflammation and fibrosis, which are harmful to tubular epithelial cells. The glomerular hypothesis postulates that obstruction of the glomerulotubular junction by local fibrosis causes tubules to be disconnected from glomeruli, resulting in tubular degeneration.4 Recently, altered HIF-1 and HIF-2 have been suggested as another possible mechanism by which glomerular injury affects the tubules.5 These studies all have focused on the glomerular lesion as an initial injury, and assessed subsequent effects on the tubulointerstitium. In these schemas, tubulointerstitial lesions are secondary and do not have an active role in the progression of glomerulosclerosis in chronic kidney disease.
Tubulointerstitial injury could also play an active, primary role in progressive nephron loss. Previously, patients who survived and had short-term return of normal renal function after acute kidney injury (AKI) were considered to have complete recovery. However, more recent epidemiologic studies show that AKI is a major risk factor for long-term CKD.6 Previous experimental studies using Six2-Cre-LoxP technology selectively activated diphtheria toxin receptor (DTR) expression in renal epithelia derived from the metanephric mesenchyme.7 These studies showed that mild tubular injury induced adaptive repair processes and few long-term consequences, while severe injury induced maladaptive repair, severe interstitial fibrosis and glomerulosclerosis. This study suggest the transition of AKI to CKD due to peritubular capillary rarefaction and other mechanisms.8 In a diabetic nephropathy experimental model, tubulointerstitial injury was not merely a result of glomerular injury, but was also a primary target of diabetic injury and even appeared to affect the progression of glomerular lesions.9 These findings raise the question of whether mild tubulointerstitial injury could sensitize to subsequent glomerular injury.
Previous studies either use models with tubular injury, such as ischemia-reperfusion, with long-term follow-up, or the injury model targeted both tubules and glomeruli, such as diabetic nephropathy. In contrast, we have now developed an animal model with distinct sequential tubular and glomerular injuries to specifically investigate novel mechanisms whereby tubular injury sensitizes to subsequent glomerular injury. We achieved proximal tubular cell-specific injury using diphtheria toxin (DT)-mediated conditional cell ablation in transgenic mice, where the DT receptor is expressed on proximal tubules. After DT injection, mice have increased BUN and creatinine, and initial polyuria with subsequent reversible oliguria. Injury is present in the proximal tubule without glomerular change, and injured tubular cells recover rapidly.10, 11 Glomerular-specific injury is possible in the so-called Nep25 mouse model, through selective podocyte injury by injection of a modified toxin, LMB2, in mice that express human CD25 only on podocytes, driven by the nephrin promotor. These mice show progressive nonselective proteinuria, with resulting edema, and focal segmental glomerulosclerosis due to podocyte injury.12 We now have generated combined double transgenic mice to investigate effects of mild, functionally recovered tubulointerstitial injury on the development of subsequent glomerular injury, and mechanisms for this crosstalk.
Results
Diphtheria toxin-induced acute tubular injury and chronic interstitial fibrosis
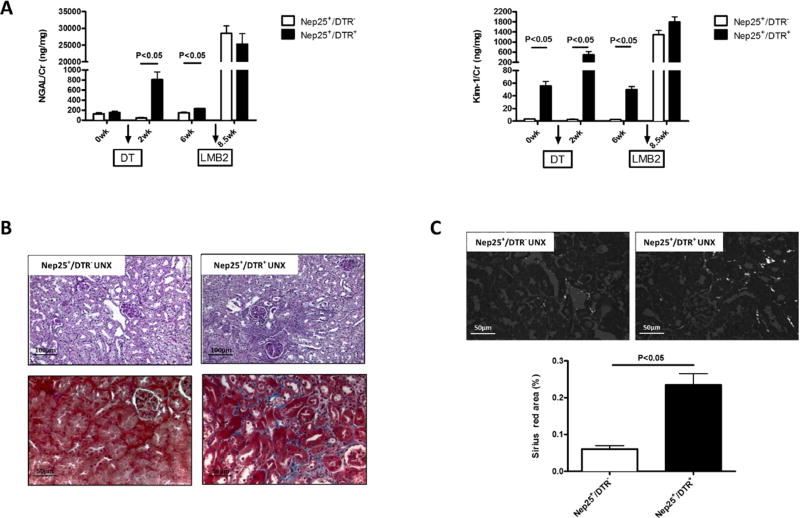
At baseline, urinary NGAL and Kim-1 excretion levels were similar in Nep25+/DTR− and Nep25+/DTR+ mice. Two weeks after DT injection, urinary NGAL and Kim-1 were markedly increased in Nep25+/DTR+ mice (NGAL 810.7±148.0 ng/mg, Kim-1 503.3±128.8 ng/mg), but not in Nep25+/DTR− mice (NGAL 46.1±9.5 ng/mg, Kim-1 2.7± 0.6 ng/mg, p<0.05 vs. Nep25+/DTR+). At week 6, urinary NGAL and Kim-1 returned towards normal in Nep25+/DTR+ mice (NGAL 233.4±8.5 ng/mg, Kim-1 49.6±5.0 ng/mg), but were still higher than Nep25+/DTR− mice (NGAL 149.8±13.7 ng/mg, Kim-1 2.5± 0.4 ng/mg, p<0.05 vs. Nep25+/DTR+) (Figure 1A).
Fig 1.
Increased tubulointerstitial injury in Nep25+/DTR+ vs. Nep25+/DTR− mice at week 6 after DT injection before induction of podocyte injury. (A) Urine NGAL and KIM-1 significantly increased at week 2 and returned to baseline at week 6 in Nep25+/DTR+, while there was no change in Nep25+/DTR− mice. After LMB2 injection, both groups of mice had similar urinary NGAL and Kim-1. (B) PAS (top) and Masson trichrome (bottom) staining show tubulointerstitial injury in Nep25+/DTR+, but not in Nep25+/DTR− mice. (C) Interstitial fibrosis was increased in Nep25+/DTR+ vs. Nep25+/DTR− mice, detected by polarized Sirius red staining.
All mice underwent uninephrectomy at week 6 after DT injection. These kidneys showed focal tubular epithelial cell regeneration and mild tubulointerstitial fibrosis only in Nep25+/DTR+ mice (Figure 1B). Interstitial fibrosis, quantified by Sirius red staining, was higher in Nep25+/DTR+ (0.235±0.031% area stained) than Nep25+/DTR− kidney (0.060±0.009%, P<0.05) (Figure 1C). Neither group showed glomerular abnormalities by light microscopy at this time point.
Thus, after recovering from acute tubular injury induced by DT injection, Nep25+/DTR+ mice developed tubulointerstitial fibrosis, while Nep25+/DTR− had no tubular injury, as expected.
Pre-existing tubulointerstitial injury amplified subsequent glomerular injury
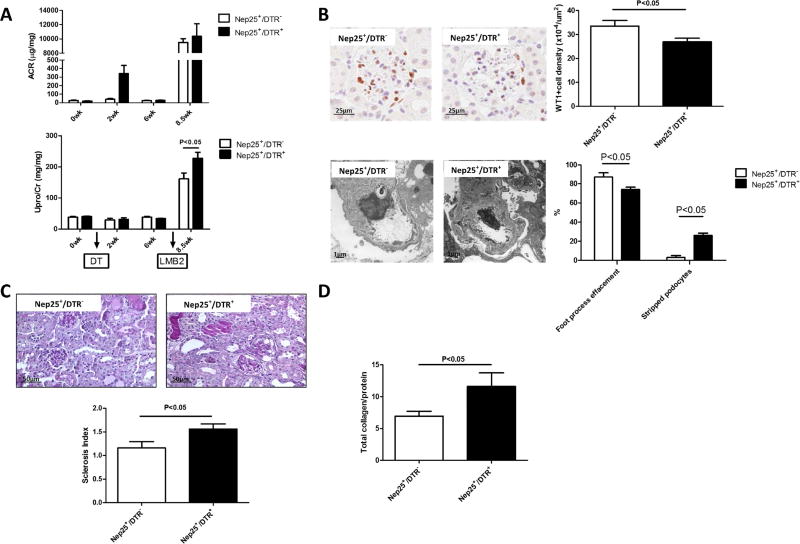
Urinary albumin increased at week 2 due to acute tubular injury, and returned to baseline at week 6 in Nep25+/DTR+ mice. Total proteinuria was also similar in Nep25+/DTR+ and Nep25+/DTR− mice at week 6 (33.85±0.69 vs. 38.73±2.37 mg/mg). LMB2 injection at week 7 injured podocytes and resulted in maximal massive albuminuria in both Nep25+/DTR+ and Nep25+/DTR− mice (10363.44±1762.80 vs. 9495.00±546.12 µ g/mg) (Figure 2A). At 10 days after LMB2 injection total proteinuria was more severe in Nep25+/DTR+ vs Nep25+/DTR− mice (228.02±19.09 vs. 161.21±19.56 mg/mg, p<0.05) (Figure 2A). Renal function, measured by BUN, was similar between Nep25+/DTR+ and Nep25+/DTR− mice at sacrifice (94.30±4.95 vs. 92.40±8.82 mg/dl).
Fig 2.
Enhanced glomerular injury in Nep25+/DTR+ vs. Nep25+/DTR− mice kidneys on day 10 after podocyte injury induced by LMB2 injection. (A) Urine albumin to creatinine ratio (ACR) increased at week 2 and returned to baseline at week 6 in Nep25+/DTR+ and ACR and urine total protein to creatinine ratios were not different between groups at week 6. However, at 10 days after LMB2 podocyte toxin injection. Urine total protein to creatinine ratio was higher in Nep25+/DTR+ than Nep25+/DTR− mice. (B) LMB2 induced more podocyte injury, shown by less glomerular WT-1+ cell density and more loss of podocytes in Nep25+/DTR+ vs Nep25+/DTR− mice. There was also more glomerulosclerosis (C) and renal fibrosis (D) in Nep25+/DTR+ vs Nep25+/DTR− mice.
Although podocyte foot process effacement was less in Nep25+/DTR+ vs. Nep25+/DTR− mice (74.0±2.2 vs. 87.0±4.4%, p<0.05), segments of denuded GBM with stripped podocytes were more extensive in Nep25+/DTR+ than Nep25+/DTR− mice (26.0±2.2 vs. 3.0±1.8%, p<0.05) (Figure 2B). Glomerular WT-1+ cell density, a marker of differentiated podocytes, was decreased in Nep25+/DTR+ vs Nep25+/DTR− mice (26.94±1.48 vs. 33.52±2.32 x10−4/µm2, p<0.05) (Figure 2B).
Nep25+/DTR+ mice also had more glomerulosclerosis than Nep25+/DTR− mice at day 10 after LMB2 (Nep25+/DTR+ 1.48±0.15 vs. Nep25+/DTR− 0.78±0.15, P<0.05) (Figure 2C). Tubular injury markers, urine NGAL and KIM-1, increased similarly in both groups after LMB2 injection (Figure 1A). Kidney fibrosis, measured as total collagen amount, was increased in Nep25+/DTR+ vs Nep25+/DTR− group at sacrifice (11.6±2.1 vs. 6.9±0.7, p<0.05) (Figure 2D).
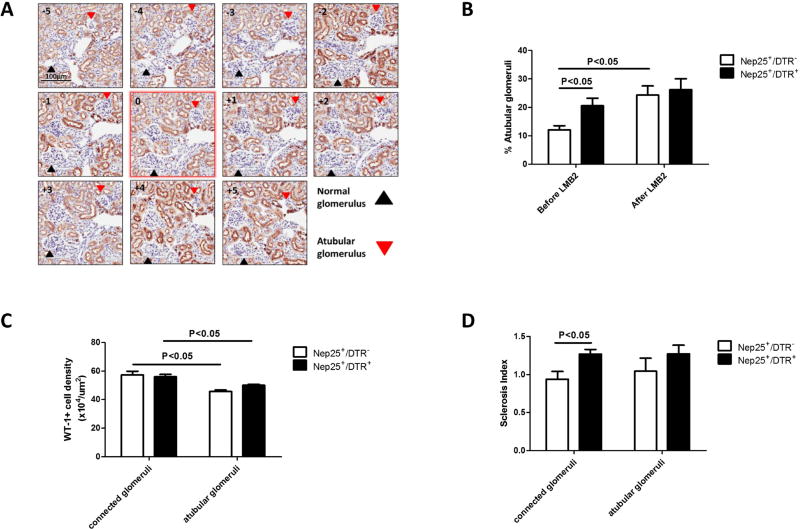
Pre-existing tubulointerstitial injury induced more atubular glomeruli and abnormal tubuloglomerular feedback
Atubular glomeruli were more frequent in Nep25+/DTR+ vs Nep25+/DTR− mice at week 6 before LMB2 injection (20.6±2.6 vs 12.1±2.4, p<0.05) (Figure 3B). WT-1+ cell density was decreased in atubular glomeruli vs tubule-connected glomeruli in both groups (Figure 3C). LMB2 injection increased the extent of atubular glomeruli in both groups (Figure 3B). Glomerulosclerosis was similar in atubular glomeruli in the two groups, while tubule-connected glomeruli showed more severe glomerulosclerosis in Nep25+/DTR+ than in Nep25+/DTR− mice (Figure 3D).
Fig 3.
Immunostaining of biotinylated lotus tetragonolobus lectin for detection of atubular glomeruli in Nep25+/DTR− and Nep25+/DTR+ mice kidneys at week 6 after DT injection. (A) Schema of method for counting atubular glomeruli. (B) Atubular glomeruli were increased in Nep25+/DTR+ vs. Nep25+/DTR− mice at week 6, but with increased frequency of atubular glomeruli after LMB2 injection. (C) WT-1+ cell density was lower in atubular glomeruli than in tubule-connected glomeruli in both groups at week 6. (D) LMB2 injection induced more severe glomerulosclerosis in tubule-connected glomeruli in Nep25+/DTR+ than in Nep25+/DTR− mice.
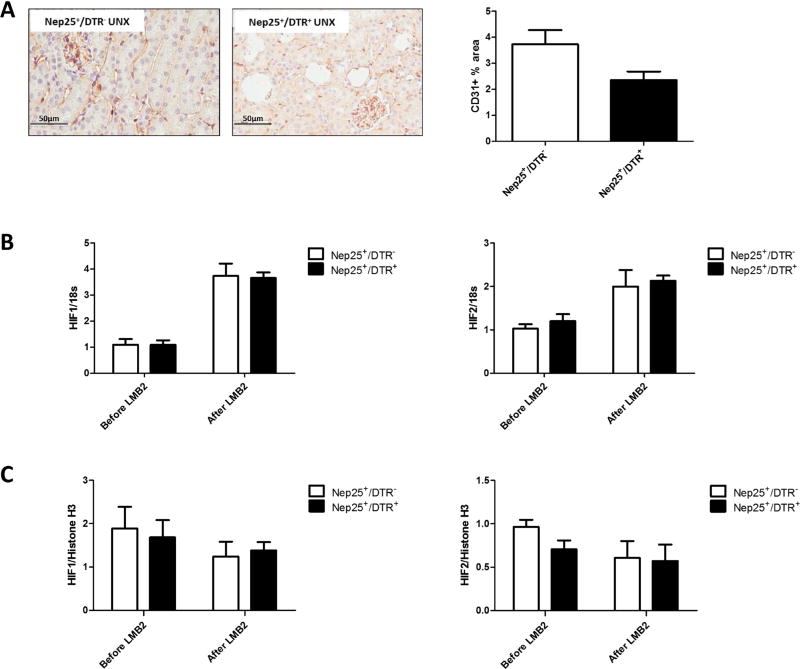
Before LMB2 injection, peritubular capillary density was only numerically reduced in Nep25+/DTR+ vs Nep25+/DTR− mice (Figure 4A). HIF1 and HIF2 mRNAs were increased similarly in Nep25+/DTR+ vs Nep25+/DTR− mice after LMB2 toxin (Figure 4B). HIF1 and HIF2 activity, measured by nuclear HIF1 and HIF2 protein levels, were not different in the groups at sacrifice (Figure 4B).
Fig 4.
Changes of peritubular capillary density and hypoxia-related factors in Nep25+/DTR− and Nep25+/DTR+ mice kidneys at week 6 after DT injection. (A) Peritubular capillary density, detected by CD31 staining, was only numerically reduced in Nep25+/DTR+ vs Nep25+/DTR− mice. (B) HIF1 and HIF2 mRNA increased after LMB2 induced glomerular injury, but were similar in Nep25+/DTR+ vs Nep25+/DTR− mice both before and after LMB2. (C) Nuclear HIF1 and HIF2 proteins were not changed by LMB2, with no difference between groups.
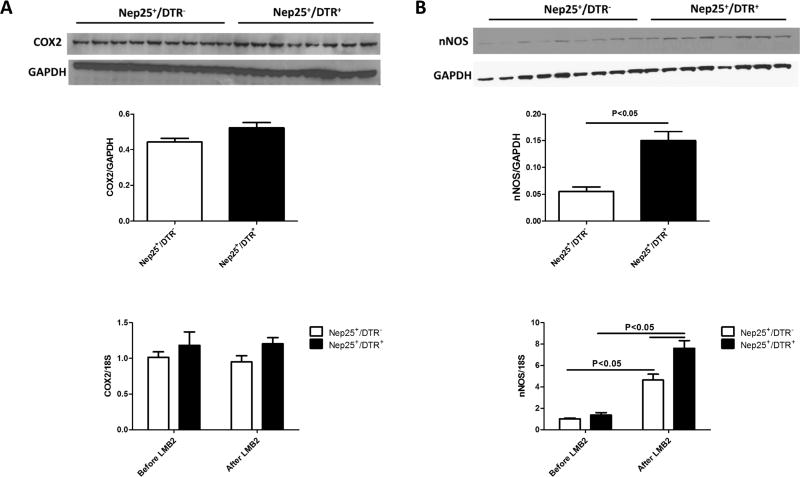
We next assessed two key modulators of tubuloglomerular feedback at the macula densa. COX2, assessed by real-time PCR and Western blot, was not significantly different in Nep25+/DTR+ vs Nep25+/DTR− mice at sacrifice (Figure 5A). In contrast, nNOS mRNA increased significantly after LMB2 injection in both groups, with higher nNOS mRNA in Nep25+/DTR+ vs Nep25+/DTR− mice with corresponding increases in protein level by Western blot (Figure 5B).
Fig 5.
COX2 and nNOS in kidney cortex in Nep25+/DTR− and Nep25+/DTR+ mice kidneys at week 6 after DT injection. (A) Nep25+/DTR+ only numerically enhanced COX2 expression vs Nep25+/DTR− mice. (B) nNOS, a key regulator of tubuloglomerular feedback at the macula densa, was stimulated by LMB2 injection, and both its mRNA and protein level were higher in Nep25+/DTR+ than Nep25+/DTR− mice after LMB2 injection.
Taken together, these data indicate that tubular injury induces increased atubular glomeruli and abnormal nNOS expression in maculae densae, which could increase glomerular susceptibility to subsequent additional injury.
Discussion
In this study, we investigated the effect of preexisting tubulointerstitial fibrosis on subsequent glomerular injury. Tubule-specific injury and glomerulus (podocyte)-specific injury were induced serially, using mice expressing two different toxin receptors on their proximal tubular epithelial cells (DTR) and podocytes (Nep25), respectively.10–12 Acute tubular injury induced by DT was allowed to recover clinically in Nep25+/DTR+ mice. Although the resulting tubulointerstitial fibrosis was very mild, the subsequent glomerular injury, induced by injection of a separate podocyte-specific toxin, was more severe in these Nep25+/DTR+ mice vs Nep25+/DTR− mice without such preexisting tubular injury.
The transition of AKI to CKD has significant clinical relevance. The possible mechanisms have been summarized in previous reviews.13, 14 In our study, we have shown that even mild tubular injury can sensitize to subsequent glomerular injury, and thus tubular injury influences and even spreads to the glomerulus. This process could involve several different pathways. First, tubulointerstitial hypoxia caused by peritubular capillary loss stimulates fibrogenesis with increased collagen I and α-smooth muscle actin, indicators of increased myofibroblasts.15, 16 Hypoxia-inducible factor (HIF)-2α target genes are upregulated in sclerosing glomeruli and there is potential signaling interaction between TGF-β and HIFs to promote renal fibrogenesis, even in normoxia.17 However, in the current study, peritubular capillary loss and HIF activation did not differ significantly between the two groups, and thus neither appears to be a major mediator of the increased glomerular injury observed.
Second, proximal tubule injury can cause atubular glomeruli that can make the kidney more sensitive to podocyte-specific injury. In many disease conditions, atubular glomeruli are associated with decreased glomerular filtration rate and disease progression.18 In the unilateral ureteral obstruction (UUO) model, proximal tubular cells became flattened, lost their affinity for Lotus lectin, and the glomerulotubular junctions became stenotic and atrophic due to cell death by apoptosis and autophagy, with concomitant remodeling of Bowman's capsule, resulting in atubular glomeruli. Although atubular glomeruli remained perfused, renin immunostaining was markedly increased along afferent arterioles, and associated maculae densae disappeared.19 In the current study, atubular glomeruli were increased in Nep25+/DTR+ vs Nep25+/DTR− mice, even before podocyte-specific injury. These atubular glomeruli could contribute to sensitization of the kidney to a second injury. Of note, the connected glomeruli in Nep25+/DTR+ mice showed significantly more severe sclerosis than connected glomeruli in Nep25+/DTR− mice, whereas atubular glomeruli had only numerically more sclerosis in the mice with preceding tubulointerstitial fibrosis vs those without such injury. Thus, the increased atubular glomeruli may lead to more sclerosis in connected glomeruli.
Third, tubular injury can stimulate maculae densae, which can induce classic tubuloglomerular feedback (TGF), and enhance profibrogenic responses to injury. Tubuloglomerular feedback is a well-known physiologic crosstalk mechanism between tubules and glomeruli, inversely regulating glomerular filtration rate according to intratubular salt concentration. 20, 21 Diabetic nephropathy shows glomerular hyperfiltration due to inappropriate TGF. Patients with diabetic nephropathy treated with a novel sodium glucose cotransporter 2 inhibitor show reduced renal hyperfiltration and decreased progressive kidney disease and cardiovascular mortality, postulated to be at least in part due to change in tubular signaling and inhibition of pathogenic TGF.22, 23 Upregulation of tubular nNOS, COX-2, and renin expression precedes, and continues after the initial manifestation of glomerulosclerotic damage in the Fawn- hooded hypertensive rat model.24 Thus, tubuloglomerular feedback can activate local paracrine mediators of glomerular disease. Because this mechanism is through local activation at the macula densa, the injury effect is expected to also be local. Our data show prominent increase of nNOS protein in kidneys of Nep25+/DTR+ vs Nep25+/DTR− mice. Of note, nNOS in the kidney originates only from maculae densae, and thus we postulate that these findings reflect maculae densae activation. Classic tubuloglomerular feedback, with afferent arteriolar vasoconstriction, is mediated by adenosine. nNOS has also been shown by Navar and others to modulate the vasoconstriction since NO is a vasodilator.25 Our results thus likely reflect failure of normal TG feedback, with persistent arteriolar vasodilation, and consequently glomerular pressure is not being appropriately modulated, similar to what occurs with hyperfiltration injury.
Fourth, tubular injury can induce expression of profibrotic cytokines. In turn, the activation of infiltrating cells results in further increase of the tubulointerstitial cytokine pool. Resident fibroblasts proliferate in response to e.g. platelet-derived growth factor, epithelial growth factor, and TGF-beta, finally resulting in their transformation to myofibroblasts.26 However, our data showed no difference of cytokine activation or myofibroblast formation in Nep25+/DTR− and Nep25+/DTR+ mice, indicating this mechanism was not a major contributor to the enhanced glomerular injury.
A previous study of mice expressing simian DTR on mesenchyme-derived renal epithelial cells, with repeated injection of sublethal doses of DT, also induced highly selective acute proximal tubular injury and subsequent tubulointerstitial fibrosis.7 These authors also observed that this repeated injury eventually induced glomerulosclerosis, and the degree of tubulointerstitial fibrosis correlated with the extent of sclerotic glomeruli. The authors postulated impaired glomerular blood flow due to capillary loss, paracrine signaling from injured tubular epithelial cells, and development of atubular glomeruli as possible mechanisms of this correlation. However, a causal relationship of these injuries could not be deduced from this study design. In our model, we used Ggt1 as a promoter, resulting in more specific expression of DTR on proximal tubular cells than in the previous study, where DTR was expressed on podocytes in addition to tubules. Thus, repeated DT injection likely also caused direct glomerular injury in those studies. In other experimental studies, injury was induced only in proximal tubules, using a transgenic mouse with DT receptor on proximal tubules. Mild acute tubular injury induced reversible fibrosis, whereas repeated DT injections caused persistent fibrosis, spread of injury to distal tubules and even glomerulosclerosis, with atubular glomeruli.27 However, these studies did not examine the effects and mechanisms of a second specific glomerular hit after initial tubule- specific injury. In contrast, our study design is characterized by sequential induction of podocyte-specific injury after establishing tubule-specific injury. Moreover, although discrete tubulointerstitial fibrosis developed after recovery from acute tubular injury at week 6, the degree of tubulointerstitial injury was very mild, and yet significantly enhanced subsequent glomerular injury.27
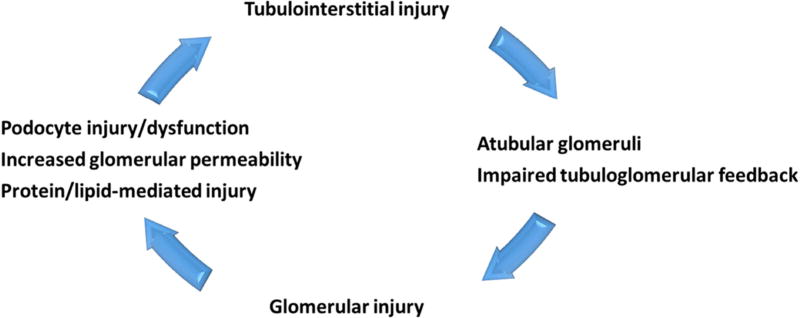
In conclusion, preexisting, even mild, tubulointerstitial injury sensitized glomeruli to subsequent podocyte-specific injury. Our study supports that increased atubular glomeruli and abnormal tubuloglomerular feedback significantly contribute to the crosstalk between the tubulointerstitium and glomeruli. These findings have important implications for progressive kidney disease. Classically, the remnant nephron theory has focused on glomerulosclerosis resulting in increased hemodynamic, and likely other stressors on remnant glomeruli, resulting in more sclerosis and completing a vicious cycle. Our data point to a key role of tubulointerstitial injury and even mild fibrosis in initiating and even perpetuating a novel vicious cycle, whereby injury to the tubules sets in motion enhanced susceptibility to a second hit on the glomerulus. The existing glomerulosclerosis then further promotes increased tubulointerstitial fibrosis (Figure 6). These data suggest that effective therapies in CKD should aim to interdict this deleterious crosstalk, and protect fibrosis and promote its regression, in addition to podocyte/glomerulosclerosis protection strategies.
Fig 6.
Schema for a proposed vicious cycle of tubulointerstitial and glomerular injury.
Methods
Animals
Nep25 mice (C57 bl/6 background), that express human CD25 on podocytes, were mated with DTR mice (C57 bl/6 background), which express γ-glutamyl transferase 1 diphtheria toxin receptor (Ggt1 DTR) on proximal tubular epithelial cells. Eight male double transgenic (Nep25+/DTR+ mice) and 9 male mice with only the Nep25 transgene (Nep25+/DTR− mice) were generated. Human diphtheria toxin (DT, Sigma, 100 ng/kg Bwt i.p.) was injected at age 10 weeks in all mice. At week 6 after the first injection (age 16 weeks), uninephrectomy was performed to evaluate tubulointerstitial injury. LMB2 was then given (8 ng/g Bwt i.v.) at week 7. All mice were sacrificed at age 19 weeks, 9 weeks after DT injection and 10 days after LMB2 injection.
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Vanderbilt University. Mice were housed under normal conditions with 20°C, 12-hour light/dark cycle, with free access to normal rodent chow (#5001, LabDiet, MO, USA) and water.
Analysis of Kidney Function
Body weight was measured at week 6 and at sacrifice. Spot urine protein to creatinine ratio (PCR) was assessed at weeks 0, 6 (before LMB2 injection) and at sacrifice by ELISA using the Pierce BCA protein assay kit (Thermo Scientific, IL, USA) and Creatinine assay kit (Abcam, MA, USA), respectively, according to the manufacturer’s instruction. Urinary albumin was determined using Albuwell M kits (Exocell, PA, USA). BUN levels were determined using the QuantiChromTM Urea Assay Kit (DIUR-500) (BioAssay Systems, Hayward, CA, USA).
Tubular injury was monitored by urinary neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (Kim-1) excretion in spot urine at baseline, week 2 (after DT), 6 (before LMB2) and at sacrifice. NGAL and Kim-1 were measured by ELISA kits (R&D Systems, MN, USA) according to the manufacturer’s instruction. The values were normalized by urinary creatinine concentration.
Morphological Assessment
Tubulointerstitial injury, assessing vacuolization, blebbing, and loss of brush border of uninephrectomized kidneys from week 6, was examined on periodic acid-Schiff (PAS) and Masson’s trichrome stained slides. For quantitative assessment of tubulointerstitial fibrosis, 3µm sections of kidney were stained with picrosirius red (0.1% Sirius Red in saturated picric acid) for 16 h, followed by 0.5% acetic acid X2, dehydrated in 100% ethanol X3, and coverslip mounting. Sections were examined by polarized light microscopy. Sirius red positive polarized area in the cortex was measured by image analyzing software (Axiovision, Carl Zeiss, CA, USA) and represented as % positive area.
Glomerular damage in kidneys was evaluated on PAS stained section by scoring the degree of glomerulosclerosis. Each glomerulus was scored from 0 to 4 according to the extent of sclerotic lesion (0; no lesion, 1; sclerosis of <25% of the glomerulus, 2; 25 to 50%, 3; 50 to 75%, and 4; >75%). The final score of each mouse was the mean of scores of all glomeruli on one kidney section.
CD31 staining was measured by image analyzing software (Axiovision) and represented as % positive area. WT-1 was assessed as number of positive nuclei per glomerular area.
Atubular glomerulus counting was performed on 7µm kidney sections. Sections at the midpoint of the series (section #6 or 7 of a total of 12 sections) were examined, and all glomeruli identified on this index section were traced through the series. Glomeruli in normal versus atubular glomeruli were readily identified by the presence of connected proximal tubules by Lotus lectin staining (see below). Atubular glomeruli were those that were included entirely within the section series and showed no connection to a proximal tubule (Figure 3A). 60 to 90 glomeruli were assessed per mouse by this approach. After glomeruli were identified as connected or atubular glomeruli, WT-1 and sclerosis index were then scored individually on a central section.
Samples of kidney cortex were routinely processed for examination with transmission electron microscopy (EM). EM study was performed in five animals from each group, assessing 1–2 glomeruli for each animal. Areas of denuded GBM with podocyte stripping and foot process effacement were semi-quantified.
Total Collagen Analysis
Relative collagen content as a percentage of total protein was calculated from the concentration of hydroxyproline, measured from portions of frozen kidney cortex (Quickzyme Bioscience, Leiden, Netherlands). Tissue (50–300mg) homogenates were hydrolyzed in Eppendorf tubes in constant boiling 6 M HCl (95°C, 20 hours). Tubes were centrifuged for 10 min at 13,000 rpm, and the supernatant assayed for collagen and total protein using an ELISA method according to the manufacturer’s instruction.
Western Blot
Frozen kidney tissue was transferred in RIPA plus buffer containing 150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5 mmol/L ethylenediamine tetraacetic acid, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 100 µg/ml phenylmethyl sulfonyl fluoride, 1:100 phosphatase inhibitor cocktail I, 1:100 phosphatase inhibitor cocktail II (Roche Diagnostics GmbH, Mannheim, Germany), 1:100 proteinase inhibitor cocktail tablet (Roche Diagnostics GmbH). Nuclear protein was extracted using Nuclear Extraction Kit (Abcam, Cambridge, MA, USA). Tissue samples were homogenized in 1.5ml of homogenizer on ice, and centrifuged at 13,000 rpm for 15 minutes at 4°C. The protein concentration was measured using Pierce BCA protein assay kit (Thermo Scientific). Eighty µg of protein were loaded and separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a 0.2µmol/L nitrocellulose membrane. Cyclo-oxygenase 2 (COX-2), neuronal nitric-oxide synthase (nNOS) and nuclear HIF-1/2 were detected using specific rabbit anti-COX-2 polyclonal antibody (Cayman Chemical, Michigan, USA), rabbit anti-nNOS polyclonal antibody (Cell Signaling Technology, MA, USA), rabbit anti-HIF-1α polyclonal antibody (Novas, CO, USA) and rabbit anti-HIF-2α (Novas, CO, USA) overnight at 4°C. After washing in Tris-buffered saline with 0.1% Tween 20 (TBS-T), horse radish peroxidase-labeled donkey anti-rabbit IgG secondary antibody (1:3,000 dilution in 5% milk TBS-T) was added and incubated at room temperature for 1hr. Protein bands on Western blots were visualized by ECL Plus (Amersham, IL, USA) according to the manufacturer’s instructions and were developed on film. The membranes were stripped with 100 mmol/L β-mercaptoethalnol, 2% sodium dodecyl sulfate, 62.5 mmol/L Tris- HCl, pH 6.7. GAPDH was detected using mouse anti-β-actin (Santa Cruz Biotechnology, TX, USA). The levels of COX-2 and nNOS expression were expressed relative to GAPDH.
Immunohistochemistry
Kidneys from uninephrectomy and sacrifice were routinely processed, and 3µ m sections were stained for Wilms’ tumor-1 antigen (WT-1), Lotus lectin, and CD31 to assess glomerular matrix, podocyte injury, atubular glomeruli, and peritubular capillary density, respectively. Briefly, antigen retrieval was performed using microwave (750W, 5 min×3) for WT-1, Lotus lectin, and CD31, and trypsin (Sigma Aldrich, St Louis, MO, USA) for collagen IV. Endogenous peroxidase was quenched by hydrogen peroxide. Nonspecific epitopes were blocked by blocking solution (Vector Laboratories, Burlingame, CA, USA). Primary antibodies were incubated overnight at 4°C by adding rabbit anti-mouse collagen IV (1:500, EMD Millipore, Darmstadt, Germany), rabbit anti-mouse WT-1 antibody (1:200, Novus Biologicals, CO, USA), biotinylated lotus tetragonolobus lectin (1:1000, Vector Laboratories, CA, USA), and rat anti-mouse CD31 (1:50, Dianova GmbH, Hamburg, Germany). The Vectastain kit (Vector Laboratories) was used as a secondary antibody for 30 min, according to primary antibody host. Diaminobenzidine (DAB) was used as a chromogen, and sections were counterstained with hematoxylin. Slides treated with nonspecific antisera instead of primary antibody were used as negative control, and known positive tissues were used as positive controls. All slides were examined without knowing group assignment.
Statistical Analysis
All data are presented as mean ± standard error of the mean. Analysis of variance (ANOVA) and post-hoc Tukey test were used to evaluate differences between the groups. Nonparametric Mann-Whitney U test was used for between-group comparisons when data were not normally distributed. All tests were two-tailed, and p < 0.05 was considered statistically significant.
Acknowledgments
This work was supported in part by NIH NIDDK DK56942-09 (Agnes B. Fogo), DK095785, DK62794 (Raymond C. Harris, Ming-Zhi Zhang) and funds from a VA Merit grant (Raymond C. Harris).
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (Ira Pastan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Motoyoshi Y, Matsusaka T, Saito A, et al. Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int. 2008;74:1262–1269. doi: 10.1038/ki.2008.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eddy AA. Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol. 1994;5:1273–1287. doi: 10.1681/ASN.V561273. [DOI] [PubMed] [Google Scholar]
- 3.Christensen EI, Verroust PJ. Interstitial fibrosis: tubular hypothesis versus glomerular hypothesis. Kidney Int. 2008;74:1233–1236. doi: 10.1038/ki.2008.421. [DOI] [PubMed] [Google Scholar]
- 4.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 5.Theilig F. Spread of glomerular to tubulointerstitial disease with a focus on proteinuria. Ann Anat. 2010;192:125–132. doi: 10.1016/j.aanat.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Heung M, Chawla LS. Acute kidney injury: gateway to chronic kidney disease. Nephron Clin Pract. 2014;127:30–34. doi: 10.1159/000363675. [DOI] [PubMed] [Google Scholar]
- 7.Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatachalam MA, Weinberg JM, Kriz W, et al. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26:1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol. 2012;32:452–462. doi: 10.1016/j.semnephrol.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldwich A, Steinkasserer A, Gessner A, et al. Impairment of podocyte function by diphtheria toxin--a new reversible proteinuria model in mice. Lab Invest. 2012;92:1674–1685. doi: 10.1038/labinvest.2012.133. [DOI] [PubMed] [Google Scholar]
- 11.Sekine M, Monkawa T, Morizane R, et al. Selective depletion of mouse kidney proximal straight tubule cells causes acute kidney injury. Transgenic Res. 2012;21:51–62. doi: 10.1007/s11248-011-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsusaka T, Xin J, Niwa S, et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16:1013–1023. doi: 10.1681/ASN.2004080720. [DOI] [PubMed] [Google Scholar]
- 13.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferenbach DA, Bonventre JV. Kidney tubules: intertubular, vascular, and glomerular cross-talk. Curr Opin Nephrol Hypertens. 2016;25:194–202. doi: 10.1097/MNH.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eardley KS, Kubal C, Zehnder D, et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74:495–504. doi: 10.1038/ki.2008.183. [DOI] [PubMed] [Google Scholar]
- 16.Palm F, Nordquist L. Renal tubulointerstitial hypoxia: cause and consequence of kidney dysfunction. Clin Exp Pharmacol Physiol. 2011;38:474–480. doi: 10.1111/j.1440-1681.2011.05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna C, Hubchak SC, Liang X, et al. Hypoxia-inducible factor-2alpha and TGF-beta signaling interact to promote normoxic glomerular fibrogenesis. Am J Physiol Renal Physiol. 2013;305:F1323–1331. doi: 10.1152/ajprenal.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol. 2008;19:197–206. doi: 10.1681/ASN.2007080862. [DOI] [PubMed] [Google Scholar]
- 19.Forbes MS, Thornhill BA, Chevalier RL. Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: a new look at an old model. Am J Physiol Renal Physiol. 2011;301:F110–117. doi: 10.1152/ajprenal.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araujo M, Welch WJ. Cyclooxygenase 2 inhibition suppresses tubuloglomerular feedback: roles of thromboxane receptors and nitric oxide. Am J Physiol Renal Physiol. 2009;296:F790–794. doi: 10.1152/ajprenal.90446.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh P, Thomson SC. Renal homeostasis and tubuloglomerular feedback. Curr Opin Nephrol Hypertens. 2010;19:59–64. doi: 10.1097/MNH.0b013e3283331ffd. [DOI] [PubMed] [Google Scholar]
- 22.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 23.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 24.Weichert W, Paliege A, Provoost AP, et al. Upregulation of juxtaglomerular NOS1 and COX-2 precedes glomerulosclerosis in fawn-hooded hypertensive rats. Am J Physiol Renal Physiol. 2001;280:F706–714. doi: 10.1152/ajprenal.2001.280.4.F706. [DOI] [PubMed] [Google Scholar]
- 25.Ichihara A, Navar LG. Neuronal NOS contributes to biphasic autoregulatory response during enhanced TGF activity. Am J Physiol. 1999;277:F113–120. doi: 10.1152/ajprenal.1999.277.1.F113. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl. 2005:S82–86. doi: 10.1111/j.1523-1755.2005.09915.x. [DOI] [PubMed] [Google Scholar]
- 27.Takaori K, Nakamura J, Yamamoto S, et al. Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015060647. [DOI] [PMC free article] [PubMed] [Google Scholar]