Abstract
Pancreatic cancer is an extremely aggressive neoplasm whose incidence equals its death rate. Despite intensive analysis, the genetic changes that mediate pancreatic cancer development and effective therapies for diminishing the morbidity associated with this disease remain unresolved. Through subtraction hybridization, we have identified a gene associated with induction of irreversible growth arrest, cancer reversion, and terminal differentiation in human melanoma cells, melanoma differentiation associated gene-7 (mda-7). Ectopic expression of mda-7 when using a recombinant adenovirus, Ad.mda-7, results in growth suppression and apoptosis in a broad spectrum of human cancers with diverse genetic defects, without exerting deleterious effects in normal human epithelial or fibroblast cells. Despite the apparently ubiquitous antitumor effects of mda-7, pancreatic carcinoma cells are remarkably refractory to Ad.mda-7 induced growth suppression and apoptosis. In contrast, the combination of Ad.mda-7 with antisense phosphorothioate oligonucleotides, which target the K-ras oncogene (a gene that is mutated in 85 to 95% of pancreatic carcinomas), induces a dramatic suppression in growth and a decrease in cell viability by induction of apoptosis. In mutant K-ras pancreatic carcinoma cells, programmed cell death correlates with expression and an increase, respectively, in MDA-7 and BAX proteins and increases in the ratio of BAX to BCL-2 proteins. Moreover, transfection of mutant K-ras pancreatic carcinoma cells with an antisense K-ras expression vector and infection with Ad.mda-7 inhibits colony formation in vitro and tumorigenesis in vivo in nude mice. These intriguing observations demonstrate that a combinatorial approach, consisting of a cancer-specific apoptosis-inducing gene and an oncogene inactivation strategy, may provide the foundation for developing an effective therapy for pancreatic cancer.
Pancreatic cancer is the fourth leading cause of cancer deaths; it is estimated that 29,200 cases will be diagnosed in the US in 2001, and 28,900 of these patients will die (1). Moreover, long-term survival for patients with organ-confined disease is only 20%, and in the majority of cases, in which the disease when diagnosed has already spread past the pancreas, survival is only 4% (2–6). These findings underscore the need for developing improved therapies for this aggressive cancer. Although they are the subjects of intensive study, the defining molecular determinants of pancreatic cancer and effective therapies for this disease remain elusive (2, 6–8). Pancreatic cancer is a complex ailment in which multiple subsets of genes undergo genetic change, either activation or inactivation, during tumor development and progression (2, 7, 8). Frequent genetic modifications in pancreatic carcinomas include activation of the K-ras oncogene (85–95%) and inactivation of the p16/RB1 (>90%), p53 (75%), and DPC4 (55%) tumor suppressor genes (2, 7, 8). These findings highlight the complexity of this cancer and may provide a partial explanation for the aggressiveness and inherent resistance of this neoplasm to conventional therapies, including chemotherapy and radiation (3–6).
The effects of modifying the expression of the K-ras oncogene that is genetically altered in pancreatic carcinoma cells on growth and viability, both in vitro and in vivo in athymic nude mice, have been examined (9–11). Antisense (AS) targeting of K-ras using a plasmid (9, 10) or with mutation-specific phosphorothioate oligodeoxynucleotides (PS ODN) (11) inhibits the growth of pancreatic cancer cells containing K-ras mutations, but not pancreatic carcinoma cells containing a wild-type (wt) K-ras gene. Specificity of the antisense mutant (mut) K-ras PS ODN approach was indicated by the absence of a growth inhibitory effect when using mutation-mismatched (MM) PS ODN, a direct reduction in the levels of K-ras p21 protein in AS treated cells, and the absence of a growth-suppressive effect in wt K-ras pancreatic cancer cells (11). Additionally, liposome-mediated in vivo gene transfer of an AS K-ras expression plasmid in animals containing AsPC-1 tumor cells, which represents a peritoneal dissemination model of pancreatic cancer, significantly suppressed tumor development in the peritoneal cavity (9). An important role for K-ras in pancreatic cancer physiology is further suggested by the ability of the dominant negative H-ras mut, N116Y, to suppress pancreatic cancer cell growth in vitro and in vivo, including tumorigenesis and metastasis to the liver of nude mice (12, 13). Although promising, these studies demonstrate that a single approach of inhibiting K-ras is not sufficient to completely eradicate pancreatic carcinoma cells (9–13).
Treatment of human melanoma cells with a combination of fibroblast IFN and the protein kinase C-activating agent mezerein results in an irreversible suppression in cell growth, loss of tumorigenic potential, and induction of terminal cell differentiation (14). Using a modified subtraction hybridization protocol, genes displaying elevated expression resulting from changes in cancer cell physiology in combination-treated melanoma cells were isolated (15). This scheme identified melanoma differentiation-associated gene-7 (mda-7), which displays elevated expression in human melanoma cells induced to terminally differentiate (16). When transfected into a wide spectrum of human cancers, growth is suppressed (17, 18). In contrast, no significant growth inhibitory effect is apparent when this gene is transfected into normal human fibroblast or epithelial cells (17, 18). Similarly, when expressed from a replication-incompetent Ad, Ad.mda-7, induction of growth suppression and apoptosis is also apparent in diverse human cancers, whereas no harmful effect is evident in normal human cells (18–21). These studies indicate that mda-7 may prove useful for the gene-based therapy of diverse human tumors.
Pancreatic cancers are recognized as one of the most therapeutically refractory neoplasms, and they are inherently resistant to ectopic expression of mda-7, which in contrast causes significant growth suppression and apoptosis in most other human cancers (18–21). The extensive genetic changes that occur in this tumor may mediate both therapeutic resistance and lack of susceptibility to mda-7. Of the genetic changes that occur in pancreatic cancers, mutations in the K-ras gene, predominantly in codon 12, are the most frequent (2, 22, 23). This observation prompted us to investigate the putative role of mut K-ras in mediating resistance to mda-7. To achieve this aim, we evaluated the consequence of a combinatorial approach involving forced expression of mda-7 and targeted K-ras gene suppression on the growth and survival of pancreatic carcinoma cells. We presently demonstrate that a single treatment of mut and wt K-ras pancreatic carcinoma cell lines with AS PS ODN or infection with Ad.mda-7 variably modifies cell growth without significantly decreasing cell survival. In contrast, the combination treatment protocol results in a striking synergistic growth inhibitory and antisurvival effect, which is apparent strictly in mut K-ras pancreatic cancer cells. Moreover, when MIA PaCa-2 cells are infected with Ad.mda-7, transfected with an AS K-ras expression vector, and then injected into athymic nude mice, tumor formation is prevented. These intriguing observations suggest that a combinatorial approach consisting of a cancer-specific apoptosis-inducing gene and an oncogene inactivation strategy could provide the basis for a new and potentially effective therapy for pancreatic cancer.
Materials and Methods
Cell Lines, Culture Conditions, and Growth Assays.
The AsPC-1, BxPC-3, MIA PaCa-2, and PANC-1 human pancreatic carcinoma cell lines (American Type Culture Collection) were grown in RPMI medium 1640 containing 10% FBS at 37°C in a 95% air, 5% CO2 humidified incubator. Cell growth and viable cell numbers were monitored by hemocytomer and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) staining as described (24).
Animal Studies.
Tumorigenicity assays were performed as described (18, 19). Briefly, MIA PaCa-2 cells were untreated or infected with 100 plaque-forming units (pfu)/cell of Ad.vec or Ad.mda-7 and then untransfected or transfected with an expression vector containing a 346-nt K-ras gene fragment (nt 172 to nt 517) cloned in a sense or AS orientation, and 1 × 106 cells were mixed with Matrigel and injected 48 h later s.c. into athymic nude mice. Animals were monitored for tumor formation, and tumor volume was determined (18).
Viral Construction, Purification, and Infectivity Assays.
The replication-defective Ad.mda-7 was created in two steps as described (17, 19). Ad.mda-7 and Ad.vec (control virus lacking the mda-7 gene) were grown in 293 cells, and recombinant structure, plaque purification, and titrations of virus were performed as described (25).
PS ODN.
Eighteen-base PS ODN were synthesized and purified by HPLC (24). AS K-ras PS ODN, CACAAGTTTATATTCAGT, were synthesized that were complementary to wt K-ras nucleotides 196–213 (adjacent to the start codon). Based on previous studies (26), MM K-ras PS ODN, CACTTGTAAATATTCAGT, and scrambled (SC) K-ras PS ODN, ACTAGCTATACTAGCTAT, to the same region (nucleotides 196–213), were synthesized.
RNA Isolation and Northern Blot Analysis.
Total RNA was isolated by the guanidinium/phenol procedure, and Northern blots were performed as described (15, 16).
DNA Extraction, Fragmentation Assays, FACS Analysis, and Annexin V, PI, and 4′,6-Diamidino-2-phenylindole Staining.
DNA was extracted, and fragmentation assays were performed as described (27) 3 days after a single or combination treatment protocol. FACS analysis and Annexin V and PI staining reactions were performed by using previously described methods (19, 24, 27, 28).
Western Blotting.
Cell extracts in RIPA buffer were prepared, and equal concentrations of proteins were evaluated for MDA-7, BCL-2, BAX, and EF-1α protein levels by Western blotting as described (11, 24, 29). Radioautograms were scanned by densitometer.
Results and Discussion
The Combination of Ad.mda-7 and AS K-ras PS ODN Synergistically Suppresses Growth in mut K-ras Expressing Human Pancreatic Carcinoma Cells.
mda-7 is a broad-spectrum cancer-specific growth-suppressing gene, which displays no apparent harmful effects in normal cells (16–21). Infection of a diverse group of human cancers with Ad.mda-7, including melanoma, glioblastoma multiforme, and osteosarcoma, and carcinomas of the breast, cervix, colon, endometrium, lung, and prostate, results in growth suppression and hypodiploidy, a cellular change frequently associated with apoptosis (17–21). In a detailed study with several breast carcinoma cell lines, the ability of Ad.mda-7 to induce growth suppression was found to be independent of p53 status and to correlate with induction of apoptosis, as monitored by DNA nucleosomal laddering, the terminal deoxynucleotidyltransferase-mediated UTP end-labeling reaction, and Annexin V staining (18, 19, 21). In contrast, growth was minimally affected, and no induction of apoptosis was apparent in early passage normal mammary epithelial cells or the normal breast epithelial cell line, HBL-100, after infection with Ad.mda-7.
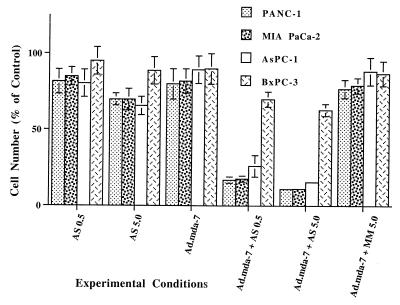
When evaluating the effect of mda-7 on diverse cancer subtypes, it was readily apparent that pancreatic carcinoma cells are inherently resistant to ectopic expression of mda-7. Infection of mut or wt K-ras expressing pancreatic carcinoma cells with 100 pfu/cell of Ad.mda-7 or Ad.vec (the Ad construct lacking the mda-7 gene insert) did not significantly effect growth, and no selective induction of apoptosis was evident (Fig. 1 and data not shown). A dose-dependent growth inhibitory effect was apparent when the different pancreatic carcinoma cells were treated with 0.1–5 μM AS K-ras PS ODN, with a maximum inhibition of ≈10–30% depending on the cell type when treated for 3 or 4 days with 5 μM AS K-ras PS ODN (Fig. 1). Growth of BxPC-3 was inhibited the least by the K-ras PS ODN. Selectivity of the AS K-ras PS ODN was suggested by the fact that treatment with either SC or MM PS ODN resulted in significantly less growth suppression than treatment with the AS K-ras PS ODN (Fig. 1 and data not shown). These studies document that a single application of Ad.mda-7 or AS K-ras PS ODN to mut or wt K-ras pancreatic carcinoma cell lines can induce variable degrees of growth suppression. However, in all cases, growth suppression was transient, and cells survived the single treatment and continued to proliferate, even when initially exposed to 5 μM PS ODN.
Figure 1.
Synergistic inhibition of growth in mut K-ras pancreatic carcinoma cells by the combination of Ad.mda-7 and AS K-ras PS ODN. Cells were treated with the indicated agents for 3 days, and viable cell counts were determined by hemocytometer. Qualitatively similar results were obtained by MTT staining. AS PS ODN, 0.5 or 5.0 μM; Ad.mda-7, 100 pfu per cell; MM PS ODN, 5.0 μM. Results are average of four plates ± SD from the mean. Qualitatively similar results were obtained in an additional experiment.
When mut K-ras pancreatic carcinoma cells were infected with Ad.mda-7 and then treated with 0.1–5.0 μM AS K-ras PS ODN, but not SC or MM PS ODN, a profound synergistic growth inhibitory effect and a decrease in cell survival were evident (Figs. 1 and 2). In contrast, no synergistic growth inhibition or decrease in cell viability was detected in wt K-ras BxPC-3 cells (Figs. 1 and 2). Additionally, no effect on growth or viability was apparent with any of the treatments in early passage normal human prostate epithelial cells or when pancreatic cancer cells were infected with an adenovirus expressing luciferase or β-galactosidase and then treated with AS K-ras PS ODN (data not shown). These results document an antisurvival effect of the combination of mda-7 and AS K-ras PS ODN in mut K-ras pancreatic carcinoma cells, but not in wt K-ras pancreatic cancer cells or normal epithelial cells.
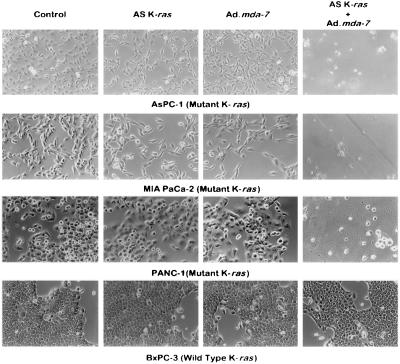
Figure 2.
The combination of Ad.mda-7 with AS K-ras PS ODN synergistically suppresses growth and decreases survival in mut K-ras pancreatic carcinoma cells. The different pancreatic carcinoma cell lines were treated as indicated, and representative microscopic fields were photographed 3 days later. Cells were untreated (control), treated with 0.5 μM AS K-ras PS ODN, infected with Ad.mda-7 (100 pfu per cell) or infected with Ad.mda-7 (100 pfu per cell) and then treated with the 0.5 μM AS K-ras PS ODN.
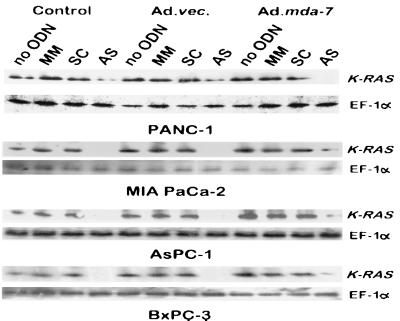
A previous study has reported that AS K-ras PS ODN that target specific point mutations in K-ras codon 12 can reduce growth in mutant pancreatic carcinoma cell lines, but not in wt K-ras BxPC-3 cells (11). This effect was greater when using appropriate mutation-matched AS PS ODN versus mutation-mismatched PS ODN. Effects on growth, although less, were also apparent when using AS PS ODN that did not correspond precisely to the mutation in K-ras codon 12 of the particular pancreatic carcinoma analyzed. This observation supports numerous previous studies indicating that AS PS ODN can induce both specific and apparently nonspecific effects in target cells (30–32). In the present study, AS K-ras PS ODN were designed to interact with the AUG start codon of the K-ras gene. Treatment of both mut and wt K-ras expressing pancreatic carcinoma cells with AS K-ras PS ODN, but not MM or SC PS ODN, reduced K-ras p21 protein levels in both mut and wt K-ras cells by > 80% within 24 h (Fig. 3). This effect was observed with and without Ad.vec or Ad.mda-7 infection, which did not consistently cause a further alteration in K-ras levels (Fig. 3). Moreover, the growth inhibitory effect of the AS K-ras PS ODN was greater in the three mut K-ras pancreatic carcinoma cells than in the wt K-ras BxPC-3 cell line (Fig. 1). As observed in the study by Kita et al. (11), growth inhibition induced by AS K-ras PS ODN, either point or start codon specific (current study), exceeded that observed when using SC or MM PS ODN. In addition, no synergistic growth inhibitory effects or decreases in cell survival were apparent in Ad.mda-7 infected pancreatic carcinoma cells that were subsequently treated with SC or MM PS ODN. These results confirm a profound synergistic growth inhibitory effect specifically in mut K-ras pancreatic carcinoma cells after infection with Ad.mda-7 and treatment with AS K-ras PS ODN.
Figure 3.
AS K-ras PS ODN inhibits K-RAS protein synthesis in pancreatic carcinoma cells. Western blot analysis of K-RAS and EF-1α protein levels in cells treated with the various agents for 3 days. The concentration of MM, SC, and AS PS ODN was 0.5 μM, and the dose of virus was 100 pfu per cell.
Plasma membrane-associated small molecular weight GTP-binding proteins are frequently used by cells in the process of signal transduction from the inner leaflet of the plasma membrane to the cytosol. The prototypical small molecular weight family of GTP-binding proteins is the ras gene family (33). K-ras is a member of the ras gene family, which consists of three members, K-ras, H-ras, and N-ras (34). When activated, the RAS proteins contain a bound GTP molecule, whereas the inactive form contains GDP (35). The process of ras activation involves an exchange of bound GDP with GTP. A common occurrence in pancreatic and other cancers involves point mutations of K-ras, which involve codon 12 (predominantly in pancreatic carcinoma) and codons 13 and 61 in other cancers (2, 22, 23). Moreover, based on the observation that K-ras mutations appear in atypical hyperplastic ducts that surround the ductal-like cancer cells (36), it is currently believed that K-ras mutations represent a very early event in pancreatic carcinogenesis. The resulting K-ras mutation induces a conformational change in the molecule and a concomitant maintenance of ras activation by decreasing hydrolysis of GTP to GDP (33, 34). When activated, K-ras can signal into the cytosol via multiple downstream signaling pathways such as the classical mitogen-activated protein kinase (MAPK) pathway, the phosphatidylinositol 3-kinase (PI3-kinase) pathway, and the c-Jun NH2-terminal kinase pathway to induce plethora cellular changes, including enhanced proliferation (37–39). In these contexts, blocking K-ras expression may alter downstream pathway activities in mut K-ras pancreatic cancer cells, rendering these cells sensitive to mda-7 induction of growth suppression and effects on cell viability. This hypothesis is currently being tested.
Infection of mut K-ras Pancreatic Carcinoma Cells with Ad.mda-7 Followed by Transfection with an AS K-ras Expression Vector Inhibits Growth in Vitro and Tumorigenesis in Vivo in Nude Mice.
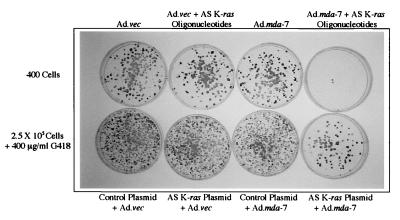
As an additional approach to inhibiting K-ras expression, a K-ras gene fragment of 346 nt (consisting of nt 172–517), which was previously shown to inhibit pancreatic cancer cell growth in vitro and in vivo when used in an AS orientation (9, 10), was isolated by PCR from BxPC-3 cells and cloned into a pcDNA3.1 (neomycin resistance) expression vector. This expression vector was then tested for effects on pancreatic carcinoma cells, when used alone or in combination with Ad.mda-7. As can be seen in Fig. 4 (Upper), infection of MIA PaCa-2 cells with Ad.vec alone or in combination with AS K-ras PS ODN did not significantly alter colony formation. Similarly, infection of MIA PaCa-2 cells with Ad.mda-7 also did not modify cloning efficiency in monolayer culture. In contrast, the combination of Ad.mda-7 with AS K-ras PS ODN dramatically inhibited colony formation (confirming previous studies using cell counting by hemocytometer and MTT staining). To test the effect of the AS K-ras plasmid in combination with Ad.mda-7 on pancreatic carcinoma cell growth, MIA PaCa-2 cells were infected with Ad.vec or Ad.mda-7 and transfected with a control or the AS K-ras plasmid, and G418-resistant colony formation was determined. As can be seen in Fig. 4 (Lower), a dramatic suppression in growth was only observed in MIA PaCa-2 cells infected with Ad.mda-7 and transfected with the AS K-ras plasmid. Qualitatively similar growth inhibitory results were obtained when the same protocols were used with AsPC-1 or PANC-1 mut K-ras pancreatic carcinoma cells but not with wt K-ras BxPC-3 cells (data not shown). These results indicate that both AS PS ODN and AS K-ras expression by plasmid transfer can synergize with mda-7 to inhibit mut K-ras pancreatic carcinoma cell growth.
Figure 4.
The combination of Ad.mda-7 plus AS K-ras PS ODN or AS K-ras plasmids synergistically inhibits colony formation in mut K-ras MIA PaCa-2 pancreatic carcinoma cells. (Upper) Effect of Ad.mda-7 plus AS K-ras PS ODN on MIA PaCa-2 colony formation. Cells were infected with 100 pfu per cell of Ad.vec or Ad.mda-7, treated with 0.5 μM AS K-ras PS ODN plus 10 μl of Lipofectamine, reseeded at a density of 400 cells per plate, and fixed and stained with Giemsa after 3 weeks. (Lower) Effect of Ad.mda-7 plus AS K-ras plasmid transfection on MIA PaCa-2 G418-resistant colony formation. Cells were infected with 100 pfu per cell of Ad.vec or Ad.mda-7, transfected with 10 μg of plasmid (either control pcDNA3.1 lacking insert or the pcDNA3.1 vector containing a 346-nt AS K-ras fragment), reseeded at a density of 2.5 × 105 cells per plate, and selected in 400 μg/ml G418, and G418-resistant colonies were fixed and stained with Giemsa after 3 weeks.
MIA PaCa-2 cells form tumors in athymic nude mice with a short latency time. Transfection with an AS K-ras plasmid or infection with Ad.mda-7 resulted in rapidly growing tumors in 80% of animals (three independent experiments; n = 26). Similarly, infection with an Ad.vec, a plasmid lacking the gene inserts, or transfection with a plasmid construct containing a 346-nt K-ras gene fragment cloned in a sense orientation did not significantly inhibit tumor formation (76% tumors; n = 17; three independent experiments). In contrast, a remarkable complete suppression in tumor formation was apparent only when MIA PaCa-2 cells were infected with Ad.mda-7 and then transfected with the AS K-ras plasmid before injecting into athymic nude mice (no tumors formed in 13 animals; three independent studies). These findings document that in mut K-ras pancreatic carcinoma cells, infection with Ad.mda-7 combined with targeting the K-ras gene for inhibition in a small subset of cells by means of transfection with an AS K-ras expression plasmid eliminates in vivo tumor formation in nude mice. Because transfection is an inherently inefficient means of introducing genes into target cells, it is possible that cells receiving the combination treatment release factor(s) that sensitize adjacent tumor cells containing mda-7 to lose viability, thereby preventing tumor formation. Further studies are necessary to mechanistically explain this provocative finding.
The Combination of Ad.mda-7 and AS K-ras PS ODN Induces Apoptosis Selectively in mut K-ras Expressing Human Pancreatic Carcinoma Cells.
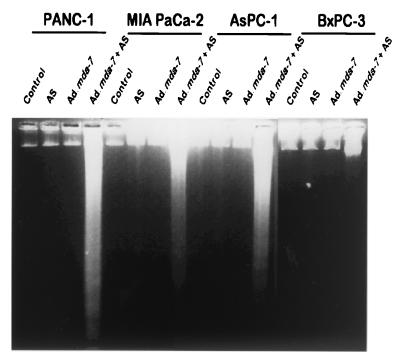
The mechanism by which mda-7 selectively decreases colony formation and growth in human cancer cells involves induction of apoptosis (18–21). To determine whether the combination treatment of K-ras mut pancreatic cancer cells decreases cell survival by induction of apoptosis, we have performed a number of assays typically used to monitor programmed cell death. In many cell types, induction of apoptosis is associated with DNA degradation, which can be monitored by generation of nucleosomal DNA ladders (19, 40, 41). As can be seen in Fig. 5, treatment of mut K-ras expressing pancreatic carcinoma cells, but not wt K-ras expressing BxPC-3, with Ad.mda-7 plus AS K-ras PS ODN results in DNA fragmentation. The specificity of this effect is further documented by the lack of nucleosomal DNA ladders in pancreatic cancer cells infected with Ad.mda-7 or treated with 5.0 μM AS K-ras PS ODN alone or in cells treated with the combination of Ad.mda-7 with 5.0 μM MM K-ras PS ODN (data not shown). Confirmation of induction of apoptosis by combination treatment in the three mut K-ras pancreatic carcinoma cells was verified by 4′,6-diamidino-2-phenylindole and by propidium iodide staining, increases in hypodiploid cells, and Annexin V staining by FACS analysis (data not shown). These results confirm that the combination of Ad.mda-7 and AS K-ras PS ODN decreases viability in mut K-ras expressing pancreatic carcinoma cells by inducing apoptosis.
Figure 5.
Ad.mda-7 plus AS K-ras PS ODN induce nucleosomal DNA degradation in K-ras mutant human pancreatic cancer cells. The indicated cell types were treated as indicated for 3 days. AS, 0.5 μM AS K-ras PS ODN; Ad.mda-7, 100 pfu per cell; Ad.mda-7 infected (100 pfu per cell) + 0.5 μM AS K-ras PS ODN. Nucleosomal ladder formation was determined as described (33).
MDA-7 Protein Is Present in mut K-ras-Expressing Human Pancreatic Carcinoma Cells Following Infection with Ad.mda-7 and Treatment with AS K-ras PS ODN.
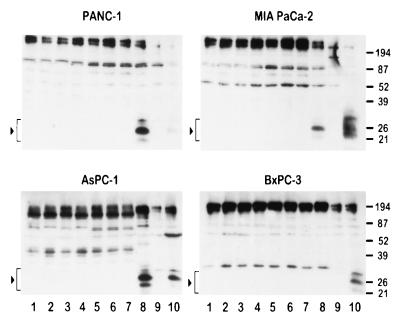
The reason pancreatic carcinoma cells are resistant to mda-7 and the mechanism by which the combination of Ad.mda-7 and AS K-ras PS ODN sensitizes specific pancreatic carcinoma cells to mda-7 induction of growth suppression and apoptosis are not known. One hypothesis is that the mut K-ras protein, or biochemical pathways modified by this protein, prevents synthesis, processing, and/or secretion of MDA-7 protein following infection with Ad.mda-7. To begin testing this possibility, we determined the effect of various treatment protocols on intracellular MDA-7 protein levels in the different pancreatic carcinoma cell lines (Fig. 6). No MDA-7 protein was detected in cell lysates from the four different pancreatic carcinomas 24 h after infection with Ad.mda-7 alone or in combination with MM or SC PS ODN. This occurred despite the production of mda-7 mRNA in all four pancreatic cancer cell lines following infection with Ad.mda-7 (Fig. 7). In contrast, MDA-7 protein was readily detected in the three K-ras mut pancreatic carcinoma cell lines after infection with Ad.mda-7 and treatment with AS K-ras PS ODN (Fig. 6). In the case of wt K-ras expressing BxPC-3, MDA-7 protein was not detected (Fig. 6). These results suggest that mut K-ras may negatively effect MDA-7 protein processing in mut K-ras pancreatic carcinoma cells. The absence of MDA-7 protein, using similar protocols, in BxPC-3 cells suggests that other pathways may be operational that modify expression and/or retention of MDA-7 protein in these pancreatic carcinoma cells. Because apoptosis only occurs in the combinatorial treated mut K-ras pancreatic carcinoma cells, the present studies demonstrate a potential correlation between presence/retention of MDA-7 protein and induction of growth suppression and programmed cell death in pancreatic carcinoma cells.
Figure 6.
MDA-7 protein is detected in mut K-ras pancreatic carcinoma cells infected with Ad.mda-7 and treated with AS K-ras PS ODN. The various cell lines were treated for 1 day as indicated: 1, control cells; 2, AS K-ras PS ODN; 3, Ad.vec; 4, Ad.vec + AS K-ras PS ODN; 5, Ad.mda-7; 6, Ad.mda-7 + MM PS ODN; 7, Ad.mda-7 + SC PS ODN; 8, Ad.mda-7 + AS K-ras PS ODN; 9, PC-3 human prostate carcinoma cells treated for 1 day with Ad.vec; and 10, PC-3 cells treated for 1 day with Ad.mda-7 (used as a positive control for MDA-7 expression). Lysates of treated cells were evaluated by Western blotting for MDA-7 and EF-1α protein as described (19, 24, 29). Arrowheads and brackets indicate MDA-7 proteins detected by Western blotting. The concentration of MM, SC, and AS PS ODN was 0.5 μM, and the dose of virus was 100 pfu per cell.
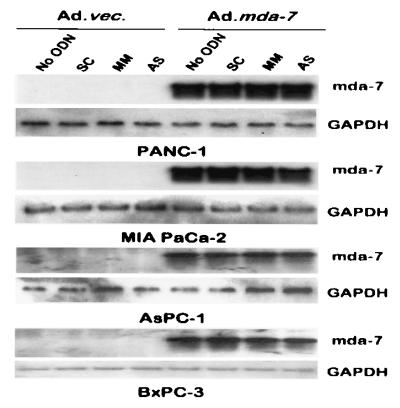
Figure 7.
Expression of mda-7 mRNA in Ad.mda-7 infected mut and wt K-ras pancreatic carcinoma cells. The indicated cell lines were treated for 3 days, and total mRNA was isolated and analyzed by Northern blotting for mda-7 and glyceraldehyde-3-phosphate dehydrogenase RNA. The concentration of SC, MM, and AS PS ODN was 0.5 μM, and the dose of virus was 100 pfu per cell.
The Combination of Ad.mda-7 and AS K-ras PS ODN Alters the Levels of Apoptosis-Associated Proteins.
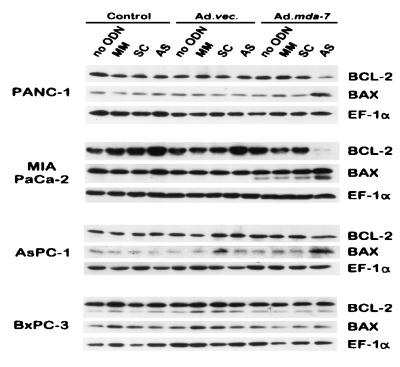
Previous studies indicate that infection of diverse cancer cells with Ad.mda-7 results in apoptosis, and in the majority of cases this process is associated with up-regulation of BAX protein and changes in the ratio of BAX to BCL-2 protein (18–21). However, the ability of Ad.mda-7 to induce apoptosis in specific cancer cells, such as DU-145 human prostate carcinoma cells that do not produce BAX protein (42), indicates that mda-7 can also mediate programmed cell death in certain cancer cells by a Bax-independent pathway (18, 21). Based on these considerations and the presence of MDA-7 protein specifically in combination-treated mut K-ras pancreatic carcinoma cells, experiments were performed to determine the levels of BAX and BCL-2 proteins in treated cells. When analyzed 3 days after combination treatment, in which the majority of K-ras mut cells were apoptotic, the levels of BAX protein were elevated in PANC-1, MIA PaCa-2, and AsPC-1 cells, ≈7.5-, ≈3-, and ≈10-fold, respectively, but not in BxPC-3 cells (Fig. 8). Moreover, the levels of BCL-2 protein were significantly reduced in PANC-1 (≈8-fold) and MIA PaCa-2 (≈13.5-fold) cells, marginally reduced in AsPC-1 cells (≈1.2-fold), and remained unchanged in BxPC-3 cells (Fig. 8). These results support a potential involvement of BAX protein and changes in the ratio of BAX to BCL-2 proteins in inducing apoptosis in combination-treated pancreatic carcinoma cells. Although further studies are necessary to define possible roles of additional apoptosis modulating molecules, the changes presently observed in BAX and the ratio of BAX to BCL-2 proteins are anticipated to induce downstream events, including induction of cytochrome c release from mitochondria and caspase activation (40, 41, 43), culminating in programmed cell death.
Figure 8.
Expression of BAX, BCL-2, and EF-1α proteins in pancreatic carcinoma cells after various treatment protocols. The different cell lines were treated for 3 days as indicated, and the levels of the respective proteins were determined by using 30 μg of total protein lysates by Western blotting using the respective antibodies as described (19, 24, 29). The concentration of MM, SC, and AS PS ODN was 0.5 μM, and the dose of virus was 100 pfu per cell.
Summary and Future Perspectives.
The present studies illustrate a fascinating phenomenon with potentially important clinical implications for the therapy of pancreatic cancer. A combinatorial approach, forced expression of the cancer growth-suppressing and apoptosis-inducing gene mda-7 plus targeted inhibition of K-ras, results in growth suppression in vitro and in vivo in athymic nude mice and programmed cell death in mut K-ras expressing human pancreatic carcinoma cells. These observations suggest future avenues for investigation that offer potential for providing effective therapeutic approaches for this invariably fatal human cancer. These include targeting K-ras suppression by using a viral (or nonviral) delivery system to deliver an AS K-ras gene construct or AS PS ODN in combination with Ad.mda-7 and applying Ad.mda-7 together with agents that inhibit downstream mut K-ras activated genes and/or biochemical pathways.
Previous studies indicate that mutation-specific AS K-ras PS ODN, an AS wt K-ras plasmid, and a virus encoding a dominant negative H-ras mutant can inhibit pancreatic carcinoma cell growth in vitro (9–13). In addition, both an AS wt K-ras plasmid and an Ad expressing a dominant negative H-ras mutant can also inhibit human pancreatic carcinoma tumorigenesis and metastasis in vivo when tested in nude mouse human pancreatic cancer models (9, 10, 12, 13). These findings and the present AS K-ras transfection results suggest that either two viruses, one expressing Ad.mda-7 and one expressing AS wt K-ras, or a recombinant bipartite Ad expressing both mda-7 and AS wt K-ras could prove beneficial for inducing pancreatic carcinoma cell death. Because the AS K-ras plasmid sequence that shows suppressive effects in mutant pancreatic carcinoma cells does not show effects in wt K-ras pancreatic cancer cells, or nonspecific toxicity in animal models, this sequence incorporated into an Ad vector should provide a means of altering K-ras levels in mut K-ras pancreatic carcinoma cells. Moreover, the present studies indicate that infection with Ad.mda-7 and then transfection with Ad.K-ras AS induces a similar synergistic growth inhibitory effect as observed with Ad.mda-7 plus AS K-ras PS ODN. In this context, of even greater clinical potential as a therapeutic reagent, an Ad that expresses both mda-7 and AS K-ras should prove more efficacious with less potential toxicity than the use of two separate viruses. Studies are presently in progress to produce these viruses and test this hypothesis.
The ability of the combination of mda-7 and AS K-ras PS ODN to synergistically inhibit growth and promote apoptosis in mut K-ras pancreatic carcinoma cells probably occurs because K-ras expression is inhibited, thereby altering downstream signaling pathway functions, resulting in gene expression changes. Because primary targets of activated K-ras are likely to be the MAPK, PI3-kinase, and c-Jun NH2-terminal kinase pathways, it may be possible, by directly altering the functions of these signaling cascades, to sensitize pancreatic carcinoma cells to mda-7-induced biological effects. Furthermore, because several studies have argued for cytoprotective signaling via MAPK and PI3-kinase (44–46), further investigations are planned to selectively target the MAPK and PI3-kinase pathways for inhibition, with appropriate pharmacological agents, and determine whether this renders pancreatic carcinoma cells responsive to induction of apoptosis by mda-7. If effective, this strategy would also provide a potentially powerful methodology for treating pancreatic cancer.
Acknowledgments
The present studies were supported in part by National Institutes of Health Grants CA35675, CA37670, CA74468, and DK52825, Department of Defense Grant BC98-0148, an award from the Samuel Waxman Cancer Foundation, and the Chernow Endowment. P.B.F. is a Michael and Stella Chernow Urological Cancer Research Scientist.
Abbreviations
- mda
melanoma differentiation-associated
- PS ODN
phosphorothioate oligodeoxynucleotides
- AS
antisense
- MM
mismatched
- SC
scrambled
- wt
wild type
- mut
mutant
- Ad
adenovirus
- MAPK
mitogen-activated protein kinase
- PI3-kinase
phosphatidylinositol 3-kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
Footnotes
See commentary on page 10028.
References
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta: ACS; 2001. [Google Scholar]
- 2.Hilgers W, Kern S E. Genes Chromosomes Cancer. 1999;26:1–12. [PubMed] [Google Scholar]
- 3.Regine W F, John W J, Mohiuddin M. Front Biosci. 1998;3:E186–E192. doi: 10.2741/a376. [DOI] [PubMed] [Google Scholar]
- 4.Blaszkowsky L. Front Biosci. 1998;3:E214–E225. doi: 10.2741/a380. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz M, Heinrich S, Staib-Sebler E, Kohne C-H, Wils J, Nordlinger B, Encke A. Eur J Cancer. 2000;36:957–965. doi: 10.1016/s0959-8049(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg L. Drugs. 2000;59:1071–1089. doi: 10.2165/00003495-200059050-00004. [DOI] [PubMed] [Google Scholar]
- 7.Perugini R A, McDade T P, Vittimberga F J, Jr, Callery M P. Crit Rev Eukaryotic Gene Expression. 1998;8:377–393. doi: 10.1615/critreveukargeneexpr.v8.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 8.Friess H, Kleeff J, Korc M, Buchler M W. Dig Surg. 1999;16:281–290. doi: 10.1159/000018737. [DOI] [PubMed] [Google Scholar]
- 9.Aoki Y, Yoshida T, Sugimura T, Terada M. Cancer Res. 1995;55:3810–3816. [PubMed] [Google Scholar]
- 10.Aoki Y, Yoshida T, Matsumoto N, Ide H, Sugimura T, Terada M. Mol Carcinogen. 1997;20:251–258. [PubMed] [Google Scholar]
- 11.Kita K-I, Saito S, Morioka C Y, Watanabe A. Int J Cancer. 1999;80:553–558. doi: 10.1002/(sici)1097-0215(19990209)80:4<553::aid-ijc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Shichionohe T, Senmaru N, Furuuchi K, Ogiso Y, Ishikura H, Yoshiki T, Takahashi T, Katoh H, Kuzumaki N. J Surg Res. 1996;142:63–71. doi: 10.1006/jsre.1996.0383. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi M, Shichionohe T, Senmaru N, Miyamoto M, Fujita H, Takimoto M, Kondo S, Katoh H, Kuzumaki N. Gene Ther. 2000;7:518–526. doi: 10.1038/sj.gt.3301125. [DOI] [PubMed] [Google Scholar]
- 14.Fisher P B, Prignoli D R, Hermo H, Jr, Weinstein I B, Pestka S. J Interferon Res. 1985;5:11–22. doi: 10.1089/jir.1985.5.11. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Fisher P B. Mol Cell Differ. 1993;1:285–299. [Google Scholar]
- 16.Jiang H, Lin J J, Su Z-z, Goldstein N I, Fisher P B. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- 17.Jiang H, Su Z-z, Lin J J, Goldstein N I, Young C S H, Fisher P B. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madireddi M T, Su Z-z, Young C S H, Goldstein N I, Fisher P B. Adv Exp Med Biol. 2000;465:239–261. doi: 10.1007/0-306-46817-4_22. [DOI] [PubMed] [Google Scholar]
- 19.Su Z-z, Madireddi M T, Lin J J, Young C S H, Kitada S, Reed J C, Goldstein N I, Fisher P B. Proc Natl Acad Sci USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saeki T, Mhashilkar A, Chada S, Branch C, Roth J A, Ramesh R. Gene Ther. 2000;7:2051–2057. doi: 10.1038/sj.gt.3301330. [DOI] [PubMed] [Google Scholar]
- 21.Mhashilkar A B, Schrock R D, Hindi M, Liao J, Sieger K, Kourouma F, Zou-Yang X H, Onishi E, Takh O, Vedvick T S, et al. Mol Med. 2001;7:271–282. [PMC free article] [PubMed] [Google Scholar]
- 22.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 23.Longnecker D S, Terhune P G. Pancreas. 1998;17:323–324. doi: 10.1097/00006676-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Lebedeva I V, Rando R, Ojwang J, Cossum P, Stein C A. Cancer Res. 2000;60:6052–6060. [PubMed] [Google Scholar]
- 25.Volkert F C, Young C S H. Virology. 1983;125:175–193. doi: 10.1016/0042-6822(83)90072-7. [DOI] [PubMed] [Google Scholar]
- 26.Sakakura C, Hagiwara A, Tsujimoto H, Ozaki K, Sakakibara T, Oyama T, Ogaki M, Imanishi T, Yamazaki J, Takahashi T. Anticancer Drugs. 1995;6:553–561. doi: 10.1097/00001813-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Su Z-z, Lin J, Prewett M, Goldstein N I, Fisher P B. Anticancer Res. 1995;15:1841–1848. [PubMed] [Google Scholar]
- 28.Martin J S, Reutelingsperger C P M, McGahon A J, Rader J A, van Schie RCAA, LaFace D M, Green D R. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su Z-z, Yemul S, Estabrook A, Zimmer S G, Friedman RM, Fisher P B. Int J Oncol. 1995;7:1279–1284. doi: 10.3892/ijo.7.6.1279. [DOI] [PubMed] [Google Scholar]
- 30.Stein C A, Cheng Y C. Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- 31.Stein C A. Trends Biotechnol. 1996;14:147–149. doi: 10.1016/0167-7799(96)20006-X. [DOI] [PubMed] [Google Scholar]
- 32.Pawlak W, Zolnierek J, Sarosiek T, Szczylik C. Cancer Treat Rev. 2000;26:333–350. doi: 10.1053/ctrv.2000.0173. [DOI] [PubMed] [Google Scholar]
- 33.Kolch W. Biochem J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 34.Reuther G W, Der C J. Curr Opin Cell Biol. 2000;12:157–165. doi: 10.1016/s0955-0674(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J. Nature (London) 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- 36.Lemoine N R, Jain S, Hughes C M, Staddon S L, Maillet B, Hall P A, Kloppel G. Gastroenterology. 1992;102:230–236. doi: 10.1016/0016-5085(92)91805-e. [DOI] [PubMed] [Google Scholar]
- 37.Dent P, Haser W, Haystead T A J, Vincent L A, Roberts T M, Sturgill T W. Science. 1992;257:1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- 38.Gire V, Marshall C, Wynford-Thomas D. Oncogene. 2000;19:2269–2276. doi: 10.1038/sj.onc.1203544. [DOI] [PubMed] [Google Scholar]
- 39.Almeida E A, Ilic D, Han Q, Hauck C R, Jin F, Kawakatsu H, Schlaepfer D D, Damsky C H. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed J C. Am J Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green D R, Reed J C. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 42.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed J C, Perucho M. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 43.Gross A, McDonnell J M, Korsmeyer S J. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 44.Dent P, Jarvis W D, Birrer M J, Fisher P B, Schmidt-Ullrich R K, Grant S. Leukemia. 1998;12:400–408. doi: 10.1038/sj.leu.2401222. [DOI] [PubMed] [Google Scholar]
- 45.Dent P, Reardon D B, Park J S, Bowers G, Logsdon C, Valerie K, Schmidt-Ullrich R K. Mol Biol Cell. 1999;10:2493–2506. doi: 10.1091/mbc.10.8.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue L, Murray J H, Tolkovsky A M. J Biol Chem. 2000;275:8817–8824. doi: 10.1074/jbc.275.12.8817. [DOI] [PubMed] [Google Scholar]