Abstract
Post-traumatic stress disorder (PTSD) is associated with immune dysregulation. We have previously shown that severe stress exposure in a preclinical animal model of the disorder, stress-enhanced fear learning (SEFL), is associated with an increase in hippocampal interleukin-1β (IL-1β) and that blocking central IL-1 after the severe stress prevents the development of SEFL. Here, we tested whether blocking hippocampal IL-1 signaling is sufficient to prevent enhanced fear learning and identified the cellular source of stress-induced IL-1β in this region. Experiment 1 tested whether intra-dorsal hippocampal (DH) infusions of interleukin-1 receptor antagonist (IL-1RA, 1.25μg per hemisphere) 24 and 48 hours after stress exposure prevents the development of enhanced fear learning. Experiment 2 used triple fluorescence immunohistochemistry to examine hippocampal alterations in IL-1β, glial fibrillary acidic protein (GFAP), an astrocyte-specific marker, and ionized calcium binding adaptor molecule -1 (Iba-1), a microglial-specific marker, 48 hours after exposure to the severe stressor of the SEFL paradigm. Intra-DH IL-1RA prevented SEFL and stress-induced IL-1β was primarily colocalized with astrocytes in the hippocampus. Further, hippocampal GFAP immunoreactivity was not altered, whereas hippocampal Iba-1 immunoreactivity was significantly attenuated following severe stress. These data suggest that hippocampal IL-1 signaling is critical to the development of SEFL and that astrocytes are a predominant source of stress-induced IL-1β.
Keywords: PTSD, SEFL, Fear, Cytokines, Microglia, Astrocytes, Hippocampus, Stress
1. Introduction
Converging evidence from both human and animal studies has suggested that psychiatric disorders involving depression and anxiety, including post-traumatic stress disorder (PTSD), involve substantial immune system dysregulation [1–5]. Several published studies have reported that PTSD is associated with elevated peripheral cytokines, such as interleukin-1β (IL-β), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) [4, 6–9]. Cohen and colleagues have even suggested IL-1 as a potential biomarker for susceptibility to PTSD [10]. Central IL-1 signaling is consistently shown to be upregulated by a variety of different stress protocols in rodents and to be critically involved in stress response mechanisms that drive behavioral outcomes [11, 12]. For example, peripheral administration of IL-1β has been shown to lead to enhanced anxiety-like behavior in the elevated plus maze (EPM) [13], and blocking IL-1 signaling centrally prevents stress-induced reductions in social interaction [14]. We recently published the finding that stress-enhanced fear learning (SEFL), a preclinical animal model of PTSD developed by Rau and colleagues [15], requires central IL-1 signaling. Our data demonstrated that the severe stressor of the SEFL paradigm (15 foot shocks) induces an increase in IL-1β in the dorsal hippocampus (DH) 24–48 hours after the stress. Furthermore, blocking IL-1 signaling in the brain through an intracerebroventricular infusion of IL-1 receptor antagonist (IL-1RA) prevents the development of enhanced fear learning [5]. Together these data suggest that central IL-1RA may be acting specifically in the hippocampus. Accordingly the first goal of the current study was to test whether hippocampal IL-1 signaling 24–48 hours after severe stress is necessary for the expression of SEFL.
The second goal of the current study was to isolate and quantify colocalization of stress-induced IL-1β with cell-specific markers glial fibrillary acidic protein (GFAP), ionized calcium binding adaptor molecule-1 (Iba-1), and neuronal nuclear antigen (NeuN) in order to isolate the cellular source of stress-induced hippocampal IL-1β. IL-1β can be expressed by many cell types in the brain, including microglia, astrocytes, and neurons [16–21]. A critical component to better understanding the mechanism through which hippocampal IL-1 might influence behavioral outcomes following stress is to identify which cell type(s) produce(s) it in response to stress. While there is evidence of IL-1 expression in neurons [17, 20, 22], there is only one report to our knowledge of an effect of stress on neuron-derived IL-1β. Kwon and colleagues reported an increase in IL-1β colocalized with neuronal nuclei following four days of restraint stress [22]. In contrast, there are several published studies to support the potential for microglia-derived or astrocyte-derived IL-1β, as described below.
Microglia are brain macrophage cells that play important roles in the healthy brain, both maintaining the cellular environment and protecting against injury or immune challenge [23–27]. A substantial population of microglia are present in the hippocampus [28], and IL-1β is just one of the proinflammatory mediators released by activated microglia [23–26]. While microglial activation and release of proinflammatory cytokines are well-established in the context of neurodegenerative diseases, there are inconsistencies regarding the timing, brain region–specificity, and direction of the effect of psychological stressors on microglia. Two independent groups reported no change in microglial gene expression in the hippocampus immediately following exposure to foot shock [29, 30]. However, Sugama and colleagues reported that microglial activation was increased one to six hours following a two hour exposure to restraint stress in the thalamus, hypothalamus, and hippocampus [31]. Interestingly, in the same report, hypothalamic microglial activation was only associated with an increase in IL-1β mRNA and immunoreactivity when induced by lipopolysaccharide (LPS), but not when induced by restraint stress. Consistent with an increase in microglial activation and proliferation in response to stress, Frank et al. reported that major histocompatibility complex II immunoreactivity (MHC II, predominantly expressed by microglia) was increased in the hippocampus 24 hours after inescapable tail shock [32], but they observed no change in either GFAP or Iba-1 immunoreactivity in the same tissue.
The final candidate for a potential source of stress-induced IL-1β is astrocytes. Though traditionally viewed merely as neuronal “glue”, astrocytes are now known to be critically involved in a diverse array of functions in development and disease [33]. Converging evidence from several laboratories using a variety of different severe stress procedures suggests that both GFAP expression and astrocyte process length are altered over time in the brain following stress [34–37]. Choi and colleagues observed an increase in the length and number of astrocyte processes but a decrease in GFAP in the DH one hour, but not 24 hours, after exposure to foot shock fear conditioning [36]. In contrast, others have observed decreases in the number of astrocyte processes either following chronic restraint stress [35] or 24–48 hours after foot shock exposure [34]. Of particular relevance here, Sugama and colleagues found that IL-1β expression was increased specifically in astrocytes, and not microglia, following cold stress [38]. The second goal of the current study was to isolate and quantify colocalization of stress-induced IL-1β with GFAP, Iba-1, and NeuN in order to isolate the cellular source of stress-induced hippocampal IL-1β.
Importantly, much of the previous literature regarding gene expression and morphology of both astrocytes and microglia focuses on early time points post-stress. Given that we have previously reported that the IL-1-dependent mechanism that attenuates the development at SEFL is specific to the later time points following stress, 24–48 hours [5, 39], here we focus on changes in the DH at 48 hours after foot shock stress. Specifically, experiment 1 tested whether IL-1 signaling in the DH is critical to the development of a PTSD-like phenotype in SEFL. Experiment 2 examined stress-induced changes in astrocytes and microglia in the DH and identified the cellular source of stress-induced IL-1β in this region. Analyses from experiment 2A replicated our previous finding of stress-induced IL-1β in the dorsal hippocampus. Analyses from experiment 2B quantified GFAP and Iba-1 immunoreactivity to examine stress-induced changes in astrocytes and microglia, respectively. Finally, analyses in experiment 2C used Bitplane Imaris software in combination with confocal microscopy to visualize the colocalization of IL-1β with GFAP, Iba-1, and NeuN following foot shock to isolate and quantify astrocyte-derived, microglia-derived, and neuron-derived IL-1β, respectively. Collectively, these experiments tested the hypotheses that IL-1 signaling in the DH is critical for the development of SEFL and that astrocytes are the predominant cellular source of hippocampal IL-1β following stress in this context.
2. Methods
2.1 Animals
Male Sprague Dawley rats (225–250 g, Charles River Laboratories, Raleigh, NC) were housed individually under a reversed 12 hour light-dark cycle. They were given ad libitum access to food and water and were handled regularly throughout all experiments. All procedures were conducted in accordance with and approval by the UNC Institutional Animal Care and Use Committee.
2.2. Experiment 1: Effect of intra-dorsal hippocampal IL-1RA on SEFL
2.2.1. Surgery
Animals were anesthetized with a 1.0 mg/kg intraperitoneal injection of 9:1 (vol:vol) ketamine hydrochloride (100mg/ml) mixed with xylazine (100 mg/ml). Guide cannulae (26 Gauge, Plastics One, Roanoke, VA) were directed bilaterally at the DH (AP −3.4 mm, ML ± 3.1 mm, DV −2.2 mm, 15 degrees, relative to bregma). Animals were given one week for postoperative recovery prior to the start of any experimental procedures. Upon completion of the experiment, correct cannula placement was verified and any animals with incorrect placement were dropped from the analysis.
2.2.2. Stress-enhanced fear learning
All animals (N = 36, n = 9) were assigned to a Context A treatment (foot shock or no foot shock) and a drug treatment (IL-1RA or vehicle) and exposed to the SEFL paradigm (Figure 1), as has been previously described [5, 39]. Briefly, on Day 1, animals were exposed to Context A (BRS/LVE, Laurel, MD; H 26.7 cm × D 24.8 cm × W 30.7 cm) which was housed in a separate room with distinct textile, olfactory, and auditory characteristics from the home cage. Animals assigned to the foot shock condition received 15×2 mA scrambled foot shocks over 90 minutes on a 6 minute variable interval schedule while control animals were exposed to the context for the same amount of time without foot shocks being delivered. Six days later, animals were exposed to Context B (Med Associates, St. Albans, VT), which was again housed in a separate room from Context A and the home cage. Context B was also associated with distinct textile, olfactory, and auditory characteristics from both Context A and the home cage. In addition, behavior in Context B was recorded using a video recording system (Sony Video Camera Model HDR-CX150). Similar to Rau and colleagues [15], animals were exposed to Context B for 30 minutes without foot shocks being delivered to allow for habituation to the new context. On Day 8, animals were placed back into Context B where all animals received a single 1 mA scrambled foot shock, 3 minutes, 12 seconds after being placed into the context. Behavior during the three minutes prior to the single shock was recorded and analyzed to test for generalization of fear between the two contexts (these data are presented as ‘baseline’ in Figure 1). On Days 9, 10, 15 and 23 (Test Days 1, 2, 7 and 14), animals were placed in Context B for 8 minutes, 32 seconds and behavior was recorded.
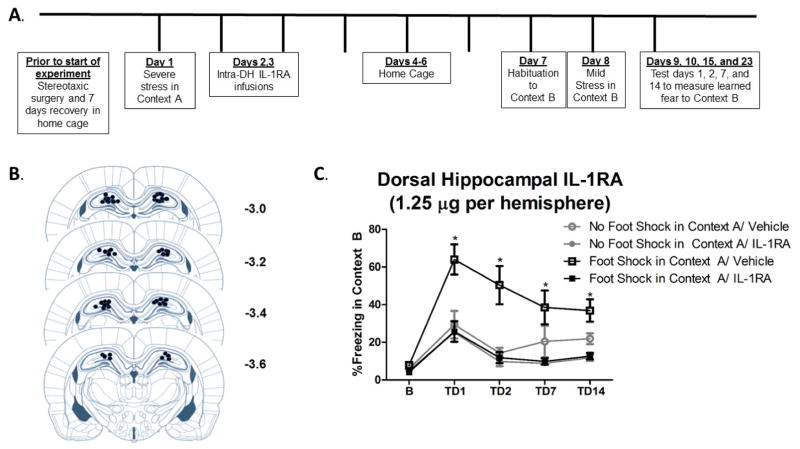
Figure 1. Intra-dorsal hippocampal IL-1RA is sufficient to prevent SEFL.
A. Schematic shows experiment 1 timeline of surgical procedures, intra-DH microinfusions, and severe stress exposure and contextual fear learning in the SEFL paradigm. B. Paxinos and Watson (2007) schematics of the rat brain show approximate cannulae placement. Coordinates −3.0 through −3.6 from Bregma are shown. Each circle represents where damage from the cannula tract was observed for all animals included in the analysis. C. DH-IL-1RA significantly attenuated SEFL. There were no differences between groups in freezing to Context B prior to the single shock. Stress-enhanced fear learning was observed within vehicle-treated groups in that rats that received foot shock in Context A followed by vehicle exhibited significantly more fear learning to Context B than rats that received no foot shock in Context A followed by vehicle. Critically, animals that received foot shock in Context A followed by IL-1RA exhibited significantly less freezing than animals that received foot shock in Context A followed by vehicle. Thus, IL-1RA prevented the expression of SEFL. * Foot Shock in Context A/Vehicle vs. Foot Shock in Context A/IL-1RA, p < 0.05.
Ethovision XT video tracking software (Noldus Information Technology Inc.) was used to analyze freezing behavior, a measure of learned fear defined as a lack of all movement except that required for breathing. Specifically, the activity analysis feature (Activity Threshold = 10) was used to calculate the percent of time each animal was inactive during each contextual fear test and at baseline. No animals in any group demonstrated significant freezing behavior to Context B prior to the single foot shock, suggesting that there was no generalization of fear between contexts (Results 3.1). Thus, any differences observed between treatment groups presented here reflect altered learning to the single foot shock in Context B.
2.2.3. IL-1 receptor antagonist
IL-1RA (GenScript, Piscataway, NJ) was reconstituted in sterile saline (2.5 μg/μl). Twenty-four hours prior to Context A exposure, animals were given a sham microinfusion to allow for habituation to microinfusion procedures. Twenty-four and 48 hours after removal from Context A, on Days 2 and 3, animals were microinfused with 1.25 μg of IL-1RA or sterile saline vehicle per hemisphere at a rate of 0.25 μl/min. Injectors were left in place for 1 minute after the infusion to allow for diffusion. These time points were based on our earlier published findings that morphine administration and intracerebroventricular IL-1RA prevent the development of SEFL when administered 48 hours after Context A [5, 39].
2.3. Experiment 2: Immunofluorescence analysis of severe stress-induced changes in hippocampal GFAP, Iba-1, NeuN, and IL-1β
2.3.1 Stress exposure
Animals (N=16, n = 8) were randomly assigned to either a foot shock or no foot shock in Context A treatment and exposed to only the initial severe stressor of the SEFL paradigm described in experiment 1. Thus, animals assigned to receive foot shocks were exposed to 15×2 mA scrambled foot shocks in Context A, an environment distinct from the home cage, while control animals were exposed to the same context without foot shocks being delivered. Forty-eight hours after removal from Context A, animals were deeply anesthetized with 9:1 (vol:vol) ketamine hydrochloride (100 mg/ml) mixed with xylazine (100 mg/ml) and transcardially perfused with cold phosphate buffer (PB; pH = 7.4) followed by 4% paraformaldehyde in 0.1M PB. Brains were extracted, post-fixed in paraformaldehyde for 4–6 hours and placed in 30% sucrose with 0.1% sodium azide at 4°C for cryoprotection. Brains were sectioned into 40 μm sections on a freezing microtome.
2.3.2. Immunohistochemistry
For colocalization analyses, tissue was stained with three primary antibodies. All primary antibodies were verified by no primary control stains in which tissue was only exposed to secondary antibodies to ensure specificity of each signal in the triple label. Tissue was first washed three times for 10 minutes in 0.1M phosphate buffer (PB, pH = 7.4). For tissue stained with anti-Iba-1 antibody, tissue was incubated in endogenous biotin and streptavidin blocks (Vector Laboratories, Burlingame, CA, USA) for 30 minutes each at room temperature, according to the manufacturer’s instructions. All tissue was incubated in 5% Normal Goat Serum (NGS) and 0.5% TritonX100 in 0.1 M PB for 3 hours at room temperature. Tissue was incubated in primary antibody, 5% NGS, and 0.5% TritonX100 in 0.1M PB overnight at 4°C, washed three times for 10 minutes in 0.1M PB, and incubated in secondary antibody, 5% NGS, and 0.5% TritonX100 in 0.1M PB for 60–120 minutes at room temperature. Each antibody was applied individually and thus the entire triple stain procedure occurred over three subsequent nights. The following primary antibodies were used: rabbit anti-IL-1β (1:500, Abcam, Cambridge, MA, Cat# Ab9722), mouse anti-GFAP (1:1000, ThermoFisher Scientific, Waltham, MA, Cat #MS-1376P), mouse anti-NeuN-Alexa 568 (1:1000, Abcam Cambridge, MA, Ab207282), and rabbit anti-Iba-1-biotinylated (1:500, Wako, Richmond, VA, Cat#016-26461). To visualize IL-1β and GFAP, Alexa Fluor conjugated secondary antibodies, goat anti-rabbit 488 (1:1000, ThermoFisher Scientific, Waltham, MA, Cat #A11008) and goat anti-GFAP Dylight 405 (1:1000, ThermoFisher Scientific, Waltham, MA, Cat # 35501BID) were used. Goat anti-rabbit Alexa488 was applied for 60 minutes and Goat anti-mouse Dylight 405 was applied for 120 minutes. To visualize Iba1, a streptavidin-conjugated Alexa Fluor 568 antibody (1:1000, ThermoFisher Scientific, Waltham, MA, Cat #S11226) was used and was applied for 60 minutes. Sections were mounted onto SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA) using Vectashield hard set mounting medium (Vector Laboratories, Burlingame, CA). Tissue from poor perfusions that yielded high nonspecific background which interfered with thresholding and colocalization calculations was dropped from the analysis, and any such decision was made blind to treatment group.
2.3.3. Confocal microscopy, Bitplane Imaris colocalization analysis, and cell counting
All image acquisition and analysis was completed by an experimenter blind to treatment group. Tissue was imaged using a Zeiss LSM800 confocal microscope with laser lines that excite at 405 nm, 488 nm, and 561 nm. Images were acquired using a 63X oil immersion lens with a 1X digital zoom factor. Z stacks of the dentate gyrus of the dorsal hippocampus (AP −3.12 mm through −3.84 mm from bregma) were acquired using a frame average of 4, 1024 by 1024 frame size, 12 bit image resolution, and 0.8 μm step size. We focused our analysis on the dentate gyrus based on our previous finding that IL-1β expression is most dense in this subregion of the DH [5].
Z stacks were deconvolved using Bitplane AutoQuant X3 (10 iterations, [40]) and exported to Bitplane Imaris software (Zurich, Switzerland). For background correction of each channel individually, absolute intensity thresholds were manually set. In the colocalization module, voxels above the threshold in both channels were included as colocalized voxels. A two-dimensional scatter plot was used to visually inspect the accuracy of colocalization thresholds. The colocalization between IL-1β and GFAP, IL-1β and Iba-1, and IL-1β and NeuN were calculated. The following values were recorded: % volume above the absolute intensity threshold selected for each channel, % IL-1β colocalized with Iba1, GFAP, and NeuN, respectively, and the Pearson’s correlation coefficient for each pair of signals. In addition to Imaris volume and colocalization analyses, the number of GFAP-positive and Iba-1-positive cells in the dentate gyrus was counted in images acquired at 10X by an experimenter blind to treatment group.
2.3.4. Statistical analyses
For experiment 1, a one way ANOVA with treatment group as the between subjects factor was used to analyze baseline freezing data during the three minutes prior to the foot shock during Context B conditioning in order to ensure there were no group differences in freezing to Context B prior to the single shock. A 2 × 2 × 4 repeated measures ANOVA with Context A treatment and drug treatment as between subject factors and test day as a within subjects factor was used to analyze freezing behavior across test days 1, 2, 7 and 14. For experiment 2, unpaired, two-tailed student’s t tests were used to test whether Context A treatment altered GFAP or Iba-1 immunoreactivity and an unpaired one-tailed student’s t test was used to test whether Context A treatment altered IL-1β immunoreactivity. For colocalization data, the percent of IL-1β colocalized and the Pearson’s correlation coefficient were subjected to a 2 × 3 ANOVA with Context A treatment and cell type specific signal analyzed as factors. Significant interactions were examined using Tukey’s post-hoc comparisons. Specifically, for experiment 1 planned comparisons included: Foot shock in Context A/Vehicle vs. Foot shock in Context A/IL-1RA and No Foot shock in Context A/Vehicle vs. Foot shock in Context A/Vehicle. For colocalization data, planned comparisons included GFAP/IL-1β colocalization parameters vs. Iba-1/IL-1β colocalization parameters and GFAP/IL-1β colocalization parameters vs. NeuN/IL-1β colocalization parameters.
3. Results
3.1 Experiment 1: Intra-dorsal hippocampal IL-RA prevents SEFL
Figure 1 shows freezing behavior across all four test days. There was no effect of Context A treatment on baseline freezing in Context B prior to the single shock, F (3, 24) = 2.674, p > 0.05, confirming that there was no generalization of fear between the two contexts. A 2 × 2 × 4 ANOVA revealed a significant main effect of Context A treatment, F (1, 23) = 5.159, p = 0.033, and a significant main effect of drug treatment, F (1, 23) = 9.354, p = 0.006. In addition, there was a significant main effect of test day, F (1, 23) = 23.344, p < 0.001, indicating that contextual fear diminished across test days. Importantly, there was a significant Context A treatment by drug treatment interaction, F (1, 23) = 4.394, p = 0.047. Tukey’s HSD post hoc comparisons revealed a significant stress-enhanced fear learning effect within vehicle-treated groups in that foot shock in Context A significantly enhanced freezing to Context B, p = 0.038. Critically, IL-1RA treatment prevented stress-enhanced fear learning within groups that received foot shock in Context A. Rats that received foot shock in Context A followed by IL-1RA exhibited significantly less freezing than rats that received foot shock in Context A followed by vehicle, p = 0.001. Further, rats that received foot shock in Context A followed by IL-1RA exhibited a comparable amount of freezing behavior (no statistically significant difference), to both control groups of rats that received no foot shock in Context A, p > 0.05.
3.2 Experiment 2A: Stress-induced increase in hippocampal IL-1β is replicated
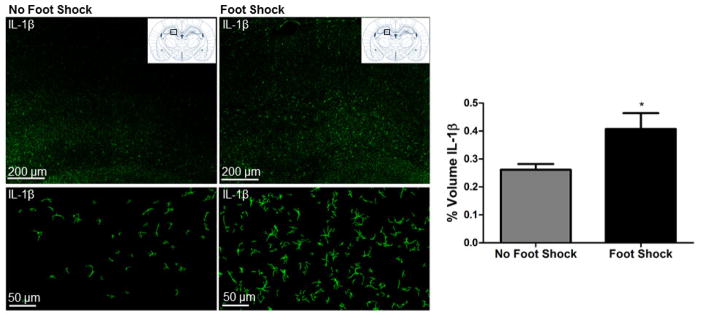
We previously reported that the severe stressor of the SEFL paradigm induces an increase in hippocampal IL-1β immunoreactivity and mRNA that emerges at 6 hours and persists through 72 hours following stress exposure [5]. Here, this effect is replicated in that exposure to the severe stressor of SEFL significantly enhanced hippocampal IL-1β immunoreactivity 48 hours later, t(10) = 2.083, p = 0.0319 (Figure 2).
Figure 2. Severe stress increases hippocampal IL-1β immunoreactivity.
The stress-induced increase in hippocampal IL-1β that we previously reported is replicated here. Representative images of IL-1β immunoreactivity in the dentate gyrus of the DH acquired at 10X are shown from stressed (Foot Shock in context A) and non-stressed (No Foot Shock in Context A) rats. Top panel shows a tiled 10X image, while bottom panel shows a single 10X image. For the bottom panel, Bitplane Imaris was used for background subtraction to better visualize individual cells presented. Paxinos and Watson (2007) schematic shows the approximate region of the DH where images were acquired, AP −3.36 from bregma. Quantification of IL-1β immunoreactivity revealed that exposure to severe stress (15 foot shocks) significantly increased IL-1β immunoreactivity in the DH 48 hours post-stress. * p < 0.05.
Experiment 2B: Severe stress attenuates Iba-1, but not GFAP, in the dorsal hippocampus
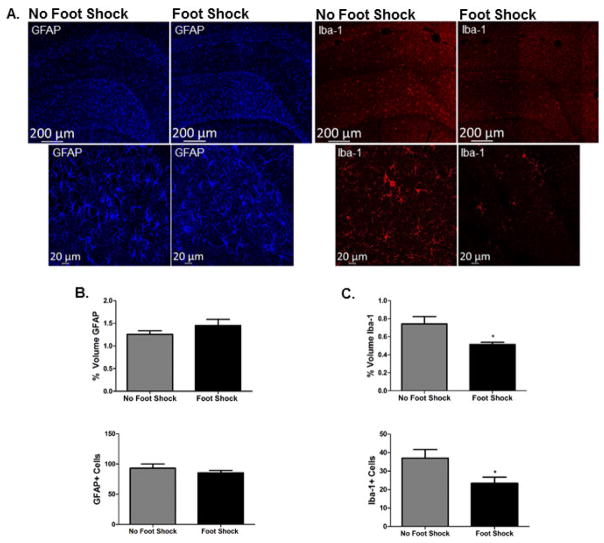
Exposure to severe stress did not alter hippocampal GFAP immunoreactivity. There was no effect of foot shock in Context A on Imaris quantification, t(9) = 1.295, p > 0.05, or the number of GFAP-positive cells t(11) = 0.9563, p > 0.05. However, exposure to severe stress significantly reduced hippocampal Iba-1 immunoreactivity. Exposure to foot shock in Context A attenuated both Imaris quantification of Iba-1 immunoreactivity, t(9) = 2.497, p = 0.0340, and the number of Iba-1-positive cells, t(10) = 2.375, p = 0.0389. Figure 3 shows representative images and quantification of hippocampal GFAP and Iba-1, respectively.
Figure 3. Dorsal hippocampal Iba-1 immunoreactivity, but not GFAP immunoreactivity, is attenuated 48 hours after severe stress.
A. Representative images of GFAP and Iba-1 immunoreactivity acquired at 10X (tiled image presented) and 20X are shown from stressed (Foot Shock in context A) and non-stressed (No Foot Shock in Context A) rats. Images were acquired in the DH, AP −3.36 from bregma. B. Both Imaris quantification and individual GFAP-positive cell counts indicated there was no effect of foot shock on GFAP immunoreactivity. C. In contrast, Imaris quantification and individual Iba-1 positive cell counts revealed that stress exposure significantly attenuated Iba-1 immunoreactivity 48 hours post-stress. * p < 0.05.
3.3 Experiment 2C: Stress-induced hippocampal IL-1β is colocalized primarily with GFAP in both stressed and non-stressed animals
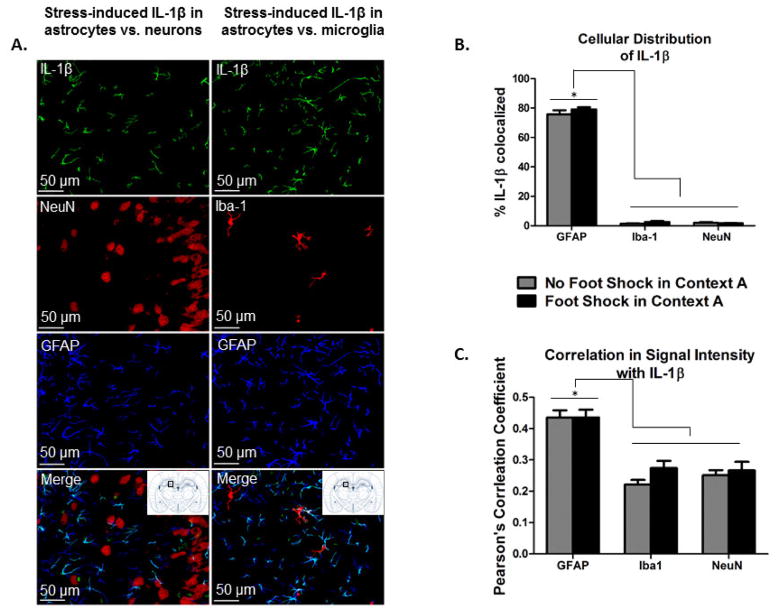
Figures 4 and 5 show that there was an overwhelming amount of IL-1β colocalized with GFAP, 75% to 79% of the IL-1β signal, and only minimal colocalization with Iba-1 or NeuN, less than 5% of the IL-1β signal. Thus, there was over 15- fold greater colocalization of IL-1β with GFAP compared to the other two cell-type markers in both stressed and non-stressed animals. As such, there was a significant main effect of cell-type analyzed in both the % IL-1β signal colocalized, F (2, 28) = 2423.859, p < 0.001, and the Pearson’s correlation coefficient, F (2, 28) = 51.166, p < 0.001. Again, regarding both the % IL-1β signal colocalized and the Pearson’s correlation coefficient, post hoc comparisons confirmed significantly more colocalization with GFAP than Iba-1, p < 0.001, and with GFAP than NeuN, p < 0.001. There was no difference between colocalization of IL-1β with Iba-1 and with NeuN, p > 0.05.
Figure 4. IL-1β signal is colocalized with GFAP, and not with Iba-1 or NeuN, in the dorsal hippocampus in stressed and non-stressed animals.
A. Representative images of IL-1β, NeuN, Iba-1, and GFAP immunoreactivity in the dentate gyrus of the DH (AP −3.36 mm from bregma) acquired at 20X are shown. Because we did not detect any differences in colocalization between stressed and non-stressed rats, all images here are taken from animals that received stress exposure. Bitplane Imaris was used for background subtraction to better visualize individual cells presented. B. Bitplane Imaris software was used to calculate the colocalization of the IL-1β signal with GFAP, Iba-1, and NeuN. Colocalization analyses revealed that the percent of the IL-1β signal colocalized with GFAP was significantly greater than the percent of the IL-1β signal colocalized with either Iba-1 or NeuN. * p < 0.05. C. Similarly, the Pearson’s correlation coefficient between the IL-1β signal intensity and the GFAP signal intensity was significantly higher than that for the IL-1β signal and Iba-1 signal or NeuN signal, respectively. * p < 0.05.
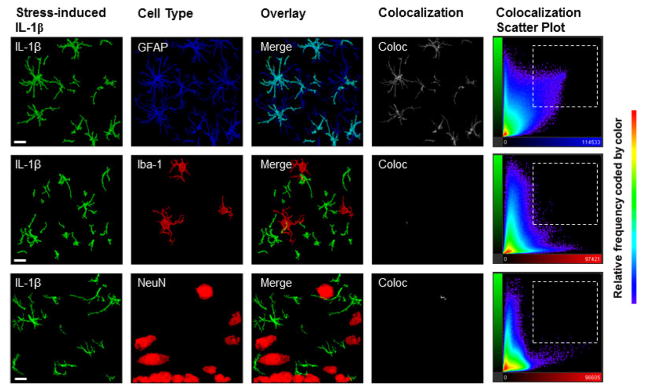
Figure 5. IL-1β signal is colocalized with GFAP, and not with Iba-1 or NeuN, in the dorsal hippocampus in stressed and non-stressed animals.
Representative images from the DH acquired at 63X (scale bar presented is 10μm) show colocalization of IL-1β with GFAP, Iba-1, and NeuN. Because we did not detect any differences in colocalization between stressed and non-stressed rats, all images here are taken from animals that received stress exposure. Colocalization panels (white, labeled ‘Coloc’) show Imaris-generated image of colocalized voxels in each Z stack image presented. Colocalization scatter plots show the signal intensity for each voxel in the Z stack. Specifically, color of each point represents the frequency, the Y axis represents IL-1β signal (Alexa-488) intensity, and the X axis represents GFAP (Alexa- 405), Iba-1 (Alexa- 568), or NeuN signal (Alexa- 568) intensity, respectively. In the top panel, the colocalization scatter plot between IL-1β and GFAP shows a high proportion of voxels that were high in both IL-1β and GFAP signal (selected region), and a high observed correlation, r = 0.3997, demonstrates that for any given voxel, as IL-1 signal increased, GFAP signal was also likely to increase. In contrast, scatter plots for both IL-1β with Iba-1 and IL-1β with NeuN show a high proportion of voxels that were high in only IL-1β or Iba-1 and NeuN signal, respectively (outside of selected region). In addition, there was a lower correlation for the IL-1β and Iba-1 signal, r = 0.1915, and IL-1β and NeuN signal, r = 0.0373, suggesting a much weaker relationship than that with GFAP.
The cellular distribution of IL-1β was not changed by exposure to severe stress (Figure 4). There was no main effect of Context A treatment on either the % IL-1β colocalized with each signal, F (1, 28) = 1.949, p > 0.05, or the Pearson’s correlation coefficient between signals, F (1, 28) = 1.749, p > 0.05.
4. Discussion
Here, we provide evidence that astrocytes are the cellular source of foot shock-induced hippocampal IL-1β, which plays a critical role in the development of SEFL, an animal model of PTSD. Experiment 1 supports our hypothesis that DH IL-1 signaling is necessary for the development of SEFL in that intra-DH IL-1RA infused 24 and 48 hours following severe stress prevented the expression of SEFL. Further, experiment 2 provides the first evidence that Iba-1 immunoreactivity is reduced 48 hours following foot shock stress and that the IL-1β signal at this critical time point for behavioral consequences of severe stress is almost exclusively colocalized with an astrocyte-specific marker, and not with microglia- or neuron-specific markers.
Our finding that hippocampal astrocytes are the predominant source of stress-induced hippocampal IL-1β is consistent with previously published data suggesting that astrocyte-dependent signaling is important in stress and anxiety-related behavior [33, 41]. Xia and colleagues identified one compound that showed promise to alleviate PTSD-like symptoms following single prolonged stress (Fibroblast growth factor-2) through an astrocyte-dependent mechanism [37]. Furthermore, Menachem-Zidon and colleagues demonstrated that introduction of neural precursor cells that ultimately differentiated into astrocytes rescued deficits in fear conditioning traditionally observed in an IL-1 receptor knock out line [42]. Gliotransmission, specifically vesicular release of astrocyte-derived ATP, also protected against a depressive-like phenotype following chronic social defeat stress [43]. Thus, while the complete mechanisms involved in each of these effects remain unclear, our data and these data converge to suggest a critical role for astrocytes in behavioral responses to stress.
One limitation of the current report is that future studies are needed to provide insight into the potential causal link between astrocyte signaling and SEFL. To test whether astrocyte-derived IL-1, specifically, is causally related to SEFL, future studies could examine the development of SEFL using an astrocyte-specific IL-1 knockout strain or test whether pharmacological or genetic astrocyte ablation influences SEFL development. Furthermore, chemogenetic tools under the control of a GFAP promoter are commercially available and will also contribute to our understanding of the role of astrocyte signaling and fear and anxiety-like behavior.
Given that microglia-derived cytokines are well-established in models of neurodegenerative disease, for example, Alzheimer’s and Parkinson’s disease [24, 44, 45], our observation of a reduction in microglia is somewhat surprising. While our data are in conflict with several reports of stress-induced increases in hippocampal microglia activation or cell count, [27, 46], we are not the first to observe a decrease in Iba-1 immunoreactivity following stress. Brzozowska and colleagues reported no change in hippocampal microglial cell count but a reduction in microglial cell count in the basolateral amygdala 30 days after stress exposure in another foot shock based rodent model of PTSD [29]. Further, Kreisel and colleagues found that multiple markers of microglia, including both Iba-1 and Cd11b mRNA expression and microglia cell count, were decreased in the dentate gyrus following five days of chronic unpredictable stress (CUS) capable of inducing a depressive-like phenotype [47]. Interestingly, they also showed that after only one day of CUS, these same measures showed an increase in microglia gene expression/immunoreactivity induced by stress. Thus, severity of stressor and a longer stress exposure or a later time point after the initial stressor may be critical to our observed effect. Nonetheless, the reduction in Iba-1 immunoreactivity observed here further supports the notion that astrocytes, not microglia, are organizing important changes in the DH following severe stress.
We hypothesized that GFAP expression would increase following stress because the IL-1β signal is increased in the hippocampus at this time point following stress, and is highly colocalized with GFAP. While this hypothesis was not confirmed here, there are several potential explanations for our observed lack of effect. First, the amount of GFAP expression in the DH (1–1.5% of the region measured) is more than six times that of IL-1β (0.2–0.3% of the region measured). Thus, an increase in IL-1β in astrocytes can easily occur without a corresponding increase in GFAP. Second, while GFAP is one of the most canonical astrocyte markers relied on in the field, GFAP is a cytoskeletal protein that is only expressed within 15% of a given astrocyte’s area and even then, only by a subset of astrocytes [48, 49]. Measures of GFAP have also yielded results that have conflicted with other measures of astrocyte reactivity. Tynan and colleagues examined stress-induced changes in astrocyte activation and showed an increase in S100β, another astrocyte-specific marker, but a decrease in GFAP following the same stressor [35]. Thus, our measure of GFAP immunoreactivity is an incomplete measure of astrocyte reactivity and future studies should take advantage of new technologies that are becoming available to study astrocyte morphology in detail. Scofield and colleagues utilized a membrane-tagged GFP under the control of a GFAP promoter to quantify morphometric properties of complete individual astrocytes, including the distal fine processes and synaptic colocalization [50]. Highly sophisticated volume analyses will provide more information regarding how severe stress alters astrocyte morphology in the future.
While the sole focus on IL-1 signaling in the current manuscript is well justified given previous work supporting the importance of IL-1 signaling in the context of behavioral responses to stress [5, 11, 12], it is important to note that IL-1β does not act in isolation and several other proinflammatory mediators may play additional vital roles. For example, both interleukin-6 (IL-6) and tumor necrosis factor- α (TNF-α) have been shown to be upregulated following stress [51, 52]. Studies to examine behavioral implications of stress-induced alterations in additional immune signaling pathways would provide more information regarding the specificity of the IL-1 mechanism or could identify additional mechanisms that play a role.
In summary, our data demonstrate that hippocampal IL-1 drives important neural changes that render animals hypersensitive to fear learning following stress. Further, our data suggest that astrocytes are an important source of hippocampal stress-induced IL-1β. These findings provide additional evidence that IL-1 signaling should be considered a target for the development of novel therapeutics to treat PTSD and suggest one mechanism through which hippocampal astrocytes may influence complex behavior.
Highlights.
Intra-dorsal hippocampal IL-1 receptor antagonist 24–48 hours after stress exposure prevents the expression of stress-enhanced fear learning, an animal model of post-traumatic stress disorder.
Hippocampal Iba-1 immunoreactivity, but not GFAP immunoreactivity, is attenuated 48 hours after stress exposure.
Stress-induced hippocampal IL-1β is colocalized primarily with GFAP; the predominant cellular source of stress-induced hippocampal IL-1β is astrocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koo JW, Duman RS. Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci Lett. 2009;456(1):39–43. doi: 10.1016/j.neulet.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman MN, et al. Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry. 2007;12(4):408–17. doi: 10.1038/sj.mp.4001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stepanichev M, et al. Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. Biomed Res Int. 2014;2014:932757. doi: 10.1155/2014/932757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill JM, et al. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45(4):262–77. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 5.Jones ME, et al. The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology. 2015;40(5):1289–96. doi: 10.1038/npp.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gola H, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passos IC, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2(11):1002–12. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- 8.Guo M, et al. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med. 2012;5(4):323–5. doi: 10.1016/S1995-7645(12)60048-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Young MR. PTSD, a Disorder with an Immunological Component. Front Immunol. 2016;7:219. doi: 10.3389/fimmu.2016.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M, et al. Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int J Psychiatry Med. 2011;42(2):117–31. doi: 10.2190/PM.42.2.b. [DOI] [PubMed] [Google Scholar]
- 11.Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30(1):30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Avital A, et al. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13(7):826–34. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- 13.Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86(4):651–9. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakawa H, Blandino P, Jr, Deak T. Central infusion of interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiol Behav. 2009;98(1–2):139–46. doi: 10.1016/j.physbeh.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29(8):1207–23. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Yabuuchi K, et al. Localization of Type-I Interleukin-1 Receptor Messenger-Rna in the Rat-Brain. Molecular Brain Research. 1994;27(1):27–36. doi: 10.1016/0169-328x(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, et al. Acute p38-mediated inhibition of NMDA-induced outward currents in hippocampal CA1 neurons by interleukin-1beta. Neurobiol Dis. 2010;38(1):68–77. doi: 10.1016/j.nbd.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Ringwood L, Li L. The involvement of the interleukin-1 receptor-associated kinases (IRAKs) in cellular signaling networks controlling inflammation. Cytokine. 2008;42(1):1–7. doi: 10.1016/j.cyto.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flannery S, Bowie AG. The interleukin-1 receptor-associated kinases: Critical regulators of innate immune signalling. Biochemical Pharmacology. 2010;80(12):1981–1991. doi: 10.1016/j.bcp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, et al. Neuron-specific effects of interleukin-1beta are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci. 2011;31(49):18048–59. doi: 10.1523/JNEUROSCI.4067-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guasch RM, et al. RhoE participates in the stimulation of the inflammatory response induced by ethanol in astrocytes. Exp Cell Res. 2007;313(17):3779–3788. doi: 10.1016/j.yexcr.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Kwon MS, et al. The repeated immobilization stress increases IL-1beta immunoreactivities in only neuron, but not astrocyte or microglia in hippocampal CA1 region, striatum and paraventricular nucleus. Neurosci Lett. 2008;430(3):258–63. doi: 10.1016/j.neulet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Mendiola AS, Cardona AE. The IL-1beta phenomena in neuroinflammatory diseases. J Neural Transm (Vienna) 2017 doi: 10.1007/s00702-017-1732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 25.Minghetti L, et al. Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Res Brain Res Rev. 2005;48(2):251–6. doi: 10.1016/j.brainresrev.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15(2):313–26. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 27.Calcia MA, et al. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 2016;233(9):1637–50. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawson LJ, et al. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–70. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 29.Brzozowska NI, et al. Genetic deletion of P-glycoprotein alters stress responsivity and increases depression-like behavior, social withdrawal and microglial activation in the hippocampus of female mice. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.05.008. [DOI] [PubMed]
- 30.Blandino P, Jr, et al. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23(7):958–68. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Sugama S, et al. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007;146(3):1388–99. doi: 10.1016/j.neuroscience.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 32.Frank MG, et al. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21(1):47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–40. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Saur L, et al. Experimental Post-traumatic Stress Disorder Decreases Astrocyte Density and Changes Astrocytic Polarity in the CA1 Hippocampus of Male Rats. Neurochem Res. 2016;41(4):892–904. doi: 10.1007/s11064-015-1770-3. [DOI] [PubMed] [Google Scholar]
- 35.Tynan RJ, et al. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126(1):75–91. doi: 10.1007/s00401-013-1102-0. [DOI] [PubMed] [Google Scholar]
- 36.Choi M, et al. Hippocampus-based contextual memory alters the morphological characteristics of astrocytes in the dentate gyrus. Mol Brain. 2016;9(1):72. doi: 10.1186/s13041-016-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia L, et al. FGF2 blocks PTSD symptoms via an astrocyte-based mechanism. Behav Brain Res. 2013;256:472–80. doi: 10.1016/j.bbr.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 38.Sugama S, et al. Immunological responses of astroglia in the rat brain under acute stress: interleukin 1 beta co-localized in astroglia. Neuroscience. 2011;192:429–37. doi: 10.1016/j.neuroscience.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 39.Szczytkowski-Thomson JL, Lebonville CL, Lysle DT. Morphine prevents the development of stress-enhanced fear learning. Pharmacol Biochem Behav. 2013;103(3):672–7. doi: 10.1016/j.pbb.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Lee JS, Wee TL, Brown CM. Calibration of wide-field deconvolution microscopy for quantitative fluorescence imaging. J Biomol Tech. 2014;25(1):31–40. doi: 10.7171/jbt.14-2501-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rial D, et al. Depression as a Glial-Based Synaptic Dysfunction. Front Cell Neurosci. 2015;9:521. doi: 10.3389/fncel.2015.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben Menachem-Zidon O, et al. Astrocytes support hippocampal-dependent memory and long-term potentiation via interleukin-1 signaling. Brain Behav Immun. 2011;25(5):1008–16. doi: 10.1016/j.bbi.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Cao X, et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med. 2013;19(6):773–7. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- 44.Rogers J, et al. Neuroinflammation in Alzheimer’s disease and Parkinson’s disease: are microglia pathogenic in either disorder? Int Rev Neurobiol. 2007;82:235–46. doi: 10.1016/S0074-7742(07)82012-5. [DOI] [PubMed] [Google Scholar]
- 45.Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson’s disease. J Neural Transm Suppl. 2006;(70):373–81. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- 46.Brevet M, et al. Chronic foot-shock stress potentiates the influx of bone marrow-derived microglia into hippocampus. J Neurosci Res. 2010;88(9):1890–7. doi: 10.1002/jnr.22362. [DOI] [PubMed] [Google Scholar]
- 47.Kreisel T, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19(6):699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 48.Benediktsson AM, et al. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141(1):41–53. doi: 10.1016/j.jneumeth.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14(11):1225–36. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scofield MD, et al. Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol Psychiatry. 2016;80(3):207–15. doi: 10.1016/j.biopsych.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Audet MC, Mangano EN, Anisman H. Behavior and pro-inflammatory cytokine variations among submissive and dominant mice engaged in aggressive encounters: moderation by corticosterone reactivity. Front Behav Neurosci. 2010:4. doi: 10.3389/fnbeh.2010.00156. [DOI] [PMC free article] [PubMed]
- 52.Barnum CJ, et al. Psychological stress in adolescent and adult mice increases neuroinflammation and attenuates the response to LPS challenge. J Neuroinflammation. 2012;9:9. doi: 10.1186/1742-2094-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]