Abstract
Despite effective treatment for chronic hepatitis C (CHC), deficiencies in diagnosis and access preclude disease elimination. Screening of baby boomers remains low. The aims of this study were to assess the impact of an electronic health record (EHR) based prompt on HCV screening rates in baby boomers in primary care, and access to specialty care and treatment among those newly diagnosed. We implemented an EHR based “Best Practice Advisory” (BPA) that prompted primary care providers (PCPs) to perform HCV screening for patients seen in primary care clinic: 1) born between 1945–1965; 2) lacked a prior diagnosis of HCV infection; and 3) lacked prior documented anti-HCV testing. The BPA had associated educational materials, order set, and streamlined access to specialty care for newly diagnosed patients. Pre and post BPA screening rates were compared and care of newly diagnosed patients analyzed. In the 3 years prior to BPA implementation, 52,660 baby boomers were seen in primary care clinics, and 28% were screened. HCV screening increased from 7.6% for patients with a PCP visit in the 6 months prior to BPA to 72% over the 1-year post BPA. Of 53 newly diagnosed patients, all were referred for specialty care, 11 had advanced fibrosis or cirrhosis, 20 started treatment and 9 achieved SVR thus far.
Conclusions
Implementation of an EHR based prompt increased HCV screening rates among baby boomers in primary care by 5 fold due to efficiency in determining needs for HCV screening and work-flow design. Streamlined access to specialty care enabled patients with previously undiagnosed advanced disease to be cured. This intervention can be easily integrated into EHR systems to increase HCV diagnosis and linkage to care.
Keywords: viral hepatitis, electronic health record, diagnosis, access to care
Introduction
Over the last few years there have been revolutionary advances in the treatment of chronic hepatitis C (CHC). Current treatments with direct-acting antiviral agents (DAAs) are highly efficacious with sustained virologic response (SVR) rates of >95% for the vast majority of patients.(1, 2) Moreover, unlike interferon-based therapies, DAAs are administered orally, have minimal side effects, and are of short duration. Thus, almost all CHC patients are candidates for treatment. Despite the dramatic advances in therapy, there remain significant barriers to hepatitis C virus (HCV) elimination, primary of which are deficiencies in screening and diagnosis of patients with CHC and subsequent linkage to care.(3) It has been estimated that only 50–65% of the estimated 3.2 million persons chronically infected with HCV in the United States are aware of their infection.(4–6) Given that risk based screening had not adequately addressed this deficiency, the Centers for Disease Control and Prevention (CDC) and the United States Preventive Services Task Force (USPSTF) recommended one-time screening for all baby boomers (adults born between 1945 and 1965) regardless of the presence of any of the traditional risk factors for HCV because this cohort has 5-fold higher prevalence of HCV than persons in other age groups.(7, 8)
Despite this evidence-based recommendation, uptake of one-time universal HCV screening among baby boomers remains low. The reported ranges for screening among this cohort varies, but in general are estimated to be <30%, even after implementing interventions specifically aimed at increasing HCV screening among baby boomers.(9–12) Various approaches have been evaluated to optimize screening rates including integration of HCV screening into other preventive health screening like colonoscopy or HIV testing, as well as screening in emergency room and inpatient settings.(13–16) In order for HCV screening to have the highest impact however, screening needs to be linked with subsequent care after test results are available. Prior estimates from outpatient and emergency room settings have shown that 30–50% of persons who test positive for HCV antibody (anti-HCV) never receive confirmatory HCV RNA testing, and among those with a confirmed diagnosis of HCV infection, only a minority (approximately 35%) are connected with specialty care.(17–19) As such, conducting HCV screening through an established primary care provider (PCP) has been hypothesized as having a higher likelihood of minimizing gaps in follow-up. These interventions need to be cognizant of minimizing work load and stretching the already overburdened PCPs.
Given the almost universal use of electronic health record (EHR) systems, there has been much interest in methods to incorporate automatic prompts into EHRs as these represent a potentially efficient and effective approach to incorporate HCV screening within the context of a busy PCP clinic visit.(20) Integrating downstream work-flow to optimize referral and ultimately access to DAA therapy would further increase the overall impact of an HCV screening program and address critical deficiencies in the HCV “care cascade” where only 7.4% of diagnosed patients are estimated to have been started on HCV treatment globally.(21) We developed a Best Practice Advisory (BPA) in our EHR to prompt HCV screening among baby boomers and designed a detailed work-flow for subsequent care management for newly diagnosed patients. The primary aim of this study was to assess the impact of our EHR based prompt on HCV screening rates in baby boomers seen in primary care clinics. Secondary aims included access to specialty care and curative treatment among patients newly diagnosed with CHC.
Methods
Assessment of Pre and Post BPA Screening Rates
In order to compare HCV screening rates and patient/provider characteristics associated with screening patterns, we evaluated all adult patients within the baby boomer cohort who had at least one visit during the prior 3 years, in primary care clinics in our health system which has clinics in 13 locations within a 30-mile radius from our main campus in Ann Arbor. All providers are employed by the University of Michigan and all clinics use the same EHR system. Primary care clinics encompass 4 different medicine specialties: general internal medicine, family medicine, medicine/pediatrics, and geriatrics. We assessed rates of both anti-HCV orders and anti-HCV tests performed to capture potential discrepancies between screening orders and screening tests completed. We additionally assessed rates of anti-HCV positivity and downstream confirmatory HCV RNA testing and results. To examine association between patient and provider characteristics and screening patterns, we collected data on patient age, gender, race/ethnicity and insurance provider, as well as clinic sites and provider specialties. The baseline screening period consisted of the 3 years prior to implementation of the BPA. The post BPA screening period consisted of 1-year post system wide implementation of the BPA. We assessed screening rates both by number of screening eligible PCP visits and by screening eligible patients (as patients had varying numbers of PCP clinic visits over the study periods of interest). This study was approved by the University of Michigan Institutional Review Board.
Design of Best Practice Advisory: PCP Clinic Visit
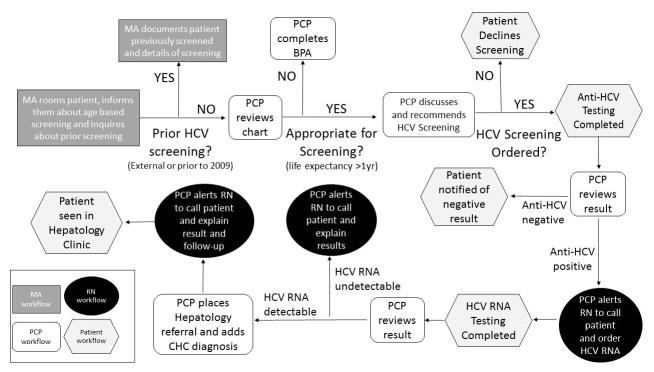
A BPA alert was developed in conjunction with our EHR Population and Health Management Ambulatory Care Services Team and consultation from several PCPs with the primary aim of increasing uptake of one-time HCV screening for baby boomers. As part of this initiative, patient educational flyers regarding rationale for HCV screening in baby boomers were posted in primary care clinics and educational materials were also provided in the electronic patient portal to raise awareness and prime patients for potential discussion about HCV screening during their visit. The BPA would “fire” for any patient seen in primary care clinic that: 1) was born between 1945–1965; 2) lacked a prior EHR ICD-9 or ICD-10 diagnosis code of HCV infection; and 3) lacked documented anti-HCV testing after 2009 in our EHR. A detailed illustration of the BPA Workflow is provided in Figure 1. To briefly review, upon rooming a patient for their appointment, the medical assistant (MA) would inform the patient about age-based HCV screening and inquire if the patient had been screened elsewhere or prior to 2009 (which would not be captured in our EHR and thus the BPA would still fire if HCV screening was done externally or prior to 2009). If patient had anti-HCV testing performed prior to 2009 or elsewhere, the MA would override the BPA. If testing had not been performed prior, then the MA would provide patient educational materials on rationale for HCV screening, what the screening consisted of, and interpretation of test results. During the clinic visit, the PCP would then discuss HCV screening with the patient and indicate if the patient agreed with or declined testing. If “patient declines” was chosen as reason to not pursue testing, the BPA would fire again at the patient’s next visit after 10 months for all patients to allow PCPs to re-discuss HCV testing. Because some patients are seen at frequent intervals, the BPA did not fire at each subsequent visit. If the patient accepted screening, then a pre-populated “Smart Set” with anti-HCV lab order and associated diagnosis code would be signed. An illustration of the BPA interface is provided in Figure 2. Of note, this BPA was not designed as a “hard stop” in that the PCP could acknowledge the BPA but was not required to either document a reason for not testing or order testing in order for them to be able to continue to work in the patient’s encounter. This decision was made given that “hard stop” BPAs were in general poorly received by providers.
Figure 1. HCV BPA Workflow.
HCV, hepatitis C virus; BPA, best practice advisory; PCP, primary care provider; MA, medical assistant; RN, registered nurse; CHC, chronic hepatitis C
Figure 2. HCV BPA Design.
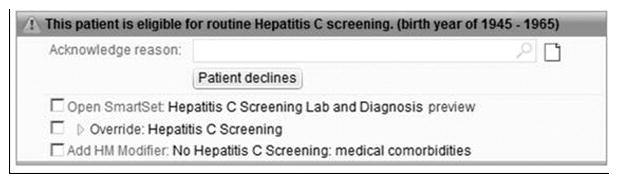
HCV, hepatitis C virus; BPA, best practice advisory
Development of BPA: Follow-up on anti-HCV test results
At the time of implementation of the BPA, our pathology lab did not have reflex testing in place for HCV RNA for samples that had reactive anti-HCV result due to concerns about potential contamination of previously used samples. Thus, patients with anti-HCV positive results were asked to return for HCV RNA testing. We developed result scripts to facilitate nurses’ (RN) follow-up with patients based on the result of their anti-HCV and HCV RNA tests. Follow-up work flow based on testing results is displayed in Figure 1. In addition, we provided guidelines on HCV consultations including recommended testing prior to referral.
Design of BPA: Pilot testing
The BPA was initially pilot-tested in three clinic sites (one general medicine, one family medicine and one medicine/pediatrics) over a 3-month period to ensure streamlined work-flow and to identify potential areas for improvement in implementation of the BPA. Feedback was solicited from patients, PCPs, RNs and MAs with regard to design and workflow. During the 3-month pilot phase, we identified the optimal timing for provision of the patient educational materials for HCV screening. Initially these materials were also made available prior to the appointment for eligible patients via the EHR patient portal, but this caused anxiety among a small number of patients and thus it was removed and provided during the clinic visit to allow PCPs to address questions/concerns in real time. During this pilot phase we also worked with our pathology department to improve the associated text that accompanied anti-HCV and HCV RNA test reports to simplify interpretation of results by both patients and providers. We additionally engaged in conversation about the possibility of reflex testing for HCV RNA among patients with anti-HCV positive results. Lastly, we were able to assess logistics and demand for referral to specialty care. The BPA was then implemented across all primary care clinics.
HCV Care Cascade
In order to assess the downstream impact of our BPA, we followed the subsequent care cascade for all patients with a detectable HCV RNA. We assessed the number of patients referred to Hepatology clinic, number of patients seen, number of patients prescribed DAA therapy, number of patients started on DAA, and number of patients who achieved SVR (assessed at week 12 after completion of therapy). We also assessed stage of liver disease for the patients seen in Hepatology clinic.
HCV Testing
Anti-HCV and HCV RNA tests were performed at the Clinical Pathology Laboratories at our main hospital for patients seen at all clinical sites. Anti-HCV was tested using a chemiluminescent immunoassay, Siemens ADVIA Centaur Immunoassay (Malvern, Pennsyvlania). A signal to cutoff ratio of 1–10.99 was categorized as weakly reactive and ≥11 was categorized as reactive. HCV RNA was tested using Abbott RealTime HCV Test (Abbott Park, Illinois) with a lower limit of detection of 12 international units/mL.
Statistical Analyses
Descriptive and bivariate analysis were performed to assess trends in testing and characteristics associated with screening. P values ≤0.05 were considered statistically significant.
Results
Screening Rates and Characteristics in Pre and Post BPA Periods
In the 3 year pre-BPA period, a total of 52,660 patients in the baby boomer cohort had a PCP visit. HCV screening was ordered in only 28% of patients over this 3-year period. HCV screening was more often performed in males (31% vs 26%), among African American and Asian patients (34% and 36% vs. 27–29%), and among patients with Medicaid or Medicare (34% and 32% vs 26–28%). Screening rates also varied substantially across clinic sites and clinic specialties as demonstrated in Table 1. The increase in screening rates and the associated patient and provider characteristics over the course of the post-BPA period are demonstrated in Table 1. Over the 1-year post BPA period, screening rates increased evenly across patient and provider characteristics, although screening rates within the single geriatrics clinic remained comparatively lower than other clinics.
Table 1.
Screening Rates for Baby Boomers followed in Primary Care Pre and Post BPA*
| Variable of Interest | Screening Rates Pre-BPA (N=52,660) |
Screening Rates Post BPA (N=52,832) |
|---|---|---|
|
| ||
| Anti-HCV ordered | 28 (14,870) | 71 (37,459) |
|
| ||
| Gender | ||
| Female | 26 | 71 |
| Male | 31 | 71 |
| P=<0.001 | ||
|
| ||
| Age | ||
| 50–59 | 28.5 | 69 |
| 60–70 | 28.5 | 73 |
|
| ||
| Race/Ethnicity | ||
| Caucasian | 27 | 72 |
| African American | 34 | 75 |
| Asian | 36 | 77 |
| Other/unknown | 29 | 71 |
| P=<0.001 | ||
|
| ||
| Insurance | ||
| Commercial | 27 | 70 |
| Medicaid | 34 | 72 |
| Medicare | 32 | 76 |
| P=<0.001 | ||
|
|
||
| Clinic Specialty | ||
| Median (range) | Range: 19–34% | Range: 50–78% |
| Gen Med | 32 (20–34) | 73 (54–81) |
| Fam Med | 28 (20–32) | 73(67–75) |
| Med-Peds^ | 25 | 75 |
| Geriatrics^ | 19 | 50 |
| P=<0.001 | ||
Represents all baby boomers considered active within UMHS primary care (having had a PCP visit within 3 years prior to BPA and within the 1 year post-BPA time frame). Data expressed as % unless otherwise noted.
only one Med-Peds Clinic and one Geriatrics Clinic
Regarding utilization of the BPA, “patient declines” was documented as a reason to not pursue screening only 4% of the time. Similarly, medical co-morbidities and prior testing elsewhere were infrequently documented as a reason why HCV screening was not pursued (1% each). There was no reason documented why HCV screening was not performed or the lab order not signed in the remaining cases. Review of baby boomer PCP clinic visits over the study period demonstrated that approximately 31% of baby boomers seen in primary care clinics did not have the BPA fire due to prior HCV screening or pre-existing diagnosis of CHC.
Diagnostic Testing Results in Pre and Post BPA Periods
We compared HCV screening and diagnostic test results in a similar number of eligible clinic visits and eligible patients in the pre and post BPA period (Table 2). In the 6 month pre-BPA period, anti-HCV was ordered in only 4.6% of eligible visits and 7.6% of eligible patients compared to 47% and 72% in the 12 month post-BPA period (p= <0.001). When ordered, anti-HCV test was performed in 99% of patients in both the pre and post BPA period. The rate of positive anti-HCV result was low in both the pre and post BPA periods (2% and 0.8% respectively). The frequency with which confirmatory HCV RNA test was ordered was higher in the post BPA period, though the difference was not statistically significant (86% vs 94%, p=0.09). It should be noted that reflex HCV RNA testing was implemented in June 2016 (approximately the midpoint of our 1 year BPA implementation).
Table 2.
Screening and Diagnostic Testing in Pre and Post BPA Periods*
| Screening: Eligible Visits | Pre-BPA N=37,289 |
Post-BPA N=42,721 |
|---|---|---|
| Anti-HCV ordered | 4.6% (1,716) | 47% (19,895) |
| Anti-HCV drawn after order | 99% (1,705) | 99% (19,847) |
| Screening: Eligible Patients |
Pre-BPA N=22,488 |
Post-BPA N=27,789 |
| Anti-HCV ordered | 7.6% (1,716) |
72% (19,895) P=<0.001 |
| Anti-HCV drawn after order | 99% (1,705) | 99% (19,847) |
| Positive Anti-HCV test | 2.1% (36) | 0.8% (178) |
| Confirmatory Testing |
Pre-BPA N=36 |
Post-BPA N=178 |
| HCV RNA ordered | 86% (31) | 94% (168) P= 0.09 |
| Detectable HCV RNA | 74% (23) | 33% (56) P=<0.001 |
Pre and Post BPA periods represent similar number of screening eligible PCP visits and patients. Pre-BPA era captured 6 months prior to BPA implementation. Post-BPA period captured 1 year most BPA implementation.
The percentage of anti-HCV positive patients with a detectable HCV RNA was lower in the post BPA period (74% vs 33%, p <0.001). Prior to implementation of reflex HCV RNA testing, anti-HCV results were reported as reactive or weakly reactive based on the signal-to-cut-off ratio, whereas after reflex testing was implemented, anti-HCV results were reported only as reactive or non-reactive without further qualification (i.e. reactive vs weakly reactive). Eighty-three patients with a positive anti-HCV but undetectable HCV RNA had testing done prior to implementation of reflex HCV RNA testing. Of these, 69 (83%) were only weakly reactive on anti-HCV test. Among patients with a positive anti-HCV, those with undetectable HCV RNA were less likely to be African American (72% vs 85%, P=0.04), but had similar age distributions (age 50–59 45% vs 34%, age 60–70 55% vs 66%, P=0.16) compared to those with detectable HCV RNA.
HCV Care Cascade
Among the 56 patients with an initial detectable HCV RNA in the post-BPA period, 3 patients had low HCV RNA levels (<12 international units/mL) that were not confirmed on subsequent testing. All 53 patients with confirmed CHC were referred to specialty care. At the time of data analysis, a total of 46 had attended their specialty clinic appointment, 1 had an upcoming appointment, 2 declined referral, and 4 were no longer seen at our center. Among these 53 patients, 6 had cirrhosis by imaging or transient elastography, 3 had advanced fibrosis and 21 had no or mild fibrosis (F0–F2) by transient elastography. Of the remaining 23 patients, 2 had aspartate aminotransferase to platelet ratio index (APRI) >1.5. Thus, in total, 11 of 53 (20.7%) patients with CHC had advanced fibrosis or cirrhosis.
At the time of writing, DAA therapy was prescribed in 31 patients of whom 20 had started treatment. For the 11 prescribed DAA but not started on treatment, 6 were still pending approval of their DAA prescription, 4 were denied access to treatment by their insurance provider, and one patient was managed at an outside facility and thus treatment status was unknown. For the 20 patients who started therapy, 9 had completed treatment and achieved SVR 12, and 11 patients had SVR labs upcoming with 9 having end of treatment response (the remaining 2 patients were still on therapy). (Figure 3) Of the 15 patients not yet prescribed DAA treatment, 7 patients required additional follow-up assessment, 4 required management of severe active alcohol or substance abuse, 2 had active malignancies (one non-hepatic and one newly diagnosed HCC), 1 did not return for follow-up, and 1 was managed by another specialist outside our system.
Figure 3. HCV Care Cascade.
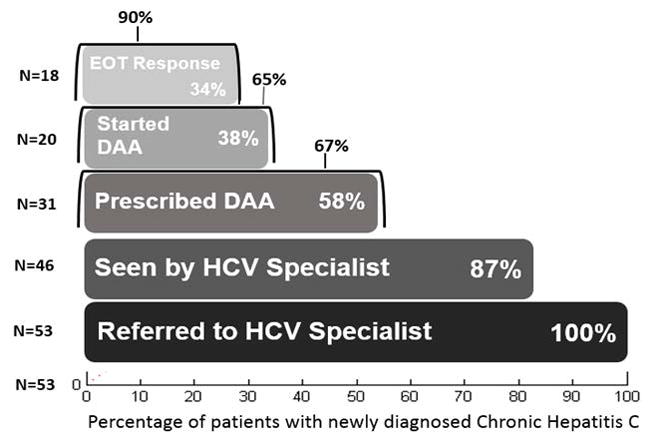
HCV, hepatitis C virus; DAA, direct acting anti-viral agent; EOT, end of treatment. Percentage outside of bar shows proportion accounting for only the number of patients eligible for that outcome.
Discussion
Despite revolutionary advances in treatment for CHC, HCV infection remains a worldwide public health problem due to the multiple systematic barriers to disease eradication. Given the potential morbidity and mortality resulting from CHC, interventions aimed at mitigating these deficiencies in the HCV care cascade are critical. The National Academies of Sciences, Engineering and Medicine recently released a report regarding a strategy to eliminate CHC with a goal to reduce new cases of HCV infection by 90% and mortality from CHC by 65% in the United States by the year 2030.(6) Key areas within this strategy focus on improvements in screening and diagnosis as well as improvement in access to specialty care and curative therapies. While various approaches have been evaluated to improve HCV screening across diverse patient care settings, most programs have resulted in only modest improvement in screening rates. Moreover, without linkage to subsequent confirmatory diagnostic testing and follow-up care, the impact of screening programs has been significantly muted. Herein we describe a low cost, efficient and highly effective EHR based HCV screening program for baby boomers in the primary care setting that resulted in a 5-fold increase in screening rates with subsequent successful linkage to specialty care and curative treatment.
The findings of our study echoed those of prior investigations demonstrating the low rate of uptake of the universal recommendation for one-time HCV screening for baby boomers with only 28% of patients having been screened over the 3-year baseline assessment period. Also of note, the practice patterns at baseline varied according to gender, race, insurance type and clinic type. These discrepant screening rates have been shown previously and in part reflect perceived varying prevalence rates of HCV infection among different patient cohorts.(22) Of note, these practice variations disappeared once the BPA implementation had been in place for 1 year, with screening rates being driven by baby boomer status and not by other demographic, insurance, or practice setting variables. HCV screening rates among baby boomers increased 5-fold to 72% during the 1-year period after implementation of the EHR-based BPA. This HCV screening rate among baby boomers is one of the highest cited in the literature to date. The success of this screening intervention is a direct result of the ease of the EHR design that was constructed based on the needs of and feedback provided by PCPs. Specifically, this BPA eliminated the burden of work previously placed on PCPs to first remember the need for HCV screening in this population and secondly to verify prior HCV testing or diagnosis of individual patients. There has been concern about “alert fatigue”, particularly on the part of PCPs who are asked to screen for numerous conditions within time constrained visits. Keeping this concern in mind, we developed the alert with input from a PCP (HC), did a pilot run for 3 months, and proactively solicited feedback from PCPs. We elected to not make the alert a “hard stop” for the clinic encounters and also decided to not have the BPA fire at each subsequent visit until it was addressed based on PCP feedback and in order to prevent impeding workflow. As such, this BPA was favorably received by PCPs. Lastly, we incorporated educational materials for both patients and the medical staff in order to help decrease anxiety related to screening, reduce work load for PCP clinics, and to make it easy for staff to provide rationale for screening and explanation of subsequent results.
In addition to the improvement in HCV screening were the associated improvements in confirmatory testing and subsequent linkage to care. Post-BPA, 94% of patients with a positive anti-HCV had a confirmatory HCV RNA ordered compared to only 86% in the pre-BPA period although these differences were not statistically significant and implementation of reflex HCV RNA testing during the latter part of the study period may also have contributed to this increase. Our rate of confirmatory testing compares favorably with estimates of only 30–50% in other studies.(17, 18) Some studies showed that reflex HCV RNA testing can improve the likelihood of confirmatory testing and would certainly minimize inconvenience to patients and decrease their anxiety while waiting for additional testing. We are pleased that with continued dialogue with our pathology colleagues, we were able to incorporate reflex testing for HCV RNA in patients who test positive for anti-HCV. Interestingly, in our cohort the prevalence of positive anti-HCV was low (2% and 0.8% in pre and post BPA period respectively). Estimates from the CDC for the baby boomer cohort cite an approximate 3.25% prevalence of anti-HCV, though similar studies have found lower rates when screening programs have been implemented for this population in non-urban primary care settings.(23–25) Our low anti-HCV positivity rate may also be reflective of the demographics of our patient population. In addition, many of our patients have been in our health system for many years and an estimated 31% of our baby boomers seen in primary care clinics did not have the BPA fire due to prior HCV screening or pre-existing diagnosis of CHC. Another interesting finding was the low rate of detectable HCV RNA in the post BPA period with only 33% of patients with positive anti-HCV having a detectable HCV RNA. Reviewing these cases in detail revealed that the majority of the patients who were anti-HCV positive but had undetectable HCV RNA had weakly reactive anti-HCV and thus were likely false positives. Similar findings had been observed in other studies when screening is conducted in larger numbers of persons with low risk of infection.(24, 26)
Contrary to other studies of birth cohort screening, we had much higher success rates with follow-up care and subsequent DAA treatment. Within 6 months of the end of the 1-year post-BPA implementation period, 100% of patients with newly diagnosed CHC were referred to specialty care with 87% already seen by a specialist and 67% of these patients prescribed DAA therapy. This success in bridging gaps in linkage to care after initial screening and diagnosis are reflective of the primary care based setting for the intervention that is more amenable to facilitating necessary follow-up. Data examining subsequent linkage to care and curative treatment from other similar EHR based interventions for HCV screening of baby boomers in primary care are limited. Our rates of visits with HCV specialists and initiation on DAA therapy were higher than other studies: 77% newly diagnosed patients seen by specialists in the study by Goel et al and 21% and 57% of newly diagnosed patients started on DAA in the studies by Mera et al and Miller et al, respectively. (11, 27, 28) While most of the newly diagnosed patients had early stage disease, 20.7% had evidence of either cirrhosis or advanced fibrosis at the time of initial referral. These patients likely would remain undiagnosed until they present with decompensated liver disease, had they not been screened as a result of the BPA. With the high rate of cure in the DAA era, early diagnosis and treatment would prevent progression of liver disease. Though preliminary, our study showed that 9 of 9 evaluable patients achieved SVR 12 and an additional 9 patients had end of treatment response.
There are several limitations to our study. These include the relatively homogenous patient population with potentially lower risk of HCV infection that may have accounted for the low anti-HCV positivity rate in this study. Secondly, while EHR has been adopted widely for clinical patient care, it may not be present in all settings and thus the applicability of this intervention would be limited to practices with EHR in place. Our hospital system uses an EPIC-based EHR which is used by many other health centers in the United States. The BPA design could easily be implemented in other EPIC based systems or adapted for other EHRs.
In conclusion, we demonstrated that implementation of an EHR based BPA for HCV screening among baby boomers in the primary care setting resulted in a 5-fold increase in screening rates and was well received by PCPs. The associated structured work-flow to address confirmatory testing and referral to specialty care was critical in minimizing gaps in the HCV care cascade. As a result of this intervention, we were able to identify patients with CHC who likely would not be diagnosed and treated until they present with cirrhosis complications. This type of EHR based intervention represents a low cost, efficient and effective means to improve HCV screening, diagnosis and access to care which ultimately can lead to mitigation of the associated morbidity and mortality of CHC.
Acknowledgments
Financial Support: Dr. Lok has received research grant funding from Bristol-Myers Squibb and Gilead. This study was funded in part by the National Institutes of Health T32DK062708 training grant and the AASLD Advanced/Transplant Hepatology Fellowship (MAK).
Abbreviations
- HCV
hepatitis C virus
- EHR
electronic health records
- DAA
direct acting antiviral agent
- SVR
sustained virologic response
- CHC
chronic hepatitis C
- PCP
primary care provider
- BPA
best practice advisory
- HCC
hepatocellular carcinoma
- MA
medical assistant
Footnotes
Conflict of interests
Dr. Lok has received research grant funding from Bristol-Myers Squibb and Gilead. The remaining authors have no conflicts to disclose.
References
- 1.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 2.Feld JJ, Zeuzem S. Sofosbuvir and Velpatasvir for Patients with HCV Infection. N Engl J Med. 2016;374:1688–1689. doi: 10.1056/NEJMc1601160. [DOI] [PubMed] [Google Scholar]
- 3.Konerman MA, Lok AS. Hepatitis C Treatment and Barriers to Eradication. Clin Transl Gastroenterol. 2016;7:e193. doi: 10.1038/ctg.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–1861. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine. National Strategy on the Elimination of Hepatitis B and C. National Academies of Sciences, Engineering and Medicine; 2016. [Google Scholar]
- 7.Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 8.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 9.Adebajo CO, Aronsohn A, Te HS, et al. Birth cohort HCVscreening is lower in the Emergency Department than the outpatient setting. Gastroenterology. 2015;148(4 AUPPL 1):S1101–S1102. [Google Scholar]
- 10.Litwin AH, Smith BD, Drainoni ML, McKee D, Gifford AL, Koppelman E, Christiansen CL, et al. Primary care-based interventions are associated with increases in hepatitis C virus testing for patients at risk. Dig Liver Dis. 2012;44:497–503. doi: 10.1016/j.dld.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Goel A, Sanchez J, Paulino L, Feuille C, Arend J, Shah B, Dieterich D, et al. A systematic model improves hepatitis C virus birth cohort screening in hospital-based primary care. J Viral Hepat. 2016 doi: 10.1111/jvh.12669. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Fedewa SA. Recent Hepatitis C Virus Testing Patterns Among Baby Boomers. Am J Prev Med. 2017 doi: 10.1016/j.amepre.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Sears DM, Cohen DC, Ackerman K, Ma JE, Song J. Birth cohort screening for chronic hepatitis during colonoscopy appointments. Am J Gastroenterol. 2013;108:981–989. doi: 10.1038/ajg.2013.50. [DOI] [PubMed] [Google Scholar]
- 14.United States Department of Health and Human Services. Combating the silent epidemic of viral hepatitis. 2011. [Google Scholar]
- 15.Allison WE, Chiang W, Rubin A, O’Donnell L, Saldivar MA, Maurantonio M, Dela Cruz J, et al. Hepatitis C virus infection in the 1945–1965 birth cohort (baby boomers) in a large urban ED. Am J Emerg Med. 2016;34:697–701. doi: 10.1016/j.ajem.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 16.Turner BJ, Taylor BS, Hanson J, Liang Y, Veerapaneni P, Villarreal R, Perez M, et al. High priority for hepatitis C screening in safety net hospitals: Results from a prospective cohort of 4582 hospitalized baby boomers. Hepatology. 2015;62:1388–1395. doi: 10.1002/hep.28018. [DOI] [PubMed] [Google Scholar]
- 17.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V., 3rd The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9:e101554. doi: 10.1371/journal.pone.0101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGibbon E, Bornschlegel K, Balter S. Half a diagnosis: gap in confirming infection among hepatitis C antibody-positive patients. Am J Med. 2013;126:718–722. doi: 10.1016/j.amjmed.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Reau N. HCV testing and linkage to care: Expanding access. Clinical Liver Disease. 2014;4:31–34. doi: 10.1002/cld.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brady JE, Liffmann DK, Yartel A, Kil N, Federman AD, Kannry J, Jordan C, et al. Uptake of hepatitis C screening, characteristics of patients tested, and intervention costs in the BEST-C study. Hepatology. 2017;65:44–53. doi: 10.1002/hep.28880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolrd Health Organization. Hepatitis C Fact Sheet. 2017 http://www.who.int/mediacentre/factsheets/fs164/en/
- 22.Backus LI, Belperio PS, Loomis TP, Mole LA. Impact of race/ethnicity and gender on HCV screening and prevalence among U.S. veterans in Department of Veterans Affairs Care. Am J Public Health. 2014;104(Suppl 4):S555–561. doi: 10.2105/AJPH.2014.302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thuluvath PJ, FH, Horowitz A, Lowe G. Screening for hepatitis C in baby boomer population using EMR pop-up and targeted mailing from primary care physicians in a single community teaching hospital. Hepatology. 2016;63(Supplement 1):409A. [Google Scholar]
- 24.Younossi ZM, LaLuna LL, Santoro JJ, Mendes F, Araya V, Ravendhran N, Pedicone L, et al. Implementation of baby boomer hepatitis C screening and linking to care in gastroenterology practices: a multi-center pilot study. BMC Gastroenterol. 2016;16:45. doi: 10.1186/s12876-016-0438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federman AD, Kil N, Kannry J, Andreopolous E, Toribio W, Lyons J, Singer M, et al. An Electronic Health Record-based Intervention to Promote Hepatitis C Virus Testing Among Adults Born Between 1945 and 1965: A Cluster-randomized Trial. Med Care. 2017;55:590–597. doi: 10.1097/MLR.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moorman AC, Drobenuic J, Kamili S. Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007–2012. J Clin Virol. 2017;89:1–4. doi: 10.1016/j.jcv.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mera J, Vellozzi C, Hariri S, Carabin H, Drevets DA, Miller A, Reilley B, et al. Identification and Clinical Management of Persons with Chronic Hepatitis C Virus Infection - Cherokee Nation, 2012–2015. MMWR Morb Mortal Wkly Rep. 2016;65:461–466. doi: 10.15585/mmwr.mm6518a2. [DOI] [PubMed] [Google Scholar]
- 28.Miller LBC, Park B, Wimberly W, Fluker SA, Okoson IS. What Happens After Screening and Linkage to Care? Examination of HCV Care Cascade Outcomes Among 5,000 Urban Baby Boomers Screened for HCV 2012–2014. AASLD Liver Meeting; 2016. [Google Scholar]