Abstract
Background
Inflammation is a key component of both acute kidney injury (AKI) and response to cardiopulmonary bypass. Since AKI poses risks to children after cardiac surgery, we investigated the value of inflammatory biomarkers interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF) for predicting AKI and other complications.
Methods
We enrolled 412 children between 1 month and 18 years old undergoing cardiopulmonary bypass for cardiac surgery. We collected blood both preoperatively and postoperatively (within 6 hours post-surgery) and measured plasma IL-8 and TNF.
Results
IL-8 and TNF did not predict AKI in children <2 years, but strongly associated with AKI in children ≥2 years. There were significant associations between biomarker levels and age (<2 or ≥2). In children ≥2, patients in the highest tertile of preoperative IL-8 and postoperative TNF had 4.9 [95% CI: 1.8–13.2]- and 3.3 [95% CI: 1.2–9.0]-fold higher odds of AKI compared to those in the lowest tertile. Children <2 with higher biomarker levels also had higher odds of AKI, but the difference was not significant. We also found that postoperative TNF levels were significantly higher in patients with longer hospital stays, and that both postoperative IL-8 and TNF levels were significantly higher in patients with longer ventilation lengths. However there was no evidence that biomarker levels mediated the association between AKI and length of ventilation; they appear to be independent predictors.
Conclusions
Preoperative IL-8 and postoperative TNF are significantly associated with higher odds of AKI and greater lengths of hospital stays and ventilator use in children ≥2.
Classifications: Cardiopulmonary bypass, CPB; Congenital heart disease CHD; Kidney, renal function failure, dialysis; Pediatric; Surgery, complications
Every year, 40,000 children are born with congenital heart disease in the United States. About 25% of these children require cardiac surgery (1). Unfortunately, acute kidney injury (AKI) is a common complication of these procedures and is associated with morbidity. As a patient’s blood flows through the extracorporeal circuit during bypass, the body reacts by mounting an immune response. This inflammation contributes to the development of AKI, which in turn propagates further inflammation (2). Because of these close relationships between AKI, CPB, and inflammation, we chose to investigate the association between inflammatory biomarkers and AKI in pediatric patients undergoing cardiopulmonary bypass.
The present investigation is a sub-study of the Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) Consortium. The goal of this multi-center prospective observational study is to investigate novel biomarkers as potential tools to help detect early AKI in patients post-cardiac surgery. Ultimately, TRIBE-AKI aims to improve outcomes and safety associated with cardiac surgery. Since 2007, over 1,500 patients across nine North American sites have participated in the cohort. This group consisted of both adult and pediatric patients enrolled in parallel. Three of the nine sites conducted the pediatric arm of the cohort, enrolling 412 children over the course of the study. Many studies examining the extensive set of resulting data on the pediatric cohort have been published so far. Some of these key findings include that serum brain natriuretic peptide helps risk-stratify patients for AKI, that postoperative neutrophil gelatinase-associated lipocalin and interleukin-18 help predict AKI, and that chronic kidney disease and hypertension are common 5 years after cardiac surgery (3–5). In the present study, we are extending the analysis of TRIBE-AKI pediatric data to two additional promising biomarkers of AKI.
Namely, we focused on interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF). IL-8 is a chemokine produced by macrophages, neutrophils, fibroblasts, and epithelial cells. This protein is a key mediator of inflammation which assists in neutrophil recruitment and degranulation (6). TNF is a proinflammatory cytokine produced primarily by monocytes but also by lymphocytes and endothelial cells. TNF acts on neutrophils and macrophages leading to cytokine secretion and cytotoxicity, and is one of the cytokines that drive the acute phase reaction (7). Both IL-8 and TNF have been associated with AKI in adult patients with various diseases. Elevated IL-8 levels have been tied to AKI risk in patients undergoing liver transplants, patients in septic shock, and patients with acute lung injury (8–11). Elevated TNF levels have been associated with AKI risk in patients developing sepsis and in patients having cardiovascular surgery (12, 13). Only one previous study has been performed measuring IL-8 and TNF in children undergoing cardiac surgery and showed that IL-8 was associated with increased risk of AKI. However the association of TNF with AKI was inconclusive, and this study was also very small, with only 18 AKI and 21 non-AKI patients (14). Thus, further evidence is needed.
Patients and Methods
Patients were part of the pediatric cohort of the TRIBE-AKI multi-center study. This sub-study consisted of 412 patients between the ages of 1 month and 18 years who were enrolled prospectively from July 2007 – December 2010. Three medical center were involved, namely the Cincinnati Children’s Hospital Medical Center, the Montreal Children’s Hospital, and the Yale-New Haven Children’s Hospital. Informed consent was obtained from all patient guardians and patients themselves when appropriate. All 412 patients received CPB surgery to correct congenital heart defects (15). This study was approved by the ethics board of each medical center.
Sample Collection
We collected blood specimens both preoperatively and postoperatively (first sample within 6 hours after surgery). Plasma aliquots were then stored at −80°C until biomarker measurement.
Biomarker Measurements
All samples were measured using the Meso Scale Discovery platform (Meso Scale Discovery, Gaithersburg, MD). IL-8 was able to be detected at a range of 0.047–516 pg/mL, and TNF at a range of 0.04–311 pg/mL. Personnel responsible for biomarker measurements were blinded to clinical outcomes.
Outcome Definitions
The two main outcomes analyzed in this paper were “any AKI” and “severe AKI.” We defined any AKI as the development of at least stage 1 AKI (composite of stage 1, stage 2, and stage 3 AKI). Severe AKI was defined as only patients with stage 2 or greater AKI. Our stage 1 AKI definition was based on the Kidney Disease: Improving Global Outcomes standard, namely a ≥50% or 0.3 mg/dL increase in serum creatinine during the first post-operative week relative to the baseline serum creatinine concentration (16). Stage 2 AKI was at least a doubling of the serum creatinine level from the baseline preoperative value (stage 3 AKI was a tripling or receipt of acute dialysis). We also measured other outcomes including length of hospital stay, length of ICU stay, and number of days on a ventilator.
Variable Definitions
In order to categorize the complexity of surgery, we used the Risk Adjustment for Congenital Heart Surgery 1 (RACHS-1) consensus-based scoring system and definitions of the Society of Thoracic Surgeons (17). Preoperative estimated glomerular filtration rate (eGFR) was determined using the updated Schwartz equation (18). Percentiles for eGFR values were obtained based on previously-published data on normal pediatric renal function (19, 20).
Statistical Analysis
For comparisons of continuous variables, we used the two-sample t test or the Wilcoxon rank sum test. For comparisons of dichotomous variables, we used the chi-squared test or Fisher’s exact test. For most analyses, we prespecified stratifying the results by age (less than two or greater than or equal to two) as the kidney does not fully mature until about age two. There is also precedent for this stratification in previous studies (4, 21). In addition, the interaction p-values between biomarker levels and age (<2 or ≥2) were all less than 0.05.
To examine associations between biomarkers and AKI, we divided patients into tertiles based on IL-8 and TNF levels for the entire cohort, and then stratified by age. We examined the association between biomarkers and development of any or severe AKI via logistic regression and estimating adjusted odds ratios (aOR) of AKI. We also assess the discrimination of IL-8 and TNF for AKI by calculating the area under the receiver operating characteristics curve (AUC-ROC) (22). We examined the evidence for biomarkers’ incremental predictive value by examining the p-value for the regression coefficient in a logistic model containing standard predictors (23). All regression analyses were adjusted for age, sex, race, surgical site, RACHS-1, and CPB time (15).
To estimate the association between biomarkers and clinical outcomes, we used Poisson regression with a log link function and robust standard error estimates. SAS version 9.4 (SAS Institute, Cary, North Carolina) was used for all analyses.
Path Analysis
Path analysis estimates associations by fitting a series of linear regressions, where each regression specifies the effect as the dependent variable and specifies the causes as independent variables. We estimated the direct and indirect (mediated) components of the associations of CPB time, TNF, IL-8, and ΔCr with length of ventilation (LOV). If the standard assumptions underlying linear regression hold, then the coefficient estimates can be used to decompose the correlation between variables into direct and indirect components (24). We used the percentile bootstrap procedure to form approximate 95% CIs for the estimated direct and indirect components of the correlations we calculated (25).
Results
Characteristics of the Study Cohort
Table 1 displays the characteristics of the 412-patient cohort by AKI status. During their hospital stays, overall 180 (44%) of participants developed any AKI (54% of those <2 and 32% of those ≥2). In addition, no patients received pre-operative steroids, and 168 patients (41%) had a prior surgery. Only two patients developed septicemia.
Table 1.
Patient Characteristics by Acute Kidney Injury Status
| Age <2 years (n=208) | Age ≥ 2 years (n=204) | |||
|---|---|---|---|---|
| Patient Characteristics | No AKI (n = 95) | Any AKI (n = 113) | No AKI (n = 138) | Any AKI (n = 66) |
| Demographic characteristics | ||||
| Age (years) | 0.61±0.45 | 0.49±0.32 | 7.4±4.38 | 6.23±4.33 |
| Male gender | 56 (59%) | 56 (50%) | 69 (50%) | 41 (62%) |
| White race (% white) | 79 (83%) | 88 (78%) | 112 (81%) | 59 (89%) |
| Preoperative characteristics | ||||
| Serum creatinine (pg/mL) | 0.36±0.08 | 0.31±0.10 | 0.51±0.16 | 0.4±0.18 |
| eGFR (mL/min per 1.73 m2) | 79.11±20.37 | 89.63±30.89 | 101.42±20.99 | 118.04±25.93 |
| eGFR (percentile)a | 60.72±33.2 9 | 70.41±32.33 | 46.22±31.34 | 66.53±30.83 |
| Operative Characteristics | ||||
| RACHS - 1 category | 2.41±0.62 | 2.45±0.65 | 2.46±0.67 | 2.73±0.66 |
| CPB Time (min) | 101.02±42.49 | 118.71±62.57 | 91.64±50.63 | 125.89±77.94 |
| Cross clamp time (min) | 54.27±36.65 | 63.33±44.67 | 38.26±43.76 | 26. 26±43.37 |
| Urgency of Surgery | ||||
| Elective | 84 (88%) | 96 (85%) | 132 (96%) | 63 (95%) |
| Urgent | 11 (12%) | 17 (15%) | 6 (4%) | 3 (5%) |
| Type of Surgery | ||||
| Septal defect repair | 42 (45%) | 43 (39%) | 41 (32%) | 13 (21%) |
| Inflow/outflow trace or valve procedure | 10 (11%) | 4 (4%) | 38 (29%) | 17 (27%) |
| Combined procedure | 41 (44%) | 62 (57%) | 50 (39%) | 33 (52%) |
| Outcomes | ||||
| Length of hospital stay (days), median (IQR) | 5 (4–8) | 8 (5–15) | 4 (3–6) | 6 (4–10) |
| Length of ICU stay (days), median (IQR) | 3 (2–3) | 4 (2–7) | 1 (1–2) | 2 (1–4) |
| Number of days on ventilator, median (IQR) | 1 (1–2) | 2 (1–5) | 1 (0–1) | 1 (1–2) |
| Number of deaths | 1 (1%) | 3 (3%) | 0 (0%) | 4 (6%) |
Values in table are presented as the mean ± standard deviation (SD) or as a number with the percentage in parentheses, unless indicated otherwise
AKI, acute kidney injury; GFR, glomerular filtration rate; IQR, interquartile range; RACHS-1, Risk Adjustment for Congenital Heart Surgery-1; CPB, cardiopulmonary bypass; ICU, intensive care unit
Percentile eGFR was calculated by quartile regression based on published normal renal function measured by nuclear medicine scan GFR in 651 children (19)
Correlations Between Preoperative Biomarker Levels, Postoperative Biomarker Levels, and AKI
There were significant correlations between preoperative IL-8 and TNF, postoperative IL-8 and TNF, and pre- and post-operative TNF levels. However, there were no significant correlations between pre- and post-operative IL-8 levels (Supplementary Table 1).
Figure 1 demonstrates biomarker levels by AKI status at both preoperative and postoperative timepoints by patient age group (<2 and ≥2). In patients ≥2, both preoperative and postoperative levels of both biomarkers were significantly higher among patients who developed postoperative AKI compared with patients who did not. This trend is less pronounced in patients <2. Supplementary Table 2 displays the numerical data shown in Figure 1. An especially notable difference was observed for postoperative levels of IL-8; patients with any AKI had 2-fold higher levels of postoperative IL-8 compared to patients with no AKI (61.57 vs 31.12 pg/mL, p<0.001).
Figure 1.
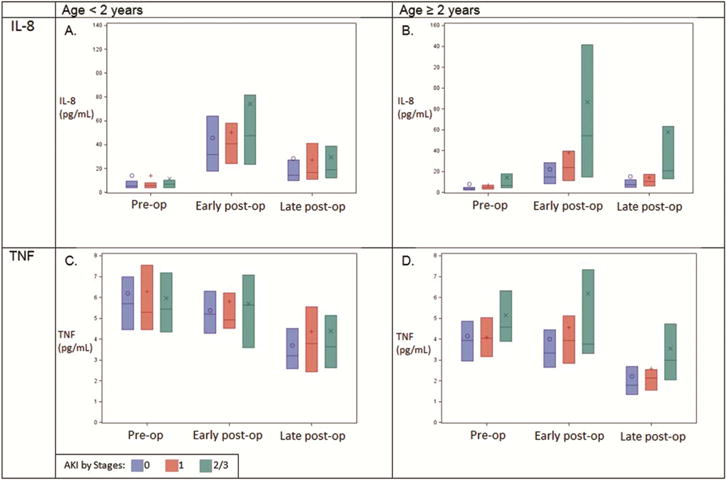
Blue bars indicate no AKI, grey bars stage 1 AKI, and orange bars stage 2/3 AKI. “Early post-op time” means 0–6 hours postoperative, and “Late post-op” means first non-missing measurement from days 2–5 postoperative. Each bar encompasses IQR of values; black lines are medians. Circles, plus-signs, and crosses are means.
Association of Preoperative Biomarkers with AKI
The association between biomarker levels and AKI differed significantly in patients <2 versus ≥2 years old. Table 2 and Supplementary Table 3 display the association of tertiles of biomarker levels with any or severe AKI. For patients under age two years, there was no statistical association between preoperative IL-8 or TNF levels and AKI. However, for patients over age two, there was a strong association of preoperative IL-8 with any AKI (adjusted odds ratio 2.61 [95% CI: 1.05–6.46] and 4.88 [95% CI: 1.81–13.15] for tertiles 2 and 3, respectively). Among children older than two, the highest tertile of preoperative IL-8 had substantially higher adjusted odds of developing severe AKI (5.06 [95% CI: 1.36–18.74]) compared with the first tertile. This association was not affected by prior surgery status or by preoperative eGFR levels. Preoperative levels of TNF were not significantly associated with AKI in patients over two. Supplementary Table 4 provides biomarker results for all ages combined.
Table 2.
Association of Biomarkers with Acute Kidney Injury (Any and Severe) (ages greater than or equal to 2 years†)
| Pre-operative | Post-operative | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tertile; Range | Any AKI | Severe AKI | Tertile; Range | Any AKI | Severe AKI | ||||
| n (%) | aOR* (95% CI) | n (%) | aOR* (95% CI) | n (%) | aOR* (95% CI); aOR + pre-op (95% CI)a | n (%) | aOR* (95% CI); aOR + pre-op (95% CI)a | ||
| IL - 8 | |||||||||
| T1; (0.7 – 3.5) n=77 | 13 (24%) | 1.0 (referent) | 4 (5%) | 1.0 (referent) | T1; (1.8 – 16.5) n=89 | 18 (20%) | 1.0 (referent); 1.0 (referent) | 8 (9%) | 1.0 (referent); 1.0 (referent) |
| T2; (3.5 – 6.4) n=53 | 21 (38%) | 2.61 (1.05, 6.46) | 8 (15%) | 2.26 (0.59, 8.63) | T2; (16.7 – 39.5) n=48 | 17 (35%) | 1.65 (0.68, 3.99); 1.33 (0.48, 3.67) | 3 (6%) | 0.56 (0.13, 2.52); 0.51 (0.10, 2.50) |
| T3; (6.4–362.9) n=41 | 21 (38%) | 4.88 (1.81,13.15) | 12 (29%) | 5.06 (1.36, 18.74) | T3; (39.8- 505.2) n=39 | 21 (38%) | 2.10 (0.79, 5.61); 1.71 (0.54, 5.45) | 14 (36%) | 2.77 (0.84, 9.16); 2.34 (0.58, 9.42) |
| TNF | |||||||||
| T1; (1.1 – 3.9) n=80 | 23 (29%) | 1.0 (referent) | 8 (10%) | 1.0 (referent) | T1; (0.7 – 3.4) n=83 | 20 (24%) | 1.0 (referent); 1.0 (referent) | 8 (10%) | 1.0 (referent); 1.0 (referent) |
| T2; (3.9 – 5.6) n=57 | 17 (30%) | 0.80 (0.31, 2.07) | 7 (12%) | 1.33 (0.34, 5.32) | T2; (3.4 – 5.3) n=59 | 20 (34%) | 2.80 (1.12, 7.04) ; 2.60 (0.92, 7.32) | 7 (12%) | 2.99 (0.77, 11.66); 3.28 (0.67,16.01) |
| T3; (5.6 – 34.4) n=34 | 15 (44%) | 1.52 (0.55, 4.18) | 9 (26%) | 3.15 (0.90, 11.04) | T3; (5.4 – 20.3) n=34 | 16 (47%) | 3.31 (1.22, 8.97); 3.67 (1.10,12.27) | 10 (29%) | 5.88 (1.57, 22.07); 8.71 (1.74,43.61) |
adjusted for age, sex, race, RACHS-1 score, CPB time, and site
adjusted for preoperative biomarker level as well as for age, sex, race, RACHS-1 score, CPB time, and site
CI, confidence interval; IL, interleukin; TNF, tumor necrosis factor alpha; T, tertile
Bolded and italicized odds ratios indicate significance; aOR, adjusted odds ratio
Interaction p-value between biomarker and age: Any AKI IL-18 pre-op, p=0.03, post-op p<0.0001, TNF pre-op p=0.004, post-op p=0.004.
Severe AKI IL-18 pre-op, p=0.04, post-op p=0.002, TNF pre-op p=0.23, post-op p=0.
Association of Postoperative Biomarkers with AKI
As shown in Supplementary Table 3, none of the biomarkers were significantly associated with AKI in patients under two. In patients over two years old, postoperative TNF was significantly associated with AKI; compared with the lowest tertile, the highest tertile was associated with an OR of 3.31 [95% CI 1.22–8.97] for any AKI and 5.88 [95% CI 1.57–22.07] for severe AKI after multivariate adjustment. This association was not affected by prior surgery status or by preoperative eGFR levels.
Diagnostic Testing
Supplementary Table 5 summarizes discrimination analysis for each biomarker. As can be seen in the table, both biomarkers yielded significant AUC values which improved diagnostic ability for both any and severe AKI beyond the clinical model alone, and preoperatively for any AKI in patients ≥2.
Biomarker Levels and Nonrenal Outcomes
Figure 2 displays the association of postoperative values of IL-8 and TNF with nonrenal outcomes for patients less than and greater than two (results for all ages in Supplementary Table 4). Figure 2 displays notable increases in length of stay from postoperative biomarker T1 to T3 in several categories, especially in patients over two.
Figure 2.
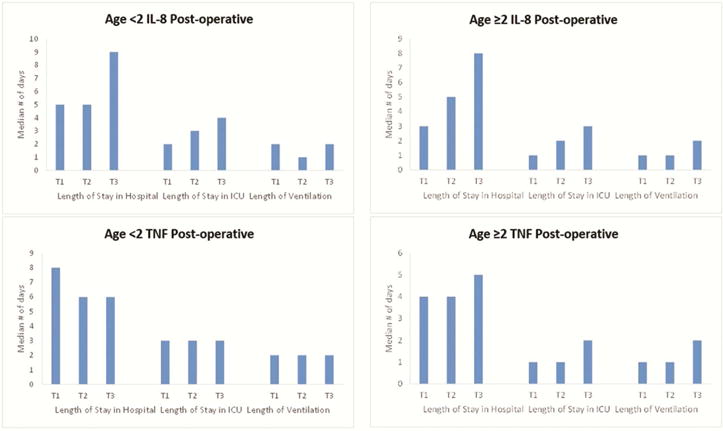
Hospital stay, ICU stay, and ventilation lengths in days by biomarker tertiles. The bars indicate median number of days per biomarker tertile.
IL, interleukin; TNF, tumor necrosis factor alpha; T, tertile
Figure 3 demonstrates the path analysis that included 175 children age 2 years and older with non-missing variable values. Supplementary Table 6 shows the sample means, standard deviations, and observation counts for the five variables represented in the path diagram; Supplementary Table 7 shows the sample correlations.
Figure 3.
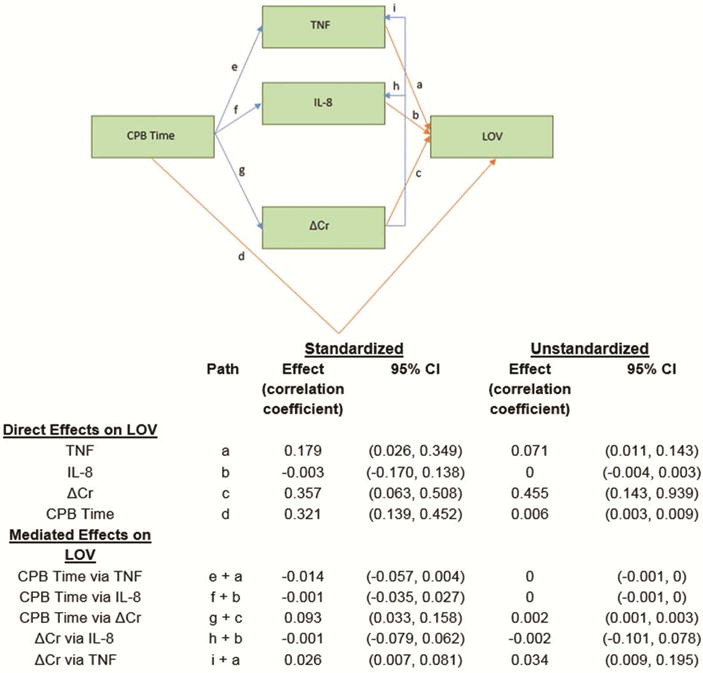
Arrows denote the effect of the term at the base on the term at the head. Indirect effects are blue, direct effects are orange. Estimated correlation coefficients are listed. “Standardized” means variables were standardized to have a mean of zero and standard deviation of one before computing estimates.
CI, confidence interval; CPB, cardiopulmonary bypass; TNF, tumor necrosis factor alpha; IL, interleukin; LOV, length of ventilation
The path analysis estimated that postoperatively, a patient’s plasma TNF level contributed 18% effect to the length of ventilation independently of AKI (Figure 3). There was no evidence of such an effect for IL-8. The analysis also estimated large direct effects of CPB time (32%) and sCr (36%) on length of ventilation, without either TNF or IL-8 mediating these relationships.
Comment
We found that elevated preoperative IL-8 levels were associated with developing both any and severe AKI. IL-8 also demonstrated strong discriminative and reclassification potential along with clinical variables. Liu et al. in 2009 also found an association between elevated IL-8 levels and an increased likelihood of developing AKI in pediatric patients after CPB, but the association was modest and the sample size was limited (14). In adults, however, elevated IL-8 levels have been shown to be associated with AKI risk in large cohorts of patients with various inflammatory conditions (8, 10). Our study provides furthers this line of work by demonstrating an association between preoperative IL-8 levels and AKI development in pediatric patients after CPB.
For plasma TNF, we found that postoperative values were associated with development of both any and severe AKI in patients ages two or older. TNF also demonstrated adequate discriminative potential when used along with clinical variables. Relevant studies in children have been completed in the past, but this is the first to demonstrate a direct relationship between TNF levels and the development of AKI. Namely, Delanghe et al. found that sepsis was diagnosable by IL-8 and TNF levels and was also associated with higher sCr levels (26). Qi et al. also found that early bedside hemofiltration in children with severe pneumonia and acute renal failure led to lower TNF (27). More direct studies have been conducted linking TNF with elevated AKI risk in adults with conditions tending to display inflammation, (12, 13). Our study had only two patients develop sepsis but still demonstrated that TNF is predictive of AKI in pediatric patients. In addition, we were likely able to find evidence for this association while Liu et al. were unable because of our large cohort and stratification of patients into age groups.
The accuracy of our plasma IL-8 and TNF data are in line with the performance of biomarkers measured in prior studies. In 2011, AUC values for diagnosing severe AKI were reported as 0.72 (standard error 0.04) for postoperative IL-18, and 0.71 (0.04) for postoperative NGAL (4). The values reported here for children over two years old are similar and perhaps even a bit stronger; namely that preoperative IL-8 had an AUC value for diagnosing severe AKI of 0.81 (0.05), and that postoperative TNF had an AUC value for diagnosing severe AKI of 0.83 (0.05).
We have demonstrated the effect of age on the association between biomarkers and AKI. Infants are born with reduced glomerular filtration and tubular functions that continue to develop postnatally until approximately age two (28). However, studies have shown great variability in the rate of this maturation. Rubin et al. showed that children can achieve adult capacity GFR within a range of two months to two years old, and that premature infants may not reach this threshold until much later (29). Thus, the range in kidney function in patients under the age of two likely introduced substantial variability into our data and obscured possible trends.
Furthermore, an infant’s immune system is not mature at birth and does not fully mature until about 6 years of age, although the majority of maturation is completed by two years (29). Differences in immune system maturation could have added further variability to our under-two group. Thus, the findings of this paper are most clinically relevant to patients over the age of two.
Our results also demonstrated associations between biomarkers and nonrenal outcomes. Specifically, there were significant differences between biomarker tertiles for postoperative TNF in association with days spent in the hospital for patients under two, for postoperative TNF in association with days on ventilator for patients greater than two, and for postoperative IL-8 in association with days on ventilator for patients under two. Our mediation analysis provided additional insight and suggested that while postoperative TNF has direct effects on length of ventilation, TNF and IL-8 do not mediate the relationship between AKI and length of ventilation.
The use of biomarker measurement in conjunction with the already-established sCr diagnosis method could improve AKI diagnostic accuracy as well as possibly predict longer ventilation and ICU stay lengths. However, our study did have several limitations. First of all, the trend in differences in nonrenal outcomes per biomarker level was somewhat unclear, likely because patients usually had only low numbers of days in the hospital, ICU, or on ventilation. Furthermore, although we collected data showing that none of our patients received pre-operative steroids, we did not collect data on intra- or postoperative steroid use. Intra-operative steroid use could have been a potential confounder and should be addressed in future projects. Furthermore, the vast majority of the patients in our study were white, and thus results may not be applicable to more diverse populations. Despite these drawbacks, this study nonetheless represents important progress in the field of pediatric AKI. In combination with the existing sCr method of detecting AKI, measuring these inflammatory biomarkers could help physicians predict and risk-stratify AKI much sooner in children before and after cardiac surgery. This clinical advance would enable pediatricians to conduct more tailored management of patients and thus reduce morbidity and mortality.
Supplementary Material
Acknowledgments
Additional members of the TRIBE-AKI Consortium are Dr Charles Edelstein, Dr Cary Passik, Dr Madhav Swaminathan, Dr Jay Koyner, Dr Amit Garg, and Catherine Krawczeski. This study was supported by the National Institutes of Health (NIH) (R01HL085757 to Dr. Parikh) to fund the TRIBE-AKI Consortium. Dr. Greenberg is supported by the NIDDK of the NIH under award number K08DK110536. Dr. Parikh is supported by the NIH (K24DK090203) and P30 DK079310–07 O’Brien Center Grant, and is also a member of the NIH-sponsored ASESS-AKI Consortium (U01DK082185). P.D. is supported by P50 DK 096418.
Abbreviations and Acronyms
- AKI
Acute Kidney Injury
- aOR
Adjusted Odds Ratio
- AUC-ROC
Area Under the Curve of a Receiver Operating Characteristics Curve
- CPB
Cardiopulmonary Bypass
- eGFR
Estimated Glomerular Filtration Rate
- ICU
Intensive Care Unit
- IL-8
Interleukin - 8
- LOV
Length of Ventilation
- RACHS- 1
Risk Adjustment for Congenital Heart Surgery 1
- sCr
Serum Creatinine
- TNF
Tumor Necrosis Factor Alpha
- TRIBE-AKI
Translational Research Investigating Biomarker Endpoints in Acu te Kidney Injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oster ME, et al. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5):e1502–1508. doi: 10.1542/peds.2012-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silver SA, et al. Acute kidney injury: Preclinical innovations, challenges, and opportunities for translation. Can J Kidney Health Dis. 2015;2:30. doi: 10.1186/s40697-015-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornik CP, et al. Serum brain natriuretic peptide and risk of acute kidney injury after cardiac operations in children. Ann Thorac Surg. 2014;97(6):2142–2147. doi: 10.1016/j.athoracsur.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh CR, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22(9):1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg JH, et al. Kidney outcomes 5 years after pediatric cardiac surgery: The TRIBE-AKI study. JAMA Pediatr. 2016;170(11):1071–1078. doi: 10.1001/jamapediatrics.2016.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch AE, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 7.Sethi G, et al. Tnf: A master switch for inflammation to cancer. Front Biosci. 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 8.Liu D, et al. Using inflammatory and oxidative biomarkers in urine to predict early acute kidney injury in patients undergoing liver transplantation. Biomarkers. 2014;19(5):424–429. doi: 10.3109/1354750X.2014.924997. [DOI] [PubMed] [Google Scholar]
- 9.Sirota JC, et al. Urine IL-18, NGAL, IL-8 and serum IL-8 are biomarkers of acute kidney injury following liver transplantation. BMC Nephrol. 2013;14:17. doi: 10.1186/1471-2369-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Pablo R, et al. Role of circulating soluble chemokines in septic shock. Med Intensiva. 2013;37(8):510–518. doi: 10.1016/j.medin.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Liu KD, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35(12):2755–2761. [PMC free article] [PubMed] [Google Scholar]
- 12.Powell TC, et al. Association of inflammatory and endothelial cell activation biomarkers with acute kidney injury after sepsis. Springerplus. 2014;3:207. doi: 10.1186/2193-1801-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kambhampati G, et al. Fluid balance and conventional and novel biomarkers of acute kidney injury in cardiovascular surgery. J Cardiovasc Surg (Torino) 2013;54(5):639–646. [PubMed] [Google Scholar]
- 14.Liu KD, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: A case-control study. Crit Care. 2009;13(4):R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: A prospective multicenter study. Crit Care Med. 2011;39(6):1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuno T, et al. KDIGO (kidney disease: Improving global outcomes) criteria could be a useful outcome predictor of cisplatin-induced acute kidney injury. Oncology. 2012;82(6):354–359. doi: 10.1159/000338664. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins KJ, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123(1):110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piepsz A, et al. Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging. 2006;33(12):1477–1482. doi: 10.1007/s00259-006-0179-2. [DOI] [PubMed] [Google Scholar]
- 20.Zappitelli M, et al. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. 2011;80(6):655–662. doi: 10.1038/ki.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zappitelli M, et al. The association of albumin/creatinine ratio with postoperative AKI in children undergoing cardiac surgery. Clin J Am Soc Nephrol. 2012;7(11):1761–1769. doi: 10.2215/CJN.12751211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janes H, Pepe MS. Adjusting for covariates in studies of diagnostic, screening, or prognostic markers: An old concept in a new setting. Am J Epidemiol. 2008;168(1):89–97. doi: 10.1093/aje/kwn099. [DOI] [PubMed] [Google Scholar]
- 23.Pepe MS, et al. Testing for improvement in prediction model performance. Stat Med. 2013;32(9):1467–1482. doi: 10.1002/sim.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacKinnon DP, et al. Statistical mediation analysis. 2012 [Google Scholar]
- 25.Efron BaT, R.J. An introduction to the bootstrap. CRC press; 1994. [Google Scholar]
- 26.Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. 2015;451(Pt A):46–64. doi: 10.1016/j.cca.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Qi GJ, et al. Effect analysis of early bedside hemo-filtration in treatment of severe pneumonia with acute renal failure of children. Eur Rev Med Pharmacol Sci. 2015;19(24):4795–4800. [PubMed] [Google Scholar]
- 28.Schmidt IM, et al. Impaired kidney growth in low-birth-weight children: Distinct effects of maturity and weight for gestational age. Kidney Int. 2005;68(2):731–740. doi: 10.1111/j.1523-1755.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 29.Rubin MI, Calcagno PL. Renal disease in infants and children; summary of round table discussion. Pediatrics. 1956;17(5):781–785. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


