Abstract
Background
As recurrence is high following pulmonary metastasectomy (PM) for soft tissue sarcoma (STS), repeat PM is commonly performed. Our objective was to define the selection criteria for repeat PM among patients experiencing recurrence and to identify factors associated with survival.
Methods
We reviewed a prospectively-maintained database of 539 patients undergoing PM for STS. Characteristics of the primary tumor, metastatic disease, treatment, and recurrence were examined. Multivariable Cox models were constructed to identify factors associated with the likelihood of operative selection following recurrence. Overall survival between patients with or without repeat PM was estimated using the Kaplan-Meier method, with prognostic factors identified using Cox models. Both analyses incorporated propensity score matching weights. Factors associated with survival after repeat PM were assessed with multivariable Cox models among those who underwent repeat PM.
Results
Following initial PM, 63% of patients (n=341) experienced pulmonary recurrence; 141 (41%) underwent repeat PM. Patients who were younger (p=0.033), underwent minimally invasive resection at first PM (p=0.041), had a longer disease-free interval following first PM (p=0.009), were without extrapulmonary disease (p<0.001), and had fewer nodules on recurrence (p<0.001) were more likely to undergo repeat PM. Comparison between the repeat and non-repeat PM groups demonstrated an increased hazard of death among patients managed nonoperatively. Factors associated with an increased hazard of death following second PM included preoperative chemotherapy (p=0.008) and R1/R2 metastasectomy (p<0.001).
Conclusions
Although operative selection occurs, when prognostic factors are controlled for, repeat PM for STS remains independently associated with prolonged overall survival.
Up to 50% of patients with soft tissue sarcoma (STS) develop lung metastases [1–5]. Effective systemic treatment for metastatic STS is limited [6]. Therapeutic pulmonary metastasectomy (PM) has been selectively used, with an associated survival benefit over no surgery, based on Level II evidence. Guidelines for the use of pulmonary metastasectomy do not currently exist [7].
Treatment of STS is further complicated by high recurrence rates following initial PM, as >50% of patients develop lung recurrence [1, 3, 8, 9]. A subset of these patients is selected for repeat PM, with the potential to undergo several operations during their disease course [10]. In the International Registry of Lung Metastases study, 33% of patients with sarcoma examined (n=642) underwent a second PM [8]. Data specific to repeat PM, however, are limited. Several studies document a longer median overall survival (OS) with an increasing number of metastasectomies [3, 8, 11–14]. Repeat PM has thus been considered an acceptable treatment for recurrent disease [3, 15]. Direct comparison between single and multiple PMs, however, has been criticized due to differences within patient characteristics in each of these groupings [16]. In particular, data on nonoperatively managed patients have been scarce [17]. Therefore, the presumed survival benefit ascribed to repeat resection has been questioned.
The objectives of this study are to (1) identify factors associated with selection for repeat PM among patients experiencing pulmonary recurrence; (2) compare OS between patients with pulmonary recurrence who undergo repeat PM and patients who do not have surgery, with a consideration of the differences in clinicopathologic variables at presentation; and (3) identify factors associated with OS among patients who undergo repeat PM.
PATIENTS AND METHODS
Patient Selection
A database of patients undergoing PM for primary STS is prospectively maintained at Memorial Sloan Kettering Cancer Center (MSK). We identified 539 patients undergoing PM with therapeutic intent for STS between September 1991 and June 2014 and have previously reported on these patients [18]. All data were collected with approval of the Institutional Review Board.
Clinicopathologic Variables
Patient demographics, primary and metastatic disease characteristics, and details of treatment, recurrence, and survival were collected. Histologic subtype, size, and grade of the primary tumor were verified by review of pathology reports. Operative reports were reviewed to identify surgical access (open or minimally invasive), as well as extent of resection (wedge resection, lobectomy, or pneumonectomy). The number of metastases identified and/or resected and “R” status at the initial PM were obtained from operative and pathologic reports. Data on chemotherapy were obtained through review of clinical documentation.
Recurrence Following Initial PM
After their initial metastasectomy, patients were monitored with periodic clinical examinations and imaging. Recurrence was defined as the first documentation of disease following initial R0 PM. Upon lung recurrence, data on synchronous extrapulmonary disease and the number of clinical lung metastases were collected. The Karnofsky Performance Status (KPS) scale, used to assess patient fitness, was obtained through documentation during office visits. For patients undergoing repeat PM, treatment details and outcomes were obtained.
Statistical Analyses
Clinicopathologic variables for patients undergoing repeat PM and those not were compared using Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. Cox proportional hazards models were constructed to identify factors associated with the probability of undergoing repeat PM. Time to event was measured from the time of recurrence to the date of repeat PM; otherwise it was censored on the date of last follow-up. Variables with a significant association in univariable analyses (p≤0.05) and clinically relevant were included in a multivariable Cox model.
Comparison of OS between patients who underwent a second PM and those who did not required attention to factors associated with selection. To address this, we used the matching weights method [19]. This approach is a weighting analogue to the 1:1 pair matching method, although shown to be more efficient, that provides better balance across covariates. Unlike 1:1 pair matching, which excludes any unmatched patients, the matching weights approach never discards any patients; instead, it only down-weights some of the patients. The matching weights approach is a variant of the inverse probability weights method; the matching weights can be considered the probability of being selected to the matched data set. With the application of the patient-level matching weights, each patient contributes a fraction of itself to the overall cohort used in the analyses.
Variables for inclusion in the logistic model were selected based on clinical factors associated with the likelihood of undergoing repeat PM. These included age at diagnosis of the primary tumor; age at first PM; sex; histologic subtype, site, and grade of the primary tumor; surgical access; extent of resection; duration between the first PM and relapse; synchronous extrapulmonary recurrence; number of recurrent nodules; and KPS scale. Performance of the matching-weight approach was assessed through evaluation of the standardized mean differences in variables between the two groups. Covariate balance was confirmed by an absolute standardized mean difference (ASMD) ≤ 0.1 for each variable following the application of matching weights. The distribution of propensity scores before and after application of the matching weights is presented as a mirror histogram, to visually assess the success of the approach [19]. Overall survival was measured from the time of first pulmonary recurrence to the time of death, or censored at the time of last follow-up. OS was estimated by the Kaplan-Meier approach and compared between groups by the log-rank test, with the application of matching weights. In addition, the association between receiving repeat PM and death was assessed using Cox models. The matching weights were incorporated as weights at the patient-level, and the second PM status was considered a time-varying covariate, where all patients initially begin in the non-repeat PM group at the time of recurrence and switch over to the repeat PM group on the date of the second PM.
Factors associated with the hazard of death following repeat PM were assessed with Cox models among patients who received repeat PM. The time to event was measured from the time of the second PM to the time of death or was censored on the date of the last follow-up. Variables that were significant in the univariable analyses (p≤0.05) and clinically relevant variables were included in a multivariable model. All analyses were conducted in R 3.1.1 (R Development Core Team, Vienna, Austria). The matching weight procedure was performed with the survey and tableone packages. All statistical tests were two-sided, and p<0.05 was considered statistically significant.
RESULTS
Patient Characteristics
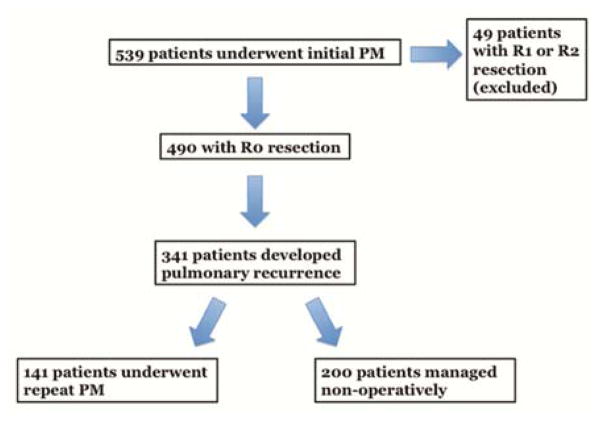
Between September 1991 and June 2014, 539 patients underwent 760 PMs with therapeutic intent for STS at MSK. Following R0 resection, 63% of the cohort (n=341) experienced pulmonary recurrence. Of these, 141 underwent repeat PM. Thirty-three patients undergoing repeat PM had bilateral disease, and of these, 14 were staged resections. The remaining 200 patients did not undergo subsequent PM (Fig. 1). Median follow-up was 23.9 months (interquartile range [IQR], 11.2 – 44.4 months) from initial PM. Table 1 demonstrates baseline clinicopathologic characteristics of these two groups as well as details on recurrence. Patients in the repeat PM group were younger (p<0.001), and a larger percentage of these patients underwent a minimally invasive procedure at their initial surgery (p=0.002). Patients undergoing repeat PM also had longer disease-free intervals (DFIs) from their initial PM (p<0.001), fewer pulmonary nodules at recurrence (p<0.001), lower occurrence of synchronous extrapulmonary metastases (p<0.001), and higher overall fitness, represented by KPS scale (p<0.001).
Figure 1.
Consort diagram for inclusion into analysis. PM, pulmonary metastasectomy.
Table 1.
Clinicopathologic Characteristics in Patients with Pulmonary Recurrence Based on Subsequent Treatment
| Variable | No Surgery n=200 (%) | Repeat PM n=141 (%) | P |
|---|---|---|---|
| Sex | 0.179 | ||
| Female | 126 (63.0) | 78 (55.3) | |
| Male | 74 (37.0) | 63 (44.7) | |
| Age at diagnosis of primary tumora | 53.0 (41.6, 62.4) | 48.0 (36.0, 56.7) | <0.001 |
| Histologic subtype of primary tumor | 0.002 | ||
| PS/MFH | 59 (29.5) | 21 (14.9) | |
| Synovial | 21 (10.5) | 32 (22.7) | |
| Leiomyosarcoma | 71 (35.5) | 42 (29.8) | |
| Liposarcoma | 10 (5.0) | 9 (6.4) | |
| Fibrosarcoma | 8 (4.0) | 14 (9.9) | |
| MPNST | 5 (2.5) | 4 (2.8) | |
| Other | 26 (13.0) | 19 (13.5) | |
| Site of primary tumor | 0.087 | ||
| Extremity | 96 (48.0) | 71 (50.4) | |
| Trunk | 25 (12.5) | 13 (9.2) | |
| Retroperitoneal/abdomen/pelvis | 24 (12.0) | 10 (7.1) | |
| Visceral-GU/GYN | 51 (25.5) | 37 (26.2) | |
| Head and neck | 4 (2.0) | 10 (7.1) | |
| Size of primary tumor | 0.107 | ||
| ≤10 cm | 97 (48.5) | 75 (53.2) | |
| >10 cm | 90 (45.0) | 50 (35.5) | |
| Unknown | 13 (6.5) | 16 (11.3) | |
| Grade of primary tumor | 0.230 | ||
| Low | 16 (8.0) | 18 (12.8) | |
| High | 180 (90.0) | 122 (86.5) | |
| Unknown | 4 (2.0) | 1 (0.7) | |
| Interval to first PM | 0.128 | ||
| Synchronous | 29 (14.5) | 17 (12.1) | |
| <12 months | 74 (37.0) | 40 (28.4) | |
| ≥12 months | 97 (48.5) | 84 (59.6) | |
| Type of surgery at first PM | 0.002 | ||
| Open | 155 (77.5) | 87 (61.7) | |
| Minimally invasive | 45 (22.5) | 54 (38.3) | |
| Extent of resection at first PM | 0.064 | ||
| Wedge | 151 (75.5) | 121 (85.8) | |
| Lobectomy | 45 (22.5) | 18 (12.8) | |
| Pneumonectomy | 4 (2.0) | 2 (1.4) | |
| Disease-free intervalb | <0.001 | ||
| <12 months | 175 (87.5) | 98 (69.5) | |
| ≥12 months | 25 (12.5) | 43 (30.5) | |
| Number pulmonary nodules at recurrence | <0.001 | ||
| 1 | 42 (21.0) | 70 (49.6) | |
| 2 | 25 (12.5) | 21 (14.9) | |
| 3 | 43 (21.5) | 15 (10.6) | |
| 4 | 24 (12.0) | 6 (4.3) | |
| 5 | 7 (3.5) | 1 (0.7) | |
| >5 | 42 (21.0) | 20 (14.2) | |
| Unknown | 17 (8.5) | 8 (5.6) | |
| Synchronous extrapulmonary disease at recurrence | <0.001 | ||
| No | 74 (37.0) | 110 (78.0) | |
| Yes | 126 (63.0) | 31 (22.0) | |
| KPS scale at recurrence | <0.001 | ||
| ≤70 | 18 (9.0) | 0 (0.0) | |
| 80 | 45 (22.5) | 12 (8.5) | |
| 90 | 77 (38.5) | 71 (50.4) | |
| 100 | 19 (9.5) | 15 (10.6) | |
| Unknown | 41 (20.5) | 43 (30.5) |
GU, genitourinary; GYN, gynecological; MPNST, malignant peripheral nerve sheath tumor; PM, pulmonary metastasectomy; PS/MFH, pleomorphic sarcoma/malignant fibrous histiocytoma; KPS, Karnofsky Performance Status.
Represented as median (25th, 75th percentile)
From first pulmonary metastasectomy to recurrence at any site.
Selection for Repeat PM
We subsequently created multivariable Cox models to identify factors significantly associated with selection for repeat PM (Table 2). Older patients had a lower likelihood of repeat PM (HR: 0.98; 95% CI: 0.97 to 1.00). Minimally invasive resection at the initial PM was associated with an increased likelihood of repeat PM (HR: 1.58; 95% CI: 1.02 to 2.45). Patients with a longer DFI from the initial PM were more likely to be selected for repeat PM (HR: 1.02; 95% CI: 1.00 to 1.04). Patients with a greater number of recurrent pulmonary nodules (HR: 0.73; 95% CI: 0.63 to 0.83) and synchronous extrapulmonary disease (HR: 0.13: 95% CI: 0.08 to 0.23), were less likely to undergo repeat PM. KPS scale (HR: 1.01; 95% CI: 0.99 to 1.06), and receipt of perioperative chemotherapy at initial PM (HR: 0.67; 95% CI: 0.41 to 1.09) were not significantly associated with selection for repeat PM. Of the 200 patients who did not undergo repeat PM, 143 (71.5%) went on to receive chemotherapy.
Table 2.
Multivariable Cox Proportional Hazards Models for Likelihood of Undergoing Repeat Pulmonary Metastasectomy (PM)
| Variable | Hazard Ratio (95% CI) | P |
|---|---|---|
| Age at diagnosis of primary tumor | 0.98 (0.97 to 1.00) | 0.033 |
| Perioperative treatment at initial PM | 0.67 (0.41 to 1.09) | 0.10 |
| Minimally invasive surgery at initial PM | 1.58 (1.02 to 2.45) | 0.041 |
| Disease-free intervala | 1.02 (1.00 to 1.04) | 0.009 |
| No. of pulmonary nodules at recurrence | 0.73 (0.63 to 0.83) | <0.001 |
| Synchronous extrapulmonary disease at recurrence | 0.13 (0.08 to 0.23) | <0.001 |
| KPS scale at recurrence (%) | 1.01 (0.99 to 1.06) | 0.14 |
KPS, Karnofsky Performance Status.
From first pulmonary metastasectomy to recurrence at any site per month.
Propensity Score Matching Weight Analysis of Repeat PM Versus No Surgery
Patients selected for repeat PM had a median OS of 44.9 months from the first recurrence at any site, compared with 14.0 months among patients without repeat PM (p<0.001). As described previously, however, several differences exist between these groups. The matching weight approach was subsequently performed to compare OS between the repeat and non-repeat PM groups among comparable patients, to reduce potential selection bias. Baseline factors pertaining to the primary tumor, characteristics of the index metastasectomy, and clinicopathologic variables on recurrence, as described in the Methods, were used in the calculation of propensity scores and corresponding matching weights for each patient.
The standardized mean differences for each parameter, before and after applying the matching weights, are displayed in Supplemental Table 1. Before applying the matching weights, values for the ASMD were >0.1, confirming known baseline differences between the groups. After applying the matching weights, the ASMD was ≤0.1 for every covariate, indicating balance across all clinically relevant factors (Supplemental Fig. 1). The distribution of propensity scores showed good overlap between the repeat and non-repeat PM groups after application of the matching weights (Supplemental Fig. 2). After applying the matching weights, the effective sample sizes were 68.6 contributions in the non-repeat PM group and 67.7 contributions in the repeat PM group.
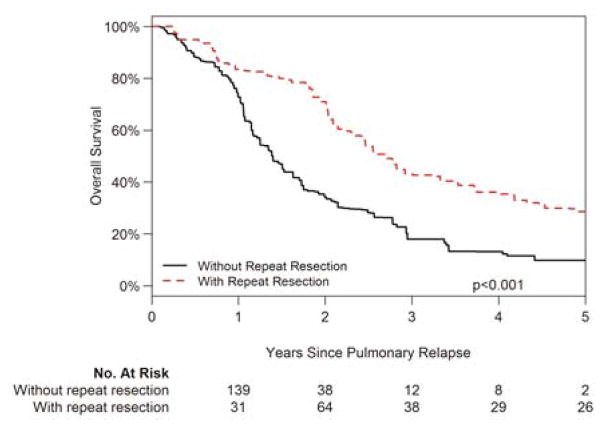
Hazard ratios for the hazard of death from the time of the first recurrence were subsequently estimated incorporating these matching weights. Patients who did not undergo repeat PM had a 2.13-fold greater hazard of death, compared with patients who did (95% CI: 1.39 to 3.26; p<0.001). Thus, when characteristics of the primary tumor and metastatic disease are controlled for, repeat PM remains associated with a survival benefit (Fig. 2).
Figure 2.
Overall survival with recurrent metastatic pulmonary soft tissue sarcoma based on treatment following weight-based propensity matching.
*P value calculated from Cox proportional hazards model incorporating matching weights, and repeat PM is considered a time-dependent variable.
**Considering repeat PM as a time-dependent variable, the number at risk reflects the number of patients who have undergone repeat PM by the indicated time point. The number may increase as patients are selected for repeat PM during their follow-up.
Factors Associated with Overall Survival Following Repeat PM
Data on patients who underwent repeat PM (n=141) are presented in Supplemental Table 2. Leiomyosarcoma and synovial sarcoma were the most commonly represented primary histologic subtypes in the repeat PM group. Thirty-two patients had synchronous extrapulmonary disease. Most patients (n=100) underwent surgery directly; a smaller fraction received chemotherapy before resection. Twenty-three percent of patients (n=33) underwent a minimally invasive procedure for repeat PM, compared with 29% for the initial PM. Wedge resection was the most commonly performed procedure (n=110). A single pulmonary metastasis was resected in sixty-nine patients. R0 resection was achieved in 86% of cases (n=121), which is comparable to the 91% R0 resection rate for first PM.
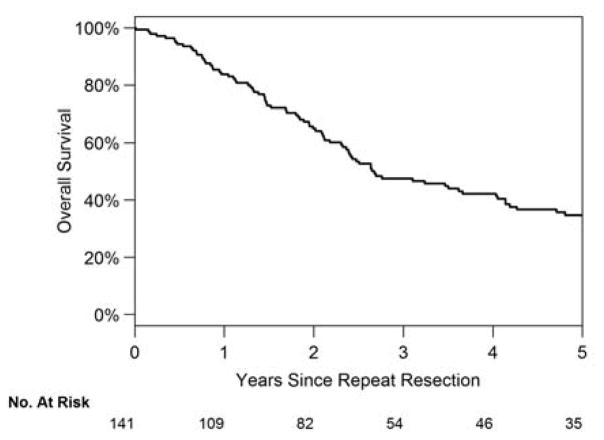
The rate of recurrence remained high, with 98 patients (70%) experiencing a recurrence at any site following an R0 second PM. Ninety patients had lung recurrence following complete resection. Median time to recurrence for these patients was 5.0 months (range, 2.0 to 9.0 months). Median OS for patients undergoing a second PM was 4.1 years from the first PM and 2.7 years from the second PM (Fig. 3). Thirty-eight patients (42%) underwent a third PM.
Figure 3.
Overall survival of patients undergoing repeat pulmonary metastasectomy from second resection.
Log-rank analyses of OS from the second PM demonstrated differences for several clinicopathologic variables. A DFI >12 months from the first PM was associated with an increased median OS of approximately 27 months (p=0.007). Likewise, the initial DFI between resection of the primary lesion and the first PM continued to have a significant association with survival in those undergoing repeat resection. Patients who received preoperative chemotherapy had shorter OS than patients treated with surgery directly (p<0.001). No differences were seen between minimally invasive and open procedures (p=0.055), and the extent of resection was also not significant (p=0.319). Patients with more recurrent nodules generally had poorer survival (p=0.025). Complete resection was associated with an increased median OS of 28 months (p<0.001).
We subsequently constructed a multivariable Cox model for the hazard of death following repeat PM (Table 3). Patients with leiomyosarcoma had a lower hazard of death (HR=0.48; 95% CI, 0.25 to 0.92) than patients with PS/MFH. Primary tumor grade was not associated with OS (p=0.14). Similarly, the DFI from the first PM was not associated with hazard of death (HR: 0.98; 95% CI: 0.96 to 1.00). Patients treated with preoperative chemotherapy had a significantly greater hazard of death (HR: 1.94; 95% CI: 1.19 to 3.18). More metastatic nodules were not associated with a greater hazard of death (HR: 1.13; 95% CI: 0.99 to 1.28). The factor most strongly associated with OS was resection outcome: patients with incomplete PM had a 4-fold greater hazard of death compared to those with complete resection (HR: 4.15; 95% CI: 2.26 to 7.62).
Table 3.
Multivariable Cox Proportional Hazards Models for the Hazard of Death After Repeat Pulmonary Metastasectomy
| Variable | Cox Hazard Ratio (95% CI) | P |
|---|---|---|
| Histologic subtype of primary tumor | ||
| PS/MFHa | 1.00 | — |
| Synovial | 0.65 (0.33 to 1.28) | 0.2 |
| Leiomyosarcoma | 0.48 (0.25 to 0.92) | 0.026 |
| Liposarcoma | 0.42 (0.14 to 1.28) | 0.13 |
| Fibrosarcoma | 0.44 (0.17 to 1.10) | 0.077 |
| MPNST | 0.70 (0.16 to 3.13) | 0.6 |
| Other | 1.04 (0.43 to 2.55) | 0.9 |
| High-grade primary tumor | 1.89 (0.81 to 4.38) | 0.14 |
| Disease-free intervalb | 0.98 (0.96 to 1.00) | 0.11 |
| Preoperative chemotherapy | 1.94 (1.19 to 3.18) | 0.008 |
| No. of pulmonary nodules | 1.13 (0.99 to 1.28) | 0.073 |
| Incomplete (R1/R2) resection | 4.15 (2.26 to 7.62) | <0.001 |
PS/MFH, pleomorphic sarcoma/malignant fibrous histiocytoma; MPNST, malignant peripheral nerve sheath tumor.
Reference group.
From first pulmonary metastasectomy to recurrence at any site per month.
COMMENT
Repeat PM is commonly used for several tumor types [3, 13, 20–23]. A survey of 146 members of the European Society of Thoracic Surgeons found that 53% of respondents would not place a limit on the number of repeat metastasectomies they would perform. [24]. A frequent criticism of the positive relationship between survival and number of PMs is the inability to differentiate the effect of selection bias from treatment benefit [16, 17, 25]; however, level I evidence of this association remains lacking.
Factors associated with selection for repeat PM in our study have been shown to be associated with improved survival in patients undergoing PM [1, 8, 9, 26, 27]. Noting the overlap of selection and prognostic factors, we performed a matching weight propensity score analysis. When potential confounders were controlled for, a statistically significant reduced risk of death was still associated with repeat PM, compared with no surgery. Previous reports on PM, initial or repeat, have not performed propensity-matched analyses which are a significant advance of this study. Our study is therefore novel in that we have attempted to evaluate the previously indefinable denominator from which patients are selected, and control for variability in baseline characteristics that might indicate a bias toward a repeat PM or nonoperative management. The result of this more rigorous analysis indicates that the survival curves for the nonsurgical and repeat PM groups remain significantly divergent.
Previous studies investigating repeat PM for sarcoma focused on relatively small series [17, 28, 29]. Our institution previously reported on 86 patients who underwent repeat PM between 1982 and 1997, where 5-year survival was 36%. [3]. In the present study, 5-year OS was similar, at 35%, with a median OS of 32.4 months. On univariable analyses, longer DFI, treatment with preoperative chemotherapy, number of recurrent pulmonary metastases, and completeness of resection were significantly associated with survival, as described in various series [3, 26, 29]. On multivariable analysis, leiomyosarcoma histologic subtype was significantly associated with longer OS as previously reported [13].
Preoperative chemotherapy was also associated with a greater risk of death on multivariable analysis. This likely represents a bias toward patients with a more-aggressive underlying disease biology being selected for multimodality treatment. It has been suggested that progression of disease while receiving chemotherapy is an independent prognostic factor for worse survival [30]. The current analyses demonstrate that, for patients undergoing repeat PM, selection for preoperative chemotherapy is itself a negative predictor, regardless of response.
Several studies have emphasized the importance of achieving an R0 resection [1, 3, 15, 29]. Complete resection was achieved in 86% of patients who underwent repeat PM, with an associated survival benefit that remained significant on multivariable analysis. Selection of patients for resection is of paramount importance, as resectability has been shown to be prognostic. Factors we found to be associated with selection for repeat PM may apply to resectability overall, and careful consideration of these factors may help to ensure that the high rate of R0 resections is maintained.
Limitations of this study include its retrospective design, and the data are derived from a single institution. Although our analysis includes weight-based propensity matching to control for factors associated with selection for surgery, the study is not a randomized controlled trial. The variables we have included for matching, although inclusive of factors associated with selection, may also not capture all of the intricacies involved in the decision-making process, which is often individualized for each patient. Our database also focuses on surgical management; other treatment modalities, such as radiofrequency ablation and stereotactic body radiation therapy that are being increasingly used were not evaluated.
Metastatic disease is frequently relegated to the category of non-operative management. Our results indicate that surgical intervention, even in cases of recurrent metastases, can be associated with a survival benefit. Multidisciplinary disease management teams ought to be used to identify these patients, as is routinely performed at our institution. Specifically, the disease-free interval from the preceding PM, the number of recurrent nodules, the presence of other synchronous sites of disease, and resectability are considered. Furthermore, we plan to use our data for the creation of a nomogram, such that patients can be appropriately identified for referral to a thoracic surgeon.
In summary, we have identified several prognostic variables associated with repeat PM for STS. The high recurrence rate after initial and repeat PM remains one of the greatest challenges in treating this disease. However, compared with patients managed without repeat PM, patients who undergo repeat PM have superior survival and improved outcomes based on matching weights propensity score analysis.
Supplementary Material
ABBREVIATIONS
- ASMD
absolute standardized mean difference
- DFI
disease-free interval
- GU
genitourinary
- GYN
gynecological
- KPS
Karnofsky performance status
- MIS
minimally invasive surgery
- MPNST
malignant peripheral nerve sheath tumor
- MSK
Memorial Sloan Kettering Cancer Center
- OS
overall survival
- PM
pulmonary metastasectomy
- PS/MFH
pleomorphic sarcoma, malignant fibrous histiocytoma
- STS
soft tissue sarcoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: Analysis of patterns of diseases and postmetastasis survival. Ann Surg. 1999;229(5):602–610. doi: 10.1097/00000658-199905000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith R, Demmy TL. Pulmonary metastasectomy for soft tissue sarcoma. Surg Oncol Clin N Am. 2012;21(2):269–286. doi: 10.1016/j.soc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Weiser MR, Downey RJ, Leung DH, Brennan MF. Repeat resection of pulmonary metastases in patients with soft-tissue sarcoma. J Am Coll Surg. 2000;191(2):184–190. doi: 10.1016/s1072-7515(00)00306-9. [DOI] [PubMed] [Google Scholar]
- 4.Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):416–421. doi: 10.1097/SLA.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Predina JD, Puc MM, Bergey MR, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol. 2011;6(5):913–919. doi: 10.1097/JTO.0b013e3182106f5c. [DOI] [PubMed] [Google Scholar]
- 6.Pang A, Carbini M, Maki RG. Contemporary therapy for advanced soft-tissue sarcomas in adults: A review. JAMA Oncol. 2016;2(7):941–947. doi: 10.1001/jamaoncol.2016.0241. [DOI] [PubMed] [Google Scholar]
- 7.Ripley RT, Downey RJ. Pulmonary metastasectomy. J Surg Oncol. 2014;109(1):42–46. doi: 10.1002/jso.23450. [DOI] [PubMed] [Google Scholar]
- 8.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113(1):37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 9.Dossett LA, Toloza EM, Fontaine J, et al. Outcomes and clinical predictors of improved survival in a patients undergoing pulmonary metastasectomy for sarcoma. J Surg Oncol. 2015;112(1):103–106. doi: 10.1002/jso.23961. [DOI] [PubMed] [Google Scholar]
- 10.Treasure T, Fiorentino F, Scarci M, Moller H, Utley M. Pulmonary metastasectomy for sarcoma: A systematic review of reported outcomes in the context of thames cancer registry data. BMJ Open. 2012;2(5) doi: 10.1136/bmjopen-2012-001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackmon SH, Shah N, Roth JA, et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg. 2009;88(3):877–884. doi: 10.1016/j.athoracsur.2009.04.144. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: Prognostic factors associated with improved outcomes. Ann Thorac Surg. 2011;92(5):1780–1786. doi: 10.1016/j.athoracsur.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 13.Burt BM, Ocejo S, Mery CM, et al. Repeated and aggressive pulmonary resections for leiomyosarcoma metastases extends survival. Ann Thorac Surg. 2011;92(4):1202–1207. doi: 10.1016/j.athoracsur.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 14.Rehders A, Hosch SB, Scheunemann P, Stoecklein NH, Knoefel WT, Peiper M. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Arch Surg. 2007;142(1):70–75. doi: 10.1001/archsurg.142.1.70. [DOI] [PubMed] [Google Scholar]
- 15.Pogrebniak HW, Roth JA, Steinberg SM, Rosenberg SA, Pass HI. Reoperative pulmonary resection in patients with metastatic soft tissue sarcoma. Ann Thorac Surg. 1991;52(2):197–203. doi: 10.1016/0003-4975(91)91336-t. [DOI] [PubMed] [Google Scholar]
- 16.Treasure T, Mineo T, Ambrogi V, Fiorentino F. Survival is higher after repeat lung metastasectomy than after a first metastasectomy: Too good to be true? J Thorac Cardiovasc Surg. 2015;149(5):1249–1252. doi: 10.1016/j.jtcvs.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 17.Hamaji M, Chen F, Miyamoto E, et al. Surgical and non-surgical management of repeat pulmonary metastasis from sarcoma following first pulmonary metastasectomy. Surg Today. 2016;46(11):1296–1300. doi: 10.1007/s00595-016-1312-x. [DOI] [PubMed] [Google Scholar]
- 18.Chudgar NPB, Munhoz RR, Bucciarelli PR, Tan KS, D’Angelo SP, Bains MS, Bott M, Huang J, Park BJ, Rusch VW, Adusumilli PS, Tap W, Singer S, Jones DR. Pulmonary metastasectomy with therapeutic intent for soft tissue sarcoma. J Thoracic Cardiovasc Surg. doi: 10.1016/j.jtcvs.2017.02.061. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat. 2013;9(2):215–234. doi: 10.1515/ijb-2012-0030. [DOI] [PubMed] [Google Scholar]
- 20.van Geel AN, Hoekstra HJ, van Coevorden F, Meyer S, Bruggink ED, Blankensteijn JD. Repeated resection of recurrent pulmonary metastatic soft tissue sarcoma. Eur J Surg Oncol. 1994;20(4):436–440. [PubMed] [Google Scholar]
- 21.Temeck BK, Wexler LH, Steinberg SM, McClure LL, Horowitz MA, Pass HI. Reoperative pulmonary metastasectomy for sarcomatous pediatric histologies. Ann Thorac Surg. 1998;66(3):908–912. doi: 10.1016/s0003-4975(98)00666-3. [DOI] [PubMed] [Google Scholar]
- 22.Park JS, Kim HK, Choi YS, et al. Outcomes after repeated resection for recurrent pulmonary metastases from colorectal cancer. Ann Oncol. 2010;21(6):1285–1289. doi: 10.1093/annonc/mdp475. [DOI] [PubMed] [Google Scholar]
- 23.Kromer DE, Tuchler H, et al. Long-term results after repeated surgical removal of pulmonary metastases. Ann Thorac Surg. 1998;65(4):909–912. doi: 10.1016/s0003-4975(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 24.Internullo E, Cassivi SD, Van Raemdonck D, Friedel G, Treasure T. Pulmonary metastasectomy: A survey of current practice amongst members of the european society of thoracic surgeons. J Thorac Oncol. 2008;3(11):1257–1266. doi: 10.1097/JTO.0b013e31818bd9da. [DOI] [PubMed] [Google Scholar]
- 25.Treasure T, Macbeth F. Doubt about effectiveness of lung metastasectomy for sarcoma. J Thorac Cardiovasc Surg. 2015;149(1):93–94. doi: 10.1016/j.jtcvs.2014.09.073. [DOI] [PubMed] [Google Scholar]
- 26.Lin AY, Kotova S, Yanagawa J, et al. Risk stratification of patients undergoing pulmonary metastasectomy for soft tissue and bone sarcomas. J Thorac Cardiovasc Surg. 2015;149(1):85–92. doi: 10.1016/j.jtcvs.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 27.Digesu CS, Wiesel O, Vaporciyan AA, Colson YL. Management of sarcoma metastases to the lung. Surg Oncol Clin N Am. 2016;25(4):721–733. doi: 10.1016/j.soc.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toussi MS, Bagheri R, Dayani M, Anvari K, Sheibani S. Pulmonary metastasectomy and repeat metastasectomy for soft-tissue sarcoma. Asian Cardiovasc Thorac Ann. 2013;21(4):437–442. doi: 10.1177/0218492312462710. [DOI] [PubMed] [Google Scholar]
- 29.Liebl LS, Elson F, Quaas A, Gawad KA, Izbicki JR. Value of repeat resection for survival in pulmonary metastases from soft tissue sarcoma. Anticancer Res. 2007;27(4c):2897–2902. [PubMed] [Google Scholar]
- 30.Stephens EH, Blackmon SH, Correa AM, et al. Progression after chemotherapy is a novel predictor of poor outcomes after pulmonary metastasectomy in sarcoma patients. J Am Coll Surg. 2011;212(5):821–826. doi: 10.1016/j.jamcollsurg.2011.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.