Abstract
Introduction
The dystonias are a group of disorders defined by over-contraction of muscles leading to abnormal movements and postures. In recent years, enormous advances have been made in elucidating the neurobiological mechanisms responsible for many types of dystonia.
Methods
A literature review was conducted focusing on evolving concepts in dystonia genetics, anatomy and physiology.
Results
The list of genes related to dystonia has grown from a relatively small number to more than 100. Concepts regarding the neuroanatomical basis for dystonia have evolved from a relatively narrow focus on dysfunction of the basal ganglia to a broader motor network model in which the basal ganglia, cerebellum, cerebral cortex, and other brain regions play a key role. Physiologically, our understanding of the core abnormalities has matured; and numerous changes in neural signaling have been revealed in the basal ganglia, cerebellum and cortex.
Conclusion
Although the dystonias share certain clinical aspects such as over-contraction of muscles leading to abnormal movements and postures, they actually comprise a very clinically and etiologically heterogeneous group of disorders. Understanding their neurobiological basis is important for devising rational therapies appropriately targeted for specific subgroups of patients.
Keywords: Neurobiology, pathogenesis, pathophysiology, neurogenetics, neuroanatomy, neurophysiology
Introduction
More than 100 years ago, Oppenheim described a series of patients who had hypo-tonic muscles at rest, but hyper-tonic muscles during attempts to move. The increased muscle tone led to movements with an appearance that was stiff, twisting, and jerky. He coined the term dys-tonia, because he believed that abnormal neural control of muscle tone was the fundamental problem in these patients.
Since Oppenheim’s original descriptions, many additional clinical manifestations of dystonia have been reported, and a defect in the control of muscle tone is no longer considered the core problem. Dystonia is now defined as a disorder of excessive muscle contraction leading to involuntary twisting or repetitive movements, often with abnormal postures [1]. The clinical picture is remarkably heterogeneous. The many different clinical manifestations are classified according to four major dimensions that include age at onset, body region affected, temporal qualities, and whether there are accompanying neurological or medical problems.
The etiologies for dystonia are similarly heterogeneous [2]. Some are known to e caused by specific gene defects, with patterns of inheritance that may be dominant, recessive, X-linked, or mitochondrial. Others are acquired, such as those due to exposure to certain drugs or chemicals, physical trauma, and those related to infections or autoimmune processes. This review focuses on how traditional concepts regarding the neurobiology of dystonia have evolved following new evidence uncovered in recent years.
Pathogenesis of dystonia
Genetics
Enormous advances have been made in understanding the genetic basis for the dystonias in the past few years [3]. Only 5–10 years ago, most reviews on dystonia genetics focused on a list of 20–30 genes that had been assigned a “DYT” number. This approach is now considered inadequate for many reasons [4]. One reason is that the DYT numbers did not identify dystonia genes, but rather chromosomal loci based on statistical association studies of families. As a result, several entries in the DYT list ultimately could not be verified, or were found to be erroneous. In other cases, more than one gene was associated with the same DYT locus, or different DYT loci were associated with the same gene. The DYT designation was also clinically misleading, because it implied that dystonia was a frequent or major feature for all the associated disorders. This was not the case for some disorders that were given a DYT number, where a different movement disorder typically predominated, such as myoclonus-dystonia (DYT11) where myoclonus is the most consistent problem. Perhaps the major limitation of the DYT list was that it neglected a very large number of dystonia genes that were discovered before the start of the DYT naming convention. A good example is Wilson’s disease, where many patients may first present with dystonia or have dystonia as a major and disabling clinical feature. Wilson’s disease never had a DYT number because the chromosomal location and gene were identified before the DYT naming system began. More than 100 inherited conditions are now recognized where dystonia may be a feature. For the majority of these disorders, dystonia is combined with other neurological or medical problems; only a small number reflect pure dystonia [2]. Only a fraction of these have a DYT number.
One advantage of the larger number of recognized dystonia genes is that it has become possible to begin to search for shared molecular pathways [5, 6]. The known genes are involved in numerous biological processes, but some common mechanisms are evident. Examples of some shared molecular and cellular pathways include disruptions in neurotransmitter signaling, calcium homeostasis, brain heavy metal accumulation, transcriptional regulation, or cell cycling. For example, dystonia is frequently associated with parkinsonism, and a number of the responsible genes result in damage or dysfunction of the basal ganglia or its dopaminergic afferents from the midbrain [7]. In other cases, dystonia is associated with ataxia and disruption of cerebellar or GABAergic pathways [8]. In the future, identification of these shared molecular and cellular pathways will be increasingly important for parsing subtypes of patients with dystonia into biologically meaningful groups, and for designing novel treatments that are targeted towards specific underlying neurobiological mechanisms.
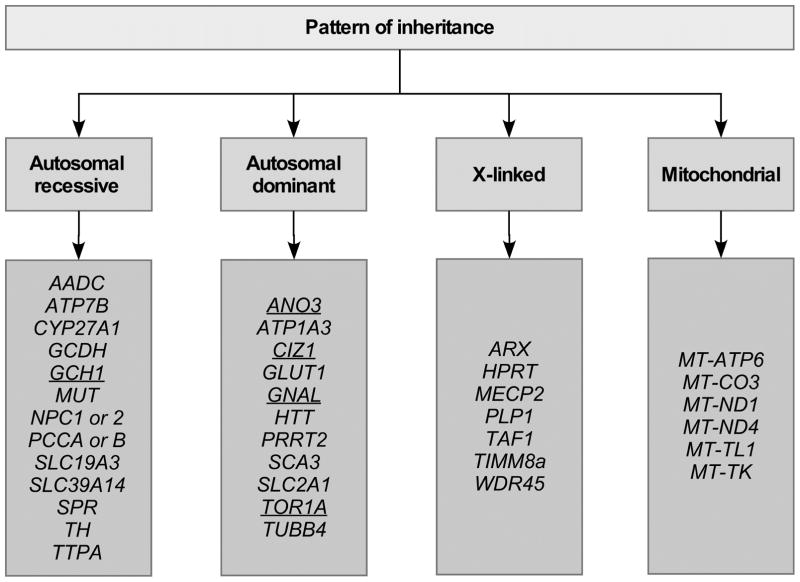
Because of the many difficulties with the traditional DYT naming convention, a new genetic nomenclature system has been proposed [4]. In the proposed system, the number is replaced by the name of the gene. In the proposed system, DYT1 is called DYT-TOR1A. In cases where the phenotype is mixed, the prefix includes other relevant designators such as PARK for parkinsonism or SCA for ataxia. For the clinician, organizing the genes according to manner of inheritance (Figure 1) may be more useful, because it aids genetic counseling. For the neurobiologist seeking to understand shared molecular pathways, organizing the genes according to biological pathways (Figure 2) is more valuable, because it aids recognition of common biological mechanisms. For both of these strategies for gene classification, a DYT prefix is not needed.
Figure 1. Patterns of inheritance.
This schematic shows a classification system for genes for monogenic dystonias based on pattern of inheritance. It is not a complete list of all genes and patterns of inheritance; only representative examples are shown. Genes shown in underlined type are those that are commonly associated with dystonia as the only or predominant clinical feature. Those in plain type are those where dystonia is often combined with other clinical features.
Figure 2. Biological pathways.
This schematic shows a classification system for genes for monogenic dystonias based on shared biological pathways. It is not a complete list of all genes and their biological pathways. Only representative examples are shown. Genes shown in underlined type are those that are commonly associated with dystonia as the only or predominant clinical feature. Those in plain type are those where dystonia is often combined with other clinical features. Some genes may fall in more than one group when their functions overlap more than one biological mechanism. In many cases the biological pathways are well established, but in some the pathways are suspected but not proven.
Neuroanatomy
Dystonia has traditionally been considered a basal ganglia disorder. This view originated from early studies showing that the basal ganglia were frequently involved in cases of hemidystonia that were acquired as a result of focal brain injury [9]. Subsequently, many studies examining other types of dystonia revealed that additional brain regions, including the cerebellum, are implicated [9, 10]. Dystonia therefore is now regarded as a disorder of a motor network that may involve the basal ganglia, cerebellum, cerebral cortex, and other regions.
There is good evidence that the basal ganglia play an important role in dystonia. One of the most illustrative examples is dopa-responsive dystonia. In both humans [11] and mice [12], defects in the synthesis of dopamine in the basal ganglia cause dystonia. Supplementation with levodopa either peripherally or directly into the striatum in mice corrects the dystonia, suggesting this dystonia is caused by a very specific defect limited to the basal ganglia. Numerous studies of other types of dystonia in humans and other animals have also implicated the basal ganglia. The cerebellum has been implicated as a source for dystonia in numerous rodent models including the Dt rat, the tottering mutant mouse, the DYT1 mouse, and a model for rapid-onset dystonia-parkinsonism [13]. In humans, the cerebellum has been implicated by imaging studies of different types of dystonia, and by increasing recognition of subclinical cerebellar signs in different types of dystonia [9, 10]. The thalamus also is a well-recognized source for jerky hand dystonia [14], and recent evidence has pointed to defects in the midbrain interstitial nucleus of Cajal for cervical dystonia [15].
Exactly how the motor network is disrupted in different types of dystonia remains uncertain, and it seems likely that some types of dystonia may involve only certain portions of this network. For example, dystonia might arise from dysfunction of one node in the network, a combination of nodes in the network, or even abnormal communication among the nodes. Elucidating the neuroanatomical basis for different types of dystonia has direct implications for surgical interventions. Deep brain stimulation targeting the internal segment of the globus pallidus provides a clearly effective therapy for some types of dystonia, but not others. Recent studies have begun to explore the role of cerebellar targets for surgery [16, 17].
Physiology
At the level of muscle activity, it is often said that co-contraction of agonist and antagonist muscles is a cardinal feature of dystonia. This concept derives from early studies of focal hand dystonia, where electromyography revealed frequent simultaneous contractions of muscles that normally oppose each other [18]. Since then, other studies have shown that co-contraction of antagonists does not occur in all forms of dystonia [19]. For example, simultaneous contraction of antagonistic muscles is not seen in many patients with blepharospasm, cervical dystonia, or laryngeal dystonia. Additionally, co-contraction of antagonistic muscles is not specific to dystonia, because it may occur in other disorders and even in voluntary isometric contractions. As a result of these additional observations, co-contraction of antagonistic muscles is no longer considered a cardinal feature of dystonia. A more broadly applicable concept is that dystonia involves muscle over-contraction leading to excessive force, and/or spread of contraction to nearby muscles. In cases where spreading occurs to muscles that normally oppose each other, then co-contraction of agonist and antagonist muscles also is observed.
At the level of motor systems physiology, three major themes have been described [20]. The first theme involves abnormalities in neural inhibition. A loss of inhibition has been described for several different types of dystonia and at multiple levels of the nervous system including the spinal cord, brainstem, and cortex. The second theme involves abnormalities of sensorimotor integration [21]. Although most patients with dystonia do not have overt sensory defects, numerous studies of different types of dystonia have revealed subclinical defects in spatial and temporal somatosensory discrimination thresholds. The third theme involves maladaptive plasticity [20]. Abnormalities of neural plasticity have also been reported for many different types of dystonia. This mechanism may be most relevant to task-specific dystonias, but may not apply to inherited metabolic disorders where dystonia arises during infancy. Because all three pathophysiologies occur in other neurological disorders with no particular specificity for dystonia, the challenge now is to determine whether the abnormalities are a cause or consequence of dystonia.
The abnormalities of neuronal signaling that ultimately lead to dystonia remain to be elucidated. This task is critically important, but very challenging because direct electrophysiological measurements of neuronal signaling in humans with dystonia is not generally feasible. Even when such measurements are feasible, for example during neuromodulation brain surgery, the types of measurements that can be made are limited, and it is impossible to distinguish causal defects from secondary changes [22]. In view of these challenges, most studies of neuronal signaling have been conducted in animals. Electrophysiological studies of primate models have implied an imbalance between the direct and indirect pathways of the basal ganglia, or a rate-dependent alteration in pallidal output [23]. Similar studies in a rodent model for DYT1 dystonia have implied defects in cortico-striatal signaling and cholinergic interneurons [24, 25]. On the other hand, electrophysiological studies of other rodent models have implied abnormal activity and particularly burst-firing of cerebellar Purkinje neurons [26–30]. Additional studies are needed to determine if the electrophysiological abnormalities observed in different animal models can independently cause dystonia, or if, like the anatomical and molecular pathways, common themes emerge.
Conclusions
Enormous advances have been made in recent years in elucidating the pathogenesis of different types dystonia in genetics, neuroanatomy, and neurophysiology. A broad conclusion that is shared across many of these studies is that the underlying neurobiological mechanisms are not the same for all types of dystonia. Although the causes and mechanisms responsible for dystonia may be quite varied, it is likely that they converge to produce a similar motor phenotype through shared molecular, anatomical, or physiological mechanisms. Delineating these shared mechanisms is important for devising rational therapies appropriately targeted for specific subgroups of patients.
Acknowledgments
This work was supported in part by a grant to the Dystonia Coalition (U54 TR001456 and NS065701) from the Office of Rare Diseases Research (ORDR) in the National Center for Advancing Translational Sciences (NCATS) and the National Institute of Neurological Disorders and Stroke (NINDS) and by NINDS grant R01 NS088528.
Footnotes
Financial disclosures related to the manuscript
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Albanese A, Bhatia K, Bressman SB, DeLong MR, Fahn S, Fung VSC, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK. Phenomenology and classification of dystonia: A consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fung VS, Jinnah HA, Bhatia K, Vidailhet M. Assessment of the patient with dystonia: An update on dystonia syndromes. Mov Disord. 2013;28:889–898. doi: 10.1002/mds.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlesworth G, Bhatia KP, Wood NW. The genetics of dystonia: new twists in an old tale. Brain. 2013;136:2017–37. doi: 10.1093/brain/awt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marras C, Lang A, van de Warrenburg BP, Sue CM, Tabrizi SJ, Bertram L, Mercimek-Mahmutoglu S, Ebrahimi-Fakhari D, Warner TT, Durr A, Assmann B, Lohmann K, Kostic V, Klein C. Nomenclature of genetic movement disorders: Recommendations of the international Parkinson and movement disorder society task force. Mov Disord. 2016;31:436–57. doi: 10.1002/mds.26527. [DOI] [PubMed] [Google Scholar]
- 5.Domingo A, Erro R, Lohmann K. Novel dystonia genes: Clues on disease mechanisms and the complexities of high-throughput sequencing. Mov Disord. 2016;31:471–7. doi: 10.1002/mds.26600. [DOI] [PubMed] [Google Scholar]
- 6.LeDoux MS, Dauer WT, Warner T. Emerging molecular pathways for dystonia. Mov Disord. 2013;15:968–981. doi: 10.1002/mds.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balint B, Bhatia KP. Isolated and combined dystonia syndromes - an update on new genes and their phenotypes. Eur J Neurol. 2015;22:610–617. doi: 10.1111/ene.12650. [DOI] [PubMed] [Google Scholar]
- 8.Nibbeling EA, Delnooz CC, de Koning TJ, Sinke RJ, Jinnah HA, Tijssen MA, Verbeek DS. Using the shared genetics of dystonia and ataxia to unravel their pathogenesis. Neurosci Biobehav Rev. 2017;75:22–39. doi: 10.1016/j.neubiorev.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neychev VK, Gross R, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35. doi: 10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wijemanne S, Jankovic J. Dopa-responsive dystonia--clinical and genetic heterogeneity. Nat Rev Neurol. 2015;11:414–24. doi: 10.1038/nrneurol.2015.86. [DOI] [PubMed] [Google Scholar]
- 12.Rose SJ, Yu XY, Heinzer AK, Harrast P, Fan X, Raike RS, Thompson VB, Pare JF, Weinshenker D, Smith Y, Jinnah HA, Hess EJ. A new knock-in mouse model of L-DOPA responsive-dystonia. Brain. 2015;138:2987–3002. doi: 10.1093/brain/awv212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakkottai VG, Batla A, Bhatia K, Dauer WT, Dresel C, Niethammer M, Eidelberg D, Raike RS, Smith Y, Jinnah HA, Hess EJ, Meunier S, Hallett M, Fremont R, Khodakhah K, LeDoux MS, Popa T, Gallea C, Lehericy S, Bostan AC, Strick PL. Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum. 2017;16(2):577–594. doi: 10.1007/s12311-016-0825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liuzzi D, Gigante AF, Leo A, Defazio G. The anatomical basis of upper limb dystonia: lesson from secondary cases. Neurol Sci. 2016;37:1393–1398. doi: 10.1007/s10072-016-2598-6. [DOI] [PubMed] [Google Scholar]
- 15.Shaikh AG, Zee DS, Crawford JD, Jinnah HA. Cervical dystonia: a neural integrator disorder. Brain. 2017;139:2590–2599. doi: 10.1093/brain/aww141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teixeira MJ, Schroeder HK, Lepski G. Evaluating cerebellar dentatotomy for the treatment of spasticity with or without dystonia. Br J Neurosurg. 2015:1–6. doi: 10.3109/02688697.2015.1025697. [DOI] [PubMed] [Google Scholar]
- 17.Sokal P, Rudas M, Harat M, Szylberg L, Zielinski P. Deep anterior cerebellar stimulation reduces symptoms of secondary dystonia in patients with cerebral palsy treated due to spasticity. Clin Neurol Neurosurg. 2015;135:62–8. doi: 10.1016/j.clineuro.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Cohen LG, Hallett M. Hand cramps: clinical features and electromyographic patterns in a focal dystonia. Neurology. 1988;38:1005–12. doi: 10.1212/wnl.38.7.1005. [DOI] [PubMed] [Google Scholar]
- 19.Malfait N, Sanger TD. Does dystonia always include co-contraction? A study of unconstrained reaching in children with primary and secondary dystonia. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0606-4. [DOI] [PubMed] [Google Scholar]
- 20.Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord. 2013;28:958–967. doi: 10.1002/mds.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13:100–12. doi: 10.1016/S1474-4422(13)70213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos VF, Pillai AS, Lungu C, Ostrem J, Starr P, Hallett M. Intraoperative neurophysiology in deep brain surgery for psychogenic dystonia. Ann Clin Transl Neurol. 2015;2:707–710. doi: 10.1002/acn3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guehl D, Cuny E, Ghorayeb I, Michelet T, Bioulac B, Burbaud P. Primate models of dystonia. Prog Neurobiol. 2009;87:118–31. doi: 10.1016/j.pneurobio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Sciamanna G, Tassone A, Mandolesi G, Puglisi F, Ponterio G, Martella G, Madeo G, Bernardi G, Standaert DG, Bonsi P, Pisani A. Cholinergic dysfunction alters synaptic integration between thalamostriatal and corticostriatal inputs in DYT1 dystonia. J Neurosci. 2012;32:11991–2004. doi: 10.1523/JNEUROSCI.0041-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martella G, Tassone A, Sciamanna G, Platania P, Cuomo D, Viscomi MT, Bonsi P, Cacci E, Biagioni S, Usiello A, Bernardi G, Sharma N, Standaert DG, Pisani A. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain. 2009;132:2336–49. doi: 10.1093/brain/awp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fremont R, Calderon DP, Maleki S, Khodakhah K. Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. J Neurosci. 2014;34:11723–32. doi: 10.1523/JNEUROSCI.1409-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hisatsune C, Miyamoto H, Hirono M, Yamaguchi N, Sugawara T, Ogawa N, Ebisui E, Ohshima T, Yamada M, Hensch TK, Hattori M, Mikoshiba K. IP3R1 deficiency in the cerebellum/brainstem causes basal ganglia-independent dystonia by triggering tonic Purkinje cell firings in mice. Front Neural Circuits. 2013;7:156. doi: 10.3389/fncir.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan X, Hughes KE, Jinnah HA, Hess EJ. Selective and sustained AMPA receptor activation in cerebellum induces dystonia in mice. J Pharmacol Exp Ther. 2012;340:733–41. doi: 10.1124/jpet.111.190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeDoux MS, Lorden JF. Abnormal cerebellar output in the genetically dystonic rat. In: Fahn S, Marsden CD, DeLong M, editors. Dystonia 3. Lippincott-Raven Publishers; Philadelphia: 1998. pp. 63–78. [PubMed] [Google Scholar]