Abstract
This study investigated the Oxytocin Receptor gene’s (OXTR) moderation of associations between exposure to a substance misuse intervention, average peer substance use, and adolescents’ own alcohol use during the 9th grade. OXTR genetic risk was measured using five Single Nucleotide Polymorphisms (SNPs) and peer substance use was based on youths’ nominated closest friends’ own reports of alcohol, cigarette and marijuana use, based on data from the PROSPER Project. Regression models revealed several findings. First, low OXTR risk was linked to affiliating with friends who reported less substance use in the intervention condition but not the control condition. Second, affiliating with high substance-using friends predicted youth alcohol risk regardless of OXTR risk or intervention condition. Third, although high OXTR risk youth in the intervention condition who associated with low substance-using friends reported somewhat higher alcohol use than comparable youth in the control group, the absolute level of alcohol use among these youth was still among the lowest in the sample.
Keywords: Intervention, Gene x Intervention Research, Alcohol Research, Oxytocin Receptor gene
Alcohol use always poses risks, but those perils are greater during early adolescence. Early adolescent drinking—at any level—is linked to sexual risk-taking and school failure in adolescence (Keyes et al., 2007; Stueve & O’Donnell, 2005), and predicts heavy drinking and problems with alcohol in young adulthood (Lo, 2000; Parker et al., 1996; Sampson et al., 1989; Werner et al., 1994). Those who drink before age 15 are at greater risk for increased frequency, bingeing, and alcohol-related problem behaviors and attitudes in young adulthood (Pikanen et al., 2005).
The higher risks associated with drinking in early adolescence occur during the years in which susceptibility to peer influence peaks (Steinberg & Monahan, 2007) and adolescents begin to spend less time with parents and more time with peers (Larson, Richards, Moneta, Holmbeck, & Duckett, 1996). The confluence of increased peer exposure and reduced time with parents increases the impact of modeling and greater alcohol use that come with proximity to substance-using peers (Osgood, Feinberg, et al., 2013; Osgood, Ragan, et al., 2013). Accordingly, many preventive interventions designed to reduce adolescent alcohol use target peer influences in early adolescence.
Preventive interventions vary in effectiveness, reducing risk behaviors among some adolescents but not others. In the last decade, some of this variability in individual responses has been shown to be associated with genetic differences—specifically, differences at particular genetic loci (Brody et al., 2009). The impact of early adolescents’ perceived peer pressure to use alcohol on their own use has been added to the list of factors moderated by specific genetic variance (i.e., candidate genes; Griffin, Cleveland, Schlomer, Vandenbergh, & Feinberg, 2015). Given that substance use preventive interventions often focus on peer influence, variability in substance use interventions’ effects may be due at least in part to genetic differences that influence susceptibility to peer factors.
In the current study, we investigated ways in which variability in the oxytocin receptor gene (OXTR) might impact affiliations with substance-using peers across control and intervention conditions of a preventive intervention trial designed to reduce adolescent substance use and the potential impact of these peer affiliations across control and intervention conditions of this preventive intervention trial. We focused on genetic variation in the oxytocin system because oxytocin has been linked to social processes such as social attachment (Carter, 1998), affiliation (Andari et al., 2010), and trust (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005) that may contribute to differences in relationships to peers and their influences on alcohol use. These oxytocin-related social processes are relevant to the two primary peer processes examined by behavioral scientists: the selection of friends and their influence on behaviors (i.e. socialization). Similarly, many interventions designed to reduce initiation and escalation of adolescent substance misuse target these peer processes. The current study addressed interventions’ impacts on selection and socialization processes relevant to adolescents’ use of alcohol in the 9th grade, and whether variation in OXTR moderates the intervention effects of these processes.
We examined three research questions. First, does OXTR variation moderate interventions’ impact on affiliations with substance-using peers? Second, does OXTR variation moderate the similarity between friends’ substance use and adolescents’ own alcohol use? Third, does OXTR variation moderate intervention’s impact on the links between affiliations between peer substance use and individual adolescents’ own alcohol use? Prior to setting out details on the approach taken to address these research questions, we review the literature on how peer processes can contribute to adolescent alcohol use; emerging evidence of how genetic variance, both general and specific, can contribute to these peer processes; how interventions address such peer processes; evidence of candidate genes moderating intervention and other environmental effects; and the importance of oxytocin generally, and the OXTR gene specifically, for the social processes examined here.
Peer Selection and Influence and the Role of Genetic Variance
Affiliating with peers who engage in risk behaviors such as substance use increases adolescents’ own risks of similar behaviors (Fisher, Miles, Austin, Camargo, & Colditz, 2007). Historically, this phenomenon has been explained by theories such as differential association, which suggests that pro-deviance messages within peer groups can lead to deviant behaviors (Sutherland 1973), and social learning, which suggests that individuals model the behaviors of others and conform to norms to gain social rewards such as acceptance (Bauman & Ennett, 1996; Cialdini & Trost, 1998).
It is important to emphasize that although adolescents may model the behaviors of distant peers and even media figures, the influence of close peers and friends appears to be stronger. In fact, even when adolescents imitate indirect social contacts, they do so mainly to impress their friends and maintain social circles (Payne & Cornwell, 2007). Thus, the context created by adolescents’ direct peers is doubly relevant. It is possible that families can influence affiliations with substance-using friends by monitoring their children’s activities and friendship choices, modeling behaviors and transmitting values that affect friendship choice, and structuring their children’s social contexts to increase access to prosocial peers. However, behavior genetic analyses suggest that adolescents select, or are selected by, friends in accordance with genetic variance. Applying a twin modeling approach to substance use scores constructed from nominated friends’ own reports, predicting 9th-grade alcohol use, Cleveland, Wiebe, and Rowe (2005) found that most of the variation (64%) in affiliations with substance-using friends was related to genetic variance, with the remainder (36%) explained by nonshared environmental factors.
Although findings that link peer affiliations to genetic variation, like all biometric results, do not address which genes, or how such genes, are involved, they demonstrate the viability of an approach that hypothesizes about specific genetic variants, such as those related to oxytocin, that might contribute to affiliations with substance-using peers. The viability of a potential role for specific genes as contributors to risky peer affiliations and their impact on drinking is further buttressed by recent candidate gene findings. For example, Griffin et al. (2015) found that carriers of at least one copy of the DRD4 7-repeat allele (7+) reported a stronger positive association between perceived peer pressure and increased alcohol use than non-carriers. Similarly, in a randomized experiment, Larsen et al. (2010) found that when exposed to heavy-drinking confederates, young adults carrying DRD4 7+ drank more than those without the allele. A second randomized study suggested a mechanism behind this interaction: Creswell et al. (2012) found that DRD4 7+ carriers were more sensitive to the social bonding effect of alcohol than 7- individuals.
Substance Misuse Interventions within the PROSPER Project
Given the importance of peer influence for substance use, many preventive interventions are designed to assist adolescents’ abilities to make decisions that reduce exposure to and influence of substance-using peers. The data used here are drawn from the PROSPER project. Intervention communities delivered both family-focused and school-based interventions (Spoth et al., 2004). All intervention communities in PROSPER delivered the Strengthening Families Program: For Parents and Youth 10-14 (SFP 10-14) as their family-focused program. Approximately 17% of all eligible families across the PROSPER project’s two study cohorts participated in the SFP 10-14.
For their school-based program, four communities selected Life Skills Training (Botvin, 2000) and Project Alert (Ellickson et al., 2003), while the remaining six chose the All Stars curriculum (McNeal et al., 2004), All 14 communities delivered their chosen program during required classes as part of the 7th-grade curriculum, so nearly all students in participating schools took part. All three school-based programs targeted social norms, decision-making, peer group affiliation, and peer pressure. Lesson activities included question-answer sessions, role-play, and small-group activities. Assignments focused on recognizing and resisting peer pressure, benefits of not using alcohol and drugs, and practicing decision-making skills. Youth in the intervention conditions of the PROSPER study reported lower levels of use across an array of substances (see Spoth et al. [2011] for review). Very high levels of implementation quality have been confirmed across family-focused and school-based interventions and cohorts (Spoth et al., 2007). For more details on each program, see Spoth et al. (2004). Youth in the intervention conditions of the PROSPER study report lower levels of use across an array of substances, however, intervention main effects on alcohol are modest (see Spoth et al., 2011 for review).
Candidate Gene Moderation of Intervention Effects
Research adding specific genetic variance to data from preventative intervention studies, including PROSPER, has demonstrated that specific genetic variance can moderate intervention effects. This line of research takes advantage of one of the defining characteristics of preventative intervention trials: Random assignment to condition, which helps this work avoid rGE confounds. Findings from this line of research, known as “candidate gene by intervention” (cGxI) research, include that adolescents who were not at genetic risk based on their 5-HTTLPR status and who participated in a family-based intervention were less likely to initiate risk behaviors (e.g., including alcohol use) than adolescents at genetic risk attributed to 5-HTTLPR status (Brody, Beach, Philibert, Chen, & Murry, 2009). It is worth noting that this finding has been replicated (see Schlomer et al., in press).
Genetic moderation of intervention effects can also be conditioned by other aspects of the environment. Predicting an alcohol measure similar to the one investigated here, Cleveland et al. (2015) found that interventions reduced alcohol use among adolescents who reported moderate to high levels of maternal involvement and who carried the DRD4 7+ allele. This cGxI finding was not limited to DRD4, as 5-HTTLPR variation was also related to alcohol use in the same conditional fashion, that is among intervention youth with moderate to high levels on maternal involvement. It is worth noting that in the above study, which used the same 9th grade alcohol use outcome considered in the current analyses, there was no main effect of intervention on alcohol use.
The Role of Oxytocin in Social Behavior
The foregoing studies involved two commonly examined genetic variants related to dopamine and serotonin regulation. These variants, associated with DRD4 and 5-HTTLPR, respectively, have been conceptualized as linked to general environmental sensitivity (see Belsky et al., 2007). When considering hypotheses about affiliations with peers and their potential impacts, a different biochemical process—oxytocin—and related genes may be more specifically relevant. Interacting with neurotransmitter systems, oxytocin is involved in the regulation of social behavior and social cognition (Donaldson & Young, 2008). Oxytocin blood levels and genetic variance related to oxytocin (i.e., the oxytocin receptor gene; OXTR) have been linked to a wide array of social behaviors and characteristics relevant to social behaviors in humans (e.g., Walum et al., 2012) and other mammals (e.g., MacLean & Hare, 2015). For example, social recognition is related to blood-level oxytocin (Feldman, Monakhov, Pratt, & Ebstein, 2015) and pair bonding to OXTR variation (Walum et al., 2012). Socially relevant characteristics including callus-unemotional traits (Cecil et al., 2014; Rice & Derish, 2015), orientations toward individualism vs. collectivism (Luo & Han, 2014), aggressiveness (Wu, Li, & Su, 2012), and low empathy (Feldman et al., 2015) have all been linked to OXTR. Oxytocin also has been linked to prosocial behaviors, but in a contextually moderated fashion (Bartz et al., 2011); enhancing prosociality in cooperative contexts but increasing aggression in competitive contexts (id.). In this way, oxytocin appears to facilitate groupism—in-group bonding and out-group distinction (see Shamay-Tsoory & Abu-Akel, 2015; also Feldman et al., 2015). Thus, the impacts of genetic variation linked to oxytocin on social behaviors are likely context-sensitive.
It should be noted that although the OXT gene contributes directly to the production of the oxytocin precursor, variation in this gene does not appear to affect behavioral outcomes and characteristics as much as variation in the receptor (e.g., OXTR gene), which encodes a receptor protein conveying oxytocin’s signal to receptive cells. To operationalize OXTR gene variance, we used multiple markers within OXTR, following the example of Schneiderman, Kanat-Maymon, Ebstein, & Feldman (2013) who studied of the association between OXTR variation and empathetic communication at the beginning of loving romantic relationships. Similar to Schneiderman and colleagues’ cumulative OXTR genetic risk score, ours is a multi-SNP single-gene index where variation in each of 5 OXTR Single Nucleotide Polymorphisms (SNPs) contributes additively to the overall gene score.
Current Study
The current study examined whether variability in the OXTR gene moderated associations among (a) the PROSPER project intervention; (b) peer substance use, as reported by nominated peers; and (c) and early adolescent alcohol use measured in the 9th grade. Although our outcome measure was alcohol use, we operationalized peer risk as substance use because it provides a broader measure of youths’ likely exposure to pro-deviance messages that encourage risky behavior (see Sutherland, 1973). Exposure to this context creates risks for youth beginning down an at-risk behavioral pathway themselves, the first steps of which would include alcohol use (see Cleveland & Wiebe, 2008).
Given that individual differences in affiliations with substance-using peers are heritable (Cleveland, Wiebe, & Rowe, 2005), it is possible that OXTR variability might influence selection of friends based on their substance use. Moreover, selection processes might be impacted by intervention. To this end, we investigated the possibility of a 2-way interaction between the intervention and OXTR variation predicting affiliations with substance-using peers. Based upon consistent findings that peer behaviors predict individual behaviors, we expected that affiliations with substance-using friends would be associated with individual adolescents’ own 9th-grade alcohol use. Without evidence to the contrary, we did not expect a direct effect of variation in the OXTR gene on youth alcohol use. Instead, we expected to see an interaction among friend substance use, intervention program participation, and OXTR genetic risk on youth’s own alcohol use. Specifically, similar to Cleveland, et al.’s (2015) finding that the intervention potentiated the impact of high maternal involvement on alcohol use only among adolescents carrying sensitive genotypes, we expected that (1) the intervention would increase the impact of low-risk friends on adolescents’ alcohol use but (2) primarily among adolescents with high OXTR scores. Also, although it was based on the subsample of PROSPER participants with in-home interview data rather than the in-school data used herein, Cleveland et al’s (2015) null finding for intervention effects on 9th grade alcohol use led us not to hypothesize a main effect of the intervention on 9th alcohol use.
Four hypotheses guided our inquiry: First, OXTR variation will moderate the association between intervention status and affiliations with substance-using peers. Second, affiliations with substance-using friends will predict 9th-grade alcohol use. Third, OXTR variation will moderate the impact of affiliations with substance-using friends on adolescents’ own alcohol use. Fourth, OXTR variation will moderate the impact of intervention status on the association between affiliations with substance-using friends and individual adolescents’ own alcohol use.
Methods
Sample and participants
The PROSPER project is an evidence-based intervention program with longitudinal data on 28 communities in Iowa and Pennsylvania randomized into 14 control and 14 intervention units (for more information on intervention design see Spoth et al., 2013; Spoth, Redmond, et al., 2007). Students completed in-school questionnaires in the fall and spring of 6th grade with annual spring follow-ups until the 12th grade. See Figure 1 for the PROSPER CONSORT diagram (for design details see Spoth et al, 2013). In-school questionnaire included a request for participants to nominate up to two best friends and five additional close friends in their grade and school who also participated in the PROSPER project. Additionally, a random sample of 2,267 families of youth in Cohort 2 of the PROSPER project were invited to participate in the in-home data collections, which consisted of interviews and parent and adolescent questionnaires in Waves 1 through 5; 979 (43%) participated.
Figure 1. In-School Survey Total Participation by Wave*.
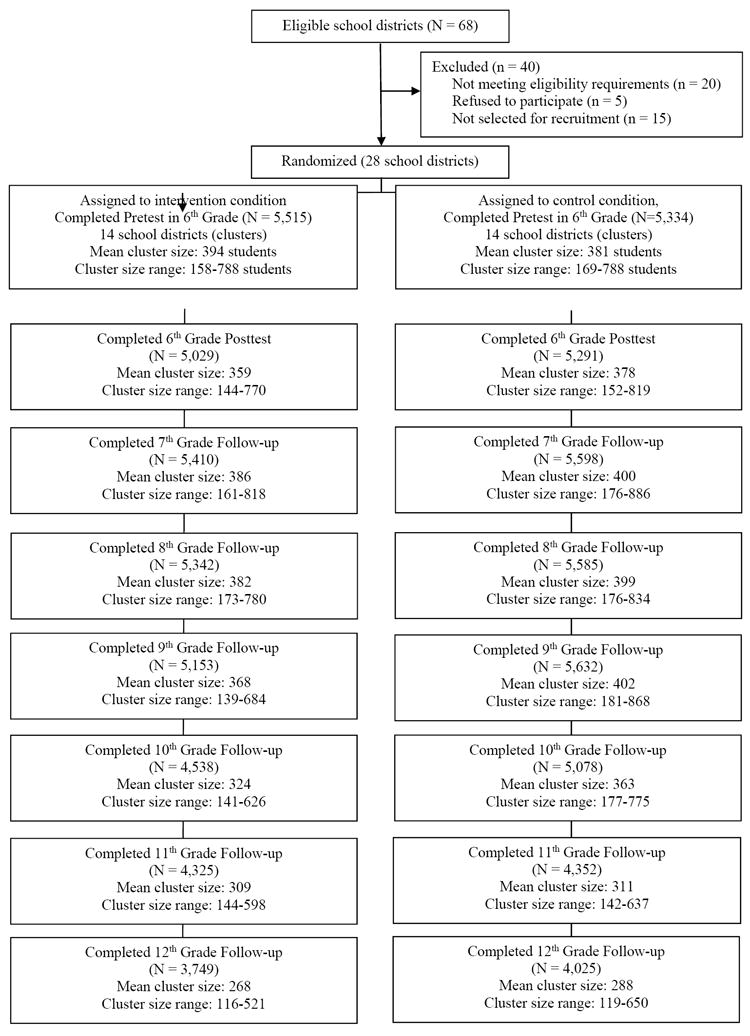
The gPROSPER sample includes 2,032 youth participating in the larger PROSPER project who provided buccal cell samples for genotyping. Of the 2,032 youth who provided buccal cell samples, 537 did so during the Wave 5 in-home assessment, and the remaining 1,495 provided samples through the mail as part of a young-adult follow-up assessment. Of the 2,032 participants who provided DNA,1,418 (69.78%) had valid peer nomination and self-report data. This reduced sample size was due to both missing data from the 9th-grade assessment (n=377), and the combined effect of genotype failures across all 5 SNPs (n= 237). The analytic sample was predominantly self-identified as Caucasian (90.6%), with fewer self-identified as Hispanics/Latino (4.4%), African-American (1.6%), Asian (1.3%), and other ethnicities (2%). About 23% of participants in the analysis sample took part in the family-focused program. Supplemental analyses were completed to confirm that the results of primary analyses do not vary based on family program participation.
Measures
Control for population stratification (PC1)
When testing for GxE (including G×I), it is important to account for differences in genotype and allele frequency distributions across geographic ancestry populations, known as population stratification. If these differences are not accounted for, spurious associations between alleles and phenotypes can result in false positives (Cardon & Palmer, 2003). We used 34 AIMs to identify geographic ancestry in a Principal Coordinate Analyses (PCoA) in which allele-sharing distances are used to extract principal coordinates (PCs) that describe the genetic ancestry of the sample (Halder et al., 2008). Based on PCoA’s of 1,922 participants (94.59% of samples from whom DNA was collected), PC1 provided an index of non-European ancestry that complemented information from self-reported ethnicity; high PC1 scores indicated less European ancestry.
OXTR Genetic Risk Score
DNA was collected using buccal swabs and genotyped using TaqMan assays on an OpenArray system (Life Technologies, part of Thermo Fisher Inc.) as described in Cleveland et al. (2015). Five Single Nucleotide Polymorphisms (SNPs) were used to calculate an OXTR genetic risk score (see Table 1). The OXTR risk score combines genetic variants chosen for their empirical relationships with what can be described as negative social behavior and related intrapersonal characteristics (e.g., aggression and low empathy). These SNPs, identified from the literature and searches of the Public Health Genomics Knowledge Base (Yu, Clyne, Khoury, & Gwinn, 2010; Yu, Gwinn, Clyne, Yesupriya, & Khoury, 2008), include rs6770632, rs53576, rs2254298, rs4686302, and rs1488467. We were unable to use the rs6770632 SNP, which has been associated frequently with social behavior (Feldman et al., 2015). However, rs7632287 is in strong linkage disequilibrium with this SNP, indicating that rs7632287 could be used as a proxy (R2 = .95;1000 Genomes Project Consortium, 2012). SNPs were coded so that 2 indicated homozygous for the risk allele (high), 1 indicated heterozygous for risk allele (medium), and 0 indicated homozygous for the non-risk allele (low). These values were summed for each individual to make up the OXTR genetic risk score (range = 2-8, M(SD) = 5.76 (1.11)).
Table 1.
Multilocus Oxytocin Score Marker Information
| RS Number | Position and Function | Alleles* | Risk | N | Citation |
|---|---|---|---|---|---|
| rs6770632 | 3’-UTR, Unk | C/C | High | 799 | Malik et al. (2012) |
| C/A | Medium | 537 | |||
| A/A | Low | 82 | |||
| rs53576 | Intron 3, Unk | A/A | High | 137 | Rodrigues et al. (2012) Kogan et al. (2011) Lucht et al. (2009) |
| A/G | Medium | 620 | |||
| G/G | Low | 661 | |||
| rs2254298 | Intron 3, Unk | G/G | High | 325 | Lucht et al. (2009) Montag et al. (2012) |
| G/C | Medium | 1093 | |||
| C/C | Low | ||||
| rs4686302 | Exon 3 | C/C | High | 1057 | Lucht et al. (2009) |
| Nonsynonymous | C/T | Medium | 339 | Wu et al. (2012) | |
| SNP (A218T) | T/T | Low | 22 | ||
| rs1488467 | 5’-flank, Unk | C/C | High | 5 | Nan, We, et al. (2012) |
| G/C | Medium | 160 | |||
| G/G | Low | 1253 |
Notes:
= Maj/Min, Risk Allele is in bold, Gene search used the Public Health Genomics Knowledge Base (https://phgkb.cdc.gov/GAPPKB/phgHome.do?action=home) (Yu et al., 2008; 2010)
Intervention Status
Of the analytic sample, 729 (51.4%) attended and participated in the school intervention, while the remaining 689 (48.6%) were in the control condition. Intervention status was coded as either “Control” (0) or “Intervention” (1).
Peer Substance Use
Social network modeling of peer substance use in these data is detailed elsewhere (Osgood, Ragan et al., 2013). Briefly, participants nominated up to two best friends and up to five other close friends in the same grade who also attended the same school. Peer substance use during the 9th grade was based on the nominated peers’ self-reported past month substance use. The substance use measure was based on the mean of four items that addressed how often during the past month the participant (1) drank alcohol, (2) drank enough alcohol to become intoxicated, (3) smoked cigarettes, and (4) used marijuana (1 = Not at all, 5 = More than once a week). Higher scores indicated higher substance use (M(SD) = 1.44(.52)).
Alcohol Use
Alcohol use by the 9th grade was assessed using three aggregated “Yes” (1) or “No” (0) items: “Have you ever had a drink of alcohol?”, “Have you ever drunk more than just a few sips of alcohol?”, and “Have you ever been drunk from drinking alcohol?” (range = 0-3, Cronbach’s α = .79, M(SD) = 1.44 (0.52)).
Statistical Analysis
To address the clustered structure of the data (i.e., children nested within schools), we fit a multilevel model of covariance in SAS PROC MIXED with the REPEATED statement and maximum likelihood estimation. The amount of variance in 9th-Grade Alcohol Use at the school district level was low (intra-class correlation = .012).
Results
Preliminary Results
Three sets of preliminary analyses were conducted to examine possible sources of bias in the data. First, we tested for differential attrition between adolescents in analysis (N = 1418) and the full genetic sample (N = 2032) on key sociodemographic characteristics collected at the baseline during the 6th grade. There was no statistical difference for baseline free and reduced lunch (F(1, 2,030) = .30, p = .583), however, there was a difference for baseline parent marital status (F(1, 2,030) = 9.88, p = .002). The Cohen’s d for the differences in parent marital status was d= 0.15, indicating a small difference. Second, using PLINK (v 1.9, Purcell et al., 2007) the Hardy-Weinberg Equilibrium of the OXTR SNPs was tested to determine whether sample allele and genotype frequencies were consistent with Mendelian patterns of inheritance. All five SNPs were in Hardy-Weinberg equilibrium (p-values > 0.05), consistent with accurate genotyping. In addition, the SNPs were not in linkage disequilibrium (r2 < 0.13), indicating that the use of these selected SNPs does not provide redundant information and combined use allows for better coverage of variation across the OXTR gene. Third, PC1 was not was related to analyses’ measures, indicating population stratification was unlikely to confound results.
Primary Analysis
To examine Hypothesis 1 analyses were conducted to determine the moderating role of OXTR risk on the relationship between Intervention Status and substance-using peer affiliations. The first model, a regression model, included main effects for Intervention Status and OXTR risk. Both intervention status (b = −0.07, p < .01) and OXTR (b = 0.03, p < .01) predicted affiliation with substance-using friends such that adolescents in the intervention had fewer substance-using friends and higher OXTR risk was related to having more substance-using friends. In the second model, a two-way interaction between Intervention Status and OXTR risk was added to the first, which was significant (b = 0.06, p < .01). The interaction remained significant after controlling for PC1. Figure 2 illustrates that OXTR variability was not associated with 9th-grade peer substance use within the control group, but had substantial effect in the intervention condition. Figure 2 shows that affiliations with substance-using friends were never higher in the intervention condition than in the control condition. In fact, the intervention appeared to provide a protective effect among low OXTR risk youth in the intervention, who affiliated with substantially lower-risk peers. In contrast, the peer affiliations of high-risk OXTR youth were not affected, for better or worse, by the intervention.
Figure 2.
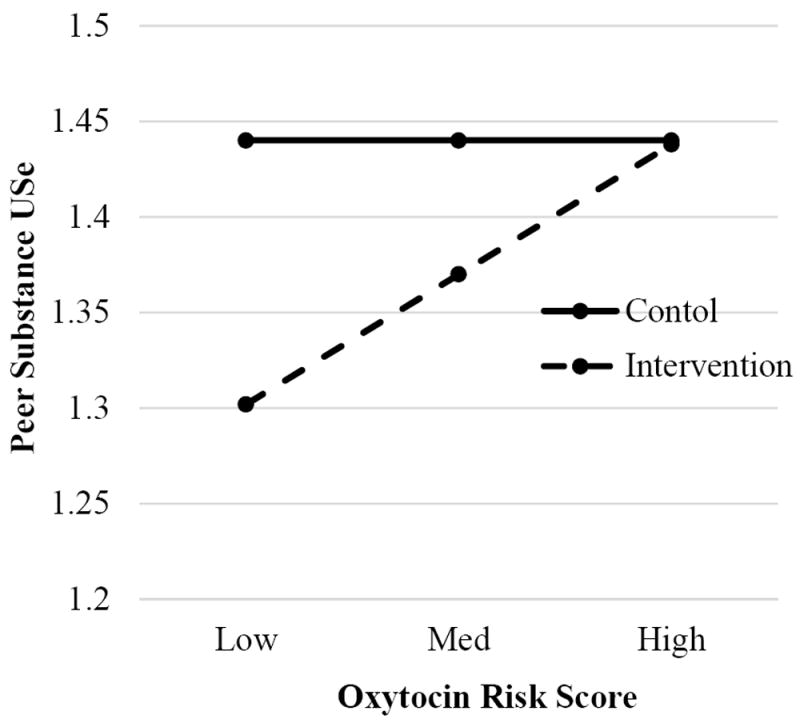
Effects of intervention on 9th-grade peer substance use by oxytocin risk score.
A series of three models predicting 9th-Grade Alcohol Use were conducted to examine Hypotheses 2 through 4. The first model included main effects of OXTR risk, Intervention Status, and Peer Substance Use; the second model added the three two-way interactions; and the last model included the three-way interaction with the subordinate two-ways and main effects retained (see Table 2 for full results). Results supported Hypothesis 2: While controlling for Intervention Status and OXTR risk, higher peer substance use was related to higher adolescent alcohol use (b = 0.82, p < .01). Main effects of intervention status (b = 0.03, ns) and OXTR (b = −0.01, ns) on alcohol use were not significant, however. None of the two-way interactions were significant when added to the model, demonstrating no support for Hypothesis 3. OXTR variation did not moderate the impact of affiliations with substance-using friends on adolescents’ own alcohol use. However, the significant three-way interaction in the final model supported Hypothesis 4 (b = -0.22, p < .05). OXTR variation did moderate the impact of intervention status on links between affiliations with substance-using friends and individual adolescents’ own alcohol use. Parameters in this last model were robust to the PC1 covariate.
Table 2.
Parameter Estimates and Standard Errors for Intervention Condition, 9th Peer Substance Use, and Oxytocin Risk Score Effects on 9th-Grade Alcohol Use Models
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| b | SE | b | SE | b | SE | |
| Main Effects | ||||||
| Intercept | 1.41** | 0.04 | 1.41** | 0.04 | 1.41** | 0.04 |
| Condition | 0.03 | 0.06 | 0.03 | 0.06 | 0.04 | 0.06 |
| Peer Use | 0.82** | 0.06 | 0.92** | 0.08 | 0.92** | 0.08 |
| Oxy Risk Score | -0.01 | 0.03 | -0.05 | 0.04 | -0.06 | 0.04 |
| Two-way Interactions | ||||||
| Cond X Peer Use | -0.21 | 0.11 | -0.15 | 0.12 | ||
| Cond X Oxy | 0.09 | 0.05 | 0.09 | 0.05 | ||
| Peer Use X Oxy | -0.01 | 0.05 | 0.11 | 0.07 | ||
| Three-way Interaction | ||||||
| Cond X Peer Use X Oxy | -0.22** | 0.09 | ||||
Notes: Cond = Condition, Oxy = Oxytocin Risk Score, Alc = Alcohol Use, Peer Use= Peer Substance Use.
p < .05,
p < .01.
The effects of the three-way interaction were probed using a series of conditional main effects tests using techniques recommended by Frazier, Tix, and Barron (2004; i.e., simple effects tests). Specifically, analyses were conducted wherein categorical variables were reverse-coded and continuous variables were re-centered ±1 SD around their mean. This method provides slopes for the association between peer substance use and alcohol use separated by intervention and control participants.
Figure 3 presents the associations between peer substance use and adolescent alcohol use in the intervention vs. control conditions across high, medium, and low levels of OXTR risk. Two important inferences can be drawn from the figure. First, across each level of OXTR and intervention level, higher substance-using peer scores were related to higher alcohol use, demonstrating the consistency of this main effect. Second, the three-way interaction among OXTR, Intervention Status, and Peer Substance Use appears to be largely due to differences in alcohol use between intervention and control youth at high OXTR risk among adolescents who associate with low substance-using friends (see the far left end of the far right panel). Among these high OXTR risk, the effect of peer substance use on adolescents’ alcohol use diverge, taking on a relatively steeper slope in the control condition than in the intervention condition. The divergence can be accounted for by lower alcohol use among adolescents who affiliate with low substance-using friends within the control condition compared to the intervention. Thus, it seems that for a subset of youth—those with high OXTR risk but who associate with low-risk friends—the intervention is associated with higher alcohol use than comparable youth in the control group. It should be noted that this effect is relatively small and occurs among youth who report the lowest levels of drinking: those who affiliate with low substance-using friends.
Figure 3.

Associations between peer substance use levels and 9th-grade alcohol use for intervention and control youth across high, medium, and low levels of oxytocin risk.
In addition, we considered whether the primary results held up when controlling for participation in the family-based intervention, the Strengthening Families Program (SFP), in which 22.63% of those in the intervention communities took part. First, there was no difference on Peer Substance Use between SFP participants and either all others, t (1415) = 1.55, ns, or intervention community members whose families did not take part in the SFP intervention, t (727) = 0.67, ns. Second, adding a dummy variable indicating SFP participation to the analyses did not change results. Specifically, compared to -0.22 (p < .05) the three-way interaction parameter was -0.22(p < .05).
Key findings from these results are that: (1) low OXTR risk youth associate with low substance-using friends in the intervention but not the control condition, (2) a main effect of substance-using friends on adolescents’ own alcohol use exists across all combinations of OXTR and intervention conditions, and (3) the three-way interaction is driven by adolescents with higher OXTR risk who associate with lower substance-using friends reporting somewhat higher alcohol use in the intervention than similar adolescents’ reports in the control group. The difference in alcohol use levels captured by this interaction was small and occurred at the low end of the alcohol use scale.
Discussion
The purpose of this study was to investigate whether OXTR variation moderated intervention effects on both affiliations with substance-using peers and the impact of these affiliations on adolescents’ own alcohol use in the 9th grade. Analyses addressing affiliation revealed a two-way interaction with OXTR variation predicting peer substance use, whereby lower OXTR risk was linked to lower levels of peer substance use among intervention youth but not control youth. The interpretation of this interaction is straightforward: Within the intervention condition OXTR variation has a strong effect on the selection of substance-using friends, and no effect on the selection of those friends in the control condition.
In contrast to this clear result, analyses addressing the possible role of OXTR variation on the link between exposure to substance using peers and adolescents’ own alcohol use and how this association might vary across intervention conditions provided more mixed results. Although the three-way interaction among the OXTR score, peer substance use, and intervention status was significant, like all adolescents affiliating with low substance-using peers, the alcohol use levels of the high OXTR risk adolescents who affiliate with low substance-using peers in the intervention, whose use drove this result, were low compared to the full sample. Rather than finding that OXTR substantially moderated the impact of peers, or the combined impact of peers and intervention, affiliations with substance-using peers were consistently linked to individuals’ own alcohol use across all combinations of OXTR variation and intervention conditions.
That affiliation with substance-using friends is similarly linked to individual adolescents’ alcohol use across all combinations of intervention and OXTR risk is important. Findings suggest that the interactive effects of OXTR variation manifest more in terms of peer selection than peer influence. The shape of the OXTR by intervention interaction is important. First, rather than crossing over, the interaction took on a fan-shape, whereby the intervention appears to facilitate the ability of low OXTR risk adolescents to select peers engaging in very low levels of substance use. Perhaps such adolescents, without the intervention, would minimize or ignore their friends’ substance use out of loyalty or empathy. Given that across all combinations of OXTR risk and intervention vs. control conditions, affiliating with peers who use fewer substances appears to be better than affiliating with higher-use peers, the ability of the intervention to positively impact this peer context is important.
Framed differently, findings also contribute to our understanding of just what OXTR variation itself may impact and the conditions under which this impact might change. The impact of OXTR variability on the affiliations with risky peers seems to be facilitated by the PROSPER interventions. Across the control and intervention conditions, youth with more OXTR ‘risk” alleles are equally prone to affiliate with high-risk peers. These youth follow the general preference for friends who engage in problem behaviors (see Osgood, Feinberg, and Ragan, 2015). In contrast, messages delivered by the intervention appear to reduce the general preference for risky friends, but only among low OXTR risk youth. It may be that having fewer “risky” OXTR alleles conveys a weaker affiliative drive. Alternatively, these less “risky” alleles might convey a greater sensitivity to the larger school context.
Limitations and Future Directions
The current study uses a cGxE framework to examine the effect of the interactive relationship among OXTR status, affiliating with substance-using peers, and substance use interventions. This framework is uniquely suited to understanding for whom and why environments matter (Schlomer et al., 2015). Some critics of this method have indicated that single-gene approaches do not reflect how genetic variance impacts behaviors. This critique is true. However, the use of single genes, whether VNTRs, such as DRD4 or 5-HTTLPR, or via multiple SNPs, as we do here, comes with the advantage of understanding the role of specific genes on specific biological processes that in turn affect behaviors via neurocognitive processes and differences in individual characteristics. Although these pathways are not by any means fully understood, the use of genes with roles in these pathways provides the opportunity to gain greater insight into whom is being affected by interventions and why. Other approaches offer advantages as well. Although they are agnostic to the identity of specific genes, twin studies provide the basis for determining the existence and extent of genetic influences on infinite phenotypes, ranging from the obvious, such as height, to the somewhat surprising, such as affiliations with substance-using peers. GWA approaches are also powerful tools for discovery, identifying specific genes rather than the variance accounted for by genetic variance. But given the large sample sizes required to make them most effective and the averaged environments across which they scour the genome for “hits”, they do not provide insight into how genes work or, of course, how environments impact behaviors. In comparison, adding assays of specific genetic variance to behavioral science data sets allows researchers the opportunity to make prior predictions for how genes with known characteristics may transact with experiences to impact human behavior. Of course, not all of these hypotheses will be correct. Our predictions for OXTR impacts on peer selection were supported. Our predictions for OXTR moderating peers’ direct effects and their interactions with interventions were not.
The current analyses predict alcohol use, but not volume or frequency. We chose this measure because drinking, regardless of level, by 9th grade is related to increased risk for a range of problem behaviors in adolescence and later alcohol problems in young adulthood (Pikanen et al., 2005). Future cGxI analyses of the PROSPER data set will no doubt focus on other alcohol outcomes, included but not limited to volume and frequency of drinking in later adolescence and problem drinking in young adulthood. In addition, it would be very useful to follow-up the most compelling finding of this study—that OXTR risk interacts with intervention status to impact the selection of risky peers—with longitudinal analyses. The current study focused on 9th grade outcomes as peers are especially relevant (Steinberg & Monahan, 2007) and drinking appears especially harmful during this time. Because PROSPER has longitudinal data on peer affiliations across each grade from 6th to 12th grade, it provides the opportunity to investigate not only what genes impact peer affiliation, but also when different genes impact these affiliations.
Another possible limitation is that we discuss our OXTR SNP score in terms of low-versus high-risk, in part to align our work with current cGxE frameworks. Understanding the relationship among OXTR, oxytocin, and behavior is a work in progress. Given what appears to be the highly contextual nature of oxytocin’s relationship to behavioral outcomes, the label “risk” may be less accurate than “affiliative tendency.” Hopefully in the future we will have a better understanding of and label for OXTR gene variation. Because they are often longitudinal and focus on measuring experiences and processes in the social domains through which interventions are designed to work, such as via parent-child and peer-youth relationships, preventative intervention data sets might prove especially valuable for uncovering how OXTR variation, as well as variation in other genes and sets of genes, affects social processes. Kurt Lewin’s phrase, “If you want truly to understand something, try to change it”, is often mentioned by prevention researchers to underscore the implications of interventions for understanding the etiology of the behaviors they are trying to change. It seems that this phrase is equally applicable to the advantage of intervention for trying to understand how and why specific genes impact behaviors.
Acknowledgments
The authors would like to thank Eric Tricou, and The Penn State Genomics Core Facility, for technical assistance. Work on this paper was supported by the National Institute on Drug Abuse (grants DA030389, DA013709, and R01-DA018225).
Footnotes
Compliance with Ethical Standards
The authors declare that they have no conflicts of interest.
Research was approved by The Pennsylvania State University Office of Research Protections. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
References
- 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences. 2010;107(9):4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bauman KE, Ennett ST. On the importance of peer influence for adolescent drug use: Commonly neglected considerations. Addiction. 1996;91(2):185–198. [PubMed] [Google Scholar]
- Brody GH, Beach SRH, Philibert Ra, Chen Y, Murry VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: Gene × environment hypotheses tested via a randomized prevention design. Child Development. 2009;80(3):645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. The Lancet. 2003;361(9357):598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Carter SC. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Cecil CAM, Lysenko LJ, Jaffee SR, Pingault J-B, Smith RG, Relton CL, Barker ED, et al. Environmental risk, Oxytocin Receptor Gene (OXTR) methylation and youth callous-unemotional traits: A 13-year longitudinal study. Molecular Psychiatry. 2014;19(10):1071–1077. doi: 10.1038/mp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini R, Trost M. Social influence: Social norms, conformity and compliance. In: Gilbert G, Fiske DT, Lindzey ST, editors. The handbook of social psychology. 4. Vol. 2. Boston: McGraw-Hill; 1998. [Google Scholar]
- Cleveland HH, Schlomer GL, Vandenbergh DJ, Feinberg ME, Greenberg M, Spoth R, Redmond C, Shriver MD, Zaidi AA, Hair KL. The conditioning of intervention effects on early adolescent alcohol use by maternal involvement and dopamine receptor D4 (DRD4) and serotonin transporter linked polymorphic region (5-HTTLPR) genetic variants. Development and Psychopathology. 2015;2:51–67. doi: 10.1017/S0954579414001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland HH, Wiebe R, Rowe DC. Genetic Influences on associations with substance using peers. Journal of Genetic Psychology. 2005;166:153–169. [PubMed] [Google Scholar]
- Cleveland HH, Wiebe R. Understanding the progression from adolescent marijuana use to young adult serious drug use: Gateway effect or developmental trajectory? Development and Psychopathology. 2008;20:615–632. doi: 10.1017/S0954579408000308. [DOI] [PubMed] [Google Scholar]
- Creswell KG, Sayette MA, Manuck SB, Ferrell RE, Hill SY, Dimoff JD. DRD4 polymorphism moderates the effect of alcohol consumption on social bonding. PLoS ONE. 2012;7(2):e28914. doi: 10.1371/journal.pone.0028914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biological Psychiatry. 2015:1–11. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Fisher LB, Miles IW, Austin SB, Camargo Ca, Colditz GA. Predictors of initiation of alcohol use among US adolescents: Findings from a prospective cohort study. Archives of Pediatrics & Adolescent Medicine. 2007;161(10):959–966. doi: 10.1001/archpedi.161.10.959. [DOI] [PubMed] [Google Scholar]
- Griffin AM, Cleveland HH, Schlomer GL, Vandenbergh DJ, Feinberg ME. Differential susceptibility: The genetic moderation of peer pressure on alcohol use. Journal of Youth and Adolescence. 2015;44(10):1841–1853. doi: 10.1007/s10964-015-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: Implications for substance abuse prevention. Psychological Bulletin. 1992;112(1):64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Keyes MA, Iacono WG, McGue M. Early onset problem behavior, young adult psychopathology, and contextual risk. Twin Research and Human Genetics. 2007;10(01):45–53. doi: 10.1375/twin.10.1.45. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Larson RW, Richards MH, Moneta G, Holmbeck G, Duckett E. Changes in adolescents’ daily interactions with their families from ages 10 to 18: Disengagement and transformation. Developmental Psychology. 1996;32(4):744–754. [Google Scholar]
- Luo S, Han S. The association between an oxytocin receptor gene polymorphism and cultural orientations. Culture and Brain. 2014;2:89–107. [Google Scholar]
- MacLean EL, Hare B. Dogs hijack the human bonding pathway. Science. 2015;348(6232):280–281. doi: 10.1126/science.aab1200. [DOI] [PubMed] [Google Scholar]
- Malik AI, Zai CC, Abu Z, Nowrouzi B, Beitchman JH. The role of oxytocin and oxytocin receptor gene variants in childhood-onset aggression. Genes, Brain and Behavior. 2012;11(5):545–551. doi: 10.1111/j.1601-183X.2012.00776.x. [DOI] [PubMed] [Google Scholar]
- Montag C, Brockmann E-M, Lehmann A, Müller DJ, Rujescu D, Gallinat J. Association between oxytocin receptor gene polymorphisms and self-rated “empathic concern” in schizophrenia. PloS One. 2012;7(12):e51882. doi: 10.1371/journal.pone.0051882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood DW, Feinberg ME, Gest SD, Moody J, Ragan DT, Spoth R, Redmond C, et al. Effects of PROSPER on the influence potential of prosocial versus antisocial youth in adolescent friendship networks. Journal of Adolescent Health. 2013;53(2):174–179. doi: 10.1016/j.jadohealth.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood DW, Feinberg ME, Ragan DT. Social networks and the diffusion of adolescent problem behavior: Reliable estimates of selection and influence from 6th through 9th grade. Prevention Science. 2015;16:832–843. doi: 10.1007/s11121-015-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood DW, Ragan DT, Wallace L, Gest SD, Feinberg ME, Moody J. Peers and the emergence of alcohol use: Influence and selection processes in adolescent friendship networks. Journal of Research on Adolescence. 2013;23(3):500–512. doi: 10.1111/jora.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DC, Cornwell B. Reconsidering peer influences on delinquency: Do less proximate contacts matter? Journal of Quanititative Criminology. 2007;23:127–149. [Google Scholar]
- Pitkänen T, Lyyra A-L, Pulkkinen L. Age of onset of drinking and the use of alcohol in adulthood: a follow-up study from age 8–42 for females and males. Addiction. 2005;100(5):652–661. doi: 10.1111/j.1360-0443.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Boergers J, Spirito A. Adolescents’ and their friends’ health-risk behavior: Factors that alter or add to peer influence. Journal of Pediatric Psychology. 2001;26(5):287–298. doi: 10.1093/jpepsy/26.5.287. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Sham PC, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice TR, Derish NE. Oxytocin and callous-unemotional traits: Towards a social-cognitive approach to forensic analysis. International Journal of Adolescent Medicine and Health. 2015;27(2):195–201. doi: 10.1515/ijamh-2015-5011. [DOI] [PubMed] [Google Scholar]
- Schlomer GL, Cleveland HH, Feinberg ME, Wolf PSA, Greenberg MT, Spoth RL, Redmond C, Tricou EP, Vandenbergh DJ. Extending previous cGxI findings on 5-HTTLPR’s moderation of intervention effects on adolescent substance misuse initiation. Child Development. doi: 10.1111/cdev.12666. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman I, Kanat-Maymon Y, Ebstein RP, Feldman R. Cumulative risk on the oxytocin receptor gene (OXTR) underpins empathic communication difficulties at the first stages of romantic love. Social Cognitive and Affective Neuroscience. 2013:1524–1529. doi: 10.1093/scan/nst142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biological Psychiatry. 2015;(26):1–9. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Spoth R, Greenberg M, Bierman K, Redmond C. PROSPER community-university partnership model for public education systems: Capacity-building for evidence-based, competence-building prevention. Prevention Science. 2004;5(1):31–39. doi: 10.1023/b:prev.0000013979.52796.8b. [DOI] [PubMed] [Google Scholar]
- Spoth R, Guyll M, Redmond C, Greenberg M, Feinberg M. Six-year sustainability of evidence-based intervention implementation quality by community-university partnerships: The PROSPER study. American Journal of Community Psychology. 2011;48(3-4):412–425. doi: 10.1007/s10464-011-9430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R, Redmond C, Shin C, Greenberg M, Clair S, Feinberg M. Substance-use outcomes at 18 months past baseline. American Journal of Preventive Medicine. 2007;32(5):395–402. doi: 10.1016/j.amepre.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R, Redmond C, Shin C, Greenberg M, Feinberg M, Schainker L. PROSPER community-university partnership delivery system effects on substance misuse through6½ years past baseline from a cluster randomized controlled intervention trial. Preventive Medicine. 2013;56:190–196. doi: 10.1016/j.ypmed.2012.12.013. http://dx.doi.org/10.1016/j.ypmed.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Monahan KC. Age differences in resistance to peer influence. Developmental Psychology. 2007;43(6):1531–1543. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Silverberg SB. The vicissitudes of autonomy in early adolescence. Child Development. 1986;57(4):841. doi: 10.1111/j.1467-8624.1986.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Stueve A, O’Donnell LN. Early alcohol initiation and subsequent sexual and alcohol risk behaviors among urban youths. American Journal of Public Health. 2005;95(5):887–893. doi: 10.2105/AJPH.2003.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JMJ, Spotts EL, Westberg L, et al. Variation in the oxytocin receptor gene (OXTR) is associated with pair-bonding and social behavior. Biol Psychiatry. 2012;71(5):419–426. doi: 10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Li Z, Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. Journal of Affective Disorders. 2012;138(3):468–472. doi: 10.1016/j.jad.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Yu W, Clyne M, Khoury MJ, Gwinn M. Phenopedia and genopedia: Disease-centered and gene-centered views of the evolving knowledge of human genetic associations. Bioinformatics. 2010;26(1):145–146. doi: 10.1093/bioinformatics/btp618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Gwinn M, Clyne M, Yesupriya A, Khoury MJ. A navigator for human genome epidemiology. Nature Genetics. 2008;40(2):124–125. doi: 10.1038/ng0208-124. [DOI] [PubMed] [Google Scholar]


