Abstract
Research on the rate at which people discount the value of future rewards has become increasingly prevalent as discount rate has been shown to be associated with many unhealthy patterns of behavior such as drug abuse, gambling, and overeating. fMRI research points to a fronto-parietal-limbic pathway that is active during decisions between smaller amounts of money now and larger amounts available after a delay. Researchers in this area have used different variants of delay discounting tasks and reported various contrasts between choice trials of different types from these tasks. For instance, researchers have compared 1) choices of delayed monetary amounts to choices of the immediate monetary amounts, 2) ‘hard’ choices made near one’s point of indifference to ‘easy’ choices that require little thought, and 3) trials where an immediate choice is available versus trials where one is unavailable, regardless of actual eventual choice. These differences in procedure and analysis make comparison of results across studies difficult. In the present experiment, we designed a delay discounting task with the intended capability of being able to construct contrasts of all three comparisons listed above while optimizing scanning time to reduce costs and avoid participant fatigue. This was accomplished with an algorithm that customized the choice trials presented to each participant with the goal of equalizing choice trials of each type. We compared this task, which we refer to here as the individualized discounting task (IDT), to two other delay discounting tasks previously reported in the literature (McClure et al., 2004; Amlung et al., 2014) in 18 participants. Results show that the IDT can examine each of the three contrasts mentioned above, while yielding a similar degree of activation as the reference tasks. This suggests that this new task could be used in delay discounting fMRI studies to allow researchers to more easily compare their results to a majority of previous research while minimizing scanning duration.
Keywords: Delay discounting, fMRI, impulsivity, task development
1. Introduction
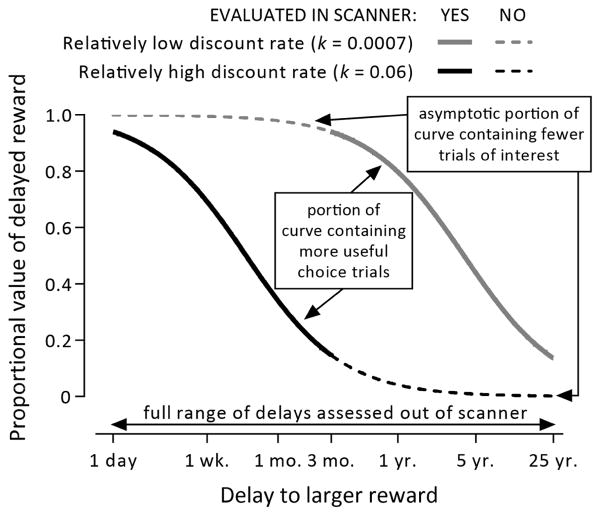
Research on the rate at which people discount the value of future rewards has become increasingly prevalent as this temporal discount rate has been shown to be associated with many unhealthy patterns of behavior such as drug abuse, gambling, and overeating (see Bickel et al., 2012; Bickel et al., 2014 for review, see MacKillop et al., 2011; Amlung et al., 2016 for meta analyses of behavior). The general procedure to characterize an individual’s discounting rate is to give them a series of forced choice questions between a smaller amount of money available after a smaller delay versus a larger amount of money available after a larger delay. Behavioral delay discounting data is often quantified with a rate parameter from a hyperbolic function first validated by Mazur (1987). Points of indifference across a series of delays expressed as a proportion of the larger delayed amount are fit to the function V=1/(1+kD) where V is the proportional present value, D is the delay, and k is the discount rate. Indifference curves fit by this function take on a characteristic ‘S’ shape when plotted on logarithmic axes, where the specific discount rate for an individual participant is determined by the transition of this curve from asymptotic values near 1.0 at lower delays to asymptotic values near 0.0 at higher delays (Figure 1). With a series of delays common in the behavioral discounting literature (ranging from days to years), this transition occurs across a consecutive series of four delays, but importantly, individual differences in discount rates result in a different set of four delays among participants.
Figure 1.
Examples of two indifference curves representing discount rates that are within the range of typical values. The shape of the curve is similar across discount rates, but is shifted left or right depending on the specific rate. After an individual’s discount rate is determined by evaluating the full range of delays in an out-of-scanner task, the Individualized Discounting Task only assesses the portion of the curve for each participant (solid lines) that is most relevant to resolving the discount rate without assessing the asymptotic portions of the curve that are less useful (dashed lines).
Across fMRI delay discounting studies, the tasks examined by researchers seem to fall into two prototypical types, which we have cataloged in Table 1. The first of these types consists of tasks similar to behavioral delay discounting assessments. In these tasks, a series of choices are presented between some amount of money available immediately and a larger amount available after a delayed amount of time (e.g. $10 now versus $50 in 3 weeks), and data are typically analyzed based on the choice made by the participant. For example, some studies have focused on contrasts between choices for the immediate option versus choices of the delayed option, while other studies have focused on ‘hard’ choices that represent a decision near that participant’s indifference curve versus ‘easy’ choices far from the indifference curve. In Table 1, studies of these response-focused tasks are described along with the type of contrast reported.
Table 1.
Previous papers examining a monetary delay discounting task with an fMRI framework. Summarized here are two categories of tasks that either consist entirely of choices between an immediate option and a delayed option or tasks that are defined by trials in which an immediate option is available versus trials between two delayed options. For each analysis category, we have indicated whether the task allowed the authors to determine participants’ discount rates in-scanner, whether the task included control trials, and whether authors reported on a significant result for each trial comparison category. For each column, we have identified if the task feature was possible and reported on (Yes), was not possible due to task limitations (No), may have been possible but was not reported on (?). Note that for many of the reports below, the goals and hypotheses of the paper did not align with the three categories of contrasts we have focused on here, and a failure to report a contrast does not necessarily mean that a particular contrast could not have been performed.
| Citation | Study n | Mean age (range) | Total choice trials | Resolve discount rate | Control choices | Contrast type | ||
|---|---|---|---|---|---|---|---|---|
| Immediate vs. delayed choices | ‘Hard’ vs. ‘easy’ choices | Immediate available vs. unavailable | ||||||
| Response-focused tasks | ||||||||
| Amlung et al., 2014 † | 25 | 22.5 (nr) | 120 | Yes | Yes | Yes | Yes | No |
| Ballard & Knutson, 2009 | 16 | 21.6 (nr) | 84 | Yes | Yes | Yes | ? | No |
| Bickel et al., 2009 | 30 | 47.1 (20–67) | 56 | Yes | Yes | ? | ? | No |
| Boettiger et al., 2007 | 19 | 28.3 (nr) | 37 | Yes | No | Yes | ? | No |
| Boettiger et al., 2009 | 19 | 28.3 (nr) | 37 | Yes | No | Yes | ? | No |
| Carlisi et al., 2016 | 32 | 15.1 (11–17) | 60 | Yes | No | Yes | ? | No |
| Chantiluke et al., 2014 | 64 | 14.6 (11–17) | 60 | Yes | No | ? | ? | No |
| Christakou et al., 2011 | 40 | 20.2 (12–32) | 60 | Yes | No | Yes | ? | No |
| Clewett et al., 2014 | 72 | 33.2 (nr) | 72 | ? | Yes | ? | Yes | No |
| Elton et al., 2017 | 95 | 25.9 (18–40) | 252 | Yes | No | ? | ? | No |
| Ersner-Hershfield et al., 2009 | 18 | nr (18–23) | 137 | Yes | Yes | Yes | ? | No |
| Fassbender et al., 2014 | 14 | 26.1 (20–36) | 62 | Yes | No | ? | ? | No |
| Hare et al., 2014 | 27 | 24.1 (19–40) | 216 | Yes | No | Yes | ? | No |
| Hinvest et al., 2011 | 34 | 23.8 (18–49) | 56 | Yes | Yes | No | Yes | No |
| Hoffman et al., 2008 | 36 | 35.7 (nr) | 160 | Yes | Yes | ? | Yes | No |
| Hu et al., 2017 | 22 | 24 (19–28) | 96 | Yes | No | ? | ? | No |
| Kable & Glimcher, 2007 | 10 | 21.2 (nr) | 144 | Yes | No | Yes | ? | No |
| King et al., 2016 | 62 | 15.9 (12–22) | 90 | Yes | No | ? | Yes | No |
| Kishinevsky et al., 2012 | 19 | 33.4 (19–50) | 160 | Yes | Yes | Yes | Yes | No |
| Li et al., 2013 | 23 | 22.8 (20–25) | 168 | Yes | Yes | ? | Yes | No |
| Liu et al., 2012 | 19 | 21.7 (19–25) | 200 | Yes | Yes | ? | ? | No |
| Luhmann et al., 2009 | 20 | 23.4 (19–30) | 58 | ? | No | Yes | ? | No |
| Luo et al., 2011 | 21 | 29.8 (22–44) | 38 | Yes | Yes | Yes | ? | No |
| MacKillop et al., 2012 | 13 | 40.2 (nr) | 54 | Yes | Yes | Yes | ? | No |
| Manning et al., 2014 | 37 | 24.9 (20–32) | 108 | Yes | No | ? | ? | No |
| Marco-Pallares et al., 2010 | 17 | 28 (nr) | 27 | Yes | No | Yes | Yes | No |
| Martin et al., 2015 | 21 | 43 (18–65) | 120 | ? | No | Yes | ? | No |
| Mavrogiorgou et al., 2016 | 20 | 31.3 (nr) | 140 | Yes | Yes | Yes | ? | No |
| Meade et al., 2011 | 39 | 48.1 (18–59) | 36 | Yes | Yes | ? | Yes | No |
| Meade et al., 2016 | 35 | 41 (18–55) | 120 | Yes | Yes | ? | Yes | ? |
| Miedl et al., 2012 | 32 | nr (21–50) | 48 | Yes | No | ? | ? | No |
| Miedl et al., 2015 | 30 | 36.5 (27–47) | 108 | Yes | No | Yes | Yes | No |
| Monterosso et al., 2007 | 29 | 32 (nr) | 27 | Yes | No | ? | Yes | No |
| Onoda et al., 2011 | 30 | 21.7 (nr) | 100 | No | No | No | No | No |
| Ortiz et al., 2015 | 21 | nr (19–45) | 49 | Yes | No | ? | ? | No |
| Peters & Buchel, 2009 | 22 | 26.3 (nr) | 48 | Yes | No | ? | ? | No |
| Peters & Buchel, 2010 | 30 | 25.4 (nr) | 48 | Yes | No | ? | ? | No |
| Pine et al., 2009 | 24 | nr (19–28) | 200 | Yes | Yes | No | Yes | No |
| Pine et al., 2010 | 14 | 21 (18–30) | 200 | Yes | Yes | No | Yes | No |
| Ripke et al., 2012 | 263 | 15.7 (13–50) | 90 | Yes | No | ? | ? | No |
| Ripke et al., 2014 | 206 | nr (13–15) | 90 | Yes | No | ? | ? | No |
| Ripke et al., 2015 | 206 | nr (13–15) | 90 | Yes | No | ? | ? | No |
| Rodriguez et al., 2015 | 23 | 24.5 (19–46) | 240 | Yes | No | ? | ? | No |
| Sasse et al., 2015 | 23 | 25.0 (21–30) | 72 | Yes | No | ? | ? | No |
| Schmaal et al., 2014 | 32 | 42.3 (nr) | 48 | Yes | No | Yes | ? | No |
| Schneider et al., 2014 | 48 | 14.3 (13–15) | 84 | Yes | No | ? | ? | No |
| Sohn et al., 2015 | 20 | 24.0 (20–29) | 60 | Yes | No | ? | ? | No |
| Stanger et al., 2013 | 30 | 15.7 (12–18) | 100 | Yes | Yes | Yes | ? | No |
| Steinbeis et al., 2014 | 20 | 9.7 (6–12) | 48 | Yes | No | ? | ? | No |
| Stoeckel et al., 2012 | 24 | nr (19–50) | 160 | Yes | Yes | ? | Yes | No |
| Taylor et al., 2016 | 129 | nr (31–53) | 27 | Yes | No | ? | ? | No |
| Vanyukov et al., 2016 | 48 | nr (46–90) | 48 | Yes | No | Yes | ? | No |
| Wang et al., 2014 | 28 | 22.1 (nr) | 256 | No | No | Yes | No | No |
| Wang et al., 2016a | 63 | 21.5 (nr) | 72 | Yes | No | ? | ? | No |
| Wang et al., 2016b | 39 | 22.6 (nr) | 72 | Yes | No | ? | ? | No |
| Weber & Huettel, 2008 | 23 | 23 (19–36) | 30 | ? | No | ? | ? | No |
| Wittmann et al., 2007 | 13 | 26 (nr) | 48 | Yes | No | Yes | ? | No |
| Wittmann et al., 2010 | 13 | 30.2 (nr) | 25 | Yes | Yes | Yes | ? | No |
| Yu et al., 2015 | 80 | 26 (nr) | 120 | No | Yes | ? | ? | No |
| Stimulus-focused tasks | ||||||||
| Albrecht et al., 2010 | 28 | nr (nr) | 42 | Yes | No | ? | ? | Yes |
| Aranovich et al., 2016 | 19 | 33.6 (nr) | 128 | Yes | No | ? | ? | ? |
| Bos et al., 2014 | 22 | 20.4 (nr) | 70 | Yes | No | ? | ? | ? |
| Decker et al., 2015 | 93 | 24.8 (nr) | 144 | Yes | No | Yes | ? | Yes |
| Eppinger et al., 2012 | 30 | 45.3 (18–80) | 42 | ? | No | ? | ? | Yes |
| Kable & Glimcher, 2010 | 25 | 22.2 (nr) | 200 | Yes | No | ? | ? | Yes |
| Kim et al., 2012 | 33 | 21.5 (nr) | 42 | ? | No | ? | ? | Yes |
| Kobiella et al., 2013 | 66 | 41.3 (30–60) | 40 | Yes | No | a | ? | Yes |
| Luo et al., 2012 | 15 | nr (nr) | 51 | ? | No | ? | ? | ? |
| McClure et al., 2004 † | 14 | 21.4 (nr) | 42 | ? | No | ? | Yes | Yes |
| McClure et al., 2007 | 34 | nr (nr) | 42 | ? | No | ? | Yes | Yes |
| Samamez-Larkin et a., 2011 | 25 | 48.7 (19–85) | 42 | Yes | No | ? | ? | Yes |
| Sellitto et al., 2016 | 89 | nr (nr) | 70 | Yes | Yes | ? | ? | Yes |
| Sripada et al., 2011 | 20 | 28.7 (nr) | 42 | ? | No | ? | Yes | Yes |
| Xu et al., 2009 | 18 | 25 (22–29) | 42 | ? | No | ? | Yes | Yes |
| Our Individualized Discounting Task | 18 | 33.7 (23–50) | 98 | Yes | Yes | Yes | Yes | Yes |
Chosen as a comparison task to the IDT.
A contrast was reported between smaller sooner trails and larger later trials, with smaller sooner included both immediate options and less delayed options. Study sample sizes represent those reported after any exclusion criteria were applied.
nr = not reported.
The second type of task focuses on the difference between choice trials, where trials consisting of two delayed options (e.g. $10 in 1 week versus $50 in 1 month) are interspersed with choices consisting of both a delayed and an immediate option, with roughly half the trials of each type. Data are typically analyzed as a function of the type of trial that was presented, contrasting on whether an immediate option was available (regardless of participant choice). We have therefore labeled these tasks as stimulus-focused. These tasks often have the appropriate trial types for the ‘hard’ versus ‘easy’ and immediate choice versus delayed choice contrasts, but these are not always reported. Inconsistent reporting of these response-focused contrasts may be because they were auxiliary to the goals of these studies or that some studies lacked power to analyze these types of contrasts since the number of choice trials that fall into the response-focused trial categories can be small or highly unbalanced.
Across different types of tasks, researchers have compared 1) choices of delayed monetary amounts to choices of the immediate monetary amounts, 2) ‘hard’ choices made near one’s point of indifference to ‘easy’ choices that require little thought, and 3) trials where an immediate choice is available versus trials where one is unavailable (regardless of the participant’s actual choice). Unfortunately, however, capturing all of these contrasts in the existing delay discounting tasks during the same imaging study is difficult, and as a result, researchers are forced to limit their eventual analyses to only a subset of these contrasts. Note that in Table 1, no task has been used to analyze all three of these contrasts. Of course, one option is to simply run both types of tasks to obtain data on both types of contrasts, but the high cost of scanner time and the increased potential for participant fatigue (which often leads to increased head motion) typically precludes this.
Thus, the goal of this project was to develop a task that could incorporate both response-focused and stimulus-focused trials into a compact fMRI task. A key insight in our approach, similar to approaches used previously (e.g., Kishinevsky et al., 2012; Manning et al., 2014), comes from the observation that existing tasks must be able to capture a wide range of individual discounting rates, and thus current tasks need to include trials that for one individual may be critical because they are near their indifference point, but are inefficient for another individual because they are near either the upper or lower asymptotic regions of their discounting curve. The crux of the task that we developed is that if a participant’s discount rate is characterized before the scanning session, the in-scanner portion of the task can be individualized to be optimal for each participant. Thus, we call this the individualized discounting task (IDT). We hypothesized that a task with trials individualized to an individual’s pre-estimated discount rate would allow us to examine the common contrasts of both response-focused and stimulus-focused discounting tasks of Table 1 with a similar scanning duration as either reference task alone. To test this assertion, we directly compared our IDT to prototypical response-focused and stimulus-focused tasks and evaluated whether our novel IDT met two criteria: 1) it yielded similar activation maps as the two comparison tasks when comparing the same contrast, and 2) it yielded similar activation maps as previous reports in the literature. To examine the tasks on these criteria, we focused on contrasts among the trial types that differentiate existing discounting tasks reported in the literature (see Table 1), but we also examined an ‘all-trials’ contrast that is not unique to any task to verify that our IDT yields similar results as other tasks with this popular contrast.
2. Materials and Methods
2.1. Participants
Participants (n = 22) were recruited from the community in and around Roanoke, VA. Participants were excluded if they met DSM-5 use disorder criteria for any drug of abuse other than nicotine (American Psychological Association, 2013) or if they had medical conditions contraindicated for an MRI scan (e.g., ferromagnetic implants, or claustrophobia). This study was conducted as part of a larger trial so inclusion/exclusion criteria and sample size based on power to detect behavioral differences in discounting were inherited from that trial. Four participants moved excessively during the imaging session and were excluded. Among the excluded participants, one had a mean maximum motion of 6.7 mm (with 10.6 mm as their maximum); the second participant fell asleep and had a mean maximum motion of 6.6 mm (with 17.7 mm as their maximum); the third participant had a mean maximum motion of 10.9 mm (with 15.7 mm as their maximum); and the fourth excluded participant had a mean maximum motion of 4.6 mm (with 8.1 mm as their maximum). The 18 participants who remained in the final analyses had a mean maximum motion of 1.7 mm (+/− 0.6 mm) and were 28% female, were 67% White and 33% African American, had a mean age of 33.7 (SD = 8.4, range 23 to 50), completed a mean of 13.9 years of education (SD = 2.1), and had a mean monthly income of $953 (median = $700, SD = $915).
2.2. Individualized Discounting Task (IDT)
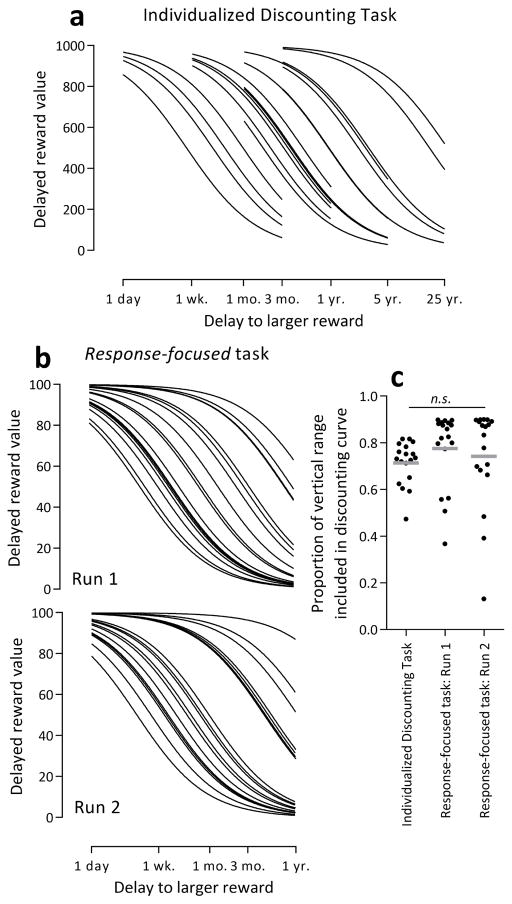
Example screens from the IDT are displayed in Figure 2. As mentioned, our IDT resulted from an examination of the shape of indifference curves and the distribution of choice trials in common discounting tasks in relation to these indifference curves. With a series of delays that is common in the behavioral discounting literature (1 day, 1 week, 1 month, 3 months, 1 year, 5 years, 25 years), the transition of indifference points occurs across a consecutive series of four delays, but the particular four delays involved differs among participants. In the examples drawn in Figure 1, individualized series of four delays capture the transition period well as long as the full delay series from 1 day to 25 years are candidates for selection. Our data suggest that these longer, multiple-year delays are necessary to fully characterize the discounting curve, as some participants do not show appreciable discounting until delays of approximately 1 year (Figure 3).
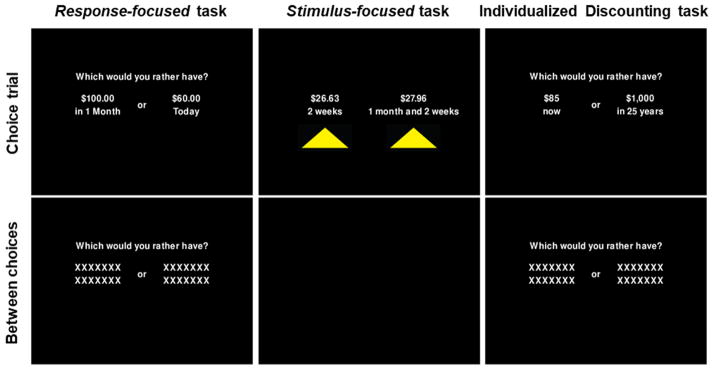
Figure 2.
Example images from each of the in-scanner tasks. Visual elements described by the source papers (Amlung et al., 2014; McClure et al., 2004) were included as to match as closely as possible, while other elements not described by the source papers (e.g., font) were kept similar across tasks. The response-focused task and IDT were very similar in appearance, while the stimulus-focused task had fewer on-screen instructions and yellow triangles under the response options. When a response was made, the yellow triangle under the selected option turned red briefly and the other disappeared. Between choice trials, the stimulus-focused task went to a blank screen, while the text elements in the response-focused task and IDT remained on the screen, but with amount and delay information temporarily replaced by a series of X’s.
Figure 3.
The individualized delay series of the IDT captured the informative delays for each participant well (a) in fewer trials than the response-focused task (b). The vertical range of the discounting curves captured by these two tasks did not differ significantly (c).
In the IDT, we focused on this individualized series of four delays to increase the proportion of ‘hard’ choices (defined here as an immediate value for that choice within 0.2 units of the indifference point at that delay where 1 unit is the delayed amount) compared to ‘easy’ choices (all other choices that are not ‘hard’) and to approximately equalize the number of immediate and delayed choices. We chose to divide ‘hard’ and ‘easy’ trials in this way to match the source article for our response-focused task (Amlung et al., 2014). We chose this definition as one of the goals of this experiment was to replicate previous results in the literature, but nothing prevents users of this task to use other methods for determining cutoffs between ‘hard’ and ‘easy’ trials (e.g., Pine et al., 2009). The out-of-scanner task (described below) passed the participant’s discounting rate (fitted k value from Mazur’s (1987) hyperbolic equation) and indifference points at each delay to the IDT task. The specific four delays assigned to each participant were determined by the k value with unit days−1, with k > 0.03542 assigned delays 1 day to 3 months, 0.03542 ≥ k > 0.0098 assigned delays 1 week to 1 year, 0.0098 ≥ k > 0. 002813 assigned delays 1 month to 5 years, and k ≤ 0.002813 assigned delays 3 months to 25 years. At each of the four assigned delays, the IDT script arranged the following specific trials, with all amounts calculated as proportions of configurable amount of a configurable commodity set at task onset: 10 trials with set immediate proportional amounts of 0.05, 0.15, 0.25, 0.35, 0.45, 0.55, 0.65, 0.75, 0.85, and 0.95; 1 trial where the immediate amount is at the participant’s predetermined indifference point for that delay; 4 trials with amounts near to the participant’s indifference point at +0.04, +0.08, −0.04, and −0.08 (logic was inserted in the task to prevent these four trials from creating amounts <0 or >1 – in these cases 0.1 was added to or subtracted from the proportional multiplier as necessary). In addition to these immediate versus delayed trials, the task contained 14 trials spread throughout the task with both options delayed to mimic the similar trials of the McClure et al. (2004) task. A number of tasks employ additional control trials with similar visual and motor response elements as the active trials, but with the choices altered such that only one logical response is available. We incorporated different forms of these trials, including 12 choices between two different amounts of immediately available money, 4 choices between the full larger-later amount available immediately versus the same amount delayed (1 trial at each delay), and 4 choices between nothing now and the full larger-later amount delayed (1 trial at each delay). The total number of trials equaled 98. For the present experiment, we set the amount and commodity to $1000 in US currency because this is the most commonly studied amount of money in the addiction literature (MacKillop et al., 2011) and more effective at distinguishing substance users from controls than smaller amounts (Mellis et al., 2017). Our IDT task script and associated out-of-scanner script can be downloaded from <https://github.com/micned/IDT>.
2.3. Comparison Discounting Tasks
To test whether our IDT was associated with similar neural activity as both stimulus-focused and response-focused tasks from Table 1, we also administered a comparison task from each type to participants. Example screens from the comparison discounting tasks are displayed in Figure 2. We chose the stimulus-focused task used in McClure et al. (2004), and the response-focused task used in Amlung et al. (2014). The two tasks were described well by the original authors including images of the visual elements, such that we were able to reprogram them to be highly similar to their original form. When a task element was not specified (e.g., specific font or measurements of screen elements), we chose options to make the three tasks as similar to each other as possible.
2.4. Procedure
After giving informed consent, participants completed a series of behavioral assessments outside of the scanner including a delay discounting task. This out-of-scanner task was used to determine which delays would be included in the IDT for that person (see below). For the out-of-scanner task, we used an adjusting-amount titration task that we have used previously (e.g., Koffarnus & Bickel, 2014), which is based on a task developed by Du et al. (2002). Briefly, this algorithm starts each set of trials based on one delay with a question between the larger-later amount and half that amount available immediately. Based on the participant’s choice, the immediate amount then adjusts up or down across a series of six choice trials to increasingly approximate the participant’s point of indifference between the immediate amount and the larger-later amount. This task consisted of 42 trials and the mean duration was 4.2 min (SD = 1.0 min). Participants then completed the fMRI portion of the study, which consisted of a structural scan and three delay discounting tasks: our IDT and the comparison response-focused and stimulus-focused tasks (see Figure 2 for task visual elements). The response-focused task was split into four parts, each 6.5 min (26 min total), which were always run consecutively in sequential order as was done in the original paper (Amlung et al., 2014). Two of these parts constituted one full run of the task, so two full runs were completed. The mean duration for the stimulus-focused task was 13.3 min (SD = 1.0 min) and the mean duration of the IDT was 15.1 min (SD = 2.7 min). The order that these tasks were presented was randomized across participants. For all discounting tasks in and out of the scanner, participants responded by pressing a button in or under their left and right hands for the option on the left and right side of the screen, respectively. All task choices were hypothetical. Hypothetical choices have been shown to produce similar results as consequated choices in both behavioral and fMRI contexts (Bickel et al., 2009; Johnson & Bickel, 2002; Lagorio & Madden, 2005; Lawyer et al., 2011; Madden et al., 2003; Madden et al., 2004). All procedures were approved by the Virginia Tech Institutional Review Board.
Structural and functional brain data were collected on a 3.0 T scanner (Siemens Tim Trio). T1-weighted anatomical volumes were acquired with a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) pulse sequence with 192 axial slices (resolution = 1 x 1 x 1 mm3, repetition time (TR) = 2,600 ms, echo time (TE) = 3.02 ms, field of view (FOV) = 256 mm2, flip angle (FA) = 8°). Functional data consisted of 33 interleaved slices collected every 2 s with an echo time of 30 ms and a 90 degree flip angle using an echo planar sequence (resolution = 3.4 x 3.4 x 4.4 mm3, FOV = 220 mm2).
2.5. Data Analyses
All analyses were conducted with 18 participants unless otherwise noted. Behavioral data were analyzed in GraphPad Prism 6. All MRI data processing and analyses were performed using AFNI (Cox, 1996). Preprocessing of functional data were performed using the afni_proc.py python script (Cox, 2012) with default settings unless otherwise specified and included slice timing correction, motion correction, spatial smoothing (6 mm FWHM) and scaling to percent signal change. Anatomical volumes were skull-stripped, aligned to the first functional volume and subsequently co-registered to the MNI 152 template. The resulting functional-to-MNI transformation matrices were applied to the statistical maps generated by the following GLM analyses. For first-level analyses, separate general linear models (GLMs) were obtained for each of the trial categories: Hard Choices, Easy Choices, Immediate Choices, Delayed Choices, Immediate Available, and Immediate Unavailable. Note that because the response-focused task always presented an immediate choice, only the stimulus-focused and IDT were analyzed with an immediate available versus immediate unavailable contrast. Time points containing motion spikes and time series outliers were censored from the analysis. Apart from baseline and task-related regressors, six additional nuisance regressors were included to account for head motion (roll, pitch, yaw, X, Y, Z). Group analyses were then performed using a mixed effects meta-analysis model with the 3dMEMA command in AFNI (Chen et. al., 2012), which accounts for both within and across participant variability. All statistical maps were thresholded at a False Discovery Rate (FDR) corrected value of 0.05 except the ‘all trials’ contrast which was thresholded at p < .001, uncorrected, 5 contiguous voxels as in McClure et al. (2004). Since the stimulus-focused task did not include any control trials, control trials from the other two tasks were also regressed out from the general linear model for this contrast. For only the immediate versus delayed choices, seven participants that did not choose any delayed choices in the stimulus-focused task were excluded from analyses with that contrast for that task and task-to-task comparisons. All contrasts were conducted bi-directionally and all statistically significant results are reported here. Beta maps from analyses reported here can be downloaded in NIfTI format from <https://github.com/micned/IDT>.
3. Results
3.1. Behavioral Data
Fits for the in-scanner discounting curves for the IDT and runs 1 and 2 from the response-focused task are shown in Figure 3a and 3b, respectively. The analogous fits for the stimulus-focused task are not easily depicted on similar coordinates because the delay of both response options are manipulated, but this task assessed a more narrow range of delays (i.e., immediate to 1 month + 2 weeks. Even though only four indifference points were assessed, the IDT assessed the relevant trials for each participant, excluding the asymptotic portion of each individual’s discounting curve. This process resulted in a similar vertical range assessed by these two tasks (Figure 3c), calculated by taking the y-value of the indifference curve at the lowest assessed delay minus the y-value of the indifference curve at the highest assessed delay. Correlations among log-transformed discount rates from the out-of-scanner adjusting amount discounting task and the in-scanner tasks were all statistically significant and generally very high (Table 2).
Table 2.
Correlation matrix of discounting rates as measured by the out-of-scanner adjusting amount task, the IDT, the two runs of the response-focused task, and the stimulus-focused task. Correlations are Pearson r values among the log(k) values determined by least-squares fits of Mazur’s (1987) hyperbola to indifference points at each delay combination. All correlations significant at p < .003.
| Task | 2. | 3. | 4. | 5. |
|---|---|---|---|---|
| 1. Out of scanner | .88 | .87 | .85 | .66 |
| 2. IDT | .88 | .90 | .75 | |
| 3. Response-focused run 1 | .95 | .75 | ||
| 4. Response-focused run 2 | .76 | |||
| 5. Stimulus-focused |
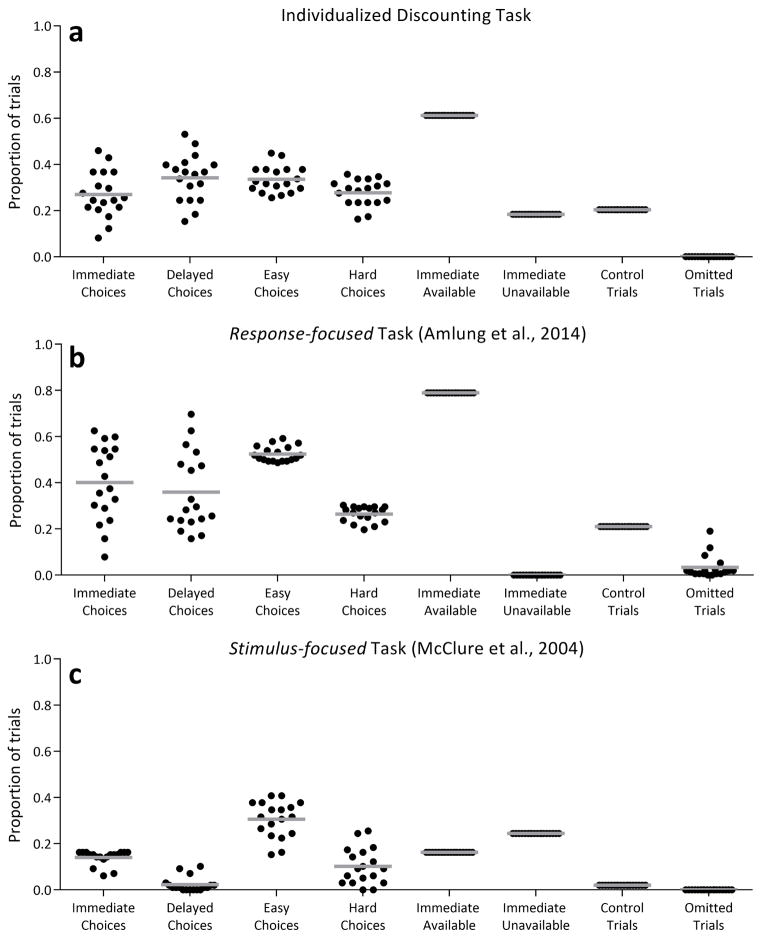
The primary goal of the IDT was to include sufficient trials of each type reported in the literature as important for understanding the neural correlates of intertemporal choice. The distribution of these trial types for each of the three tasks, expressed as a proportion of all trials, is shown in Figure 4. The distribution of immediate versus delayed choices (first two columns) was approximately equal in the IDT (Figure 4a, t(17) = 1.53, p = .1, dz = 0.36) and in the response-focused task (Figure 4b, t(17) = 0.54, p = .6, dz = 0.13), but more immediate choices were made in the stimulus-focused task with our sample (Figure 4c, t(17) = 7.82, p < .001, dz = 1.84). The differences in immediate versus delayed trials across tasks was significant with a one-way ANOVA (F(2, 53) = 4.4, p = .03), with the IDT having a significantly more equitable distribution than the stimulus-focused task (Tukey-adjusted p < .001) and the response-focused task not different from the other two. In all three tasks, significantly more ‘easy’ choice trials were made than ‘hard’ choice trials, but this difference was substantially smaller in the IDT (Figure 4a, t(17) = 2.16, p = .046, dz = 0.51) than in the response-focused (Figure 4b, t(17) = 16.34, p < .001, dz = 3.85) and stimulus-focused (Figure 4c, t(17) = 5.54, p < .001, dz = 1.31) tasks. These differences among tasks were significant (F(2, 53) = 17.5, p < .001) with the IDT having a significantly more equitable distribution of ‘hard’ and ‘easy’ trials than both the response-focused (p < .001) and stimulus-focused (p = .006) tasks, which were not significantly different from each other. By design, trials with only delayed options presented were only included in the IDT (18.4% of trials) and stimulus-focused task (24.5% of trials), and appreciable numbers of control trials were only included in the IDT (20.5% of trials) and response-focused (21.1% of trials). For the purposes of Figure 4, control trials and other trial types are mutually exclusive.
Figure 4.
The obtained distribution of trial types for the IDT task (a), response-focused task (b), and stimulus-focused task (c). The IDT was designed to equalize the number of immediate and delayed choices and the number of ‘easy’ and ‘hard’ choices while including immediate unavailable trials and control trials.
3.2. fMRI Findings for Each Task
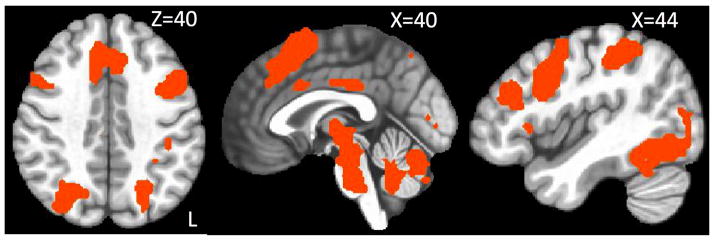
The IDT was designed to optimize the availability of contrasts comparing specific trial types within the task to one another, but we also examined an ‘all-trials’ contrast that is commonly reported in the literature to determine if this novel task reproduces associated effects. Each individual statistical map for all trials versus baseline produces widespread activation in a pattern characteristic to this contrast, and the overlap map obtained by intersecting these individual maps is displayed in Figure 5. Areas of activation include the visual cortex, bilateral dorsolateral prefrontal cortex (DLPFC), premotor area (PMA), supplementary motor area (SMA), bilateral superior and inferior parietal lobules, and bilateral ventrolateral prefrontal cortex. A repeated-measures ANOVA was also performed to compare the activation maps across tasks. The stimulus-focused task showed significantly higher activation in the visual cortex compared to the other two tasks for the all trials contrast, reflective of additional visual stimuli and more visual transitions in this task paradigm (see Figure 2). No other significant differences were observed among the tasks.
Figure 5.
Overlap of significant statistical maps for all trials independent of delay for all three in-scanner tasks. Regions in the overlap are preferentially activated compared to baseline during any choice trial (control trials regressed out). These areas include the visual cortex, bilateral dorsolateral prefrontal cortex (DLPFC), premotor area (PMA), supplementary motor area (SMA), bilateral superior and inferior parietal lobules and bilateral ventrolateral prefrontal cortex.
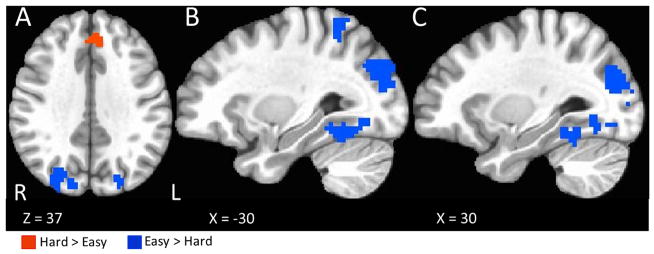
For the contrast between ‘hard’ and ‘easy’ choices (those choices near and far to that individual’s indifference point for that delay combination, respectively), the analysis for each task used a three-regressor model (hard choices, easy choices, and control trials). The common areas of activation between the IDT and response-focused tasks included medial prefrontal cortex and anterior cingulate for hard > easy (Table 3, Figure 6). No significant activations were found for the stimulus-focused task, which is likely due to the limited number of trials fitting the ‘hard’ criteria in many participants (see trial type distributions in Figure 4).
Table 3.
Areas of activation overlap for the contrast of hard choices versus easy choices between the response-focused task and the IDT. Foci are in MNI coordinates and reflect the peak t-value for voxels with p < 0.05 after FDR correction. Only clusters with at least 20 voxels were considered.
| Region | IDT task results | t value of same response-focused task area | |||||
|---|---|---|---|---|---|---|---|
| voxels | BA | x | y | z | t value | ||
| Hard > Easy | |||||||
| Bilateral Medial Prefrontal, L Anterior Cingulate | 46 | 9 | −4 | 36 | 32 | 3.36 | 3.01 |
| Easy > Hard | |||||||
| R Lingual, Fusiform, & Parahippocampal | 654 | 18 | 28 | −46 | −16 | −5.09 | −4.75 |
| L Middle Occipital | 305 | 19 | −32 | −84 | 18 | −5.90 | −5.46 |
| L Lingual, Parahippocampal | 274 | 19 | −30 | −64 | −8 | −3.97 | −4.89 |
| R Superior and Inferior Parietal Lobes | 58 | 7/40 | 28 | −52 | 60 | −4.63 | −6.40 |
| L Inferior Occipital | 21 | 18 | −34 | −78 | −10 | −5.87 | −3.65 |
Figure 6.
Areas of activation overlap for the contrast of ‘hard’ choices minus ‘easy’ choices between the response-focused task and the IDT.
For the contrast of delayed choices versus immediate choices, the analysis used a four-regressor model (delayed choices, immediate choices, control, and immediate unavailable). No areas of activation were statistically significant after FDR error correction (p < 0.05) for any of the three tasks examined. Only 11 participants could be included for this contrast in the stimulus-focused task due to exclusive or nearly exclusive responding on the immediate option.
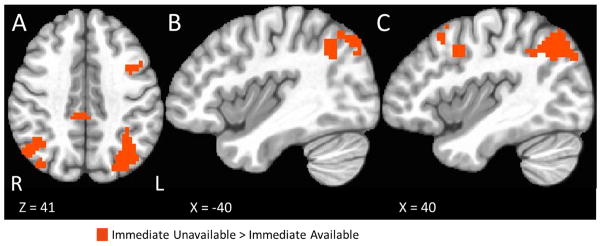
Immediate unavailable trials presenting a choice between two delayed options could only be compared to trials with an immediate option available in the stimulus-focused task and IDT because these were the only tasks that contained immediate unavailable trials. This comparison used a three-regressor model (immediate available, immediate unavailable, and control). In our sample, we observed no significant activations associated with this contrast with the stimulus-focused task, but noted a number of areas with the IDT including Brodmann areas 47, 8, and 6 in the left middle frontal cortex (Table 4, Figure 7).
Table 4.
Areas of greater activation during immediate unavailable trials than immediate available trials for the IDT. No significant areas of activation were observed in the stimulus-focused task. Foci are in MNI coordinates and reflect the peak t-value for voxels with p < 0.05 after FDR correction. Only clusters with at least 20 voxels were considered.
| Region | IDT task results | |||||
|---|---|---|---|---|---|---|
| voxels | BA | x | y | z | t value | |
| L Superior Parietal Lobule | 310 | 7 | −34 | −78 | 50 | 4.36 |
| R Superior Parietal Lobule | 187 | 7 | 36 | −76 | 50 | 4.87 |
| L Middle Frontal | 88 | 47 | −52 | 42 | −6 | 5.01 |
| L Middle Frontal | 41 | 6 | −48 | 12 | 50 | 4.02 |
| L Lingual | 37 | 17 | −10 | −98 | −10 | 4.46 |
| L Middle Frontal | 30 | 8 | −40 | 20 | 50 | 4.02 |
| L Cerebellum (Declive) | 29 | 45 | −10 | −84 | −28 | 5.78 |
| Bilateral Paracentral Lobule/Cingulate | 24 | 31 | 2 | −34 | 38 | 4.27 |
| R Lentiform Nucleus | 20 | 47 | 14 | 8 | 2 | 4.13 |
Figure 7.
Map of immediate unavailable versus immediate available trials for the IDT (FDR corrected at p < .05). Note, no significant regions were observed for the stimulus-focused task.
Regions of interest (ROIs) were also identified from each area reported as significant in one or both of the comparison task papers (Amlung et al., 2014; McClure et al., 2004) for each of these four contrasts. Activation associated with our implementation of the comparison tasks and the IDT were compared for each ROI, the results of which are detailed in Supplementary Material.
3.3. Comparisons between Tasks
When proposing a new behavioral measure, it is important to consider its equivalence to previous measures. Therefore, to determine if areas of activation differed as a function of task, statistical maps for each of the three pairs of trial types were compared across tasks in second-level analyses using a two-way ANOVA with the task as the fixed effect and the participants as the random effect. For the ‘all-trials’ contrast, the stimulus-focused task was associated with greater visual cortex activity than the other two tasks, but this was the only difference noted. This task included colored triangles below the response options and more visual element transitions (Figure 2), which may account for this increased visual cortex activity. For each of the three within-task contrasts examined in this study, no significant main effect of task on activation in any area of the brain was observed, indicating that similar neural activity was associated with the analogous trial types in each of the three tasks. This comparison included all three tasks for immediate choices versus delayed choices (n = 11 only due to nearly exclusive responding for the immediate option in many participants on the stimulus-focused task) and for ‘hard’ choices versus ‘easy’ choices (n = 18). For the contrast of immediate available versus immediate unavailable trials (n = 18), only the IDT and stimulus-focused task could be compared since the response-focused task did not have immediate unavailable trials. Furthermore, when the beta coefficients associated with each of the ROIs reported in the comparison task papers (Amlung et al., 2014; McClure et al., 2004) were compared among the tasks, no significant task-associated effect for any area was detected (see Supplementary Material).
4. Discussion
By focusing the choice trials in the in-scanner task on those delays most important for resolving that individual’s discount rate and distributing choice trials more evenly among the different trial types, the IDT was able to maximize scanner time while producing activation maps that did not significantly differ from either of the comparison tasks for each of three contrasts commonly performed in the literature and only differed in the visual cortex in a fourth common contrast. Conclusions about some of these contrasts should be interpreted with caution due to the lack of activation with any of the tasks in our sample, but overall, our results suggest that the IDT produces similar activation as two comparison tasks. The IDT differs from existing tasks in two key ways. First, it contains each of the trial types that have been highlighted in other intertemporal choice tasks in sufficient numbers to measure associated neural activation. Second, it maintains a reasonable duration by tailoring the specific questions asked to the individual to equalize the distribution of trial types, effectively optimizing scanner time.
4.1. Similarity of the Individualized Discounting Task Activation Maps to Comparison Tasks
The first basis on which we set out to evaluate the IDT was the degree to which it produced the same activation maps as the comparison tasks for the same contrast. No regions were significantly correlated with immediate choices or delayed choices for any of the tasks, indicating that we may have not had sufficient power with our sample size to detect activations associated with this contrast. Perhaps unsurprisingly, these activation maps were also not significantly different among tasks. For the contrasts with significant neural correlates, the activation maps from the IDT were also not significantly different from either of the comparison tasks. The ‘all-trials’ contrast produced maps that look highly similar to those in McClure et al. (2004) and ROI analyses (see Supplementary Material) confirm that the IDT did not differ from either of the other two tasks in these key areas. Visual cortex activation was significantly different among tasks, but this is likely explained by the extra visual content on the screen during the stimulus-focused task (see Figure 2). For the immediate available versus unavailable contrast, only the stimulus-focused task and IDT could be compared, and activation maps were not significantly different and ROI analyses did not reveal any differences between the tasks (see Supplementary Material). This pattern suggests that individuals completing the IDT recruit similar neural resources when completing the same trial types as each of the two reference tasks. The individual activation maps for these contrasts were not always identical, although significant overlap in the ‘hard’ versus ‘easy’ contrast was observed (Figure 6). However, the lack of statistical significance in the second-level comparisons and similar activation in ROIs identified in Amlung et al. (2014) indicate that these apparent differences were perhaps due to power differences among the tasks, not due to significantly different patterns of activity.
4.2. Similarity of the Individualized Discounting Task Activation Maps to the Published Literature
The second basis on which we evaluated the IDT was in comparison to previous literature. Looking at activation associated with all choice trials grouped together, all three tasks were associated with a pattern of activation characteristic of intertemporal choice that includes activation of the visual cortex, bilateral DLPFC, PMA, SMA, bilateral superior and inferior parietal lobules and bilateral ventrolateral prefrontal cortex (Figure 5; also see McClure et al., 2004; 2007; Wesley & Bickel, 2014). Both the response-focused task and IDT were associated with activation of the medial prefrontal cortex and anterior cingulate during ‘hard’ choices (Table 3). Activation in the anterior cingulate is commonly associated with ‘hard’ trials, including in the original paper for our reference response-focused task (Amlung et al., 2014; Monterosso et al., 2007; Pine et al., 2009; McClure et al., 2007). The IDT was associated with a number of areas during immediate unavailable trials (Table 4). Others, including the original paper for our reference stimulus-focused task, have reported similar activations, albeit often with responding in general (i.e., not restricted to one trial type, see McClure et al., 2004; McClure et al., 2007; Xu et al., 2009; Eppinger et al., 2012; Sripada et al., 2011; Kim et al., 2012). See Supplementary Material for further discussion of ROI analyses.
4.3. Conclusions
The IDT was the only one of the three tested that was able to simultaneously 1) produce activation maps that did not differ from the other two tasks, 2) examine all three commonly discussed contrasts in all participants, and 3) resolve each participant’s discounting rate in-scanner. For these reasons, we consider this task an improved method for studying the neural correlates of intertemporal choice behavior.
Supplementary Material
Acknowledgments
Funding: This research was supported by National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health grant R01 AA021529 to Warren K. Bickel and Stephen M. LaConte. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht K, Volz KG, Sutter M, Laibson DI, Von Cramon DY. What is for me is not for you: brain correlates of intertemporal choice for self and other. Social cognitive and affective neuroscience. 2010:nsq046. doi: 10.1093/scan/nsq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- Amlung M, Sweet LH, Acker J, Brown CL, MacKillop J. Dissociable brain signatures of choice conflict and immediate reward preferences in alcohol use disorders. Addiction biology. 2014;19(4):743–753. doi: 10.1111/adb.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. Steep Delay Discounting and Addictive Behavior: A Meta-Analysis of Continuous Associations. Addiction. 2016 doi: 10.1111/add.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45(1):143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacology & therapeutics. 2012;134(3):287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral-and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76:518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJ. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. The Journal of Neuroscience. 2009;29(27):8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Kelley EA, Mitchell JM, D'esposito M, Fields HL. Now or Later? An fMRI study of the effects of endogenous opioid blockade on a decision-making network. Pharmacology Biochemistry and Behavior. 2009;93(3):291–299. doi: 10.1016/j.pbb.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158Val/Val genotype. The Journal of Neuroscience. 2007;27(52):14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Connectivity strength of dissociable striatal tracts predict individual differences in temporal discounting. The Journal of Neuroscience. 2014;34(31):10298–10310. doi: 10.1523/JNEUROSCI.4105-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisi CO, Chantiluke K, Norman L, Christakou A, Barrett N, Giampietro V, … Rubia K. The effects of acute fluoxetine administration on temporal discounting in youth with ADHD. Psychological Medicine. 2015;46(06):1197–1209. doi: 10.1017/s0033291715002731. [DOI] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Nath AR, Beauchamp MS, Cox WR. FMRI group analysis combining effect estimates ant their variances. NeuroImage. 2012;60(1):767–765. doi: 10.1016/j.neuroimage.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantiluke K, Christakou A, Murphy CM, Giampietro V, Daly EM, Ecker C, … Rubia K. Disorder-specific functional abnormalities during temporal discounting in youth with Attention Deficit Hyperactivity Disorder (ADHD), Autism and comorbid ADHD and Autism. Psychiatry Research: Neuroimaging. 2014;223(2):113–120. doi: 10.1016/j.pscychresns.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54(2):1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Clewett D, Luo S, Hsu E, Ainslie G, Mather M, Monterosso J. Increased functional coupling between the left fronto-parietal network and anterior insula predicts steeper delay discounting in smokers. Human brain mapping. 2014;35(8):3774–3787. doi: 10.1002/hbm.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: What a long strange trip it’s been. NeuroImage. 2012;62(2):743–747. doi: 10.1016/j.neuroimage.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JH, Figner B, Steinglass JE. On Weight and Waiting: Delay Discounting in Anorexia Nervosa Pretreatment and Posttreatment. Biological Psychiatry. 2015;78(9):606–614. doi: 10.1016/j.biopsych.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Green L, Myerson J. Cross-cultural comparisons of discounting delayed and probabilistic rewards. The Psychological Record. 2002;52(4):479. doi: 10.1037/0033-2909.130.5.769. [DOI] [Google Scholar]
- Elton A, Smith CT, Parrish MH, Boettiger CA. Neural systems underlying individual differences in intertemporal decision-making. Journal of Cognitive Neuroscience. 2017;29(3):467–479. doi: 10.1162/jocn_a_01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Nystrom LE, Cohen JD. Reduced sensitivity to immediate reward during decision-making in older than younger adults. PloS one. 2012;7(5):e36953. doi: 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersner-Hershfield H, Wimmer GE, Knutson B. Saving for the future self: Neural measures of future self-continuity predict temporal discounting. Social Cognitive and Affective Neuroscience. 2009;4(1):85–92. doi: 10.1093/scan/nsn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Houde S, Silver-Balbus S, Ballard K, Kim B, Rutledge KJ, … McClure SM. The decimal effect: behavioral and neural bases for a novel influence on intertemporal choice in healthy individuals and in ADHD. Journal of Cognitive Neuroscience. 2014;26(11):2455–2468. doi: 10.1162/jocn_a_00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Hakimi S, Rangel A. Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Frontiers in Neuroscience. 2014;8:50. doi: 10.3389/fnins.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinvest NS, Elliott R, McKie S, Anderson IM. Neural correlates of choice behavior related to impulsivity and venturesomeness. Neuropsychologia. 2011;49(9):2311–2320. doi: 10.1016/j.neuropsychologia.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201(2):183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Kleinschmidt H, Martin JA, Han Y, Thelen M, Meiberth D, … Weber B. A Reduction in Delay Discounting by Using Episodic Future Imagination and the Association with Episodic Memory Capacity. Frontiers in Human Neuroscience. 2017:10. doi: 10.3389/fnhum.2016.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within3subject comparison of real and hypothetical money rewards in delay discounting. Journal of the experimental analysis of behavior. 2002;77(2):129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature neuroscience. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. An “as soon as possible” effect in human intertemporal decision making: behavioral evidence and neural mechanisms. Journal of Neurophysiology. 2010;103(5):2513–2531. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Sung YS, McClure SM. The neural basis of cultural differences in delay discounting. Phil Trans R Soc B. 2012;367(1589):650–656. doi: 10.1098/rstb.2011.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Geisler D, Bernardoni F, Ritschel F, Böhm I, Seidel M, … Ehrlich S. Altered Neural Efficiency of Decision Making During Temporal Reward Discounting in Anorexia Nervosa. Journal of the American Academy of Child & Adolescent Psychiatry. 2016;55(11):972–979. doi: 10.1016/j.jaac.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW, Weller RE. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 2012;58(2):582–592. doi: 10.1016/j.appet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Kobiella A, Ripke S, Kroemer NB, Vollmert C, Vollstädt-Klein S, Ulshöfer DE, Smolka MN. Acute and chronic nicotine effects on behaviour and brain activation during intertemporal decision making. Addiction biology. 2014;19(5):918–930. doi: 10.1111/adb.12057. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Bickel WK. A 5-trial adjusting delay discounting task: Accurate discount rates in less than one minute. Experimental and Clinical Psychopharmacology. 2014;22:222–228. doi: 10.1037/a0035973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: steady-state assessments, forced-choice trials, and all real rewards. Behavioural processes. 2005;69(2):173–187. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lawyer SR, Schoepflin F, Green R, Jenks C. Discounting of hypothetical and potentially real outcomes in nicotine-dependent and nondependent samples. Experimental and clinical psychopharmacology. 2011;19(4):263. doi: 10.1037/a0024141. [DOI] [PubMed] [Google Scholar]
- Li N, Ma N, Liu Y, He XS, Sun DL, Fu XM, … Zhang DR. Resting-state functional connectivity predicts impulsivity in economic decision-making. The Journal of Neuroscience. 2013;33(11):4886–4895. doi: 10.1523/JNEUROSCI.1342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Feng T, Wang J, Li H. The neural dissociation of subjective valuation from choice processes in intertemporal choice. Behavioural brain research. 2012;231(1):40–47. doi: 10.1016/j.bbr.2012.02.045. [DOI] [PubMed] [Google Scholar]
- Luhmann CC, Chun MM, Yi DJ, Lee D, Wang XJ. Neural dissociation of delay and uncertainty in intertemporal choice. The Journal of Neuroscience. 2008;28(53):14459–14466. doi: 10.1523/JNEUROSCI.5058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Ainslie G, Monterosso J. The behavioral and neural effect of emotional primes on intertemporal decisions. Social cognitive and affective neuroscience. 2014;9(3):283–291. doi: 10.1093/scan/nss132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Ainslie G, Pollini D, Giragosian L, Monterosso JR. Moderators of the association between brain activation and farsighted choice. Neuroimage. 2012;59(2):1469–1477. doi: 10.1016/j.neuroimage.2011.08.004. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216(3):305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Wier LM, David SP, Ray LA, Bickel WK, Sweet LH. The neuroeconomics of nicotine dependence: A preliminary functional magnetic resonance imaging study of delay discounting of monetary and cigarette rewards in smokers. Psychiatry Research: Neuroimaging. 2012;202(1):20–29. doi: 10.1016/j.pscychresns.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Experimental and Clinical Psychopharmacology. 2003;11(2):139. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Raiff BR, Lagorio CH, Begotka AM, Mueller AM, Hehli DJ, Wegener AA. Delay discounting of potentially real and hypothetical rewards: II. Between-and within-subject comparisons. Experimental and Clinical Psychopharmacology. 2004;12(4):251. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- Manning J, Hedden T, Wickens N, Whitfield-Gabrieli S, Prelec D, Gabrieli JD. Personality influences temporal discounting preferences: Behavioral and brain evidence. NeuroImage. 2014;98:42–49. doi: 10.1016/j.neuroimage.2014.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Pallarés J, Mohammadi B, Samii A, Münte TF. Brain activations reflect individual discount rates in intertemporal choice. Brain Research. 2010;1320:123–129. doi: 10.1016/j.brainres.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Martin LE, Pollack L, McCune A, Schulte E, Savage CR, Lundgren JD. Comparison of obese adults with poor versus good sleep quality during a functional neuroimaging delay discounting task: A pilot study. Psychiatry Research: Neuroimaging. 2015;234(1):90–95. doi: 10.1016/j.pscychresns.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrogiorgou P, Enzi B, Klimm A-K, Köhler E, Roser P, Norra C, Juckel G. Serotonergic modulation of orbitofrontal activity and its relevance for decision making and impulsivity. Human Brain Mapping. 2016 doi: 10.1002/hbm.23468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analysis of behavior (vol 5) the effect of delay and of intervening events on reinforcement value. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. The Journal of Neuroscience. 2007;27(21):5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Meade CS, Cordero DM, Hobkirk AL, Metra BM, Chen NK, Huettel SA. Compensatory activation in fronto-parietal cortices among HIV-infected persons during a monetary decision-making task. Human Brain Mapping. 2016;37(7):2455–2467. doi: 10.1002/hbm.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Lowen SB, MacLean RR, Key MD, Lukas SE. fMRI brain activation during a delay discounting task in HIV-positive adults with and without cocaine dependence. Psychiatry Research: Neuroimaging. 2011;192(3):167–175. doi: 10.1016/j.pscychresns.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellis AM, Woodford AE, Stein JS, Bickel WK. A second type of magnitude effect: Reinforcer magnitude differentiates delay discounting between substance users and controls. Journal of the experimental analysis of behavior. 2017;107(1):151–160. doi: 10.1002/jeab.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedl SF, Peters J, Büchel C. Altered neural reward representations in pathological gamblers revealed by delay and probability discounting. Archives of General Psychiatry. 2012;69(2):177–186. doi: 10.1001/archgenpsychiatry.2011.1552. [DOI] [PubMed] [Google Scholar]
- Miedl SF, Wiswede D, Marco-Pallarés J, Ye Z, Fehr T, Herrmann M, Münte TF. The neural basis of impulsive discounting in pathological gamblers. Brain Imaging and Behavior. 2015;9(4):887–898. doi: 10.1007/s11682-015-9352-1. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Human brain mapping. 2007;28(5):383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Kunisato Y, Aoyama S, Shishida K, Okada G, … Yamawaki S. Inter-individual discount factor differences in reward prediction are topographically associated with caudate activation. Experimental brain research. 2011;212(4):593–601. doi: 10.1007/s00221-011-2771-3. [DOI] [PubMed] [Google Scholar]
- Ortiz N, Parsons A, Whelan R, Brennan K, Agan ML, O’Connell R, … Garavan H. Decreased frontal, striatal and cerebellar activation in adults with ADHD during an adaptive delay discounting task. Acta Neurobiol Exp. 2015;75:326–338. [PubMed] [Google Scholar]
- Peters J, Büchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. The Journal of Neuroscience. 2009;29(50):15727–15734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66(1):138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Pine A, Seymour B, Roiser JP, Bossaerts P, Friston KJ, Curran HV, Dolan RJ. Encoding of marginal utility across time in the human brain. The Journal of Neuroscience. 2009;29(30):9575–9581. doi: 10.1523/JNEUROSCI.1126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. The Journal of Neuroscience. 2010;30(26):8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Hübner T, Mennigen E, Müller KU, Li SC, Smolka MN. Common neural correlates of intertemporal choices and intelligence in adolescents. Journal of cognitive neuroscience. 2014 doi: 10.1162/jocn_a_00698. [DOI] [PubMed] [Google Scholar]
- Ripke S, Hübner T, Mennigen E, Müller KU, Rodehacke S, Schmidt D, … Smolka MN. Reward processing and intertemporal decision making in adults and adolescents: the role of impulsivity and decision consistency. Brain research. 2012;1478:36–47. doi: 10.1016/j.brainres.2012.08.034. [DOI] [PubMed] [Google Scholar]
- Ripke S, Hübner T, Mennigen E, Müller KU, Li SC, Smolka MN. Common Neural Correlates of Intertemporal Choices and Intelligence in Adolescents. Journal of Cognitive Neuroscience. 2015;27(2):387–399. doi: 10.1162/jocn_a_00698. [DOI] [PubMed] [Google Scholar]
- Rodriguez CA, Turner BM, Van Zandt T, McClure SM. The neural basis of value accumulation in intertemporal choice. European Journal of Neuroscience. 2015;42(5):2179–2189. doi: 10.1111/ejn.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Mata R, Radu PT, Ballard IC, Carstensen LL, McClure SM. Age differences in striatal delay sensitivity during intertemporal choice in healthy adults. Frontiers in Neuroscience. 2011;16(2011):5. doi: 10.3389/fnins.2011.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse LK, Peters J, Büchel C, Brassen S. Effects of prospective thinking on intertemporal choice: The role of familiarity. Human Brain Mapping. 2015;36(10):4210–4221. doi: 10.1002/hbm.22912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Goudriaan AE, Joos L, Dom G, Pattij T, van den Brink W, Veltman DJ. Neural substrates of impulsive decision making modulated by modafinil in alcohol-dependent patients. Psychological Medicine. 2014;44(13):2787–2798. doi: 10.1017/s0033291714000312. [DOI] [PubMed] [Google Scholar]
- Schneider S, Peters J, Peth JM, Büchel C. Parental inconsistency, impulsive choice and neural value representations in healthy adolescents. Translational psychiatry. 2014;4(4):e382. doi: 10.1038/tp.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto M, Ciaramelli E, Mattioli F, di Pellegrino G. Reduced Sensitivity to Sooner Reward During Intertemporal Decision-Making Following Insula Damage in Humans. Frontiers in Behavioral Neuroscience. 2016;9 doi: 10.3389/fnbeh.2015.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JH, Kim HE, Sohn S, Seok JW, Choi D, Watanuki S. Effect of emotional arousal on inter-temporal decision-making: an fMRI study. Journal of Physiological Anthropology. 2015;34(1):8. doi: 10.1186/s40101-015-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Gonzalez R, Luan Phan K, Liberzon I. The neural correlates of intertemporal decision-making: Contributions of subjective value, stimulus type, and trait impulsivity. Human brain mapping. 2011;32(10):1637–1648. doi: 10.1002/hbm.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Elton A, Ryan SR, James GA, Budney AJ, Kilts CD. Neuroeconomics and adolescent substance abuse: individual differences in neural networks and delay discounting. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(7):747–755. doi: 10.1016/j.jaac.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeis N, Haushofer J, Fehr E, Singer T. Development of Behavioral Control and Associated vmPFC–DLPFC Connectivity Explains Children’s Increased Resistance to Temptation in Intertemporal Choice. Cerebral Cortex. 2014;26(1):32–42. doi: 10.1093/cercor/bhu167. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Murdaugh DL, Cox JE, Cook EW, III, Weller RE. Greater impulsivity is associated with decreased brain activation in obese women during a delay discounting task. Brain imaging and behavior. 2013;7(2):116–128. doi: 10.1007/s11682-012-9201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EM, Murphy A, Boyapati V, Ersche KD, Flechais R, … Elliott R. Impulsivity in abstinent alcohol and polydrug dependence: a multidimensional approach. Psychopharmacology. 2016;233(8):1487–1499. doi: 10.1007/s00213-016-4245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov PM, Szanto K, Hallquist MN, Siegle GJ, Reynolds CF, Forman SD, … Dombrovski AY. Paralimbic and lateral prefrontal encoding of reward value during intertemporal choice in attempted suicide. Psychological Medicine. 2015;46(02):381–391. doi: 10.1017/s0033291715001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Luo S, Monterosso J, Zhang J, Fang X, Dong Q, Xue G. Distributed value representation in the medial prefrontal cortex during intertemporal choices. The Journal of Neuroscience. 2014;34(22):7522–7530. doi: 10.1523/JNEUROSCI.0351-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu L, Wang L, Zhang Y, Du X, Dong G. Impaired decision3 making and impulse control in Internet gaming addicts: evidence from the comparison with recreational Internet game users. Addiction biology. 2016a doi: 10.1111/adb.12458. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu L, Zhou H, Lin X, Zhang Y, Du X, Dong G. Impaired executive control and reward circuit in Internet gaming addicts under a delay discounting task: independent component analysis. European Archives of Psychiatry and Clinical Neuroscience. 2016b doi: 10.1007/s00406-016-0721-6. [DOI] [PubMed] [Google Scholar]
- Weber BJ, Huettel SA. The neural substrates of probabilistic and intertemporal decision making. Brain research. 2008;1234:104–115. doi: 10.1016/j.brainres.2008.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biological psychiatry. 2014;75(6):435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Experimental Brain Research. 2007;179(4):643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Lovero KL, Lane SD, Paulus MP. Now or later? Striatum and insula activation to immediate versus delayed rewards. Journal of neuroscience, psychology, and economics. 2010;3(1):15. doi: 10.1037/a0017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Liang ZY, Wang K, Li S, Jiang T. Neural mechanism of intertemporal choice: from discounting future gains to future losses. Brain research. 2009;1261:65–74. doi: 10.1016/j.brainres.2008.12.061. [DOI] [PubMed] [Google Scholar]
- Yu P, Chen X, Zhao W, Zhang Z, Zhang Q, Han B, … Chen C. Effect of rs1063843 in theCAMKK2gene on the dorsolateral prefrontal cortex. Human Brain Mapping. 2016;37(7):2398–2406. doi: 10.1002/hbm.23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.