Abstract
Renal fibrosis is a common pathway leading to the progression of chronic kidney disease and bone marrow derived fibroblasts contribute significantly to the development of renal fibrosis. However, the signaling mechanisms underlying the activation of these fibroblasts are not completely understood. Here, we examined the role of IL-4 receptor α (IL-4Rα) in the activation of myeloid fibroblasts in two experimental models of renal fibrosis. Compared with wild-type mice, IL-4Rα knockout mice accumulated fewer bone marrow derived fibroblasts and myofibroblasts in their kidneys. IL-4Rα deficiency suppressed the expression of α-smooth muscle actin, extracellular matrix proteins and the development of renal fibrosis. Furthermore, IL-4Rα deficiency inhibited the activation of signal transducer and activator of transcription 6 (STAT6) in the kidney. Moreover, wild-type mice engrafted with bone marrow cells from IL-4Rα knockout mice exhibited fewer myeloid fibroblasts in the kidney and displayed less severe renal fibrosis following ureteral obstructive injury compared with wild-type mice engrafted with wild-type bone marrow cells. In vitro, IL-4 activated STAT6 and stimulated expression of α-smooth muscle actin and fibronectin in mouse bone marrow monocytes. This was abolished in the absence of IL-4Rα. Thus, IL-4Rα plays an important role in bone marrow-derived fibroblast activation resulting in extracellular matrix protein production and fibrosis development. Hence, the IL-4Rα/STAT6 signaling pathway may serve as a novel therapeutic target for chronic kidney disease.
Keywords: Chronic kidney disease, Fibrosis, Cytokines, Fibroblast, Extracellular matrix
INTRODUCTION
Chronic kidney disease (CKD) is a leading cause of death and a serious global health challenge. The prevalence of CKD continues to rise worldwide1. Therefore, new strategies for prevention and treatment of CKD are much needed to reduce its morbidity and mortality. Renal fibrosis is a pathological hallmark of CKD, which is manifested by extensive fibroblasts activation. Activated fibroblasts produce a large amount of extracellular matrix (ECM) leading to destruction of renal parenchyma and progressive loss of kidney function2–4. Despite improvement in the knowledge of diverse aspects related to CKD, the cellular and molecular events leading to development of renal fibrosis and eventually renal failure are not fully understood. Therefore, a better understanding of the pathogenesis of renal fibrosis is essential for developing novel therapeutic strategies to treat chronic fibrotic kidney disease and prevent its progression.
Traditionally, activated fibroblasts are thought to arise from resident fibroblasts5, 6. Recently, mounting evidence indicates that bone marrow-derived fibroblast precursors contribute significantly to the population of activated fibroblasts and the development of renal fibrosis7–10. Bone marrow-derived fibroblast precursors termed fibrocytes are spindle-shaped cells that express hematopoietic markers such as CD45 and CD11b as well as mesenchymal markers such as platelet-derived growth factor receptor-β (PDGFR-β) and vimentin11–13. Our previous studies have demonstrated that bone marrow–derived fibroblast precursors contribute substantially to development of renal interstitial fibrosis13–16. However, the molecular mechanisms underlying the activation of these fibroblasts are not completely understood.
Activation of myeloid fibroblasts is regulated by cytokines that are produced locally by T helper (Th) cells. Th1 cells produce anti-fibrotic cytokines such as interferon γ (IFN-γ) that suppress myeloid fibroblasts differentiation, while Th2 cells produce the profibrotic cytokines such as IL-4 and IL-13 that promote myeloid fibroblasts differentiation17–19. IL-4 receptor α (IL-4Rα) is a key component of Th2 cytokine receptor, which mediates Th2 cytokines/signal transducer and activator of transcription 6 (STAT6) signaling pathway transduction and subsequent effector functions20–22. Previously, we have demonstrated that Th2 cytokines activate JAK3/STAT6 signaling pathway to stimulate myeloid fibroblast activation, which play a crucial role in the development of renal fibrosis13. Therefore, we hypothesized that IL-4Rα mediates the signaling transduction of Th2 cytokines/STAT6 in myeloid fibroblasts activation and renal fibrosis. Here, we investigated the role of IL-4Rα signaling in the activation of bone marrow–derived fibroblast precursors in murine models of renal fibrosis induced by folic acid or unilateral ureteral obstruction (UUO). Our results have demonstrated that IL-4Rα deficiency inhibits activation of bone marrow–derived fibroblasts and suppresses development of renal fibrosis.
RESULTS
IL-4Rα Deficiency Impairs Myeloid Fibroblast Accumulation
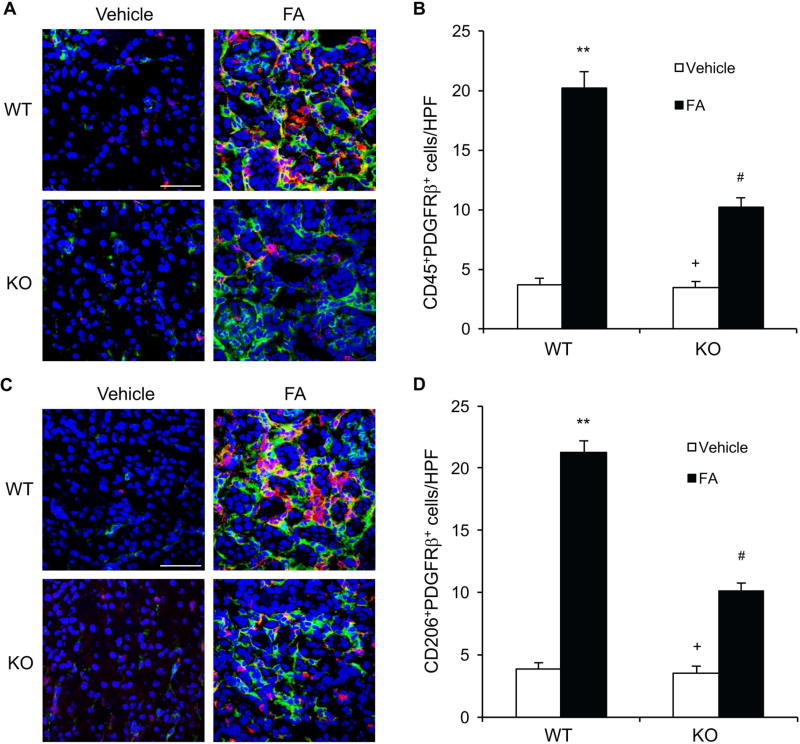
Myeloid fibroblast precursors contribute substantially to the pathogenesis of renal fibrosis6, 13, 14, 23. To investigate the role of IL-4Rα in the activation of bone marrow–derived fibroblasts, wild-type (WT) and IL-4Rα knockout (KO) mice were treated with vehicle or folic acid. Kidney sections were stained for both CD45, a hematopoietic marker, and PDGFR-β, a mesenchymal marker, to identify bone marrow–derived fibroblasts. The number of CD45+ and PDGFR-β+ cells was significantly increased in the kidneys of WT mice after folic acid treatment. On the other hand, the number of CD45+ and PDGFR-β+ cells was markedly reduced in the kidneys of IL-4Rα KO mice following folic acid treatment (Figure 1, A–B). These data indicate that IL-4Rα plays an important role in the activation of bone marrow–derived fibroblast precursors in the kidneys after folic acid administration.
Figure 1. IL-4Rα Deficiency Suppresses Myeloid Fibroblast Accumulation and Macrophage Polarization in Folic Acid Nephropathy.
A. Representative photomicrographs of kidney sections from WT and IL-4Rα KO mice at 2 weeks after vehicle or folic acid treatment stained for CD45 (red), PDGFR-β (green), and DAPI (blue). Scale bar, 50 μm. B. Quantitative analysis of CD45+ and PDGFR-β+ fibroblasts in kidneys. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group. C. Representative photomicrographs of kidney sections stained for CD206 (red), PDGFR-β (green), and DAPI (blue). Scale bar, 50 μm. D. Quantitative analysis of CD206+ and PDGFR-β+ fibroblasts in kidneys. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group.
IL-4Rα Deficiency Attenuates M2 Macrophage Polarization
IL-4Rα signaling pathway plays an important role in M2 macrophage polarization24. Recently, we have shown that myeloid fibroblasts are derived from monocytes through M2 macrophage polarization in a mouse model of renal fibrosis9. Therefore, we determined whether IL-4Rα regulates macrophage polarization and myeloid fibroblast formation in folic acid nephropathy. Kidney sections were stained for CD206, a M2 macrophage marker, and PDGFR-β. The number of CD206+ and PDGFR-β+ cells was substantially increased in the kidneys of WT mice following folic acid administration. The number of CD206+ and PDGFR-β+ cells was significantly decreased in the kidneys of IL-4Rα KO mice after folic acid treatment (Figure 1, C–D). These results indicate that bone marrow-derived fibroblasts are derived from monocytes through M2 macrophage polarization and this process is driven by IL-4Rα signaling pathway.
IL-4Rα Deficiency Suppresses Myofibroblast Transformation
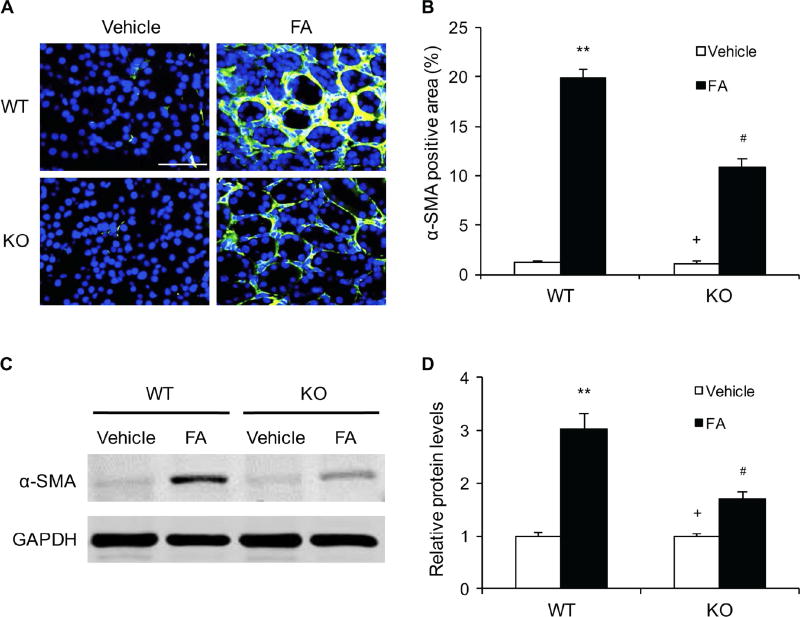
We next examined if IL-4Rα signaling affects myofibroblast formation in the kidneys. Kidney sections were stained for α-smooth muscle actin (α-SMA). The number of α-SMA-positive myofibroblasts was markedly increased in the kidneys of WT mice after folic acid treatment. In contrast, the number of myofibroblasts was significantly reduced in the kidneys of IL-4Rα KO mice treated with folic acid. (Figure 2, A–B). Consistent with these findings, the protein levels of a-SMA were significantly decreased in the kidneys of IL-4Rα KO mice following FA administration compared with WT. (Figure 2, C–D). These results indicate that IL-4Rα signaling stimulates myofibroblast transformation in the kidneys during the development of renal fibrosis.
Figure 2. IL-4Rα Deficiency Inhibits Myofibroblast Formation in Folic Acid Nephropathy.
A. Representative photomicrographs of kidney sections stained for α-SMA (green) and counterstained with DAPI (blue). Scale bar, 50 μm. B. Quantitative analysis of α-SMA-positive area in kidneys. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group. C. Representative Western blots show the levels of α-SMA protein expression in the kidneys. D. Quantitative analysis of α-SMA protein expression in the kidneys. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group.
IL-4Rα Deficiency Reduces Renal Fibrosis
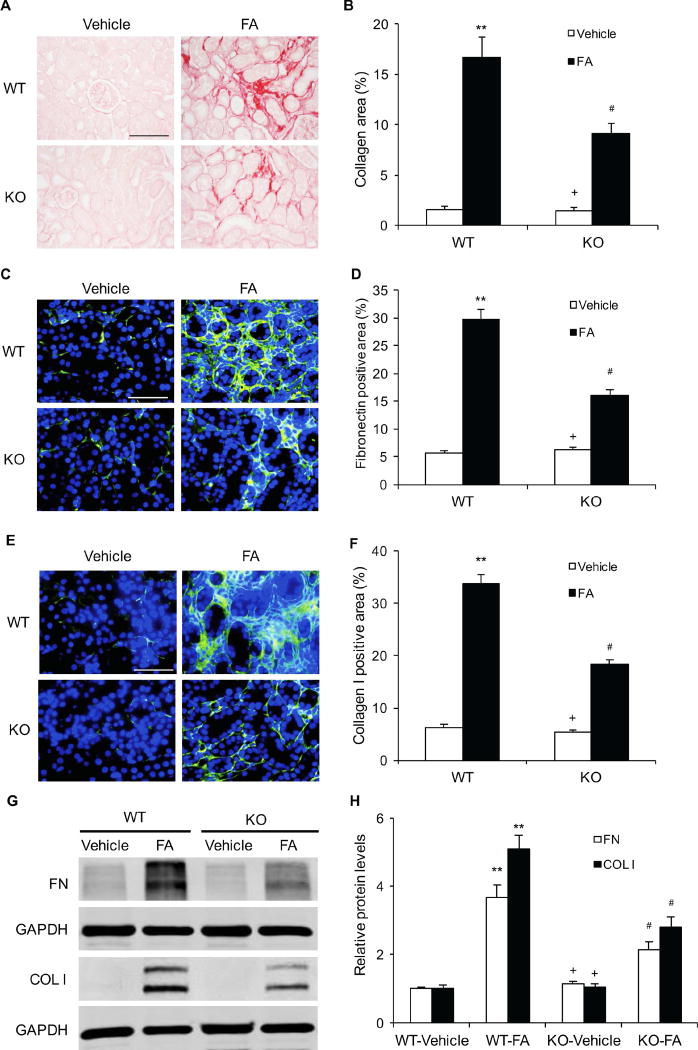
To examine the role of IL-4Rα in the development of renal fibrosis, Sirius red staining was performed to detect total collagen deposition in kidneys. WT mice treated with folic acid developed significant collagen deposition in the kidneys compared with WT mice treated with vehicle. The degree of collagen deposition was markedly reduced in the kidneys of IL-4Rα KO mice following folic acid administration. These data suggest that IL-4Rα plays a crucial role in the pathogenesis of renal fibrosis (Figure 3, A–B).
Figure 3. IL-4Rα Deficiency Attenuates Renal Fibrosis and Expression of ECM Protein in Folic Acid Nephropathy.
A. Representative photomicrographs of kidney sections stained with sirius red for evaluation of total collagen deposition in the kidneys. Scale bar, 50 μm. B. Quantitative analysis of renal interstitial collagen deposition in the kidneys. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group. C. Representative photomicrographs of kidney sections stained for fibronectin (green) and counterstained with DAPI (blue). Scale bar, 50 μm. D. Quantitative analysis of fibronectin-positive area in kidneys. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group. E. Representative photomicrographs of kidney sections stained for collagen I (green) and counterstained with DAPI (blue). Scale bar, 50 μm. F. Quantitative analysis of collagen-positive area in kidneys. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group. G. Representative Western blots show protein expression of fibronectin and collagen I in the kidneys. H. Quantitative analysis of protein levels of fibronectin and collagen I in the kidneys. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group.
To evaluate the effect of IL-4Rα deficiency on ECM protein expression, immunofluorescence staining was carried out to detect the expression levels of fibronectin and collagen I, two major ECM proteins. The protein levels of fibronectin and collagen I were significantly increased in the kidneys of folic acid-treated WT mice. The protein levels of these ECM proteins were significantly reduced in the kidneys of IL-4Rα KO mice after folic acid administration (Figure 3, C–F). Consistent with immunofluorescence staining, western blot analysis demonstrated that IL-4Rα deficiency inhibited the expression levels of fibronectin and collagen I in the kidneys after folic acid administration (Figure 3, G–H). These data indicate that IL-4Rα signaling contributes significantly to ECM protein expression in the kidney following folic acid nephropathy.
IL-4Rα Deficiency Inhibits STAT6 Activation in Folic Acid Nephropathy
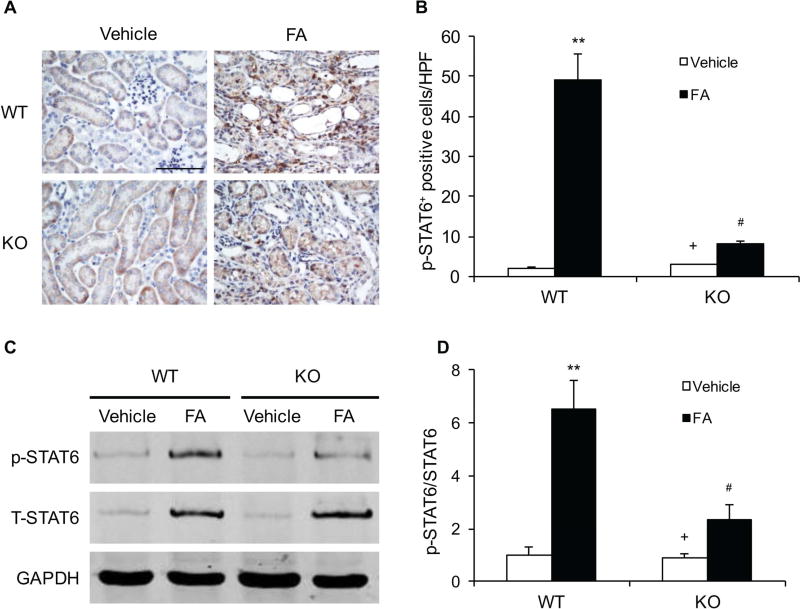
We have recently shown that STAT6 signaling regulates Th2 cytokines-induced myeloid fibroblast activation and fibrosis development13. We then investigated whether IL-4Rα mediates the activation of STAT6 signaling in the kidney. Kidney sections were stained with a phospho-STAT6 (Tyr641) antibody. Prominent nuclear staining of phosphorylated STAT6 was mainly detected in interstitial cells of WT mice after folic acid treatment. IL-4Rα deficiency significantly inhibited the levels of phospho-STAT6 in the kidneys following folic acid administration (Figure 4, A–B). In agreement with these results, Western blot analysis showed that the protein levels of phospho-STAT6 in the kidneys of IL-4Rα KO mice were reduced markedly compared with WT mice following folic acid administration (Figure 4, C–D).
Figure 4. IL-4Rα Deficiency Inhibits STAT6 Activation in Folic Acid Nephropathy.
A. Representative photomicrographs of kidney sections stained for phosphorylated STAT6 (brown) and counterstained with hematoxylin (blue) at 2 weeks after vehicle or folic acid treatment. Scale bar, 50 μm. B. Quantitative analysis of phosphorylated STAT6 cells. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group. C. Representative Western blots show the effect of IL-4Rα Deficiency on STAT6 activation in kidneys. D. Quantitative analysis of STAT6 phosphorylation. ** P<0.01 vs WT-Vehicle, # P <0.05 vs WT-FA, + P <0.05 vs KO-FA. n=6 in each group.
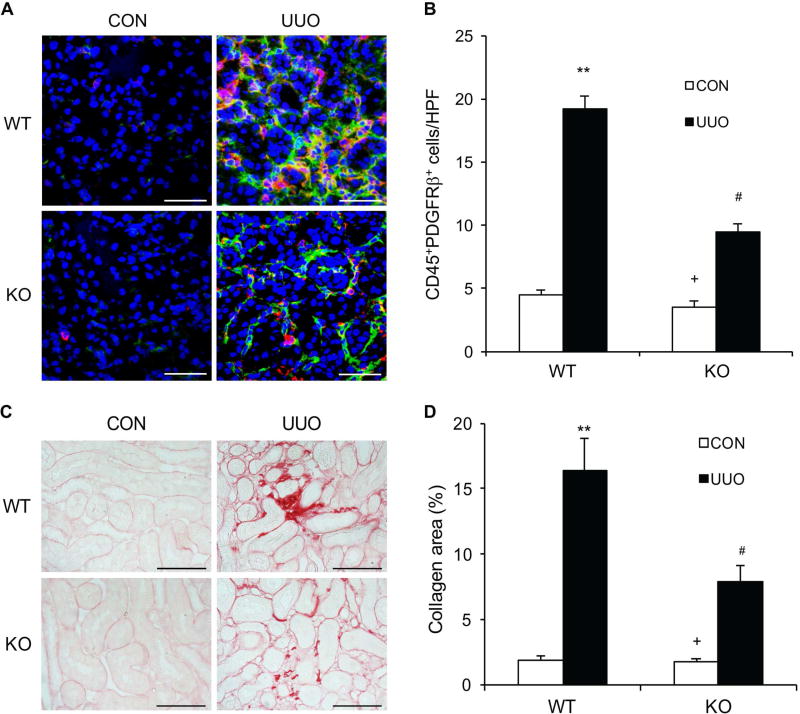
IL-4Rα Deficiency Suppresses Myeloid Fibroblast Activation and Renal Fibrosis after UUO Injury
We have shown that IL-4Rα plays a key role in activation of CD45+ and PDGFR-β+ myeloid fibroblasts in the kidney and development of renal fibrosis following folic acid nephropathy. We then examined whether IL-4Rα mediates bone marrow-derived fibroblast activation and fibrosis development in another model of renal fibrosis induced by UUO. Kidney sections were stained for CD45 and PDGFR-β to identify bone marrow–derived fibroblasts. IL-4Rα KO mice accumulated significantly fewer CD45+ and PDGFR-β+ myeloid fibroblasts in the kidneys after UUO compared with WT mice (Figure 5, A–B). We next examined if IL-4Rα signaling affects bone marrow-derived myofibroblast formation in the kidneys. Kidney sections were stained for CD45 and α-SMA and examined with a confocal microscope. The number of CD45+ and α-SMA+ myofibroblasts was significantly increased in the kidneys of WT mice with obstructive injury. In contrast, the number of CD45+ and α-SMA+ myofibroblasts was considerably reduced in the obstructed kidneys of IL-4Rα KO mice (Figure S1). To further quantify the number of myeloid fibroblasts in the kidney, freshly isolated kidney cells were stained for CD45 and collagen I or CD45 and α-SMA, and examined by flow cytometry. IL-4Rα deficiency significantly reduced CD45+ and collagen I+ cell accumulation and CD45+ and α-SMA+ cell formation in obstructed kidneys compared with WT mice with obstructive injury (Figure S2). Consistent with these findings, IL-4Rα deficiency attenuated interstitial collagen deposition in the kidneys in response to UUO (Figure 5, C–D). There results indicate that IL-4Rα has a crucial role in myeloid fibroblast activation and renal fibrosis development in response to obstructive injury.
Figure 5. IL-4Rα Deficiency Suppresses Myeloid Fibroblast Accumulation and Renal Fibrosis in Obstructive Nephropathy.
A. Representative photomicrographs of kidney sections from WT and IL-4Rα KO mice at 2 weeks after UUO stained for CD45 (red), PDGFR-β (green), and DAPI (blue). Scale bar, 50 μm. B. Quantitative analysis of CD45+ and PDGFR-β+ fibroblasts in the kidneys. ** P<0.01 vs WT-CON, # P <0.05 vs WT-UUO, + P <0.05 vs KO-UUO. n=6 in each group. C. Representative photomicrographs of kidney sections stained with Sirius red for evaluation of total collagen deposition in the kidneys. Scale bar, 50 μm. D. Quantitative analysis of renal interstitial collagen deposition in the kidneys. ** P<0.01 vs WT-CON, # P <0.05 vs WT-UUO, + P <0.05 vs KO-UUO. n=6 in each group.
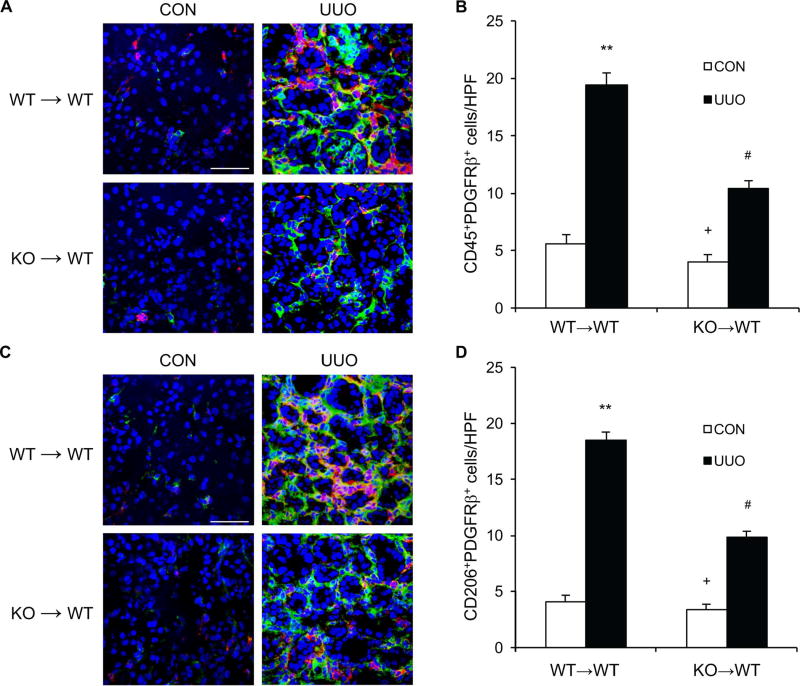
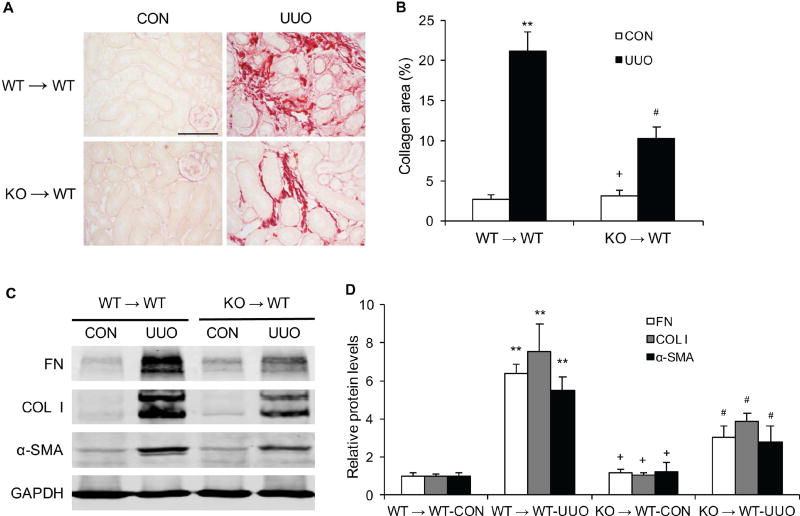
IL-4Rα Deficiency in Bone Marrow–Derived Fibroblasts Inhibits Myeloid Fibroblast Accumulation and Renal Fibrosis
To determine the role of IL-4Rα in bone marrow–derived cells in the development of renal fibrosis, we performed bone marrow transplantation of WT mice with IL-4Rα+/+ or IL-4Rα−/− bone marrow cells. Eight weeks after bone marrow transplantation, chimeric mice were subjected to UUO. The genotype of bone marrow–derived cells from the chimeric mice was confirmed by PCR of DNA extracted from peripheral blood cells (Figure S3). WT mice transplanted with IL-4Rα−/− bone marrow cells exhibited fewer CD45+ and PDGFR-β+ bone marrow–derived fibroblasts in the obstructed kidneys compared with WT mice transplanted with IL-4Rα+/+ bone marrow cells (Figure 6, A–B). Furthermore, there was a significant decrease in the number of CD206+ and PDGFR-β+ fibroblasts in the obstructed kidneys of WT mice transplanted with IL-4Rα−/− bone marrow cells compared with WT mice transplanted with IL-4Rα+/+ bone marrow cells (Figure 6, C–D). WT mice engrafted with IL-4Rα−/− bone marrow cells developed less severe interstitial fibrosis and produced much lower levels of α-SMA and ECM proteins in the kidneys (Figure 7, A–D). These data indicate that IL-4Rα signaling in bone marrow–derived cells plays an important role in the activation of myeloid fibroblasts and the development of renal interstitial fibrosis. These results reinforce the notion that myeloid fibroblasts are derived from monocytes through M2 macrophage polarization, which is regulated by IL-4Rα signaling pathway.
Figure 6. IL-4Rα Deficiency in Bone Marrow–Derived Cells Impairs Myeloid Fibroblast Accumulation and Macrophage Polarization.
A. Representative photomicrographs of kidney sections from WT→WT and KO→WT mice at 10 days after vehicle or UUO treatment stained for CD45 (red), PDGFR-β (green), and DAPI (blue). Scale bar, 50 μm. B. Quantitative analysis of CD45+ and PDGFR-β+ fibroblasts in kidneys. ** P<0.01 vs WT→WT CON, # P <0.05 vs WT→WT UUO, + P <0.05 vs KO→WT UUO. n=6 in each group. C. Representative photomicrographs of kidney sections stained for CD206 (red), PDGFR-β (green), and DAPI (blue). Scale bar, 50 μm. D. Quantitative analysis of CD206+ and PDGFR-β+ fibroblasts in kidneys. ** P<0.01 vs WT→WT CON, # P <0.05 vs WT→WT UUO, + P <0.05 vs KO→WT UUO. n=6 in each group.
Figure 7. IL-4Rα Deficiency in Bone Marrow–Derived Cells Reduces Renal Fibrosis and ECM Protein Expression.
A. Representative photomicrographs of kidney sections from WT→WT and KO→WT mice stained with Sirius red for evaluation of total collagen deposition in the kidneys at 10 days after vehicle or UUO treatment. Scale bar, 50 μm. B. Quantitative analysis of renal interstitial collagen deposition in the kidneys. ** P<0.01 vs WT→WT CON, # P <0.05 vs WT→WT UUO, + P <0.05 vs KO→WT UUO. n=6 in each group. C. Representative Western blots show protein expression of fibronectin, collagen I, and α-SMA in the kidneys. D. Quantitative analysis of protein levels of fibronectin, collagen I, and α-SMA in the kidneys. ** P<0.01 vs WT→WT CON, # P <0.05 vs WT→WT UUO, + P <0.05 vs KO→WT UUO. n=6 in each group.
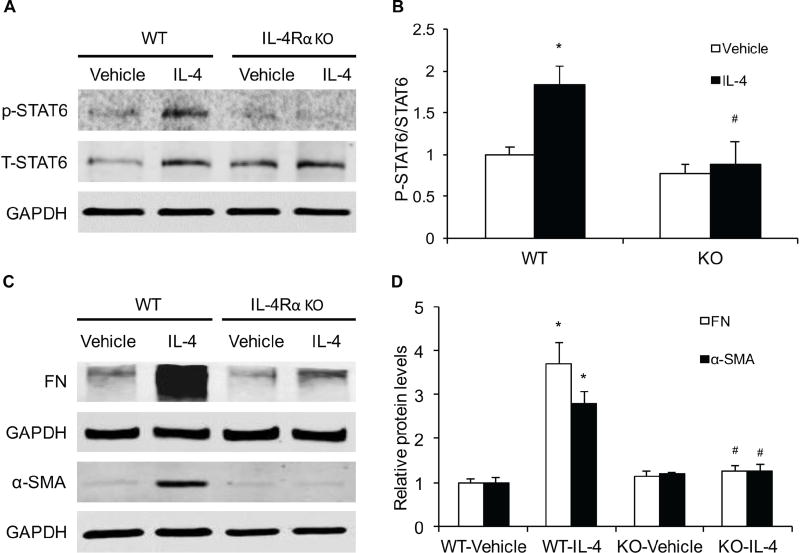
IL-4Rα Deficiency Inhibits STAT6 activation and α-SMA and ECM Protein Expression in Bone Marrow–Derived Monocytes
As IL-4Rα signaling regulates IL-4 - induced STAT6 activation and STAT6 is involved in myeloid fibroblast activation13, 25, we then explored whether IL-4Rα mediates IL-4-induced STAT6 activation in mouse bone marrow–derived monocytes. IL-4 treatment increased the levels of phosphorylated STAT6 in mouse bone marrow–derived monocytes. IL-4Rα deficiency abolished IL-4-indcued STAT6 phosphorylation (Figure 8, A–B). These data indicate that IL-4-induced STAT6 activation is mediated by IL-4Rα in bone marrow–derived monocytes.
Figure 8. IL-4Rα Deficiency Suppresses IL-4-induced α-SMA and ECM Protein Expression and STAT6 Activation in Bone Marrow–Derived Monocytes.
A. Representative Western blots show IL-4-induced STAT6 activation in cultured bone marrow–derived monocytes of WT or IL-4Rα KO mice after IL-4 (50 ng/ml) treatment for 24 hours. B. Quantitative analysis of STAT6 phosphorylation in monocytes. * P<0.05 vs WT Vehicle, # P <0.05 vs WT IL-4. n=6 in each group. C. Representative Western blots show the expression of fibronectin and α-SMA in cultured bone marrow–derived monocytes of WT or IL-4Rα KO mice after IL-4 (50 ng/ml) treatment for 24 hours. D. Quantitative analysis of protein levels of fibronectin and α-SMA in monocytes. * P<0.05 vs WT-Vehicle, # P <0.05 vs WT-IL-4. n=6 in each group.
We next investigated if IL-4Rα deficiency affects IL-4-induced monocyte-to-fibroblast transition and ECM protein expression in mouse bone marrow–derived monocytes. The protein levels of fibronectin and α-SMA of mouse bone marrow-derived monocytes were increased substantially after IL-4 treatment, which were reduced significantly in the absence of IL-4Rα (Figure 8, C–D). These results indicate that IL-4Rα signaling mediates IL-4-induced bone marrow-derived fibroblast activation and ECM protein production.
DISSCUSSION
Recent studies have demonstrated that bone marrow–derived fibroblast precursors contribute substantially the development of renal fibrosis6, 9, 13–16, 23. However, the molecular mechanisms underlying activation of myeloid fibroblasts during development of renal fibrosis are not fully elucidated. We have recently demonstrated that Th2 cytokines stimulate myeloid fibroblast activation via JAK3/STAT6 signaling pathway13. Although it is well-established that the biological effects of Th2 cytokines are regulated by IL-4Rα signaling17, 18, the precise role of IL-4Rα in activating myeloid fibroblasts and promoting renal fibrosis remains to be determined. In the present study, we have demonstrated that genetic disruption of IL-4Rα inhibits the bone marrow–derived fibroblast accumulation and myofibroblast formation in the kidney and reduces the development of renal fibrosis. In cultured bone marrow–derived monocytes, IL-4Rα deficiency inhibits STAT6 activation and monocyte-to-fibroblast transition and ECM protein production following IL-4 treatment. These results indicate that IL-4Rα signaling via STAT6 plays a critical role in bone marrow-derived fibroblast activation and renal fibrosis development.
Th2 cytokines exert their biological functions by interacting with specific type I and type II receptors on the cell surface. Both type I and type II receptors contain the IL-4Rα chain. When IL-4 binds IL4Rα with high affinity, the IL-4Rα chain interacts with the common γ chain to form the type I IL-4 receptor. Type II IL-4 receptor consists of IL-4Rα chain and IL-13Rα1 chain. IL-13 is only able to bind type II IL-4 receptor, whereas both type I and type II IL-4 receptor can bind IL-426–28. By binding IL-4Rα, IL-4 interaction with the type I and type II IL-4 receptor induces downstream signaling including JAK3/STAT6 pathways29–31. Recent evidence indicates that IL-4/IL-4Rα signaling may contribute to fibrotic diseases. It is reported that IL-4Rα has profibrotic effects via activating macrophage during skin repair in mice32. In the present study, we have shown that IL-4Rα deficiency significantly suppresses bone marrow-derived fibroblast accumulation and myofibroblast formation in the kidney. It is widely accepted that activated fibroblasts are responsible for the production of an excessive amount of ECM proteins such as fibronectin and collagen, leading to renal interstitial fibrosis2. In the present study, we have demonstrated that IL-4Rα deficiency inhibits markedly the expressions of ECM proteins and the development of folic acid-induced renal fibrosis. Consistent with the results of murine models of renal fibrosis, absence of IL-4Rα abolished IL-4-indcued expression of ECM proteins and α-SMA in cultured bone marrow-derived monocytes. These data indicate that IL-4Rα plays an important role in the activation of bone marrow–derived fibroblasts and the development of renal fibrosis.
Tissue damage leads to the activation of innate immunity, including recruitment of monocytes. Cells of the monocyte-macrophage lineage are characterized by considerable plasticity. Monocytes are able to differentiate into two distinct polarized phenotypes, which depends on cytokines produced in the local microenvironment. Classical activation of M1-polarized macrophages can be promoted by bacterial lipopolysaccharide and Th1 cytokines such as IFN-γ. M1 macrophages produce typical proinflammatory cytokines and chemokines, which initiate and drive inflammation. By contrast, Th2 cytokines such as IL-4 favor macrophage polarization toward profibrotic M2 phenotypes33. M2 macrophages secrete large amounts of profibrotic cytokines, which contribute to tissue fibrosis34–36. We have previously demonstrated that M2 macrophages produce collagen I and ECM proteins, indicating myeloid fibroblasts are derived from monocytes through M2 macrophage polarization9, 13. In this study, we have shown that IL-4Rα KO mice exhibit a substantial reduction in the number of CD206+ and PDGFR-β+ cells in kidneys and displayed less severe renal fibrosis following folic acid administration. These data indicate that IL-4Rα mediates M2 macrophage polarization and monocyte-to-fibroblast transition resulting in renal fibrosis.
STAT6 signaling pathway plays a key role in macrophages phenotypic polarization37, 38. Growing evidence indicates that STAT6-mediated activation of M2 macrophage polarization contributes to tissue fibrosis39–41. We have recently shown that disruption of STAT6 impairs M2 macrophage polarization and the development of renal fibrosis13. In the present study, we have demonstrated that IL-4Rα deficiency significantly inhibits the activation of STAT6 in the kidney following folic acid treatment, which is associated with a significant reduction in the number of M2 macrophage-derived fibroblasts and the degree of renal fibrosis. Furthermore, we have shown that IL-4Rα deficiency suppresses STAT6 activation and ECM protein expression in cultured bone marrow-derived monocytes after IL-4 treatment. These data indicate that IL-4Rα mediates monocyte-to-fibroblast transition via M2 macrophage polarization through activating STAT6 signaling pathway.
As IL-4Rα plays a key role in the activation of myeloid fibroblasts in the kidney following folic acid treatment, we further examined whether IL-4Rα mediates bone marrow-derived fibroblast activation, myofibroblast formation, and fibrosis development in another fibrosis model induced by UUO. Consistent with the results of folic acid nephropathy, IL-4RαKO mice accumulate a significant fewer bone marrow– derived fibroblasts and myofibroblasts, and develop less severe interstitial fibrosis in the kidneys following UUO. These results indicate that IL-4Rα plays a key role in activation of myeloid fibroblasts and development of renal fibrosis in obstructive nephropathy. Using bone marrow chimeric model, we provide strong evidence that IL-4Rα in bone marrow–derived cells play a fundamental role in myeloid fibroblast activation, myofibroblasts formation, and renal fibrosis development after obstructive injury. Furthermore, IL-4Rα deficiency in bone marrow–derived cells impairs M2 macrophage polarization and monocyte-to-fibroblast transition, which support the notion that IL-4Rα mediates myeloid fibroblast activation via M2 macrophage polarization during the development of renal fibrosis.
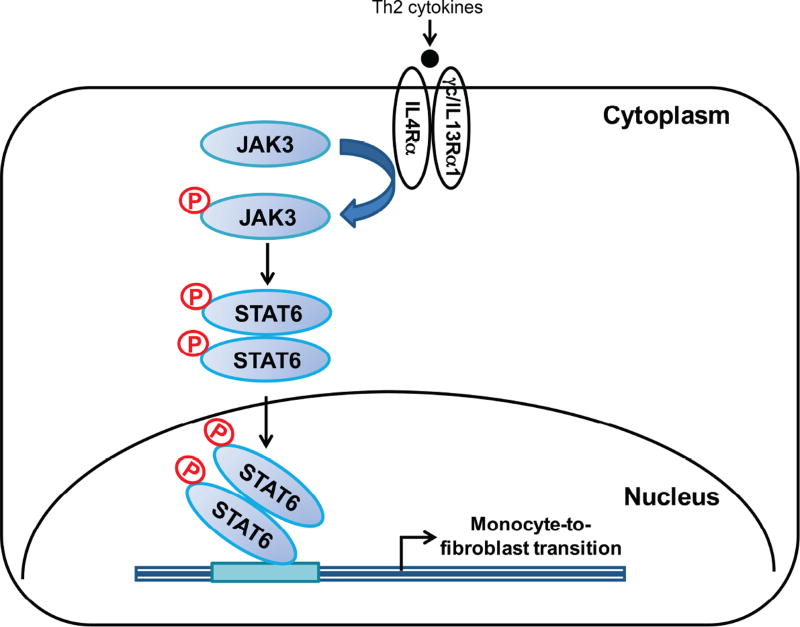
In summary, our study identifies a fundamental role of IL-4Rαin bone marrow– derived fibroblast activation and renal fibrosis development. In response to injury, locally produced Th2 cytokines such as IL-4 and IL-13 activate IL-4Rα/STAT6 signaling resulting in myeloid fibroblast activation and fibrogenesis (Figure 9). These data indicate that inhibition of IL-4Rα/STAT6 signaling may constitute a novel therapeutic strategy for chronic kidney disease.
Figure 9. Proposed model for the role of IL-4Rα/STAT6 signaling in Th2 cytokines-induced myeloid fibroblast activation and renal fibrosis.
Th2 cytokines such as IL-4 and IL-13 activate IL-4Rα/STAT6 signaling resulting in monocyte-to-fibroblast transition and renal fibrosis.
MATERIAL AND METHODS
Animals
WT BALB/C mice and IL-4Rα KO mice on a BALB/C background were purchased from the Jackson Laboratory. Mice were bred and maintained in the animal care facility of Baylor College of Medicine and had access to food and water ad libitum. All animal procedures were in accordance with national and international animal care and ethical guidelines and have been approved by the institutional animal welfare committee. Mice at 8–10 weeks old, weighing 20–30 g were used for the study. For folic acid model, WT and IL-4Rα KO mice were administrated folic acid at a dose of 250 mg/kg (125 mg folic acid and 252 mg NaHCO3 were dissolved in 10 ml H2O) by intraperitoneal injection. Controls mice were given NaHCO3 by intraperitoneal injection. For UUO model, male WT and IL-4Rα KO mice were anesthetized by intraperitoneal injection of ketamine and xylazine. Through a flank incision, the left ureter was exposed and completely ligated using fine suture material (4-0 silk) at two points as described9, 13, 14, 42–44.
Bone Marrow Transplantation
Bone marrow transplantation was performed as previously described13, 14, 45. Briefly, bone marrow cells (5× 106) from WT or IL-4Rα KO mice were transferred to lethally irradiated WT mice. Chimeric mice were allowed to recuperate for 8 weeks before UUO surgery.
Bone Marrow Monocyte Culture
Bone marrow monocytes of WT or IL-4Rα mice were isolated and cultured as described9, 13. Briefly, bone marrow monocytes were isolated from the femur and tibia of 8–10 weeks old mice, passed through a 40-mm cell strainer (Corning, Tewksbury, MA), and cultured in RPMI medium containing 10% FBS, 10% L929-conditioned medium, 1% glutamine, 1% MEM vitamins, and 1% penicillin/streptomycin in a humid incubator at 37°C and 5% CO 2. Fresh medium was replaced every 2–3 days. For IL-4 treatment, cells were starved with RPMI 1640 containing 2% FBS and 2% L929-conditioned medium for 24 hours and then treated to vehicle or IL-4 (PeproTech).
Renal Morphology
Mice were perfused with PBS to remove the blood. A portion of kidney tissue was embedded in paraffin, and cut at 4-μm thickness. After deparaffinization and rehydration, sections were stained with Sirius red to evaluate collagen fibers. The Sirius red-stained sections were scanned using a microscope equipped with a digital camera (Nikon Instruments), and quantitative evaluation was performed using NIS-Elements Br 3.0 software as described15, 46. The Sirius red-stained area was calculated as a percentage of the total area.
Immunohistochemistry
Immunohistochemical staining was performed on paraffin sections. Antigen retrieval was performed with antigen unmasking solution (Vector Laboratories) or proteinase K. Endogenous peroxidase activity was quenched with 3% H2O2 for 10 min. After blocking with 5% normal serum, slides were incubated with primary antibodies in a humidified chamber overnight. After washing, slides were incubated with appropriate secondary antibodies and ABC solution sequentially according to the ABC kit (Vector Laboratories). Slides were then visualized by incubation in diaminobenzidine solution for an appropriate period of time. Nuclear staining was performed with hematoxylin. The slides were dehydrated, cleared, and mounted. The images from these slides were obtained and analyzed by NIS Element software (Nikon Instruments) with Nikon microscope image system (Nikon Instruments).
Immunofluorescence
Fibronectin, Collagen I, and α-SMA staining were performed on paraffin sections. After fixation and antigen retrieval, nonspecific binding was blocked with protein block (Dako). Kidney sections were then incubated with primary antibody followed by Alexa-488 conjugated secondary antibody. Slides were mounted with medium containing DAPI. Fluorescence intensity was visualized using a microscope equipped with a digital camera (Nikon Instruments). The fluorescence positive area was calculated as a percentage of the total area as described9, 13, 14, 46. For double immunofluorescence, kidney sections were fixed and stained with anti-PDGFR-β or anti-α-SMA (Santa Cruz Biotechnology) and rat anti-CD45 (BD Biosciences) or anti-CD206 (Bio Rad Laboratories) followed by appropriate secondary antibodies sequentially. Slides were mounted with medium containing DAPI. Confocal Z-stack scanning was performed using Zeiss LSM 780 confocal microscope with Plan-Apochromat 63x/1.4 Oil DIC M27 Objective. Z stacks were created at 0.55 μm intervals throughout the section. Orthogonal views from different planes (x/y, x/z or y/z) of the confocal microscope images were obtained to show the co-expression of CD45 and α-SMA in the kidneys.
Western Blot Analysis
Protein was extracted using RIPA buffer containing cocktail proteinase inhibitors and quantified with a Bio-Rad protein assay. An equal amount of protein was separated on SDS-polycrylamide gels in Tris/SDS buffer system, and then transferred onto nitrocellulose membranes. The membranes were incubated with primary antibodies overnight, followed by incubation with appropriate fluorescence-conjugated secondary antibodies. The proteins of interest were analyzed using an Odyssey IR scanner (LI-COR Biosciences), and signal intensities were quantified using NIH Image/J software (National Institutes of Health).
Statistical Analysis
Data were expressed as mean ± SEM. Multiple group comparisons were performed by ANOVA followed by the Bonferroni procedure for comparison of means. A P value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants R01DK95835 (to Yanlin Wang), R01AR63686 (to Z Hu), and R37DK37175 (to WE Mitch), US Department of Veterans Affairs grant I01BX02650 (to Yanlin Wang), and an American Heart Association grant 11BGIA7840054 (to Yanlin Wang). J Yan was supported in part by a NIH T32 training grant T32DK62706.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have declared that no conflict of interest exists.
References
- 1.Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 2.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 5.Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2006;17:2992–2998. doi: 10.1681/ASN.2006050420. [DOI] [PubMed] [Google Scholar]
- 6.Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015;87:297–307. doi: 10.1038/ki.2014.287. [DOI] [PubMed] [Google Scholar]
- 7.Sakai N, Wada T. T Helper 2 Cytokine Signaling in Bone Marrow-Derived Fibroblasts: A Target for Renal Fibrosis. J Am Soc Nephrol. 2015;26:2896–2898. doi: 10.1681/ASN.2015040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y, Yang M, Zhang J, et al. Depletion of CD8+ T Cells Exacerbates CD4+ T Cell-Induced Monocyte-to-Fibroblast Transition in Renal Fibrosis. Journal of immunology. 2016 doi: 10.4049/jimmunol.1501232. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Lin SC, Chen G, et al. Adiponectin promotes monocyte-to-fibroblast transition in renal fibrosis. J Am Soc Nephrol. 2013;24:1644–1659. doi: 10.1681/ASN.2013030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niedermeier M, Reich B, Rodriguez Gomez M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A. 2009;106:17892–17897. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog EL, Bucala R. Fibrocytes in health and disease. Experimental hematology. 2010;38:548–556. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J, Zhang Z, Yang J, et al. JAK3/STAT6 Stimulates Bone Marrow-Derived Fibroblast Activation in Renal Fibrosis. J Am Soc Nephrol. 2015;26:3060–3071. doi: 10.1681/ASN.2014070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen G, Lin SC, Chen J, et al. CXCL16 recruits bone marrow-derived fibroblast precursors in renal fibrosis. J Am Soc Nephrol. 2011;22:1876–1886. doi: 10.1681/ASN.2010080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Y, Jin X, Yan J, et al. CXCR6 plays a critical role in angiotensin II-induced renal injury and fibrosis. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1422–1428. doi: 10.1161/ATVBAHA.113.303172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Y, Yan J, Jin X, et al. The chemokine receptor CXCR6 contributes to recruitment of bone marrow-derived fibroblast precursors in renal fibrosis. Kidney international. 2014;86:327–337. doi: 10.1038/ki.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schurigt U, Pfirschke C, Irmler IM, et al. Interactions of T helper cells with fibroblast-like synoviocytes: up-regulation of matrix metalloproteinases by macrophage migration inhibitory factor from both Th1 and Th2 cells. Arthritis and rheumatism. 2008;58:3030–3040. doi: 10.1002/art.23904. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins SJ, Ruckerl D, Cook PC, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 20.Quentmeier H, Kolsdorf K, Zaborski M, et al. IL-4R alpha and gamma chain expression in LPS- and IL-4-stimulated MONO-MAC-6 cells. Leukemia. 1995;9:336–340. [PubMed] [Google Scholar]
- 21.Kohanbash G, McKaveney K, Sakaki M, et al. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-alpha. Cancer research. 2013;73:6413–6423. doi: 10.1158/0008-5472.CAN-12-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbert DR, Orekov T, Perkins C, et al. IL-4R alpha expression by bone marrow-derived cells is necessary and sufficient for host protection against acute schistosomiasis. Journal of immunology. 2008;180:4948–4955. doi: 10.4049/jimmunol.180.7.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SJ, Lee KP, Kang S, et al. Sphingosine 1-phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cellular signalling. 2014;26:2249–2258. doi: 10.1016/j.cellsig.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Vento-Tormo R, Company C, Rodriguez-Ubreva J, et al. IL-4 orchestrates STAT6-mediated DNA demethylation leading to dendritic cell differentiation. Genome biology. 2016;17:4. doi: 10.1186/s13059-015-0863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirey KA, Pletneva LM, Puche AC, et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal immunology. 2010;3:291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ripple MJ, You D, Honnegowda S, et al. Immunomodulation with IL-4R alpha antisense oligonucleotide prevents respiratory syncytial virus-mediated pulmonary disease. Journal of immunology. 2010;185:4804–4811. doi: 10.4049/jimmunol.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ennaciri J, Girard D. IL-4R(alpha), a new member that associates with Syk kinase: implication in IL-4-induced human neutrophil functions. Journal of immunology. 2009;183:5261–5269. doi: 10.4049/jimmunol.0900109. [DOI] [PubMed] [Google Scholar]
- 29.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Souza PP, Brechter AB, Reis RI, et al. IL-4 and IL-13 inhibit IL-1beta and TNF-alpha induced kinin B1 and B2 receptors through a STAT6-dependent mechanism. British journal of pharmacology. 2013;169:400–412. doi: 10.1111/bph.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livraghi A, Grubb BR, Hudson EJ, et al. Airway and lung pathology due to mucosal surface dehydration in {beta}-epithelial Na+ channel-overexpressing mice: role of TNF-{alpha} and IL-4R{alpha} signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. Journal of immunology. 2009;182:4357–4367. doi: 10.4049/jimmunol.0802557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knipper JA, Willenborg S, Brinckmann J, et al. Interleukin-4 Receptor alpha Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity. 2015;43:803–816. doi: 10.1016/j.immuni.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L, Yang T, Li L, et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nature communications. 2014;5:4696. doi: 10.1038/ncomms5696. [DOI] [PubMed] [Google Scholar]
- 34.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 35.Pechkovsky DV, Prasse A, Kollert F, et al. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clinical immunology. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Cao Q, Wang Y, Harris DC. Macrophage heterogeneity, phenotypes, and roles in renal fibrosis. Kidney international supplements. 2014;4:16–19. doi: 10.1038/kisup.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto H, Katsuyama E, Miyauchi Y, et al. An essential role for STAT6-STAT1 protein signaling in promoting macrophage cell-cell fusion. The Journal of biological chemistry. 2012;287:32479–32484. doi: 10.1074/jbc.M112.358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji Y, Sun S, Xu A, et al. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. The Journal of biological chemistry. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards JK. Renal fibrosis: Activation of JAK3/STAT6 contributes to the development of renal fibrosis. Nat Rev Nephrol. 2015;11:445. doi: 10.1038/nrneph.2015.97. [DOI] [PubMed] [Google Scholar]
- 40.Yukawa K, Kishino M, Goda M, et al. STAT6 deficiency inhibits tubulointerstitial fibrosis in obstructive nephropathy. International journal of molecular medicine. 2005;15:225–230. [PubMed] [Google Scholar]
- 41.He C, Larson-Casey JL, Gu L, et al. Cu,Zn-SOD-mediated Redox Regulation of Jmjd3 Modulates Macrophage Polarization and Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2015-0183OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia Y, Entman ML, Wang Y. CCR2 Regulates the Uptake of Bone Marrow-Derived Fibroblasts in Renal Fibrosis. PLoS One. 2013;8:e77493. doi: 10.1371/journal.pone.0077493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia Y, Yan J, Jin X, et al. The chemokine receptor CXCR6 contributes to recruitment of bone marrow-derived fibroblast precursors in renal fibrosis. Kidney Int. 2014;86:327–337. doi: 10.1038/ki.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Chen J, Yan J, et al. Effect of interleukin 6 deficiency on renal interstitial fibrosis. PLoS One. 2012;7:e52415. doi: 10.1371/journal.pone.0052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin X, Chen J, Hu Z, et al. Genetic deficiency of adiponectin protects against acute kidney injury. Kidney Int. 2013;83:604–614. doi: 10.1038/ki.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia Y, Entman ML, Wang Y. Critical Role of CXCL16 in Hypertensive Kidney Injury and Fibrosis. Hypertension. 2013;62:1129–1137. doi: 10.1161/HYPERTENSIONAHA.113.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.