Abstract
Background
Acute myocardial ischemia is a common cause of ventricular arrhythmias, yet recent ECG methods predicting susceptibility to ventricular tachyarrhythmia have not been fully evaluated during spontaneous ischemia. We sought to evaluate the clinical utility of alternans and non-alternans components of repolarization variability from the standard 10-second 12-lead ECG signals to risk stratify patients with acute chest pain.
Methods
We enrolled consecutive, non-traumatic, chest pain patients transported through Emergency Medical Services (EMS) to three tertiary care hospitals with cardiac catheterization lab capabilities in Pittsburgh, PA. ECG signals were manually annotated by an electrophysiologist, then automatically processed using a custom-written software. Both T wave alternans (TWA) and non-alternans repolarization variability (NARV) were calculated using the absolute RMS differences over the repolarization window between odd/even averaged beats and between consecutive averaged pairs, respectively. The primary study outcome was the presence of acute myocardial infarction (AMI) documented by cardiac angiography.
Results
After excluding patients with secondary repolarization changes (n=123) and those with excessive noise (n=90), our final sample included 537 patients (age 57±16 years, 56% males). Patients with AMI (n=47, 9%) had higher TWA and NARV values (p<0.01). Mean RR correlated with TWA, and noise measures correlated with TWA and NARV, after adjusting for potential confounders. There was a high collinearity between TWA and NARV, and each was separately predictive of AMI after controlling for number of analyzed beats, noise measures, and other clinical variables.
Conclusions
Despite limitations imposed by signal quality, TWA and NARV are higher in patients with AMI, even after correction for potential confounders. The clinical value of TWA and NARV derived from standard ECG using our time-domain RMS method is questionable due to small number of beats and significant noise.
Keywords: Ischemia, repolarization, T wave lability, 12-lead ECG, Emergency Medical Services
INTRODUCTION
The 12-lead ECG remains the principal tool for the initial emergency evaluation of acute myocardial ischemia and infarction in patients with chest pain. ECG changes associated with acute myocardial ischemia include alterations in the amplitude of the ST-segment, and/or the amplitude and spatial-temporal characteristics of the T-wave.[1] Our knowledge of these latter characteristics is still incomplete. Given that more than 60% of patients with acute myocardial infarction (AMI) manifest little or no ST elevation on their presenting ECG, [2] novel methods to quantify the ischemia-induced T-wave changes from the standard 12-lead ECG might have important clinical applications.
Since acute myocardial ischemia is strongly associated with the onset of ventricular arrhythmias, [3] we hypothesized that ECG methods quantifying ventricular repolarization heterogeneity for the task of arrhythmia prediction might also be useful for the task of ischemia detection. As such, we previously demonstrated that the principal components of the T wave, T loop morphology, and wavefront direction metrics correlate with acute myocardial ischemia.[4–6] One of the best researched methods for arrhythmia prediction that has already made its way to clinical practice is T-wave alternans (TWA). TWA has been associated with risk of ventricular arrhythmia in a wide variety of clinical settings.[7] More interestingly, Martinez et al. [8] showed that experimental myocardial ischemia induced abnormal TWA in more than 33% of patients within 1–2 minutes of balloon inflation. However, the impact of spontaneous ischemia on TWA is unknown. The clinical value of TWA from short ECG recordings is also questionable. In contrast, we recently showed that non-alternans repolarization variability (NARV) also correlates with spontaneous ventricular tachycardia and is more robust than TWA when small number of beats is analyzed.[9] Accordingly, we sought to evaluate the presence of TWA and NARV in standard 10-second 12-lead ECG signal during spontaneous myocardial ischemia in patients evaluated at the emergency department for chest pain.
METHODS
Study Population
This is a secondary analysis of the Electrocardiographic Methods for the Prompt Identification of Coronary Events Study (EMPIRE). The methods of EMPIRE have been previously described in detail.[10] In short, EMPIRE is an ongoing prospective, observational cohort study designed to risk stratify patients with chest pain transported by ambulance to three tertiary care UPMC-affiliated hospitals in Pittsburgh, PA. This study is approved by the institutional review board of the University of Pittsburgh, with a waiver of informed consent to enroll eligible patients. Inclusion criteria include age >18; chief complaint of non-traumatic chest pain; and availability of prehospital 12-lead ECG. Patients are followed during hospitalization to collect American College of Cardiology’s recommended key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes.[11] This analysis is based on the first cohort recruited between May 2013 and August 2014 (n=750).
Outcome Data
The primary study outcome was the presence of AMI defined as a spontaneous myocardial infarction related to ischemia due to a primary coronary event (i.e., acute culprit lesion). AMI was identified according to the universal definition of MI, [12] plus the presence of >70% occlusion in at least one main coronary artery during cardiac catheterization performed in the indexed visit. This outcome was coded by a reviewer who was independent from the investigative team and who was blinded from the ECG analyses of TWA and NARV. To make proper assessments, the reviewer was granted full access to patient index and discharge records, serial ECG reports, results of cardiac diagnostic tests (e.g., lab values, perfusion imaging scans, and catheterization lab reports), and other information pertinent to the course of hospitalization (e.g., interventions, procedures, and prescribed medications). This primary outcome was coded as yes or no.
ECG Analysis
Analysis was based on the prehospital 10-sec 12-lead ECGs, which were obtained by paramedics in the field using HeartStart MRx defibrillators with DXL 12-lead ECG algorithm (Philips Healthcare, NV, USA). Philips Healthcare exported the raw ECG signal into XML file format (500 samples per second, 5 μV per least significant bit; 0.05–150 Hz). Then, a cardiac electrophysiologist (J.N.) blinded to the clinical data manually annotated each ECG file. ECG files with pacing, or predominant rhythm other than sinus rhythm were excluded (n=123/750, 16%). ECG files non-analyzable due to excessive noise or artifact were also excluded (n=90/750, 12%). Obvious atrial or ventricular ectopic beats were manually deleted. At least 5 consecutive sinus beats of acceptable quality were required for inclusion.
Next, the ECGs were analyzed using a custom-written software created for this purpose in C++ (Microsoft Visual Studio, Microsoft Corp., Redmond, WA, USA) by one of the investigators (J.N.).[9] Briefly, the signals were manually reviewed for quality, ectopic beats were deleted, and consecutive normal QRS-T complexes were manually selected for analysis. After R peak detection, the signal was low-pass filtered at 20 Hz with a 3-pole Butterworth filter. The baseline wander was minimized by subtraction of smooth cubic spline passing through fiducial points in the PR segments (80 ms before R wave peak in most cases). TWA was calculated by separately averaging odd and even beats, then taking the root-mean-square (RMS) of the differences between the two averaged-beats over the repolarization window (Figure 1, A). NARV was calculated by first averaging each pair of consecutive beats, then taking the RMS of differences between these averaged pairs over the same repolarization window (Figure 1, B). Averaging consecutive pairs eliminates alternans variability from NARV and removes contamination by TWA. Both TWA and NARV values were normalized to mean QRS amplitude. TWA and NARV values were computed in each of the 12 ECG leads, the reviewer selected and reported the largest values from the ECG lead with least noise and artifact. It is worth noting that our methods depends on the time-domain RMS of differences between odd/even averaged beats or consecutive averaged pairs, which is different from the FDA-cleared Modified Moving Average (MMA) and Spectral Methods for TWA analysis.[13]
Figure 1. Calculating Alternans and Non-Alternans Time-Domain T Wave Variability.
This simplified diagram illustrates the differences between computing alternans (A) and non-alternans (B) variability of the T wave. In alternans variability (A), odd and even beats are streamed into two separate bins (green and black bins). An averaged beat is created for each bin, and the averaged bins were then superimposed. The root-mean-square (RMS) of the differences between these two averaged beats is computed over the entire T wave period. The average RMS over this window is reported as the time-domain TWA. In non-alternans variability (B), consecutive pairs are averaged first, then the RMS of the differences between averaged pairs is computed. We selected and reported the TWA and NARV from the ECG lead with largest liability values with least noise level.
To control for residual noise in the signal, we computed and reported the HF noise (i.e. high-frequency noise - RMS of the difference between the raw signal and the low-pass filtered signal), fiducial point lability (i.e., RMS of fiducial point signal amplitudes), and R peak lability (i.e., RMS of signal amplitudes at R wave maxima). These noise measures were used as covariates in statistical analyses. In addition, to account for residual baseline error caused by respiration artifact and to distinguish such error from beat-to-beat repolarization changes, we computed and reported the vital signs-derived heart rate-to-breathing ratio (HR/BR), including odd vs. even ratio, and controlled for this ratio in the multivariate analyses.
Statistical Analysis
Analysis was performed using SPSS; a two-tailed p-value of 0.05 was set for hypothesis testing. Values were reported as mean ± SD or n (%). Groups were compared using chi-square or independent samples t-test. Collinearity between continuous variables were evaluated using Pearson’s r correlation coefficient, and R2 was reported for significant correlations. Logistic regression was used to test univariate and multivariate predictors of primary outcome. Odds ratio and 95% CI were reported for each significant variable at the univariate level, and those significant at p<0.10 were selected for multivariate analysis. To test the independent prognostic values of TWA and NARV, three multivariate models were developed: one with TWA alone, one with NARV alone, and one with both variables. Goodness of fit of each model was evaluated using Hosmer-Lemeshow test.
RESULTS
Baseline Characteristics
Final sample included 537 patients aged 57 ± 16 years, 56% males, and 55% whites. Nearly two thirds had hypertension and more than one third had at least one other major cardiovascular risk factor (e.g., diabetes, hyperlipidemia, smoking, known CAD, past MI). Overall, 47 patients (9%) had a positive outcome. Table 1 compares the baseline demographic and clinical characteristics between groups. Those with AMI were more likely to be white, and had slower heart rate and faster breathing rate (vital signs during first medical contact). AMI group had lower HR/BR ratio. On the presenting ECG, patients with AMI also had longer mean RR, higher HF noise and fiducial point lability, and higher TWA and NARV values.
Table 1.
Baseline Demographic and Clinical Characteristics
|
|
||||
|---|---|---|---|---|
| Variable | All Patients (n=537) |
Acute Myocardial Infarction
|
p | |
| YES (n=47, 9%) | NO (n=490, 91%) | |||
|
| ||||
| Demographics | ||||
| Age (years) | 57 ± 16 | 60 ± 15 | 57 ± 17 | .145 |
| Sex (Male) | 302 (56%) | 26 (55%) | 276 (56%) | .894 |
| Race (White) | 297 (55%) | 40 (85%) | 257 (52%) | .000 |
|
| ||||
| Past Medical History | ||||
| Hypertension | 354 (66%) | 27 (57%) | 327 (68%) | .160 |
| Diabetes | 131 (24%) | 13 (28%) | 118 (24%) | .236 |
| Dyslipidemia | 190 (35%) | 18 (38%) | 172 (36%) | .706 |
| Ever Smoker | 311 (58%) | 24 (51%) | 287 (59%) | .457 |
| Coronary Artery Disease | 166 (31%) | 13 (28%) | 153 (32%) | .577 |
| Myocardial Infarction | 135 (25%) | 9 (19%) | 126 (26%) | .301 |
| Heart Failure | 77 (14%) | 6 (13%) | 71 (15%) | .723 |
| Prior PCI/Stent | 122 (23%) | 15 (30%) | 107 (22%) | .003 |
| Prior CABG | 43 (8%) | 6 (13%) | 37 (8%) | .169 |
|
| ||||
| Presenting Vital Signs | ||||
| Heart Rate (bpm) | 86 ± 23 | 78 ± 18 | 87 ± 24 | .011 |
| Breathing Rate (breath/min) | 18 ± 3 | 20 ± 4 | 18 ± 3 | .013 |
| Systolic BP (mmHg) | 147 ± 30 | 147 ± 34 | 147 ± 29 | .933 |
| Diastolic BP (mmHg) | 87 ± 18 | 90 ± 21 | 87 ± 18 | .247 |
| Oxygen Saturation (%) | 98 ± 5 | 98 ± 3 | 98 ± 5 | .898 |
|
| ||||
| Laboratory Values at ED | ||||
| Initial Positive Troponin | 68 (13%) | 13 (28%) | 54 (11%) | .001 |
| Sodium | 138 ± 3 | 138 ± 3 | 138 ± 3 | .985 |
| Potassium | 3.9 ± 0.5 | 3.8 ± 0.5 | 3.9 ± 0.5 | .234 |
| Calcium | 9.0 ± 0.6 | 8.8 ± 0.7 | 9.0 ± 0.6 | .021 |
| Magnesium | 1.9 ± 1.2 | 1.8 ± 0.3 | 1.9 ± 1.3 | .681 |
| Glucose | 135 ± 70 | 145 ± 43 | 134 ± 72 | .289 |
| Creatinine | 1.2 ± 1.3 | 1.1 ± 0.9 | 1.2 ± 1.4 | .584 |
|
| ||||
| ECG Variables | ||||
| Mean RR | 768 ± 199 | 834 ± 239 | 761 ± 194 | .017 |
| # of beats analyzed | 13 ± 4 | 12 ± 3 | 13 ± 4 | .035 |
| HR/BR Ratio (median [IQR]) | 4.2 (3.4–5.1) | 3.8 (3.0–4.3) | 4.3 (3.6–5.3) | .034 |
| HR/BR Ratio (odd vs. even) | 49% vs. 51% | 47% vs. 53% | 49% vs. 51% | .735 |
| High Frequency Noise | 6.4 ± 3.8 | 7.5 ± 5.0 | 6.3 ± 3.7 | .032 |
| Fiducial Point Lability | 8.5 ± 6.7 | 10.7 ± 8.0 | 8.3 ± 6.5 | .050 |
| R Peak Lability | 14.3 ± 8.4 | 13.0 ± 7.0 | 14.4 ± 8.5 | .264 |
| TWA (mV) | .0109 ± .0098 | .0163 ± .0144 | .0103 ± .0091 | .007 |
| NARV (mV) | .0179 ± .0119 | .0225 ± .0167 | .0174 ± .0113 | .005 |
Precordial leads were selected 80% of the time (n=432/537), and limb leads were selected 20% of the time (n=106/537). The ECG leads that were most frequently selected for analysis were V4 (n=121, 23%), V3 (n=88, 16%), V2 (n=73, 14%), V5 (n=70, 13%), and V6 (n=53, 10%). The leads that were least likely selected for analysis were lead aVF (n=11, 2%), III (~1%) and aVL (~1%). To account for the potential noise artifact induced by limb leads, we compared TWA and NARV values obtained from limb leads vs. precordial leads. In patients with AMI, there were no differences between TWA and NARV obtained from limb vs. precordial leads (0.017 vs. 0.016, p=0.80, and 0.030 vs. 0.028, p=0.11). In patients with no AMI, there were no differences in TWA as well (0.012 vs. 0.010, p=0.53), but NARV was marginally higher in limb leads (0.20 vs. 0.17, p=0.04), suggesting that some of the NARV pattern seen in this study might relate to noise artifact associated with the limb leads.
Assessment of Confounders
We explored the potential confounding effect of clinically important variables on TWA and NARV, including the effects of HR/BR, mean RR, HF noise, and fiducial point lability.
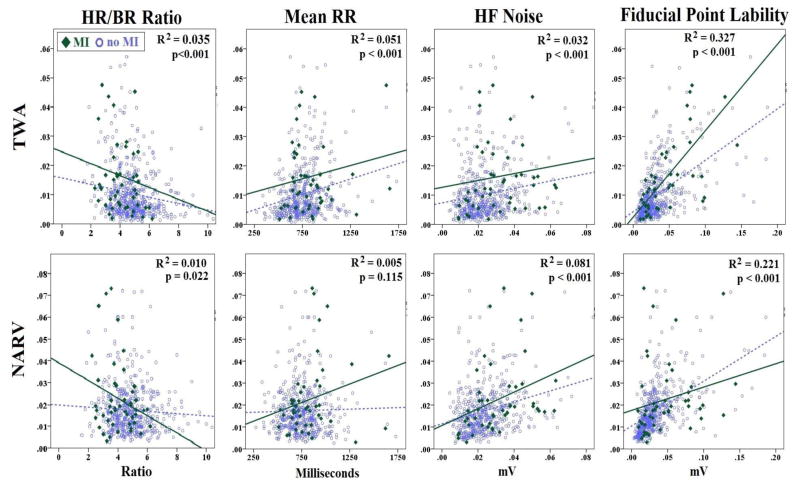
HR/BR Ratio: Patients with AMI had a smaller HR/BR ratio (Table 1), which is likely influenced by a faster respiration in this group. Both TWA and NARV were significantly associated with smaller HR/BR ratio (Figure 2, left), suggesting potential contamination with baseline error. There was an interaction between HR/BR ratio and MI group; patients with AMI had a steeper ratio/NARV slope compared to those without AMI.
Mean RR: heart rate had a significant effect on TWA in both MI groups (Figure 2, center). Surprisingly, larger TWA were observed at slower, rather than faster, heart rate. The effect of heart rate on TWA was independent from age and presence or absence of MI (adjusted R2=0.07, F=15.2, p<0.001 using linear regression). Mean RR did not have a significant effect on NARV in the overall sample, but there was an interaction between mean RR and the presence of MI (interaction term F=1.8, p=0.009 using GLM). This means that slower heart rate was associated with larger NARV in the MI group alone.
High-Frequency noise: there is a direct relationship between HF noise and TWA and NARV; higher HF noise unsurprisingly leads to more alternans and non-alternans variabilities (Figure 3, center). After controlling for MI (MI group has higher HF noise), there is still a significant relationship between HF noise and both TWA (adjusted R2=0.05) and NARV (adjusted R2=0.04).
Fiducial point lability: there is a direct and strong relationship between fiducial point lability and both TWA and NARV; larger baseline wander unsurprisingly explains more than 25% of variability in alternans and non-alternans variables of interest (Figure 2, right). There is a small positive relationship between HF noise and fiducial point lability (r=0.15), but each remains an independent predictor of TWA or NARV after correcting for the collinearity effect.
Figure 2. Correlation of TWA and NARV with other important covariates.
These are scatter plots of the correlation between each of Heart Rate/Breathing Rate (HR/BR) ratio, mean RR, high-frequency (HF) noise, and fiducial point lability with TWA (upper row) and NARV (bottom row). Linear regression lines are shown separately for subjects with myocardial infarction (MI) confirmed by angiography and the other subjects. The reported R2 and p values are based on all patients in each respective scatter plot.
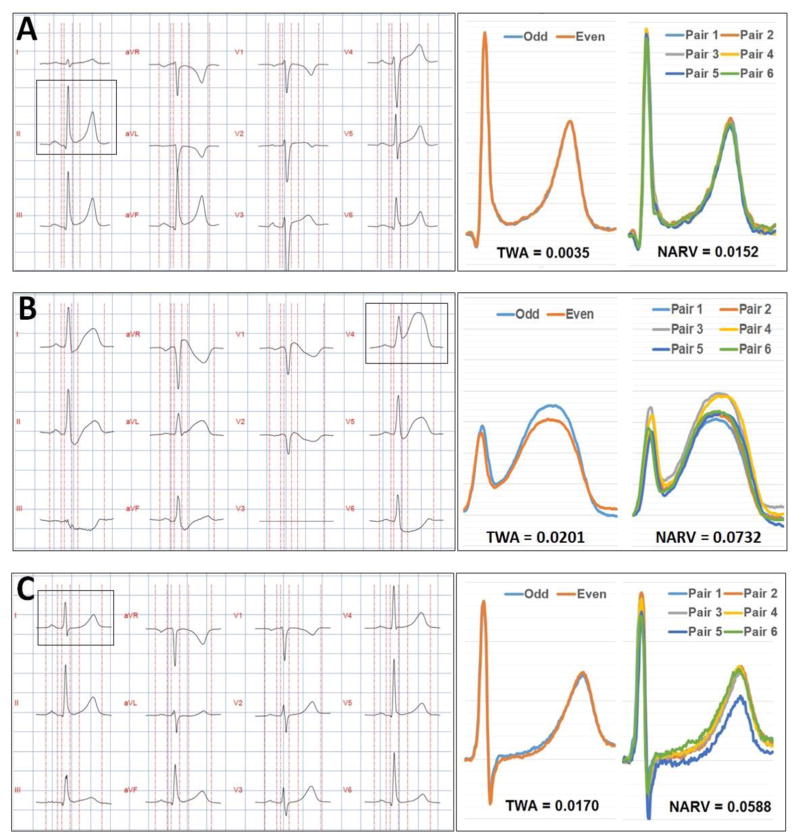
Figure 3. Selected examples of 12-lead ECG and corresponding lability measurements.
(A) This is a 52-year-old white male with known CAD who presented with chest pain and shortness of breath. Serial troponin levels were negative. Patient discharged from ED with a final diagnosis of musculoskeletal pain.
(B) This is a 53-year-old white male with no significant past medical history who presented with chest pain. The initial troponin was 0.33 and the angiogram revealed 100% occlusion of the left anterior descending artery and 30% occlusion of the right coronary artery.
(C) This is a 57-year-old white male with a past medical history of hypertension who presented with chest pain. Initial troponin was negative, but second troponin was 3.23. The angiogram revealed 99% occlusion of the right coronary artery and 60% occlusion of the left anterior descending artery.
Prediction of Primary Outcome
Table 2 summarizes the univariate and multivariate predictors of AMI. As shown in this table, TWA was a significant predictor of AMI after controlling for mean RR, HR/BR ratio, noise measures, and other clinical variables (Model 1). NARV was also a significant predictor of AMI after controlling for mean RR, HR/BR ratio, noise measures, and other clinical variables (Model 2). TWA, but not NARV, remains an independent predictor in Model 3.
Table 2.
Univariate & Multivariate Predictors of Culprit Artery
| PREDICTOR | UNIVARIATE ANALYSIS | MULTIVARIATE MODELS | |||
|---|---|---|---|---|---|
|
| |||||
| OR (95% CI) | P value | Model 1 * | Model 2 ** | Model 3 *** | |
| White Race | 5.2 (2.3–11.8) | <0.001 | <0.001 | <0.001 | <0.001 |
| Presenting HR (per 1 bmp) | 0.98 (0.97–0.99) | 0.001 | – – – | – – – | – – – |
| Breathing Rate (per 1 b/min) | 1.1 (1.0–1.2) | 0.007 | – – – | – – – | – – – |
| HR/BR Ratio | 0.6 (0.4–0.8) | <0.001 | <0.001 | <0.001 | <0.001 |
| Initial Positive Troponin | 3.1 (1.5–6.2) | 0.002 | 0.005 | 0.004 | 0.005 |
| Mean RR (per 10 millisecond) | 1.02 (1.01–1.03) | 0.019 | 0.191 | 0.268 | 0.211 |
| HF Noise | 1.07 (1.04–1.14) | 0.036 | 0.004 | 0.006 | 0.003 |
| Fiducial Point Lability | 1.04 (1.01–1.07) | 0.026 | 0.747 | 0.145 | 0.705 |
| TWA | 1.6 (1.2–2.0) | <0.001 | 0.011 | – – – | 0.038 |
| NARV | 1.3 (1.1–1.6) | 0.006 | – – – | 0.041 | 0.274 |
TWA: T wave alternans, NARV: non-alternans repolarization variability. Presenting HR is not included in multivariate analyses due to high collinearity with mean RR.
NARV is not included in this model. Goodness-of-fitness test X2 = 10.68, p=0.221
TWA is not included in this model. Goodness-of-fitness test X2 = 9.87, p=0.274
Goodness-of-fitness test X2 = 10.99, p=0.202
DISCUSSION
In this observational study, we evaluated the alternans and non-alternans components of repolarization variability from the standard 10-sec 12-lead ECG of patients evaluated for AMI at the emergency department. We found that patients with AMI have higher TWA and NARV, after controlling for potential confounders. This finding is similar to a previous report by Martinez et al. that showed that experimental ischemia induces TWA during balloon inflation in the catheterization lab.[8] To the best of our knowledge, this is the first study to demonstrate that acute ischemia also induces non-alternans repolarization variability as well.
However, our findings need to be interpreted with caution; both alternans and non-alternans variabilities were significantly influenced by noise and baseline wander. Interpreting this correlation is difficult because patients with AMI also had higher levels of noise and baseline wander on their ECG (potentially due to hyperventilation or perspiration related to the acuity of their condition during the acquisition of the prehospital ECG). The non-alternans component was especially sensitive to noise artifact induced by limb leads, which is a finding that was previously demonstrated by Nearing et al.[14] Given that TWA and NARV remained significant predictors after controlling for noise level, it is plausible that the observed alternans and non-alternans variabilities are in fact physiological signals induced by the acute myocardial ischemia. It should be noted that TWA and NARV were only marginally significant in multivariable models, and only in certain of the multiple models assessed.
The finding that TWA increases with decreasing HR also has to be interpreted carefully. Most of the clinical and experimental literature on TWA, especially microvolt TWA, indicate that it is a tachycardia-dependent phenomenon, and the positive correlation between TWA and mean RR interval is thus unexpected. A possible explanation is that in recordings of fixed duration (10 s in this case), there is a tight correlation between the mean RR interval and the number of analyzed beats (though not perfect dependence, since many beats were manually deleted). We [9] have previously shown that the TWA values calculated from short data segments are contaminated by noise and NARV to a degree which is inversely proportional to the number of analyzed beats. In this dataset with relatively high levels of noise and low number of analyzed beats, this could explain at least part of this unexpected effect.
Few reliable data are available on the NARV dependence on HR. In patients with implantable defibrillators, NARV tended to be higher during elevated high rates [9]. We have also seen different NARV/RR slopes between pre-VT and control samples, and that the difference in NARV was more pronounced during slow HR.[9] In this study, there was no overall correlation between HR and NARV, but MI patients with slower HR had higher NARV. It remains to be determined if the difference in NARV is due to true difference between the two patient groups, or rather due to some sort of artifact.
Ischemia and T wave Variability
Repolarization alternans in the setting of myocardial ischemia has been reported in multiple animal models, usually associated with alternans of intracellular Ca2+ transient.[13] The experimental work evaluating the relationship between action potential alternans and Ca2+ transient alternans mostly indicate that the latter is driving the former, [15, 16] although the causality has been rarely studied directly in the setting of spontaneous ischemia. The work by Kapur et al. [17] shows discordant calcium alternans in adjacent cells and discordant subcellular calcium alternans during ischemia, suggesting that calcium cycling abnormality drives the voltage abnormality in this setting also. Several phases of myocyte Ca2+ handling depend on the presence of adequate concentration of intracellular ATP either directly (Ca2+ transport from cytoplasm to sarcoplasmic reticulum by SERCA) or indirectly (transport from cytoplasm to extracellular fluid by NCX1). It is probable that ischemia results in slowing of these transport processes, producing the well-known prolongation of Ca2+ transient, which contrasts with simultaneous action potential shortening caused by IKATP augmentation.[18] The lower Ca2+ concentration in junctional sarcoplasmic reticulum during ischemia and the nonlinear dependence of Ca2+ release on junctional sarcoplasmic reticulum Ca2+ load provides a probable explanation for ischemia-induced alternans of Ca2+ transient.
Previous studies have shown that TWA is concentrated in the first half of the T wave during myocardial ischemia.[13] However, we have computed TWA value across the entire T wave, which might introduce a dilution effect and might explain the small TWA values observed in this study. Moreover, previous studies have shown that TWA shows regional distribution among different precordial leads, with the maximum TWA monitored from the anterolateral precordial lead V5 [19]. In this study, we found that the maximum TWA was most frequently observed in the anterolateral precordial lead V4. Within the context of chest pain, this observation might be explained by the expected severe myocardial ischemia that can be induced in the anterior wall by disease of the left anterior descending artery.
Non-alternans repolarization variability has been initially reported in congenital long QT syndrome [20] and it is also related to abnormal myocardial calcium handling in animal experiments [21–23]. It is uncertain if NARV is a prominent feature of acute myocardial ischemia, though it seems to increase prior to onset of ventricular arrhythmias in patients with coronary artery disease [9, 24].
Clinical Implications of TWA and NARV in Chest Pain Evaluation
Figure 3 shows three selected case studies from our data. Figure 3-A shows the 12-lead ECG of a 52-year-old white male with known CAD who presented with chest pain and shortness of breath. Serial troponin levels were negative and the patient was discharged from the ED with a final diagnosis of musculoskeletal pain. The ECG shows no significant ST-T abnormalities, and the corresponding beat-to-beat analysis shows very small T wave variability. Figure 3-B shows the 12-lead ECG of a 53-year-old white male with no significant past medical history who presented with chest pain. The initial ECG shows massive ST elevation in the anterolateral wall, and the corresponding beat-to-beat analysis shows larger T wave variability. This patient was sent to the catheterization lab directly and there was 100% occlusion of the left anterior descending artery. Figure 3-C is more interesting, it shows the 12-lead ECG of a 57-year-old white male with a past medical history of hypertension who presented with chest pain. The ECG showed no significant ST-T abnormalities and the initial troponin was negative. A repeat troponin 3 hours later was 3.23. The patient was admitted and scheduled for angiography next morning, which revealed 99% occlusion of the right coronary artery and 60% occlusion of the left anterior descending artery. This latter example demonstrates how TWA and NARV could have value in triaging chest pain patients. The utility of such method needs to be evaluated in future prospective studies.
Limitations
There were 2 major limitations of this work. First, the ECG signal was contaminated with noise due to the nature of the prehospital setting in which these ECGs were obtained. To account for this issue, we manually reviewed each ECG strip and excluded “bad” recordings, as well as selected proper beats for analysis. We also included noise measures in all multivariate analyses. A second limitation was the small number of analyzed beats due to the fixed duration of signal analyzed (10-second ECGs). We used mean RR as a covariate in our analyses and evaluated the relationship between this measure and variables of interest. Our findings need to be validated in an independent population with longer duration recordings (few minutes).
CONCLUSIONS
Despite limitations imposed by signal quality, TWA and NARV are higher in patients with AMI, even after correction for potential confounders. The clinical value of TWA and NARV derived from standard ECG using our time-domain RMS method is questionable due to small number of beats and significant noise.
Acknowledgments
Funding: The project described was supported by the National Institutes of Health through Grant Number UL1TR001857.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wagner GS, et al. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram: Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):1003–1011. doi: 10.1016/j.jacc.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Khera S, et al. Non-ST-elevation myocardial infarction in the United States: contemporary trends in incidence, utilization of the early invasive strategy, and in-hospital outcomes. Journal of the American Heart Association. 2014;3(4):e000995. doi: 10.1161/JAHA.114.000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cascio WE, et al. Ischemia-induced arrhythmia: the role of connexins, gap junctions, and attendant changes in impulse propagation. Journal of electrocardiology. 2005;38(4):55–59. doi: 10.1016/j.jelectrocard.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Al-Zaiti S, Runco K, Carey M. Increased T-Wave Complexity Can Indicate Subclinical Myocardial Ischemia in Asymptomatic Adults. Journal of Electrocardiology. 2011;44(6):684–688. doi: 10.1016/j.jelectrocard.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Zaiti S, et al. Clinical Utility of Ventricular Repolarization Dispersion for Real-Time Detection of Non-ST Elevation Myocardial Infarction in Emergency Departments. Journal of the American Heart Association. 2015;4(7):e002057. doi: 10.1161/JAHA.115.002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Zaiti S, et al. Spatial Indices of Repolarization Correlate with Non-ST Elevation Myocardial Ischemia in Patients with Chest Pain. Medical & Biological Engineering & Computing. 2017;55 doi: 10.1007/s11517-017-1659-1. In Press. [DOI] [PubMed] [Google Scholar]
- 7.Gehi AK, et al. Microvolt T-Wave Alternans for the Risk Stratification of Ventricular Tachyarrhythmic Events: A Meta-Analysis. Journal of the American College of Cardiology. 2005;46(1):75–82. doi: 10.1016/j.jacc.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 8.Martínez JP, et al. Characterization of repolarization alternans during ischemia: time-course and spatial analysis. IEEE Transactions on Biomedical Engineering. 2006;53(4):701–711. doi: 10.1109/TBME.2006.870233. [DOI] [PubMed] [Google Scholar]
- 9.Krokhaleva Y, et al. Increased Nonalternans Repolarization Variability Precedes Ventricular Tachycardia Onset in Patients with Implantable Defibrillators. Pacing Clin Electrophysiol. 2016;39(2):140–8. doi: 10.1111/pace.12785. [DOI] [PubMed] [Google Scholar]
- 10.Al-Zaiti SS, et al. Rationale, development, and implementation of the Electrocardiographic Methods for the Prehospital Identification of Non-ST Elevation Myocardial Infarction Events (EMPIRE) J Electrocardiol. 2015;48(6):921–26. doi: 10.1016/j.jelectrocard.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes: A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, American College of Emergency Physicians, American Heart Association, Cardiac Society of Australia & New Zealand, National Heart Foundation of Australia, Society for Cardiac Angiography and Interventions, and the Taiwan Society of Cardiology. Journal of the American College of Cardiology. 2001;38(7):2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, et al. Universal definition of myocardial infarction: Statement from ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. European Heart Journal. 2007;28(20):2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 13.Verrier RL, et al. Microvolt T-Wave Alternans: Physiological Basis, Methods of Measurement, and Clinical Utility—Consensus Guideline by International Society for Holter and Noninvasive Electrocardiology. Journal of the American College of Cardiology. 2011;58(13):1309–1324. doi: 10.1016/j.jacc.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia induced vulnerability by precordial T wave alternans analysis in dog and human. Cardiovascular research. 1994;28(9):1440–1449. doi: 10.1093/cvr/28.9.1440. [DOI] [PubMed] [Google Scholar]
- 15.Walker ML, et al. Hysteresis effect implicates calcium cycling as a mechanism of repolarization alternans. Circulation. 2003;108:2704–2709. doi: 10.1161/01.CIR.0000093276.10885.5B. [DOI] [PubMed] [Google Scholar]
- 16.Chudin E, et al. Intracellular Ca(2+) dynamics and the stability of ventricular tachycardia. Biophys J. 1999;77:2930–2941. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapur S, et al. Acidosis and ischemia increase cellular Ca2+ transient alternans and repolarization alternans susceptibility in the intact rat heart. Am J Physiol Heart Circ Physiol. 2009;296(5):H1491–512. doi: 10.1152/ajpheart.00539.2008. [DOI] [PubMed] [Google Scholar]
- 18.Lakireddy V, et al. Contrasting effects of ischemia on the kinetics of membrane voltage and intracellular calcium transient underlie electrical alternans. Am J Physiol Heart Circ Physiol. 2005;288(1):H400–7. doi: 10.1152/ajpheart.00502.2004. [DOI] [PubMed] [Google Scholar]
- 19.Leino J, et al. Importance of regional specificity of T-wave alternans in assessing risk for cardiovascular mortality and sudden cardiac death during routine exercise testing. Heart Rhythm. 2011;8(3):385–390. doi: 10.1016/j.hrthm.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Nemec J, et al. Catecholamine-provoked microvoltage T wave alternans in genotyped long QT syndrome. Pacing Clin Electrophysiol. 2003;26:1660–1667. doi: 10.1046/j.1460-9592.2003.t01-1-00249.x. [DOI] [PubMed] [Google Scholar]
- 21.Nemec J, Kim JJ, Salama G. The link between abnormal calcium handling and electrical instability in acquired long QT syndrome--Does calcium precipitate arrhythmic storms? Prog Biophys Mol Biol. 2016;120(1–3):210–21. doi: 10.1016/j.pbiomolbio.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemec J, et al. Calcium oscillations and T-wave lability precede ventricular arrhythmias in acquired long QT type 2. Heart Rhythm. 2010;7(11):1686–94. doi: 10.1016/j.hrthm.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JJ, et al. Synchronous systolic subcellular Ca2+-elevations underlie ventricular arrhythmia in drug-induced long QT type 2. Circ Arrhythm Electrophysiol. 2015;8(3):703–12. doi: 10.1161/CIRCEP.114.002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shusterman V, Goldberg A, London B. Upsurge in T-wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation. 2006;113(25):2880–2887. doi: 10.1161/CIRCULATIONAHA.105.607895. [DOI] [PubMed] [Google Scholar]