Abstract
The heat shock response (HSR) was originally discovered as a transcriptional response to elevated temperature shock and led to the identification of heat shock proteins and Heat Shock Factor 1 (HSF1). Since then, HSF1 has been shown to be important for combating other forms of environmental perturbations as well as genetic variations that cause proteotoxic stress. The HSR has long been thought to be an absolute response to conditions of cell stress and the primary mechanism by which HSF1 promotes organismal health by preventing protein aggregation and subsequent proteome imbalance. Accumulating evidence now shows that HSF1, the central player in the HSR, is regulated according to specific cellular requirements through cell-autonomous and non-autonomous signals, and directs transcriptional programs distinct from the HSR during development and in carcinogenesis. Here, we discuss these ‘non-canonical’ roles of HSF1, and its regulation in diverse conditions of development, reproduction, metabolism, and aging, and posit that HSF1 serves to integrate diverse biological and pathological responses.
Keywords: HSF1, heat shock response (HSR), proteostasis, cell proliferation, metabolism, organismal health
HSF1 directs the dynamic HSR for stress adaptation and proteostasis
The function of HSF1 that has been studied most extensively is its role in the heat shock response (HSR) (BOX 1), an evolutionarily conserved cellular defense mechanism which protects cells against proteotoxicity associated with misfolding, aggregation, and proteome mismanagement [1]. Protein quality control machinery is comprised of the proteostasis network (PN) and is essential for all aspects of protein biogenesis, cellular robustness and function, and organismal lifespan and stress resilience [1]. HSF1 and its transcriptional targets in the HSR, principally molecular chaperones and components of the protein degradation machinery, are critical to protect against diverse forms of environmental stress [2], the accumulation of aggregation-prone proteins [3, 4], errors in protein synthesis [5, 6], and various genetic perturbations that promote proteotoxic stress [7, 8]. Thus, the HSR provides a mechanism that constantly monitors the flux of misfolded protein species and coordinate different arms of the PN to prevent the accumulation of aggregates and other non-native intermediates.
BOX 1. HSF1 is essential for the HSR and proteostasis.
The heat shock response (HSR) was initially discovered as a transcriptional response to elevated temperatures [63], and is best known for the rapid and robust transcriptional induction by the evolutionarily conserved Heat Shock Factor 1 (HSF1). The well-studied target genes of HSF1 in the HSR include molecular chaperones, that are essential for protein folding, to prevent misfolding and to restore the native conformation of misfolded proteins, and components of the ubiquitin proteasome system, that degrade damaged proteins and recycle amino acids. The subset of chaperones whose expression is heat shock inducible are also historically called heat shock proteins. The coordinated actions of these protein quality control genes restores protein homeostasis (proteostasis) when disrupted by heat shock [1]. While induction of the HSR is specific to elevated temperature stress, a closely related form of cell stress response involving HSF1 is induced when cells are exposed to other forms of environmental stress such as oxidants, heavy metals, and xenobiotics that cause protein damage and misfolding [2], or by genetic perturbations that lead to errors in protein biogenesis and proteome imbalance [5–8]. Likewise, the cellular response to heat shock includes diverse transcriptional and post-transcriptional events, many of which are independent of HSF1 [46, 54, 64]. Since its initial discovery, the HSR has evolved from its broad historical definition to a more specific terminology referring to the transcriptional program regulated by HSF1 in response to acute proteotoxic stress as occurs upon heat shock. While the full scope and connectivity of the HSR in eukaryotes is still not fully understood, it is clear that the HSF1-regulated HSR provides a cellular defense mechanism against protein damage, misfolding and aggregation in the cytoplasm and nucleus in parallel to the unfolded protein responses of the mitochondrion and endoplasm reticulum.
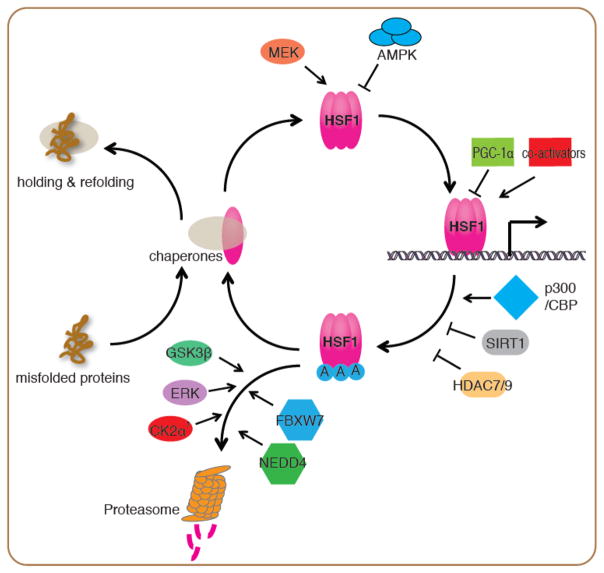
The HSR has dynamic properties as a highly tuned transcriptional response, in which HSF1 is regulated by numerous protein-protein interactions and extensive post-translational modifications (Figure 1). The activation and attenuation cycle of HSF1 in the HSR includes at least four steps: (1) De-repression & trimerization: upon cell stress, the influx of misfolded proteins prevents HSP70, HSP90, TRiC and perhaps other chaperones from binding to HSF1 monomers. This alleviates the repression of HSF1 by chaperones and is followed by conversion of the monomer to DNA binding competent trimers [9–11], (2) Translocation to the nucleus: HSF1 can exist either constitutively in the nucleus or in the cytoplasm. Upon protein misfolding, HSF1 translocates to the nucleus and drives transcription [2]. Two protein kinases, MEK and AMPK have been shown to promote or inhibit HSF1 nuclear translocation, respectively [12, 13], (3) DNA binding and transcriptional activation: HSF1 DNA binding ability is impaired by acetylation in the DNA binding domain by the acetyltransferase p300/CBP leading to attenuation of the HSR [8, 14, 15]. The deacetylases SIRT1, HDAC7 and HDAC9 enhance the HSR by increasing the dwell time of HSF1 on DNA [14, 16]. Co-activators including the Mediator complex, and repressors such as PGC-1α have been proposed to promote or suppress HSF1 transcriptional activity at its target promoters [17–19], and (4) Protein stability: The E3 ligases FBXW7 and NEDD4 have been shown to target HSF1 for degradation by the ubiquitin proteasome system [20, 21], with FBXW7 mediated degradation being promoted by phosphorylation of HSF1 by the ERK, GSK3β and CK2 α′ kinases [20, 22].
Figure 1.
Regulation of the HSF1 activation and attenuation cycle in the HSR
Upon stress, misfolded proteins dissociate chaperones from HSF1 and allow HSF1 to form DNA-binding competent trimers. MEK promotes HSF1 nuclear translocation and transcriptional activity through phosphorylation at Ser326. Conversely, AMPK inhibits HSF1 nuclear translocation through phosphorylation at Ser121. HSF1 transcriptional activity is regulated by co-activators such as the mediator complex, and repressors including PGC-1α at its target promoters. Attenuation of the HSR is controlled by acetylation of HSF1 at its DNA binding domain by p300/CBP. The histone deacetylases SIRT1, HDAC7 and HDAC9 prevent this acetylation and stabilize HSF1 DNA binding. The E3 ligases FBXW7 and NEDD4 target HSF1 for degradation through the ubiquitin proteasome system, with FBXW7 mediated degradation being promoted by phosphorylation of HSF1 by GSK3β, ERK and CK2 α′ kinases.
Because the HSR has been characterized predominantly in yeast and dissected tissues or tissue culture cells of animals, and expression of HSF1 is ubiquitous, it was widely thought that the magnitude, kinetics, and duration of the HSR are determined solely by the stress conditions encountered. Likewise, HSF1 has been generally considered to function only in the classical HSR to protect cellular health and tissue physiology. However, accumulating evidence in metazoans have challenged these traditional views and suggest that the HSR is tailored to specific cellular needs and regulated by cell-non-autonomous control through communication between tissues [23–25]. Further, HSF1 directs transcriptional programs in development and metabolism [26, 27] that are distinct from the classical HSR. These non-canonical HSF1 transcriptional programs influence the PN and other cellular functions in aging and disease in addition to the HSR [25, 28].
Regulation of HSF1 by the proliferative and metabolic states of the cell
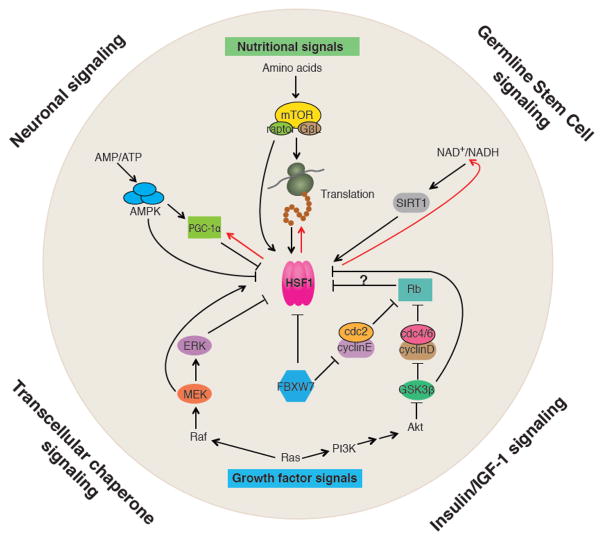
Among the known regulators of HSF1 are proteins that also function in growth factor signaling or nutrient sensing pathways (Figure 2), suggesting that HSF1 and the HSR is influenced by the proliferative and metabolic states of the cell, thus providing a means for the cell to tailor the activity of HSF1 and the potency of the HSR to specific needs and cellular context. For example, MEK kinase of the RAS/MAPK pathway phosphorylates HSF1 at Ser326, which promotes both HSF1 nuclear translocation and its activity in the HSR [12]. The RAS/MAPK pathway also regulates other transcription factors including C-MYC, which is important for the cell cycle, and the 40S ribosomal protein S6 kinase, which regulates translation, supporting a critical role for RAS/MAPK signaling to coordinate aspects of cell proliferation, protein biosynthesis and proteostasis. Likewise, the turnover of nuclear HSF1 and therefore the duration of the HSR are affected by the E3 ubiquitin ligase FBXW7 [20], which also controls the levels of multiple cell cycle regulators including Cyclin E [29]. Ubiquitylation of HSF1 by FBXW7 is primed by phosphorylation of HSF1 at Ser303 and Ser307 by GSK3β and ERK1 respectively [20, 30, 31], kinases involved in growth factor signaling (Figures 1&2), providing further support for the relationship between HSF1 and cell proliferation. Collectively, these observations reveal that cell proliferation and the demands for protein synthesis are directly coordinated via HSF1 to prevent misfolded species from persisting in the cell.
Figure 2.
Intercellular and intracellular signaling pathways converging on HSF1
At the organismal level, HSF1 is subject to cell non-autonomous regulation by neuronal signaling (serotonin), insulin/IGF-1 signaling, germline stem cell (GSC) signaling and transcellular chaperone signaling. Within the cell, HSF1’s activity is regulated by signal pathways that sense nutrients and control cell proliferation. The three major metabolic sensors, AMPK, mTORC1 and SIRT1 control HSF1’s activity directly through post-translational modifications. AMPK also inhibits HSF1 indirectly through PGC-1α. mTORC1 activates translation, and the nascent polypeptides and newly synthesized proteins titrate chaperones from HSF1, leading to de-repression of HSF1. HSF1 also influence metabolism by activating expression of PGC-1α, promoting protein synthesis through co-translational folding, and maintaining cellular levels of NAD+ via the NAD+ salvage pathway (indicated by the red arrows). HSF1 is also regulated by key components of the RAS/MAPK and RAS/PI3K pathways including MEK, ERK and GSK3β through phosphorylation. The pocket protein Rb, an important cell cycle regulator, may also influence HSF1 activity considering it is a repressor of E2F/DP that serves as a co-activator of HSF1 in C. elegans larval development.
In addition to cell proliferation, regulation of the HSR is influenced by the metabolic status of the cell. For example, in senescent human cells, the repression of the HSR is linked to reduced levels of the NAD+-dependent lysine deacetylase SIRT1. In the absence of SIRT1 activity, HSF1 is rapidly acetylated by p300/CBP resulting in the release of HSF1 from heat shock promoters thereby dampening the HSR [8, 14]. Additional evidence for the regulation of HSF1 by the metabolic state of the cell has emerged with AMP-activated protein kinase (AMPK), which phosphorylates HSF1 at Ser121 in conditions of metabolic stress therefore reducing the activity of the HSR by restricting nuclear entry of HSF1 [13]. AMPK can also regulate the HSR indirectly through its substrate PGC1α, a regulator of mitochondrial biogenesis that interacts with HSF1 during fasting in mouse livers and primary hepatocytes [17], thereby repressing the HSR when energy availability is low. Furthermore, HSF1 is inactivated by amino acid deprivation [32]. A potential mechanism involves mTORC1, a key regulator of translation that depends on amino acid levels, which promotes HSF1 activity through phosphorylation at Ser326 [33]. Together, these findings provide evidence for a regulatory network that responds to the cellular NAD+/NADH, AMP/ATP and amino acid levels to link the potency of the HSR to energy availability and protein biogenesis (Figure 2).
HSR is subject to cell non-autonomous regulation in metazoans
Since the HSR is influenced by the proliferative and metabolic states of the cells, how do animals coordinate HSF1 activity among different cell types and tissues to achieve organismal protection against cell stress conditions? Studies in Caenorhabditis elegans (C. elegans) provided the initial evidence for the spatial and temporal control of HSF1 at the organismal level by cell non-autonomous regulation by neuronal signaling. For example, animals deficient in thermosensory neuron function are incapable of inducing the HSR in other somatic tissues, thus leaving the organism vulnerable to environmental stress [24]. The neuronal regulation of the HSR is mediated by serotonin, thereby coupling stress sensing and neurotransmitter activity with movement, fecundity, and the response to food [34] (Figure 2, neuronal signaling). The role of intertissue communication in organismal proteostasis is further supported by observations that misfolding of metastable proteins expressed in muscle cells induces the expression of the chaperone HSP90 in distal tissues, and that over-expression of HSP90 in neurons or intestinal cells is sufficient to suppress protein misfolding in muscle cells [35] (Figure 2, Transcellular chaperone signaling). The tissue-selective HSR and cell-non-autonomous regulation of proteostasis has also been observed in the Drosophila flight motor system [36]. The flight motor composed of specific muscle cells, motor neurons and glia cells is more vulnerable to heat-stress induced degeneration compared to the corresponding cells in the leg motor. Over-expression of the HSP23 chaperone, a canonical HSR gene, specifically in the muscle cells of the flight motor was shown to protect the muscle as well as the neurons and glia cells, consistent with a cell-non-autonomous regulation of the HSR.
Additional support for cell non-autonomous control of the HSR comes from studies on the relationship between aging and the HSR. In C. elegans, the organismal HSR declines precipitously in early adulthood at reproductive maturity by signals from the germ stem cells (GSCs) [23, 37] (Figure 2, Germline stem cell signaling). Repression of the HSR involves the placement of the repressive H3K27me3 chromatin mark and reduced chromatin accessibility at the promoters of heat shock genes [23]. This programed repression of the HSR leaves cells vulnerable to stress conditions and protein misfolding. The timing of this repression, which impairs protein quality control, has been proposed to be among the earliest molecular events affecting cellular healthspan and longevity. Similarly, the organismal HSR is regulated by endocrine signals. For example, C. elegans lifespan is nearly doubled and the animals exhibit a more robust HSR when the insulin/IGF-1 signaling is impaired [38, 39] (Figure 2, insulin/IGF-1 signaling).
These observations reveal that metazoans utilize intertissue communication to transmit signals from cells that are proximal to conditions of proteotoxic stress in order to prime distal cells and safeguard them against impending adverse conditions. This phenomenon is observed in response to protein misfolding within specific tissues and is not restricted only to those cells that directly sense proteotoxic perturbations [35]. The relationship between reproduction and inducibility of the HSR observed in animals at reproductive maturity suggests that the age-associated events of cellular failure and loss of tissue robustness during aging are not a random chaotic process but rather a highly regulated event, perhaps to ensure that somatic tissues decline post reproduction [23]. By far, most of these intertissue signaling pathways have been discovered in invertebrate model systems. An important future direction is to link them with the evolutionarily conserved growth factor and nutritional signals that affect the demands on the PN and varies among tissues in diverse invertebrate and vertebrate animal models. It will also be important to understand how these intercellular and intracellular signaling pathways communicate through HSF1 at different life stages to determine organismal health.
HSF1 directs transcriptional programs that are uncoupled from the HSR
As HSF1 and the HSR are closely intertwined, it is not unexpected that new roles for HSF1 in growth, development, reproduction and longevity have therefore been attributed to the HSR. However, there is now increasing evidence from a number of biological systems that HSF1 is important for diverse “non-stress” conditions including development [26, 40], energy metabolism [27], programmed cell death [41, 42] and carcinogenesis [28, 43]. For these non-heat shock conditions, the transcriptomes regulated by HSF1 are distinct from that of acute heat shock [26, 40]. It is, therefore, important to understand mechanistically how HSF1 regulates these alternative transcriptional programs to influence long-term cellular health.
HSF1 is a single copy gene in C. elegans and Drosophila and is essential for growth and development [26, 44]. In addition, HSF1 is the major regulator of the HSR among multiple HSFs expressed in mammals, and is a maternal factor required for gametogenesis in mice [45]. It was not known whether this was because the HSR was essential for development or because HSF1 regulated the expression of a distinct set of target genes. The latter proposal is now supported by observations that HSF1 regulates oocyte maturation in mammals by directly activating genes that function in the meiotic cell cycle but not the canonical genes induced by the HSR [40]. Likewise, during C. elegans development, HSF1 directs a pro-growth transcriptional program distinct from the HSR [26]. While a subset of chaperone genes are direct targets of HSF1 under both development and upon acute heat shock, the developmental program of HSF1 is not a variant or subset of the HSR, but rather a distinct HSF1-regulated developmental program. This conclusion is highlighted by the analysis of HSC70 and HSP90 transcription in C. elegans, the two major ATP-dependent chaperones, where HSF1 controls the developmental expression and heat shock induction through separate promoters [26]. This strategy may provide an efficient means to control and alter the transcription rate in development and the HSR through the use of distinct regulatory mechanisms.
Even with limited mechanistic understanding, there is growing support that the pro-growth transcriptional program of HSF1 is evolutionarily conserved. Comparison of HSF1 targets in yeast under normal growth conditions identified a transcriptional program that corresponds closely with the HSF1 developmental targets in C. elegans required for proteostasis [46]. Likewise, in cancer cell lines, HSF1 is constitutively active and essential for the malignant transformation [28]. Comparison of the genomic distribution of HSF1 binding and HSF1 regulated transcriptome in human cancer cells [28] and in C. elegans development [26] revealed similar profiles enriched for genes that promote protein biogenesis, protein folding and anabolic metabolism. Further, both of the pro-growth programs of HSF1 are linked with proliferation and metabolic states, in which HSF1 occupancy at most loci is reduced in cells of low-malignant potential compared to cells with high-malignant potential [28], and in young adult C. elegans compared to developing larvae [26]. In both examples, the binding profiles of HSF1 are strikingly different in the presence or absence of heat shock. Based on these observations, we propose that signals from growth or stress engage HSF1 in distinct transcriptional programs for the increased influx of nascent proteins in growth, or elevated levels of misfolded, damaged, and aggregated proteins during stress.
An additional intriguing role for HSF1 in cell growth is in energy metabolism. HSF1 is required to maintain NAD+ and ATP levels in hepatic cells through the HSF1-dependent transcription of nicotinamide phosphoribosyl transferase in the NAD+ salvage pathway [47]. It has also been proposed that increased levels and/or activity of HSF1 promotes the expression of PGC1α in brown adipose tissue (BAT), inguinal white adipose tissue, and skeletal muscle, leading to the induction of transcriptional programs related to mitochondrial function and maintenance of BAT, thereby ameliorating metabolic dysfunction [27]. These findings together with the observations that the HSR is dampened in metabolic stress [13], raise the intriguing possibility that HSF1 activity is linked to both energy availability and expenditure, and that HSF1 influences and is influenced by oscillations in the metabolic demands of different tissues and cell types.
It is noteworthy that, other than the pro-growth program of HSF1 in proteostasis and energy metabolism in diverse model systems, HSF1 appears to be involved in cell and tissue type specific transcriptional programs that are uncoupled from the HSR. For example, synaptic proteins in primary neurons are expressed upon pharmacological activation of HSF1 [48] and sex chromosomal multi-copy genes are regulated by HSF1 in testis [49]. Whether these are direct or indirect targets of HSF1 will require further studies, nevertheless the transcriptional regulation by HSF1 during diverse biological conditions distinct from heat shock reveals that HSF1 may bring together diverse pathways under common control.
Activation and regulation of HSF1 in the absence of cell stress conditions
How is HSF1 activated in the absence of proteotoxic stress conditions of the HSR? In cancer cells, the genomic occupancy of HSF1 decreases when translation is inhibited [50]. Similar to the proposed role for misfolded proteins in de-repression of HSF1, increased influx of nascent polypeptides and newly synthesized proteins in cancer cells could also titrate chaperones away from HSF1 for nascent folding and maturation, thereby releasing HSF1 to activate transcription. Many of the regulators of HSF1 in the activation and attenuation cycle of the HSR (Figure 1) could have roles in the pro-growth transcriptional program, and HSF1 activation in cancer cells is likely achieved through coordinated action of multiple regulators. In addition to the requirements for high levels of protein synthesis and chaperones, the E3 ubiquitin ligase FBXW7 is frequently mutated or transcriptionally down-regulated in melanomas. FBXW7 has been suggested to stabilize nuclear-localized HSF1 for transcription in the absence of stress, thus promoting the metastatic potential of cancer cells [20]. On the contrary, PGC1α, which co-occupies with HSF1 at non HSR loci and represses HSF1 transcriptional activity, [17] decreases metastasis in prostate cancer [51]. It will therefore be of interest to determine how HSF1 is involved in the metabolic regulation of cancer by PGC1α, and together with other identified HSF1 regulators and interaction partners, to understand how HSF1 regulates the pro-growth program in development, metabolism and cancer.
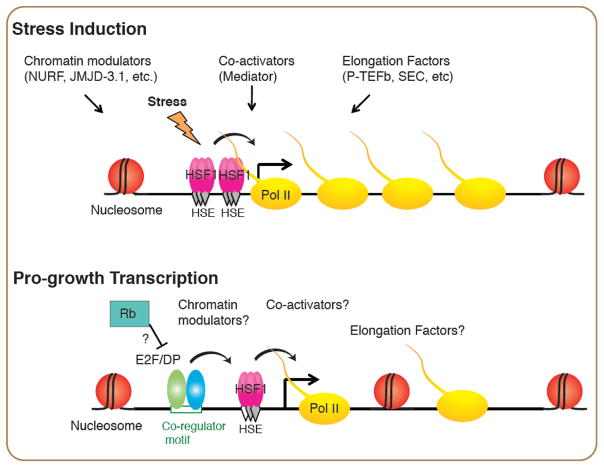
How is HSF1 recruited to selective genomic loci in each cellular context to perform distinct functions? Recent work on HSF1 in C. elegans larval development shed lights on this question, and suggests the specificity can be established through co-activators. During larval development, HSF1 binds preferentially to promoters that have a degenerate heat shock element (HSE) and an adjacent binding site for the E2F/DP heterodimer [26] (Figure 3). This unique promoter architecture underlies the selective binding of HSF1 in the absence of cell stress at its developmental gene targets over the canonical HS-inducible promoters that are comprised of tandem HSEs and with higher intrinsic affinity for HSF1. Since E2F/DP is also an essential regulator of the cell cycle under the control of Rb and Cyclins D&E [52] (Figure 2), the partnership between E2F/DP and HSF1 reveals direct evidence to link the growth control program regulated by E2F/DP with the regulation of proteostasis and protein biogenesis by HSF1. Further, the degenerate HSE utilized at the HSF1 developmental sites provides flexibility for this regulatory partnership such that a robust HSR can overwrite the developmental binding upon stress. We propose that the HSF1 developmental switch may provide a paradigm for other HSR-independent transcriptional programs regulated by HSF1.
Figure 3.
Models of HSF1 in stress-induced and pro-growth transcription
Binding of HSF1 induced by stress is through cooperative binding of HSF1 at clusters of canonical HSEs, which accessibility is controlled by chromatin modulators such as the chromatin remodeler NURF [65] and the histone demethylase JMJD-3.1. HSF1 cooperates with Mediator, and transcription elongation factors such as the P-TEFb kinase and the Super Elongation Complex to robustly induce the HSR. Binding of HSF1 in the pro-growth transcriptional program, however, relies on co-regulators such as the active E2F/DP heterodimer that binds to the same promoters with HSF1 in C. elegans larval development. How the co-activators, chromatin modulators and elongation factors in the HSR contribute to the pro-growth transcriptional program of HSF1 are of interests for future studies.
Many years of study on the HSR have revealed detailed mechanisms underlying robust transcriptional activation by HSF1. However it is largely unknown how HSF1 regulates transcription in alternative transcriptional programs. Transcriptional activation by HSF1 in the HSR is achieved in cooperation with chromatin modulators and transcription elongation factors [53, 54]. It is known that epigenetic modulators including chromatin remodelers and histone modification enzymes are frequently mutated in cancer cells and underlies malignancy [55]. Similarly, elongation factors including components of the Super Elongation Complex [56] are abnormally recruited to genes in cancer cells. An important future direction is to test whether HSF1 serves as an adaptor between these two classes of factors and consequently contributes to the abnormal activation of pro-growth genes in cancer. As has been reported for the HSR, the alternative transcriptional programs of HSF1 are also subject to cell-type specific regulation with HSF1 directing the expression of different sets of genes in cancer cells and cancer-associated fibroblasts [43]. Currently, it is unclear how these cell-type specific HSF1 programs are coordinated but future work should reveal how different combinations of co-factors and alternate chromatin structures generate diverse transcriptomes of HSF1.
The alternate transcriptional programs regulated by HSF1, may share common features including expression of a small set of key chaperones and metabolic regulators, in addition to condition specific targets. Defining these HSF1 regulated programs under conditions distinct from acute heat shock, and comparing them with the HSR will provide a new system level understanding of how biological systems evolved to meet environmental and physiological challenges.
Concluding remarks
Cellular and organismal health requires optimal proteostasis, for which HSF1 has an essential role to respond and protect against proteotoxic damage. Protein misfolding and aggregation underlies the pathologies of many age-related human diseases, most notably neurodegenerative and metabolic disorders [1]. For these diseases, the ability to restore and enhance different arms of the PN to prevent imbalance and suppress aggregation and proteotoxicity may show promise. However, a greater challenge will be to achieve healthy proteostasis over long periods of life, which requires careful monitoring and managing of the quality control machinery through the use of folding and degradation sensors.
It is noteworthy that for certain protein conformational diseases, such as Huntington’s disease, the HSR is dysregulated [57]. The inability to respond to aggregation by activation of the HSR has been proposed to exacerbate disease progression through a feed forward mechanism [58]. It is well established that genetic or pharmacological activation of HSF1 can suppress proteotoxicity and ameliorate symptoms in various disease models [59], and it is clear that in this context, increased HSF1 activity should be beneficial if carefully titrated. In addition to activation of HSF1, another potential strategy to engage the protective mechanism of HSF1 could be through the regulators of HSF1 that are affected in neurodegenerative diseases. For example, in mouse models of Huntington’s Disease and Parkinson’s Disease, the levels of the CK2 α′ kinase and NEDD4 E3 ligase increase respectively in the presence of disease-related proteins [21, 22], which causes degradation of HSF1. Therefore, stabilizing HSF1 through pharmacological inhibition of CK2 α′ and NEDD4 could offer a complementary strategy to restore HSF1 and the HSR in disease.
The observations in cancer show that diverse tumors exhibit constitutive activation of HSF1 and elevated levels or altered interactions of chaperones. These changes have been suggested to contribute to oncogenesis and ability of cancer cells to compensate for genomic instability and elevated load of mutations in the expressed proteome [60, 61]. Thus for cancer, a strategy would be to reduce HSF1 activity [62]. Inhibition of HSF1 has been shown to increase the vulnerability of rapidly dividing tumor cells to proteotoxic stress conditions [12]. Comparison of the pro-growth program of HSF1 in C. elegans development with that of cancer cells reveals significant functional overlap, which suggests that the HSF1 developmental program may be re-engaged in cancer cells to support the high metabolic demands and high protein load through ‘non-canonical’ targets in multiple cellular pathways. Yet, in normal cells the inhibition of HSF1 renders them vulnerable to protein misfolding and aggregation, and accelerates aging. Thus, to establish the specificity for cancer, it may be potentially useful to develop small molecules that specifically block the interaction between HSF1 and its cell-proliferation related cofactors. This class of HSF1 inhibitors could dampen the pro-growth program driven by HSF1, while leaving the HSR intact, thereby negating potentially harmful effects on post-mitotic cells such as neurons. While challenging, such efforts to characterize the molecular basis of HSF1 function in cell growth and development, and to identify the regulatory components distinct from those in the HSR may be fruitful (see Outstanding Questions).
Outstanding questions.
What are the biological roles of distinct HSF1 transcriptional programs in normal physiology and upon exposure to diverse forms of cell stress?
How many types of ‘non-canonical’ transcriptional programs of HSF1 are utilized in biology? Which features are common, and to what extent is the function and regulation of HSF1 tailored to specific cell types and tissues, to nutrients, aging and stress?
What are the molecular switches that distinguish different HSF1 transcriptional programs?
What regulatory mechanisms and machineries are shared or unique to the HSR and the ‘non-canonical’ transcriptional programs of HSF1?
What are the contributions of the HSR and ‘non-canonical’ transcriptional programs of HSF1 in different diseases?
Trends.
The activity of HSF1 is tuned to diverse cellular conditions that include the acute response to heat shock and other forms of cell stress conditions and extends to the proliferation and metabolic status of the cell.
The HSR can be regulated in a cell-non-autonomous manner through intertissue signaling to communicate stress signals and to ensure a coordinated organismal wide proteostatic response.
During development and in carcinogenesis, HSF1 directs transcriptional programs that are distinct from the HSR.
The transcriptional regulatory mechanism employed by HSF1 in development is uncoupled from the HSR; through partnership with other transcription factors.
Acknowledgments
The authors thank the members of the Morimoto Laboratory for their support and helpful discussion of the manuscript. J.L was supported by postdoctoral awards from the National Ataxia Foundation, the BrightFocus Foundation for Alzheimer’s Disease Research, and the Chicago Biomedical Consortium, and the Northwestern University Lung Sciences Training Program. J.P.L was supported by a Postdoctoral Fellowship from the ALS Association and a BBSRC David Phillips Fellowship. R.I.M. was supported by grants from the National Institutes of Health (National Institute on Aging), the Ellison Medical Foundation, the Glenn Foundation, the Chicago Biomedical Consortium, and the Daniel F. and Ada L. Rice Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–64. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akerfelt M, et al. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11(8):545–55. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Zvi A, et al. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A. 2009;106(35):14914–9. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen E, et al. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313(5793):1604–10. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 5.Brandman O, et al. A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell. 2012;151(5):1042–54. doi: 10.1016/j.cell.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe YJ, et al. Failure of RQC machinery causes protein aggregation and proteotoxic stress. Nature. 2016;531(7593):191–5. doi: 10.1038/nature16973. [DOI] [PubMed] [Google Scholar]
- 7.Guisbert E, et al. Identification of a tissue-selective heat shock response regulatory network. PLoS Genet. 2013;9(4):e1003466. doi: 10.1371/journal.pgen.1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raychaudhuri S, et al. Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell. 2014;156(5):975–85. doi: 10.1016/j.cell.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 9.Zou J, et al. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94(4):471–80. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, et al. Evidence for a mechanism of repression of heat shock factor 1 transcriptional activity by a multichaperone complex. J Biol Chem. 2001;276(49):45791–9. doi: 10.1074/jbc.M105931200. [DOI] [PubMed] [Google Scholar]
- 11.Neef DW, et al. A direct regulatory interaction between chaperonin TRiC and stress-responsive transcription factor HSF1. Cell Rep. 2014;9(3):955–66. doi: 10.1016/j.celrep.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Z, et al. MEK guards proteome stability and inhibits tumor-suppressive amyloidogenesis via HSF1. Cell. 2015;160(4):729–44. doi: 10.1016/j.cell.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai S, et al. Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J. 2015;34(3):275–93. doi: 10.15252/embj.201489062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westerheide SD, et al. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323(5917):1063–6. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh SK, et al. Negative elongation factor accelerates the rate at which heat shock genes are shut off by facilitating dissociation of heat shock factor. Mol Cell Biol. 2011;31(20):4232–43. doi: 10.1128/MCB.05930-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelin E, Freeman BC. Lysine deacetylases regulate the heat shock response including the age-associated impairment of HSF1. J Mol Biol. 2015;427(7):1644–54. doi: 10.1016/j.jmb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minsky N, Roeder RG. Direct link between metabolic regulation and the heat-shock response through the transcriptional regulator PGC-1alpha. Proc Natl Acad Sci U S A. 2015;112(42):E5669–78. doi: 10.1073/pnas.1516219112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JM, et al. Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol Cell. 2001;8(1):9–19. doi: 10.1016/s1097-2765(01)00296-9. [DOI] [PubMed] [Google Scholar]
- 19.Ni Z, et al. P-TEFb is critical for the maturation of RNA polymerase II into productive elongation in vivo. Mol Cell Biol. 2008;28(3):1161–70. doi: 10.1128/MCB.01859-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kourtis N, et al. FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat Cell Biol. 2015;17(3):322–32. doi: 10.1038/ncb3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim E, et al. NEDD4-mediated HSF1 degradation underlies alpha-synucleinopathy. Hum Mol Genet. 2016;25(2):211–22. doi: 10.1093/hmg/ddv445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Pastor R, et al. Abnormal degradation of the neuronal stress-protective transcription factor HSF1 in Huntington’s disease. Nat Commun. 2017;8:14405. doi: 10.1038/ncomms14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labbadia J, Morimoto RI. Repression of the Heat Shock Response Is a Programmed Event at the Onset of Reproduction. Mol Cell. 2015;59(4):639–50. doi: 10.1016/j.molcel.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prahlad V, et al. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320(5877):811–4. doi: 10.1126/science.1156093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglas PM, et al. Heterotypic Signals from Neural HSF-1 Separate Thermotolerance from Longevity. Cell Rep. 2015;12(7):1196–204. doi: 10.1016/j.celrep.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, et al. E2F coregulates an essential HSF developmental program that is distinct from the heat-shock response. Genes Dev. 2016;30(18):2062–2075. doi: 10.1101/gad.283317.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, et al. Celastrol Protects against Obesity and Metabolic Dysfunction through Activation of a HSF1-PGC1alpha Transcriptional Axis. Cell Metab. 2015;22(4):695–708. doi: 10.1016/j.cmet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Mendillo ML, et al. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150(3):549–62. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, et al. Tumor suppressor functions of FBW7 in cancer development and progression. FEBS Lett. 2012;586(10):1409–18. doi: 10.1016/j.febslet.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu B, et al. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem. 1996;271(48):30847–57. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- 31.Knauf U, et al. Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev. 1996;10(21):2782–93. doi: 10.1101/gad.10.21.2782. [DOI] [PubMed] [Google Scholar]
- 32.Hensen SM, et al. Heat shock factor 1 is inactivated by amino acid deprivation. Cell Stress Chaperones. 2012;17(6):743–55. doi: 10.1007/s12192-012-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou SD, et al. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS One. 2012;7(6):e39679. doi: 10.1371/journal.pone.0039679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatum MC, et al. Neuronal serotonin release triggers the heat shock response in C. elegans in the absence of temperature increase. Curr Biol. 2015;25(2):163–74. doi: 10.1016/j.cub.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Oosten-Hawle P, et al. Regulation of organismal proteostasis by transcellular chaperone signaling. Cell. 2013;153(6):1366–78. doi: 10.1016/j.cell.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawasaki F, et al. Small heat shock proteins mediate cell-autonomous and -nonautonomous protection in a Drosophila model for environmental-stress-induced degeneration. Dis Model Mech. 2016;9(9):953–64. doi: 10.1242/dmm.026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shemesh N, et al. Germline stem cell arrest inhibits the collapse of somatic proteostasis early in Caenorhabditis elegans adulthood. Aging Cell. 2013;12(5):814–22. doi: 10.1111/acel.12110. [DOI] [PubMed] [Google Scholar]
- 38.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15(2):657–64. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu AL, et al. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–5. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 40.Le Masson F, et al. Identification of heat shock factor 1 molecular and cellular targets during embryonic and adult female meiosis. Mol Cell Biol. 2011;31(16):3410–23. doi: 10.1128/MCB.05237-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benderska N, et al. DAPK-HSF1 interaction as a positive-feedback mechanism stimulating TNF-induced apoptosis in colorectal cancer cells. J Cell Sci. 2014;127(Pt 24):5273–87. doi: 10.1242/jcs.157024. [DOI] [PubMed] [Google Scholar]
- 42.Kinet MJ, et al. HSF-1 activates the ubiquitin proteasome system to promote non-apoptotic developmental cell death in C. elegans. Elife. 2016:5. doi: 10.7554/eLife.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scherz-Shouval R, et al. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell. 2014;158(3):564–78. doi: 10.1016/j.cell.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jedlicka P, et al. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J. 1997;16(9):2452–62. doi: 10.1093/emboj/16.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christians E, et al. Maternal effect of Hsf1 on reproductive success. Nature. 2000;407(6805):693–4. doi: 10.1038/35037669. [DOI] [PubMed] [Google Scholar]
- 46.Solis EJ, et al. Defining the Essential Function of Yeast Hsf1 Reveals a Compact Transcriptional Program for Maintaining Eukaryotic Proteostasis. Mol Cell. 2016;63(1):60–71. doi: 10.1016/j.molcel.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao A, et al. The transcriptional regulator of the chaperone response HSF1 controls hepatic bioenergetics and protein homeostasis. J Cell Biol. 2017;216(3):723–741. doi: 10.1083/jcb.201607091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, et al. Hsp90 chaperone inhibitor 17-AAG attenuates Abeta-induced synaptic toxicity and memory impairment. J Neurosci. 2014;34(7):2464–70. doi: 10.1523/JNEUROSCI.0151-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akerfelt M, et al. Heat shock transcription factor 1 localizes to sex chromatin during meiotic repression. J Biol Chem. 2010;285(45):34469–76. doi: 10.1074/jbc.M110.157552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santagata S, et al. Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science. 2013;341(6143):1238303. doi: 10.1126/science.1238303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torrano V, et al. The metabolic co-regulator PGC1alpha suppresses prostate cancer metastasis. Nat Cell Biol. 2016;18(6):645–656. doi: 10.1038/ncb3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadasivam S, DeCaprio JA. The DREAM complex: master coordinator of cell cycle-dependent gene expression. Nat Rev Cancer. 2013;13(8):585–95. doi: 10.1038/nrc3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuda NJ, et al. Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature. 2009;461(7261):186–92. doi: 10.1038/nature08449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duarte FM, et al. Transcription factors GAF and HSF act at distinct regulatory steps to modulate stress-induced gene activation. Genes Dev. 2016;30(15):1731–46. doi: 10.1101/gad.284430.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feinberg AP, et al. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat Rev Genet. 2016;17(5):284–99. doi: 10.1038/nrg.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin C, et al. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell. 2010;37(3):429–37. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Labbadia J, Morimoto RI. Huntington’s disease: underlying molecular mechanisms and emerging concepts. Trends Biochem Sci. 2013;38(8):378–85. doi: 10.1016/j.tibs.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim YE, et al. Soluble Oligomers of PolyQ-Expanded Huntingtin Target a Multiplicity of Key Cellular Factors. Mol Cell. 2016;63(6):951–64. doi: 10.1016/j.molcel.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Neef DW, et al. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov. 2011;10(12):930–44. doi: 10.1038/nrd3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodina A, et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature. 2016;538(7625):397–401. doi: 10.1038/nature19807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai C, et al. Loss of tumor suppressor NF1 activates HSF1 to promote carcinogenesis. J Clin Invest. 2012;122(10):3742–54. doi: 10.1172/JCI62727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai C, et al. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130(6):1005–18. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritossa F. New puffs induced by temperature shock, DNP and salicylate in salivary chromosomes of D. melanogaster. Drosophila Info Serv. 1963;37:122–123. [Google Scholar]
- 64.Mahat DB, et al. Mammalian Heat Shock Response and Mechanisms Underlying Its Genome-wide Transcriptional Regulation. Mol Cell. 2016;62(1):63–78. doi: 10.1016/j.molcel.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsukiyama T, Wu C. Purification and properties of an ATP-dependent nucleosome remodeling factor. Cell. 1995;83(6):1011–20. doi: 10.1016/0092-8674(95)90216-3. [DOI] [PubMed] [Google Scholar]