Abstract
The mammalian fetus develops in a largely sterile environment, and direct exposure to a complex microbiota does not occur until birth. We took advantage of this to examine the effect of the microbiota on brain development during the first few days of life. The expression of anti- and pro-inflammatory cytokines, developmental cell death, and microglial colonization in the brain were compared between newborn conventionally colonized mice and mice born in sterile, germ-free (GF) conditions. Expression of the pro-inflammatory cytokines interleukin 1β and tumor necrosis factor α was markedly suppressed in GF newborns. GF mice also had altered cell death, with some regions exhibiting higher rates (paraventricular nucleus of the hypothalamus and the CA1 oriens layer of the hippocampus) and other regions exhibiting no change or lower rates (arcuate nucleus of the hypothalamus) of cell death. Microglial labeling was elevated in GF mice, due to an increase in both microglial cell size and number. The changes in cytokine expression, cell death and microglial labeling were evident on the day of birth, but were absent on embryonic day 18.5, approximately one-half day prior to expected delivery. Taken together, our results suggest that direct exposure to the microbiota at birth influences key neurodevelopmental events and does so within hours. These findings may help to explain some of the behavioral and neurochemical alterations previously seen in adult GF mice.
Keywords: germ-free, activated caspase 3, Iba1, prenatal, neonatal, arcuate nucleus, paraventricular nucleus, CA1 oriens, cytokines
1. Introduction
Birth is an inflammatory event. The onset of labor depends on a state of ‘sterile inflammation,’ marked by a surge of maternal inflammatory cytokines (Golightly et al., 2011; Kobayashi, 2012; Thomson et al., 1999) that may reach the fetus (Dahlgren et al., 2006; Zaretsky et al., 2004) and stimulate brain cytokine expression (Dammann and Leviton, 1997). At parturition the fetus transitions from the relatively sterile environment of the womb to one teeming with microorganisms. Neonatal blood leukocyte populations expand rapidly following birth, likely due to both the stress of birth and sudden antigenic stimulation from maternal and environmental microbes (Marchini et al., 2000; Steinborn et al., 1999; Yektaei-Karin et al., 2007). Whether and how this microbial colonization affects the perinatal brain remains to be determined. However, effects of the microbiota on adult brain physiology and behavior have been reported in a variety of species including humans (Bravo et al., 2011; Clarke et al., 2013; Messaoudi et al., 2011).
One approach to address the question of the effects of the microbiota on perinatal brain development is to examine neonates born into germ-free (GF) conditions, because any role of microbial exposure would be absent in these animals. Neuronal cell death is a major neurodevelopmental event occurring around the time of birth in mice. Roughly 50% of the neurons that are initially produced are eliminated by apoptosis (Oppenheim, 1991). This large-scale pruning of neurons occurs primarily during the first week of life (Ahern et al., 2013) and is crucial for sculpting neuronal circuits. Although the importance of neuronal cell death is widely recognized, some surprisingly basic questions remain, such as what initiates the cell death period, or what accounts for the large regional differences in the magnitude of cell death. We recently found that cell death peaks just after birth in most forebrain regions of C57BL/6 mice (Mosley et al., 2017), suggesting that birth triggers or amplifies cell death.
Microglia are the resident immune cells of the brain and have been causally linked to developmental neuronal cell death (Marín-Teva et al., 2011). Microglia respond to perturbations by initiating an immune response involving the release of pro-inflammatory cytokines such as interleukin (IL) -6, IL-1β and tumor necrosis factor α (TNF-α; Olson and Miller, 2004), and are also activated by peripherally produced cytokines that reach the brain (Chen et al., 2012; Dantzer et al., 2008; Dilger and Johnson, 2008; Godbout et al., 2005; Qin et al., 2007). Microglial number increases during the first few weeks of postnatal life (Crain et al., 2013; Dalmau et al., 2003; Sharaf et al., 2013), and perinatal microglia have a relatively activated morphology and gene expression profile (Christensen et al., 2014; Crain et al., 2013; Lai et al., 2013; Schwarz et al., 2012; Strahan et al., 2017). Microglia may actively promote developmental cell death in the hippocampus and cerebellum (Marín-Teva et al., 2004; Wakselman et al., 2008), but enhance neuronal survival in the cerebral cortex (Arnoux et al., 2014; Ueno et al., 2013).
Adult GF mice have increased microglial numbers and altered microglial morphology (Erny et al., 2015), but whether effects of the microbiota on microglia are present early in life is unknown. Here, we investigated whether the normal exposure to microorganisms that occurs at birth influences cytokine expression, cell death, or microglial colonization of the newborn brain. Compared to conventionally colonized (CC) mice, we found markedly reduced levels of pro-inflammatory cytokine expression in the brains of GF mice on the day of birth and three days later. This was associated with brain-region specific changes in developmental neuronal cell death, and increased microglial density in GF mice. None of these changes were seen in the brains of GF embryos 12 hours prior to expected birth. Together, our results suggest that the microbiota plays an important, region-specific role in brain development, and does so within hours of birth.
2. Methods
2.1. Animals
Swiss Webster mice (GF and CC) were obtained from our breeding program at Georgia State University. GF mice were kept under sterile conditions in a Park Bioservices isolator, as previously described (Chassaing et al., 2015), and CC mice were housed under conventional conditions. Mice were maintained in a 12:12 light dark cycle with ad libitum access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee at Georgia State University and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Brain collection
Breeding pairs were checked twice daily for births. Pups were collected on the day of birth, postnatal day (P) 0, or three days later (P3). Pups were weighed, sexed, rapidly decapitated, and their brains were harvested and bisected with a coronal cut rostral to the cerebellum. The forebrain was fixed in 5% acrolein for 24 h, transferred to 30% sucrose, and processed for immunohistochemistry. The caudal portion of each brain, containing the hindbrain and caudal midbrain, was frozen on dry ice and stored at −80°C for analyses of cytokine expression.
In a follow-up study, we examined potential prenatal differences between GF and CC groups. Timed-pregnancies were established in CC and GF Swiss Webster dams by introducing males within an hour of lights off and removing them the next morning, 1–2 h after lights on; this day was designated as embryonic day (E) 0. On E18.5 (one-half day prior to expected delivery), pregnant dams were briefly exposed to 2% CO2 (< 2 minutes) and decapitated. An abdominal incision exposed the uterine horns, and fetuses were extruded, rapidly decapitated, and their brains collected as above.
2.3. Immunohistochemistry (IHC)
Brains were frozen-sectioned into four, 40 μm, coronal series. Sections were collected into cryoprotectant solution and stored at −20°C until processed for the detection of cleaved caspase 3 (AC3) or ionized calcium binding adaptor molecule 1 (Iba1) to identify dying cells and microglia, respectively. For both AC3 and Iba1 staining, epitope retrieval was performed with 0.05 M sodium citrate and unreacted aldehyde blockade with 0.1 M glycine. Tissue was then incubated in a blocking solution (20% normal goat serum and 1% hydrogen peroxide), followed by an overnight incubation with the primary antibody: rabbit anti-AC3 (Cell Signaling, Beverly, MA, USA; Antibody Registry ID: AB_2341188; 1:20 000) or rabbit anti-Iba1 (Wako, Chuo-Ku, Osaka, Japan; AB_839504; 1:10 000), and treated with goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA, USA; AB_2313606; 1:500 for AC3 and 1:1 000 for Iba1), and the avidin-biotin method (Vector Laboratories; 1:500 for AC3 and 1:1 000 for Iba1) with 3,3′-diaminobenzidine tetrahydrochloride and nickel sulfate as chromogens and hydrogen peroxide as substrate. Sections were mounted onto gelatin-coated slides, dehydrated and coverslipped. AC3 stained tissue was counterstained with thionin.
2.4. Brain regions examined
We first analyzed cell death and microglial labeling in large brain areas including the septum, hippocampus and hypothalamus. Fine-grained analyses were later performed on the paraventricular nucleus of the hypothalamus (PVN), the CA1 oriens layer of the hippocampus, and the arcuate nucleus of the hypothalamus (ARC).
For the septum, we included sections from the point at which the medial border of the anterior commissure was immediately ventral to the ventral tip of the lateral ventricle (Plate 57 in Paxinos et al., 2007) to the midline crossing of the anterior commissure (Plate 59). For the hippocampus and CA1 oriens layer, we analyzed sections from the rostral-most appearance of the dentate gyrus (Plate 65) to the point where the hippocampus starts to tip ventrally (Plate 70), as previously described (Mosley et al., 2017). For the hypothalamus, sections ranged from the crossing of the anterior commissure (Plate 59) to the caudal-most appearance of the ARC (Plate 76). All sections containing the PVN (Plates 63–68) and ARC (Plates 69–76) were included in the analyses of these cell groups. Because the neonatal mouse PVN is located slightly more ventral to the dorsal border of the third ventricle than in the adult, we confirmed the location of the PVN in P0 and P3 brains stained with vasopressin using a rabbit anti-vasopressin primary antibody (Peninsula Laboratories LLC, San Carlos, CA, USA; AB_518673; 1:32 000). For this, we followed the protocol described for AC3 (section 2.3.), but without blocking unreacted aldehydes and with a 1:250 dilution of the secondary antibody.
All regions were analyzed bilaterally and analyses were performed by an experimenter blind to treatment group.
2.5. Quantification of AC3 and Iba1 labeling
AC3 immunostaining was quantified using StereoInvestigator software (MBF Biosciences, Williston, VT, USA). For each region of interest, contours were drawn around the region in each relevant section (2–7 sections in each animal, depending on brain region) and the number of AC3 positive cells within the contours was recorded. The sum of AC3+ cell counts across all sections was divided by total volume sampled to obtain the density of AC3+ cells per mm3 for each animal. For the Iba1 stained tissue, photomicrographs were taken of the relevant brain regions, and contours were drawn around the regions of interest using Image J software (National Institutes of Health, Bethesda, MD, USA). We then quantified the area covered by immunostaining above threshold within the region of interest, using pre-set algorithms in Image J, and data were expressed as # of Iba1+ pixels per volume (mm3) sampled in each animal. In addition, total number of Iba1+ cells and Iba1+ cells falling into four different morphological categories were quantified manually from photomicrographs of the PVN. Microglia were categorized as being amoeboid, stout, transitioning or fully ramified, as in previous studies (Schwarz et al., 2012; Strahan et al., 2017). The number of animals per group were as follows – for P0 and P3: AC3, 8–13 mice/group (4–7 of each sex), Iba1, 5–13 mice/group (2–7 of each sex); for E18.5: AC3, 10–12 mice/group (4–6 of each sex), Iba1, 9–12 mice/group (4–6 of each sex).
2.6. Overall forebrain size
To estimate overall brain size, we outlined the left side of the forebrain in alternate sections of the AC3-stained tissue, starting from the point where the medial border of the anterior commissure is located ventral to the tip of the lateral ventricle (Plate 57; Paxinos et al., 2007) and finishing at the rostral most appearance of the dorsomedial nucleus of the hypothalamus (Plate 70). The forebrain was chosen because it includes all areas covered by our histological analyses.
2.7. Reverse transcription PCR for brain cytokine levels
Tissue (mid- and hindbrain) was homogenized in trizol (Invitrogen, Carlsbad, CA, USA). RNA was precipitated, and concentration and purity determined using standard methods. Reverse transcription was performed with an AMV First-Stand Synthesis Kit (Invitrogen) in a thermal cycler (Applied Biosystems Inc., Foster City, CA, USA) and real time PCR was performed in the LightCycler 96 System (Roche, Mannheim, Germany) using FastStart Essential DNA Green Master Kit (Roche) according to the manufacturer’s instructions. Primers used were IL-10, IL-1β, TNF-α, IL-6, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; all validated primers from Qiagen Inc., Valencia, CA, USA). Each reaction was run in duplicate, and negative controls were run for each cytokine. Quantitative cycle (Cq) values from duplicate samples were averaged, then cytokine expression relative to expression of the control gene (GAPDH) was calculated for each animal using the following formula: 2(− (Cq cytokine of interest − Cq GAPDH)). Fold change values were obtained by dividing each experimental value by the average value for the CC P0 group (control group for all PCR analyses). Number of animals per group were 18–24 for P0 and P3 (8–12 of each sex) and 7–12 per group (1–6 of each sex) for E18.5.
2.8. Statistics
We first performed three-way ANOVAs (microbiota status-by-age-by-sex) to compare P0 and P3 groups for all dependent measures with the exception of microglial morphology. Because no significant effects of sex were identified for any measure, data from males and females were combined, and two-way ANOVAs are reported below. For the analysis of microglial morphology, we used a mixed design ANOVA with microbiota status and age as between subject factors and microglial phenotype (amoeboid, stout, transitioning, ramified) as a within-subjects factor. Post-hoc comparisons were performed using Bonferroni’s test. Pearson’s correlation coefficients were computed to test for relationships between cell death and microglial labeling. Two-tailed independent t-tests were used to compare cell death and microglial labeling in CC and GF animals on E18.5.
3. Results
3.1. The microbiota increases the expression of inflammatory cytokines in the neonatal brain
We examined the expression of anti- (IL-10) and pro-inflammatory (IL-1β, IL-6, and TNF-α) cytokines in the mid- and hind-brain on P0 and P3. While there was no effect of microbiota status on the expression of IL-10 (F 1, 76 = 0.18, p = 0.67; Figure 1A), expression of the pro-inflammatory cytokines was markedly suppressed in the neonatal GF brain: IL-1β was reduced by 87% (F 1, 76 = 11.38, p < 0.002; Figure 1B) and TNF-α by 90% compared to CC mice (F 1, 76 = 11.06, p < 0.002; Figure 1C). There was also an 83% reduction in mean IL-6 expression in GF mice, but this did not reach significance (F 1, 76 = 3.68, p = 0.06; Figure 1D). There was no effect of age, or microbiota status-by-age interaction, for any of the cytokines analyzed.
Figure 1.
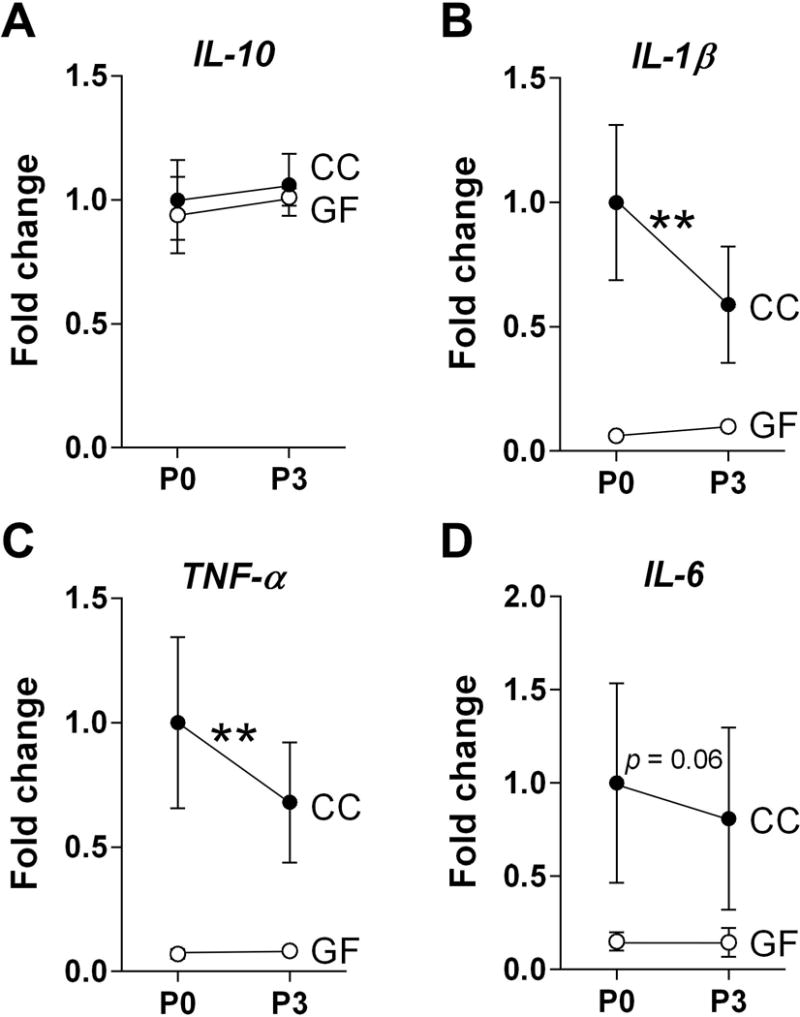
The microbiota upregulates the expression of pro-inflammatory cytokines in the neonatal mouse brain. (A) Expression levels of anti-inflammatory IL-10 did not differ between conventionally colonized (CC; filled circles) and germ-free (GF; open circles) neonates. (B–D) In contrast, pro-inflammatory cytokines: IL-1β, TNF-α, and IL-6 were reduced in GF mice on postnatal day (P) 0 and P3. Data are expressed relative to levels of CC mice on P0. Error bars are smaller than symbols for the GF group in B and C. Asterisks represent main effect of microbiota status. **p < 0.01. N = 18 – 22 mice per group.
3.2. The absence of a microbiota does not grossly interfere with developmental neuronal cell death
We next examined whether the microbiota influences perinatal neuronal cell death, which peaks in many forebrain regions around the time of birth (Mosley et al., 2017). The activated form of caspase-3 (AC3) was used to identify dying cells (Hengartner, 2000; Porter and Janicke, 1999; Srinivasan et al., 1998). We observed widespread AC3 labeling across the brain at P0 and P3 in both GF and CC groups, indicating that the absence of the microbiota does not grossly interfere with perinatal neuronal cell death. Quantitative analyses of large brain areas confirmed this observation: we found no main effect of microbiota status and no microbiota status-by-age interaction on cell death density in the septum, hippocampus or hypothalamus (Figure 2). There was a main effect of age on cell death density in the hypothalamus and hippocampus (F 1, 45 = 8.95, p < 0.005; F 1, 40 = 6.30, p = 0.02; respectively), with more cell death at P3 than at P0, but this was not significant in the septum (Figure 2).
Figure 2.
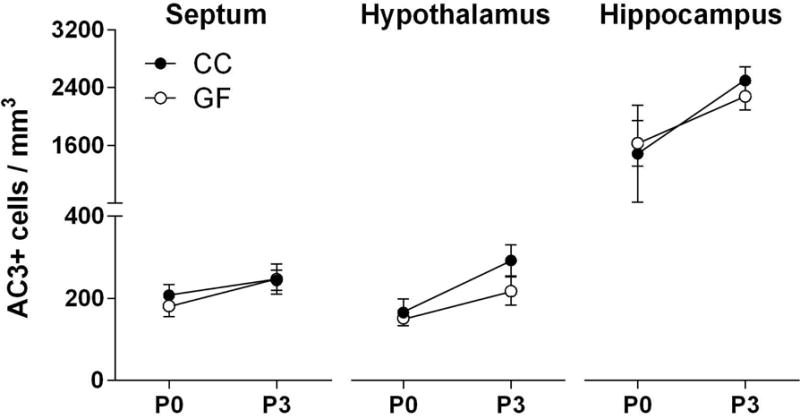
The absence of a microbiota does not grossly interfere with developmental neuronal cell death in the septum (left), hypothalamus (middle) or hippocampus (right) in neonatal conventionally colonized (CC; filled circles) and germ-free (GF; open circles) mice on postnatal day (P) 0 or P3. N = 8 – 13 mice per group.
3.3. The microbiota alters cell death in the hypothalamus and hippocampus in a subregion-specific manner
The hypothalamus and hippocampus are heterogeneous structures with subregions serving different functions, containing different cell types, and exhibiting different patterns of developmental cell death. We therefore performed more fine-grained analyses in subregions of the hypothalamus and hippocampus where we had previously quantified changes in cell death around the time of birth (Ahern et al., 2013; Mosley et al., 2017). In the PVN, a critical site for the stress response and brain-immune interactions (Buller, 2003), we found a significant main effect of microbiota status (F 1, 41 = 17.37, p < 0.0002). GF mice had more than twice as many dying cells as CC mice, with no effect of age (F 1, 41 = 0.58, p = 0.45) or microbiota status-by-age interaction (F 1, 41 = 0.12, p = 0.73; Figure 3).
Figure 3.
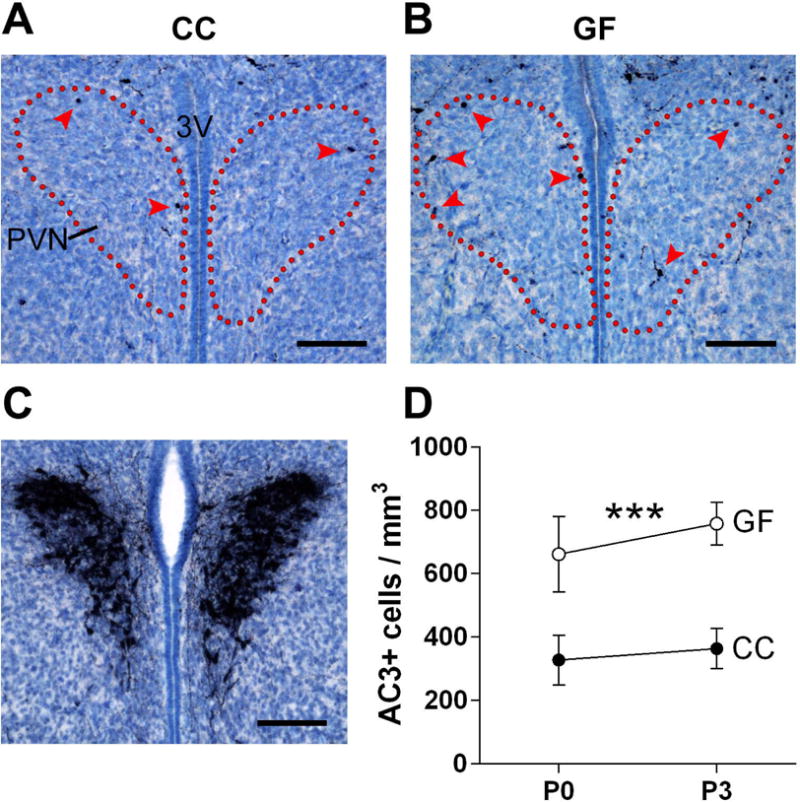
The absence of a microbiota increases cell death in the neonatal mouse paraventricular nucleus of the hypothalamus (PVN). (A, B) Photomicrographs of AC3-labeled tissue (counterstained with thionin) in representative conventionally colonized (CC) and germ-free (GF) mice on the day of birth. Dotted lines indicate the outlines of the PVN and arrowheads indicate AC3 labeled cells. 3V, third ventricle. Scale bars = 100 μm. (C) Vasopressin immunoreactivity was used to confirm the location of the PVN in the neonatal mouse brain. Scale bar = 100 μm. (D) Cell death density in CC (filled circles) and GF (open circles) mice at postnatal day (P) 0 and P3. Asterisks represent main effect of microbiota status. *** p < 0.001. N = 10 – 12 mice per group.
The CA1 oriens layer of the hippocampus has an unusually high rate of cell death in newborn C57BL/6 mice (Mosley et al., 2017), and this was confirmed here in newborn CC Swiss Webster mice. We found even greater cell death in the absence of a microbiota: GF pups had higher cell death density in the CA1 oriens than CC mice (F 1, 39 = 23.79, p < 0.0001), with no significant effect of age (F 1, 39 = 0.08, p = 0.78) and no microbiota status-by-age interaction (F 1,39 = 3.43, p = 0.07; Figure 4A–C).
Figure 4.
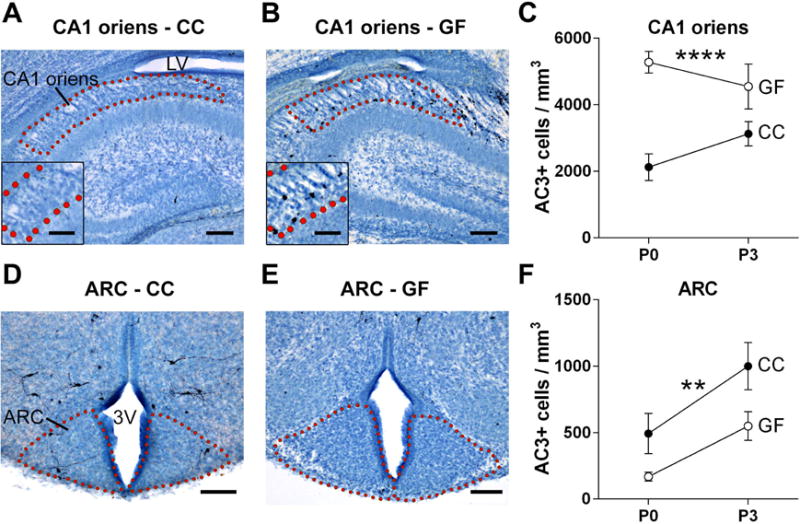
The absence of a microbiota alters developmental cell death in subregions of the hippocampus and hypothalamus. (A, B, D, and E) Photomicrographs of AC3-labeled tissue (counterstained with thionin) in sections containing the CA1 oriens layer of the hippocampus and arcuate nucleus of the hypothalamus (ARC) of conventionally colonized (CC) and germ-free (GF) mice on postnatal day (P) 0. Dotted lines outline the regions of interest; insets are a higher magnification view of the region shown above. 3V, third ventricle; LV, lateral ventricle. Scale bars = 100 μm (A,B,D,E) and 50 μm (insets A, B). (C, F) Cell death density in the CA1 oriens and ARC in CC (filled circles) and GF (open circles) mice at P0 and P3. Asterisks represent main effect of microbiota status. ** p < 0.01; **** p < 0.0001. N = 10 – 12 mice per group.
In the ARC, a brain region that receives metabolic information from the gut and plays an important role in the control of food intake (Heijboer et al., 2006; Joly-Amado et al., 2014), we found an effect of microbiota status (F 1, 41 = 9.00, p < 0.005), but unlike the PVN and CA1 oriens, AC3 cell density was reduced in GF mice (Figure 4D–F). We also identified a significant effect of age (F 1, 41 = 11.81, p < 0.002) with more cell death at P3 than at P0, with no microbiota status-by-age interaction.
We also analyzed the suprachiasmatic nucleus of the hypothalamus (SCN), and found no effect on cell death in this region (not shown). Thus, subregions of the hypothalamus and hippocampus showed striking effects of the microbiota on cell death density. The presence and direction of the effect differed by region, which may explain why differences in cell death are not identified in analyses of gross brain regions.
3.4. The absence of a microbiota increases microglial labeling in whole hypothalamus and in hypothalamic and hippocampal subregions
In gross analyses of large brain regions, we found no effect of microbiota status on Iba1 labeling in the septum or hippocampus, but increased labeling in the hypothalamus of GF mice (F 1, 39 = 10.99, p = 0.002; Figure 5). We also found a microbiota status-by-age interaction in the hypothalamus (F 1, 39 = 11.14, p < 0.002), with GF animals having increased Iba1 labeling on P3 (p = 0.0002; Figure 5), but not on P0. We found significant main effects of age (P3>P0) on Iba1 labeling in all three gross brain regions examined (Figure 5), which is consistent with previous reports of an increase in microglial colonization during the first postnatal week (Dalmau et al., 2003; Schwarz et al., 2012; Sharaf et al., 2013).
Figure 5.
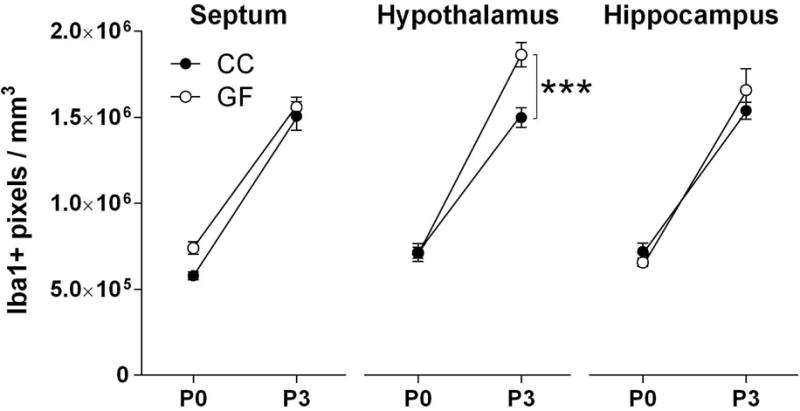
The absence of a microbiota causes higher microglial labeling in the hypothalamus (center) but not in the septum (left) or hippocampus (right) of neonatal conventionally colonized (CC; filled circles) and germ-free (GF; open circles) mice. Asterisks represent a significant difference between GF and CC at postnatal day (P) 3. *** p < 0.001. N = 11 – 13 mice per group, except N = 6 mice for GF-P3.
We next examined region-specific effects on microglial labeling. GF mice had more Iba1 labeling than CC animals in the PVN (F 1, 38 = 26.98, p < 0.0001; Figure 6A–C), CA1 oriens (F 1, 32 = 17.98 p = 0.0002; Figure 6D–F) and ARC (F 1, 38 = 15.09, p = 0.0004; Figure 6G–I), with no microbiota status-by-age interactions. The effect of age was only significant for the ARC. We also observed higher Iba1 labeling in the SCN of GF mice (not shown).
Figure 6.
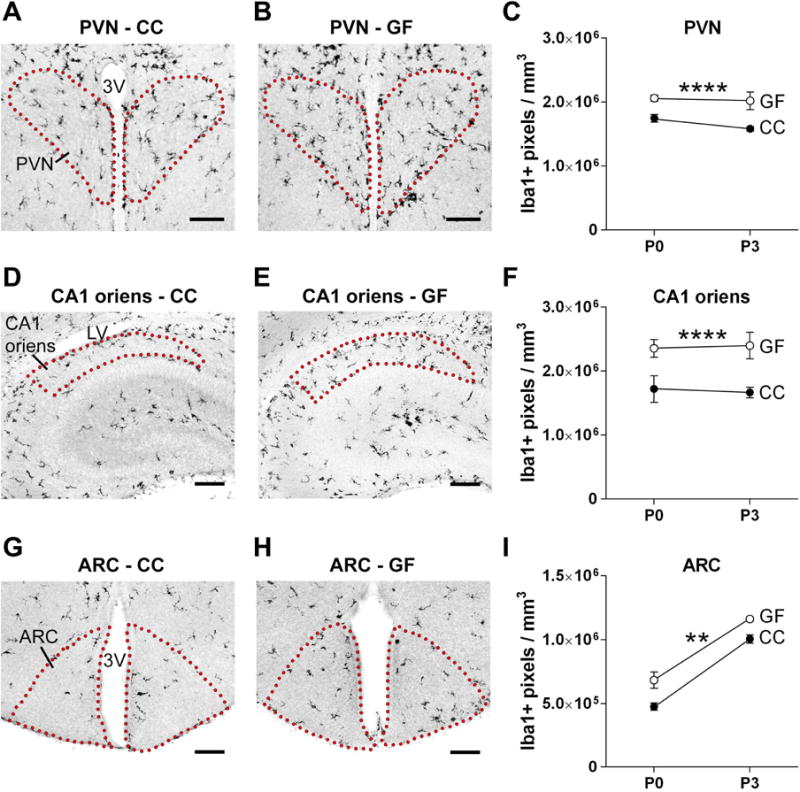
The absence of a microbiota causes increased microglial labeling in subregions of the hypothalamus and hippocampus in the neonatal mouse brain. (A, B, D, E, G, H) Photomicrographs of Iba1 labeled sections containing the PVN (A, B), CA1 oriens (D, E) and ARC (G, H) in conventionally colonized (CC) and germ-free (GF) mice at postnatal day (P) 0. Dotted lines outline the regions of interest. 3V, third ventricle; LV, lateral ventricle. Scale bars = 100 μm. (C, F, I) Microglial labeling density in the PVN, CA1 oriens, and ARC in CC (filled circles) and GF (open circles) mice at P0 and P3. Error bars are smaller than symbols for the GF group in C and I. Asterisks represent main effect of microbiota status. ** p < 0.01; **** p < 0.0001. N = 913 mice per group, except N = 5 mice for GF-P3.
Thus, effects of the microbiota on cell death and microglial labeling did not always go in the same direction: both Iba1 and AC3 labeling were increased in GF mice in the PVN and CA1 oriens, but increased Iba1 labeling was associated with decreased or unchanged cell death in the ARC and SCN. This suggested that effects of GF status on cell death and microglial labeling might be independent. To further examine this, we calculated Pearson’s correlation coefficients between AC3 cell density and Iba1 cell density within each group. None of the correlation coefficients were significant for any of the brain regions examined.
3.5. The absence of a microbiota increases the number and size of microglia
The effects of the microbiota on microglial labeling (section 3.4.) may be the result of altered microglial number, size, morphology, or a combination. To address this further, we counted Iba1+ cells and categorized each cell as amoeboid, stout, transitioning, or fully ramified. Average microglial cell size was determined by dividing the total microglial labeling in our thresholding analysis by total microglial cell number. The PVN was selected for this analysis because it showed a strong effect of microbiota on Iba1 labeling, and because it plays a key role in the stress response and brain-immune interactions (reviewed in Wrona, 2006).
We found an effect of microbiota status, with no microbiota status-by-age interaction, for both microglial cell density and cell size. GF mice had a modest (5%), but significant, increase in microglial cell density (F 1, 38 = 4.29, p = 0.045; Figure 7A) and a more sizeable (28%) increase in cell size (F 1, 38 = 15.21, p = 0.0004; Figure 7B) compared to CC mice. There was also an effect of age on these measures, with greater cell density and cell size on P3 than on P0 (density: F 1, 38 = 44.50, p < 0.0001; cell size: F 1, 38 = 33.73, p < 0.0001; Figure 7A–B).
Figure 7.
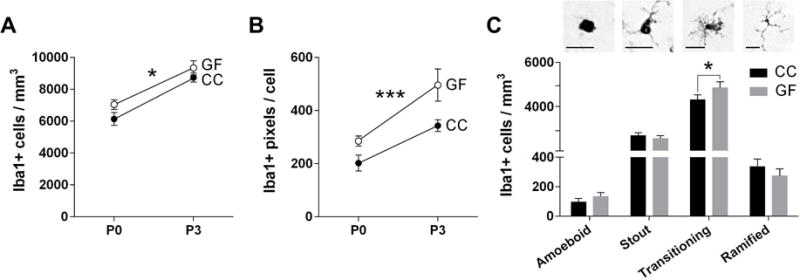
The absence of a microbiota alters the number, size and morphology of microglia in the neonatal mouse paraventricular nucleus of the hypothalamus (PVN). (A) Total microglia density in conventionally colonized (CC; filled circles) and germ-free (GF; open circles) at postnatal day (P) 0 and P3. (B) Average size of microglia in the PVN (in pixels). Asterisks represent main effect of microbiota status (A,B). (C) Top: Photomicrographs of amoeboid, stout, transitioning, and ramified microglia. Scale bar = 20 μm. Bottom: Density of microglial phenotypes in CC (black bars) and GF (grey bars) at P0 and P3 (ages combined for this analysis). Asterisk represent effect of microbiota status on transitioning microglia. * p < 0.05 and *** p = 0.0004. N = 11 – 13 mice per group except N = 6 mice GF-P3 (A,B); N= 18 – 24 mice per group (C).
Consistent with previous observations in neonatal mice (Schwarz et al., 2012; Strahan et al., 2017), most microglia in both CC and GF mice were stout or transitioning, with very few amoeboid or fully ramified cells (Figure 7C). We found a main effect of microbiota status (F 1, 38 = 4.29, p = 0.045), as well as a microbiota status-by-phenotype interaction (F 1.75, 66.66 = 6.42, p = 0.0004; Figure 7C) on microglial morphology. The interaction was due to a modest (12%), but significant (p = 0.04), increase in the number of transitioning microglia in GF mice, with no effect on other morphologies (Figure 7C).
3.6. The maternal microbiota does not alter fetal brain cytokine expression, cell death or microglial colonization
Our findings that brain cytokine levels, cell death, and microglial labeling were affected by microbiota status on the day of birth raised the question of whether these effects might be due to the maternal microbiota, and already be present in utero. To address this, we collected fetal brains at E18.5 from timed pregnancies of CC and GF mice, and analyzed cytokine expression as above (section 3.1.). Samples from the P0 CC group were included in the PCR run, so that the E18.5 CC and GF groups could be expressed relative to the P0 CC control. We found no differences among the three groups (E18.5 CC, E18.5 GF, P0 CC) for IL-10, L-1β, or IL-6 (F 2, 42 = 0.44, p = 0.64; F 2, 43 = 0.86, p = 0.43; F 2, 25 = 2.65, p = 0.09; respectively; Figure 8A,B,D). Expression of TNF-α was higher in P0 CC than for the in utero groups (p = 0.03 and p = 0.009 vs. CC and GF, respectively), with no difference between the two E18.5 groups (p > 0.99; Figure 8C).
Figure 8.
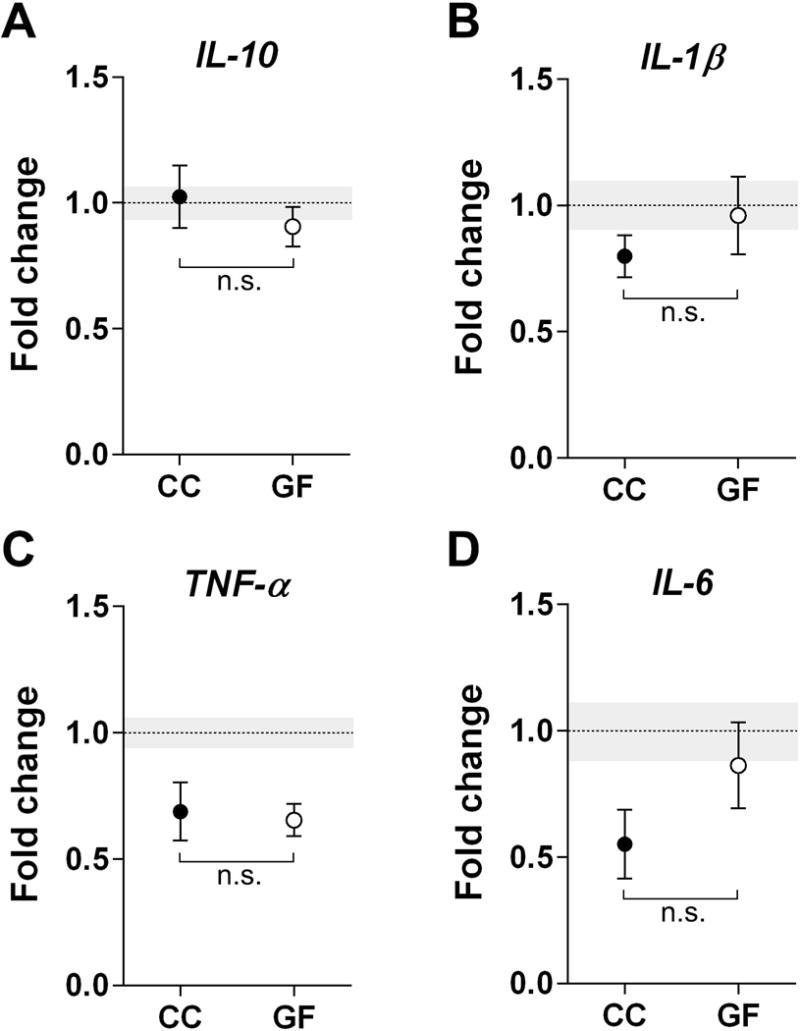
The absence of a microbiota does not affect cytokine expression in the prenatal mouse brain. Expression levels of anti-inflammatory IL-10 (A) and pro-inflammatory cytokines, IL-1β, TNF-α, and IL-6 (B-D), did not differ between conventionally colonized (CC; filled circles) and germ-free (GF; open circles) embryos. Data are expressed relative to levels of CC mice on P0. The mean of the control group is represented by a dotted line and its SEM by grey shading. n.s., non-significant. N = 12 –21 mice per group for (A); N = 12 – 22 mice per group for (B); N = 820 mice per group for (C); N = 7 – 14 mice per group for (D).
There also were no significant differences between GF and CC fetuses in any of the regions examined for cell death (PVN: t22 = 1.64, p = 0.12; CA1 oriens: t20 = 1.37, p = 0.19; ARC: t20 = 0.52, p = 0.61; Figure 9A,C,E) or microglial labeling (PVN: t21 = 1.85, p = 0.08; CA1 oriens: t19 = 1.46, p = 0.16; ARC: t18 = 0.51, p = 0.61; Figure 9B,D,F). Thus, differences between GF and CC offspring were not observed prior to birth.
Figure 9.
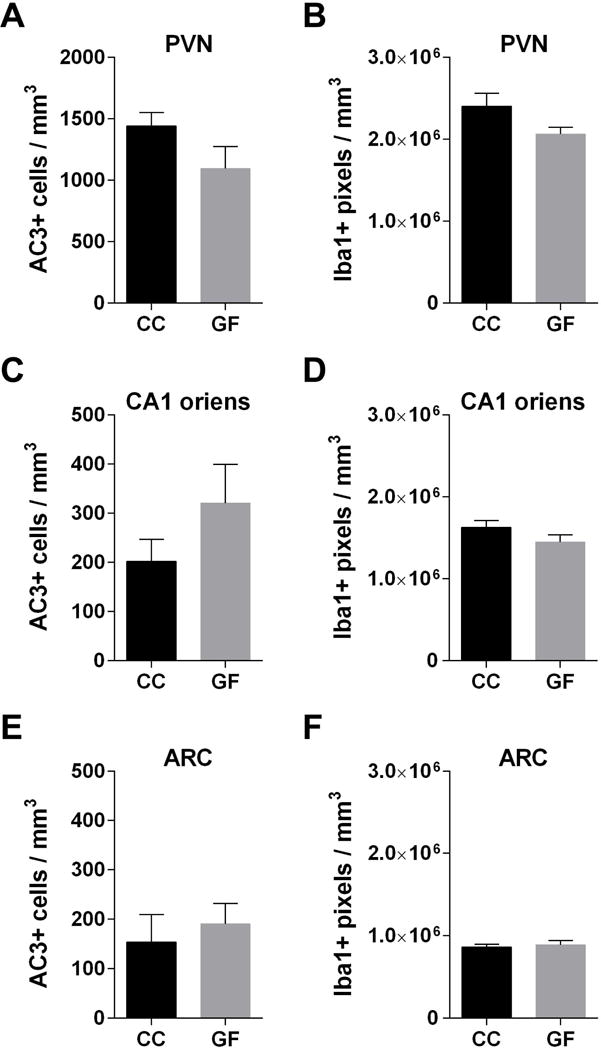
The absence of a microbiota does not affect cell death or microglial labeling in the prenatal hippocampus and hypothalamus. (A, C, E) AC3 and (B, D, F) Iba1 labeling in the PVN (A, B), CA1 oriens (C, D), and ARC (E, F) in conventionally colonized (CC) and germ-free (GF) mice on embryonic day (E) 18.5. N = 10 – 12 mice per group (A, C, E); N = 9 – 12 mice per group (B, D, F). - Effects of the absence of a microbiota were examined in the neonatal mouse brain.
3.7. The microbiota influences body weight and brain size at P3
The effects seen above in GF newborns could be influenced by altered overall development or brain size. Previous research on the relationship between a GF environment and body weight has variously been associated with decreased, increased, or no change in body weight in adult mice (Bäckhed et al., 2004; Fleissner et al., 2010; Khosravi et al., 2015; Selwyn et al., 2015). In perinatal animals, we found a microbiota status-by-age interaction on body weight (F 2, 123 = 3.71, p = 0.03): GF and CC animals did not differ on E18.5 (p=0.86) or P0 (p>0.99), but GF weighed more than CC pups at P3 (p = 0.002; Table 1). Given the body weight difference, we next assessed whether overall forebrain size was affected by microbiota status at P3. Indeed, volume of the forebrain of GF mice was 17% larger than in CC mice at P3 (t(15) = 4.10, p = 0.001; Table 1). Thus, because density measures are reported above (AC3 cells or Iba1 labeling per unit area), total AC3+ and total Iba1+ label may be underestimated in P3 GF mice. If so, then the effects of GF status would be even larger than those noted above for microglial labeling (all areas) and cell death in the PVN and CA1; the opposite would be true for cell death density in the ARC (the one measure that was lower in GF mice).
Table 1.
Body weight and brain size in perinatal conventionally colonized (CC) and germ-free (GF) mice.
| Age | Microbiota Status | |
|---|---|---|
|
| ||
| CC | GF | |
| Body weight (g) | ||
| E18.5 | 1.35 ± 0.02 | 1.43 ± 0.03 |
| P0 | 1.56 ± 0.03 | 1.59 ± 0.07 |
| P3 | 2.28 ± 0.06 | 2.58 ± 0.07*** |
| Forebrain volume (mm3) | ||
| P3 | 12.67 ± 0.36 | 14.89 ± 0.40** |
Mean ± SEM. N = 17 – 27 mice per group for body weight; N = 7 – 10 mice per group for forebrain volume.
p = 0.001,
p= 0.0002.
4. Discussion
We find that lack of a microbiota alters neonatal brain development. Previously, altered brain physiology and behavior have been reported in adult GF mice. Compared to CC mice, GF adults exhibit a behavioral phenotype including reduced anxiety (Diaz Heijtz et al., 2011; Neufeld et al., 2011), a hyper-responsive stress response (Clarke et al., 2013; Sudo et al., 2004), impaired social behavior (Desbonnet et al., 2014), and impaired memory consolidation (Gareau et al., 2011). The production of neurotransmitters and neuropeptides is also altered in GF adults (Clarke et al., 2013; Diaz Heijtz et al., 2011; Neufeld et al., 2011; Peters et al., 2016). Some of these differences are normalized by exposing GF animals to microbes prior to puberty, but other changes persist (Clarke et al., 2013; Desbonnet et al., 2014; Peters et al., 2016; Sudo et al., 2004), suggesting that exposure to a microbiota early in development has programming effects on brain function and behavior. Here we have identified changes in brain development in newborn GF mice that may contribute to these effects.
4.1. Postnatal brain cytokine expression
Recent reports suggest that the mammalian fetus may be exposed to a low number of bacterial species from the placenta and amniotic fluid (Collado et al., 2016; Jimenez et al., 2008), although this is still controversial (Hornef and Penders, 2017; Lauder et al., 2016). Regardless, the situation changes dramatically at birth, as the neonate is rapidly colonized by microbial flora from the mother’s vagina, feces, skin and the environment (Tamburini et al., 2016; van Best et al., 2015). Parturition is associated with an acute phase inflammatory response in the newborn (Kaiser et al., 2014; Marchini et al., 2000) but it was unknown whether this response is triggered by microbial exposure at birth, and whether it extends to the central nervous system. Because peripheral inflammation elicits coordinated responses in the brain (Dantzer et al., 2008), we hypothesized that birth may normally be associated with brain cytokine expression, even in the absence of pathology or infection, and that this would be prevented or attenuated in GF animals. Our findings support this prediction.
The expression of pro-inflammatory cytokines, especially IL-1β and TNF-α, was profoundly attenuated in the brains of neonatal GF mice. Brain cytokine expression has not previously been examined in perinatal GF mice. However, our findings are consistent with reports of measurable inflammatory cytokines in the normal newborn rodent brain (Garay et al., 2013; Pendyala et al., 2017), and demonstrate that this expression depends on the microbiota. IL-1 β and TNF-α have been associated with both increased neuronal cell death, as well as with neuroprotection (Correale and Villa, 2004; Lambertsen et al., 2009; Sedel et al., 2004), and these dual roles may explain why cell death was increased in some brain regions but decreased in others of GF mice.
The source of the cytokine expression we measured is unknown; microglia are the main immunocompetent cells in the central nervous system, but cytokines are also produced by astrocytes and, in some cases (e.g., TNF-α), neurons of neonatal rodents (Faustino et al., 2011; Loram et al., 2012; Semple et al., 2010). One limitation of our study is that cytokine measures were restricted to the mid- and hind-brain. Differences in cytokine expression between brain regions have been described (Garay et al., 2013), so we cannot assume that all brain regions of CC and GF mice show differential cytokine expression at birth.
4.2. The microbiota and patterns of cell death
Neonatal GF mice had higher levels of cell death than CC mice in the PVN and CA1 oriens, lower levels in the ARC, and no change in the SCN. The regional specificity of the effects of the microbiota on cell death, and the fact that no differences were seen prenatally in any of the subregions analyzed, allow us to rule out certain trivial explanations. It is not the case, for example, that GF mice express a generalized neuropathology that induces higher cell death.
Although the cell type undergoing death cannot be determined from AC3 labeling alone, the large majority of AC3+ cells in the neonatal brain are neurons (Zuloaga et al., 2011), which is consistent with the size and neuron-like morphology of the majority of the AC3+ cells we observed in both CC and GF mice here. We do not know whether the changes in cell death we observed neonatally would lead to lasting differences in cell number or, alternatively, are compensated for later in development. Long-term effects of altered cell death in the PVN, CA1 oriens and ARC could contribute to functional changes previously described in GF adults. For example, the PVN controls the stress response and brain-immune interactions, both of which are altered in adult GF mice (Clarke et al., 2013; Erny et al., 2015; Sudo et al., 2004). Functions carried out by the CA1 oriens and ARC – memory consolidation and food intake, respectively – also are impaired in GF adults (Bäckhed et al., 2004; Bravo et al., 2011; Gareau et al., 2011). GF mice show altered oxytocin labeling in the PVN (Peters et al., 2016), which could be due to changes in the number of neurons expressing these peptides. In future studies, it will be important to determine whether the microbiota affects the survival of specific subsets of phenotypically-identified neurons.
The mechanisms responsible for altered cell death in GF mice remain to be determined. Classic explanations for what controls the magnitude of developmental neuronal cell death have focused on interactions with target cells, innervating afferents, neighboring glia, or neural activity (Marín-Teva et al., 2011; Oppenheim, 1985, 1991). Our findings demonstrate that “non-self” cells – i.e., the microbiota – influence developmental cell death, and do so in a brain region-specific manner. The microbiota communicates with the brain via both metabolic (e.g., production of short chain fatty acids) and neural pathways (Bercik et al., 2011; Bravo et al., 2011; Desbonnet et al., 2014) to influence brain physiology, including apoptosis. In adult rats, for example, probiotic administration after myocardial infarction abolishes caspase 3 activation in the amygdala, and this effect is prevented by vagotomy (Malick et al., 2015; Wann et al., 2006). Infection of the gastrointestinal tract with pathological bacteria activates the PVN, and this also requires signaling by the vagus nerve (Wang et al., 2002). Vagal stimulation alters cytokine levels in the brain as well as microglial numbers (Meneses et al., 2016), which may in turn influence cell survival. Although technically challenging, it would be interesting to determine whether neonatal vagotomy prevents the changes in cell death observed here in GF mice.
4.3. The microbiota and patterns of microglial colonization
One possible mechanism for how the microbiota might alter developmental cell death considered here was via effects on microglia. We found higher levels of microglial labeling in the hypothalamus as a whole, and in all of the subregions analyzed in the hypothalamus and hippocampus in GF neonates. As revealed in our detailed analysis of the PVN, this appears to be due to both increased microglial number and cell size. Although greater microglial labeling in GF mice might seem counterintuitive, our findings are consistent with a report of more microglia, and more branched microglia with longer processes, in several brain regions of adult GF mice (Erny et al., 2015). Altered gene expression was also observed in microglia isolated from GF adults, which reflected an immature gene expression profile; despite increased microglial labeling and size, GF mice appear to have a reduced innate immune response (Erny et al., 2015). Similarly, in the neonatal rat, higher microglial cell numbers were associated with lower brain cytokine levels following acute neonatal ischemia (Faustino et al., 2011), and changes in cytokine expression can be independent of changes in microglial morphology in mice (Norden et al., 2016).
We did not find sex differences in Iba1 labeling in any of the brain regions examined. This is in agreement with our previous study in C57BL/6 mice (Strahan et al., 2017), but contrasts with work in rats, in which neonatal males are reported to have more microglia than females in hippocampal subregions, including the CA1 (Schwarz et al., 2012). The discrepancies may be related to species differences or differences in the developmental time points analyzed.
4.4. Are changes in cell death and microglia labeling causally related?
Microglia have long been associated with neuronal cell death (Marín-Teva et al., 2011), and dying neurons are often found in close contact with microglia, which phagocytose cell corpses (Ashwell, 1990; Ferrer et al., 1990). Recently, a more active role for microglia in controlling cell death has been suggested. Several studies report that microglia participate in the killing of neurons (Marín-Teva et al., 2011), whereas others argue for neuroprotective effects of microglia during the perinatal cell death period (Arnoux et al., 2014; Ueno et al., 2013). Whether changes in cell death were caused by the changes in microglia in our study or, conversely, the microglial changes were caused by altered cell death cannot be definitively determined. In the PVN and CA1 oriens, we saw higher levels of cell death together with higher Iba1 labeling. However, lower cell death in the ARC and no change in cell death in the SCN was also associated with greater microglial labeling. A dissociation of cell death and microglial labeling was also supported by our correlational analysis, which found no significant relationship between AC3 cell density and microglial labeling in any of the brain regions analyzed. This suggests that the microbiota may independently affect cell death and microglial labeling.
4.5. Effects of the maternal microbiota
Differences between GF and CC mice were already present on the day of birth, which suggested that the microbiota may exert effects on brain development in utero. Although direct exposure to microbes in CC and GF should be similar before birth, the maternal microbiota can influence the development of the fetal immune system via microbial byproducts that cross the placenta (Gomez de Agüero et al., 2016). However, we did not find any differences in cytokine expression, cell death, or microglial labeling between CC and GF mice on E18.5. It remains possible that the maternal microbiota exerts effects in regions not included in our analyses, or interacts with the stress of parturition to influence neurodevelopment in the offspring. The most parsimonious explanation, however, is that the effects we observed require the differential exposure to microbes encountered postnatally in GF and CC pups. If so, this exposure influences brain development within hours. Although we do not know the precise hour of birth for our P0 groups, we estimate that they were between 10 and 14 h old at sacrifice, based on time of brain collection and previous studies of the timing of parturition in mice (Roizen et al., 2007). The PVN is activated 6 hours after oral inoculation of adult GF mice with bacteria (Sudo et al., 2004), demonstrating that gut microbiota can affect the brain within the time-frame examined here.
Relatively high levels of IL-1β and IL-6 were seen in the brains of both CC and GF fetuses on E18.5. This may be related to the inflammatory events that precede labor and include an increase in maternal uterine immune cells, as well as increased proinflammatory cytokines in the uterus, amniotic fluid and fetal membranes (Lindstrom and Bennett, 2005; Orsi et al., 2006). This so-called ‘sterile inflammation’ is not dependent on the presence of pathogens and therefore is expected to be similar in GF and CC dams. Maternal cytokines may cross the placenta (Dahlgren et al., 2006; Zaretsky et al., 2004) and the blood brain barrier (reviewed in Banks et al., 2002), inducing the expression of pro-inflammatory cytokines in the fetal brain (reviewed in Dammann and Leviton, 1997). In mice, the inflammatory events preceding labor occur during the last day or two of gestation (Condon et al., 2004; Montalbano et al., 2013; Payne et al., 2012) and therefore would have been present at E18.5. After birth, only CC newborns are exposed to immune stimulation by microbes, which may explain why high cytokine levels were maintained in CC mice and dropped to low levels in GF newborns.
Conclusions
Taken together, our study highlights the importance of microbial exposure for neonatal brain development. Recently, differential exposure to microbiota at birth has been associated with the development of psychological disorders, such as autism (Curran et al., 2015). Millions of years of evolution have shaped the symbiotic relationship between mammalian species and their microbiota, so it is not surprising that our microbial symbionts influence key developmental processes. Moreover, because mode of birth (vaginal versus cesarean), neonatal hygiene practices, and maternal diet are all reported to alter the neonatal microbiota (Dominguez-Bello et al., 2010; Dominguez-Bello et al., 2016; Romano-Keeler and Weitkamp, 2015), our findings suggest that such practices have the potential to influence early brain development.
-
-
Pro-inflammatory cytokine expression was decreased in germ-free mice.
-
-
Microbiota influenced cell death and microglial labeling a region specific manner.
-
-
These effects were seen on the day of birth but not a day before.
-
-
Our results highlight the importance of the microbiota on early brain development.
Acknowledgments
We thank Geert de Vries, Mary Holder, Carla Cisternas, Nicole Peters, and Laura Cortes for critical comments on earlier versions of this manuscript. We also thank Daniel Cox and Atit Patel for RT-PCR training and Lucie Etienne-Mesmin for technical assistance. Supported by the National Science Foundation IOS-1743673 and the National Institutes of Health R21-MH108345 (to N.G.F). B.C. is a recipient of the Research Fellowship award from the Crohn’s and Colitis Foundation of America (CCFA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern TH, Krug S, Carr AV, Murray EK, Fitzpatrick E, Bengston L, McCutcheon J, De Vries GJ, Forger NG. Cell death atlas of the postnatal mouse ventral forebrain and hypothalamus: effects of age and sex. J Comp Neurol. 2013;521:2551–2569. doi: 10.1002/cne.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoux I, Hoshiko M, Sanz Diez A, Audinat E. Paradoxical effects of minocycline in the developing mouse somatosensory cortex. Glia. 2014;62:399–410. doi: 10.1002/glia.22612. [DOI] [PubMed] [Google Scholar]
- Ashwell K. Microglia and cell death in the developing mouse cerebellum. Brain Res Dev Brain Res. 1990;55:219–230. doi: 10.1016/0165-3806(90)90203-b. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. 609 e591–593. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller KM. Neuroimmune stress responses: reciprocal connections between the hypothalamus and the brainstem. Stress. 2003;6:11–17. doi: 10.1080/1025389031000092313. [DOI] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012;32:11706–11715. doi: 10.1523/JNEUROSCI.0730-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LB, Woods TA, Carmody AB, Caughey B, Peterson KE. Age-related differences in neuroinflammatory responses associated with a distinct profile of regulatory markers on neonatal microglia. J Neuroinflammation. 2014;11:70. doi: 10.1186/1742-2094-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004;101:4978–4983. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J, Villa A. The neuroprotective role of inflammation in nervous system injuries. J Neurol. 2004;251:1304–1316. doi: 10.1007/s00415-004-0649-z. [DOI] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran EA, O’Neill SM, Cryan JF, Kenny LC, Dinan TG, Khashan AS, Kearney PM. Research review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2015;56:500–508. doi: 10.1111/jcpp.12351. [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Samuelsson AM, Jansson T, Holmang A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006;60:147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Vela JM, Gonzalez B, Finsen B, Castellano B. Dynamics of microglia in the developing rat brain. J Comp Neurol. 2003;458:144–157. doi: 10.1002/cne.10572. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera-Vinas JI, Mendez K, Knight R, Clemente JC. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22:250–253. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, Vexler ZS. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Bernet E, Soriano E, del Rio T, Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39:451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr. 2010;104:919–929. doi: 10.1017/S0007114510001303. [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Golightly E, Jabbour HN, Norman JE. Endocrine immune interactions in human parturition. Mol Cell Endocrinol. 2011;335:52–59. doi: 10.1016/j.mce.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- Heijboer AC, Pijl H, Van den Hoek AM, Havekes LM, Romijn JA, Corssmit EP. Gut-brain axis: regulation of glucose metabolism. J Neuroendocrinol. 2006;18:883–894. doi: 10.1111/j.1365-2826.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hornef M, Penders J. Does a prenatal bacterial microbiota exist? Mucosal Immunol. 2017;10:598–601. doi: 10.1038/mi.2016.141. [DOI] [PubMed] [Google Scholar]
- Jimenez E, Marin ML, Martin R, Odriozola JM, Olivares M, Xaus J, Fernandez L, Rodriguez JM. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159:187–193. doi: 10.1016/j.resmic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Joly-Amado A, Cansell C, Denis RG, Delbes AS, Castel J, Martinez S, Luquet S. The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Pract Res Clin Endocrinol Metab. 2014;28:725–737. doi: 10.1016/j.beem.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi Y, Seow SW, Amoyo AA, Chiow KH, Tan TL, Wong WY, Poh QH, Sentosa IM, Bunte RM, Pettersson S, Loke MF, Vadivelu J. Helicobacter pylori infection can affect energy modulating hormones and body weight in germ free mice. Sci Rep. 2015;5:8731. doi: 10.1038/srep08731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. The entry of fetal and amniotic fluid components into the uterine vessel circulation leads to sterile inflammatory processes during parturition. Front Immunol. 2012;3:321. doi: 10.3389/fimmu.2012.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Dibal CD, Armitage GA, Winship IR, Todd KG. Distinct activation profiles in microglia of different ages: a systematic study in isolated embryonic to aged microglial cultures. Neuroscience. 2013;254:185–195. doi: 10.1016/j.neuroscience.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder AP, Roche AM, Sherrill-Mix S, Bailey A, Laughlin AL, Bittinger K, Leite R, Elovitz MA, Parry S, Bushman FD. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4:29. doi: 10.1186/s40168-016-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–581. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malick M, Gilbert K, Daniel J, Arseneault-Breard J, Tompkins TA, Godbout R, Rousseau G. Vagotomy prevents the effect of probiotics on caspase activity in a model of postmyocardial infarction depression. Neurogastroenterol Motil. 2015;27:663–671. doi: 10.1111/nmo.12540. [DOI] [PubMed] [Google Scholar]
- Marchini G, Berggren V, Djilali-Merzoug R, Hansson LO. The birth process initiates an acute phase reaction in the fetus-newborn infant. Acta Paediatr. 2000;89:1082–1086. doi: 10.1080/713794557. [DOI] [PubMed] [Google Scholar]
- Marín-Teva JL, Cuadros MA, Martin-Oliva D, Navascues J. Microglia and neuronal cell death. Neuron Glia Biol. 2011;7:25–40. doi: 10.1017/S1740925X12000014. [DOI] [PubMed] [Google Scholar]
- Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Meneses G, Bautista M, Florentino A, Diaz G, Acero G, Besedovsky H, Meneses D, Fleury A, Del Rey A, Gevorkian G, Fragoso G, Sciutto E. Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J Inflamm. 2016;13:33. doi: 10.1186/s12950-016-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Montalbano AP, Hawgood S, Mendelson CR. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154:483–498. doi: 10.1210/en.2012-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley M, Shah C, Morse KA, Miloro SA, Holmes MM, Ahern TH, Forger NG. Patterns of cell death in the perinatal mouse forebrain. J Comp Neurol. 2017;525:47–64. doi: 10.1002/cne.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–264, e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–316. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Naturally occurring cell death during neural development. Trends Neurosci. 1985;8:487–493. [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Orsi NM, Gopichandran N, Ekbote UV, Walker JJ. Murine serum cytokines throughout the estrous cycle, pregnancy and post partum period. Anim Reprod Sci. 2006;96:54–65. doi: 10.1016/j.anireprosci.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang HQ. Atlas of the developing mouse brain at E17.5, P0 and P6. San Diego: Elsevier; 2007. [Google Scholar]
- Payne KJ, Clyde LA, Weldon AJ, Milford TA, Yellon SM. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012;87:106. doi: 10.1095/biolreprod.112.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala G, Chou S, Jung Y, Coiro P, Spartz E, Padmashri R, Li M, Dunaevsky A. Maternal immune activation causes behavioral impairments and altered cerebellar cytokine and synaptic protein expression. Neuropsychopharmacology. 2017;42:1435–1446. doi: 10.1038/npp.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NV, Paul MJ, Chassaing B, Dunn J, Gewirtz AT, de Vries GJ. Increased oxytocin immunoreactivity in male and female germ-free Swiss Webster mice. San Diego, CA: Society for Neuroscience; 2016. (Program No. 445.07. 2016 Neuroscience Meeting Planner). 2016. Online. [Google Scholar]
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizen J, Luedke CE, Herzog ED, Muglia LJ. Oxytocin in the circadian timing of birth. PLoS One. 2007;2:e922. doi: 10.1371/journal.pone.0000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-Keeler J, Weitkamp JH. Maternal influences on fetal microbial colonization and immune development. Pediatr Res. 2015;77:189–195. doi: 10.1038/pr.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedel F, Bechade C, Vyas S, Triller A. Macrophage-derived tumor necrosis factor α, an early developmental signal for motoneuron death. J Neurosci. 2004;24:2236–2246. doi: 10.1523/JNEUROSCI.4464-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn FP, Csanaky IL, Zhang Y, Klaassen CD. Importance of large intestine in regulating bile acids and glucagon-like peptide-1 in germ-free mice. Drug Metab Dispos. 2015;43:1544–1556. doi: 10.1124/dmd.115.065276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Frugier T, Morganti-Kossmann MC. CCL2 modulates cytokine production in cultured mouse astrocytes. J Neuroinflammation. 2010;7:67. doi: 10.1186/1742-2094-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf A, Krieglstein K, Spittau B. Distribution of microglia in the postnatal murine nigrostriatal system. Cell Tissue Res. 2013;351:373–382. doi: 10.1007/s00441-012-1537-y. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, Tomaselli KJ. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- Steinborn A, Sohn C, Sayehli C, Baudendistel A, Huwelmeier D, Solbach C, Schmitt E, Kaufmann M. Spontaneous labour at term is associated with fetal monocyte activation. Clin Exp Immunol. 1999;117:147–152. doi: 10.1046/j.1365-2249.1999.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahan JA, Walker WH, 2nd, Montgomery TR, Forger NG. Minocycline causes widespread cell death and increases microglial labeling in the neonatal mouse brain. Dev Neurobiol. 2017;77:753–766. doi: 10.1002/dneu.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22:713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- van Best N, Hornef MW, Savelkoul PH, Penders J. On the origin of species: Factors shaping the establishment of infant’s gut microbiota. Birth Defects Res C Embryo Today. 2015;105:240–251. doi: 10.1002/bdrc.21113. [DOI] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. 2002;8:540–545. doi: 10.3748/wjg.v8.i3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wann BP, Boucher M, Kaloustian S, Nim S, Godbout R, Rousseau G. Apoptosis detected in the amygdala following myocardial infarction in the rat. Biol Psychiatry. 2006;59:430–433. doi: 10.1016/j.biopsych.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Wrona D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172:38–58. doi: 10.1016/j.jneuroim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Yektaei-Karin E, Moshfegh A, Lundahl J, Berggren V, Hansson LO, Marchini G. The stress of birth enhances in vitro spontaneous and IL-8-induced neutrophil chemotaxis in the human newborn. Pediatr Allergy Immunol. 2007;18:643–651. doi: 10.1111/j.1399-3038.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103:546–550. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner. Neuroscience. 2011;199:535–547. doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


