Abstract
Seizure-provoking factors circulate late in gestation during normal pregnancy, but do not readily gain access to the brain due to the protective nature of the blood-brain barrier. In particular, efflux transporters are powerful ATP-driven pumps that actively prevent unwanted compounds from entering the brain. We hypothesized that acute inhibition of efflux transporters at the blood-brain barrier would result in spontaneous seizures in pregnant rats. We further hypothesized that the blood-brain barrier protects the maternal brain from seizure by increasing expression and/or activity of p-glycoprotein (P-gp), a major efflux transporter. Main blood-brain barrier efflux transporters were inhibited in-vivo in nonpregnant (Nonpreg) and pregnant (Preg; d19) Sprague Dawley rats (n = 8/group). Seizures were monitored in conscious animals for 8 hours via chronically implanted electroencephalography (EEG) electrodes in the hippocampus and motor cortex and time-synced video. P-gp activity was measured via a calcein accumulation assay in freshly isolated cortical and hippocampal capillaries from Preg (d20) and Nonpreg rats (n = 8–16/group), to assess regional susceptibility to transporter inhibition. P-gp expression, capillary density, and microglial activation as a measure of neuroinflammation were quantified using immunohistochemistry (n = 4–6/group). Efflux transporter inhibition elicited hippocampal seizures within 1 hour in 100% of Preg rats that was not associated with neuroinflammation or elevated tumor necrosis factor alpha (TNFα) or vascular endothelial growth factor (VEGF), but negatively correlated with levels of estradiol. Hippocampal seizures were considerably less prevalent in Nonpreg rats. However, behavioral seizures in the motor cortex developed of similar severity in both groups of rats, demonstrating regional heterogeneity in response to efflux transporter inhibition. Basal P-gp activity was similar between groups, however, exposure to serum from Preg rats significantly decreased P-gp activity in the hippocampus, but not cortex, compared to serum from Nonpreg rats (0.29±0.1 units/sec in Preg vs. 0.06±0.02 units/sec in Nonpreg rats; p < 0.05) that was not associated with elevated TNFα or VEGF. Thus, pregnancy differentially increased the susceptibility of the hippocampus to seizures in response to blood-brain barrier efflux transporter inhibition that may be due to the inhibitory effect of circulating factors in pregnancy on P-gp activity in the hippocampus.
Keywords: P-glycoprotein, Seizure, Blood-Brain Barrier, Pregnancy, Eclampsia
1. Introduction
Preeclampsia is a hypertensive complication of pregnancy during which de novo seizures can occur, known as eclampsia. Eclamptic seizures remain unpredictable, and are a leading cause of maternal morbidity and mortality worldwide.1,2 Importantly, studies report that up to ~40 % of eclampsia occurs in women with seemingly uncomplicated pregnancies, i.e. in the absence of the diagnosis of preeclampsia or even hypertension, suggesting that normal pregnancy may be a state of increased seizure susceptibility.3 In support of this theory, we previously reported that normal pregnancy was associated with lower seizure threshold compared to the nonpregnant state.4 In addition, seizure-provoking factors circulate late in gestation, a time point at which eclampsia occurs most often.3,5 Using an organotypic hippocampal slice culture model, direct exposure to serum from late-pregnant rats resulted in neuroinflammation and network hyperexcitability via a tumor necrosis factor alpha (TNFα)-mediated mechanism.5 The inflammatory response to serum exposure did not occur when hippocampal slices were exposed to nonpregnant serum.5 However, under normal conditions, inflammatory serum factors do not readily gain access to the brain due to the presence and protective nature of the blood-brain barrier (BBB). In fact, the seizure-provoking serum was collected from pregnant animals that were not seizing, suggesting the BBB has a critical role in protecting the hyperexcitable maternal brain from circulating seizure-provoking inflammatory factors.
ATP-binding cassette (ABC) transporters are a family of integral membrane proteins that form active efflux pumps in several organs including the liver, intestine and brain.6–8 In the brain, efflux transporters contained within the BBB provide a specialized interface that is critical to regulating passage of blood constituents into and out of the central nervous system (CNS).8–11 P-glycoprotein (P-gp), one of the best-characterized and most abundant ABC transporters, is expressed mainly on the luminal side of the BBB and exhibits significant polyspecificity for a large number of exogenous and endogenous substrates.6,8,12 The expression and activity of P-gp has been studied in response to various medications, inflammatory mediators, hormones, and xenobiotics.12–14 In fact, P-gp expression and activity at the BBB can be either rapidly increased or decreased in response to several different stimuli, including inflammatory mediators and growth factors such as TNFα and vascular endothelial growth factor (VEGF).14–17 Both TNFα and VEGF rapidly inhibit P-gp activity; however, TNFα can stimulate upregulation of P-gp expression and activity over time.15,17 This dynamic response of P-gp to circulating factors makes P-gp highly adaptive and effective at maintaining cerebral homeostasis.12–14
Pregnancy has been shown to be associated with a mild systemic inflammatory response and has been reported to be a state of elevated serum levels of pro-inflammatory cytokines (e.g. TNFα) that may affect P-gp activity and expression.16–20 Further, growth factors (e.g. VEGF) and hormones (e.g. estradiol) are significantly elevated during normal pregnancy.21–23 Estradiol is an endogenous efflux transporter substrate that has pro-convulsive actions upon passage into the brain.24–26 However, the role of pregnancy-induced circulating factors in P-gp activity and/or expression changes, and how that may relate to the susceptibility of the maternal brain to seizure remain unclear.
In the current study, we investigated the role of efflux transporters at the BBB in protecting the maternal brain from seizure late in gestation when seizure-provoking factors are circulating, inflammatory mediators are elevated, and seizure threshold is decreased.4,5 We hypothesized that acute inhibition of efflux transporters would lead to spontaneous seizures in pregnant rats due to the passage of seizure-provoking factors, such as estradiol, into the hyperexcitable maternal brain. We further hypothesized that the BBB would undergo a protective adaptation by increasing P-gp expression and activity in response to increased circulating inflammatory factors. To test this hypothesis, electroencephalography (EEG) electrodes were implanted into the hippocampus, a brain region with high susceptibility to seizures, and the primary motor cortex, a brain region involved in the physical convulsions of seizures, of pregnant and nonpregnant rats.27,28 Continuous and simultaneous EEG and time-synched video were performed to monitor for spontaneous seizures in response to inhibition of efflux transporters at the BBB. We also investigated pregnancy-induced changes in P-gp expression, and in basal and serum-induced P-gp activity at the BBB using a calcein accumulation assay in freshly isolated capillaries from both the hippocampus and cortex. Lastly, activation of microglia as a measure of neuroinflammation was investigated as an underlying mechanism by which spontaneous seizures may be potentiated in pregnancy in response to efflux transporter inhibition.
2. Materials and Methods
2.1 Animals
All experiments were conducted using virgin, nonpregnant (Nonpreg) or timed-pregnant (Preg) Sprague Dawley rats between 14 and 16 weeks of age (Charles River, Canada). Pregnant rats were used late in gestation (day 19–20 of a 22 day gestation) when seizure-provoking factors are circulating.5 Rats were housed singly with environmental enrichment and kept in the University of Vermont Animal Care Facility, an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facility. All procedures were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All euthanasia was under isoflurane anesthesia according to NIH guidelines.
2.2 In-vivo efflux transporter inhibition with continuous EEG and video monitoring
2.2.1 EEG electrode implantation surgery
To determine the role of BBB efflux transporters in seizure prevention in pregnancy, spontaneous seizures were monitored for 8 hours post-efflux transporter inhibition in freely moving rats (n = 8/group). Nonpreg and Preg rats (day 12–14 of pregnancy) were implanted with a custom-made EEG implant under isoflurane anesthesia. A short range of gestational time points was necessary for EEG implantation due to the timed-nature of pregnancy such that all rats could consistently be used experimentally on day 19 of gestation. EEG electrodes were placed within the primary motor cortex at coordinates: 2.0 mm anterior, 2.0 mm lateral, and 2.5 mm ventral to Bregma, and in the dorsal CA3 region of the hippocampus at coordinates: −3.8 mm posterior, 3.0 mm lateral, 3.5 mm ventral to Bregma (Supplementary Figure 1A and 1B).29 In preliminary studies, we confirmed appropriate placement of EEG electrodes using fluorescence microscopy (Supplementary Figure 1C and 1D). See Supplementary Material for more information regarding EEG implant placement.
2.2.2 In-vivo electrophysiology and video monitoring
After a 5–7 day recovery period (gestational day 19 for Preg rats), continuous intracranial EEG from the primary motor cortex and the hippocampus was performed for 8 hours together with video monitoring. Baseline EEG recordings were taken for 2 hours before animals were treated with PSC833 (10 mg/kg s.c., dissolved in 65% kolliphor and 35% ethanol), a P-gp inhibitor, and probenecid (200 mg/kg i.p., dissolved in NaOH), a broad spectrum multidrug resistance protein (Mrp), breast cancer resistance protein (BCRP), and organic anion transporter (OAT) inhibitor.30–34 An additional group of Nonpreg rats (n = 3) received vehicle control injections of kolliphor and ethanol (65%/35%) s.c. and NaOH i.p. EEG and time-synced video monitoring were recorded for 8 hours post-treatment. After recording, animals were anesthetized with isoflurane anesthesia and euthanized. Serum was collected via cardiac puncture and stored at −80 °C. See Supplementary Material for details regarding EEG and video monitoring, and assessment of seizure activity.
2.3 In-vitro electrophysiology
In order to test the effect of probenecid and PSC833 themselves on neuronal excitability, hippocampal slice electrophysiology was performed. A separate group of Nonpreg rats (n = 4) were anesthetized with isoflurane (3 % in oxygen) and euthanized by decapitation. Brains were immediately removed and put into ice-cold artificial cerebrospinal fluid (aCSF) bubbled with carbogen gas at pH 7.3. Hippocampi were quickly dissected out of the brain and 400 μm coronal sections were visualized with an upright Nikon Eclipse E600 FN microscope and a bipolar stimulating electrode placed in the dentate gyrus and recording electrode in CA3 (Supplementary Figure 2A). Please refer to Supplementary Material for details on acquisition of hippocampal slices and electrophysiological recordings. Evoked responses confirmed slice viability prior to exposure to aCSF containing 4.16 μM PSC833 and 100 μM probenecid, or vehicle, and network activity recorded for 30 minutes (n = 3/group). The concentration of PSC833 was chosen based on the IC50 being 4.16 μM reported to inhibit transporters at the BBB in mice.35 In addition, 100 μM of probenecid was chosen because it is the maximum concentration found to be present in the interstitial fluid of the rat hippocampus after an acute intravenous infusion.36 Three hundred 500 ms sweeps were averaged at the beginning, middle and end of the 30-minute recording period for each treatment condition and network activity compared by determining the presence or absence of spikewave activity of amplitude > 2 standard deviations from baseline.
2.4 Measurement of P-gp activity in freshly isolated capillaries
Efflux transporter activity was measured using a calcein accumulation assay that has been previously described with modifications.17 To compare P-gp activity between Nonpreg and Preg rats, cortical (n = 13–16/group) and hippocampal (n = 8/group) capillaries were isolated from separate groups of Nonpreg and Preg rats. Capillaries were transferred to a 6-well glass-bottom plate containing pre-warmed 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered physiological salt solution. Capillaries were equilibrated for 15 minutes at 37 °C in HEPES buffer. Experiments measuring basal efflux transporter activity had calcein-AM (25 nM) added to the bath and calcein fluorescence intensity was measured in the capillary wall using a fluorescent microscope at 40X with an attached photomultiplier tube (IonOptix, Milton, MA) for 30 minutes. Some capillaries were incubated with the P-gp inhibitor zosuquidar (90 nM) for 15 minutes prior to the addition of calcein-AM as a positive control for calcein accumulation. To determine the effect of circulating factors in pregnancy on P-gp activity, cortical and hippocampal capillaries were isolated from Nonpreg rats and incubated in 20% serum from either Nonpreg or Preg rats (pooled from 12 rats/group and collected previously) for 15 min prior to performing the calcein accumulation assay. The order of capillary exposure to different serum types was randomized for each animal using an online randomizing tool (random.org). Serum clouded the bath and prevented fluorescence readings and therefore was not present during the recordings. The rate of accumulation of calcein was calculated 1200s after the addition of calcein-AM for cortical and hippocampal capillaries. See Supplementary Material for more details on the measurement of P-gp activity in isolated capillaries.
2.5 Quantification of P-gp (Mdr1) expression and capillary density in the cortex and hippocampus by immunohistochemistry
Rats (n = 5–6/group) were deeply anesthetized using isoflurane (3%) in O2, immediately decapitated and brains removed. Trunk blood was collected and serum was stored at −80°C. Coronal brain slices that were 3 mm were taken of the motor cortex anterior to the location of the middle cerebral artery, and hippocampus 4 mm posterior to Bregma in the posterior cerebral cortex and fixed in 10 % buffered formalin overnight at 4° C then transferred to 0.1 M phosphate-buffered saline (PBS) and slices paraffin embedded. P-gp expression and capillary density in the cortex and CA3 region of the hippocampus were determined by immunohistochemical staining for P-gp and collagen IV, respectively. A reviewer blinded to group performed all assessments. For detailed information regarding the immunohistochemical protocol and confocal microscopy please see Supplementary Material.
2.6 Measurement of circulating factors via enzyme-linked immunosorbent assays (ELISAs)
To determine the relationship between circulating estradiol and latency to seizures, a commercially available ELISA kit for estradiol (Caymen Chemical Company, Ann Arbor, MI) was used to measure serum estradiol in each animal (N = 12; n = 5 Nonpreg and n = 7 Preg). To determine the potential role of circulating VEGF and TNFα in susceptibility to seizures in response to efflux transporter inhibition, commercially available rat ELISA kits for VEGF and TNFα were purchased from R&D Systems (Minneapolis, MN). Serum levels of VEGF and TNFα were measured in rats that underwent in-vivo BBB efflux transporter inhibition with chronically implanted EEG electrodes and in pooled serum samples used in the calcein accumulation assay. Samples were measured undiluted and in duplicate.
2.7 Quantification and morphological assessment of microglia
Separate groups of Nonpreg and Preg rats (n = 5/group) were euthanized under isoflurane anesthesia, and brains immediately removed. Coronal sections of the posterior cerebral cortex were fixed and immunohistochemical staining for ionized calcium-binding adapter molecule 1 (Iba1) performed as previously described.37 For each brain section, six micrographs of the hippocampus (two from dentate gyrus, two from CA3, and two from CA1) were captured using an Olympus BX50 microscope at 20X magnification. Morphology of each Iba1+ cell was determined and used to rank its activation state using a previously established scale from 1 (relatively inactive) to 4 (relatively active).4,37 To assess microglia, two analyses were performed: the percentage of cells in each activation state was calculated for each micrograph and averaged per group, and total number of Iba1+ cells were counted per mm2 and averaged for each group. Images were randomized and all morphological assessments completed by an evaluator blinded to group.
2.8 Drugs and solutions
PSC833, probenecid, calcein-AM, HEPES and MOPS were purchased from Sigma-Aldrich (St. Louis, MO). PSC833 was dissolved in 65% kolliphor and 35% ethanol and stored at −20 °C until use.38 Probenecid was dissolved in 1.5 mL 0.1M NaOH and the pH was brought to 7.4 with HCl and was made fresh daily.31 Calcein-AM and zosuquidar were diluted in dimethyl sulfoxide. Please see Supplementary Material for details on buffers used in in-vitro electrophysiological experiments and in-vitro calcein accumulation assays.
2.9 Statistical analyses
The number of animals used for in-vivo and in-vitro experiments were justified by statistical power calculations based upon our previous seizure study using pregnant and nonpregnant rats,4 pilot data, and our previous studies using similar methodology.4,39 All data presented are mean ± standard error. To compare latency to seizure, total number of seizures and seizure duration between Nonpreg and Preg rats, Mann-Whitney test was used. Serum estradiol concentrations from Nonpreg and Preg rats were compared using Mann-Whitney test. To determine if there was a relationship between serum estradiol and latency to seizure, a linear regression analysis and Pearson’s correlation was performed using GraphPad 6 (GraphPad Software Inc., La Jolla, CA). For the calcein-accumulation assay, the rates of accumulation of fluorescence at 1200s were calculated by the equation: rate = Δy/Δx, where Δy is the change in fluorescence intensity between 1195–1205s for each treatment per capillary, and Δx is the change in time (10s). Rates were then averaged within treatment groups, and compared between groups using Mann-Whitney test. Changes in expression of P-gp, capillary density, number of microglial, and microglial activation states were compared between Preg and Nonpreg rats using Mann-Whitney test. Differences were considered significant at p < 0.05.
3. Results
3.1 The effect of BBB efflux transporter inhibition on spontaneous hippocampal seizures
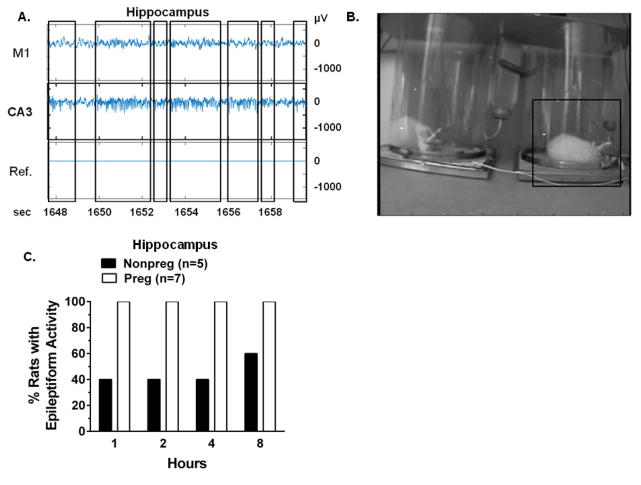
To provide proof-of-principle evidence that BBB efflux transporters protect the maternal brain from seizure-provoking factors, we inhibited BBB efflux transporters in-vivo in Nonpreg and Preg rats and monitored for spontaneous seizures. Inhibition of efflux transporters induced hippocampal epileptiform activity, as seen in a representative raw EEG tracing from a Preg rat 2 hours post-efflux transporter inhibition (Figure 1A). Evident high frequency (40–60 Hz), high amplitude rhythmic spike bursts occurred in the hippocampal EEG that were not associated with changes in primary motor cortex EEG (Figure 1A). The corresponding time-synced video recording showed no changes in behavior during hippocampal epileptiform activity. A video image corresponding to the raw EEG tracing showed that the rat experiencing hippocampal epileptiform activity had no apparent physical manifestations of convulsion (Figure 1B). Within 1 hour post-efflux transporter inhibition, 100% of Preg rats exhibited epileptiform discharges on hippocampal EEG, whereas only 40% of Nonpreg rats had epileptiform activity in the hippocampus (Figure 1C). This pattern continued and by 8 hours post-inhibition only 60% of Nonpreg rats had hippocampal epileptiform activity compared to the sustained presence of hippocampal epileptiform activity in all Preg rats (Figure 1C). The disposition of hippocampal epileptiform activity to occur in Preg rats was also apparent in the latency to discharge onset. Hippocampal seizures began rapidly in all Preg rats, with a latency to onset being 23 ± 3 minutes, compared to a slower, more variable onset in the three Nonpreg rats that developed spontaneous hippocampal discharges with a latency of 172 ± 138 minutes (p = 0.117). Two Nonpreg rats were excluded due to technical issues with baseline EEG recording and one Nonpreg rat died during the electrode implant surgery. One Preg rat was excluded because it exhibited epileptiform discharges during baseline EEG recording. There was no hippocampal epileptiform activity in rats that were treated with vehicle (data not shown), verifying that the discharges measured were not due to the electrode implants, but rather an effect of efflux transporter inhibition. Thus, the hippocampus was more susceptible to seizures in response to efflux transporter inhibition during pregnancy.
Figure 1. Inhibition of blood-brain barrier efflux transporters caused hippocampal seizure activity predominantly in pregnant rats.
(A) Representative raw EEG tracings from a pregnant (Preg) rat 2 hours post-inhibition of efflux transporters showing high amplitude fast epileptiform spikes in the CA3 region of the hippocampus (boxed insets, middle trace). Epileptiform activity did not correspond to changes in EEG signal in the primary motor cortex M1 (top trace) or in the reference electrode (Ref., bottom trace). (B) Time-synced video image shows the rat on the right (boxed inset) demonstrating no behavioral changes associated with the epileptiform activity exhibited in CA3 in panel A. (C) Quantification of the percent of nonpregnant (Nonpreg, n = 5) and Preg (n = 7) rats exhibiting hippocampal epileptiform activity 1, 2, 4 and 8 hours post-inhibition of efflux transporters. 100% of Preg rats demonstrated epileptiform activity in the hippocampus within 1 hour after efflux transporter inhibition that was sustained throughout the 8 hour recording time. 40% of Nonpreg rats exhibited epileptiform activity at 1, 2 and 4 hours post-inhibition, and 60% after 8 hours.
To determine that the seizure-promoting effects were due to efflux transporter inhibition and not to PSC833 and probenecid passing into the brain and causing neuronal excitability themselves, we acutely exposed hippocampal slices to aCSF containing PSC833 and probenecid and measured network excitability for 30 minutes. Supplementary Figure 2B shows representative electrophysiological recordings of an evoked response to confirm slice viability (top panel), and network activity during exposure to probenecid and PSC833, and vehicle. There was no difference in network activity between baseline conditions or after treatment with probenecid and PSC833, or vehicle. The oscillations in response to stimulation confirm that the hippocampal slices were healthy. Thus, the presence of probenecid and/or PSC833 in the hippocampus did not elicit epileptiform activity, supporting that epileptiform discharges in the hippocampus were in response to efflux transporter inhibition and not secondary effects of PSC833 or probenecid on neuronal/glial activity.
3.2 The effect of BBB efflux transporter inhibition on spontaneous primary motor seizures
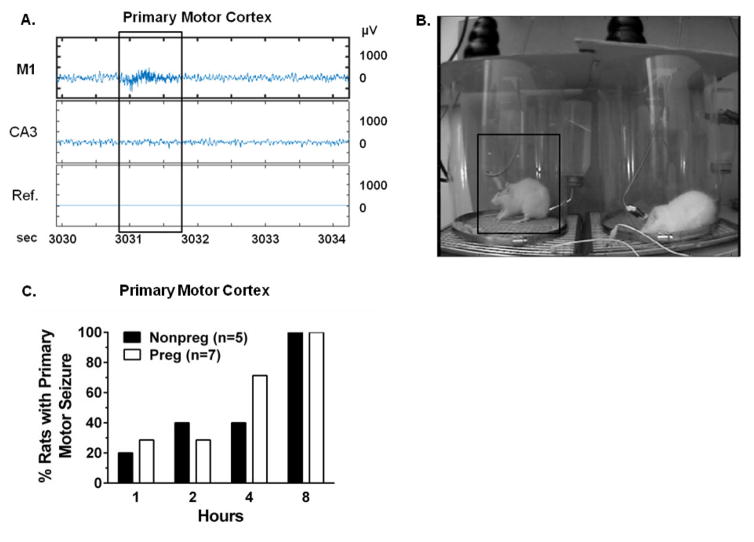
Inhibition of efflux transporters resulted in epileptiform activity in the primary motor cortex, as seen in a representative raw EEG tracing from a Nonpreg rat 2 hours post-efflux transporter inhibition (Figure 2A). The boxed inset highlights a region of high frequency epileptiform activity (> 200 Hz) in the primary motor cortex that was not present in the hippocampus. Time-synced video showed behavioral changes consisting of bilateral forelimb tone (Racine score of 4) in this rat that was associated with epileptiform discharge on EEG (Figure 2B). Both Nonpreg and Preg rats demonstrated epileptiform activity in the primary motor cortex that progressed over time after efflux transporter inhibition. By 4 hours post-transporter inhibition, 40% of Nonpreg and ~70% of Preg rats had behavioral seizures, and within 8 hours after efflux transporter inhibition, 100% of rats in both groups exhibited epileptiform discharges in the primary motor cortex on EEG that were associated with physical manifestation of a behavioral seizure (Figure 2C). Temporal development of seizures after efflux transporter inhibition did not differ between Nonpreg and Preg rats, as the latency to EEG epileptiform discharges in the primary motor cortex coupled with physical manifestation of seizure was similar between groups (250 ± 72 vs. 190 ± 48 minutes, respectively; p > 0.05). Within the 8-hour recording period after efflux transporter inhibition, Nonpreg rats had 6 ± 2 seizures and Preg rats had 4 ± 2 seizures (p > 0.05) that were of similar severity, with average Racine scores being 2.9 ± 0.5 for Nonpreg and 3.0 ± 0.2 for Preg rats (p > 0.05). The duration of epileptiform activity in the primary motor cortex post-efflux transporter inhibition was also similar between Nonpreg and Preg rats, with epileptiform activity lasting 2.36 ± 0.48 sec in Nonpreg rats and 2.15 ± 0.20 sec in Preg rats (p > 0.05). No epileptiform activity in the primary motor cortex was evident on EEG in rats treated with vehicle confirming seizure activity was not due to the electrode implantation (data not shown).
Figure 2. Blood-brain barrier efflux transporter inhibition caused behavioral seizures in the primary motor cortex.
(A) Representative raw EEG tracings of a nonpregnant (Nonpreg) rat 2 hours after efflux transporter inhibition. A high frequency epileptiform discharge was present in the M1 EEG (top trace inset) that was absent from the hippocampal EEG (middle trace) with no change in reference signal (Ref.; bottom trace). (B) Time-synced video image associated with the epileptiform activity in the primary motor cortex from panel A. The rat on the left demonstrated bilateral tonic extension of the forelimbs (boxed inset). (C) Quantification of the incidence of behavioral seizures in Nonpreg (n = 5) and pregnant (Preg, n = 7) rats 1, 2, 4 and 8 hours post-efflux transporter inhibition. The percent of Nonpreg and Preg rats exhibiting behavioral seizures increased over time, with 100% of rats exhibiting behavioral seizures 8 hours after efflux transporter inhibition.
3.3 Relationship between serum estradiol levels and seizure onset
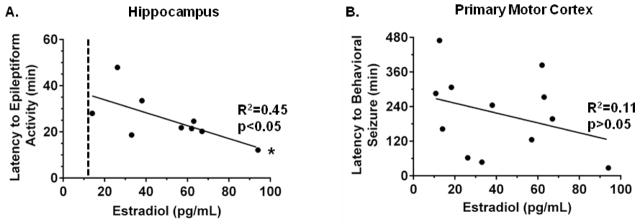
Estradiol has pro-convulsive effects and is also an efflux transporter substrate that can inhibit transporter activity.6,24–26,40 We therefore measured estradiol levels and assessed the relationship between estradiol and latency to seizure. As expected, estradiol levels were higher during pregnancy with a serum concentration of 52.7 ± 8.7 pg/mL in Preg rats compared to 25.0 ± 8.4 pg/mL in Nonpreg rats (p < 0.05). Since all rats exhibited seizures when efflux transporters were inhibited, the relationship between serum estradiol and the latency to seizures was determined (Figure 3). There was a significant negative correlation between serum estradiol concentration and latency to hippocampal epileptiform activity (R2 = 0.45, p < 0.05; Figure 3A), indicating that rats with higher levels of serum estradiol exhibited hippocampal epileptiform discharges sooner after efflux transporter inhibition. In fact, the only two Nonpreg rats that demonstrated hippocampal epileptiform activity in response to efflux transporter inhibition had the highest serum estradiol levels within the Nonpreg group (26.2 pg/mL and 57.0 pg/mL). Nonpreg rats that did not develop hippocampal epileptiform activity had estradiol levels of < 12.8 pg/mL (Figure 3A). In the cortex, however, there was a negative correlation between estradiol and latency to behavioral seizures but this did not reach statistical significance (R2 = 0.11, p > 0.05; Figure 3B). One rat was excluded from the comparison of latency to epileptiform activity to serum estradiol level because its latency to onset was a statistical outlier (> 2 standard deviations from the mean).
Figure 3. Serum estradiol levels correlated with latency to epileptiform activity in the hippocampus.
(A) Correlation of serum estradiol concentrations and latency to hippocampal epileptiform activity after efflux transporter inhibition (n = 9). The higher the serum levels of estradiol, the shorter the latency to epileptiform discharges within the hippocampus in response to efflux transporter inhibition. The dotted line represents an estimated estradiol serum threshold; Nonpreg rats with estradiol levels below threshold did not develop epileptiform discharges. (B) Correlation of serum estradiol concentrations and latency to behavioral seizure onset after efflux transporter inhibition (n = 12). * p < 0.05 by linear regression analysis and Pearson’s correlation.
3.4 The effect of pregnancy on basal P-gp activity in cortical and hippocampal capillaries
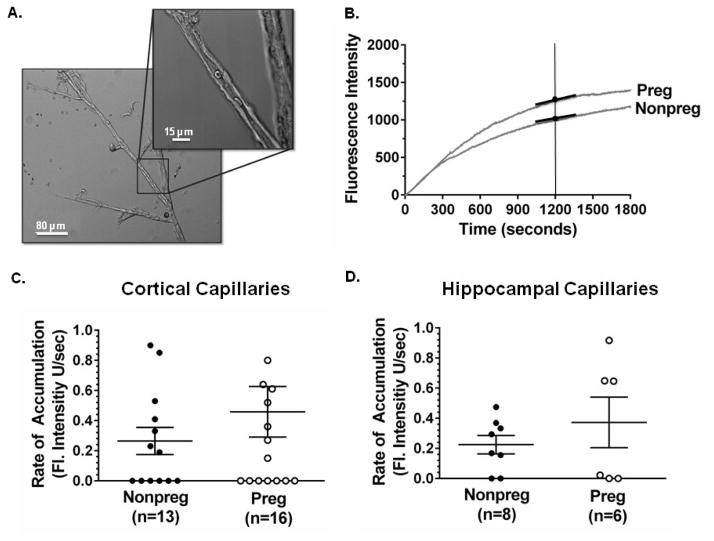
To determine if pregnancy-induced changes in P-gp activity at the BBB contributed to the increased susceptibility of the hippocampus to seizures in response to efflux transporter inhibition, basal P-gp activity of freshly isolated hippocampal capillaries from Preg and Nonpreg rats was measured using a calcein accumulation assay and compared to that of the cortex. Figure 4A shows a photomicrograph of a capillary tree isolated from the cerebral cortex of a Nonpreg rat used for the calcein accumulation assay. All hippocampal and cortical capillaries were less than 8 μm in lumen diameter and similar between groups (data not shown). Hippocampal capillaries from two Preg rats in the calcein group were excluded due to technical difficulties. Because P-gp is an efflux transporter, increased calcein accumulation and fluorescence intensity indicated reduced P-gp activity. Capillaries that were exposed to the P-gp inhibitor zosuquidar had a significantly increased rate of accumulation compared to control capillaries (Supplementary Figure 3), confirming functional P-gp in isolated capillaries and that the assay was sensitive enough to detect changes in P-gp activity. Figure 4B shows representative tracings of fluorescence intensity of capillaries from Preg and Nonpreg rats over the recording time of 1800 seconds. The rate of accumulation of calcein was calculated at 1200 seconds as a quantification of basal P-gp activity. The rate of accumulation of calcein was similar in Preg and Nonpreg rats in cortical capillaries (Figure 4C) as well as hippocampal capillaries (Figure 4D), suggesting regionally similar basal levels of P-gp activity in the pregnant and nonpregnant states.
Figure 4. Basal P-gp activity of cortical and hippocampal capillaries in pregnancy.
(A) Photomicrograph of a capillary tree isolated from the cerebral cortex of a nonpregnant (Nonpreg) rat. Boxed inset represents the capillary segment used for the calcein accumulation assay. (B) Representative tracings of calcein fluorescence accumulation in capillaries from Nonpreg and pregnant (Preg) rats. The rate of accumulation at 1200s (slope of the tangent) was used to determine P-gp activity. As P-gp is an efflux transporter, the greater the fluorescence accumulation, the less P-gp activity. (C) Graph showing the rate of accumulation of fluorescence as a measure of P-gp activity in cortical capillaries from Nonpreg (n = 13) and Preg (n = 16) rats. P-gp activity was similar between capillaries from Nonpreg and Preg rats. (D) Graph showing the rate of accumulation of fluorescence as a measure of P-gp activity in hippocampal capillaries from Nonpreg (n = 8) and Preg (n = 6) rats. P-gp activity was similar in capillaries from Nonpreg and Preg rats.
3.5 The effect of circulating factors during pregnancy on P-gp activity
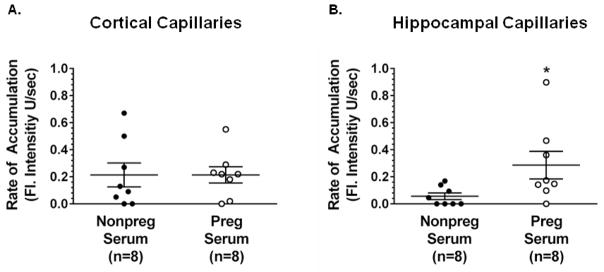
In addition to being seizure-provoking, serum constituents that are elevated during pregnancy have inhibitory effects on P-gp activity that could potentially put the maternal brain at risk.15,16,20,41 To determine the effect of circulating factors during pregnancy on P-gp activity, we quantified P-gp activity after acute exposure to serum from Preg and Nonpreg rats. Figure 5A shows the rate of accumulation of calcein in cortical capillaries from Nonpreg rats that have been exposed to either serum from Nonpreg or Preg rats. There was no difference in P-gp activity between cortical capillaries exposed to serum from Nonpreg or Preg rats. However, hippocampal capillaries exposed to serum from Preg rats had a significantly higher rate of accumulation of calcein, indicating significantly decreased P-gp activity compared to capillaries exposed to serum from Nonpreg rats (Figure 5B). Thus, serum from Preg rats had a differential effect on activity of P-gp in cortical versus hippocampal capillaries such that there was an inhibitory effect on P-gp activity in the hippocampus.
Figure 5. P-gp activity in cortical and hippocampal capillaries after exposure to serum from nonpregnant (Nonpreg) or pregnant (Preg) rats.
(A) Graph showing the rate of accumulation of fluorescence as a measure of P-gp activity in cortical capillaries from Nonpreg rats exposed to serum from Nonpreg or Preg rats (n = 8/group). P-gp activity was similar between cortical capillaries exposed to serum from Nonpreg and Preg rats. (B) Graph showing the rate of accumulation of fluorescence as a measure of P-gp activity in hippocampal capillaries from Nonpreg rats exposed to serum from Nonpreg or Preg rats. P-gp activity was significantly lower in capillaries exposed to serum from Preg rats compared to those exposed to serum from Nonpreg rats. *p < 0.05 by Mann-Whitney test.
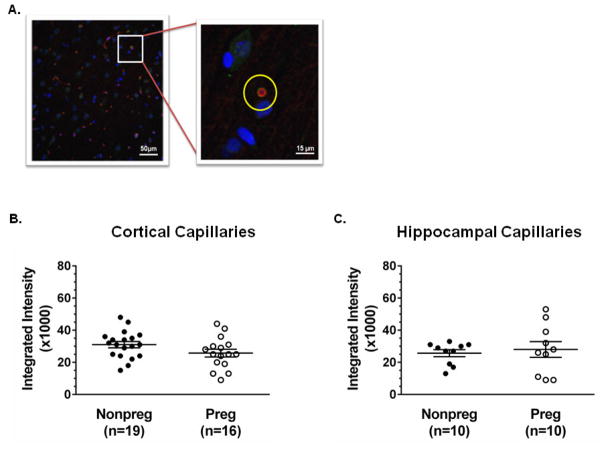
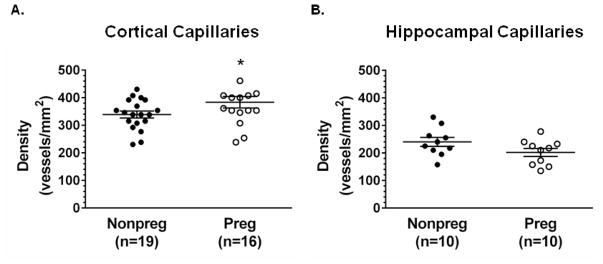
3.6 P-gp expression and capillary density in the cortex and hippocampus in pregnancy
To determine if pregnancy-induced changes in P-gp expression at the BBB occurred in a region-specific manner that contributed to increased susceptibility of the hippocampus to seizures, we quantified P-gp expression in both cortical and hippocampal capillaries from Nonpreg and Preg rats. Figure 6A shows a representative photomicrograph depicting a brain section stained for P-gp, collagen IV and DAPI (94′, 6-diamidino-2-phenylindole). The inset depicts an example of a P-gp+/collagen IV+ capillary in cross-section used to quantify P-gp expression in relation to the luminal diameter of the capillary. P-gp expression was similar between groups in both cortical capillaries (Figure 6B) and capillaries in the CA3 region of the hippocampus (Figure 6C). However, capillary density was significantly increased in the cerebral cortex during pregnancy compared to the nonpregnant state (Figure 7A). In the CA3 region of the hippocampus, however, capillary density was not different in Preg compared to Nonpreg rats (Figure 7B).
Figure 6. P-gp expression in cortical and hippocampal capillaries.
(A) Representative confocal micrograph of the cerebral cortex of a nonpregnant (Nonpreg) rat stained for P-gp (red), collagen IV (green), and DAPI (blue). The yellow circle (inset) represents a region of interest assigned to a capillary in cross-section used for fluorescent intensity measurements. (B) Quantification of integrated intensity of P-gp staining of cortical capillaries in Nonpreg and pregnant (Preg) rats (n = 16–19 images from 8–10 rats/group). There was no change in P-gp expression in capillaries from Preg compared to Nonpreg rats; p = 0.099 by Mann-Whitney test. (C) Quantification of integrated intensity of P-gp staining of capillaries in the CA3 region of the hippocampus of Nonpreg and Preg rats (n = 10 images from 5 rats/group). P-gp expression was similar in the hippocampus between groups.
Figure 7. Changes in capillary density in the cortex and hippocampus during pregnancy.
(A) The density of capillaries was significantly increased in the cortex of pregnant (Preg) rats compared to nonpregnant (Nonpreg) rats (n = 16–19 images from 8–10 rats/group). (B) Capillary density was similar in the CA3 region of the hippocampus of Preg compared to Nonpreg rats (n = 10 images from 5 rats/group). * p < 0.05 by Mann-Whitney test.
3.7 Circulating levels of TNFα and VEGF in pregnancy
To determine if elevated circulating VEGF and/or TNFα in pregnancy contributed to the susceptibility of the hippocampus to seizure in response to efflux transporter inhibition, or the inhibitory effect of Preg serum on P-gp activity in hippocampal capillaries, serum levels of TNFα and VEGF were measured via ELISA. Serum levels of TNFα from Preg and Nonpreg rats that underwent efflux transporter inhibition in-vivo were below the detection limit of the ELISA (< 5 pg/ml). Further, TNFα levels in serum pooled from Preg and Nonpreg rats that were used in the calcein accumulation assay were also below the detection limit of the ELISA. Serum VEGF levels of Nonpreg rats that had BBB efflux transporters inhibited in-vivo was 23.28 ± 2.43 pg/mL, however, VEGF levels in Preg rats were below the detection limit of the ELISA (< 8 pg/mL). Further, VEGF levels in the pooled serum samples used in the calcein-accumulation assay were 18.38 ± 2.73 pg/mL in serum from Nonpreg rats, but below the detection limit of the ELISA for serum from Preg rats. Thus, it is unlikely that circulating TNFα or VEGF were contributors to the pregnancy-specific effects on hippocampal seizures in response to efflux transporter inhibition, or the acute inhibitory effects of Preg serum on P-gp activity in hippocampal capillaries.
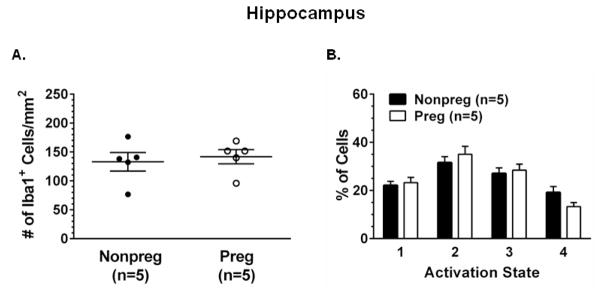
3.8 The effect of pregnancy on neuroinflammation
Lastly, to determine if neuroinflammation contributed to the susceptibility to spontaneous hippocampal seizures in response to BBB efflux transporter inhibition in pregnancy, we determined the activation state of microglia in the hippocampus of Preg and Nonpreg rats. The density of Iba1+ microglia (Figure 8A) and the percent of microglia in each activation state (Figure 8B) were similar between Preg and Nonpreg rats.
Figure 8. Basal activation state of microglia in the hippocampus of nonpregnant (Nonpreg) and pregnant (Preg) rats.
(A) Quantification of the average number of Iba1+ microglia in the hippocampus of Nonpreg and Preg rats (n = 5 rats/group) showed similar number of microglial between groups. (B) The percent of Iba1+ microglia in each activation state was similar in the hippocampus of Nonpreg and Preg rats.
4. Discussion
The main finding of the current study was that acute inhibition of efflux transporters resulted in spontaneous seizures that were more pronounced in the hippocampus during pregnancy and not due to neuroinflammation. The increased sensitivity of the hippocampus to seizures in response to efflux transporter inhibition may be due to an inhibitory affect of circulating factors in pregnancy on P-gp activity. Although basal P-gp activity and expression were similar between the pregnant and nonpregnant states, serum factors present late in gestation exerted an inhibitory effect on P-gp activity in capillaries specifically within the hippocampus, this effect was absent in capillaries from the cerebral cortex. These results importantly provide proof-of-principle evidence that BBB efflux transporters are critical in seizure prevention in pregnancy and suggest that serum constituents present late in pregnancy affect P-gp activity and have the ability to promptly initiate seizures when their passage into the hippocampus is unregulated.
The regional difference in seizure susceptibility during pregnancy in response to efflux transporter inhibition is not clear, but may be related to regional differences in sensitivity to efflux transporter inhibition. Using freshly isolated capillaries, we found an inhibitory effect of serum from Preg rats occurred only in capillaries within the hippocampus, and not the cerebral cortex. Based on this finding, we speculate that the hippocampus was prone to seizure in response to efflux transporter inhibition during pregnancy at least partially due to greater sensitivity of hippocampal efflux transporters to inhibition. Region-specific differences in P-gp activity, both basally and in response to inhibition, have been reported in the rodent brain.42,43 For example, P-gp in the hippocampus was more susceptible to pharmacological inhibition with elacridar, resulting in greater passage of verapamil into the hippocampus than other brain regions as detected via positron emission tomography imaging, indicating overall lower P-gp activity in the hippocampus.43 In the current study, increased susceptibility of hippocampal P-gp to inhibition did not appear to be due to regional differences in basal activity or expression, but only in response to inhibition by serum from pregnant animals. While further studies are needed to specifically determine the mechanism by which there is regional sensitivity to efflux transporter inhibition in response to Preg serum, the regional effect could promote increased susceptibility of the hippocampus to seizures in response to efflux transporter inhibition.
BBB efflux transporters have endogenous substrates that are elevated during pregnancy, including TNFα and growth factors such as VEGF.15,16,20,41 In the current study, circulating levels of TNFα were below the detectable limit, potentially due to the presence of soluble TNFα receptors during pregnancy.44,45 Thus, it is unlikely that TNFα contributed to the increased susceptibility of the hippocampus to seizures in response to efflux transporter inhibition during normal pregnancy. However, unlike TNFα that acutely inhibits P-gp activity but induces upregulation and activity of P-gp at the BBB,16,17 VEGF rapidly and reversibly inhibits P-gp activity without affecting P-gp expression, and is also elevated in pregnancy.15,21,22 Thus, VEGF could exert an inhibitory effect on P-gp and increase the sensitivity to efflux transporter inhibition in pregnancy, thereby potentiating the onset of hippocampal seizures. However, the bioavailability of circulating VEGF during pregnancy is regulated by soluble receptors soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble fetal liver kinase 1 (sFlk-1) that may limit its inhibitory effects on P-gp.46 Interestingly, in the current study, serum VEGF levels from Preg rats, but not Nonpreg rats, were undetectably low, supporting that soluble VEGF receptors are elevated in pregnancy. As VEGF exerts its inhibitory effects on P-gp through interaction with its receptor VEGFR-2 (Flk-1), the sequestering of VEGF by sFlk-1 and sFlt-1 likely eliminates the potential of VEGF to inhibit P-gp activity and increase the susceptibility of the hippocampus to seizures in response to BBB efflux transporter inhibition during pregnancy. Although the current study did not identify the specific serum constituent(s) during pregnancy responsible for P-gp inhibition in the hippocampus, these factors may be potential biomarkers of women who may be at greater risk of acute failure of BBB efflux transporters and de novo seizures.
In addition to inflammatory and growth factors, neuroactive steroids are elevated in pregnancy that can affect efflux transporters and hippocampal excitability that may also contribute to the pregnancy-specific increase in susceptibility of the hippocampus to seizures. For example, estrogen is increased 10-fold in late-pregnancy and is a substrate inhibitor of the BBB efflux transporter BCRP.23,40,47,48 In addition, acute application of estradiol rapidly (within seconds to minutes) increases neuronal inward Na+ currents and attenuates outward voltage-gated K+ currents in an estrogen receptor-independent manner by acting as an open-channel block and depolarizing hippocampal pyramidal neurons.26,49,50 The findings of the current study that estradiol levels significantly correlated to latency of hippocampal seizures supports the pro-convulsive nature of estradiol. Thus, the overall inhibitory effect of circulating factors in pregnancy on hippocampal BBB efflux transporters, that may include estrogen and other growth factors and inflammatory mediators, may also increase the passage of pro-convulsive factors such as estradiol into the hyperexcitable maternal hippocampus.
That BBB efflux transporter inhibition caused spontaneous seizures provides the first evidence that we are aware of that de novo seizure can occur during pregnancy in the absence of injury or edema formation. Disruption of the BBB is linked to seizure onset in several pathologic conditions including traumatic brain injury and hypertensive encephalopathy (HE).51–54 Cerebral edema is the hallmark of these conditions indicative of BBB disruption that is thought to contribute to seizure onset.55 However, there are a few conditions in which de novo seizure occurs as an isolated event in a non-progressive manner (i.e., not a form of epilepsy) and without clear etiology, and pregnancy is one of them. Further, we have previously shown that pregnancy is not associated with basal changes in BBB tight junction expression, increased BBB permeability, or cerebral edema.5,56,57 In some cases, eclampsia may be considered to be a form of HE in that acute hypertension associated with preeclampsia leads to BBB disruption, vasogenic edema formation and seizure.58,59 However, eclampsia has been reported to occur in up to ~ 40% of women with seemingly uncomplicated pregnancies, that is without hypertension or brain edema.3 Under these conditions, impaired function of efflux transporters at the BBB may represent one mechanism by which eclamptic seizure occurs during seemingly uncomplicated pregnancies, as well as in preeclampsia, that is not associated with cerebral edema.
5. Conclusions
The current study provides proof-of-principal evidence that BBB efflux transporters are important in seizure prevention. Seizure occurred during efflux transporter inhibition independent of BBB tight junction disruption, edema, elevated VEGF, TNFα or neuroinflammation and was more pronounced in the hippocampus during pregnancy. Thus, efflux transporter inhibition may represent a mechanism by which eclamptic seizure occurs in seemingly uncomplicated pregnancies and preeclampsia in the absence of cerebral edema. While BBB efflux transporters are an obstacle in therapeutic drug delivery to the CNS,60 their role in seizure onset may be important, as it suggests that conditions in which efflux transporters are inhibited are at increased risk for seizures. Understanding the role efflux transporters play in seizure prevention, and how their function may be compromised in physiological and pathological states, may provide new insight into the etiology of seizure.
6. Acknowledgements
We thank Nicole Bishop in the Microscopy Imaging Center at the University of Vermont for her technical expertise in performing immunohistochemistry.
7. Funding
This work was supported by the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) grants R01 NS045940 (to MJC), R01 NS073083 (to GLH) and R01 NS078279 (to GLH), the Preeclampsia Foundation (to ESH and MJC), the Totman Medical Research Trust, and the Cardiovascular Research Institute of Vermont.
Supplementary Material
Highlights.
Blood-brain barrier efflux transporter inhibition caused spontaneous seizures
Efflux transporter inhibition differentially affected the hippocampus in pregnancy
Circulating factors in pregnancy inhibited p-glycoprotein activity in the hippocampus
Neuroinflammation did not contribute to seizure susceptibility in pregnancy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindheimer MD, Taylor RN, Roberts JM, Cunningham FG, Chesley LC. Introduction, History, Controversies, and Definitions. In: Taylor RN, Roberts JM, Cunningham FG, Lindheimer MD, editors. Chesley’s Hypertensive Disorders in Pregnancy. 4. Boston: Academic Press/Elsevier; 2014. [Google Scholar]
- 2.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309:1395–400. doi: 10.1136/bmj.309.6966.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson AC, Nagle KJ, Tremble SM, Cipolla MJ. The Contribution of Normal Pregnancy to Eclampsia. PloS one. 2015;10:e0133953. doi: 10.1371/journal.pone.0133953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cipolla MJ, Pusic AD, Grinberg YY, Chapman AC, Poynter ME, Kraig RP. Pregnant serum induces neuroinflammation and seizure activity via TNFalpha. Exp Neurol. 2012;234:398–404. doi: 10.1016/j.expneurol.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10:1295–312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 7.Borges-Walmsley MI, McKeegan KS, Walmsley AR. Structure and function of efflux pumps that confer resistance to drugs. Biochem J. 2003;376:313–38. doi: 10.1042/BJ20020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glavinas H, Krajcsi P, Cserepes J, Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv. 2004;1:27–42. doi: 10.2174/1567201043480036. [DOI] [PubMed] [Google Scholar]
- 9.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–77. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–17. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenstermacher J, Gross P, Sposito N, Acuff V, Pettersen S, Gruber K. Structural and functional variations in capillary systems within the brain. Ann N Y Acad Sci. 1988;529:21–30. doi: 10.1111/j.1749-6632.1988.tb51416.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends in pharmacological sciences. 2010;31:246–54. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:2723–33. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer B, Hartz AM, Fricker G, Miller DS. Modulation of p-glycoprotein transport function at the blood-brain barrier. Experimental biology and medicine (Maywood, NJ) 2005;230:118–27. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins BT, Sykes DB, Miller DS. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30:1417–25. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartz AM, Bauer B, Fricker G, Miller DS. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–70. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- 17.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–75. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 18.Szarka A, Rigo J, Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aagaard-Tillery KM, Silver R, Dalton J. Immunology of normal pregnancy. Semin Fetal Neonatal Med. 2006;11:279–95. doi: 10.1016/j.siny.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 21.Tandon V, Hiwale S, Amle D, Nagaria T, Patra PK. Assessment of Serum Vascular Endothelial Growth Factor Levels in Pregnancy-Induced Hypertension Patients. J Pregnancy. 2017;2017:3179670. doi: 10.1155/2017/3179670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004;25:103–13. doi: 10.1016/j.placenta.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 23.O’Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clinical chemistry. 1991;37:667–72. [PubMed] [Google Scholar]
- 24.Reddy DS. Pharmacology of endogenous neuroactive steroids. Critical reviews in neurobiology. 2003;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- 25.Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–8. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 26.Druzin M, Malinina E, Grimsholm O, Johansson S. Mechanism of estradiol-induced block of voltage-gated K+ currents in rat medial preoptic neurons. PloS one. 2011;6:e20213. doi: 10.1371/journal.pone.0020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 28.Racine RJ. Modification of seizure activity by electrical stimulation: cortical areas. Electroencephalogr Clin Neurophysiol. 1975;38:1–12. doi: 10.1016/0013-4694(75)90204-7. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Amsterdam; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- 30.Ortega I, Rodriguez M, Suarez E, Perez-Ruixo JJ, Calvo R. Modeling methadone pharmacokinetics in rats in presence of P-glycoprotein inhibitor valspodar. Pharm Res. 2007;24:1299–308. doi: 10.1007/s11095-007-9251-2. [DOI] [PubMed] [Google Scholar]
- 31.Vamos E, Pardutz A, Fejes A, Tajti J, Toldi J, Vecsei L. Modulatory effects of probenecid on the nitroglycerin-induced changes in the rat caudal trigeminal nucleus. Eur J Pharmacol. 2009;621:33–7. doi: 10.1016/j.ejphar.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 32.May K, Minarikova V, Linnemann K, et al. Role of the multidrug transporter proteins ABCB1 and ABCC2 in the diaplacental transport of talinolol in the term human placenta. Drug Metab Dispos. 2008;36:740–4. doi: 10.1124/dmd.107.019448. [DOI] [PubMed] [Google Scholar]
- 33.Watson CP, Dogruel M, Mihoreanu L, et al. The transport of nifurtimox, an anti-trypanosomal drug, in an in vitro model of the human blood-brain barrier: evidence for involvement of breast cancer resistance protein. Brain Res. 2012;1436:111–21. doi: 10.1016/j.brainres.2011.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab Dispos. 2006;34:690–5. doi: 10.1124/dmd.105.008219. [DOI] [PubMed] [Google Scholar]
- 35.Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. Influence of breast cancer resistance protein (Abcg2) and p-glycoprotein (Abcb1a) on the transport of imatinib mesylate (Gleevec) across the mouse blood-brain barrier. J Neurochem. 2007;102:1749–57. doi: 10.1111/j.1471-4159.2007.04808.x. [DOI] [PubMed] [Google Scholar]
- 36.Deguchi Y, Nozawa K, Yamada S, Yokoyama Y, Kimura R. Quantitative evaluation of brain distribution and blood-brain barrier efflux transport of probenecid in rats by microdialysis: possible involvement of the monocarboxylic acid transport system. J Pharmacol Exp Ther. 1997;280:551–60. [PubMed] [Google Scholar]
- 37.Johnson AC, Tremble SM, Chan SL, et al. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PloS one. 2014;9:e113670. doi: 10.1371/journal.pone.0113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan CS, Wong IL, Chan KF, et al. A New Class of Safe, Potent, and Specific P-gp Modulator: Flavonoid Dimer FD18 Reverses P-gp-Mediated Multidrug Resistance in Human Breast Xenograft in Vivo. Molecular pharmaceutics. 2015;12:3507–17. doi: 10.1021/mp500770e. [DOI] [PubMed] [Google Scholar]
- 39.Johnson AC, Cipolla MJ. Altered hippocampal arteriole structure and function in a rat model of preeclampsia: potential role in impaired seizure-induced hyperemia. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16676287. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imai Y, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Estrone and 17beta-estradiol reverse breast cancer resistance protein-mediated multidrug resistance. Jpn J Cancer Res. 2002;93:231–5. doi: 10.1111/j.1349-7006.2002.tb02162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anthony FW, Evans PW, Wheeler T, Wood PJ. Variation in detection of VEGF in maternal serum by immunoassay and the possible influence of binding proteins. Annals of clinical biochemistry. 1997;34(Pt 3):276–80. doi: 10.1177/000456329703400309. [DOI] [PubMed] [Google Scholar]
- 42.Rizzi M, Caccia S, Guiso G, et al. Limbic seizures induce P-glycoprotein in rodent brain: functional implications for pharmacoresistance. J Neurosci. 2002;22:5833–9. doi: 10.1523/JNEUROSCI.22-14-05833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuntner C, Bankstahl JP, Bankstahl M, et al. Dose-response assessment of tariquidar and elacridar and regional quantification of P-glycoprotein inhibition at the rat blood-brain barrier using (R)-[(11)C]verapamil PET. Eur J Nucl Med Mol Imaging. 2010;37:942–53. doi: 10.1007/s00259-009-1332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Austgulen R, Liabakk NB, Lien E, Espevik T. Increased levels of soluble tumor necrosis factor-alpha receptors in serum from pregnant women and in serum and urine samples from newborns. Pediatr Res. 1993;33:82–7. doi: 10.1203/00006450-199301000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Sedger LM, McDermott MF. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine & growth factor reviews. 2014;25:453–72. doi: 10.1016/j.cytogfr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Espinoza J, Uckele JE, Starr RA, Seubert DE, Espinoza AF, Berry SM. Angiogenic imbalances: the obstetric perspective. Am J Obstet Gynecol. 2010;203:17e1–8. doi: 10.1016/j.ajog.2009.10.891. [DOI] [PubMed] [Google Scholar]
- 47.Hartz AM, Mahringer A, Miller DS, Bauer B. 17-beta-Estradiol: a powerful modulator of blood-brain barrier BCRP activity. J Cereb Blood Flow Metab. 2010;30:1742–55. doi: 10.1038/jcbfm.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 49.Kow LM, Devidze N, Pataky S, Shibuya I, Pfaff DW. Acute estradiol application increases inward and decreases outward whole-cell currents of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2006;1116:1–11. doi: 10.1016/j.brainres.2006.07.104. [DOI] [PubMed] [Google Scholar]
- 50.Wong M, Moss RL. Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17 beta-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Res. 1991;543:148–52. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]
- 51.Tomkins O, Feintuch A, Benifla M, Cohen A, Friedman A, Shelef I. Blood-brain barrier breakdown following traumatic brain injury: a possible role in posttraumatic epilepsy. Cardiovasc Psychiatry Neurol. 2011;2011:765923. doi: 10.1155/2011/765923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seiffert E, Dreier JP, Ivens S, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–36. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marchi N, Tierney W, Alexopoulos AV, Puvenna V, Granata T, Janigro D. The etiological role of blood-brain barrier dysfunction in seizure disorders. Cardiovasc Psychiatry Neurol. 2011;2011:482415. doi: 10.1155/2011/482415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hesdorffer DC, Hauser WA, Annegers JF, Rocca WA. Severe, uncontrolled hypertension and adult-onset seizures: a case-control study in Rochester, Minnesota. Epilepsia. 1996;37:736–41. doi: 10.1111/j.1528-1157.1996.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 55.Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–9. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 56.Cipolla MJ, Sweet JG, Chan SL. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J Appl Physiol. 2011;110:329–39. doi: 10.1152/japplphysiol.01159.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Euser AG, Cipolla MJ. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension. 2007;49:334–40. doi: 10.1161/01.HYP.0000255791.54655.29. [DOI] [PubMed] [Google Scholar]
- 58.Cipolla MJ, Kraig RP. Seizures in Women with Preeclampsia: Mechanisms and Management. Fetal Matern Med Rev. 2011;22:91–108. doi: 10.1017/S0965539511000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz RB, Jones KM, Kalina P, et al. Hypertensive encephalopathy: findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 1992;159:379–83. doi: 10.2214/ajr.159.2.1632361. [DOI] [PubMed] [Google Scholar]
- 60.Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:1663–74. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.