Abstract
Background
While disruption of acid-base homeostasis has been pathoetiologically implicated in panic disorder (PD), the mechanism by which pH imbalance is translated to panic pathophysiology is poorly understood. Recently, in a translational rodent model of PD, we reported a role of microglial acid sensing Gprotein coupled receptor, T cell death associated gene-8 (TDAG8) in panic-associated behavior and physiology. However, the clinical validity of the TDAG8 receptor has not been investigated.
Objective
To assess TDAG8 in PD, we evaluated TDAG8 receptor expression in adolescents and young adults with PD and healthy comparison subjects.
Methods
Relative expression of TDAG8 mRNA was determined in peripheral blood mononuclear cells from patients with PD, and compared to expression in healthy subjects. Linear models were utilized to evaluate the relationship between TDAG8 expression and panic disorder symptom severity scale (PDSS) score as well as other potential explanatory variables (e.g., CRP, body mass index, sex, age). Models were refined based on the estimated parameter significance, evidence of omitted variable bias and Bayesian/Akaike information criteria.
Results
Relative to healthy comparison subjects (n=17), expression of TDAG8 mRNA was significantly increased in patients with PD (n=15) (1.60±0.65 vs. 1.01±0.50, p=0.008). TDAG8 mRNA expression predicted PD symptom severity in a fixed effect model incorporating age and sex (p=0.003).
Conclusions
Collectively, our results suggest greater TDAG8 expression in patients with PD compared to healthy subjects and directly link TDAG8 expression and the severity of the PD symptoms. Further investigation of the TDAG8 receptor in panic pathophysiology is warranted.
Keywords: pH, panic, TDAG8, expression, PDSS, SSRI, treatment
1. Introduction
Panic disorder (PD), a syndrome characterized by spontaneous and recurrent episodes of incapacitating anxiety and physiologic hyperarousal, affects approximately 5% of Americans (Kessler et al., 2005) and typically emerges during adolescence or early adulthood (Beesdo et al., 2010). PD is second only to major depressive disorder in terms of debility among psychiatric conditions in the United States (Gadermann et al., 2012) and increases the risk of suicidality (Nepon et al., 2010) and represents a risk factor for the development of other anxiety disorders and secondary mood disorders (Bittner et al., 2004).
Despite significant progress, panic pathophysiology is not well understood. There is significant evidence from clinical studies, that homeostatic triggers such as acidosis may be associated with panic disorder (see Vollmer et al., 2015; Wemmie, 2011). Administration of CO2 (Rassovsky and Kushner, 2003) or intravenous lactate (Pitts and Mcclure, 1967) to patients with PD precipitates panic attacks, supporting acid-base homeostasis in the pathoetiology of PD. Additionally, neuroimaging studies in patients with PD suggest dysregulated acid-base buffering (Friedman et al., 2006) and increased plasma and brain lactate responses to metabolic challenges (Dager et al., 1997; Maddock et al., 2008). Collectively, these observations support the relevance of homeostatic acid base regulatory systems in panic pathophysiology.
Recent preclinical studies from our group suggest a potential relevance of acid-sensing T cell death associated gene-8 (TDAG8) receptor in panic disorder. TDAG8, a G protein coupled receptor, regulated fear and cardiovascular responses to CO2 inhalation, a clinical panicogen (Vollmer et al., 2016). Accordingly, TDAG8 deficient mice elicited attenuated CO2-evoked responses, suggesting recruitment of the receptor in translating acidosis to panic relevant responses. Interestingly, we localized selective TDAG8 expression in microglia (Vollmer et al., 2016), innate immune cells in the brain that are recruited in physiological responses to homeostatic fluctuations (Kettenmann et al., 2011). This is consistent with an immune-enriched expression profile of TDAG8 reported in earlier studies (Choi et al., 1996; Kyaw et al., 1998). TDAG8-dependent microglial activation and inflammatory responses were required for CO2-evoked fear (Vollmer et al., 2016), suggesting that TDAG8-associated innate immune mechanisms may be relevant to panic pathophysiology. Our observations, although novel and intriguing have not been validated in the clinic and the relevance of TDAG8 as a pathophysiological target in PD remains to be established.
With these considerations in mind, the current study sought to evaluate expression of the TDAG8 receptor, in peripheral blood mononuclear cells (PBMCs) collected from a cohort of adolescent and young adult patients with PD and healthy comparison subjects. Adolescents and young adults were specifically recruited to facilitate the exploration of TDAG8 expression in patients close to the onset of PD and to address any age-related bias or developmental changes that could affect study findings. Our objectives were to establish whether TDAG8 expression was different between the two groups and from an exploratory standpoint, we sought to characterize the association of TDAG8 expression with PD symptom severity and differential treatment response. Based on our preclinical data we hypothesized elevated expression of the TDAG8 receptor in individuals with PD (compared to healthy subjects) and its association with panic symptom severity.
2. Materials and methods
2.1 Participants
Participants included 15 individuals, aged 15.7–44 years of age (mean age: 21.7±8.8 years) with a DSM-5 (American Psychiatric Association, 2013) diagnosis of PD and 17 healthy controls aged 18–44 years (mean age: 27.7±8.6 years). Patients and controls were recruited via referrals from the outpatient clinics of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center as well as from ongoing longitudinal and treatment studies within the UC Anxiety Disorders Research Program. Healthy comparison subjects were recruited by word-of-mouth and from ongoing longitudinal studies within the Department of Psychiatry. An honorarium was provided to patients and healthy control subjects. Study participants were administered the MINI or MINI-KID (Kaufman et al., 1997) by a psychiatrist who is board-certified in general and child & adolescent psychiatry (JRS). Additionally, all participants were evaluated by a physician and a complete medical history and medical review of systems was obtained. Patients or healthy comparison subjects with a past medical history of inflammatory disease (e.g., rheumatoid arthritis, inflammatory bowel disease, chronic obstructive pulmonary disease [COPD]) or those with acute infections were excluded and patients could not have taken a non-steroidal anti-inflammatory medication or systemic corticosteroids within 5 days of participation.
2.2 Measures
Anxiety symptom severity and PD symptom severity were assessed with the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959) and the Panic Disorder Severity Scale (PDSS) (Furukawa et al., 2009; Shear et al., 1997; Shear et al., 2001), respectively. The Clinical Global Impression Severity Scale (CGI) (Guy, 1976) was used to rate the general severity of PD symptoms. Depressive symptoms were evaluated with the Quick Inventory of Depressive Symptoms (QIDS) and scores were used to evaluate co-occurring depressive symptoms. Exclusionary criteria for patient participants were: an IQ < 70, a lifetime diagnosis of bipolar disorder, schizophrenia, or a pervasive developmental disorder (e.g., autism spectrum disorder), and current diagnosis of major depressive disorder. Healthy comparison subjects were free of lifetime diagnosis of DSM-5 disorders. Legal guardians and participants provided written, informed consent and assent (in the case of participants <18 years of age) and this study was approved by the University of Cincinnati Institutional Review Board.
2.3 Sample Collection, TDAG8 Receptor Expression and C-Reactive Protein Determination
Venous blood for enriched monocyte RNA expression assays was aseptically collected from basilic or cephalic veins into BD Vacutainer® CPT Mononuclear Cell Preparation Tubes (Becton, Dickinson and Company, New Jersey). Nearly all samples (88%) were collected between 8:10 h and 13:00 h, however, 3 samples (9%) were collected between 1300 h and 1500 h and one sample (3%) was obtained at 1600 h. Patients and healthy comparison subjects were not fasted so as to reduce hunger-related stress and so that samples would reflect basal conditions. Samples were immediately centrifuged for 20 minutes at 1500Xg at room temperature; the buffy coat was aspirated, aliquoted and centrifuged to yield a cell pellet. RNA was isolated using TRI Reagent (Molecular Research Center Inc., Cincinnati, OH, USA) and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to manufacturer’s instructions. Real Time Polymerase Chain Reaction (RT-PCR) was utilized to synthesize first-strand cDNA from purified RNA (SuperScript® IV First-Strand Synthesis System for RT-PCR, Invitrogen, Carlsbad, CA, USA). Quantitative real-time PCR was performed using TaqMan Fast Universal Master Mix and 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) as previously described with modifications (Loyd et al., 2016). TaqMan gene expression assays for TDAG8, Hs00269247_s1, and GAPDH, Hs02786624_g1, were used according to manufacturer’s instructions. Relative expression for each TDAG8 was calculated by extrapolation to a standard curve individually run on each plate and derived from serial dilutions of a pooled sample of reference cDNA, and normalized to relative expression of GAPDH. To provide a maker of acute inflammation, C-Reactive Protein (CRP) was determined in all patients and healthy controls using a standard immunometric assay in a CLIA-certified clinical laboratory at the University of Cincinnati Medical Center and assays were performed by certified medical laboratory technologists.
2.4 Statistical Analysis
Statistical analyses were performed using R-Studio/R (R version 3.1.3). In addition to descriptive statistics, χ2 and Welch two sample t-tests were used to compare patients with PD and healthy comparison subjects with regard to demographic and clinical features, measures of symptom severity and TDAG8 mRNA expression. To evaluate the relationship between TDAG8 expression and symptom severity in the total sample and in patients with PD, clinical and demographic variables were incorporated into a multiple regression model. This regression model set was refined, as previously described (Mills and Prasad 1992; Strawn et al. 2017), based on the fit parameters in addition to p-values, the Bayesian Information Criterion (BIC = −2 · lnL̂ +k · ln((n)) and the Akaike’s Information Criterion (AIC= −2 · lnL̂ +2k) where k is the number of regressors, including the intercept, and L is the maximized value of the likelihood function for the model. The models were evaluated for omitted variables bias and for the inclusion of irrelevant variables as further decision criteria in determining the relevance of each explanatory variable. For measures of CRP, values at the detection limit (1 mg/L) were imputed at 0.5 mg/L given the distributional assumption that the true value was bounded by 0 and 1.
3. Results
3.1 Sample Characteristics and Demographics
Of the patients and healthy comparison subjects who were screened, two healthy controls were excluded (one because of a family history of anxiety disorder in 2 first degree relatives and one because of a history of MDD). Additionally, phlebotomy could not be performed in one patient with PD. Of the patients with PD, 6 were recruited from ongoing studies (40%), 3 were recruited from referrals to the study (20%), and 6 were recruited from outpatient clinics (40%). Of the healthy comparison subjects, 4 were recruited from ongoing studies where they were enrolled as healthy controls (23.5%) and 13 were recruited from the local community (76.5%). No group differences were observed for sex, age or race, although, symptom scores were significantly higher in the anxious patients than healthy comparison subjects for both anxiety symptoms inventories (PDSS and HAM-A) and for the QIDS (Table 1).
Table 1.
Demographic and clinical features of patients with panic disorder (PD) and healthy comparison subjects.
| Healthy Controls n=17 |
PD Patients* n=15 |
Significance | |
|---|---|---|---|
| Age (years) | 27.5 ± 8.6 | 21.7 ± 8.8 | p=0.079 |
| Sex (♂) | 10 | 4 | p=0.250 |
| χ2=1.321 | |||
| Race (white) | 15 (79%) | 15 (94%) | p=0.410 |
| χ2=0.678 | |||
| Body mass index (BMI) (kg/m2) | 26.3±5.7 | 30.69±12.5 | p=0.241 |
| Comorbidity | |||
| Generalized Anxiety Disorder | 0 | 12 | |
| Separation Anxiety Disorder | 0 | 0 | |
| Social Anxiety Disorder | 0 | 4 | |
| Agoraphobia | 0 | 7 | |
| Unspecified depressive disorder | 0 | 1 | |
| Persistent depressive disorder | 0 | 0 | |
| ADHD | 0 | 0 | |
| Substance use disorder, in remission | 0 | 1 | |
| History of major depressive disorder | 0 | 2 | |
| PDSS composite score | 0.04 ± 0.10 | 1.56 ± 0.81 | p<0.0001 |
| HAM-A score | 1.24 ± 1.52 | 21.87 ± 10.9 | p<0.0001 |
| QIDS score | 2.5 ± 2.4 | 16.87 ± 7.8 | p<0.0001 |
Among patients with PD who were receiving anxiety-related pharmacotherapy (n=9), treatment consisted of selective serotonin (norepinephrine) reuptake inhibitors (n=4), benzodiazepines (n=6 [one patient treated with an SSRI and an adjunctive benzodiazepine]), soporific medications (zolpidem, n=2) and an adjunctive second generation antipsychotic in one patient ([aripiprazole 2 mg q̄HS] n=1).
3.2 TDAG8 Receptor Expression and CRP
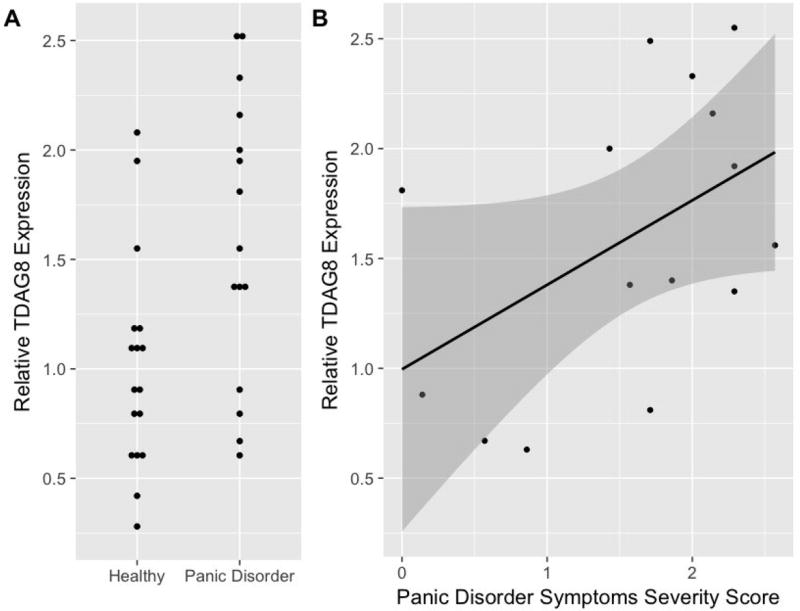
In comparison to healthy subjects, patients with PD exhibited significantly higher expression of TDAG8 mRNA (1.60±0.65 vs. 1.01±0.50, p=0.008, effect size = 0.45, Figure 1). CRP did not differ between patients with PD and healthy comparison subjects (4.85±6.40 mg/L vs. 2.36±2.41 mg/L, p=0.188) and did not correlate with TDAG8 expression in patients with PD (R=-0.074, p=0.853) or in healthy comparison subjects (R=-0.071, p=0.930).
Figure 1. TDAG8 Expression in Patients with Panic Disorder (PD).
Relative TDAG8 expression is increased in patients with PD compared to healthy comparison subjects (p=0.030) (A) and is associated with the severity of PD symptoms ( , F(3,28)=5.97, p=0.003). (B). The dark gray band in panel B depicts the 95% prediction limit for the linear model.
Linear models revealed TDAG8 expression to be strongly associated with PD symptom severity and the most parsimonious model incorporated age and sex as fixed effects. In this model, TDAG8 mRNA expression was strongly associated with PD symptom severity ( , F(3, 27)= 7.32, p<0.001). Alternate models incorporating additional variables (e.g., BMI and CRP) suggested the inclusion of irrelevant variables and were associated with higher AIC and BIC (Supplemental Results) than the most parsimonious model, described above. With regard to age, the two oldest patients in the PD group, were evaluated as outliers, given that their age was >1.5 time the interquartile range for the sample. TDAG8 expression for these patients was within 1 standard deviation of the mean TDAG8 expression in the patients with PD. In patients with PD, the linear model incorporating sex and age, as fixed effects, also revealed a strong association between TDAG8 and PD symptom severity ( , p=0.003) (Figure 1). Finally, as a preliminary analysis (Supplemental Figure 1), in patients with PD who were receiving treatment, a post-hoc analysis of TDAG8 expression and response to treatment suggested TDAG8 expression was lower in remitters (n=4, MeanTDAG8=1.00±0.55, range: 0.63–1.81) compared to non-remitters, based on a PDSS score ≤7 (Furukawa et al., 2009), although this difference only trended toward statistical significance (n=5, MeanTDAG8 =1.83±0.71, range: 0.81–2.49, p=0.08)
4. Discussion
This pilot study—the first to evaluate the T cell death associated gene 8 receptor in patients with PD— reveals significantly increased TDAG8 mRNA expression in mononuclear cells from patients with PD relative to healthy controls, as well as an association with TDAG8 mRNA expression and PD symptom severity in addition to treatment response. As such, our data, although restricted to a small sample, strengthen previous preclinical findings from our group in a rodent model of panic-associated behaviors. Collectively, these observations suggest a role of the TDAG8 receptor in panic physiology and implicate dysregulation of TDAG8 receptor expression in PD. Further, this examination of TDAG8 in a clinical population provides a scaffold for subsequent studies of TDAG8 and associated genetic and inflammatory processes in both lower animal models of the disorder and in the clinic, while contemporaneously supporting the role of acid-sensing mechanisms in the pathophysiology of PD.
The TDAG8 receptor is highly expressed in immune cells of the brain and periphery (Radu et al., 2006; Vollmer et al., 2016). Like other immune cells, microglia can transform rapidly from a resting to a pro-inflammatory activated state upon sensing subtle imbalance in ionic homeostasis (Kettenmann et al., 2011), in accordance with their role in maintenance of CNS microenvironment. Interestingly, microglial TDAG8 receptor is abundant in sensory circumventricular organs (CVOs) such as the subfornical organ (SFO), an area devoid of a blood brain barrier, well known for its role in homeostatic sensing of systemic and CNS milieu. The SFO is well connected to forebrain limbic and brain stem areas regulating emotional, cardiovascular and respiratory responses (Benarroch, 2011) Thus, TDAG8 acid sensing represents a unique mechanism whereby an interoceptive acid-base fluctuations can be translated to behavioral and physiological fear responses via microglia.
Currently, cellular and regional expression of TDAG8 in human brain has not been characterized. For this pilot study we used peripheral blood mononuclear cells as a surrogate tissue for measuring TDAG8 expression. Although, this cannot be directly extrapolated to the brain, our data suggest that alterations in TDAG8 expression (and potentially function) may exist in innate immune cells of patients with PD. Our data also raise the possibility of altered inflammatory tone in PD. There have been limited studies in this regard. In our sample, concentrations of the acute phase reactant, CRP did not significantly differ between healthy subjects and patients with PD subjects. However, broad spectrum of cytokine abnormalities in other samples of patients with PD have been observed (Hoge et al., 2009), including elevated IL-1b (Brambilla et al., 1994) and IL-6 (Belem da Silva et al., 2017) concentrations. The contribution of inflammatory mechanisms in PD needs further investigation in a larger study.
The relationship between TDAG8 expression and the severity of PD symptoms in patients with PD is intriguing, and of interest for several reasons. First, this finding is consistent with neuroimaging studies that suggest that T1 relaxation in the rotating frame (T1ρ), a marker of pH sensitivity (Magnotta et al., 2012), is increased in patients with PD and positively correlates with the severity of anxiety symptoms (Magnotta et al., 2014). Second, a recent clinical trial involving patients with PD (N=37) and utilizing biofeedback with capnometry suggested that when patients increased end tidal pCO2, panic attack symptoms attenuated (Meuret et al., 2008). Third, our observation would be consistent with: (1) normalization of blood gas CO2 and indirect measures of acid base balance is following successful treatment in patients with PD (Gorman et al., 1985) and (2) blockade of CO2 induced panic attacks in patients with PD by successful psychopharmacologic treatment (Bellodi et al., 1998).
While our study adds to the developing literature concerning acid-sensing mechanisms in PD and represents the first examination of TDAG8 in a clinical sample of patients with PD, there are several important limitations. First, this pilot study only assessed TDAG8 mRNA expression in peripheral mononuclear cells and did so in a small sample of patients; thus, there are inherent concerns related to generalizability of the findings. Second, while we controlled for age and sex in our model of the relationship between PD symptoms and relative TDAG8 expression and explored the effects of CRP and BMI, other factors or interactions among factors that may be relevant (e.g., genetic loading, chronicity of illness, influence of menstrual-related hormonal changes, prior history of depressive disorders, proximity to the most recent panic attack) could not be resolved in these analyses. Further, heterogeneity in terms of prior diagnoses within clinical samples is an important consideration. For example, one patient had a history of a substance use disorder in remission and while he had been abstinent from marijuana for >6 months, we cannot exclude that this history affected TDAG8 physiology. However, removal of this patient’s data did not affect the relationships between TDAG8 and PDSS described herein. Future studies including within subject longitudinal measurements and TDAG8 expression in proximity to panic attacks will be required to clarify whether TDAG8 expression represents a state versus trait marker. Third, depressive and anxiety symptoms syndromically overlap (Calkins et al., 2015), as suggested by the QIDS scores in the patients with PD and thus the degree to which the QIDS scores reflect this syndromic overlap versus mild depressive symptoms is difficult to explore in such a small sample. Thus, it is possible that mild depressive symptoms may have moderated the relationship between TDAG8 and other clinical and demographic variables. Fourth, in this pilot study, we have not evaluated the potential genetic factors that may have contributed to differences in TDAG8 mRNA expression. Fifth, although our data may have functional implications we did not assess functional outcomes of increased TDAG8 expression such as pro-inflammatory cytokines. Additionally, while a statistically significant difference in CRP (representative of general systemic inflammation) was not observed in our sample, we cannot rule out the possibility that this reflects type II error, particularly in light of our small sample size. Finally, although most patients were treatment-seeking or in treatment, we cannot exclude the possibility that there may have been selection bias in the sample.
Taken together, these preliminary findings (1) extend a translational model of TDAG8 in mice (Vollmer et al., 2016) to a clinical sample, (2) directly link increased TDAG8 expression with the severity of the panic symptoms and (3) raise the possibility that TDAG8 expression may reflect symptomatic remission status in treated patients with PD. These results provide a preliminary foundation for future studies aimed at understanding the functional relevance of TDAG8 and associated inflammatory processes as well as other acid sensors in patients with PD and compel further exploration of the role of TDAG8 in predicting treatment response. Additionally, these findings of elevated TDAG8 expression, even in young patients close to the onset of their illness, set the stage for a host of complimentary genetic and neuroimaging studies in patients at high risk for development of PD, such as patients with a family history of anxiety disorders (including PD), or youth with psychological risk factors for the development of PD. As such, if the TDAG8-associated abnormalities described herein are present before the onset of illness, they might serve as predictors for the development of PD as well as and thus may inform primary prevention strategies for these conditions. Such neurobiologically-informed prevention strategies could not only reduce the morbidity associated with PD in adolescents and young adults, but could circumvent the development of secondary disorders (e.g., major depressive disorder) and their sequelae (e.g., suicidality).
Supplementary Material
Highlights.
TDAG8 expression is increased in patients with PD compared to healthy subjects.
TDAG8 expression, in PD, is linked to symptom severity.
Data suggest a potential role of TDAG8 in PD pathophysiology
Acknowledgments
The authors would like to acknowledge support from National Institute of Mental Health (NIMH) grants MH106037 (JRS) and MH093362 (RS) and VA Merit grant 2I01BX001075-04 (RS) in addition to a Pilot Translational Research Program (PTRP) grant from the Department of Psychiatry & Behavioral Neuroscience (JRS, RS). LLV and KJM acknowledge support from NIH postdoctoral training grant T32DK059803. The authors thank Drs. James Herman, Robert McCullumsmith and Melissa DelBello for their thoughtful comments and suggestions.
Disclosures: Dr. Strawn has received research support from the National Institutes of Health, Eli Lilly, Edgemont, Forest Research Laboratories/Allergan, Lundbeck, Neuronetics, and Shire. He has received material support from Assurex/Genesight and receives royalties, from Springer Publishing, for the publication of two textbooks. Dr. Sah has received research support from the National Institutes of Health and the Veterans Affair Central Office (VACO). The other authors report no biomedical conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
References
- American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Press; Washington, DC: 2013. [Google Scholar]
- Belem da Silva CT, Costa M de A, Bortoluzzi A, Pfaffenseller B, Vedana F, Kapczinski F, Manfro GG. Cytokine Levels in Panic Disorder: Evidence for a Dose-Response Relationship. Psychosom. Med. 2017;79:126–132. doi: 10.1097/PSY.0000000000000384. [DOI] [PubMed] [Google Scholar]
- Bellodi L, Perna G, Caldirola D, Arancio C, Bertani A, Di Bella D. CO2-induced panic attacks: A twin study. Am. J. Psychiatry. 1998;155:1184–1188. doi: 10.1176/ajp.155.9.1184. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Circumventricular organs: Receptive and homeostatic functions and clinical implications. Neurology. 2011;77:1198–1204. doi: 10.1212/WNL.0b013e31822f04a0. [DOI] [PubMed] [Google Scholar]
- Bittner A, Goodwin RD, Wittchen H-U, Beesdo K, Höfler M, Lieb R. What characteristics of primary anxiety disorders predict subsequent major depressive disorder? J. Clin. Psychiatry. 2004;65:618–26. doi: 10.4088/jcp.v65n0505. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G, Bertani A, Panerai A, Sacerdote P. Plasma interleukin-1 beta concentrations in panic disorder. Psychiatry Res. 1994;54:135–142. doi: 10.1016/0165-1781(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Choi JW, Lee SY, Choi Y. Identification of a putative G protein-coupled receptor induced during activation-induced apoptosis of T cells. Cell. Immunol. 1996;168:78–84. doi: 10.1006/cimm.1996.0051. [DOI] [PubMed] [Google Scholar]
- Dager SR, Richards T, Strauss W, Artru A. Single voxel 1H-MRS investigation of brain metabolic changes during lactate-induced panic. Psychiatry Res. Neuroimaging. 1997;76:89–99. doi: 10.1016/s0925-4927(97)00066-8. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Mathis CM, Hayes C, Renshaw P, Dager SR. Brain pH response to hyperventilation in panic disorder: Preliminary evidence for altered acid-base regulation. Am. J. Psychiatry. 2006;163:710–715. doi: 10.1176/ajp.2006.163.4.710. [DOI] [PubMed] [Google Scholar]
- Furukawa TA, Shear MK, Barlow DH, Gorman JM, Woods SW, Money R, Etschel E, Engel RR, Leucht S. Evidence-based guidelines for interpretation of the panic disorder severity scale. Depress. Anxiety. 2009;26:922–929. doi: 10.1002/da.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadermann AM, Alonso J, Vilagut G, Zaslavsky AM, Kessler RC. Comorbidity and disease burden in the National Comorbidity Survey Replication (NCS-R) Depress. Anxiety. 2012;29:797–806. doi: 10.1002/da.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Fyer AJ, Ross DC, Cohen BS, Martinez JM, Liebowitz MR, Klein DF. Normalization of venous pH, pCO2, and bicarbonate levels after blockade of panic attacks. Psychiatry Res. 1985;14:57–65. doi: 10.1016/0165-1781(85)90089-7. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual. 1976. CGI Clinical Global Impressions; pp. 217–222. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Katherine Shear M, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, Gorman JM, Papp LA. Multicenter collaborative panic disorder severity scale. Am. J. Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions’ of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kyaw H, Zeng Z, Su K, Fan P, Shell BK, Carter KC, Li Y. Cloning, characterization, and mapping of human homolog of mouse T-cell death-associated gene. DNA Cell Biol. 1998;17:493–500. doi: 10.1089/dna.1998.17.493. [DOI] [PubMed] [Google Scholar]
- Loyd C, Magrisso IJ, Haas M, Balusu S, Krishna R, Itoh N, Sandoval DA, Perez-Tilve D, Obici S, Habegger KM. Fibroblast growth factor 21 is required for beneficial effects of exercise during chronic high-fat feeding. J. Appl. Physiol. 2016;121:687–698. doi: 10.1152/japplphysiol.00456.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH, Copeland LE, Richards aL. Elevated brain lactate responses to neural activation in panic disorder: A dynamic 1H-MRS study. Mol Psychiatry. 2008;14:537–45. doi: 10.1038/sj.mp.4002137. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Heo H-YY, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ, Wemmie JA. Detecting activity-evoked pH changes in human brain. Proc. Natl. Acad. Sci. U.S.A. 2012;109:8270–3. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotta VA, Johnson CP, Follmer R, Wemmie JA. Functional T1?? imaging in panic disorder. Biol. Psychiatry. 2014;75:884–891. doi: 10.1016/j.biopsych.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. J. Psychiatr. Res. 2008;42:560–568. doi: 10.1016/j.jpsychires.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepon J, Belik SL, Bolton J, Sareen J. The Relationship Between Anxiety Disorders and Suicide Attempts: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Depress. Anxiety. 2010;27:791–798. doi: 10.1002/da.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts FNJ, Mcclure JNJ. Lactate metabolism in anxiety neurosis. N. Engl. J. Med. 1967;277:1336. doi: 10.1056/NEJM196712212772502. [DOI] [PubMed] [Google Scholar]
- Radu CG, Cheng D, Nijagal A, Riedinger M, McLaughlin J, Yang LV, Johnson J, Witte ON. Normal immune development and glucocorticoid-induced thymocyte apoptosis in mice deficient for the T-cell death-associated gene 8 receptor. Mol. Cell. Biol. 2006;26:668–77. doi: 10.1128/MCB.26.2.668-677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y, Kushner MG. Carbon dioxide in the study of panic disorder: Issues of definition, methodology, and outcome. J. Anxiety Disord. 2003 doi: 10.1016/s0887-6185(02)00181-0. [DOI] [PubMed] [Google Scholar]
- Shear MK, Rucci P, Williams J, Frank E, Grochocinski V, Vander Bilt J, Houck P, Wang T. Reliability and validity of the Panic Disorder Severity Scale: replication and extension. J. Psychiatr. Res. 35:293–6. doi: 10.1016/s0022-3956(01)00028-0. [DOI] [PubMed] [Google Scholar]
- Vollmer LL, Ghosal S, McGuire JL, Ahlbrand RL, Li K-Y, Santin JM, Ratliff-Rang CA, Patrone LGA, Rush J, Lewkowich IP, Herman JP, Putnam RW, Sah R. Microglial Acid Sensing Regulates Carbon Dioxide-Evoked Fear. Biol. Psychiatry. 2016;80:541–551. doi: 10.1016/j.biopsych.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer LL, Strawn JR, Sah R. Acid-base dysregulation and chemosensory mechanisms in panic disorder: a translational update. Transl. Psychiatry. 2015;5:e572. doi: 10.1038/tp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA. Neurobiology of panic and pH chemosensation in the brain. Dialogues Clin. Neurosci. 2011;13:475–83. doi: 10.31887/DCNS.2011.13.4/jwemmie. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.